Air Temperature Intermittency and Photofragment Excitation
Abstract
1. Introduction
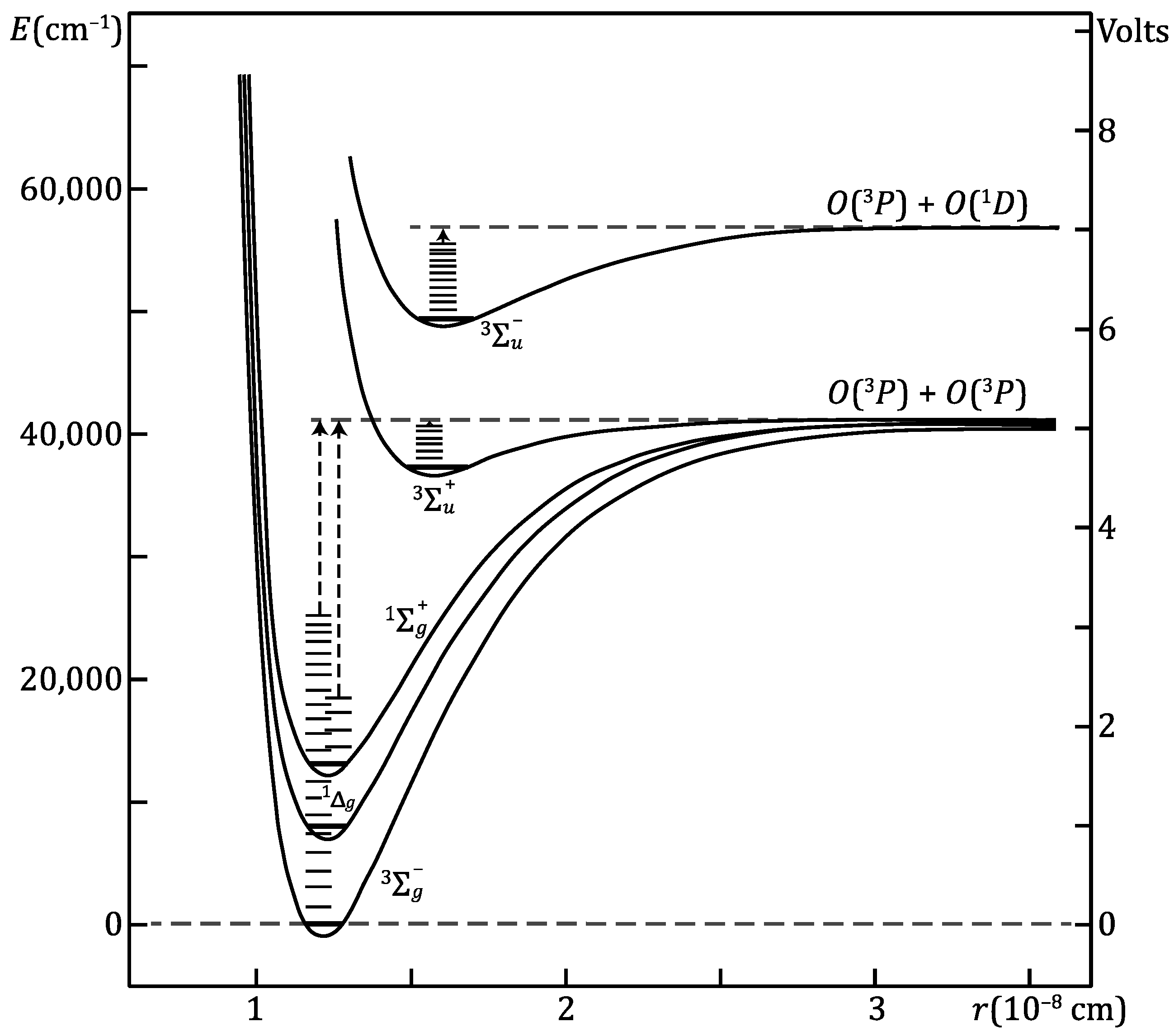
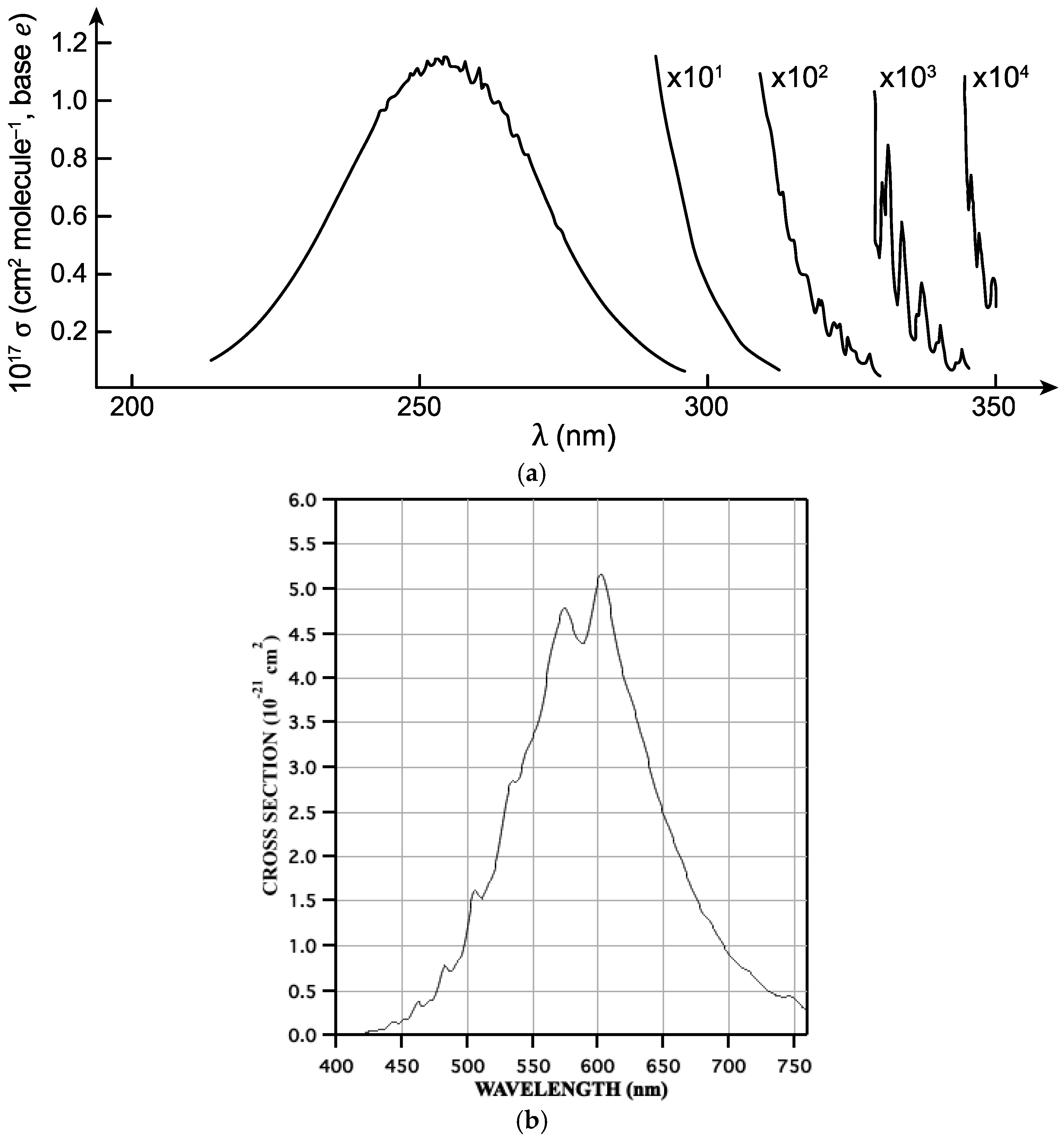
2. Photodissociation
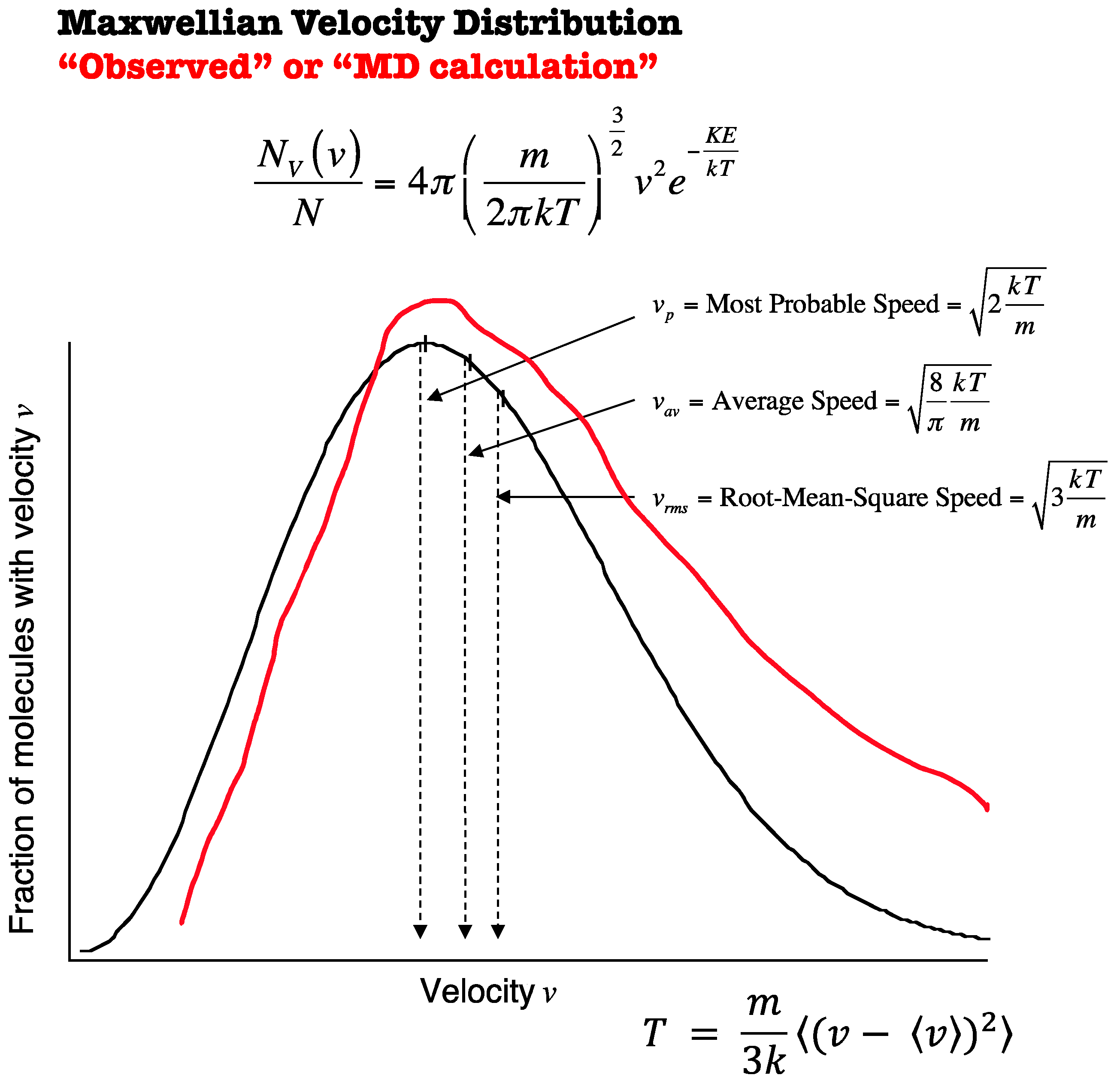
3. Temperature
4. Atmospheric Ozone
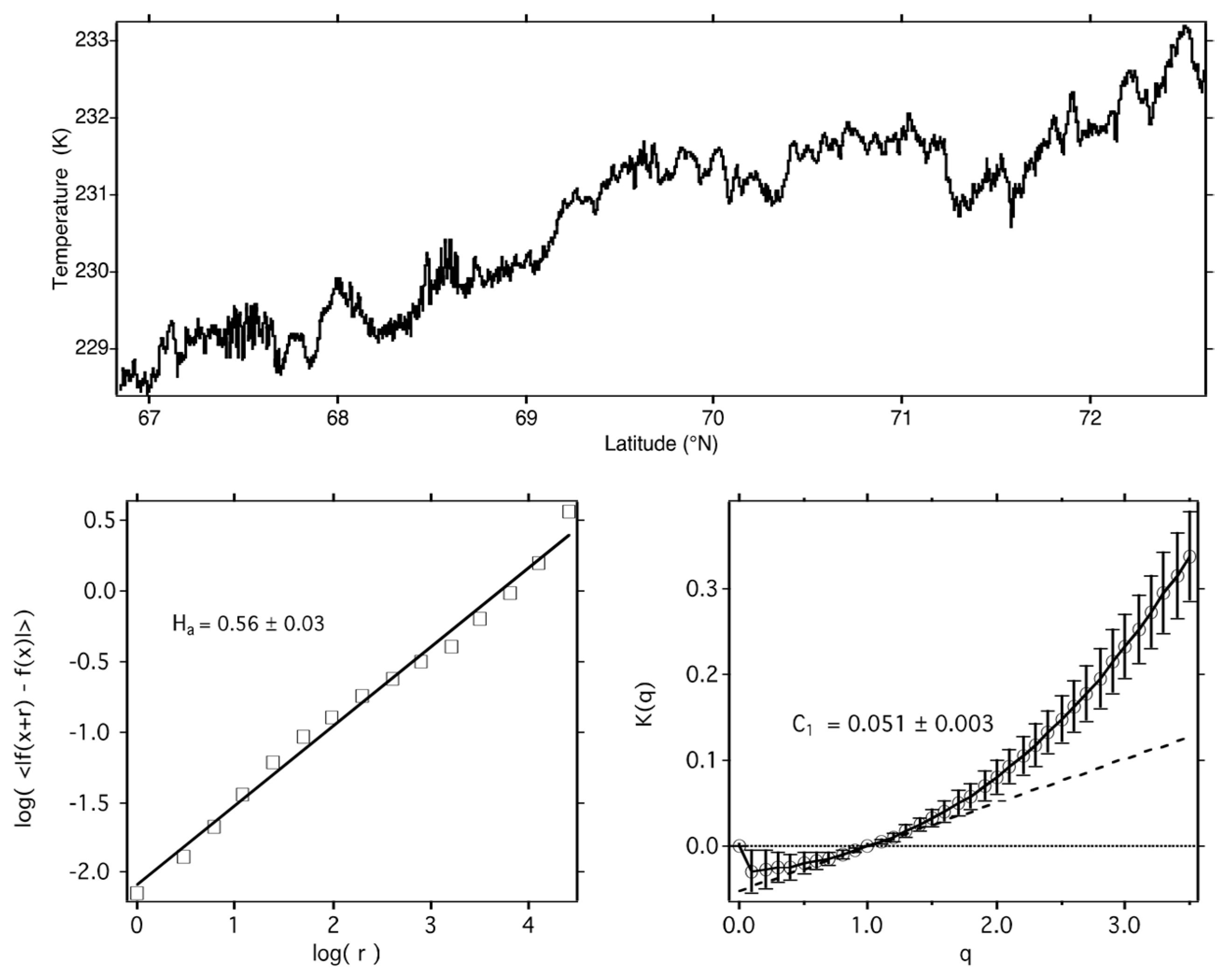
5. Statistical Multifractals
6. Aircraft Observations
7. Fluid Mechanical Effects
8. Langevin Equation
9. Chemical and Radiative Consequences
9.1. Isotope Distributions
9.2. Chemical Kinetics Effects
9.3. Radiative Effects
10. Meteorological Implications
11. Observational Tests
11.1. Laboratory Tests of Photodissociation in Nonequilibrium Air
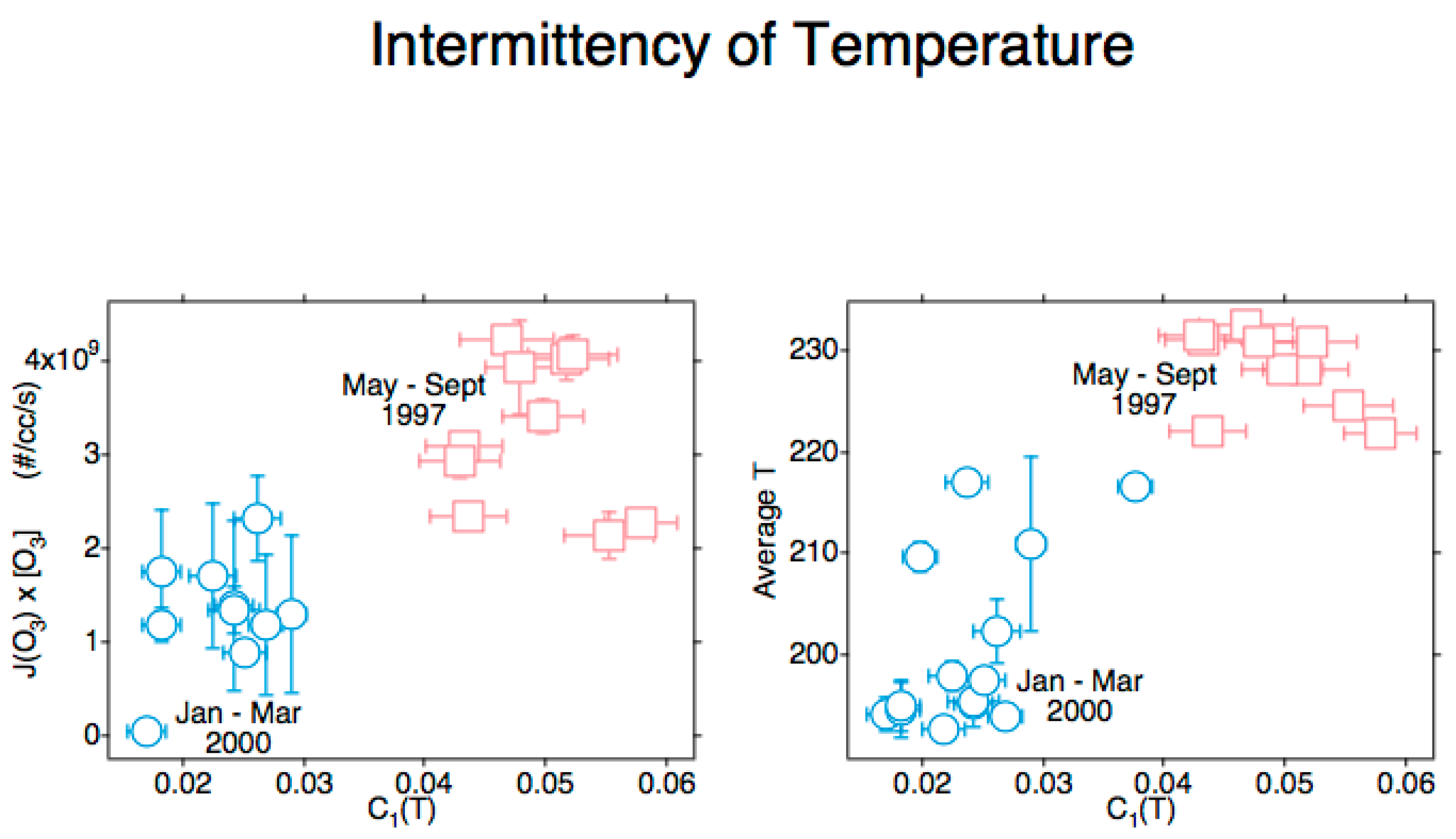
11.2. Airborne Observational Tests of the J[O3] vs. C1(T) Correlation
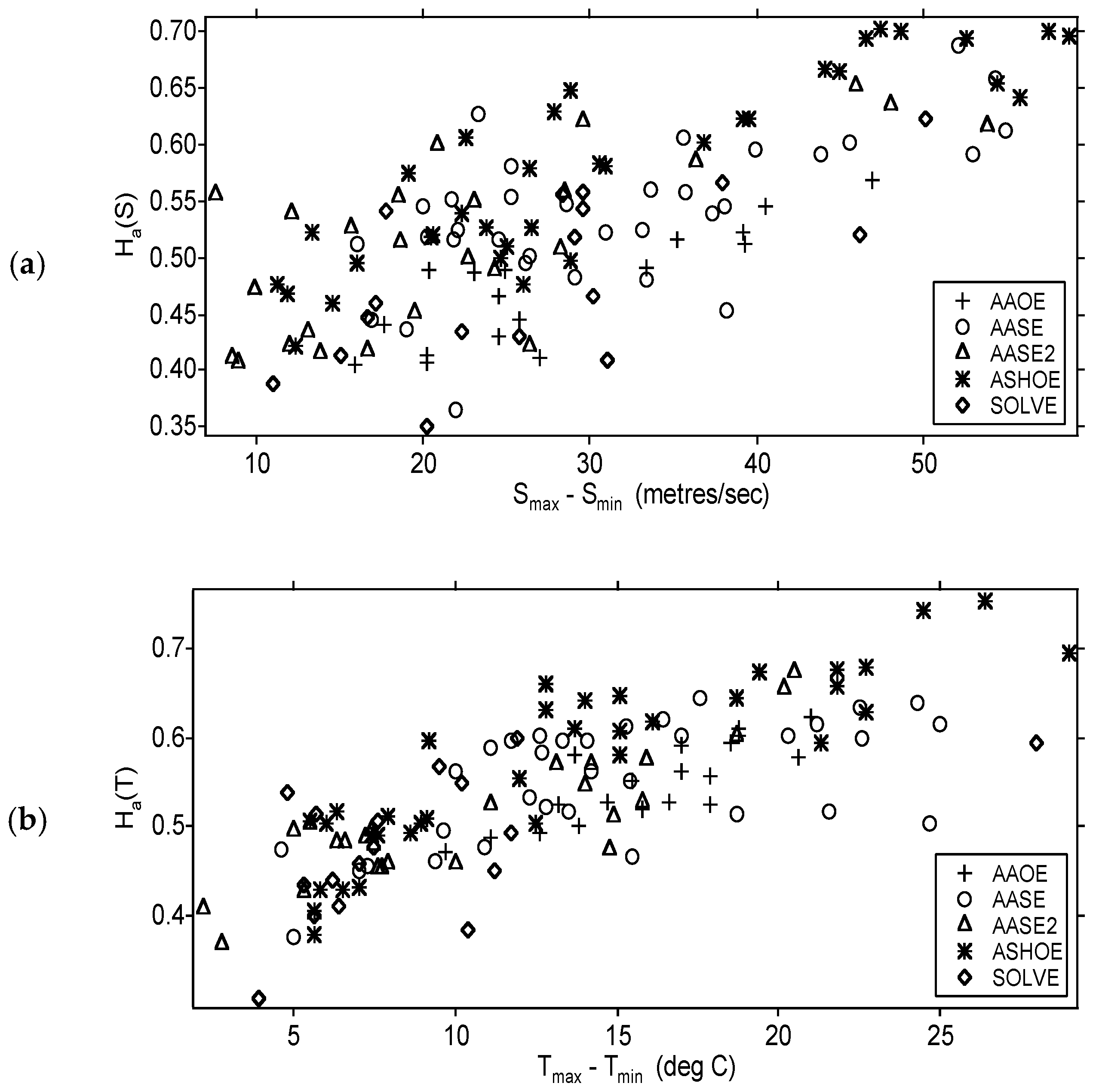
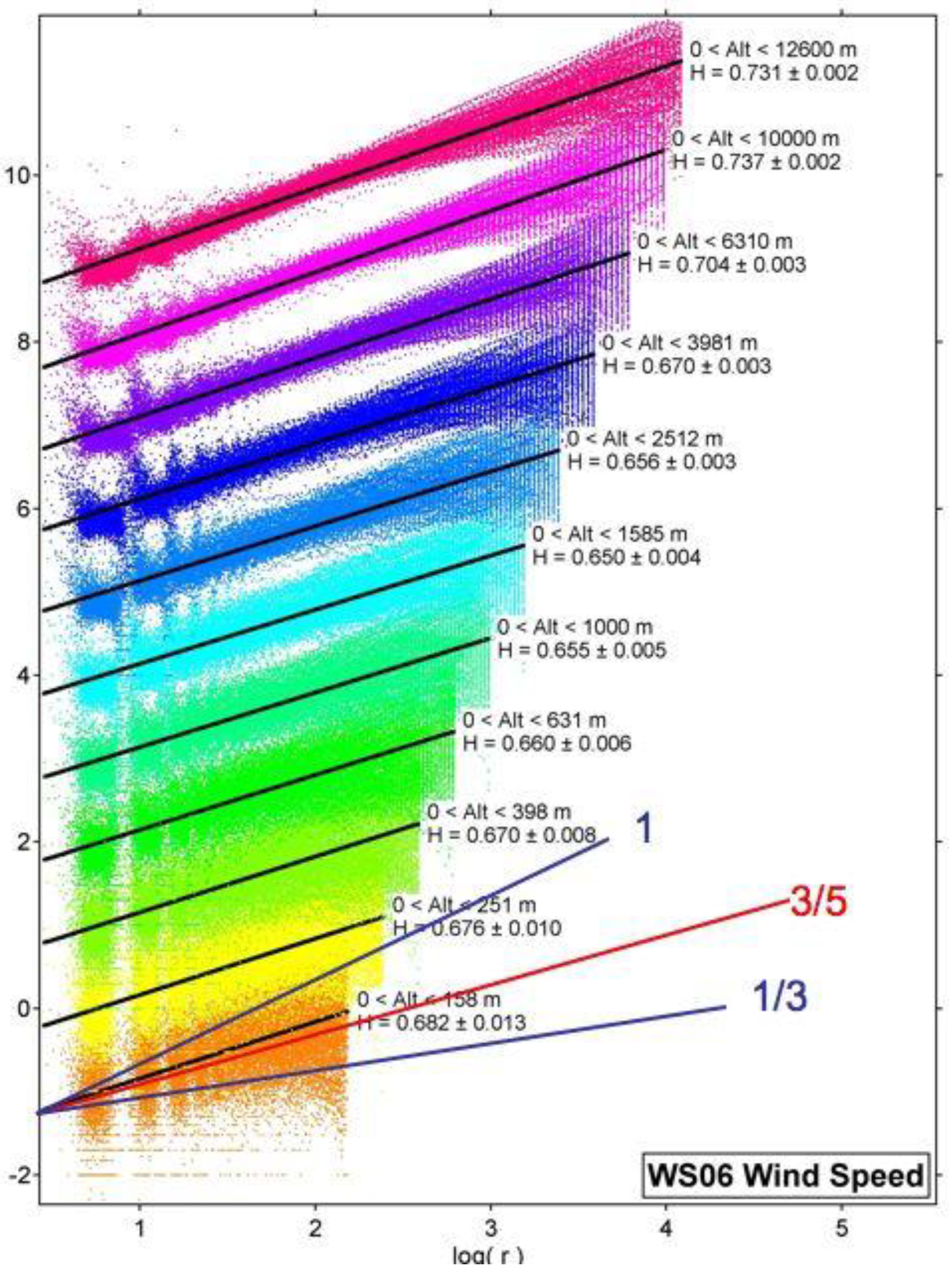
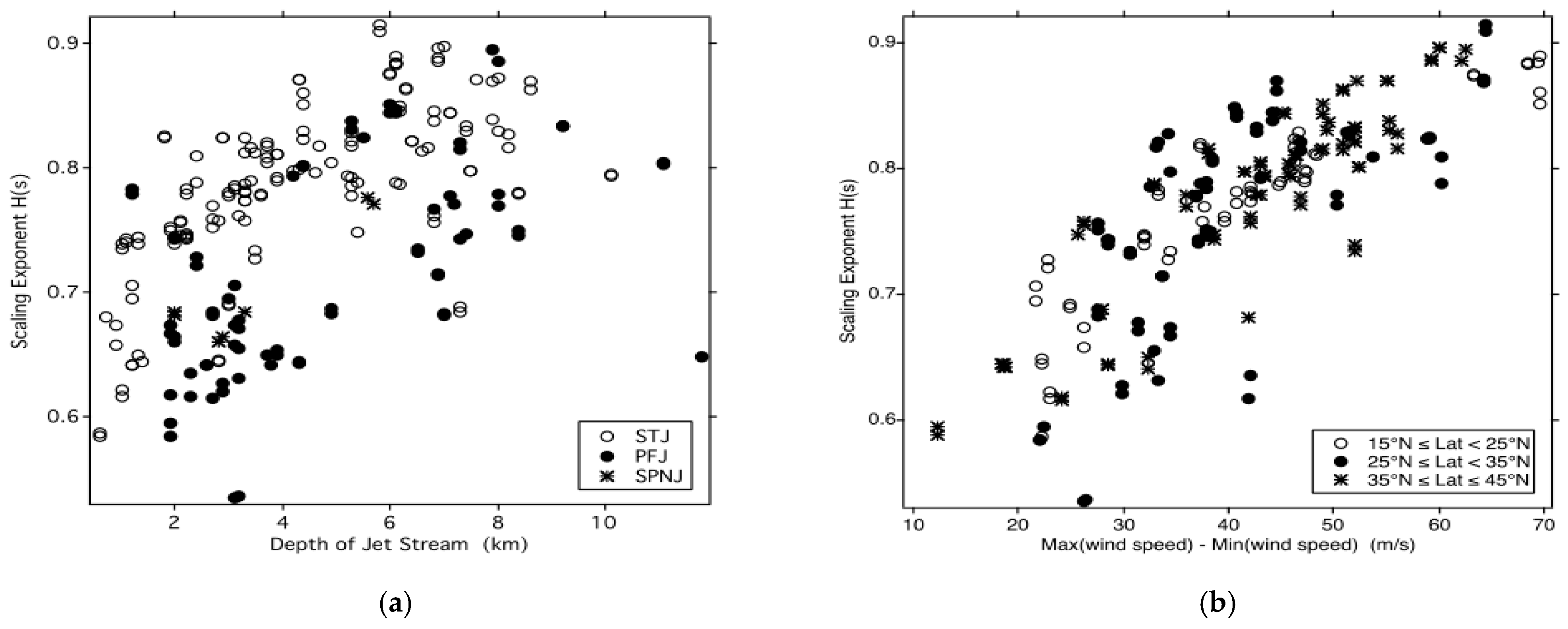
11.3. Airborne Observations of H(s) and H(T) along and across Jet Streams
11.4. Translational and Rotational Energies of N2 and O2
12. Discussion and Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Zare, R.N. Molecular Fluorescence and Photodissociation. Ph.D. Thesis, Harvard University, Cambridge, MA, USA, 1964. [Google Scholar]
- Zare, R.N.; Herschbach, D.R. Atomic and molecular fluorescence excited by photodissociation. Appl. Opt. 1965, 4, 193–200. [Google Scholar] [CrossRef][Green Version]
- Busch, G.E.; Wilson, K.R. Triatomic photofragment spectra 1. Energy partitioning in NO2 photodissociation. J. Chem. Phys. 1972, 56, 3626–3635. [Google Scholar] [CrossRef]
- Tuck, A.F. Molecular beam studies of ethyl nitrite photodissociation. J. Chem. Soc. Farad. Trans. II 1977, 73, 689–708. [Google Scholar] [CrossRef]
- Schinke, R. Photodissociation Dynamics: Spectroscopy and Fragmentation of Small Polyatomic Molecules; Cambridge University Press: Cambridge, UK, 1993. [Google Scholar]
- Barnett, J.J.; Houghton, J.T.; Pyle, J.A. The temperature dependence of the ozone concentration near the stratopause. Q. J. R. Meteorol. Soc. 1975, 101, 245–257. [Google Scholar] [CrossRef]
- Farman, J.C.; Hamilton, R.A. Measurements of Atmospheric Ozone at the Argentine Islands and Halley Bay, 1957–1972; British Antarctic Survey Atmospheric Sciences Division: Edinburgh, UK, 1975. [Google Scholar]
- Tuck, A.F. Numerical model studies of the effect of injected nitrogen oxides on stratospheric ozone. Proc. R. Soc. Lond. A 1977, A355, 267–299. [Google Scholar]
- Groves, K.S.; Mattingly, S.R.; Tuck, A.F. Increased atmospheric carbon dioxide and stratospheric ozone. Nature 1978, 273, 711–715. [Google Scholar] [CrossRef]
- Haigh, J.D.; Pyle, J.A. A two-dimensional calculation including atmospheric carbon dioxide and stratospheric ozone. Nature 1979, 279, 222–224. [Google Scholar] [CrossRef]
- Groves, K.S.; Tuck, A.F. Simultaneous effects of CO2 and chlorofluoromethanes on stratospheric ozone. Nature 1979, 280, 127–129. [Google Scholar] [CrossRef]
- Groves, K.S.; Tuck, A.F. Stratospheric O3—CO2 coupling in a photochemical-radiative column model: With chlorine chemistry. Q. J. R. Meteorol. Soc. 1980, 106, 141–157. [Google Scholar] [CrossRef]
- Haigh, J.D.; Pyle, J.A. Ozone perturbation experiments in a two-dimensional circulation model. Q. J. R. Meteorol. Soc. 1982, 108, 551–574. [Google Scholar] [CrossRef]
- Crutzen, P.J.; Schmailzl, U. Chemical budgets of the stratosphere. Planet. Space Sci. 1983, 31, 1009–1032. [Google Scholar] [CrossRef]
- Froidevaux, L.; Allen, M.; Yung, Y.L. A critical analysis of ClO and O3 in the mid-latitude stratosphere. J. Geophys. Res. 1985, 90, 12999–13029. [Google Scholar] [CrossRef]
- Austin, J.; Pallister, R.C.; Pyle, J.A.; Tuck, A.F.; Zavody, A.M. Photochemical model comparisons with LIMS observations in a stratospheric trajectory coordinate system. Q. J. R. Meteorol. Soc. 1987, 113, 361–392. [Google Scholar] [CrossRef]
- Logan, J.A.; McElroy, M.B. Distribution functions for energetic oxygen atoms in the earth’s lower atmosphere. Planet Space Sci. 1977, 25, 117–122. [Google Scholar] [CrossRef]
- Anderson, J.G. The absolute concentration of O(3P) in the earth’s stratosphere. Geophys. Res. Lett. 1975, 2, 231–234. [Google Scholar] [CrossRef]
- Anderson, J.G. The stratosphere1981: Theory and measurements. In WMO Global Ozone Research and Monitoring Project No.11; World Meteorological Organization: Geneva, Switzerland, 1982; pp. 1–60. [Google Scholar]
- Miller, R.L.; Suits, A.G.; Houston, P.L.; Toumi, R.; Mack, J.A.; Wodtke, A.M. The ozone deficit problem: O2(X, v≥26) + O(3P) from 226 nm ozone photodissociation. Science 1994, 265, 1831–1838. [Google Scholar] [CrossRef]
- Sakazaki, T.; Fujiwara, M.; Mitsuda, C.; Imai, K.; Manag, N.; Naito, Y.; Nakamura, T.; Akiyoshi, H.; Kinnison, D.; Sano, T.; et al. Diurnal ozone variations in the stratosphere revealed in observations from the Superconducting Submillimeter-Wave Limb-Emission Sounder (SMILES) on board the International Space Station (ISS). J. Geophys. Res. 2013, 118, 2991–3006. [Google Scholar] [CrossRef]
- Sauvageat, E.; Klemens, H.; Maillard Barras, E.; Shenyi, H.; Errera, Q.; Haefele, A.; Murk, A. Microwave radiometer observations of the ozone diurnal cycle and its short-term variability over Switzerland. Atmos. Chem. Phys. 2023, 23, 7321–7345. [Google Scholar] [CrossRef]
- Tuck, A.F.; Hovde, S.J.; Richard, E.C.; Gao, R.-S.; Bui, T.P.; Swartz, W.H.; Lloyd, S.A. Molecular velocity distributions and generalized scale invariance in the turbulent atmosphere. Faraday Discuss 2005, 130, 181–193. [Google Scholar] [CrossRef]
- Tuck, A.F. Scaling up: Molecular to meteorological via symmetry breaking and statistical multifractality. Meteorology 2022, 1, 4–28. [Google Scholar] [CrossRef]
- Marlton, G.; Charlton-Perez, A.; Harrison, G.; Polichtchouk, I.; Hauchecorne, A.; Keckhut, P.; Wing, R.; Leblanc, T.; Steinbrecht, W. Using a network of temperature lidars to identify temperature biases in the upper stratosphere in ECMWF reanalyses. Atmos. Chem. Phys. 2021, 21, 6079–6092. [Google Scholar] [CrossRef]
- Thiemens, M.H.; Jackson, T.; Zipf, E.C.; Erdman, P.W.; Van Egmond, C. Carbon dioxide and oxygen isotope anomalies in the mesosphere and stratosphere. Science 1995, 270, 969–972. [Google Scholar] [CrossRef]
- Herzberg, G. Molecular Structure and Molecular Spectra. III. Electronic Spectra and Electronic Structure of Polyatomic Molecules; Van Nostrand: Princeton, NJ, USA, 1967. [Google Scholar]
- Herzberg, G. Molecular Structure and Molecular Spectra. I. Spectra of Diatomic Molecules, 2nd ed.; Van Nostrand: Princeton, NJ, USA, 1950. [Google Scholar]
- Finlayson-Pitts, B.J.; Pitts, J.N., Jr. Chemistry of the Upper and Lower Atmosphere; Academic Press: San Diego, CA, USA, 1999. [Google Scholar]
- Wayne, R.P. The photochemistry of ozone. Atmos. Environ. 1987, 21, 1683–1694. [Google Scholar] [CrossRef]
- Shamsuddin, S.M.; Inagaki, Y.; Matsumi, Y.; Kawasaki, M. O(3Pj) atom formation from photodissociation of ozone in the visible and ultraviolet region. Can. J. Chem. 1994, 72, 637–642. [Google Scholar] [CrossRef]
- Baloitcha, E.; Balint-Kurti, G.G. The theory of the photodissociation of ozone in the Hartley continuum: Effect of vibrational excitation and O(1D) atom velocity distribution. Phys. Chem. Chem. Phys. 2005, 7, 3829–3833. [Google Scholar] [CrossRef]
- Tuck, A.F. Atmospheric Turbulence: A Molecular Dynamics Perspective; Oxford University Press: Oxford, UK, 2008. [Google Scholar]
- Tuck, A.F. Proposed empirical entropy and Gibbs energy based on observations of scale invariance in open nonequilibrium systems. J. Phys. Chem. A 2017, 121, 6620–6629. [Google Scholar] [CrossRef]
- Lovejoy, S.; Schertzer, D. The Weather and Climate: Emergent Laws and Multifractal Cascades; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- Austin, J.; Horowitz, L.W.; Schwarzkopf, M.D.; Wilson, R.J.; Levy II, H. Stratospheric ozone and temperature simulated from the preindustrial era to the present day. J. Clim. 2013, 26, 3528–3543. [Google Scholar] [CrossRef]
- Tuck, A.F.; Browell, E.V.; Danielsen, E.F.; Holton, J.R.; Hoskins, B.J.; Johnson, D.R.; Kley, D.; Krueger, A.J.; Mégie, G.; Newell, R.E.; et al. Stratosphere-Troposphere Exchange, Chapter 5. In Atmospheric Ozone 1985; World Meteorological Organization: Geneva, Switzerland, 1986. [Google Scholar]
- Bamber, D.J.; Healey, P.G.W.; Jones, B.M.R.; Penkett, S.A.; Tuck, A.F.; Vaughan, G. Vertical profiles of tropospheric gases: Chemical consequences of stratospheric intrusions. Atmos. Environ. 1984, 18, 1759–1766. [Google Scholar] [CrossRef]
- Parrish, D.D.; Derwent, R.G.; Steinbrecht, W.; Stübi, R.; Van Malderen, R.; Steinbacher, M.; Trickl, T.; Ries, L.; Xu, X. Zonal similarity of long-term changes and seasonal cycles of baseline ozone at northern midlatitudes. J. Geophys.Res. Atmos. 2020, 125, e2019JD031908. [Google Scholar] [CrossRef]
- Schertzer, D.; Lovejoy, S. Physical modeling and analysis of rain and clouds by multiplicative processes. J. Geophys. Res. Atmos. 1987, 92, 9693–9714. [Google Scholar] [CrossRef]
- Batchelor, G.K.; Townsend, A.A. The nature of turbulent wave motion at large wavenumbers. Proc. R. Soc. Lond. A 1949, 199, 238–255. [Google Scholar]
- Newman, P.A.; Fahey, D.W.; Brune, W.H.; Kurylo, M.J. Preface. J. Geophys. Res. Atmos. 1999, 104, 481–495. [Google Scholar]
- Proffitt, M.H.; Steinkamp, M.J.; Powell, J.A.; McLaughlin, R.J.; Mills, O.A.; Schmeltekopf, A.L.; Thompson, T.L.; Tuck, A.F.; Tyler, T.; Winkler, R.H.; et al. In situ ozone measurements within the 1987 Antarctic ozone hole from a high-altitude ER-2 aircraft. J. Geophys. Res. 1989, 94, 16547–16555. [Google Scholar] [CrossRef]
- Richard, E.C.; Aikin, K.C.; Andrews, A.E.; Daube, B.C., Jr.; Gerbig, C.; Wofsy, S.C.; Romashkin, P.A.; Hurst, D.F.; Ray, E.A.; Moore, F.L.; et al. Severe chemical ozone loss inside the Arctic polar vortex during winter 1999–2000 inferred from in situ airborne measurements. Geophys. Res. Lett. 2001, 28, 2197–2200. [Google Scholar] [CrossRef]
- McElroy, C.T. A spectroradiometer for the measurement of direct and scattered solar irradiance from on-board the NASA ER-2 high-altitude research aircraft. Geophys. Res. Lett. 1995, 22, 1361–1364. [Google Scholar] [CrossRef]
- Chan, K.R.; Scott, S.G.; Bui, T.P.; Bowen, S.W.; Day, J. Temperature and horizontal wind measurements on the ER-2 aircraft during the 1987 Airborne Antarctic Ozone Experiment. J. Geophys. Res. 1989, 94, 11573–11587. [Google Scholar] [CrossRef]
- Newman, P.A.; Harris, N.R.P.; Adriani, A.; Amanatidis, G.T.; Anderson, J.G.; Braathen, G.O.; Brune, W.H.; Carslaw, K.C.; Craig, M.S.; DeCola, P.L.; et al. An overview of the SOLVE/THESEO 2000 campaign. J. Geophys. Res. Atmos. 2002, 107, 8259. [Google Scholar] [CrossRef]
- Alder, B.J.; Wainwright, T.E. Decay of the velocity autocorrelation function. Phys. Rev. A 1970, 1, 18–21. [Google Scholar] [CrossRef]
- Zwanzig, R. Nonequilibrium Statistical Mechanics; Oxford University Press: New York, NY, USA, 2001. [Google Scholar]
- Stutz, J.; Ravishankara, A.R.; Tuck, A.F.; Evans, M.J.; Herrmann, H.; Chipperfield, M.P.; Pyle, J.A.; Zellner, R.; Roscoe, H.; Shallcross, D.E.; et al. General Discussion. Faraday Discuss. 2005, 130, 125–151. [Google Scholar]
- Kadau, K.; Barber, J.L.; Germann, T.C.; Holian, B.L.; Alder, B.J. Atomistic methods in fluid simulation. Philos. Trans. R. Soc. A 2012, 368, 1547–1560. [Google Scholar] [CrossRef]
- Yeung, L.Y.; Murray, L.T.; Ash, J.L.; Young, E.D.; Boering, K.A.; Atlas, E.L.; Schauffler, S.M.; Lueb, R.A.; Langenfelds, R.L.; Krummel, P.B.; et al. Isotopic ordering in atmospheric O2 as a tracer of ozone photochemistry and the tropical atmosphere. J. Geophys. Res. D 2016, 121, 12541–12559. [Google Scholar] [CrossRef]
- Gao, Y.Q.; Marcus, R.A. Strange and unconventional isotope effects in ozone formation. Science 2001, 293, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.-C.; Chen, Y.-C.; Gao, Y.-Q.; Zhang, X.; Yung, Y.L. Atmospheric effects on the isotopic composition of ozone. Atmos. 2021, 12, 1673. [Google Scholar] [CrossRef]
- Chapman, S.; Cowling, T.G. The Mathematical Theory of Non-Uniform Gases, 3rd ed.; Cambridge University Press: Cambridge, UK, 1970; pp. 93–96+327. [Google Scholar]
- Tuck, A.F. Theoretical chemistry and the calculation of the atmospheric state. Atmosphere 2021, 12, 727. [Google Scholar] [CrossRef]
- Tuck, A.F.; Hovde, S.J.; Gao, R.-S.; Richard, E.C. Law of mass action in the Arctic lower stratospheric polar vortex: ClO scaling and the calculation of ozone loss rates in a turbulent fractal medium. J. Geophys. Res. D 2003, 108, 4451. [Google Scholar] [CrossRef]
- Varotsos, C.A.; Lovejoy, S.; Sarlis, N.V.; Tzanis, C.G.; Efstathiou, M.V. On the scaling of the solar incident flux. Atmos. Chem. Phys. 2015, 15, 7301–7306. [Google Scholar] [CrossRef]
- Townes, C.H.; Schawlow, A.L. Microwave Spectroscopy; McGraw Hill: New York, NY, USA, 2013; Chapter 13. [Google Scholar]
- Goody, R.M.; Yung, Y.L. Atmospheric Radiation: Theoretical Basis, 2nd ed.; Oxford University Press: New York, NY, USA, 1989; Chapter 5; ISBN 0-19-505134-3. [Google Scholar]
- Hovde, S.J.; Tuck, A.F.; Lovejoy, S.; Schertzer, D. Vertical scaling of temperature, wind and humidity fluctuations: Dropsondes from 13 km to the surface of the Pacific Ocean. Int. J. Remote Sens. 2011, 32, 5891–5918. [Google Scholar] [CrossRef]
- Tuck, A.F. Turbulence: Vertical shear of the horizontal wind, jet streams, symmetry breaking, scale invariance and Gibbs free energy. Atmosphere 2021, 12, 1414. [Google Scholar] [CrossRef]
- Tuck, A.F. Synoptic and chemical evolution of the Antarctic vortex in late winter and early spring, 1987. J. Geophys. Res. Atmos. 1989, 94, 11687–11737. [Google Scholar] [CrossRef]
- Tuck, A.F.; Davies, T.; Hovde, S.J.; Noguer-Alba, M.; Fahey, D.W.; Kawa, S.R.; Kelly, K.K.; Murphy, D.M.; Proffitt, M.H.; Margitan, J.J.; et al. Polar stratospheric cloud processed air and potential vorticity in the Northern Hemisphere lower stratosphere at mid-latitudes during winter. J. Geophys. Res. Atmos. 1992, 97, 7883–7904. [Google Scholar] [CrossRef]
- Tuck, A.F.; Hovde, S.J.; Kelly, K.K.; Mahoney, M.J.; Proffitt, M.H.; Richard, E.C.; Thompson, T.L. Exchange between the upper tropical troposphere and lower stratosphere studied with aircraft observations. J. Geophys. Res. Atmos. 2003, 108, 4734. [Google Scholar] [CrossRef]
- O’Neill, A.; Oatley, C.L.; Charlton-Perez, A.J.; Mitchell, D.M.; Jung, T. Vortex splitting on a planetary scale in the stratosphere by cyclogenesis on a subplanetary scale in the troposphere. Q. J. R. Meteorol. Soc. 2017, 143, 691–705. [Google Scholar] [CrossRef]
- Tuck, A.F.; Hovde, S.J.; Bui, T.P. Scale invariance in jet streams: ER-2 data around the lower-stratospheric polar night vortex. Q. J. R. Meteorol. Soc. 2004, 130, 2423–2444. [Google Scholar] [CrossRef]
- Francis, J.A.; Vavrus, S.J. Evidence for a wavier jet stream in response to rapid Arctic warming. Environ. Res. Lett. 2015, 10, 014005. [Google Scholar] [CrossRef]
- Overland, J.E.; Ballinger, T.E.; Cohen, J.; Francis, J.A.; Hanna, E.; Jaiser, R.; Kim, B.-M.; Kim, S.-J.; Ukita, J.; Vihma, T.; et al. How do intermittency and simultaneous processes obfuscate the Arctic influence on midlatitude extreme weather events? Environ. Res. Lett. 2021, 16, 043002. [Google Scholar] [CrossRef]
- Steudel, M.; Francis, J.; White, R.; Williams, P.D.; Woollings, T. The Jet Stream and Climate Change. In Climate Change 2021, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2021; Chapter 15. [Google Scholar]
- NOAA SABRE Mission. Available online: https://csl.noaa.gov/projects/sabre/wb57/payload.html (accessed on 1 July 2023).
- Vaughan, G.; Tuck, A.F. Aircraft measurements near jet streams. In Atmospheric Ozone; D. Reidel Publishing Company: Dordrecht, The Netherlands, 1985; pp. 572–579. [Google Scholar]
- Gary, B.L. Observational results using the Microwave Temperature Profiler during the Airborne Antarctic Ozone Experiment. J. Geophys. Res. Atmos. 1989, 94, 11223–11231. [Google Scholar] [CrossRef]
- Palmer, T.; Williams, P. Preface. In Stochastic Physics and Climate Modelling; xi–xv; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar][Green Version]
- Vaughan, G. Diurnal variation of mesospheric ozone. Nature 1982, 296, 133–135. [Google Scholar] [CrossRef]
- IPCC. Climate Change: The Scientific Basis. In Third Assessment Report; Cambridge University Press: Cambridge, UK, 2001; Chapter 8. [Google Scholar][Green Version]

| 3Σg− | 1Δg | 1Σg+ | 3Σu+ | 3Σu− | |
|---|---|---|---|---|---|
| 3P | 1180 | 612 | 463 | 230 | 173 |
| 1D | 411 | 310 | 267 | 168 | 136 |
| 1S | 237 | 199 | 181 | 129 | 109 |
| Variable | Statistical Thermodynamics | Scaling Equivalent |
|---|---|---|
| Temperature | T | 1/qkBoltzmann |
| Partition function | f | e−K(q) |
| Energy | E | γ |
| Entropy | −S(E) | c(γ) |
| Gibbs free energy | −G | K(q)/q |
| Mission [Reference] | Dates (yyyymmdd) | Location | Instruments [References] | Measured Variables |
|---|---|---|---|---|
| POLARIS [42] | 19970502–19970908 | 65° N, 148° W | [43,44,45,46] | O3, J, T |
| SOLVE [47] | 20000111–20000318 | 67.86° N, 20.21° E | [43,44,45,46] | O3, J, T |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tuck, A.F. Air Temperature Intermittency and Photofragment Excitation. Meteorology 2023, 2, 445-463. https://doi.org/10.3390/meteorology2040026
Tuck AF. Air Temperature Intermittency and Photofragment Excitation. Meteorology. 2023; 2(4):445-463. https://doi.org/10.3390/meteorology2040026
Chicago/Turabian StyleTuck, Adrian F. 2023. "Air Temperature Intermittency and Photofragment Excitation" Meteorology 2, no. 4: 445-463. https://doi.org/10.3390/meteorology2040026
APA StyleTuck, A. F. (2023). Air Temperature Intermittency and Photofragment Excitation. Meteorology, 2(4), 445-463. https://doi.org/10.3390/meteorology2040026






