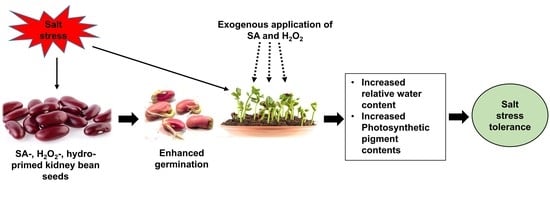
Alleviation of Salt-Inhibited Germination and Seedling Growth of Kidney Bean by Seed Priming and Exogenous Application of Salicylic Acid (SA) and Hydrogen Peroxide (H2O2)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site of Experiment, Treating Conditions, and Germination Indices Measurement
2.2. Pot Experiment
2.3. Relative Water Content Determination
2.4. Estimation of Photosynthetic Pigments
2.5. Statistical Analysis
3. Results
3.1. Priming Boosts Germination Indices and Traits of Seedlings under Salt Stress
3.2. Exogenous SA and H2O2 Enhance Growth and RWC of Plants under Salt Stress
3.3. Pretreatment of SA and H2O2 Regulate Photosynthetic Pigment of Kidney under Salt Stress
3.4. Estimation of Treatment-Variable Interactions through Heatmap and PCA
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Finch-Savage, W.E.; Bassel, G.W. Seed vigour and crop establishment: Extending performance beyond adaptation. J. Exp. Bot. 2016, 67, 567–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rhaman, M.S.; Kibria, M.G.; Hoque, A. Climate Change and Its Adverse Impacts on Plant Growth in South Asia: Current Status and Upcoming Challenges. Phyton 2022, 91, 695. [Google Scholar]
- Arun, M.N.; Hebbar, S.S.; Senthivel, T.; Nair, A.K.; Padmavathi, G.; Pandey, P.; Singh, A. Seed Priming: The Way Forward to Mitigate Abiotic Stress in Crops. In Plant Stress Physiology-Perspectives in Agriculture. In Plant Stress Physiology-Perspectives in Agriculture; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Isayenkov, S.V.; Maathuis, F.J. Plant salinity stress: Many unanswered questions remain. Front. Plant Sci. 2019, 10, 80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gopalakrishnan, T.; Kumar, L. Modeling and mapping of soil salinity and its impact on Paddy Lands in Jaffna Peninsula, Sri Lanka. Sustainability 2020, 12, 8317. [Google Scholar] [CrossRef]
- Machado, R.M.A.; Serralheiro, R.P. Soil Salinity: Effect on Vegetable Crop Growth. Management Practices to Prevent and Mitigate Soil Salinization. Horticulturae 2017, 3, 30. [Google Scholar] [CrossRef]
- Rhaman, M.S.; Kibria, M.G.; Hossain, M.; Hoque, M.A. Effects of organic manure and bio-slurries with chemical fertilizers on growth and yield of rice (cv. BRRI dhan28). Int. J. Expt. Agric. 2016, 6, 36–42. [Google Scholar]
- Rhaman, M.S.; Rauf, F.; Tania, S.S.; Karim, M.M.; Sagar, A.; Robin, A.H.K.; Murata, Y. Seed priming and exogenous application of salicylic acid enhance growth and productivity of okra (Abelmoschus esculentus L.) by regulating photosynthetic attributes. J. Exp. Biol. Agric. Sci. 2021, 9, 759–769. [Google Scholar] [CrossRef]
- Abdel Latef, A.A.H.; Tahjib-Ul-Arif, M.; Rhaman, M.S. Exogenous auxin-mediated salt stress alleviation in faba bean (Vicia faba L.). Agronomy 2021, 11, 547. [Google Scholar] [CrossRef]
- Rasel, M.; Tahjib-Ul-Arif, M.; Hossain, M.A.; Hassan, L.; Farzana, S.; Brestic, M. Screening of salt-tolerant rice landraces by seedling stage phenotyping and dissecting biochemical determinants of tolerance mechanism. J. Plant Growth Regul. 2021, 40, 1853–1868. [Google Scholar] [CrossRef]
- Tania, S.S.; Rhaman, M.S.; Hossain, M.M. Hydro-priming and halo-priming improve seed germination, yield and yield contributing characters of okra (Abelmoschus esculentus L.). Trop. Plant Res. 2020, 7, 86–93. [Google Scholar] [CrossRef]
- Rhaman, M.S.; Rauf, F.; Tania, S.S.; Khatun, M. Seed priming methods: Application in field crops and future perspectives. Asian J. Res. Crop Sci. 2020, 5, 8–19. [Google Scholar] [CrossRef]
- Zhu, Z.H.; Sami, A.; Xu, Q.Q.; Wu, L.L.; Zheng, W.Y.; Chen, Z.P.; Jin, X.Z.; Zhang, H.; Li, Y.; Yu, Y.; et al. Effects of seed priming treatments on the germination and development of two rapeseed (Brassica napus L.) varieties under the co-influence of low temperature and drought. PLoS ONE 2021, 16, e0257236. [Google Scholar] [CrossRef] [PubMed]
- Rhaman, M.S.; Imran, S.; Rauf, F.; Khatun, M.; Baskin, C.C.; Murata, Y.; Hasanuzzaman, M. Seed priming with phytohormones: An effective approach for the mitigation of abiotic stress. Plants 2021, 10, 37. [Google Scholar] [CrossRef] [PubMed]
- Pastor, V.; Luna, E.; Mauch-Mani, B.; Ton, J.; Flors, V. Primed plants do not forget. Environ. Exp. Bot. 2013, 94, 46–56. [Google Scholar] [CrossRef]
- Borges, A.A.; Jiménez-Arias, D.; Expósito-Rodríguez, M.; Sandalio, L.M.; Pérez, J.A. Priming crops against biotic and abiotic stresses: MSB as a tool for studying mechanisms. Front. Plant. Sci. 2014, 5, 642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azooz, M.M. Salt stress mitigation by seed priming with salicylic acid in two faba bean genotypes differing in salt tolerance. Int. J. Agric. Biol. 2009, 11, 343–350. [Google Scholar]
- Roy, P.R.; Tahjib-Ul-Arif, M.; Polash, M.A.; Hossen, M.; Hossain, M.A. Physiological mechanisms of exogenous calcium on alleviating salinity-induced stress in rice (Oryza sativa L.). Physiol. Mol. Biol. Plants 2019, 25, 611–624. [Google Scholar] [CrossRef]
- Khan, W.; Prithiviraj, B.; Smith, D.L. Photosynthetic responses of corn and soybean to foliar application of salicylates. J. Plant Physiol. 2003, 160, 485–492. [Google Scholar] [CrossRef]
- Koo, Y.M.; Heo, A.Y.; Choi, H.W. Salicylic acid as a safe plant protector and growth regulator. Plant Pathol. J. 2020, 36, 1. [Google Scholar] [CrossRef]
- Uchida, A.; Jagendorf, A.T.; Hibino, T.; Takabe, T. Effects of hydrogen peroxide and nitric oxide on both salt and heat stress tolerance in rice. Plant Sci. 2002, 163, 515–523. [Google Scholar] [CrossRef]
- Silva, P.C.; AzevedoNeto, A.D.; Gheyi, H.R.; Ribas, R.F.; Silva, C.R.; Cova, A.M. Seed priming with H2O2 improves photosynthetic efficiency and biomass production in sunflower plants under salt stress. Arid. Land Res. Manag. 2021, 5, 1–5. [Google Scholar] [CrossRef]
- Roy, P.R.; Tahjib-Ul-Arif, M.; Akter, T.; Ray, S.R.; Sayed, M.A. Exogenous ascorbic acid and hydrogen peroxide alleviates salt-induced oxidative stress in rice (Oryza sativa L.) by enhancing antioxidant enzyme activities and proline content. Adv. Environ. Biol. 2016, 10, 148–155. [Google Scholar]
- Hemalatha, G.; Renugadevi, J.; Eevera, T. Studies on seed priming with hydrogen peroxide for mitigating salt stress in rice. Int. J. Curr. Microbiol. App. Sci. 2017, 6, 691–695. [Google Scholar] [CrossRef] [Green Version]
- Damalas, C.A.; Koutroubas, S.D.; Fotiadis, S. Hydro-priming effects on seed germination and field performance of faba bean in spring sowing. Agriculture 2019, 9, 201. [Google Scholar] [CrossRef] [Green Version]
- Tania, S.S.; Rahaman, M.; Rauf, F.; Afroj Suborna, M.; Humayun Kabir, M.; Hoque, A.; Rhaman, M.S. Seed priming with Salicylic Acid (SA) and Hydrogen Peroxide (H2O2) Improve Germination and Seedling Growth of Wheat (Triticum aestivum L.) under Salt Stress. Asian J. Res. Crop Sci. 2021, 6, 60–69. [Google Scholar] [CrossRef]
- Fatema, R.; Rahman, J.; Shozib, H.B.; Nazrul, M.I.; Fatima, K. Genetic diversity and nutritional components evaluation of Bangladeshi germplasms of kidney bean (Phaseolus vulgaris L.). J. Genet. Res. 2019, 5, 83–96. [Google Scholar]
- Mena, E.; Leiva-Mora, M.; Jayawardana, E.K.; García, L.; Veitía, N.; Bermúdez-Caraballoso, I.; Collado, R.; Ortíz, R.C. Effect of salt stress on seed germination and seedlings growth of Phaseolus vulgaris L. Cult. Trop. 2015, 36, 71–74. [Google Scholar]
- USDA (National Nutrient Database). Cut Green Beans. 29 August 2017. Available online: https://ndb.nal.usda.gov/ (accessed on 1 April 2022).
- Azooz, M.M.; Alzahrani, A.M.; Youssef, M.M. The potential role of seed priming with ascorbic acid and nicotinamide and their interactions to enhance salt tolerance in broad bean (‘Vicia faba’L.). Aust. J. Crop Sci. 2013, 7, 2091–2100. [Google Scholar]
- Nouairi, I.; Jalali, K.; Zribi, F.; Barhoumi, F.; Zribi, K.; Mhadhbi, H. Seed priming with calcium chloride improves the photosynthesis performance of faba bean plants subjected to cadmium stress. Photosynthetica 2019, 57, 438–445. [Google Scholar] [CrossRef] [Green Version]
- Mostofa, M.G.; Fujita, M. Salicylic acid alleviates copper toxicity in rice (Oryza sativa L.) seedlings by up-regulating antioxidative and glyoxalase systems. Ecotoxicology 2013, 22, 959–973. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic bio-membranes. Meth. Enzymol. 1987, 148, 350–382. [Google Scholar]
- Shiade, S.R.; Boelt, B. Seed germination and seedling growth parameters in nine tall fescue varieties under salinity stress. Acta Agric. Scand.-B Soil Plant Sci. 2020, 17, 485–494. [Google Scholar] [CrossRef]
- Muhei, S.H. Seed priming with phytohormones to improve germination under dormant and abiotic stress conditions. Adv. Crop Sci. Technol. 2018, 6, 403–409. [Google Scholar] [CrossRef]
- Farooq, M.; Basra, S.M.A.; Rehman, H.; Saleem, B.A. Seed priming enhances the performance of late sown wheat (Triticum aestivum L.) by improving chilling tolerance. J. Agron. Crop. Sci. 2008, 194, 55–60. [Google Scholar] [CrossRef]
- Ashraf, M.; Rauf, H. Inducing salt tolerance in maize (Zea mays L.) through seed priming with chloride salts: Growth and ion transport at early growth stages. Acta Physiol. Plant. 2001, 23, 407–414. [Google Scholar] [CrossRef]
- Tahjib-Ul-Arif, M.; Afrin, S.; Polash, M.A.S.; Akter, T.; Ray, S.R.; Hossain, M.; Hossain, M.A. Role of exogenous signaling molecules in alleviating salt-induced oxidative stress in rice (Oryza sativa L.): A comparative study. Acta Physiol. Plant. 2019, 41, 1–14. [Google Scholar] [CrossRef]
- Muhammad, I.; Kolla, M.; Volker, R.; Günter, N. Impact of nutrient seed priming on germination, seedling development, nutritional status and grain yield of maize. J. Plant Nutr. 2015, 38, 1803–1821. [Google Scholar] [CrossRef]
- Adhikari, B.; Dhital, P.R.; Ranabhat, S.; Poudel, H. Effect of seed hydro-priming durations on germination and seedling growth of bitter gourd (Momordica charantia). PLoS ONE. 2021, 16, e0255258. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Agarwal, R.M. Salinity stress induced alterations in antioxidant metabolism and nitrogen assimilation in wheat (Triticum aestivum L.) as influenced by potassium supplementation. Plant Physiol. Biochem. 2017, 115, 449–460. [Google Scholar] [CrossRef]
- Li, Y.; Li, H.; Li, Y.; Zhang, S. Improving water-use efficiency by decreasing stomatal conductance and transpiration rate to maintain higher ear photosynthetic rate in drought-resistant wheat. Crop J. 2017, 5, 231–239. [Google Scholar] [CrossRef]
- Pattanagul, W.; Thitisaksakul, M. Effect of salinity stress on growth and carbohydrate metabolism in three rice (Oryza sativa L.) cultivars differing in salinity tolerance. Indian J. Exp. Biol. 2008, 46, 736–742. [Google Scholar] [PubMed]
- Saboon, R.I.; Ahmad, N.; Ilyas, N.; Batool, N.; Gul, S. Salicylic acid enhances wheat plant growth under water stress conditions. Int. J. Biol. Biotech. 2015, 12, 329–336. [Google Scholar]
- Tahjib-Ul-Arif, M.; Siddiqui, M.N.; Sohag, A.A.; Sakil, M.A.; Rahman, M.M.; Polash, M.A.; Mostofa, M.G.; Tran, L.S. Salicylic acid-mediated enhancement of photosynthesis attributes and antioxidant capacity contributes to yield improvement of maize plants under salt stress. J. Plant Growth Regul. 2018, 37, 1318–1330. [Google Scholar] [CrossRef]
- Hernández, J.A.; Díaz-Vivancos, P.; Acosta-Motos, J.R.; Barba-Espín, G. Potassium nitrate treatment is associated with modulation of seed water uptake, Antioxidative Metabolism and Phytohormone Levels of Pea Seedlings. Seeds 2022, 1, 5–15. [Google Scholar] [CrossRef]
- Parvin, K.; Hasanuzzaman, M.; Bhuyan, M.H.; Nahar, K.; Mohsin, S.M.; Fujita, M. Comparative physiological and biochemical changes in tomato (Solanum lycopersicum L.) under salt stress and recovery: Role of antioxidant defense and glyoxalase systems. Antioxidants 2019, 8, 350. [Google Scholar] [CrossRef] [Green Version]
- Farzana, S.; Rasel, M.; Tahjib Ul Arif, M.; Hossain, M.A.; Azam, M.G.; Al Galib, M.A.; Mahamud, A.S.U.; Hossain, M.A. Salicylic acid and thiourea ameliorate the negative impact of salt stress in wheat (Triticum aestivum L.) seedlings by up-regulating photosynthetic pigments, leaf water status, and antioxidant defense system. J. Phytol. 2021, 13, 130–145. [Google Scholar] [CrossRef]
- Amin, A.A.; AEK, A.A.; Abouziena, H.F.; El-Awadi, M.; Gharib, F.A. Effects of benzoic acid and thiourea on growth and productivity of wheat (Triticum aestivum L.) plants. Int. Sci. Res. J. 2016, 72, 132–149. [Google Scholar]
- Ma, X.; Zheng, J.; Zhang, X.; Hu, Q.; Qian, R. Salicylic acid alleviates the adverse effects of salt stress on Dianthus superbus (Caryophyllaceae) by activating photosynthesis, protecting morphological structure, and enhancing the antioxidant system. Front. Plant Sci. 2017, 8, 600. [Google Scholar] [CrossRef]




| Treatment | Denoted |
|---|---|
| Control | C |
| 150 mM NaCl | SS |
| Hydro- primed seed | Hp |
| Hydro-primed + 150 mM NaCl-treated seed | HpSS |
| 1 mM SA primed seed | SA1 |
| 1 mM SA + 150 mM NaCl-treated seed | SA1SS |
| 2 mM SA primed seed | SA2 |
| 2 mM SA + 150 mM NaCl-treated seed | SA2SS |
| 0.1 mM H2O2 primed seed | HP1 |
| 0.1 mM H2O2 + 150 mM NaCl-treated seed | HP1SS |
| 0.15 mM H2O2 primed seed | HP2 |
| 0.15 mM H2O2 + 150 mM NaCl-treated seed | HP2SS |
| Treatments | SL (cm) | RL (cm) | SFW (gm) | SDW (gm) | RFW (gm) | RDW (gm) |
|---|---|---|---|---|---|---|
| C | 27 a | 7.5 bcde | 4.97 c | 0.46 cde | 0.577 c | 0.07 abcd |
| SS | 23 d | 5.5 f | 3.27 f | 0.303 f | 0.323 g | 0.03 e |
| Hp | 26.33 bc | 7.83 bcd | 5.38 b | 0.523 bc | 0.553 cd | 0.06 bcde |
| HpSS | 24.76 bcd | 7.23 bcde | 4.07 e | 0.413 de | 0.423 f | 0.04 de |
| SA1 | 30.33 a | 12 a | 6.77 a | 0.673 a | 0.867 a | 0.09 a |
| SA1SS | 25.23 bcd | 6.26 def | 4.30 d | 0.426 cde | 0.52 cde | 0.06 bcde |
| SA2 | 26.5 bc | 8 bc | 5.42 b | 0.516 bcd | 0.68 b | 0.084 ab |
| SA2SS | 25.83 bc | 6.5 cdef | 4.30 d | 0.42 cde | 0.48 def | 0.05 cde |
| HP1 | 26.5 bc | 8.5 b | 5.5 b | 0.61 ab | 0.697 b | 0.08 abc |
| HP1SS | 25.33 bcd | 6.5 cdef | 4.2 de | 0.37 ef | 0.447 ef | 0.06 bcde |
| HP2 | 26.5 bc | 7.5 bcde | 5.16 c | 0.6 ab | 0.58 c | 0.072 abcd |
| HP2SS | 24.06 cd | 6.1 ef | 4.25 de | 0.423 cde | 0.423 f | 0.035 e |
| SE | 0.52 | 0.48 | 0.26 | 0.03 | 0.04 | 0.005 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tania, S.S.; Rhaman, M.S.; Rauf, F.; Rahaman, M.M.; Kabir, M.H.; Hoque, M.A.; Murata, Y. Alleviation of Salt-Inhibited Germination and Seedling Growth of Kidney Bean by Seed Priming and Exogenous Application of Salicylic Acid (SA) and Hydrogen Peroxide (H2O2). Seeds 2022, 1, 87-98. https://doi.org/10.3390/seeds1020008
Tania SS, Rhaman MS, Rauf F, Rahaman MM, Kabir MH, Hoque MA, Murata Y. Alleviation of Salt-Inhibited Germination and Seedling Growth of Kidney Bean by Seed Priming and Exogenous Application of Salicylic Acid (SA) and Hydrogen Peroxide (H2O2). Seeds. 2022; 1(2):87-98. https://doi.org/10.3390/seeds1020008
Chicago/Turabian StyleTania, Shaila Shermin, Mohammad Saidur Rhaman, Farjana Rauf, Md. Moklasur Rahaman, Muhammad Humayun Kabir, Md. Anamul Hoque, and Yoshiyuki Murata. 2022. "Alleviation of Salt-Inhibited Germination and Seedling Growth of Kidney Bean by Seed Priming and Exogenous Application of Salicylic Acid (SA) and Hydrogen Peroxide (H2O2)" Seeds 1, no. 2: 87-98. https://doi.org/10.3390/seeds1020008
APA StyleTania, S. S., Rhaman, M. S., Rauf, F., Rahaman, M. M., Kabir, M. H., Hoque, M. A., & Murata, Y. (2022). Alleviation of Salt-Inhibited Germination and Seedling Growth of Kidney Bean by Seed Priming and Exogenous Application of Salicylic Acid (SA) and Hydrogen Peroxide (H2O2). Seeds, 1(2), 87-98. https://doi.org/10.3390/seeds1020008