Effect of Annealing Temperature on Microstructure and Properties of Solid Solution Extruded Mg–2.0Zn–1.0Y–0.5Zr Alloys
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material Preparatione
2.2. Microstructure Characterization
2.3. Tensile Testing
2.4. Immersion Experiment
2.5. Electrochemical Testing
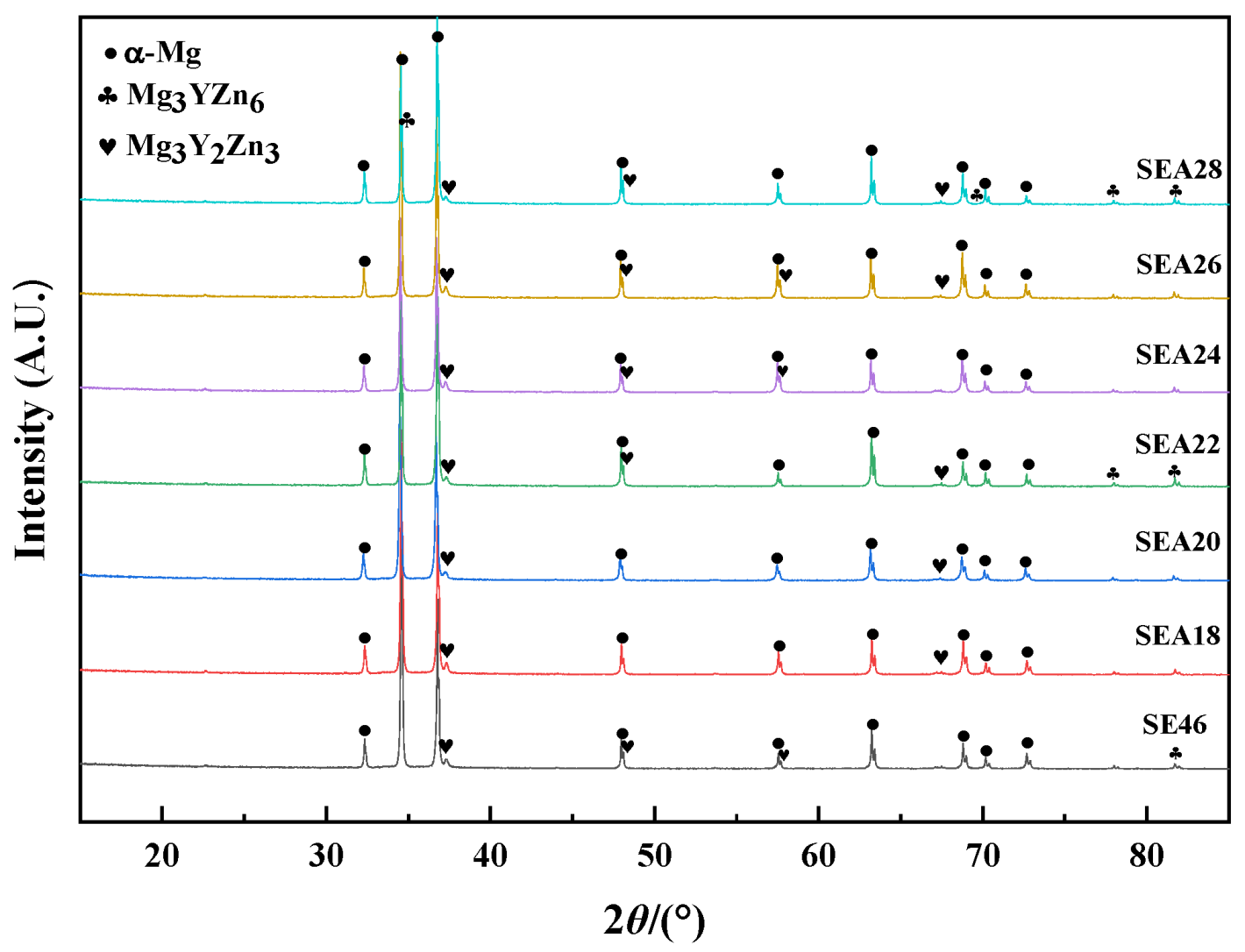
3. Results
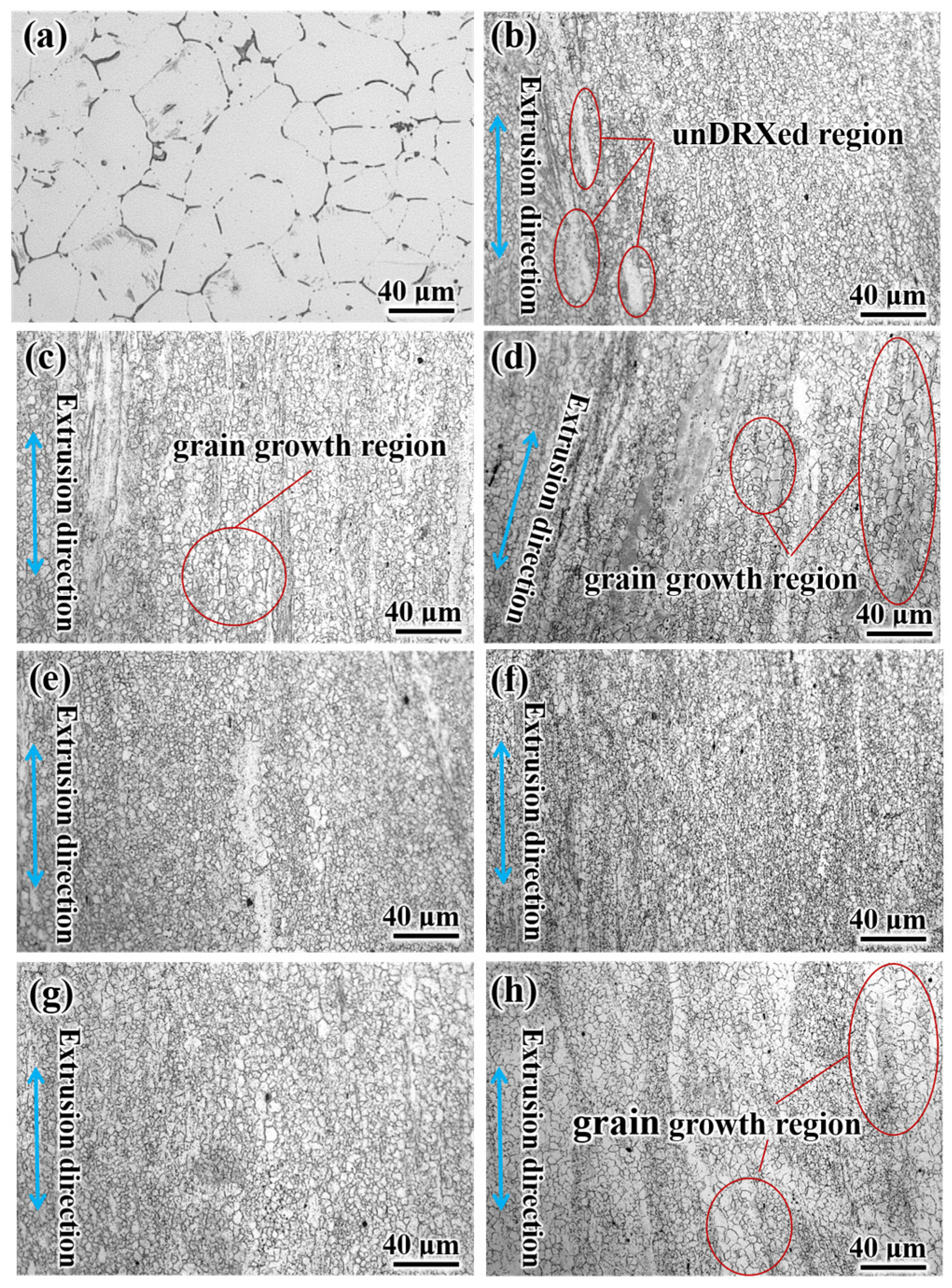
3.1. Subsection
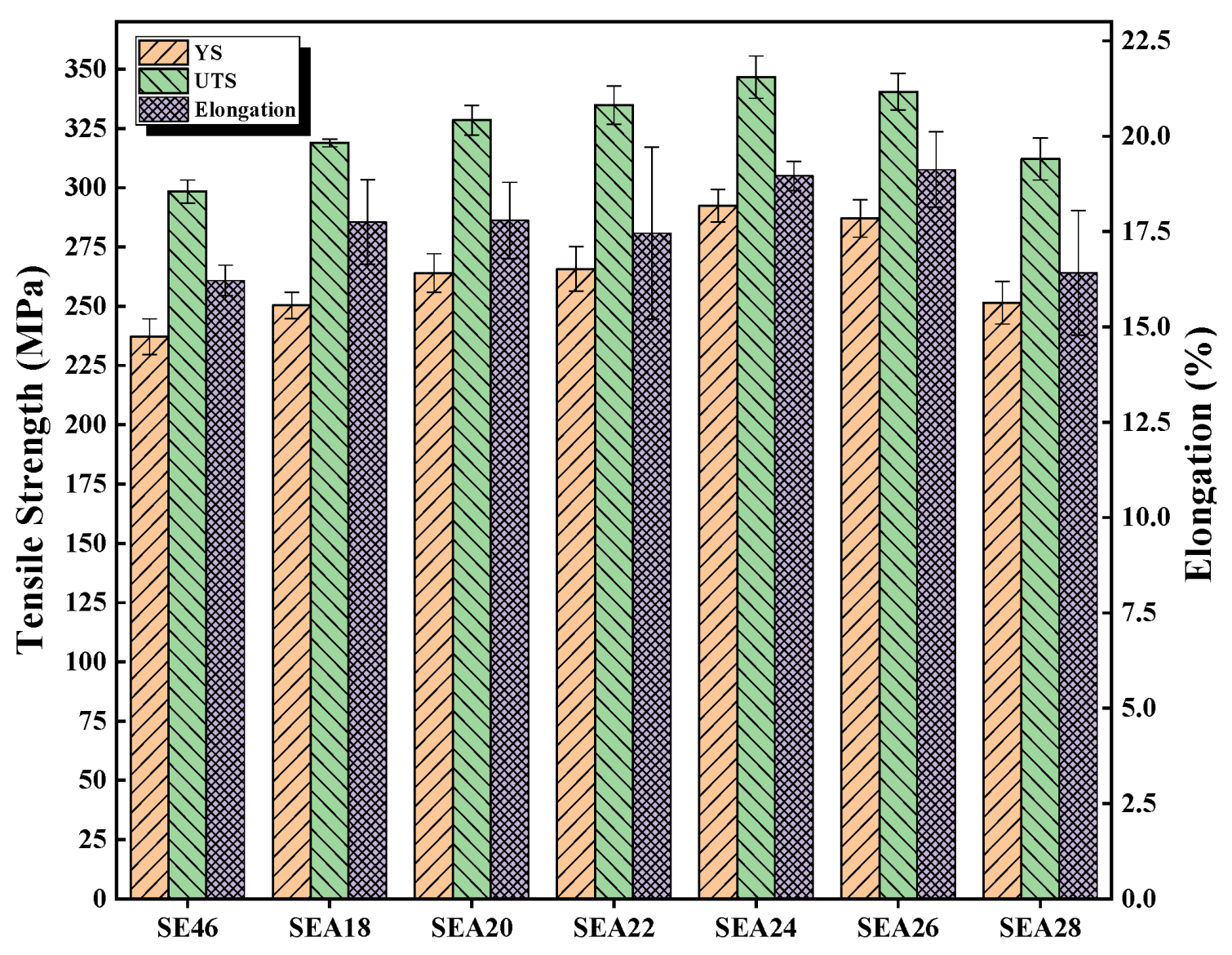
3.2. Mechanical Properties
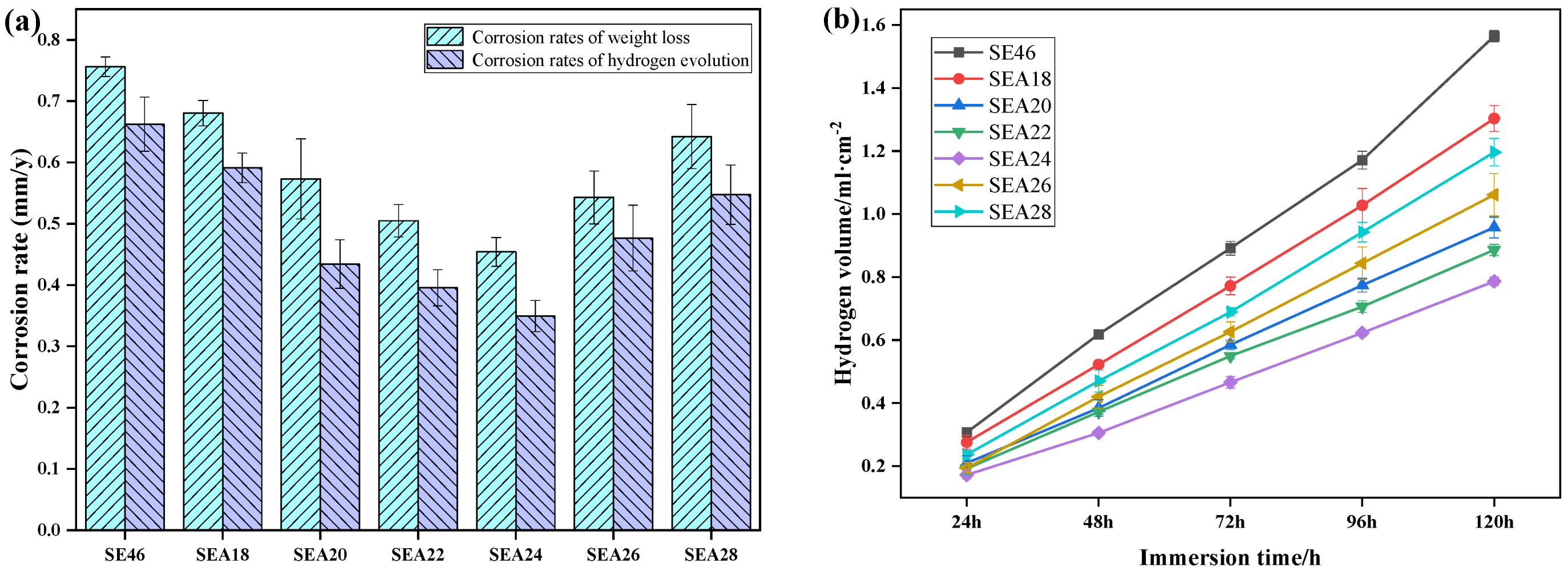
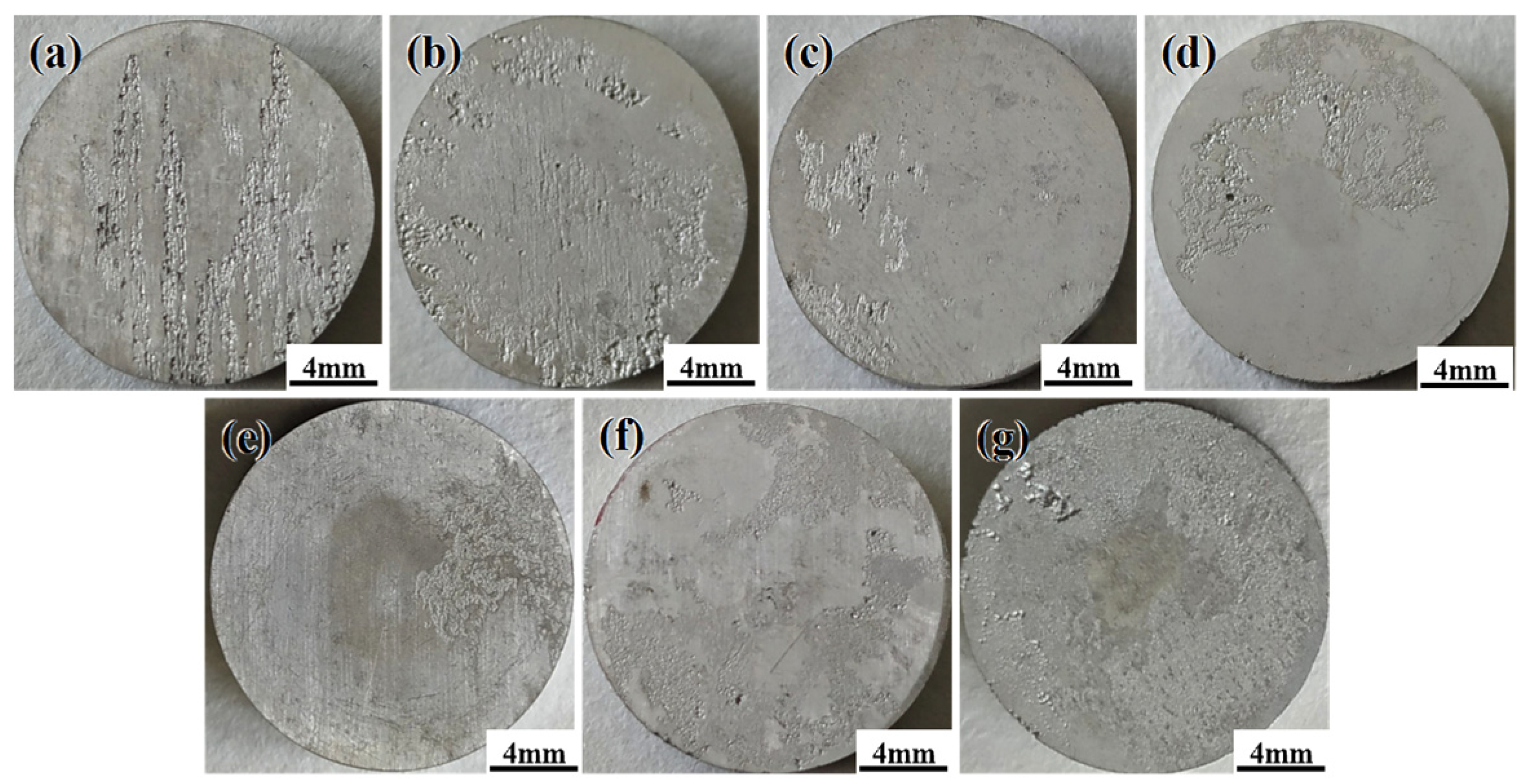
3.3. Corrosion Resistance
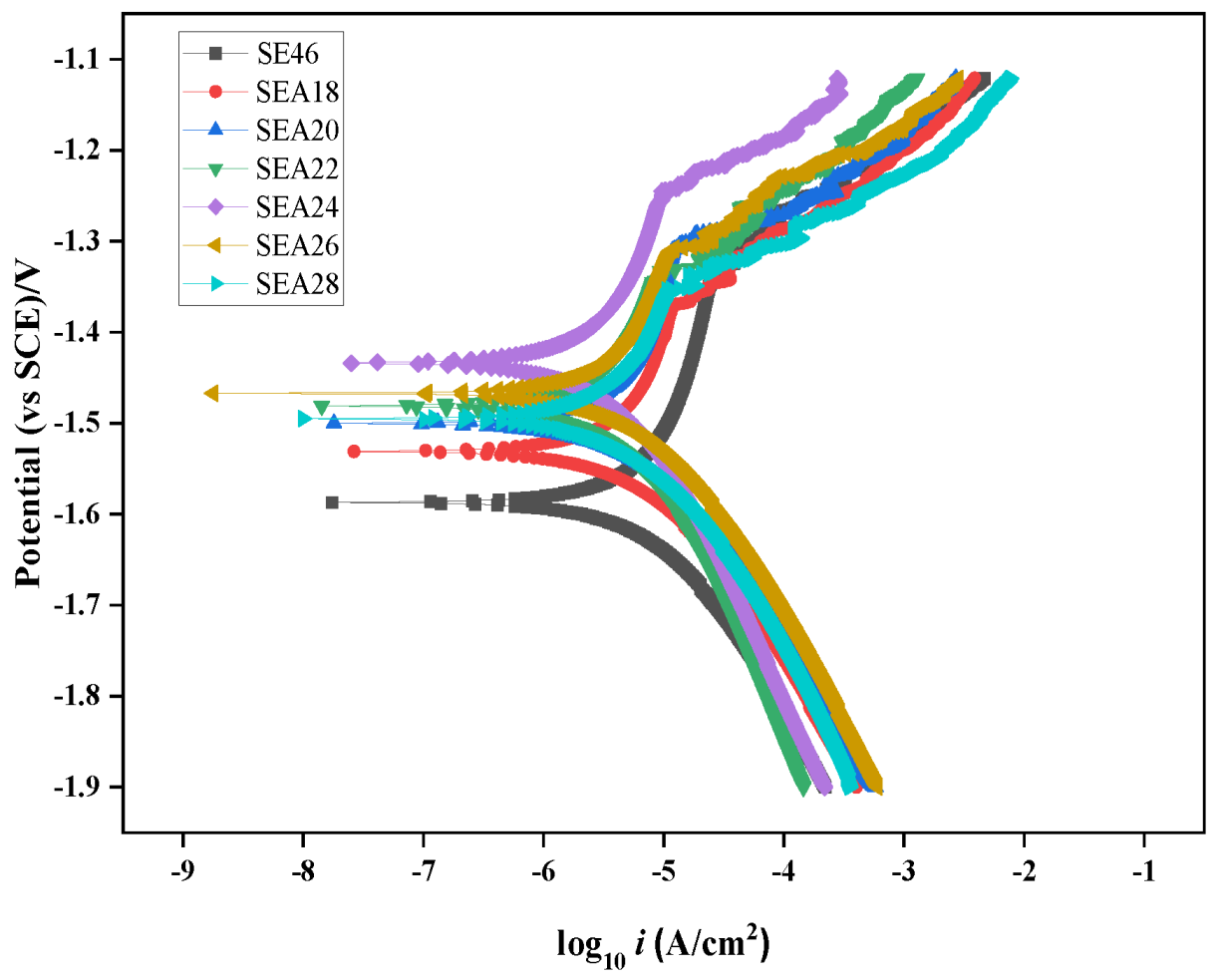
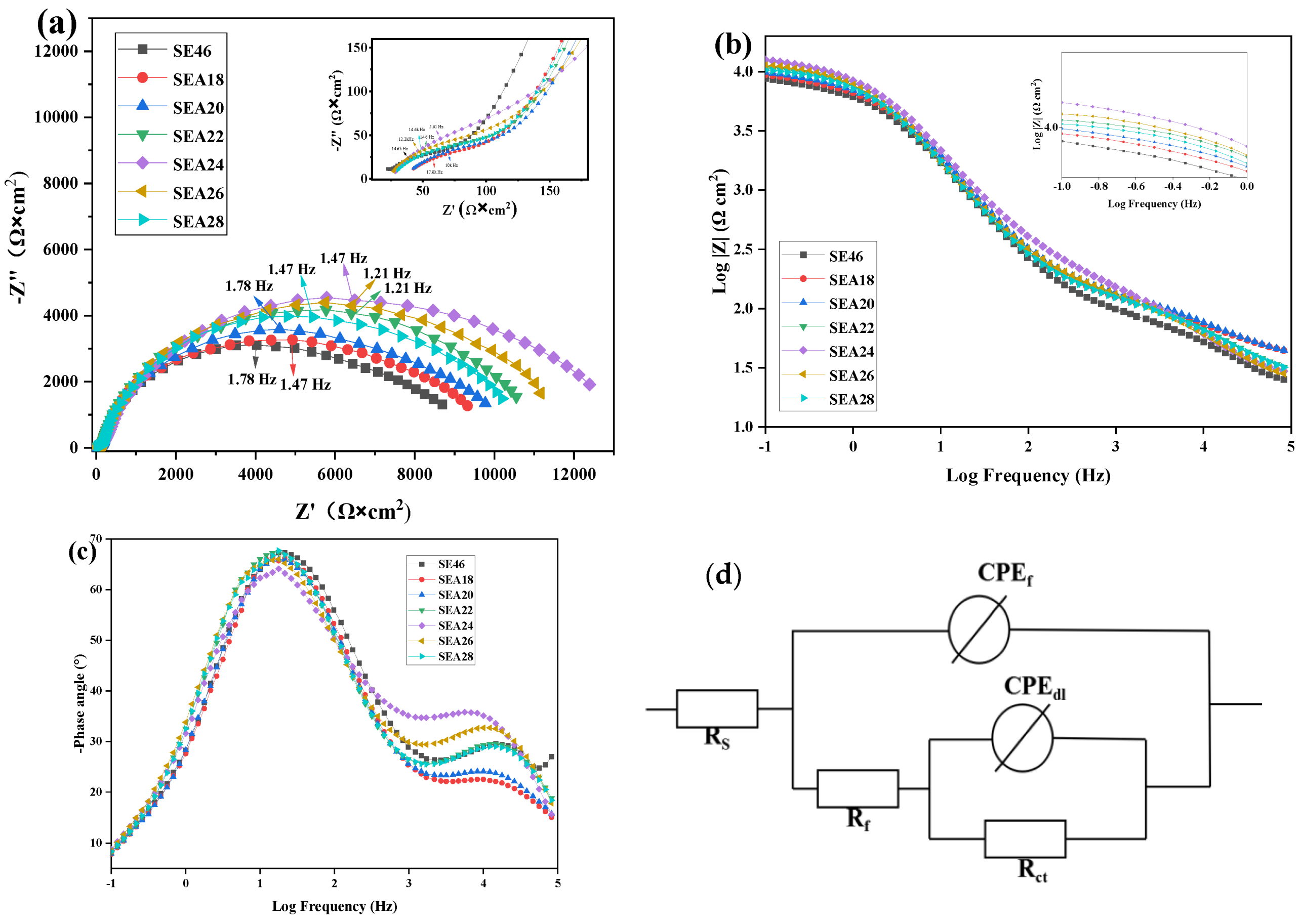
3.4. Electrochemical Properties
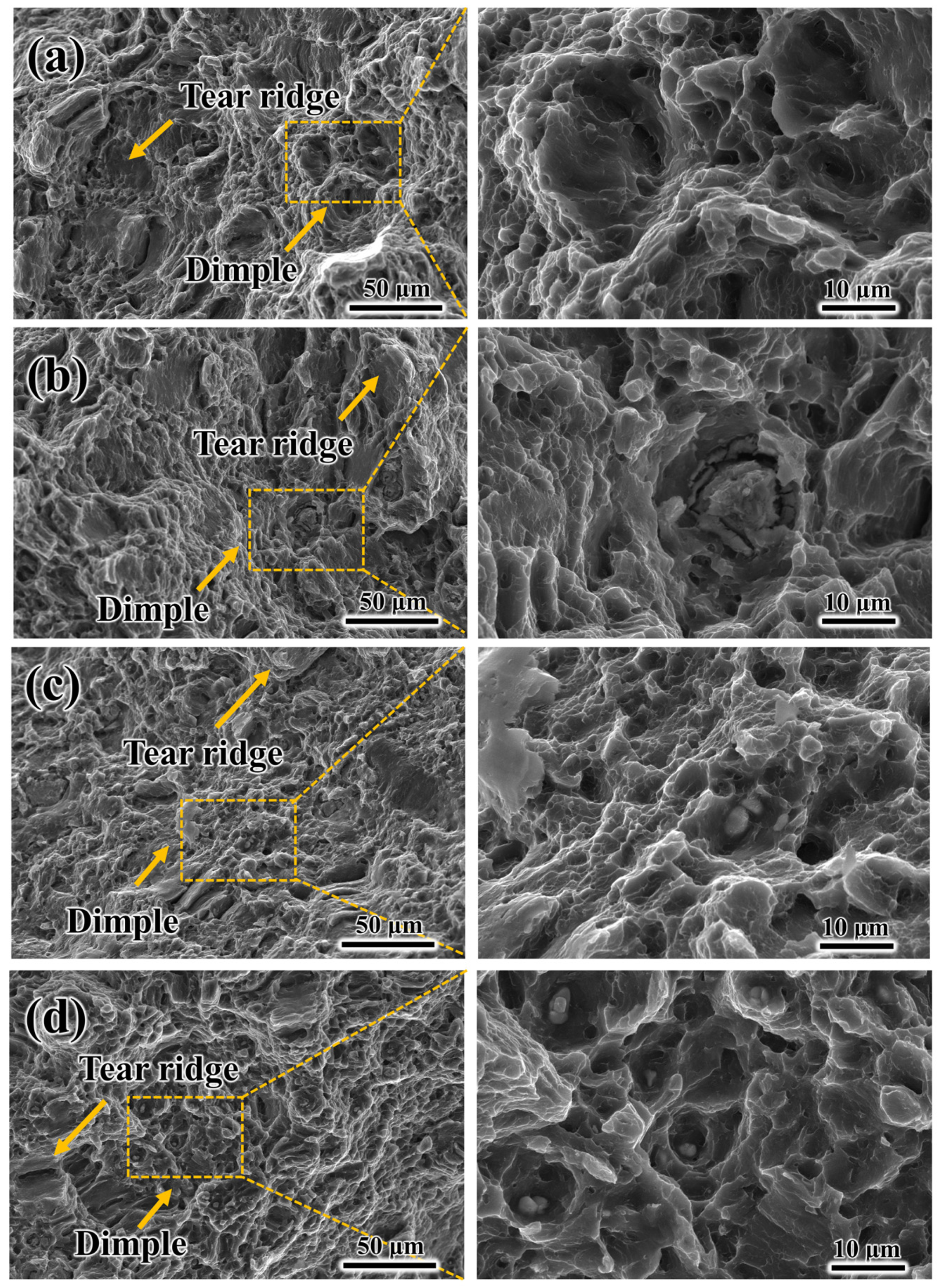
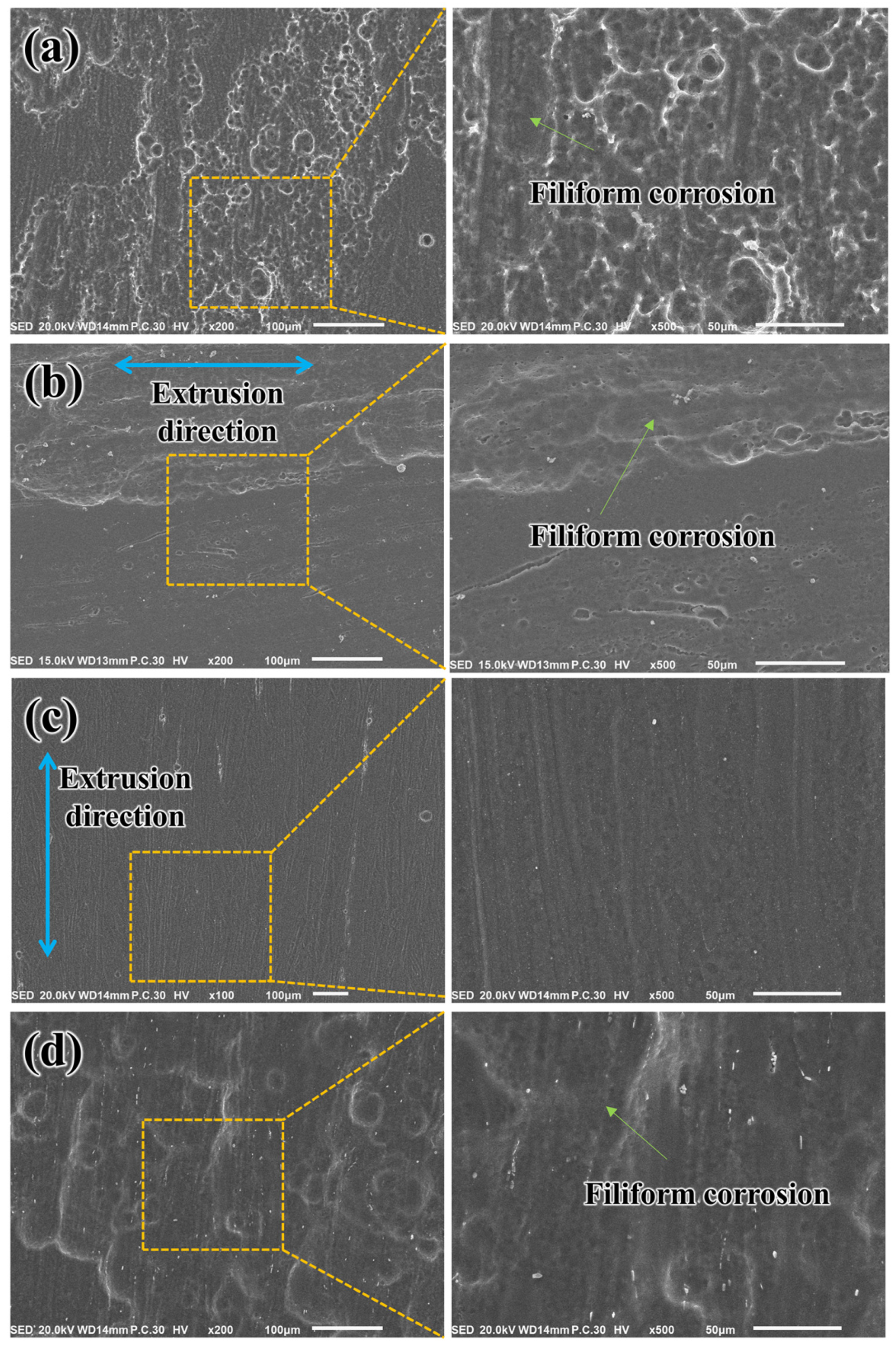
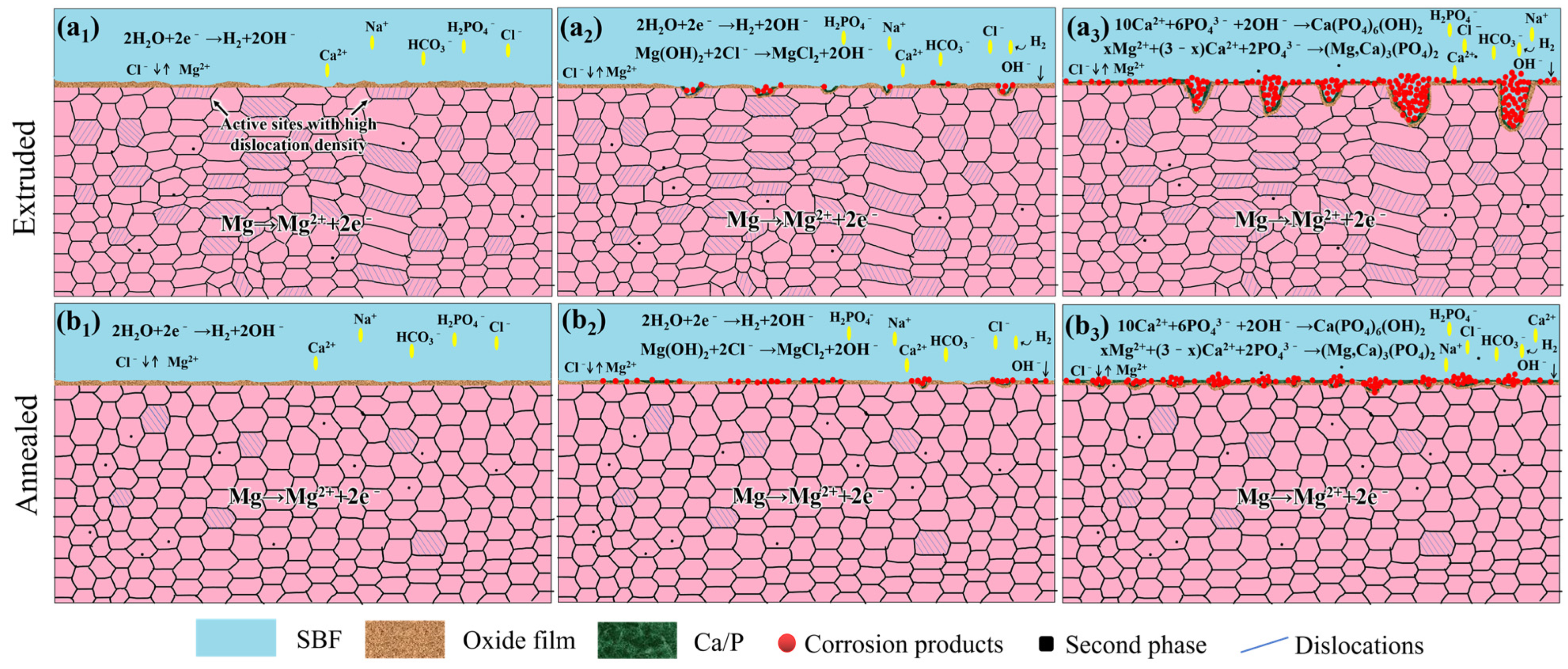
3.5. Corrosion Behavior
4. Conclusions
- (1)
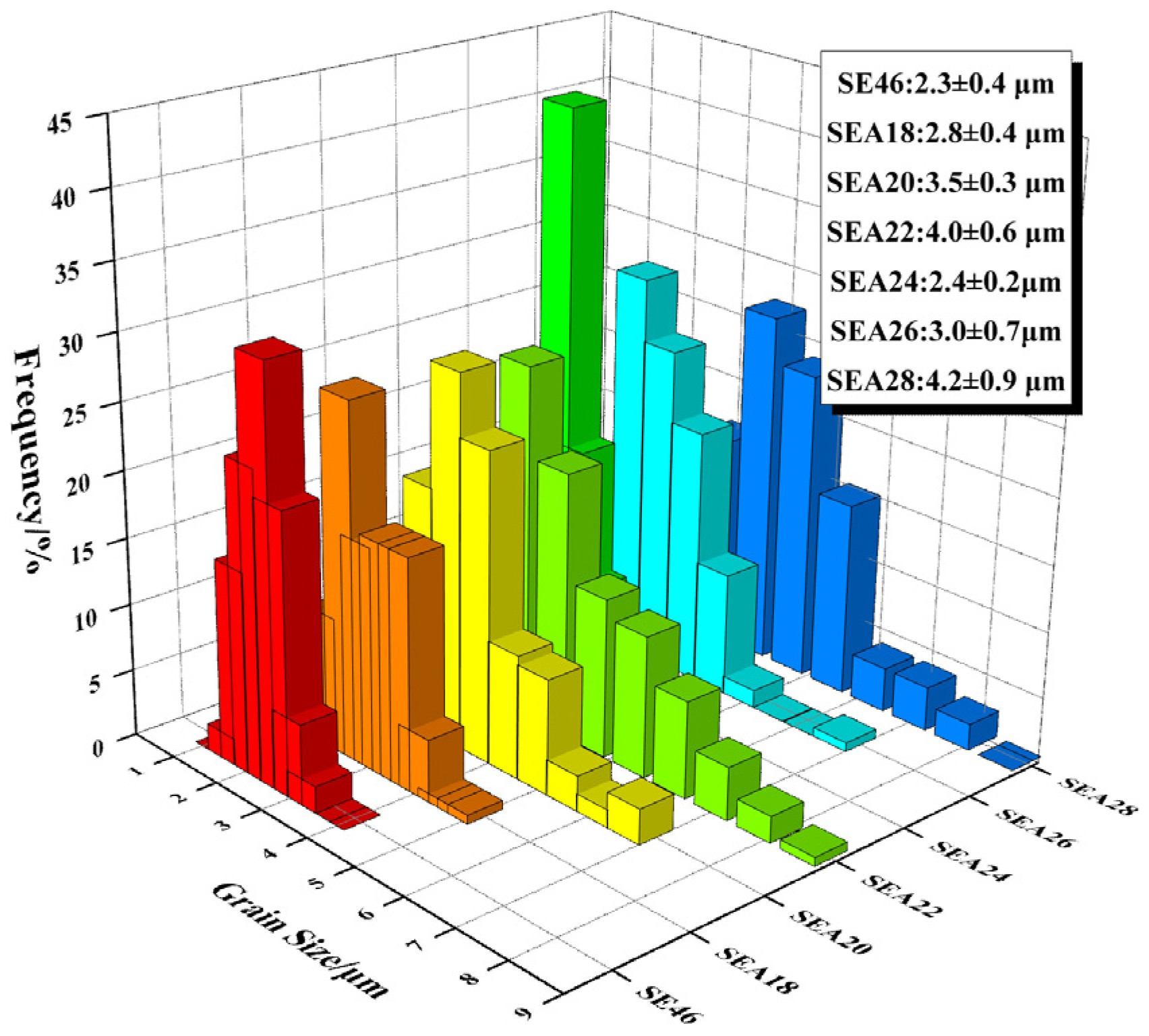
- After annealing at different temperatures for 4 h, the recrystallization degree and grain size of extruded Mg–2.0Zn–1.0Y–0.5Zr alloys change with increasing temperature. When the annealing temperature is 220 °C, the grain size increases by 1.7 μm compared with the extruded alloy. When the annealing temperature is 240 °C, recrystallization is complete and the equiaxed grain size is 2.4 ± 0.2 μm. When the temperature rises to 280 °C, the grain size gradually grows to 4.2 ± 0.9 μm.
- (2)
- The mechanical properties and corrosion resistance of annealed alloys are enhanced due to grain refinement and the reduced dislocation density. When the annealing temperature is 240 °C, the alloy has the highest corrosion resistance and best mechanical properties, with an Rw, UTS, YS, and EL of 0.454 ± 0.023 mm/y, 346.7 ± 8.9 MPa, 292.4 ± 6.9 MPa, and 19.0 ± 0.4%, respectively.
- (3)
- With the increase in the annealing temperature, Icorr first decreases and then increases, Ecorr first shifts to positive values and then to negative values, and the capacitive arc modulus first increases and then decreases. The alloy annealed at 240 °C has the maximal arc radius, the lowest Icorr value, and the maximal Ecorr value.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nagels, J.; Stokdijk, M.; Rozing, P.M. Stress Shielding and Bone Resorption in Shoulder Arthroplasty. J. Shoulder Elb. Surg. 2003, 12, 35–39. [Google Scholar] [CrossRef]
- Zhou, J.; Li, Q.; Zhang, H.; Chen, F. Corrosion Behavior of AZ91D Magnesium Alloy in Three Different Physiological Environments. J. Mater. Eng Perform. 2014, 23, 181–186. [Google Scholar] [CrossRef]
- Li, J.; Tan, L.; Wan, P.; Yu, X.; Yang, K. Study on Microstructure and Properties of Extruded Mg–2Nd–0.2Zn Alloy as Potential Biodegradable Implant Material. Mater. Sci. Eng. C 2015, 49, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Wu, D.; Dong, W.; Ding, J.; Ye, Z.; Zeng, P.; Gao, Y. Design and Validation of a Nonparasitic 2R1T Parallel Hand-Held Prostate Biopsy Robot With Remote Center of Motion. J. Mech. Robot. 2023, 16, 051009. [Google Scholar] [CrossRef]
- Li, H.; Wen, J.; Liu, Y.; He, J.; Shi, H.; Tian, P. Progress in Research on Biodegradable Magnesium Alloys: A Review. Adv. Eng. Mater. 2020, 22, 2000213. [Google Scholar] [CrossRef]
- Farraro, K.E.; Kim, K.E.; Woo, S.L.-Y.; Flowers, J.R.; McCullough, M.B. Revolutionizing Orthopaedic Biomaterials: The Potential of Biodegradable and Bioresorbable Magnesium-Based Materials for Functional Tissue Engineering. J. Biomech. 2014, 47, 1979–1986. [Google Scholar] [CrossRef]
- Munir, K.; Lin, J.; Wen, C.; Wright, P.F.A.; Li, Y. Mechanical, Corrosion, and Biocompatibility Properties of Mg-Zr-Sr-Sc Alloys for Biodegradable Implant Applications. Acta Biomater. 2020, 102, 493–507. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Dou, J.; Yu, H.; Chen, C. Degradable Magnesium-Based Alloys for Biomedical Applications: The Role of Critical Alloying Elements. J. Biomater. Appl. 2019, 33, 1348–1372. [Google Scholar] [CrossRef]
- Zhou, W.; Shen, T.; Aung, N.N. Effect of Heat Treatment on Corrosion Behaviour of Magnesium Alloy AZ91D in Simulated Body Fluid. Corros. Sci. 2010, 52, 1035–1041. [Google Scholar] [CrossRef]
- Caceres, C.H.; Mann, G.E.; Griffiths, J.R. Grain Size Hardening in Mg and Mg-Zn Solid Solutions. Metall. Mater. Trans. A-Phys. Metall. Mater. Sci. 2011, 42A, 1950–1959. [Google Scholar] [CrossRef]
- Choudhary, L.; Raman, R.K.S. Magnesium Alloys as Body Implants: Fracture Mechanism under Dynamic and Static Loadings in a Physiological Environment. Acta Biomater. 2012, 8, 916–923. [Google Scholar] [CrossRef]
- Zhang, E.; Yin, D.; Xu, L.; Yang, L.; Yang, K. Microstructure, Mechanical and Corrosion Properties and Biocompatibility of Mg–Zn–Mn Alloys for Biomedical Application. Mater. Sci. Eng. C 2009, 29, 987–993. [Google Scholar] [CrossRef]
- Song, Y.; Han, E.-H.; Shan, D.; Yim, C.D.; You, B.S. The Effect of Zn Concentration on the Corrosion Behavior of Mg–xZn Alloys. Corros. Sci. 2012, 65, 322–330. [Google Scholar] [CrossRef]
- Ding, Y.; Wen, C.; Hodgson, P.; Li, Y. Effects of Alloying Elements on the Corrosion Behavior and Biocompatibility of Biodegradable Magnesium Alloys: A Review. J. Mater. Chem. B 2014, 2, 1912–1933. [Google Scholar] [CrossRef]
- Xing, F.; Guo, F.; Su, J.; Zhao, X.; Cai, H. The Existing Forms of Zr in Mg-Zn-Zr Magnesium Alloys and Its Grain Refinement Mechanism. Mater. Res. Express 2021, 8, 066516. [Google Scholar] [CrossRef]
- Singh Raman, R.K. The Role of Microstructure in Localized Corrosion of Magnesium Alloys. Metall. Mater Trans. A 2004, 35, 2525–2531. [Google Scholar] [CrossRef]
- Li, Z.; Sun, S.; Chen, M.; Fahlman, B.D.; Liu, D.; Bi, H. In Vitro and in Vivo Corrosion, Mechanical Properties and Biocompatibility Evaluation of MgF 2 -Coated Mg-Zn-Zr Alloy as Cancellous Screws. Mater. Sci. Eng. C 2017, 75, 1268–1280. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, Y.; Huang, S.; Wei, Y.; Xi, X.; Cai, K.; Pan, F. Effects of Y on the Microstructure, Mechanical and Bio-Corrosion Properties of Mg–Zn–Ca Bulk Metallic Glass. J. Mater. Sci. Technol. 2014, 30, 1255–1261. [Google Scholar] [CrossRef]
- Neil, W.C.; Forsyth, M.; Howlett, P.C.; Hutchinson, C.R.; Hinton, B.R.W. Corrosion of Magnesium Alloy ZE41—The Role of Microstructural Features. Corros. Sci. 2009, 51, 387–394. [Google Scholar] [CrossRef]
- Sun, H.; Li, C.; Fang, W. Evolution of Microstructure and Mechanical Properties of Mg–3.0Zn–0.2Ca–0.5Y Alloy by Extrusion at Various Temperatures. J. Mater. Processing Technol. 2016, 229, 633–640. [Google Scholar] [CrossRef]
- Gui, Z.; Kang, Z.; Li, Y. Mechanical and Corrosion Properties of Mg-Gd-Zn-Zr-Mn Biodegradable Alloy by Hot Extrusion. J. Alloys Compd. 2016, 685, 222–230. [Google Scholar] [CrossRef]
- Zhang, X.; Yuan, G.; Niu, J.; Fu, P.; Ding, W. Microstructure, Mechanical Properties, Biocorrosion Behavior, and Cytotoxicity of as-Extruded Mg–Nd–Zn–Zr Alloy with Different Extrusion Ratios. J. Mech. Behav. Biomed. Mater. 2012, 9, 153–162. [Google Scholar] [CrossRef]
- Yao, H.; Li, H.; Liu, Y.; Shi, H. Enhanced Mechanical and Corrosion Properties of Grain Refined Mg-2.0Zn-0.5Zr-3.0Gd Alloy. Met. Mater./Kov. Mater. 2021, 58, 409–421. [Google Scholar] [CrossRef]
- Zhang, T.; Shao, Y.; Meng, G.; Cui, Z.; Wang, F. Corrosion of Hot Extrusion AZ91 Magnesium Alloy: I-Relation between the Microstructure and Corrosion Behavior. Corros. Sci. 2011, 53, 1960–1968. [Google Scholar] [CrossRef]
- Chen, L.; Sheng, Y.; Wang, X.; Zhao, X.; Liu, H.; Li, W. Effect of the Microstructure and Distribution of the Second Phase on the Stress Corrosion Cracking of Biomedical Mg-Zn-Zr-xSr Alloys. Materials 2018, 11, 551. [Google Scholar] [CrossRef]
- Cao, X.; Zhang, Z.; Xu, C.; Ren, C.; Yang, W.; Zhang, J. Micro-Galvanic Corrosion Behavior and Mechanical Properties of Extruded Mg–2Y–1Zn-0.4Zr-0.3Sr Alloys with Different Extrusion Temperament Immersed in Simulated Body Fluids. Mater. Chem. Phys. 2021, 271, 124928. [Google Scholar] [CrossRef]
- Yasi, J.A.; Hector, L.G.; Trinkle, D.R. First-Principles Data for Solid-Solution Strengthening of Magnesium: From Geometry and Chemistry to Properties. Acta Mater. 2010, 58, 5704–5713. [Google Scholar] [CrossRef]
- Choi, H.Y.; Kim, W.J. Effect of Thermal Treatment on the Bio-Corrosion and Mechanical Properties of Ultrafine-Grained ZK60 Magnesium Alloy. J. Mech. Behav. Biomed. Mater. 2015, 51, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Tan, L.; Yang, K. Effect of Heat Treatment on Mechanical and Biodegradable Properties of an Extruded ZK60 Alloy. Bioact. Mater. 2017, 2, 19–26. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhu, J.; Nie, Y.; Li, X.; Cheng, J.; Zhang, X. Effects of Annealing on Mechanical and Corrosion Properties of As-Extruded NQZ310K Alloy. J. Mater. Eng. Perform. 2020, 29, 925–932. [Google Scholar] [CrossRef]
- Lian, Y.; Ji, P.; Zhang, J.; Yuan, X.; Xu, W.; Zhao, Y.; Mo, J.; Zheng, L.; Dou, S. Effect of Homogenization Annealing on Internal Residual Stress Distribution and Texture in ME21 Magnesium Alloy Extruded Plates. J. Magnes. Alloys 2019, 7, 186–192. [Google Scholar] [CrossRef]
- Volkov, A.Y.; Kliukin, I.V. Improving the Mechanical Properties of Pure Magnesium through Cold Hydrostatic Extrusion and Low-Temperature Annealing. Mater. Sci. Eng. A 2015, 627, 56–60. [Google Scholar] [CrossRef]
- Song, B.; Guo, N.; Xin, R.; Pan, H.; Guo, C. Strengthening and Toughening of Extruded Magnesium Alloy Rods by Combining Pre-Torsion Deformation with Subsequent Annealing. Mater. Sci. Eng. A 2016, 650, 300–304. [Google Scholar] [CrossRef]
- Lu, Y.; Bradshaw, A.R.; Chiu, Y.L.; Jones, I.P. Effects of Secondary Phase and Grain Size on the Corrosion of Biodegradable Mg-Zn-Ca Alloys. Mater. Sci. Eng. C-Mater. Biol. Appl. 2015, 48, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; He, J.; Wen, J.; Ren, X.; Wang, Y.; Zeng, T. Effects of Solution Treatment on Microstructure, Mechanical Properties and Corrosion Behavior of Mg-2Zn-1Y-0.5Zr Alloy. Mater. Res. Express 2019, 6, 125411. [Google Scholar] [CrossRef]
- Liu, Y.; Wen, J.; Yao, H.; He, J.; Li, H. Enhancing the Corrosion Resistance Performance of Mg-1.8Zn-1.74Gd-0.5Y-0.4Zr Biomaterial via Solution Treatment Process. Materials 2020, 13, 836. [Google Scholar] [CrossRef] [PubMed]
- Ding, W. Opportunities and Challenges for the Biodegradable Magnesium Alloys as Next-Generation Biomaterials. Regen. Biomater. 2016, 3, 79–86. [Google Scholar] [CrossRef] [PubMed]
- National Standards—National Standard Information Public Service Platform. Available online: https://std.samr.gov.cn/gb/search/gbDetailed?id=D4BEFFF4EB12B241E05397BE0A0AF581%20 (accessed on 12 December 2023).
- Gollapudi, S. Grain Size Distribution Effects on the Corrosion Behaviour of Materials. Corros. Sci. 2012, 62, 90–94. [Google Scholar] [CrossRef]
- Cheng, W.; Bai, Y.; Wang, L.; Wang, H.; Bian, L.; Yu, H. Strengthening Effect of Extruded Mg-8Sn-2Zn-2Al Alloy: Influence of Micro and Nano-Size Mg2Sn Precipitates. Materials 2017, 10, 822. [Google Scholar] [CrossRef]
- He, J.; Xu, D.; Wen, J.; Shi, H.; Wang, Y.; Ren, X. Microstructure, Mechanical Properties and Corrosion Behavior of Biodegradable As-extruded Mg-2Zn-1Y-0.5Zr Alloys. Rare Met. Mater. Eng. 2022, 51, 474–483. Available online: https://kns.cnki.net/kcms2/article/abstract?v=z5VdU6XQV3XMa37BoO1exMBzTiSz9HvSD3__1iARi2bChyXd94rcqEx1xACQeFxsgkF6IwJwNMjgfvknvCGmPa1Nt2hFghVSJjbvpuz1_DT8wAOd3y6hV6WFKbIDCS6YZp4WWEbqRTs=&uniplatform=NZKPT&language=CHS (accessed on 19 May 2024).
- Lei, L.; Cui, Z.; Pan, H.; Pang, K.; Wang, X.; Cui, H. Effect of Extrusion on the Microstructure and Corrosion Behavior of Mg-Zn-Mn-(0, 1.5)Sr Alloys in Hank’s Solution. Corros. Sci. 2022, 195, 109975. [Google Scholar] [CrossRef]
- Li, L.; Zhang, C.; Lv, H.; Liu, C.; Wen, Z.; Jiang, J. Texture Development and Tensile Properties of Mg–Yb Binary Alloys during Hot Extrusion and Subsequent Annealing. J. Magnes. Alloys 2022, 10, 249–265. [Google Scholar] [CrossRef]
- Daidong, Z.; Xiaowei, H.; Daqing, F.; Yuesheng, C. Effects of Heat Treatment on Microstructure and Mechanical Properties of As-Extruded Mg-9Sn-1.5Y-0.4Zr Magnesium Alloy. Rare Met. Mater. Eng. 2016, 45, 2208–2213. [Google Scholar] [CrossRef]
- Zhang, B.P.; Geng, L.; Huang, L.J.; Zhang, X.X.; Dong, C.C. Enhanced Mechanical Properties in Fine-Grained Mg–1.0Zn–0.5Ca Alloys Prepared by Extrusion at Different Temperatures. Scr. Mater. 2010, 63, 1024–1027. [Google Scholar] [CrossRef]
- He, J.H.; Jin, L.; Wang, F.H.; Dong, S.; Dong, J. Mechanical Properties of Mg-8Gd-3Y-0.5Zr Alloy with Bimodal Grain Size Distributions. J. Magnes. Alloys 2017, 5, 423–429. [Google Scholar] [CrossRef]
- Jeong, Y.S.; Kim, W.J. Enhancement of Mechanical Properties and Corrosion Resistance of Mg–Ca Alloys through Microstructural Refinement by Indirect Extrusion. Corros. Sci. 2014, 82, 392–403. [Google Scholar] [CrossRef]
- Oh-ishi, K.; Mendis, C.L.; Homma, T.; Kamado, S.; Ohkubo, T.; Hono, K. Bimodally Grained Microstructure Development during Hot Extrusion of Mg–2.4 Zn–0.1 Ag–0.1 Ca–0.16 Zr (at.%) Alloys. Acta Mater. 2009, 57, 5593–5604. [Google Scholar] [CrossRef]
- Jiang, M.G.; Xu, C.; Yan, H.; Lu, S.H.; Nakata, T.; Lao, C.S.; Chen, R.S.; Kamado, S.; Han, E.H. Correlation between Dynamic Recrystallization and Formation of Rare Earth Texture in a Mg-Zn-Gd Magnesium Alloy during Extrusion. Sci. Rep. 2018, 8, 16800. [Google Scholar] [CrossRef]
- Fu, W.; Wang, R.; Zhang, J.; Wu, K.; Liu, G.; Sun, J. The Effect of Precipitates on Voiding, Twinning, and Fracture Behaviors in Mg Alloys. Mater. Sci. Eng. A 2018, 720, 98–109. [Google Scholar] [CrossRef]
- Esmaily, M.; Svensson, J.E.; Fajardo, S.; Birbilis, N.; Frankel, G.S.; Virtanen, S.; Arrabal, R.; Thomas, S.; Johansson, L.G. Fundamentals and Advances in Magnesium Alloy Corrosion. Prog. Mater. Sci. 2017, 89, 92–193. [Google Scholar] [CrossRef]
- Olugbade, T.O.; Omiyale, B.O.; Ojo, O.T. Corrosion, Corrosion Fatigue, and Protection of Magnesium Alloys: Mechanisms, Measurements, and Mitigation. J. Mater. Eng. Perform. 2022, 31, 1707–1727. [Google Scholar] [CrossRef]
- Qiao, Z.; Shi, Z.; Hort, N.; Zainal Abidin, N.I.; Atrens, A. Corrosion Behaviour of a Nominally High Purity Mg Ingot Produced by Permanent Mould Direct Chill Casting. Corros. Sci. 2012, 61, 185–207. [Google Scholar] [CrossRef]
- Atrens, A.; Song, G.; Liu, M.; Shi, Z.; Cao, F.; Dargusch, M.S. Review of Recent Developments in the Field of Magnesium Corrosion. Adv. Eng. Mater. 2015, 17, 400–453. [Google Scholar] [CrossRef]
- Chen, J.; Tan, L.; Yu, X.; Yang, K. Effect of Minor Content of Gd on the Mechanical and Degradable Properties of As-Cast Mg-2Zn-xGd-0.5Zr Alloys. J. Mater. Sci. Technol. 2019, 35, 503–511. [Google Scholar] [CrossRef]
- Cai, C.; Song, R.; Wang, L.; Li, J. Surface Corrosion Behavior and Reaction Product Film Deposition Mechanism of Mg-Zn-Zr-Nd Alloys during Degradation Process in Hank’s Solution. Surf. Coat. Technol. 2018, 342, 57–68. [Google Scholar] [CrossRef]
- Wang, L.-X.; Song, R.-B.; Cai, C.-H.; Li, J.-Y. Enhanced Strength and Corrosion Resistance of Mg–2Zn–0.6Zr Alloy with Extrusion. Acta Metall. Sin. (Engl. Lett.) 2019, 32, 10–22. [Google Scholar] [CrossRef]
- She, J.; Peng, P.; Xiao, L.; Tang, A.T.; Wang, Y.; Pan, F.S. Development of High Strength and Ductility in Mg–2Zn Extruded Alloy by High Content Mn-Alloying. Mater. Sci. Eng. A 2019, 765, 138203. [Google Scholar] [CrossRef]
- Karakulak, E. A Review: Past, Present and Future of Grain Refining of Magnesium Castings. J. Magnes. Alloys 2019, 7, 355–369. [Google Scholar] [CrossRef]
- Ralston, K.D.; Birbilis, N. Effect of Grain Size on Corrosion: A Review. Corrosion 2010, 66, 075005. [Google Scholar] [CrossRef]
- Liu, D.; Guo, C.; Chai, L.; Sherman, V.R.; Qin, X.; Ding, Y.; Meyers, M.A. Mechanical Properties and Corrosion Resistance of Hot Extruded Mg–2.5Zn–1Ca Alloy. Mater. Sci. Eng. B 2015, 195, 50–58. [Google Scholar] [CrossRef]
- Song, D.; Ma, A.; Jiang, J.; Lin, P.; Yang, D.; Fan, J. Corrosion Behavior of Equal-Channel-Angular-Pressed Pure Magnesium in NaCl Aqueous Solution. Corros. Sci. 2010, 52, 481–490. [Google Scholar] [CrossRef]
- Hamu, G.B.; Eliezer, D.; Wagner, L. The Relation between Severe Plastic Deformation Microstructure and Corrosion Behavior of AZ31 Magnesium Alloy. J. Alloys Compd. 2009, 468, 222–229. [Google Scholar] [CrossRef]
- Kayani, S.H.; Park, S.; Euh, K.; Seol, J.B.; Kim, J.G.; Sung, H. Dislocation-Aided Electrochemical Behavior of Precipitates in Stress Corrosion Cracking of Al–Zn–Mg–Cu Alloys. Mater. Charact. 2022, 190, 112019. [Google Scholar] [CrossRef]
- Xiong, Y.; Yang, Z.; Zhu, T.; Jiang, Y. Effect of Texture Evolution on Corrosion Resistance of AZ80 Magnesium Alloy Subjected to Applied Force in Simulated Body Fluid. Mater. Res. Express 2020, 7, 015406. [Google Scholar] [CrossRef]
- Song, D.; Li, C.; Liang, N.; Yang, F.; Jiang, J.; Sun, J.; Wu, G.; Ma, A.; Ma, X. Simultaneously Improving Corrosion Resistance and Mechanical Properties of a Magnesium Alloy via Equal-Channel Angular Pressing and Post Water Annealing. Mater. Des. 2019, 166, 107621. [Google Scholar] [CrossRef]
- Liu, Y.; Wen, J.; Li, H.; He, J. Effects of Extrusion Parameters on the Microstructure, Corrosion Resistance, and Mechanical Properties of Biodegradable Mg–Zn–Gd–Y–Zr Alloy. J. Alloys Compd. 2022, 891, 161964. [Google Scholar] [CrossRef]
- Wen, J.; He, J.; Lu, X. Influence of Silicon on the Corrosion Behaviour of Al–Zn–In–Mg–Ti Sacrificial Anode. Corros. Sci. 2011, 53, 3861–3865. [Google Scholar] [CrossRef]
- Wang, J.; Li, J.-Y.; Zhang, Y.; Yu, W.-M. Effects of the Addition of Micro-Amounts of Calcium on the Corrosion Resistance of Mg-0.1Mn-1.0Zn-xCa Biomaterials. J. Mater. Eng. Perform. 2019, 28, 1553–1562. [Google Scholar] [CrossRef]
- Ralston, K.D.; Birbilis, N.; Davies, C.H.J. Revealing the Relationship between Grain Size and Corrosion Rate of Metals. Scr. Mater. 2010, 63, 1201–1204. [Google Scholar] [CrossRef]
- Yeh, T.-C.; Huang, T.-C.; Huang, H.-Y.; Huang, Y.-P.; Cai, Y.-T.; Lin, S.-T.; Wei, Y.; Yeh, J.-M. Electrochemical Investigations on Anticorrosive and Electrochromic Properties of Electroactive Polyurea. Polym. Chem. 2012, 3, 2209. [Google Scholar] [CrossRef]
- Shi, Z.; Atrens, A. An Innovative Specimen Configuration for the Study of Mg Corrosion. Corros. Sci. 2011, 53, 226–246. [Google Scholar] [CrossRef]
- King, A.D.; Birbilis, N.; Scully, J.R. Accurate Electrochemical Measurement of Magnesium Corrosion Rates; a Combined Impedance, Mass-Loss and Hydrogen Collection Study. Electrochim. Acta 2014, 121, 394–406. [Google Scholar] [CrossRef]
- Du, Y.Z.; Qiao, X.G.; Zheng, M.Y.; Wu, K.; Xu, S.W. The Microstructure, Texture and Mechanical Properties of Extruded Mg–5.3Zn–0.2Ca–0.5Ce (Wt%) Alloy. Mater. Sci. Eng. A 2015, 620, 164–171. [Google Scholar] [CrossRef]
- Cao, F.; Shi, Z.; Hofstetter, J.; Uggowitzer, P.J.; Song, G.; Liu, M.; Atrens, A. Corrosion of Ultra-High-Purity Mg in 3.5% NaCl Solution Saturated with Mg(OH)2. Corros. Sci. 2013, 75, 78–99. [Google Scholar] [CrossRef]
- Gomes, M.P.; Costa, I.; Pébère, N.; Rossi, J.L.; Tribollet, B.; Vivier, V. On the Corrosion Mechanism of Mg Investigated by Electrochemical Impedance Spectroscopy. Electrochim. Acta 2019, 306, 61–70. [Google Scholar] [CrossRef]
- Baril, G.; Galicia, G.; Deslouis, C.; Pébère, N.; Tribollet, B.; Vivier, V. An Impedance Investigation of the Mechanism of Pure Magnesium Corrosion in Sodium Sulfate Solutions. J. Electrochem. Soc. 2007, 154, C108. [Google Scholar] [CrossRef]
- Pan, H.; Pang, K.; Cui, F.; Ge, F.; Man, C.; Wang, X.; Cui, Z. Effect of Alloyed Sr on the Microstructure and Corrosion Behavior of Biodegradable Mg-Zn-Mn Alloy in Hanks’ Solution. Corros. Sci. 2019, 157, 420–437. [Google Scholar] [CrossRef]
- Chang, C.-H.; Huang, T.-C.; Peng, C.-W.; Yeh, T.-C.; Lu, H.-I.; Hung, W.-I.; Weng, C.-J.; Yang, T.-I.; Yeh, J.-M. Novel Anticorrosion Coatings Prepared from Polyaniline/Graphene Composites. Carbon 2012, 50, 5044–5051. [Google Scholar] [CrossRef]



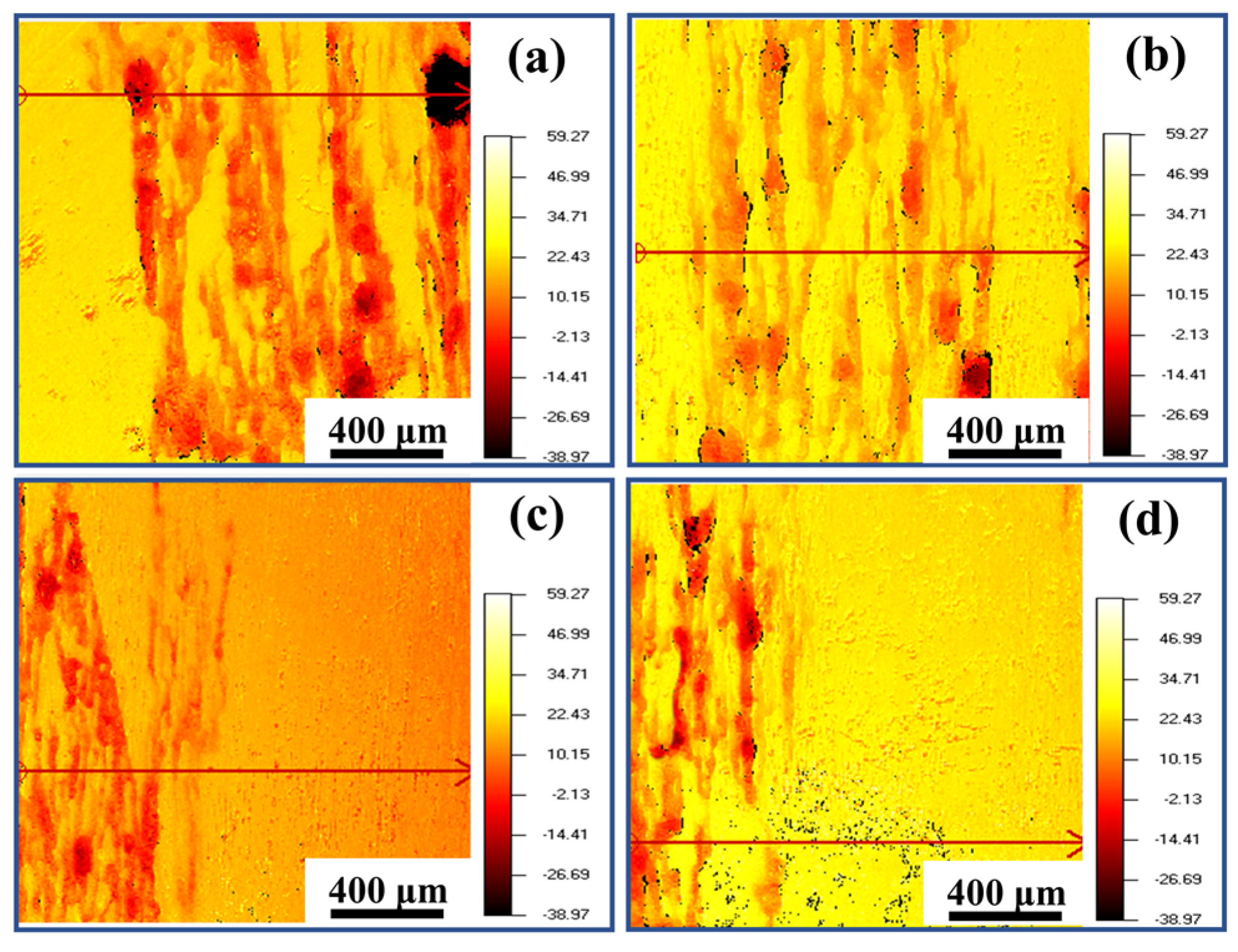
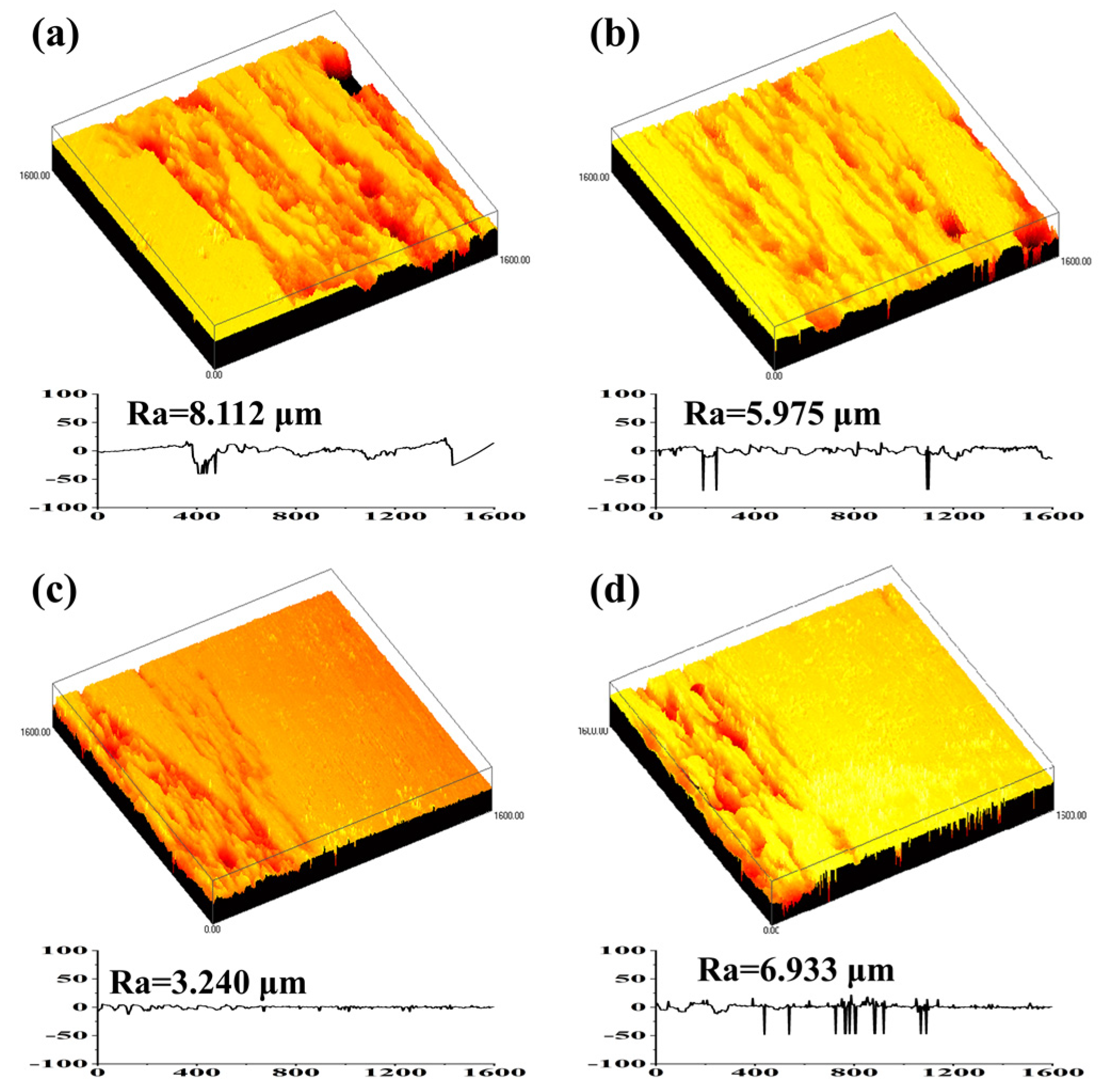

| NaCl | CaCl2 | KCl | NaHCO3 | MgCl2·6H2O | C6H12O6 | Na2HPO4·12H2O | KH2PO4 | MgSO4·7H2O |
|---|---|---|---|---|---|---|---|---|
| 8.00 | 0.14 | 0.40 | 0.35 | 0.10 | 1.00 | 0.06 | 0.06 | 0.06 |
| Position | Element (wt%) | ||||
|---|---|---|---|---|---|
| O | Ca | P | C | Mg | |
| A | 49.1 | 18.8 | 13.6 | 8.6 | 9.9 |
| B | 57.9 | 11.5 | 9.6 | 11.2 | 9.8 |
| Material | Ecorr (V/SCE) | Eb (V/SCE) | Icorr (μA/cm2) | ba (mV/dec) | bc (mV/dec) | Pi (mm/y) |
|---|---|---|---|---|---|---|
| SE46 | −1.592 | −1.328 | 5.427 | 60 | −155 | 0.132 |
| SEA18 | −1.533 | −1.369 | 4.301 | 55 | −143 | 0.104 |
| SEA20 | −1.498 | −1.310 | 3.837 | 52 | −143 | 0.093 |
| SEA22 | −1.483 | −1.332 | 2.853 | 49 | −163 | 0.069 |
| SEA24 | −1.440 | −1.244 | 2.454 | 47 | −155 | 0.060 |
| SEA26 | −1.465 | −1.308 | 3.456 | 55 | −143 | 0.084 |
| SEA28 | −1.489 | −1.354 | 3.798 | 54 | −155 | 0.092 |
| Samples | Rs (Ω·cm2) | CPEf (Ω−1·cm−2·s−n) | n1 | Rf (Ω·cm2) | CPEdl (Ω−1·cm−2·s−n) | n2 | Rct (Ω·cm2) | Rp (Ω·cm2) | IEIS (μA·cm−2) | Pi/EIS (mm·y−1) |
|---|---|---|---|---|---|---|---|---|---|---|
| SE46 | 17 | 1.14 × 10−5 | 0.68 | 109.1 | 9.22 × 10−6 | 0.91 | 8.69 × 103 | 8.80 × 103 | 4.878 | 0.118 |
| SEA18 | 36.28 | 1.10 × 10−6 | 0.67 | 125.9 | 7.77 × 10−6 | 0.92 | 9.34 × 103 | 9.47 × 103 | 4.105 | 0.100 |
| SEA20 | 36.03 | 9.79 × 10−7 | 0.68 | 141.2 | 7.98 × 10−6 | 0.91 | 9.76 × 103 | 9.90 × 103 | 3.588 | 0.087 |
| SEA22 | 24.48 | 7.53 × 10−6 | 0.70 | 140.7 | 1.00 × 10−5 | 0.90 | 1.10 × 104 | 1.11 × 104 | 2.734 | 0.066 |
| SEA24 | 24.01 | 8.62 × 10−6 | 0.70 | 244 | 7.97 × 10−6 | 0.87 | 1.27 × 104 | 1.29 × 104 | 2.266 | 0.055 |
| SEA26 | 22.04 | 7.96 × 10−6 | 0.71 | 166.7 | 1.04 × 10−5 | 0.88 | 1.17 × 104 | 1.19 × 104 | 3.275 | 0.079 |
| SEA28 | 22.97 | 7.7 × 10−5 | 0.70 | 124.8 | 2.78 × 10−5 | 0.71 | 1.04 × 104 | 1.05 × 104 | 3.423 | 0.083 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, J.; Cheng, Z.; Wen, J.; Tian, P.; Feng, W.; Zheng, X.; Gong, Y. Effect of Annealing Temperature on Microstructure and Properties of Solid Solution Extruded Mg–2.0Zn–1.0Y–0.5Zr Alloys. Alloys 2024, 3, 140-160. https://doi.org/10.3390/alloys3020008
He J, Cheng Z, Wen J, Tian P, Feng W, Zheng X, Gong Y. Effect of Annealing Temperature on Microstructure and Properties of Solid Solution Extruded Mg–2.0Zn–1.0Y–0.5Zr Alloys. Alloys. 2024; 3(2):140-160. https://doi.org/10.3390/alloys3020008
Chicago/Turabian StyleHe, Junguang, Zhenfei Cheng, Jiuba Wen, Peiwu Tian, Wuyun Feng, Xiangyang Zheng, and Yuan Gong. 2024. "Effect of Annealing Temperature on Microstructure and Properties of Solid Solution Extruded Mg–2.0Zn–1.0Y–0.5Zr Alloys" Alloys 3, no. 2: 140-160. https://doi.org/10.3390/alloys3020008




