Impact of Niobium Reduction on the Microstructure and Properties of Alloy 625 Weld Overlay Claddings: A Review
Abstract
1. Introduction
2. Historical Development and Alloy Design
3. Metallurgical Challenges of Alloy 625
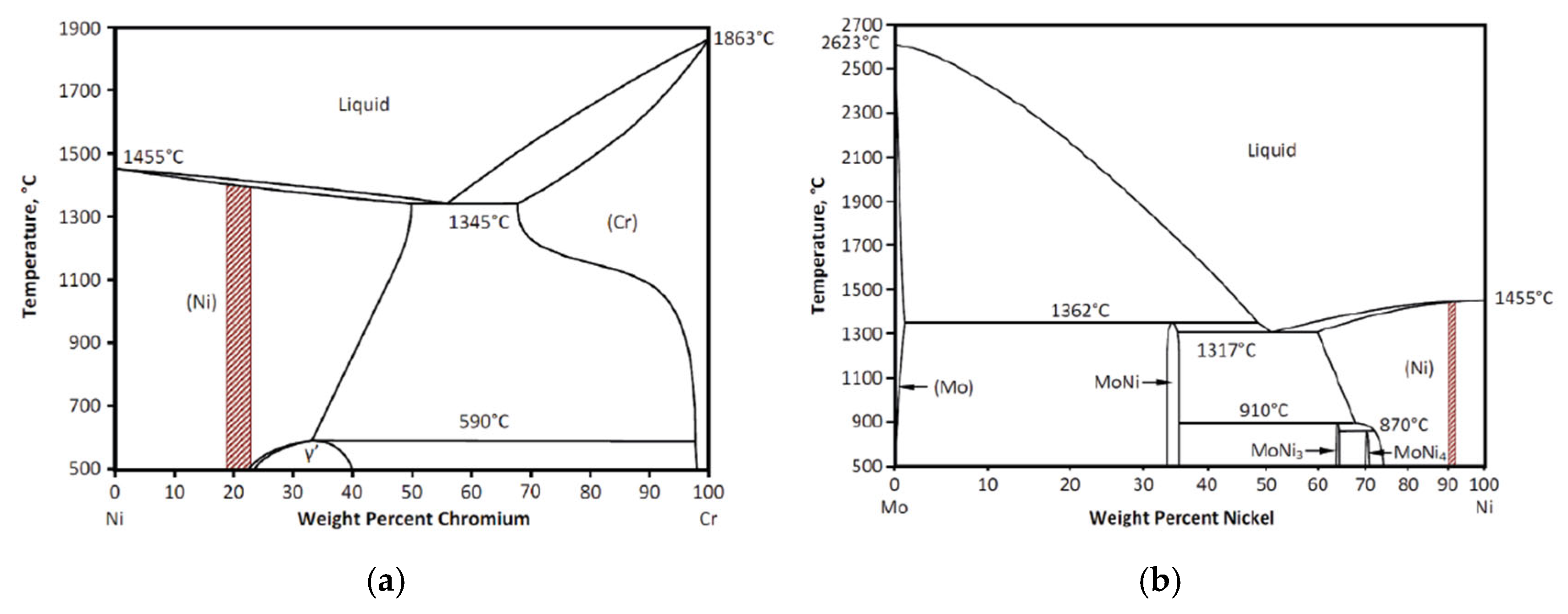
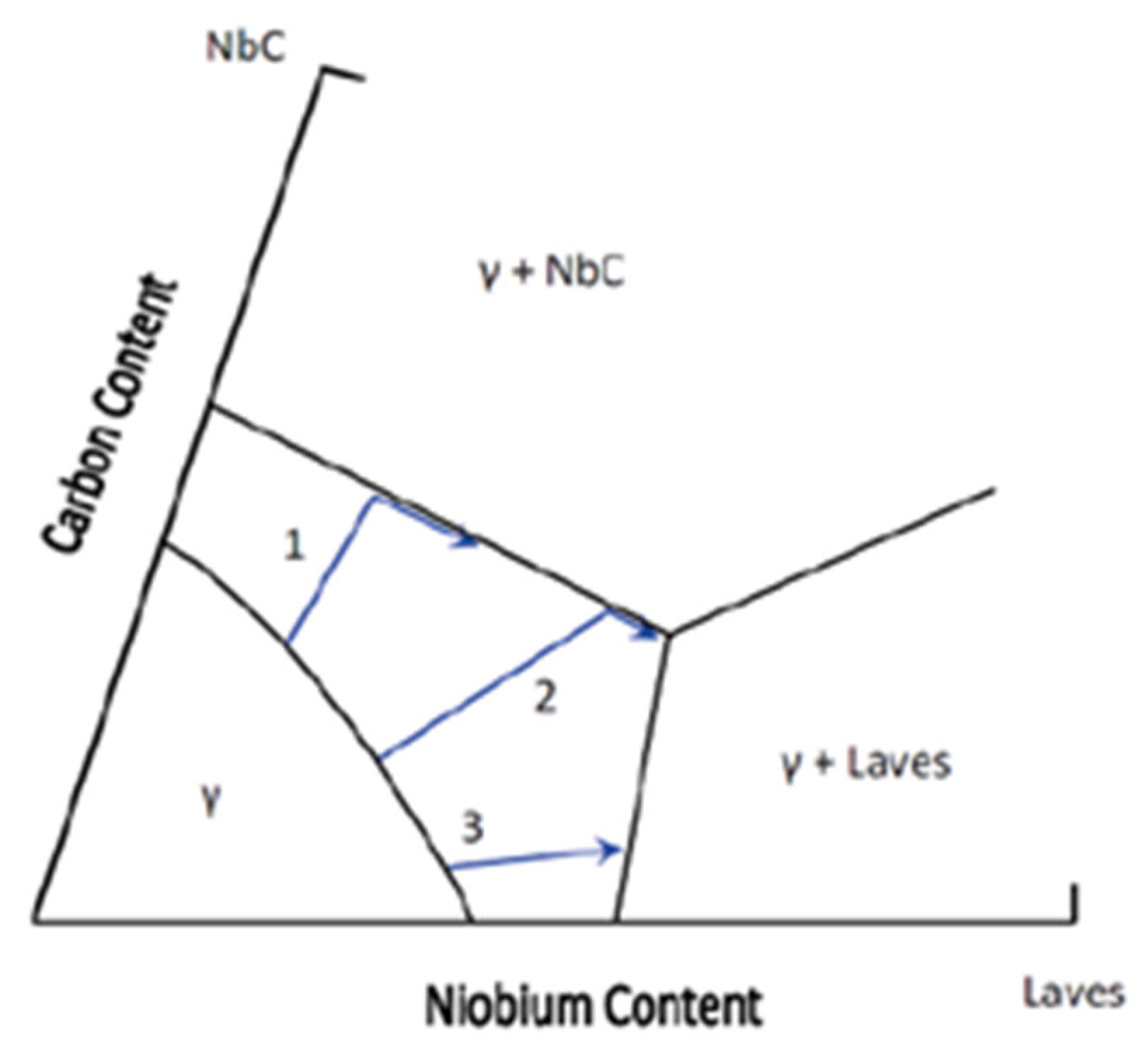
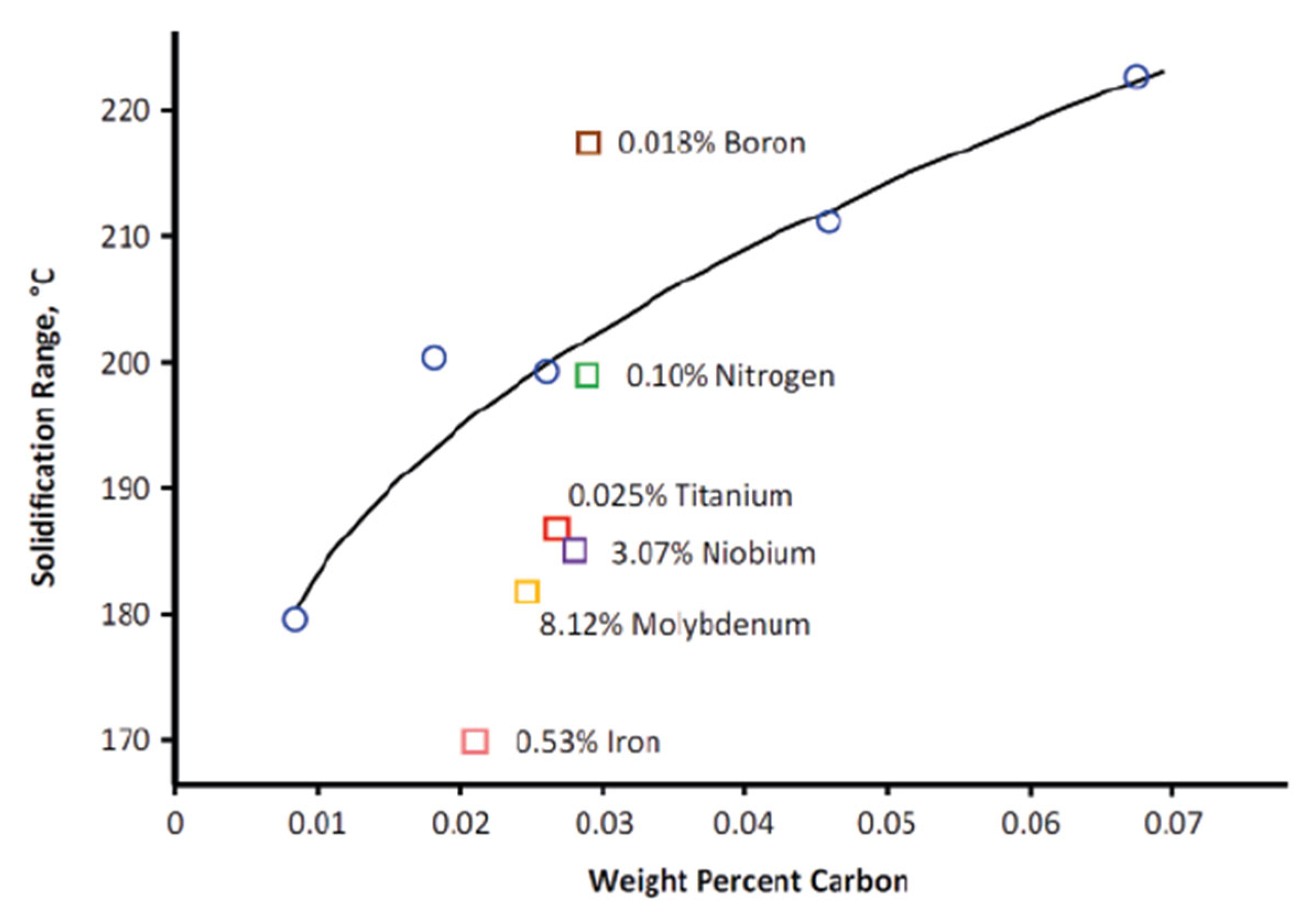
Solidification Behavior
- High C/Nb ratio: Austenite (γ) and NbC (niobium carbide) form, without the Laves phase.
- Intermediate C/Nb ratio: Initially forms γ and NbC, followed by the Laves phase during the final stages.
- Low C/Nb ratio: Results in γ and Laves phase formation, without NbC.
4. Welding Processes and Dilution Effects
- GTAW (TIG) uses a non-consumable tungsten electrode and an inert gas shield (typically argon) to produce high-quality, precise welds, especially suited for thin sections of stainless steel and non-ferrous metals [43].
- GMAW (MIG) employs a continuously fed consumable wire electrode and a shielding gas, offering high productivity and ease of automation [44].
- FCAW is a semi-automatic or automatic process that uses a tubular wire filled with flux, which may or may not require an external shielding gas, offering good penetration and high deposition rates, even in outdoor or windy conditions [45].
4.1. Mechanical Benefits [46,47]
- Weld overlay cladding can enhance mechanical properties, including high strength and wear resistance.
- It has been found that weld overlay can produce better adhesion between the substrate and clads to enhance mechanical and tribological characteristics.
- The use of weld overlay on medium-strength low alloy steel has been shown to improve both the strength and corrosion resistance.
4.2. Corrosion Resistance Benefits [45]
- Weld overlay offers clear advantages in areas of wear and corrosion protection for pipeline systems and oil and gas equipment.
- Nickel-based weld overlays are commonly used in the oil and gas industry while preserving the based metal strength, toughness, and compatibility with the external medium, including cathodic protection to improve corrosion resistance without significantly increasing manufacturing costs.
- Cladding with corrosion-resistant alloys provides compact and tightly bonded layers on less expensive materials, offering protection against corrosion in various industries such as oil and gas, chemical, and energy.
- The use of weld overlay has been widely employed to protect components from wear and corrosion, providing good resistance to general corrosion in coal-fired power plants.
4.3. Economic Benefits [37]
- Weld overlay is a recognized cost-saving technology that offers practical and financial benefits by increasing the life expectancy of marine equipment and components, both offshore and onshore, in the oil and gas industry.
- Weld cladding offers a cost-effective solution for achieving corrosion resistance in various industrial applications. By significantly reducing the cost of materials and improving the durability of components, it provides a practical alternative to using solid alloys.
- The technology of weld overlay provides a heavy-duty metallurgically bonded protective layer, avoiding the need to produce entire components from expensive corrosion-resistant materials, thus resulting in cost savings.
4.4. Environmental Benefits [48]
- Weld overlay cladding can be used to protect equipment in aggressive environments, such as those exposed to acids and chlorides, thereby reducing the impact of erosion due to sand and extending the service life of the equipment.
- The use of weld overlay cladding in petroleum refinery processing has been shown to provide protection against sulfidation attack, contributing to the reduction of high-temperature corrosion issues in the industry.
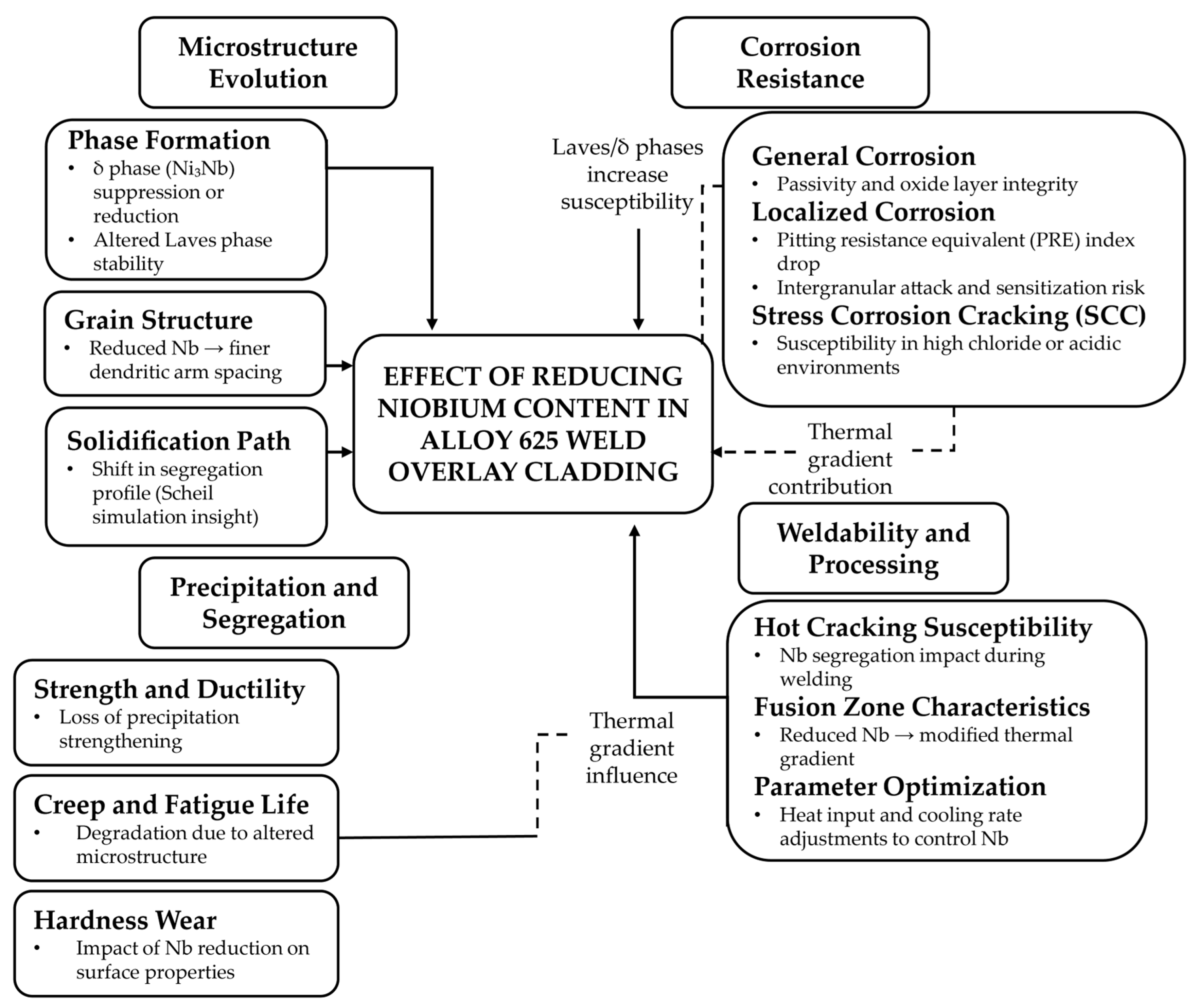
5. Effect of Niobium Reduction on Alloy 625 Cladding
5.1. Microstructure of Alloy 625 Weld Overlay Cladding
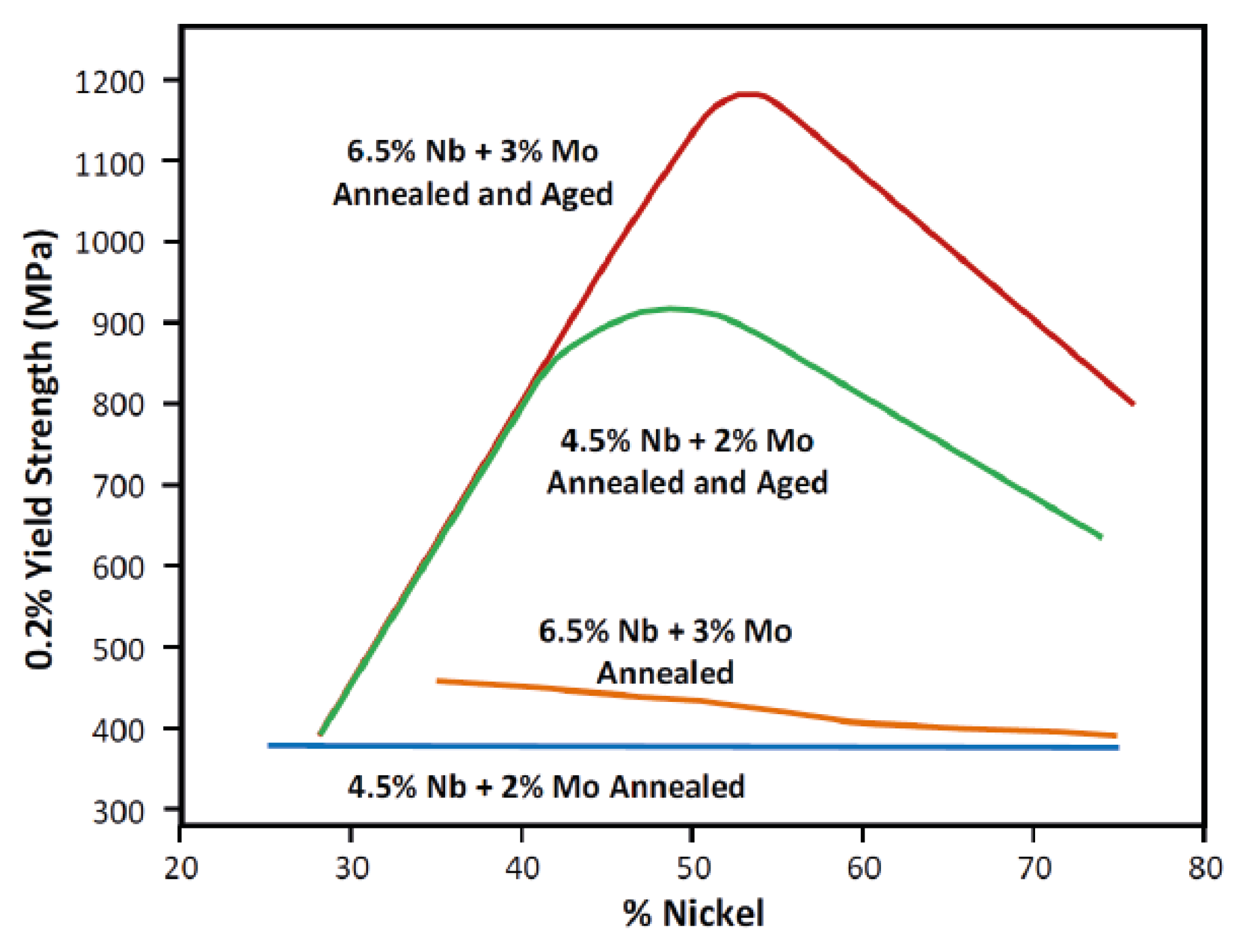
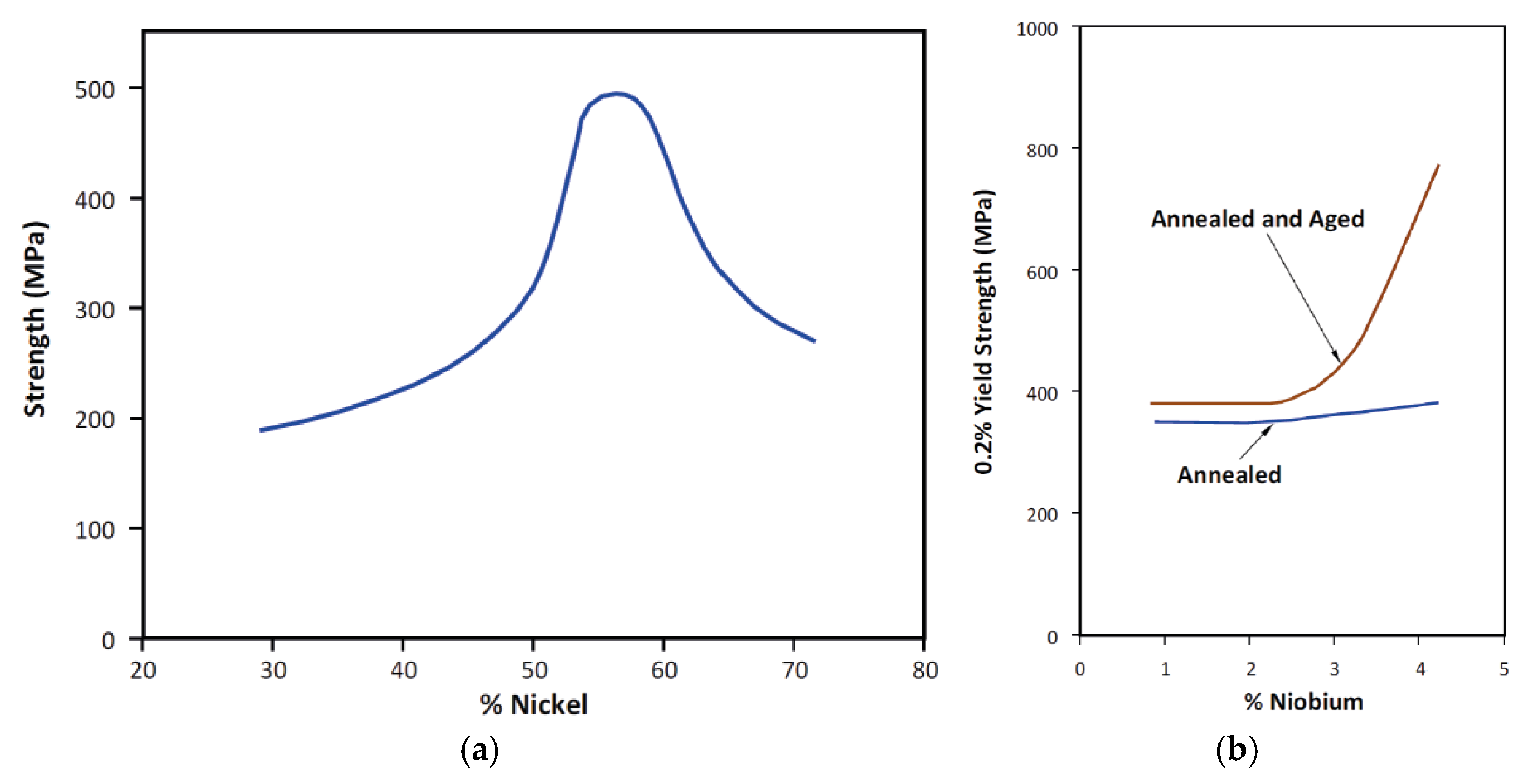
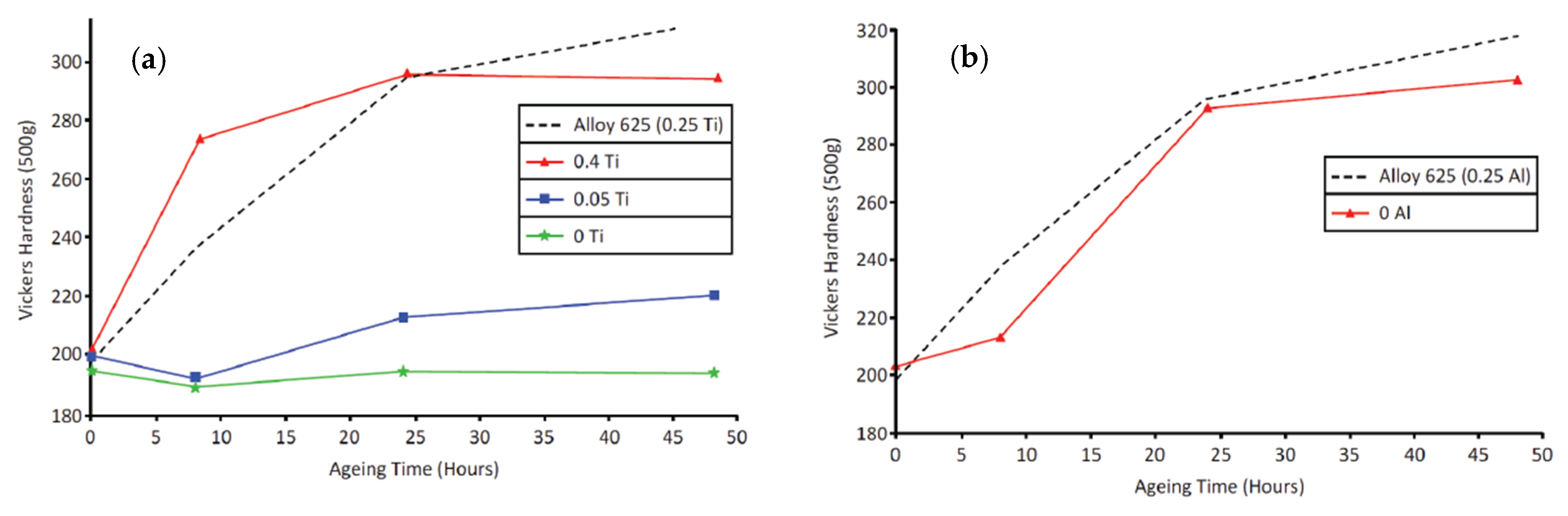
5.2. Mechanical Properties of Alloy 625 Weld Overlay
5.3. Corrosion Resistance of Alloy 625 Weld Overlay
5.4. Weldability
5.5. Processability and Performance Summary
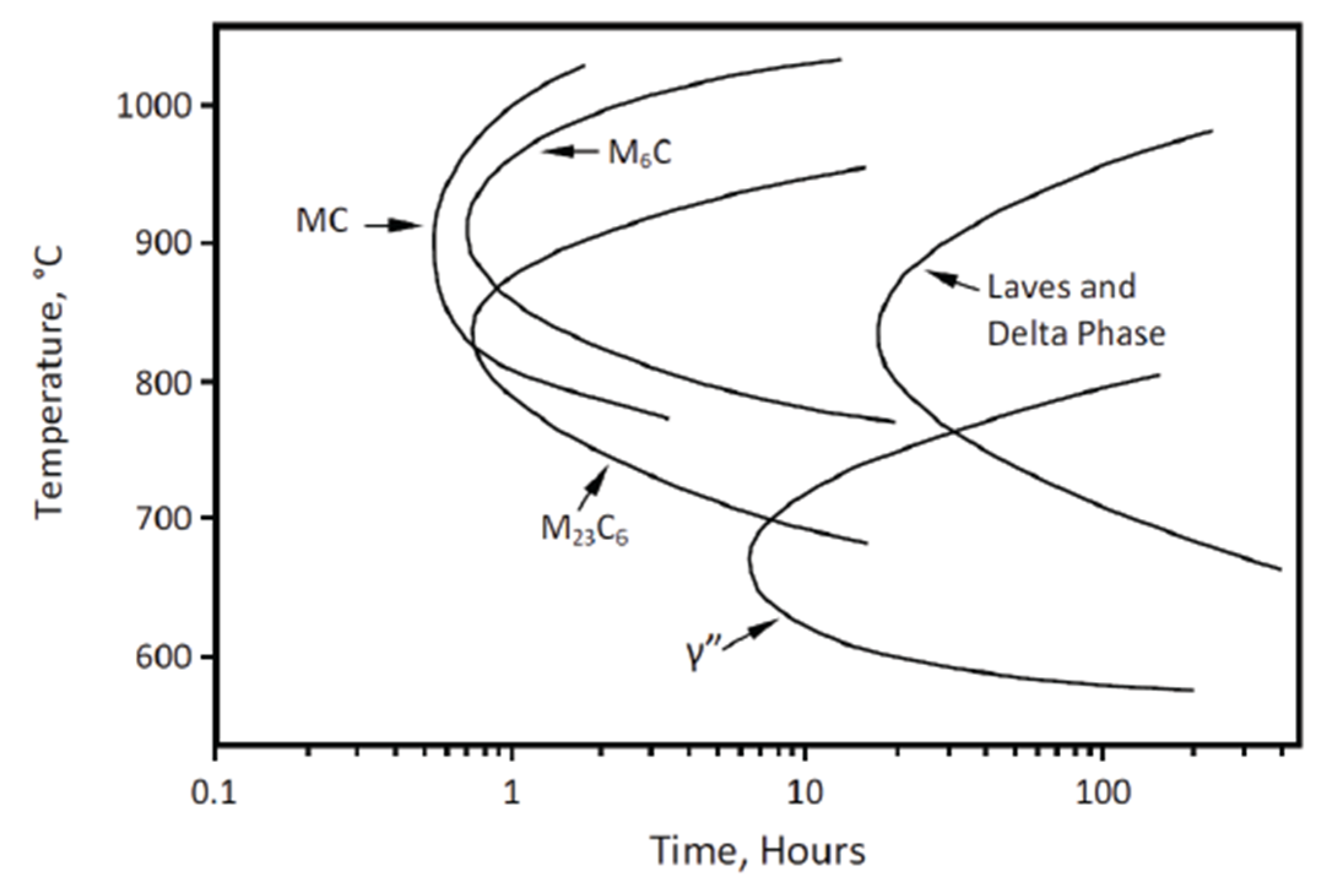
6. Phase Transformations and Nb Influence
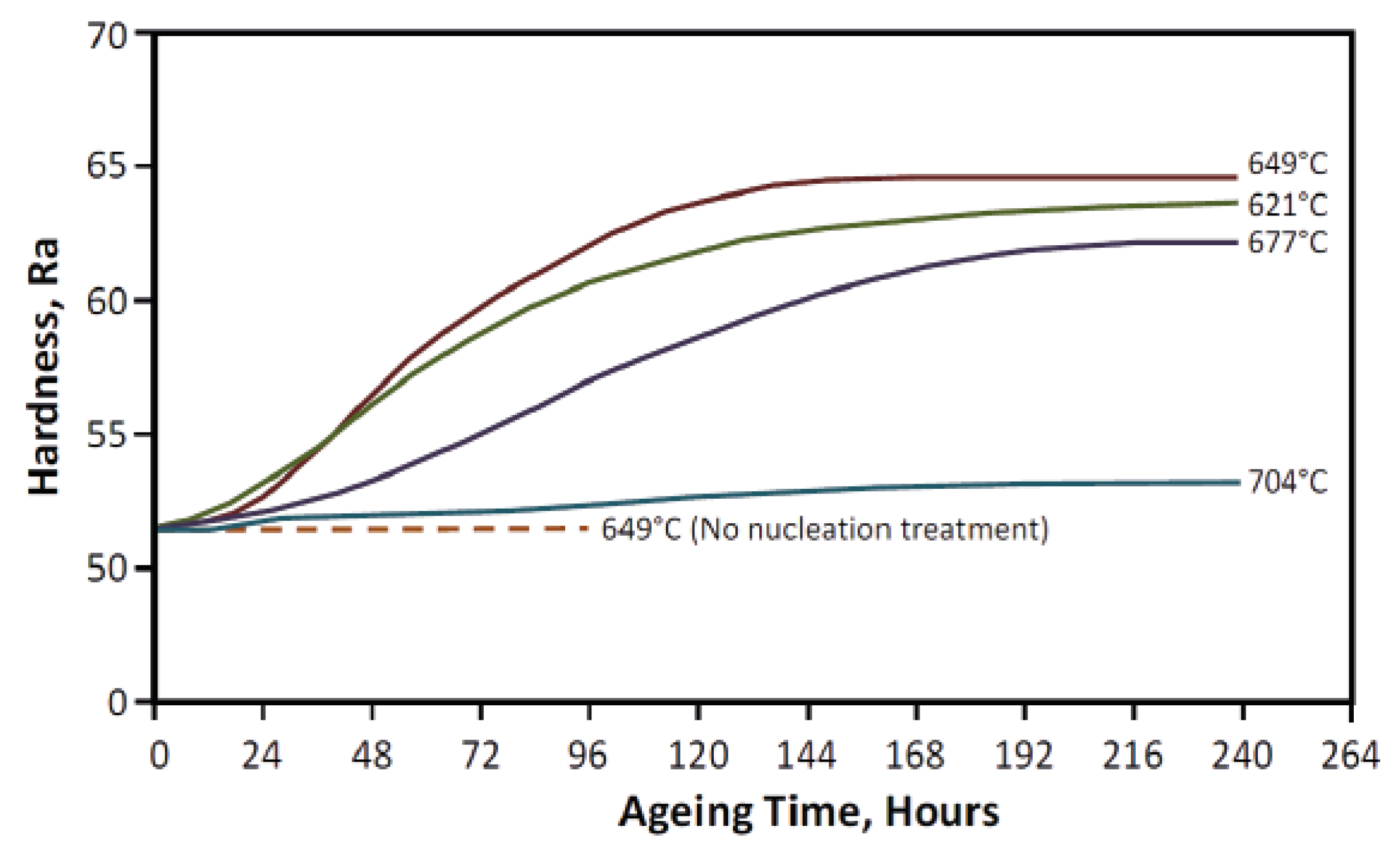
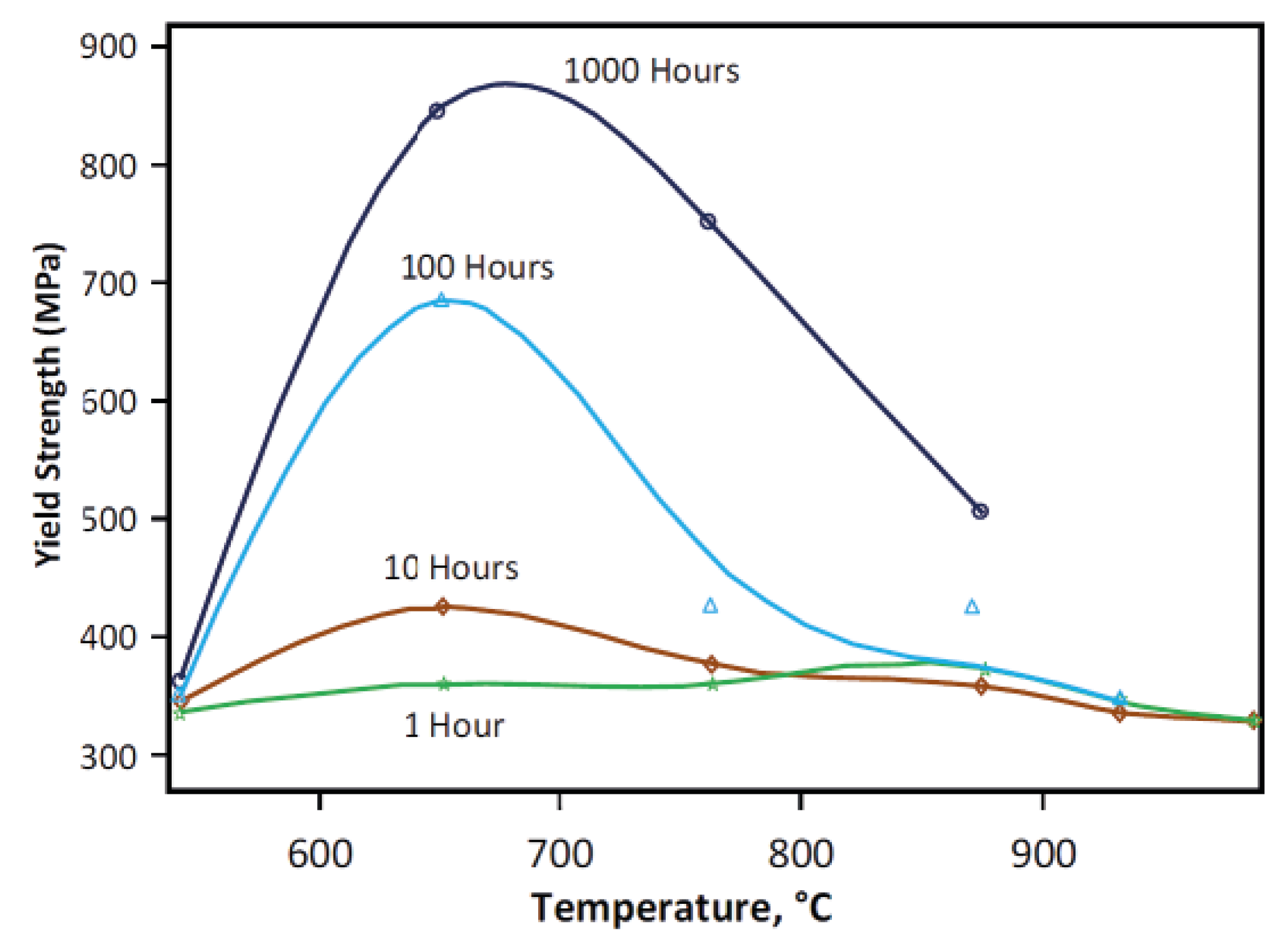
6.1. γ” Precipitation
6.2. Carbides
6.3. Intermetallics
7. Compositional Modification
8. Discussion
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Floreen, S.; Fuchs, G.; Yang, W. The Metallurgy of Alloy 625. In Superalloys 718, 625, 706 and Various Derivatives; Loria, E.A., Ed.; The Minerals, Metals & Materials Society: Pittsburgh, PA, USA, 1994. [Google Scholar] [CrossRef]
- Eiselstein, H.; Tillack, D. The Invention and Definition of Alloy 625. In Superalloys 718, 625 and Various Derivatives; Loria, E.A., Ed.; The Minerals, Metals & Materials Society: Pittsburgh, PA, USA, 1991. [Google Scholar] [CrossRef]
- NACE MR0175/ISO 15156; Petroleum and Natural Gas Industries—Materials for Use in H2S-Containing Environments in Oil and Gas Production. NACE International: Houston, TX, USA, 2024; p. 24.
- Eiselstein, H.L. Metallurgy of a columbium-hardened nickel-chromium-iron alloy. In Advances in the Technology of Stainless Steels and Related Alloys; ASTM: West Conshohocken, PA, USA, 1965; pp. 62–79. [Google Scholar] [CrossRef]
- DuPont, J.N.; Lippold, J.C.; Kiser, S.D. Welding Metallurgy and Weldability of Nickel-Base Alloys; Wiley: Hoboken, NJ, USA, 2009. [Google Scholar] [CrossRef]
- Bowman, R. Superalloys: A primer and history. In Proceedings of the 9th International Symposium on Superalloys, Warrendale, PA, USA, 17–21 September 2000; p. 6. [Google Scholar]
- Dupont, J.N.; Robino, C.V.; Marder, A.R. Solidification and weldability of Nb-bearing superalloys. Weld. J. 1998, 77, 417–431. [Google Scholar]
- Kumara, C.; Balachandramurthi, A.R.; Goel, S.; Hanning, F.; Moverare, J. Toward a better understanding of phase transformations in additive manufacturing of Alloy 718. Materialia 2020, 13, 100862. [Google Scholar] [CrossRef]
- DeArdo, A.J. Niobium in modern steels. Int. Mater. Rev. 2003, 48, 371–402. [Google Scholar] [CrossRef]
- Pasiowiec, H.; Dubiel, B.; Dziurka, R.; Bała, P.; Ledwig, P.; Wróbel, M.; Gajewska, M.; Ziaja, W.; Poręba, M. Effect of creep deformation on the microstructure evolution of Inconel 625 nickel-based superalloy additively manufactured by laser powder bed fusion. Mater. Sci. Eng. A 2023, 887, 145742. [Google Scholar] [CrossRef]
- Singh, J.B. (Ed.) Physical Metallurgy of Alloy 625. In Alloy 625: Microstructure, Properties and Performance; Chapter 3; Springer Nature: Singapore, 2022; pp. 67–110. [Google Scholar] [CrossRef]
- American Welding Society (AWS). AWS A5.14: Specification for Nickel and Nickel-Alloy Bare Welding Electrodes and Rods; AWS: Miami, FL, USA, 2024. [Google Scholar]
- Silva, C.C.; de Miranda, H.C.; Motta, M.F.; Farias, J.P.; Afonso, C.R.M.; Ramirez, A.J. New insight on the solidification path of an alloy 625 weld overlay. J. Mater. Res. Technol. 2013, 2, 228–237. [Google Scholar] [CrossRef]
- Zhao, S.; Xie, X.; Smith, G.D.; Patel, S.J. Microstructural stability and mechanical properties of a new nickel-based superalloy. Mater. Sci. Eng. A 2003, 355, 96–105. [Google Scholar] [CrossRef]
- Belan, J.; Kuchariková, L.; Vaško, A.; Tillová, E.; Chalupová, M.; Matvija, M. The SEM and TEM Analysis of IN718 Alloy after Fatigue Push-Pull Loading at 700 °C. Defect Diffus. Forum 2020, 405, 288–293. [Google Scholar] [CrossRef]
- Petrzak, P.; Kowalski, K.; Blicharski, M. Analysis of Phase Transformations in Inconel 625 Alloy during Annealing. Acta Phys. Pol. A 2016, 130, 1041–1044. [Google Scholar] [CrossRef]
- Lippold, J.C. Welding Metallurgy and Weldability; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar]
- Bunaziv, I.; Olden, V.; Akselsen, O.M. Metallurgical Aspects in the Welding of Clad Pipelines—A Global Outlook. Appl. Sci. 2019, 9, 3118. [Google Scholar] [CrossRef]
- Silva, C.C.; de Albuquerque, V.H.C.; Miná, E.M.; Moura, E.P.; Tavares, J.M.R.S. Mechanical Properties and Microstructural Characterization of Aged Nickel-based Alloy 625 Weld Metal. Met. Mater. Trans. A 2018, 49, 1653–1673. [Google Scholar] [CrossRef]
- Maltin, C.A.; Galloway, A.M.; Mweemba, M. Microstructural Evolution of Inconel 625 and Inconel 686CPT Weld Metal for Clad Carbon Steel Linepipe Joints: A Comparator Study. Met. Mater. Trans. A 2014, 45, 3519–3532. [Google Scholar] [CrossRef]
- Gibson, C.; Kelebek, S.; Aghamirian, M. Niobium oxide mineral flotation: A review of relevant literature and the current state of industrial operations. Int. J. Miner. Process. 2015, 137, 82–97. [Google Scholar] [CrossRef]
- Bayraktar, E.; Gatamorta, F.; Enginsoy, H.M.; Polis, J.E.; Miskioglu, I. New Design of Composites from Fresh Scraps of Niobium for Tribological Applications. In Mechanics of Composite, Hybrid and Multifunctional Materials: Conference Proceedings of the Society for Experimental Mechanics Series; Springer: Cham, Switzerland, 2021; pp. 35–43. [Google Scholar] [CrossRef]
- Kirkwood, P. Welding Niobium Bearing HSLA Steels “Myths and Magic”. In HSLA Steels 2015, Microalloying 2015 & Offshore Engineering Steels 2015: Conference Proceedings; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015. [Google Scholar] [CrossRef]
- Procario, J.R.; Melfi, T. Submerged arc welding solutions for niobium micro-alloyed pipe steel—Weld metal alloy systems. In Proceedings of the Rio Pipeline Conference & Exposition 2011, Rio de Janeiro, Brazil, 20–22 September 2011. Technical Papers. [Google Scholar]
- Almatani, R.A.; DeArdo, A.J. Rational Alloy Design of Niobium-Bearing HSLA Steels. Metals 2020, 10, 413. [Google Scholar] [CrossRef]
- Hariharan, K.; Iams, A.D.; Zuback, J.S.; Palmer, T.A.; Sridhar, N.; Alazemi, R.M.; Frankel, G.S.; Schindelholz, E.J. Enhanced localized corrosion resistance of Ni-based alloy 625 processed by directed energy deposition additive manufacturing. Corros. Sci. 2024, 230, 111945. [Google Scholar] [CrossRef]
- Khaple, S.; Prakash, U.; Golla, B.R.; Prasad, V.V.S. Effect of Niobium Addition on Microstructure and Mechanical Properties of Fe–7Al–0.35C Low-Density Steel. Met. Microstruct. Anal. 2020, 9, 127–139. [Google Scholar] [CrossRef]
- Hojerslev, C.; Tiedje, N.; Hald, J. Segregation Effects and Phase Developments during Solidification of Alloy 625. Mater. Sci. Forum 2006, 508, 373–378. [Google Scholar] [CrossRef]
- Yamamoto, K.; Kimura, Y.; Mishima, Y. Effect of Matrix Substructures on Precipitation of the Laves Phase in Fe-Cr-Nb-Ni System. ISIJ Int. 2003, 43, 1253–1259. [Google Scholar] [CrossRef]
- Baker, H. (Ed.) Alloy Phase Diagrams, 10th ed.; ASM Handbook; ASM International: Novelty, OH, USA, 1992; Volume 3. [Google Scholar]
- Nash, P. Ni-Cr System; ASM International: Novelty, OH, USA, 1991. [Google Scholar]
- Singleton, M.F.; Nash, P. Ni-Mo System; ASM International: Novelty, OH, USA, 1991. [Google Scholar]
- DuPont, J.N. Solidification of an alloy 625 weld overlay. Met. Mater. Trans. A 1996, 27, 3612–3620. [Google Scholar] [CrossRef]
- DuPont, J.N.; Babu, S.; Liu, S. Welding of materials for energy applications. Metall. Mater. Trans. A 2013, 44, 3385–3410. [Google Scholar] [CrossRef]
- Ogborn, J.S.; Olson, D.L.; Cieslak, M.J. Influence of solidification on the microstructural evolution of nickel base weld metal. Mater. Sci. Eng. A 1995, 203, 134–139. [Google Scholar] [CrossRef]
- Perricone, M.J.; DuPont, J.N.; Cieslak, M.J. Solidification of hastelloy alloys: An alternative interpretation. Met. Mater. Trans. A 2003, 34, 1127–1132. [Google Scholar] [CrossRef]
- Farkade, N.V.; Ravanan, P.M. Modification in Weld Overlay for Productivity and Corrosion Resistance. Int. J. Sci. Technol. Eng. 2015, 2, 37–41. Available online: https://www.semanticscholar.org/paper/5b2743a78674ec6b4f57319dfa50641e6dee3520 (accessed on 28 April 2025).
- Reda, A.; Noor, M.Z.Z.; Karrech, A. CRA Clad Pipes: Do Their Benefits Justify Sole Selection? J. Pipeline Sci. Eng. 2024, 100245. [Google Scholar] [CrossRef]
- Reda, A.; Kaspary, T.; Chimbli, S.; Montague, P.; Goodier, S.; Shahin, M.A. Material Review of Geothermal Resources Extracted from Deep Pilot Wells in the Salton Sea. In Proceedings of the AMPP Annual Conference + Expo 2025, Nashville, TN, USA, 6–10 April 2025. [Google Scholar] [CrossRef]
- Reda, A.; Shahin, M.A.; Montague, P. Review of Material Selection for Corrosion-Resistant Alloy Pipelines. Eng. Sci. 2025, 33, 1373. [Google Scholar] [CrossRef]
- Badisch, E.; Roy, M. Hardfacing for wear, erosion and abrasion. In Surface Engineering for Enhanced Performance Against Wear; Springer: Vienna, Austria, 2013. [Google Scholar] [CrossRef]
- Brown, A. Increasing the life expectancy of marine equipment. Mar. Eng. Rev. 2007, 32–33. [Google Scholar]
- Zhang, Y.M.; Lu, W.; Liu, Y.C. Guided Arc Enhances GTAW. Weld. J. 2003, 82, 40–45. [Google Scholar]
- Rajkumar, V.; Arjunan, T.V.; Kannan, A.R. Metallurgical and mechanical investigations of Inconel 625 overlay welds produced by GMAW-hardfacing process on AISI 347 pipes. Mater. Res. Express 2019, 6, 076534. [Google Scholar] [CrossRef]
- Alvarães, C.P.; Madalena, F.C.A.; de Souza, L.F.G.; Jorge, J.C.F.; Araújo, L.S.; Mendes, M.C. Performance of the INCONEL 625 alloy weld overlay obtained by FCAW process. Matéria 2019, 24, e12290. [Google Scholar] [CrossRef]
- Mohammed, R.; Kumar, E.N.; Ram, G.J.; Kamaraj, M.; Reddy, G.M.; Rao, K.S. Microstructure, Mechanical and Corrosion Behaviour of Weld Overlay Cladding of DMR 249A steel with AISI 308L. Mater. Today Proc. 2019, 15, 2–10. [Google Scholar] [CrossRef]
- Wang, Z.L.; Ding, X. Mechanical Property and Corrosion Resistance of the E309L Buffer Layer in Weld Overlay. Appl. Mech. Mater. 2014, 668–669, 39–42. [Google Scholar] [CrossRef]
- Kumar, R.; Bose, S. Influence of process parameters on mechanical properties in weld cladding. In Recent Advancements in Computational Intelligence and Design Engineering; CRC Press: Boca Raton, FL, USA, 2024; pp. 399–402. [Google Scholar] [CrossRef]
- Singh, J. Corrosion Behavior of Alloy 625. In Alloy 625. Materials Horizons: From Nature to Nanomaterials; Springer: Singapore, 2022; pp. 241–291. [Google Scholar] [CrossRef]
- Yu, H.; Wu, K.; Dong, B.; Yu, L.; Liu, J.; Liu, Z.; Xiao, D.; Jing, X.; Liu, H. Effect of Niobium Content on the Microstructure and Mechanical Properties of Simulated Coarse-Grained Heat-Affected Zone (CGHAZ) of High-Strength Low-Alloy (HSLA) Steels. Materials 2022, 15, 3318. [Google Scholar] [CrossRef] [PubMed]
- Jie, G.; Qingchao, M.; Yan, S.; Kangning, W.; Qiang, S.; Canming, W. Effect of Nb content on microstructure and corrosion resistance of Inconel 625 coating formed by laser cladding. Surf. Coat. Technol. 2023, 458, 129311. [Google Scholar] [CrossRef]
- Tinoco, J.; Fredriksson, H. Solidification of a Modified Inconel 625 Alloy under Different Cooling Rates. High Temp. Mater. Process. 2011, 23, 13–24. [Google Scholar] [CrossRef]
- Qi, Z.; Li, Y.; Qi, Y.; Fan, J.; Ma, C.; Tian, Z. Effect of Nb content variations on the microstructure and mechanical properties of NiCrMo welded joints. Mater. Charact. 2024, 212, 113974. [Google Scholar] [CrossRef]
- Mishra, R.; Pandit, D.; Imam, M. Microstructure, mechanical and corrosion study of friction stir processed Inconel 625 additive layers deposited via wire arc direct energy deposition. Addit. Manuf. 2024, 86, 104193. [Google Scholar] [CrossRef]
- Smith, G.D.; Eisinger, N.C. The effect of niobium on the corrosion resistance of nickel-base alloys. In Proceedings of the International Symposium on Niobium for High Temperature Applications, Araxa, Brazil, 1–3 December 2003; pp. 23–34. [Google Scholar]
- Guo, L.; Xiao, F.; Wang, F.; Wei, W.; He, Y.; Luo, F. Influence of heat treatments on microstructure, mechanical properties and corrosion resistance of Inconel 625 overlay cladded using PTIG. Mater. Res. Express 2020, 7, 096517. [Google Scholar] [CrossRef]
- Tayactac, R.G.; Ang, E.B. Assessment of Corrosion Resistant Alloy (CRA) Clad Material for Geothermal Wellhead Piping System. ASEAN Eng. J. 2022, 12, 207–219. [Google Scholar] [CrossRef]
- Nandhakumar, S.; Kumar, K.G.; Arivazhagan, N.; Manikandan, M.; Jose, B.; Renangi, S. Technology Development of Novel Autogenous Double Pulse Tungsten Insert Gas Welding Technique to Evaluate the Depth of penetration, Micro segregation via Machine learning and Desirability Function Approach. Mater. Today Commun. 2024, 40, 109614. [Google Scholar] [CrossRef]
- Moradi, M.J.; Ketabchi, M. Corrosion resistance and microstructure of alloy 625 weld overlay on ASTM A516 grade 70. Mater. Test. 2015, 58, 48–55. [Google Scholar] [CrossRef]
- Han, J.W.; Jung, S.H.; Lee, H.W. Corrosion Characteristics of Newly Designed Inconel Alloy Weldments Using Varying Nb Content in Heat Treatment. Met. Mater. Int. 2022, 28, 733–744. [Google Scholar] [CrossRef]
- Moura, B.B.; Souza, D.; Silva, M.d.O.; Osorio, A.G. Effect of Inert and Active Shielding Gases in the Corrosion Resistance of IN625 Weld Overlays. J. Mater. Eng. Perform. 2022, 31, 5886–5897. [Google Scholar] [CrossRef]
- Alexandrov, B.T.; Lippold, J.C.; Sowards, J.W.; Hope, A.T.; Saltzmann, D.R. Fusion boundary microstructure evolution associated with embrittlement of Ni–base alloy overlays applied to carbon steel. Weld. World 2013, 57, 39–53. [Google Scholar] [CrossRef]
- Liu, D.; Cheng, X.; Zhang, X.; Ding, Y. Effects of heating and hot extrusion process on microstructure and properties of Inconel 625 alloy. J. Wuhan Univ. Technol. Sci. Ed. 2016, 31, 1368–1376. [Google Scholar] [CrossRef]




| Ni | Cr | Mo | Nb | Fe | C | Si | Al | Ti | Mn | S |
|---|---|---|---|---|---|---|---|---|---|---|
| 58.0 min | 19.0–23.0 | 8.0–10.0 | 3.15–4.15 | 5.0 max | 0.10 max | 1.0 max | 0.4 max | 0.4 max | 0.5 max | 0.015 max |
| Aspect | High Nb Content | Reduced Nb Content |
|---|---|---|
| Microstructure | Coarse, segregated, brittle phases | Fine-grained, homogeneous |
| Mechanical Strength | High strength, low ductility | Moderate strength, improved ductility |
| Corrosion Resistance | Risk of localized corrosion | Balanced passivation, fewer defects |
| Weldability | Higher cracking tendency | Improved weldability, fewer defects |
| Processability | Complex welding procedures required | Simplified welding, cost efficiency |
| Element (Minimize) | Minimize Formation of Niobium Carbide During Solidification | Minimize Formation of Laves Phase During Solidification | Minimize Carbide Precipitation During Heat Treatment | Minimize Laves and Delta Precipitation During Heat Treatment | Avoid γ” Precipitation |
|---|---|---|---|---|---|
| Nb | Positive Effect | Positive Effect | Positive Effect | Positive Effect | Positive Effect |
| Fe | No Effect | Positive Effect | No Effect | No Effect | No Effect |
| Mo | No Effect | Positive Effect | Positive Effect | Positive Effect (2) | No Effect |
| Al and Ti | No Effect | No Effect | No Effect | No Effect | Positive Effect |
| C | Positive Effect | No Effect (1) | Positive Effect | No Effect | No Effect |
| Si | No Effect | Positive Effect | Negative Effect | Positive Effect (2) | No Effect |
| Element (Minimize) | Strength | Corrosion Resistance | Weldability |
|---|---|---|---|
| Nb | Negative Effect | No Effect | Positive Effect |
| Fe | Negative Effect | No Effect | Positive Effect |
| Mo | Negative Effect | Negative Effect | Positive Effect |
| Al and Ti | Negative Effect (1) | No Effect | Negative Effect (2) |
| C | No Effect | Negative Effect (3) | Positive Effect |
| Si | No Effect | Negative Effect (3) | Positive Effect |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tayactac, R.G.; Manuel, M.C.E.; Honra, J.P.; Kaspary, T.B.; de Medeiros, R.C. Impact of Niobium Reduction on the Microstructure and Properties of Alloy 625 Weld Overlay Claddings: A Review. Alloys 2025, 4, 12. https://doi.org/10.3390/alloys4030012
Tayactac RG, Manuel MCE, Honra JP, Kaspary TB, de Medeiros RC. Impact of Niobium Reduction on the Microstructure and Properties of Alloy 625 Weld Overlay Claddings: A Review. Alloys. 2025; 4(3):12. https://doi.org/10.3390/alloys4030012
Chicago/Turabian StyleTayactac, Reylina Garcia, Mark Christian E. Manuel, Jaime P. Honra, Tiago Bohn Kaspary, and Raimundo Cabral de Medeiros. 2025. "Impact of Niobium Reduction on the Microstructure and Properties of Alloy 625 Weld Overlay Claddings: A Review" Alloys 4, no. 3: 12. https://doi.org/10.3390/alloys4030012
APA StyleTayactac, R. G., Manuel, M. C. E., Honra, J. P., Kaspary, T. B., & de Medeiros, R. C. (2025). Impact of Niobium Reduction on the Microstructure and Properties of Alloy 625 Weld Overlay Claddings: A Review. Alloys, 4(3), 12. https://doi.org/10.3390/alloys4030012