Abstract
Seaweeds are used in traditional agriculture practices because of their beneficial effects. Recently, the rising demand for organically grown foods has amplified the use of organic fertilizers such as seaweed extracts. Despite their beneficial effects, few studies have reported information about compounds in seaweed extracts that are responsible for these benefits. Thus, the aim of this study was to evaluate the potential relationships between the components and secondary metabolites in four seaweed liquid extracts from Eisenia arborea, Macrocystis pyrifera, Padina caulescens, and Sargassum horridum and their biostimulant activity through changes in the germination, growth, and protein content of tomato seedlings (Solanum lycopersicum). The E. arborea and S. horridum extracts showed similar compositions (ash, organic carbon, bicarbonates, and chlorides), minerals (Ca, Fe, and Cu) and secondary metabolites (triterpenes and saponins), albeit with different component concentrations. The chemical composition of the P. caulescens extract was significantly different from those of the other extracts; it was characterized by high levels of total nitrogen, phenols, and carbohydrates. Almost all seaweed extracts had beneficial effects on seed germination and seedling length, except the S. horridum extract that inhibits germination. The hierarchical clustering plots and principal component analysis indicated that germination and protein content are related to the presence of sterol. Shoot length was closely related to mineral levels (K, Zn, B, Na) and the C:N ratio, whereas radicle length was closely related to the content of nitrogen, carbohydrates, phenols, and flavonoids in the seaweed extracts. However, the underlying mechanisms are still unclear and require further studies.
1. Introduction
Many organisms benefit from low doses of bioactive compounds, especially the seedlings of agricultural plants. Indeed, seaweed extracts, which contain distinct bioactive compounds, are widely accepted as plant biostimulants and commonly used in agriculture and horticulture [1]. Macroalgae species differ in their chemical compositions. Thus, their extracts exhibit different levels of biological activity, which also depend on the dosage. However, few studies have reported which compounds are responsible for the beneficial effects of seaweed extracts. The action mechanisms of seaweed extracts are complex, and understanding them requires a multidisciplinary approach to untangle the various interactions among different bioactive compounds within each extract [1,2].
In Mexico, tomato is the second most economically important vegetable crop and the most economically important worldwide [3]. In 2020, Mexico exported 3,461,766.43 metric tonnes of tomato (Solanum lycopersicum L.), with an estimated value of USD 1908 million [4]. According to data from Faostat the world produced 186.821 million metric tonnes of tomatoes on 5,051,983 hectares in 2020, achieving an average yield of 37.1 metric tonnes/hectare [5].
Recently, in the country, in horticultural crops, there has been a growing interest in using biostimulants such as seaweed extracts as crop management strategy to influenced vegetative growth and improve the yield of fruits without any negative effects on plant quality [6]. However, in Mexico, without legislation on biostimulants, seaweed extracts are considered type 1 growth regulators [7]. This strategy allows for increasing biomass production as well as increased tomato fruit nutritional quality [8,9]. Seaweed extract application enhances nutritional quality through direct plant provision of both macro- and micronutrients [10].
The delivery of seaweed extracts via foliar, soil drench, or seed coating (i.e., seed soaking or priming) applications has been widely evaluated [11]. These delivery systems have all been deemed suitable to enhance germination rates and the subsequent establishment of seedlings [10]. In seed germination experiments employing different species of brown algae, the application of seaweed extracts has been shown to increase germination [11,12,13]. In addition, seaweed extracts have been found to stimulate growth by inducing multiple physiological processes in plants, even at low concentrations [14,15,16,17].
The beneficial effects of seaweed extracts can be attributed to chemical compounds, including minerals, amino acids, vitamins, and phytohormones which enrich soils, highlighting their desirable and potential use in agriculture [18,19]. For example, multiple compounds, such as polysaccharides, have been linked to growth-promoting activities or found to elicit defensive mechanisms in plants [20,21,22]. The compounds within seaweed extracts biochemically alter plants by playing vital roles in many biological activities such as seed germination, plant growth, and fruit production. These bioactive compounds include a variety of highly diverse signal molecules and metabolites (e.g., polyphenolic compounds, carotenoids, minerals, vitamins, phlorotannins, peptides, tocotrienols, proteins, tocopherols, phytohormones, betaines, amino acid, carotenoids, vitamins, polyamines, and polysaccharides) that differ among seaweed species [1,18].
Interest in the secondary metabolites of seaweed extracts stems from their vast biological applications [23]. Brown marine algae are rich in secondary metabolites, as can be observed by their levels of phenolic compounds (e.g., phenols and flavonoids), terpenes, sterols, saponins, coumarins, glycosides, and small acetogenins [24,25]. Indeed, in brown algae, phlorotannin, phytosterol, and polyphenol are prominent secondary metabolites [26]. Secondary metabolites such as these can act as antioxidants, osmoprotectants, antimicrobial and antiviral agents, biostimulants, metabolic enhancers, and defense response elicitors [27,28].
Thus, in the present study, it was hypothesized that the different chemical compositions of four brown seaweed extracts (Eisenia arborea, Macrocystis pyrifera, Padina caulescens, and Sargassum horridum) act as plant biostimulant but in different ways. The aim of this study was to analyze the seaweed extracts components and their relationship with the seed germination, growth and protein content of tomato seedlings.
2. Materials and Methods
2.1. Sample Collection and Seaweed Extract Preparation
Seaweeds Macrocystis pyrifera, Eisenia arborea, and Sargassum horridum were harvested from wild populations of Temperate Northern Pacific along the coast of the Baja California Peninsula from March to July 2020. The seaweeds were sun-dried to a 10% moisture content and were milled with a manual mill to a 40-mesh size (0.38 mm2). The algae powder dry was provided by the Interdisciplinary Center for Marine Sciences of the National Polytechnic Institute of Mexico (CICIMAR-IPN; Baja California Sur, Mexico). Padina caulescens was collected by hand from wild populations of Tropical Eastern Pacific, along the coast of Jalisco, Mexico in intertidal zones from July 2021, and the samples were transported to the laboratory in plastic bags, washed with tap water to remove surface salt, oven-dried for 72 h at 60 °C, and then ground in an electric mill (IKA-M 20) to a size of less than 0.50 mm. The algal biomass was proceeded on the Biotechnology Research Laboratory of the University of Guadalajara (Guadalajara, Jalisco, Mexico). The seaweed extracts were elaborated according to the protocol of Hernández-Herrera et al. [14]. A dry alga sample (10 g) was added to 1 L of distilled water with constant stirring for 15 min followed by autoclaving at 121 °C for 1 h at 1.21 kg cm−2. Then, the hot extracts were filtered through a Whatman No. 40 filter paper and stored at −4 °C for use on the following day.
2.2. Physicochemical Composition of the Seaweed Extracts
The chemical composition of the dried seaweeds (Supplementary Table S1) and secondary metabolites and physicochemical composition of the dried seaweeds and their extracts were determined in the Biotechnology Research and Chemical Laboratories of the University of Guadalajara in Jalisco, Mexico. For each seaweed liquid extract, pH, electro-conductivity, and total solids were measured using a HI2550 multi-parameter benchtop meter (HANNA Instruments, Woonsocket, RI, USA). The amount of organic matter was calculated by subtracting the ash content from the total dried sample (100%). The nitrogen content of the seaweed liquid extracts was determined by the micro-Kjeldahl technique (method 976.05). Mineral content (K, Ca, P, Zn, Cu, and B) and other components, such as macronutrients (organic carbon, total nitrogen, the C:N ratio, total potassium, total calcium, total sodium, chlorites, and bicarbonates), were analyzed via atomic absorption spectrophotometry. Phosphorus content was determined by the colorimetric method.
To identify the presence or absence of various phytoconstituents in the seaweed extracts, they were characterized via the colorimetric method by employing standard protocols [29]. Test for phenols: Ferric chloride test: Aqueous plant extracts were treated with 3–4 drops of a 0.1% ferric chloride solution. The formation of a bluish green or black color confirmed a positive presence of phenols. Test for tannins: About 0.5 mg of crude plant extracts was added to 10 mL of their freshly prepared solvents (methanol, distilled water) in a test tube and shaken to dissolve. A few (2–5) drops of 0.1% ferric chloride were added, and brownish green or blue–black coloration was produced, which confirmed the presence of tannins. Test for triterpenes and sterols: Salkowski test: The crude extract (5 mL) was separately shaken with 2 mL of chloroform and followed by careful addition of concentrated sulfuric acid (2 mL) along the test tube to form a layer. A reddish-brown coloration of the interface formed, confirming a positive presence of terpenoids. Test Flavonoids: Alkaline reagent test: Exactly 0.5 g of plant extract was first dissolved with solvent (methanol and distilled water) up to 10 mL in a test tube, and second, an aqueous solution of the extracts was treated with a 10% ammonium hydroxide solution. The yellow fluorescence indicated a positive test for flavonoids. Test for Saponins: About 0.5 g of crude plant extracts was dissolved with distilled water (10 mL) in a test tube. The suspension was shaken in a test tube for about 10–15 min. A 2 cm layer of foam was taken as preliminary evidence for saponins. The layer of foam was used to determine the concentration level of saponins, whereby the higher level of foam indicated a higher level of saponins. Test for alkaloids: Dragendorff’s test: By adding 1 mL of Dragendorff’s reagent to 2 mL of extract, an orange–red precipitate was formed, indicating the presence of alkaloids. Mayer’s test: A few drops of Mayer’s reagent were added to 1 mL of extract. A yellowish or white precipitate was formed, indicating the presence of alkaloids. Test for proteins: Ninhydrin assay: Two drops of a 0.2% freshly prepared ninhydrin solution were added to 1 mL of extract. Production of purple color showed the presence of proteins. Test for carbohydrates: Molish assay: A few drops of an alcoholic a-naphthol solution were added to 2 mL of extract. Later, a few drops of concentrated H2SO4 were added along the walls of the test tube. At the junction of two liquids, a violet color ring appeared, indicating that carbohydrates were present. Tests for glycosides: Keller Killiani assay: A solution of 0.5 mL, containing glacial acetic acid and 2–3 drops of ferric chloride, was mixed with 2 mL of extract. Later, 1 mL of concentrated H2SO4 was added along the walls of the test tube. The appearance of deep blue color at the junction of two liquids indicated the presence of cardiac glycosides. All determinations were conducted in triplicate.
2.3. Bioassays: Germination and Growth under Laboratory Conditions
Experiments were conducted using certified tomato seeds (Solanum lycopersicum cv. Rio Fuego; Kristen seed®, San Diego, CA, USA). Germination was observed daily over 8 days according to the methods of the Association of Official Seed Analysts [30]. Two groups of 100 seeds were tested for germination per treatment according to the guidelines of the International Seed Testing Association [31]. Experimental units were arranged in a randomized complete block design. Before treatment with a seaweed extract, the tomato seeds were surface sterilized in a 4% sodium hypochlorite solution for 10 min and triple rinsed in sterile distilled water. Then, the tomato seeds were placed on Whatman No. 5 filter paper (Whatman International Ltd., Maidstone, UK) in sterilized 90 mm Petri dishes and treated with 5 mL of distilled water (control) or varying concentrations of the liquid extracts at 1.0%. The plates were incubated at 25 ± 1 °C with a 16:8 h light/dark regime. Germination was considered to have occurred once radicle protrusion was >2 mm and observed daily over 8 d according to the methods of the Association of Official Seed Analysts [30]. After 12 d, the tomato seedlings were weighed and photographed, and their growth characteristics (i.e., shoot and radicle length) were measured using ImageJ v. 1.52a. The protein content of the tomato seedlings was determined according to the AOAC method 954.04 [32].
2.4. Statistical Analysis
All data were analyzed for significant differences (5% level) by an analysis of variance (ANOVA) with mean separation based on the least significant difference (LSD). To identify patterns among the chemical compositions of the seaweed extracts, joint principal component analysis (PCA) and cluster analysis were performed on the normalized data (i.e., pH, electrical conductivity, total solids, total soluble salts, density, organic matter, ash, and macro and micronutrients) of each seaweed extract, and the secondary metabolites in each seaweed extract were determined. A second PCA and a cluster analysis were performed to establish whether any relationships existed between the physicochemical composition of the seaweed extracts and any growth benefits observed in the tomato plants (i.e., improved germination, growth, and protein content). The cluster groups, PCA plots, correlation values, and statistical analyses were generated using Statgraphics® Centurion XVI. II. StatPoint Technologies, Inc. The Plains, VA, USA.
3. Results
3.1. Chemical Composition of the Seaweed Extracts
Table 1 shows the physicochemical composition of each seaweed extract. The pH of the E. arborea and S. horridum extracts (7.5 and 7.20) was neutral, and their electric conductivity was low (0.68 and 0.86 dS m−1, respectively). The M. pyrifera and P. caulescens extracts showed low pH values (5 and 5.9, respectively), with electrical conductivity values of 2.8 and 2.5 dS m−1, respectively. The biochemical properties of the extracts from E. arborea, M. pyrifera, and S. horridum were similar, whereas the P. caulescens extract exhibited the lowest values. Overall, carbon content ranged from 0.014% to 0.228% (g/100 mL), whereas nitrogen content varied from 0.007% to 0.019%. Potassium level reached 1.60%, making this element the most abundant in the seaweed extracts, followed by bicarbonates (HCO3; up to 0.80%) and calcium (up to 0.074%). Micronutrient (e.g., iron and boron) content was also high in all seaweed extracts.

Table 1.
Physicochemical composition of seaweed liquid extracts from Eisenia arborea (EA), Macrocystis pyrifera (MP), Padina caulescens (PC), and Sargassum horridum (SH).
The qualitative test results for the seaweed extracts confirmed the presence of different secondary metabolites. These substances contained phenols, tannins, triterpenes, sterols, flavonoids, saponins, tannins, coumarines, carbohydrates, and glycosides (Table 2). Phenols, tannins, carbohydrates, and glycosides were dominant in the seaweed extract of P. caulescens. In contrast, the M. pyrifera extract did not include metabolites such as triterpenes, sterols, flavonoids, and coumarines. Alkaloids and proteins were not detected in any seaweed extract.

Table 2.
Phytochemical analysis of seaweed liquid extracts from Eisenia arborea (EA), Macrocystis pyrifera (MP), Padina caulescens (PC), and Sargassum horridum (SH).
3.2. Germination, Growth, and Protein Content of Tomato Seedlings Treated with Seaweed Extracts
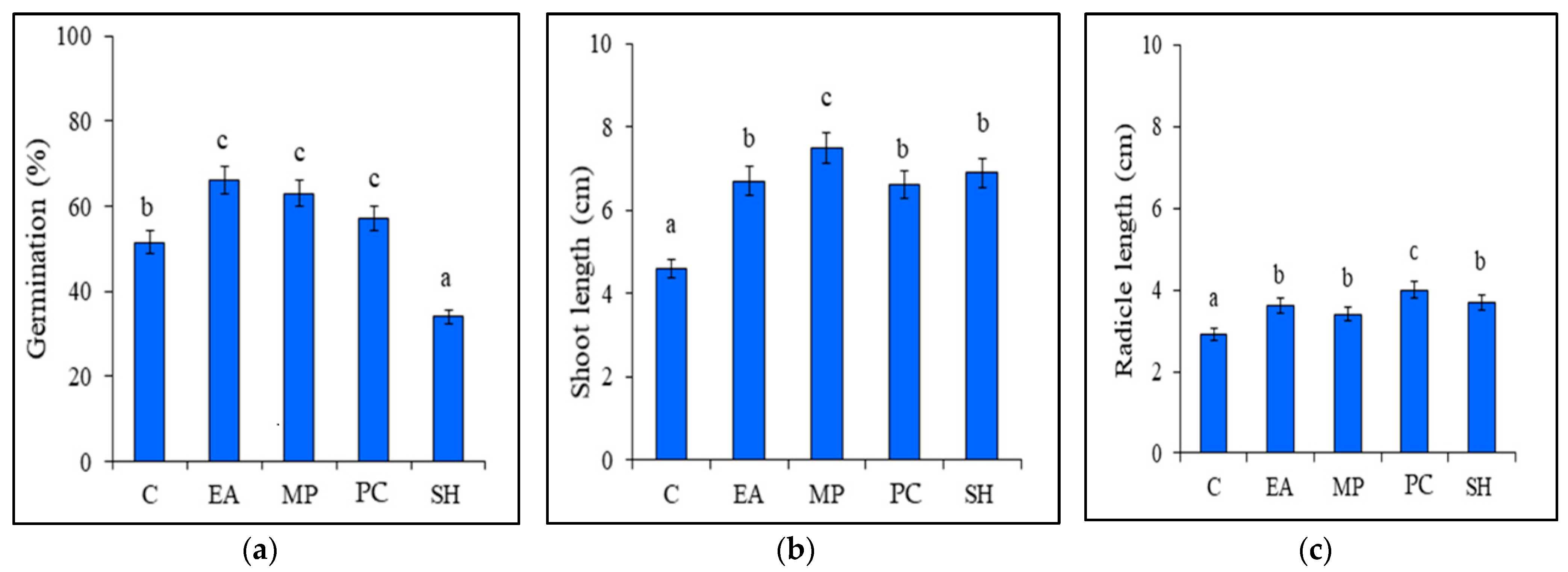
Tomato seed germination occurred in most of the treatments after 3 d. The highest positive effect on seed germination (66%) was observed for seeds treated with the seaweed extract from E. arborea, followed by the M. pyrifera extract (63% germination) and the P. caulescens extract (57% germination), the effects of which were significantly higher than those of the germination of the control (51%). In contrast, the S. horridum extract inhibited the germination of tomato seeds (34%), resulting in significantly higher gemination in the control plants (Figure 1a).
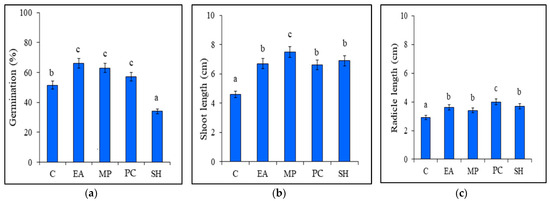
Figure 1.
Effects of seaweed extracts on (a) tomato seed germination, (b) shoot length, and (c) radicle length. Treatments: Eisenia arborea (EA), Macrocystis pyrifera (MP), Padina caulescens (PC), and Sargassum horridum (SH) and Control (C). Bars with different letters are significantly different between treatments according to the post hoc LSD mean comparison test (p ≤ 0.05). Bars represent average values ± SD (n = 300 seeds or seedlings).
All seaweed extracts had significant stimulatory effects (p ≤ 0.05) on shoot and radicle length. Tomato seedlings treated with the E. arborea extract showed greater shoot and radicle lengths (up to 6.7 and 3.6 cm, respectively) compared to those of the control. Application of the M. pyrifera extract resulted in the greatest (p < 0.05) average shoot length (up to 7.5 cm) and radicle length (3.4 cm) compared to those of the control (Figure 1b). Similarly, application of the P. caulescens extract increased shoot length (6.6 cm) and radicle length (4.0 cm) compared to those of the control. Lastly, applying the S. horridum extract resulted in an increase in shoot length (up to 6.7%) and radicle length (up to 3.6%) compared to those of the control (Figure 1c).
The protein content of the tomato seedlings was significantly higher (p < 0.05) following the application of the seaweed extracts. Seedlings treated with the E. arborea extract showed an increase in protein of up to 45%. In comparison, those treated with the M. pyrifera and P. caulescens extracts exhibited 19% and 15.4% higher protein compared to that of the control, respectively. Application of the S. horridum extract also resulted in increased protein levels in seedlings, although this increase was only 9.14% higher than that of the control seedlings (Table 3).

Table 3.
Protein content of tomato seedlings treated with seaweed extracts (mg/100g DW) from Eisenia arborea (EA), Macrocystis pyrifera (MP), Padina caulescens (PC), and Sargassum horridum (SH).
3.3. Understanding Treatment–Variable Interactions through Hierarchical Clustering and PCA
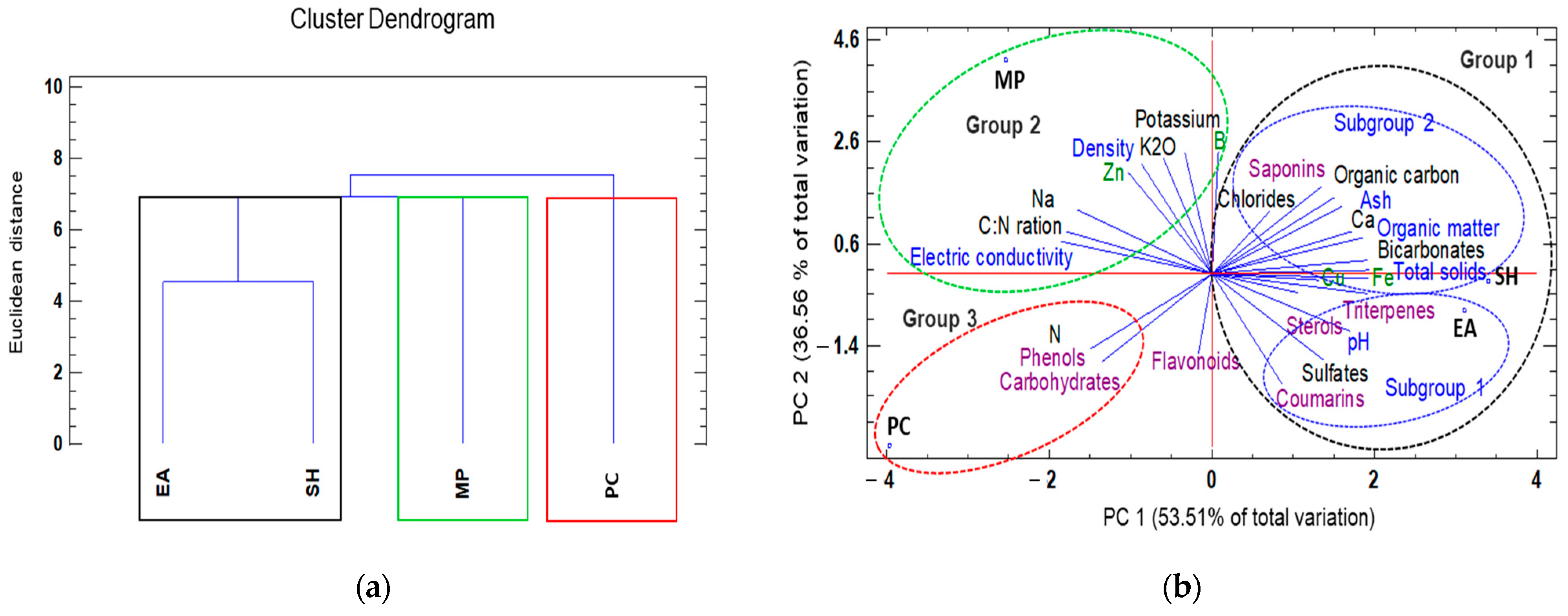
A dendrogram classification based on seaweed extract type, physicochemical composition, and the presence of secondary metabolites resulted in the formation of three groups: (1) E. arborea and S. horridum extracts, (2) M. pyrifera extract, and (3) P. caulescens extract with similar chemical characteristics at a Euclidean distance of 2.0 (Figure 2a). By relating the physicochemical variables via PCA (Figure 2b), we found that two factors explained 90.07% of the total variance. Factor 1 (PC1) explained 53.51% of the variance and was negatively correlated with electrical conductivity, the C:N ratio, and total sodium, and positively correlated with total solids, bicarbonates, iron content, and triterpenes. Factor 2 (PC2) explained 36.56% of the variance and was negatively correlated with total nitrogen, carbohydrates, and phenols, and positively correlated with density, potassium, zinc, and boron (Figure 2b).
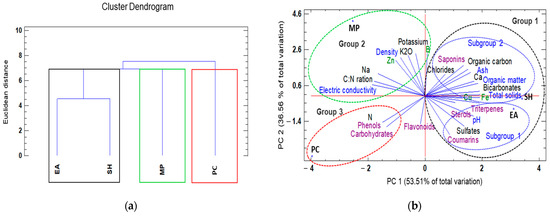
Figure 2.
Hierarchical clustering and principal components analysis (PCA) used to understand treatment-variable relationships in the different seaweed liquid extracts from Eisenia arborea (EA), Macrocystis pyrifera (MP), Padina caulescens (PC), and Sargassum horridum (SH). (a) The mean values of different parameters were clustered in three groups, group 1 was the highlighted outline in black, group 2 (green), and group 3 (red), and (b) all data were analyzed via a principal component analysis (PCA). The lines originating from the central point of the biplots indicate positive or negative correlations of different variables; their closeness indicates the correlation strength with a particular treatment. The variables include characteristics physic (in blue) characteristic chemical (green and black) and secondary metabolites (purple) and the type of seaweed extract (abbreviations of capital letters in black) at 1.0% concentration.
According to the PCA results, the three seaweed extracts differed significantly in the amounts of various compounds (Figure 2b). By plotting the data according to PC1 and PC2, clusters were clearly separated based on physicochemical variables. The first group was composed of E. arborea and S. horridum extracts, of which the former was mainly characterized by neutral pH sterols, triterpenes, coumarins and large quantities of sulphates. The S. horridum extract showed higher organic matter and total solid levels, as well as higher concentrations of elevated chlorides and bicarbonates, Fe, Cu, and saponins. The second group comprised the M. pyrifera extract, which exhibited high electric conductivity, density, C:N ratio, potassium, boron, and zinc. The third group comprised the P. caulescens extract, which was characterized by high levels of total nitrogen, phenols, and carbohydrates (Figure 2b).
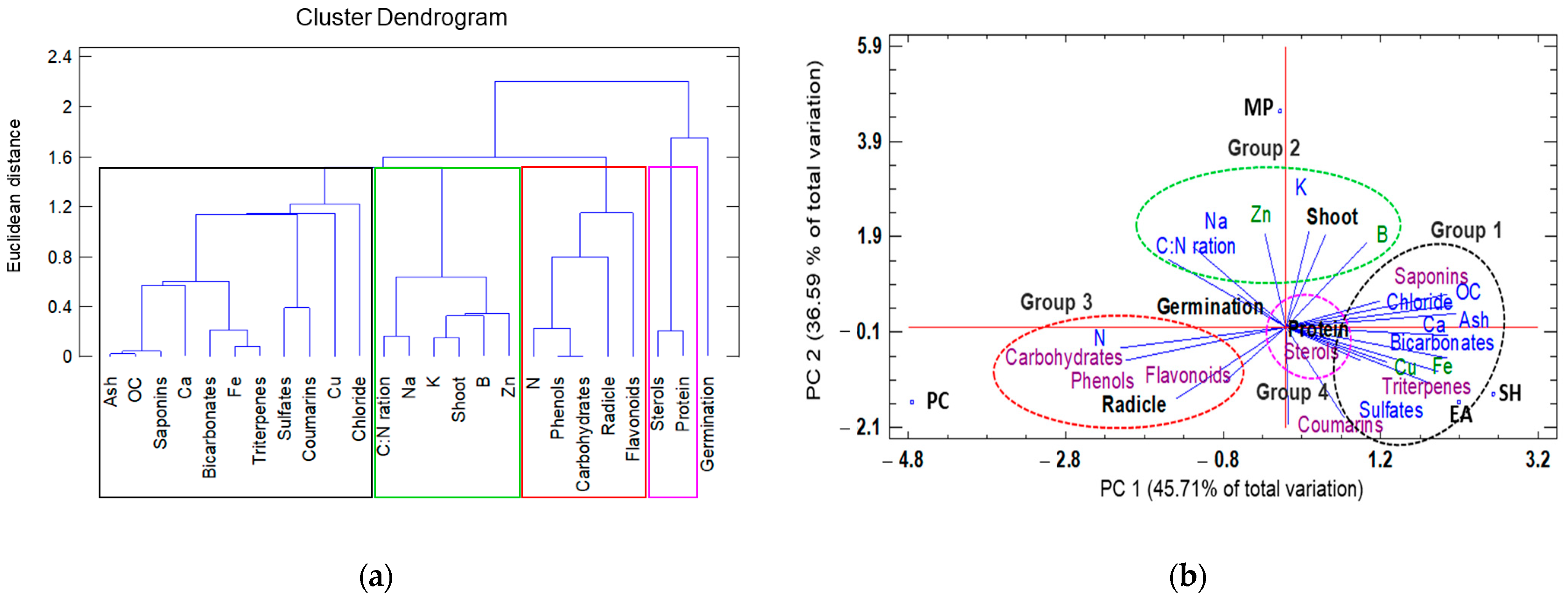
A second dendrogram classification was based on the physicochemical composition and presence of secondary metabolites in the seaweed extracts, in addition to germination, shoot length, radicle length, and protein content. This dendrogram resulted in the formation of four groups at a Euclidian distance of 1.5 (Figure 3a).
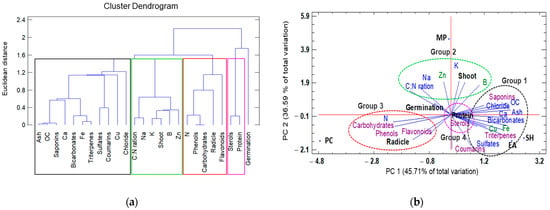
Figure 3.
Hierarchical clustering and principal component analysis (PCA) to understand treatment–variable relationships in seaweed liquid extracts from Eisenia arborea (EA), Macrocystis pyrifera (MP), Padina caulescens (PC), and Sargassum horridum (SH). (a) The mean values of different parameters were clustered in four groups, group 1 was the highlighted outline in black, group 2 (green), group 3 (red) and group 4 (pink), and (b) all data were analyzed via a principal component analysis (PCA). The lines originating from the central point of the biplots indicate positive or negative correlations of different variables; their closeness indicates the correlation strength with a particular treatment. The variables include characteristics physicochemical (in blue and green) and secondary metabolites (purple) and the type of seaweed extract (abbreviations of capital letters in black) at 1.0% concentration.
By relating growth parameters (germination, shoot, root length, and protein content) to the physicochemical composition and secondary metabolites of the seaweed extracts via a PCA, two factors were found to explain 82.3% of the total variation (Figure 3b). Factor 1 (PC1) explained 45.7% of the variation and was negatively correlated with total nitrogen, carbohydrates, and phenols, and positively correlated with ash, organic carbon, Ca, and saponin levels. Factor 2 (PC2) explained 36.59% of the variation and was negatively correlated with sulphates, coumarins, and triterpenes, and positively correlated with the C:N ratio, total potassium, zinc, and boron. By plotting the data according to PC1 and PC2, clusters were identified that were clearly separated based on the physicochemical variables of the different seaweed extracts and by the length and protein values. The first group exhibited similar chemical composition (ash, organic carbon, bicarbonates, and chlorides), minerals (Ca, Fe, and Cu) and secondary metabolites (triterpenes and saponins) to those of the group formed by the E. arborea and S. horridum extracts. The second group was defined by the C:N ratio, sodium, potassium, boron, zinc, and tomato seedling shoot length. The third group was characterized by high total nitrogen, carbohydrates, other organic components, phenols, and flavonoids, and was closely related to great radicle length. The presence of sterols and high seedling protein content characterized the fourth group (Figure 3b).
4. Discussion
In the present study, we evaluated the physicochemical properties of seaweed extracts, which act as biostimulants when applied to tomato plants. Based on our analysis, seaweeds contain considerable amounts of macro- and micronutrients, supporting their use as organic plant biostimulants. Indeed, previous studies have found that seaweed extracts can be applied conveniently to soils and plants, resulting in superior performance compared to that of conventional chemical fertilizers [33,34,35,36]. Seaweed liquid extracts contain compounds (e.g., plant growth-promoting hormones, polysaccharides, proteins, amino acids, vitamins, and macro- and micronutrients) that promote nutrient uptake and increase abiotic stress tolerance, thereby enhancing crop quality [11]. As growth biostimulants, the positive effects of seaweed extracts are based on the synergistic action of their macro- and micronutrients, amino acids, vitamins, and other components, which affect cellular metabolism, although the associated mode of action remains unknown [18,37].
Differences in the chemical composition of the seaweed extracts can be ascribed to the species of seaweed and sampling location. For example, the E. arborea and S. horridum extracts collected in Temperate Northern Pacific (Baja California Peninsula) exhibited similar mineral compositions and also contained proportions comparable to those reported by other authors on these seaweeds on the same region [38,39,40]. In contrast, P. caulescens, which was collected in Tropical Eastern Pacific, exhibited a different chemical composition from those of the three species analyzed in this study while showing mineral levels similar to those of Padina gymnospora collected in the similar zone of the tropical region [14,17].
The heterogeneous nature of algal biomass makes it difficult to identify direct correlations between general morphological features such as growth and the chemical components of seaweed extracts [41,42]. In this study, the E. arborea (EA) extract was characterized by a large amount of sterol. According to previous studies, plant sterols and their derivative brassinosteroid serve important physiological functions in plant growth and development such as seed germination [17,18].
As was observed by Di Filippo-Herrera et al. [38] with mung bean treated with an E. arborea extract, the greatest benefits to germination in this study were observed in seeds treated with the E. arborea extract, which exhibited 18% more germination than those of the control. A possible explication could be that E. arborea contains considerable amounts of cholesterol, β-sitosterol, ergosterol, stigmasterol, and fucosterol, [43,44]. In contrast, the S. horridum extract appeared to inhibit germination in this study, which may be due to the presence of solids resulting from the level of hydrolysis employed during extract production that could influence its osmotic potential [16,45]. The seeds in this treatment may not have been able to efficiently imbibe water, which would account for their observed germination failure. Moreover, the poor germination performance of the seedlings in this treatment may have been due to the presence of sterols or saponins in the S. horridum extract. Indeed, some steroidal saponins have been found to exhibit hormesis profiles in different plant species, including tomatoes, with the inhibition of seed germination at high concentrations and growth stimulation at low concentrations [46]. In this study, the negative effects on seed germination treated with S. horridum extract could also be due to an excess of particular components contained in seaweed biostimulants [47]. For example, Sun et al. [48] found that compounds such as fucosterol, 24-hydroperoxy-24-vinylcholesterol, and saringosterol, which were identified in S. fusiforme, showed allelopathic activity.
Moreover, the application of the M. pyrifera extract to tomato seedlings increased shoot length. The M. pyrifera extract exhibited high levels of potassium, boron, and zinc, which were closely related to tomato seedling shoot length. The potassium contained in seaweed extracts could help seedlings regulate their water balance while helping them improve meristematic growth and photosynthesis [49]. Trace element B plays a role in carbohydrate metabolism, sugar translocation, hormone reactions, and the normal growth and functions of the apical meristem and membrane [50,51]. According to Dell and Huang [52], enhanced trace element B is a requirement for young growing tissues, as it plays essential roles in cell division and elongation. In addition, Zn, which was present in considerable amounts in the seaweed extracts, facilitates respiration and photosynthesis, promoting the reduction in nitrates and sulphates and stimulating cation-activated enzymes [49].
In this study, the P. caulescens extract was characterized by high levels of total nitrogen, carbohydrates, and phenols and beneficially affected radicle length. This benefit may be in part because nitrogen is an essential macronutrient and a major component of the essential organic compounds required for normal plant growth; it is also a constituent of proteins, nucleic acids, and other indispensable organic compounds [53]. Many studies showed that compounds such as polysaccharides from Padina spp. increase the root and shoot length of plants [54,55,56]. In addition, Hernández-Herrera et al. [45] assessed the effects of polysaccharide-enriched extracts from P. gymnospora on the growth of tomato and mung bean plants and found that the extracts resulted in significantly higher radicle length in tomato and mung bean plants. Seaweed polysaccharides and their derived oligosaccharides can stimulate plant growth by enhancing carbon and nitrogen assimilation, plant cell division, and basal metabolism [57]. Overall, seaweed extracts are excellent sources of carbohydrate polymers (e.g., fucoidans, laminarins, and alginates) [35]. Due to their negatively charged cell walls, they can easily retain cations or positively charged molecules from seawater [58]. In agriculture, these seaweed carbohydrates have attracted interest as novel sources of environmentally friendly products to improve plant growth [8]. At this time, the most likely candidates responsible for the biostimulant effects of seaweed extracts are complex carbohydrate compounds.
Seaweeds also contain secondary metabolites, with the phenol content in seaweeds ranging from 1 to 4% (dry weight) [59]. Phenolic compounds are known for their bioactivity. In brown algae, phlorotannins are produced via secondary metabolism and consist of phloroglucinol polymers with molecular skeletons composed of eight phenol rings. In a study by Rengasamy et al. [60], eckol and phloroglucinol, which were isolated from Ecklonia maxima, increased Vigna mungo seedling length, root number, and weight. In addition, treatment with eckol phloroglucinol resulted in significantly greater lengths of the shoots and roots of maize seedlings when compared to treatment at 0.4% with Kelpak® Kelp Products (Pty) Ltd. Cape Town, Western Cape, South Africa, and promoted α-amylase activity by increasing the starch clearance zone in the maize scutellum [60].
Finally, we recognize that the relationships between the composition of seaweed extracts and their biostimulant activity is complex, and progress in unravelling this relationship requires more comprehensive experiments that assess the effects of their major and minor components.
However, these findings further our understanding of the importance of the composition of seaweed extracts to their biostimulant potential. The composition profile of each extract was influenced by the seaweed species, highlighting the different effects of each seaweed extract on key variables indicative of tomato seedling development.
5. Conclusions
Recent studies have demonstrated that mixtures of major and minor constituents can stimulate complex biological activities at low concentrations, with seaweed extracts modulating gene expression and inducing metabolic changes in treated plants. In doing so, seaweed extracts enhance nutrient use efficiency, abiotic stress tolerance, germination, and growth. However, very little data are available that link the chemical composition of a seaweed extract with its biostimulant activity or the observable morphological changes in treated plants. We observed significant differences in the levels of carbohydrates, minerals, metabolites, and bioactivity of extracts from four seaweed species, highlighting the complexity of these extracts. In addition, the presence of micro- and macronutrients, trace elements, carbohydrates, secondary metabolites, and other organic compounds in the seaweed extracts significantly enhanced the growth of tomato plants, showcasing the biostimulant potential of seaweed extracts.
Almost all seaweed extracts had beneficial effects on seed germination and seedling length. The hierarchical clustering plots and principal component analysis indicated that germination and protein content are related to the presence of sterol. Shoot length was closely related to mineral levels (K, Zn, B, Na) and the C:N ratio, whereas radicle length was closely related to the content of nitrogen, carbohydrates, phenols, and flavonoids in the seaweed extracts. The S. horridum extract contained chemical compounds (i.e., saponins and triterpenes) that may inhibit tomato seedling growth. In addition, the functional groups (e.g., carbohydrates, phenols, and flavonoids) present in the P. caulescens extract affected beneficially seedling root length. The minerals K, Zn, and B, which were present in the M. pyrifera extract, positively affected shoot length. While these results advance our understanding of how extract components influence plant germination and seedling growth, the underlying mechanisms require further study.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/seeds2040033/s1, Table S1: Proximate chemical analysis of seaweed (g 100 g−1 dry weight).
Author Contributions
Conceptualization, R.M.H.-H. and. A.P.V.-R.; methodology, M.F.G.-G. and S.F.V.-R.; software, R.M.H.-H.; validation, F.S.-R., J.F.Z.-N. and A.P.V.-R.; formal analysis, R.M.H.-H.; investigation, R.M.H.-H.; resources, R.M.H.-H.; writing—original draft preparation, R.M.H.-H.; writing—review and editing F.S.-R., visualization, R.M.H.-H. and A.P.V.-R.; supervision, F.S.-R. and J.F.Z.-N.; project administration, R.M.H.-H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are available in the manuscript file. For more detail of data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ali, O.; Ramsubhag, A.; Jayaraman, J. Biostimulant properties of seaweed extracts in plants: Implications towards sustainable crop production. Plants 2021, 10, 531. [Google Scholar] [CrossRef]
- EL Boukhari, M.E.M.; Barakate, M.; Bouhia, Y.; Lyamlouli, K. Trends in Seaweed Extract Based Biostimulants: Manufacturing Process and Beneficial Effect on Soil-Plant Systems. Plants 2020, 9, 359. [Google Scholar] [CrossRef]
- García-Estrada, R.S.; Diaz-Lara, A.; Aguilar-Molina, V.H.; Tovar-Pedraza, J.M. Viruses of Economic Impact on Tomato Crops in Mexico: From Diagnosis to Management—A Review. Viruses 2022, 14, 1251. [Google Scholar] [CrossRef]
- SIAP. Servicio de Información Agroalimentaria y Pesquera. Atlas-Agroalimentario. 2020. Consulted October 2021. Available online: https://nube.siap.gob.mx/gobmx_publicaciones_siap/pag/2020/Atlas-Agroalimentario-2020 (accessed on 12 October 2022).
- Branthôme, F.-X. Worldwide (Total Fresh) Tomato Production Exceeds 187 Million Tonnes in 2020. Available online: https://www.tomatonews.com/en/worldwide-total-fresh-tomato-production-exceeds-187-million-tonnes-in-2020_2_1565.html (accessed on 12 October 2022).
- Sariñana-Aldaco, O.; Benavides-Mendoza, A.; Robledo-Olivo, A.; González-Morales, S. The Biostimulant Effect of Hydroalcoholic Extracts of Sargassum spp. in Tomato Seedlings under Salt Stress. Plants 2022, 11, 3180. [Google Scholar] [CrossRef]
- Diario Oficial de la Federación (DOF). NORMA Oficial Mexicana NOM-182-SSA1-2010, Etiquetado de Nutrientes Vegetales. Available online: https://www.dof.gob.mx/normasOficiales/4371/salud1a1.htm#:~:text=1.1%20Esta%20norma%20establece%20las,regladores%20de%20crecimiento%20tipo%203 (accessed on 5 March 2023).
- Kocira, A.; Świeca, M.; Kocira, S.; Złotek, U.; Jakubczyk, A. Enhancement of yield, nutritional and nutraceutical properties of two common bean cultivars following the application of seaweed extract (Ecklonia maxima). Saudi J. Biol. Sci. 2018, 25, 563–571. [Google Scholar] [CrossRef]
- Mannino, G.; Campobenedetto, C.; Vigliante, I.; Contartese, V.; Gentile, C.; Bertea, C.M. The application of a plant biostimulant based on seaweed and yeast extract improved tomato fruit development and quality. Biomolecules 2020, 10, 1662. [Google Scholar] [CrossRef]
- Amirkhani, M.; Mayton, H.S.; Netravali, A.N.; Taylor, A.G. A seed coating delivery system for bio-based biostimulants to enhance plant growth. Sustainability 2019, 11, 5304. [Google Scholar] [CrossRef]
- Du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in plant science: A global perspective. Front. Plant Sci. 2017, 7, 2049. [Google Scholar] [CrossRef]
- Carvalho, M.E.A.; Castro, P.R.C.; Novembre, A.D.C.; Chamma, H.M.C.P. Seaweed extract improves the vigor and provides the rapid emergence of dry bean seeds. Am. Eurasian J. Agric. Environ. Sci. 2013, 13, 1104–1107. [Google Scholar]
- Hernández-Herrera, R.M.; Santacruz-Ruvalcaba, F.; Ruíz-López, M.A.; Norrie, J.; Hernández-Carmona, G. Effect of liquid seaweed extracts on growth of tomato seedlings (Solanum lycopersicum L.). J. Appl. Phycol. 2014, 26, 619–628. [Google Scholar] [CrossRef]
- Hernández-Herrera, R.M.; Virgen-Calleros, G.; Ruiz-López, M.A.; Zañudo-Hernández, J.; Délano-Frier, J.P.; Sánchez-Hernández, C. Extracts from green and brown seaweeds protect tomato (Solanum lycopersicum) against the necrotrophic fungus. Alternaria Solani. J. Appl. Phycol. 2014, 26, 1607–1614. [Google Scholar] [CrossRef]
- Castellanos-Barriga, L.G.; Santacruz-Ruvalcaba, F.; Hernández-Carmona, G.; Ramírez-Briones, E.; Hernández-Herrera, R.M. Effect of seaweed liquid extracts from Ulva lactuca on seedling growth of mung bean (Vigna radiata). J. Appl. Phycol. 2017, 29, 2479–2488. [Google Scholar] [CrossRef]
- González-González, M.F.; Ocampo-Alvarez, H.; Santacruz-Ruvalcaba, F.; Sánchez-Hernández, C.V.; Casarrubias-Castillo, K.; Becerril-Espinosa, A.; Castañeda-Nava, J.J.; Hernández-Herrera, R.M. Physiological, ecological, and biochemical implications in tomato plants of two plant biostimulants: Arbuscular mycorrhizal fungi and seaweed extract. Front. Plant Sci. 2020, 11, 999. [Google Scholar] [CrossRef]
- Khan, W.; Rayirath, U.P.; Subramanian, S.; Jithesh, M.N.; Rayorath, P.; Hodges, D.M.; Critchley, A.T.; Cragie, S.; Norrie, J.; Prithiviraj, B. Seaweed extracts as biostimulants of plant growth and development. J. Plant Growth Regul. 2009, 28, 386–399. [Google Scholar] [CrossRef]
- Osuna-Ruiz, I.; López-Saiz, C.M.; Burgos-Hernández, A.; Velázquez, C.; Nieves-Soto, M.; Hurtado-Oliva, M.A. Antioxidant, antimutagenic and antiproliferative activities in selected seaweed species from Sinaloa, Mexico. Pharm. Biol. 2016, 54, 2196–2210. [Google Scholar] [CrossRef]
- Jönsson, M.; Allahgholi, L.; Sardari, R.R.; Hreggviðsson, G.O.; Nordberg Karlsson, E. Extraction and modification of macroalgal polysaccharides for current and next-generation applications. Molecules 2020, 25, 930. [Google Scholar] [CrossRef]
- Pacheco, D.; Cotas, J.; Rocha, C.P.; Araújo, G.S.; Figueirinha, A.; Gonçalves, A.M.M.; Bahcevandziev, K.; Pereira, L. Seaweeds’ carbohydrate polymers as plant growth promoters. Carbohydr. Polym. Technol. Appl. 2021, 2, 100097. [Google Scholar] [CrossRef]
- Benítez-García, I.; Dueñas-Ledezma, A.K.; Martínez-Montaño, E.; Salazar-Leyva, J.A.; Carrera, E.; Osuna Ruiz, I. Identification and quantification of plant growth regulators and antioxidant compounds in aqueous extracts of Padina durvillaei and Ulva lactuca. Agronomy 2020, 10, 866. [Google Scholar] [CrossRef]
- Salehi, B.; Venditti, A.; Sharifi-Rad, M.; Kręgiel, D.; Sharifi-Rad, J.; Durazzo, A.; Lucarini, M.; Santini, A.; Souto, E.B.; Novellino, E.; et al. The therapeutic potential of apigenin. Int. J. Mol. Sci. 2019, 20, 1305. [Google Scholar] [CrossRef]
- Magallanes, C.; Córdova, C.; Orozco, R. Actividad antibacteriana de extractos etanólicos de macroalgas marinas de la costa central del Perú. Rev. Peru Biol. 2003, 10, 125–132. [Google Scholar] [CrossRef]
- Mascheck, J.A.; Baker, B.J. Macroalgal chemical defenses and their roles in structuring tropical marine communities. In Algal Chemical Ecology; Asmler, C.D., Ed.; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Hakim, M.M.; Patel, I.C. A review on phytoconstituents of marine brown algae. Futur. J. Pharm. Sci. 2020, 6, 129. [Google Scholar] [CrossRef]
- Agarwal, P.K.; Dangariya, M.; Agarwal, P. Seaweed extracts: Potential biodegradable, environmentally friendly resources for regulating plant defence. Algal Res. 2021, 58, 102363. [Google Scholar] [CrossRef]
- Asimakis, E.; Shehata, A.A.; Eisenreich, W.; Acheuk, F.; Lasram, S.; Basiouni, S.; Emekci, M.; Ntougias, S.; Taner, G.; May-Simera, H.; et al. Algae and their metabolites as potential bio-pesticides. Microorganisms 2022, 10, 307. [Google Scholar] [CrossRef]
- Harborne, J.B. Methods of plant analysis. In Phytochemical Methods: A Guide to Modern Techniques of Plant Analysis; Springer: Dordrecht, The Netherlands, 1973; pp. 1–32. [Google Scholar]
- AOSA (Association of Official Seed Analysts). Rules for Testing Seed; AOSA: Las Cruces, NM, USA, 2005; pp. 4–113. [Google Scholar]
- ISTA (International Seed Testing Association). International rules for seed testing. Seed Sci. Technol. 1999, 27, 333. [Google Scholar]
- AOAC (Association of Official Analytical Chemists). Official Methods of Analysis, 16th ed.; AOAC: Washington, DC, USA, 1996. [Google Scholar]
- Silva, L.D.; Bahcevandziev, K.; Pereira, L. Production of bio-fertilizer from Ascophyllum nodosum and Sargassum muticum (Phaeophyceae). J. Oceanol. Limnol. 2019, 37, 918–927. [Google Scholar] [CrossRef]
- Craigie, J.S. Seaweed extract stimuli in plant science and agriculture. J. Appl. Phycol. 2011, 23, 371–393. [Google Scholar] [CrossRef]
- Chojnacka, K.; Saeid, A.; Witkowska, Z.; Tuhy, L. Biologically active compounds in seaweed extracts the prospects for the application. Open Conf. Proc. J. 2012, 3, 20–28. [Google Scholar] [CrossRef]
- Soares, C.; Švarc-Gajić, J.; Oliva-Teles, M.T.; Pinto, E.; Nastić, N.; Savić, S.; Almeida, A.; Delerue-Matos, C. Mineral composition of subcritical water extracts of Saccorhiza polyschides, a brown seaweed used as fertilizer in the north of Portugal. J. Mar. Sci. Eng. 2020, 8, 244. [Google Scholar] [CrossRef]
- Pohl, A.; Kalisz, A.; Sekara, A. Seaweed extracts’ multifactorial action: Influence on physiological and biochemical status of Solanaceae plants. Acta Agrobot. 2019, 72, 1. [Google Scholar] [CrossRef]
- Di Filippo-Herrera, D.A.; Muñoz-Ochoa, M.; Hernández-Herrera, R.M.; Hernández-Carmona, G. Biostimulant activity of individual and blended seaweed extracts on the germination and growth of the mung bean. J. Appl. Phycol. 2018, 31, 2025–2037. [Google Scholar] [CrossRef]
- Di Filippo-Herrera, D.A.; Hernández-Herrera, R.M.; Ocampo-Álvarez, H.; Sánchez-Hernández, C.V.; Muñoz-Ochoa, M.; Hernández-Carmona, G. Seaweed liquid extracts induce hormetic growth responses in mung bean plants. J. Appl. Phycol. 2021, 33, 1263–1272. [Google Scholar] [CrossRef]
- Di Filippo-Herrera, D.A.; Arvizu-Higuera, D.L.; Rodríguez-Montesinos, Y.E.; Muñoz-Ochoa, M.; Hernández-Herrera, R.M.; Hernández-Carmona, G. Fucoidan and alginate on mung bean growth. Hidrobiológica 2022, 32, 353–363. [Google Scholar] [CrossRef]
- Buera, P.; Schebor, C.; Elizalde, B. Effects of carbohydrate crystallization on stability of dehydrated foods and ingredient formulations. J. Food. Eng. 2005, 67, 157–165. [Google Scholar] [CrossRef]
- Baltrusch, K.; Flórez-Fernández, N.; Illera, M.; Torres, M.D.; López-Mosquera, M.E.; Domínguez, H. Potential use of Sargassum muticum as source of plant biostimulants after three different drying methods. J. Appl. Phycol. 2023, 35, 921–933. [Google Scholar] [CrossRef]
- Sohn, S.I.; Rathinapriya, P.; Balaji, S.; Jaya Balan, D.; Swetha, T.K.; Durgadevi, R.; Alagulakshmi, S.; Singaraj, P.; Pandian, S. Phytosterols in seaweeds: An overview on biosynthesis to biomedical applications. Int. J. Mol. Sci. 2021, 22, 12691. [Google Scholar] [CrossRef]
- Ito, M.; Koba, K.; Hikihara, R.; Ishimaru, M.; Shibata, T.; Hatate, H.; Tanaka, R. Analysis of functional components and radical scavenging activity of 21 algae species collected from the Japanese coast. Food Chem. 2018, 255, 147–156. [Google Scholar] [CrossRef]
- Hernández-Herrera, R.M.; Santacruz-Ruvalcaba, F.; Zañudo-Hernández, J.; Hernández-Carmona, G. Activity of seaweed extracts and polysaccharide-enriched extracts from Ulva lactuca and Padina gymnospora as growth promoters of tomato and mung bean plants. J. Appl. Phycol. 2016, 28, 2549–2560. [Google Scholar] [CrossRef]
- Durán, A.G.; Calle, J.M.; Butrón, D.; Pérez, A.J.; Macías, F.A.; Simonet, A.M. Steroidal Saponins with Plant Growth Stimulation Effects; Yucca schidigera as a Commercial Source. Plants 2022, 11, 3378. [Google Scholar] [CrossRef]
- Battacharyya, D.; Babgohari, M.Z.; Rathor, P.; Prithiviraj, B. Seaweed extracts as biostimulants in horticulture. Sci. Hortic. 2015, 196, 39–48. [Google Scholar] [CrossRef]
- Sun, S.; Hu, S.; Zhang, B.; Sun, X.; Xu, N. Allelopathic effects and potential allelochemical of Sargassum fusiforme on red tide microalgae Heterosigma akashiwo. Mar. Pollut. Bull. 2021, 170, 112673. [Google Scholar] [CrossRef]
- Pramanick, B.; Brahmachari, K.; Ghosh, A. Effect of seaweed saps on growth and yield improvement of green gram. Afr. J. Agric. Res. 2013, 8, 1180–1186. [Google Scholar]
- Howe, P.D. A review of boron effects in the environment. Biol. Trace Elem. Res. 1998, 66, 153–166. [Google Scholar] [CrossRef]
- Broadley, M.; Brown, P.; Cakmak, I.; Rengel, Z.; Zhao, F. Chapter 7—Function of Nutrients: Micronutrients. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Marschner, P., Ed.; Academic Press: San Diego, CA, USA, 2012; pp. 191–248. [Google Scholar]
- Dell, B.; Huang, L.B. Physiological response of plants to low boron. Plant Soil 1997, 193, 103–120. [Google Scholar] [CrossRef]
- Ohyama, T. Nitrogen as a Major Essential Element of Plants. In Nitrogen Assimilation in Plants; Ohyama, T., Sueyoshi, K., Eds.; Research Singpot: Trivandrum, India, 2010; pp. 1–18. [Google Scholar]
- Drira, M.; Mohamed, J.B.; Hlima, H.B.; Hentati, F.; Michaud, P.; Abdelkafi, S.; Fendri, I. Improvement of Arabidopsis thaliana salt tolerance using a polysaccharidic extract from the brown algae Padina pavonica. Algal Res. 2021, 56, 102324. [Google Scholar] [CrossRef]
- Attia, E.Z.; Youssef, N.H.; Saber, H.; Rushdi, M.I.; Abdel-Rahman, I.A.; Darwish, A.G.; Abdelmohsen, U.R. Halimeda opuntia and Padina pavonica extracts improve growth and metabolic activities in maize under soil-saline conditions. J. Appl. Phycol. 2022, 34, 3189–3203. [Google Scholar] [CrossRef]
- Thriunavukkarasu, R.; Joseph, J.; Aruni, W. Effect of seaweed on seed germination and biochemical constituents of Capsicum annuum. Biocatal. Agric. Biotechnol. 2020, 29, 101761. [Google Scholar]
- González, A.; Castro, J.; Vera, J.; Moenne, A. Seaweed oligosaccharides stimulate plant growth by enhancing carbon and nitrogen assimilation, basal metabolism, and cell division. J. Plant Growth Regul. 2013, 32, 443–448. [Google Scholar] [CrossRef]
- Alba, K.; Kontogiorgos, V. Pectin at the oil-water interface: Relationship of molecular composition and structure to functionality. Food Hydrocoll. 2016, 68, 2011–2218. [Google Scholar] [CrossRef]
- Holdt, S.L.; Kraan, S. Bioactive compounds in seaweed: Functional food applications and legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Rengasamy, K.R.R.; Kulkarni, M.G.; Stirk, W.A.; Van Staden, J. Eckol—A new plant growth stimulant from the brown seaweed Ecklonia maxima. J. Appl. Phycol. 2015, 27, 581–587. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).