Germination Kinetics of Ferula communis L. Seeds, a Potentially Multipurpose-Use Wild Species
Abstract
:1. Introduction
2. Materials and Methods
2.1. Seed Material and Collection Site
2.2. Germination Test
2.3. Statistical Analysis
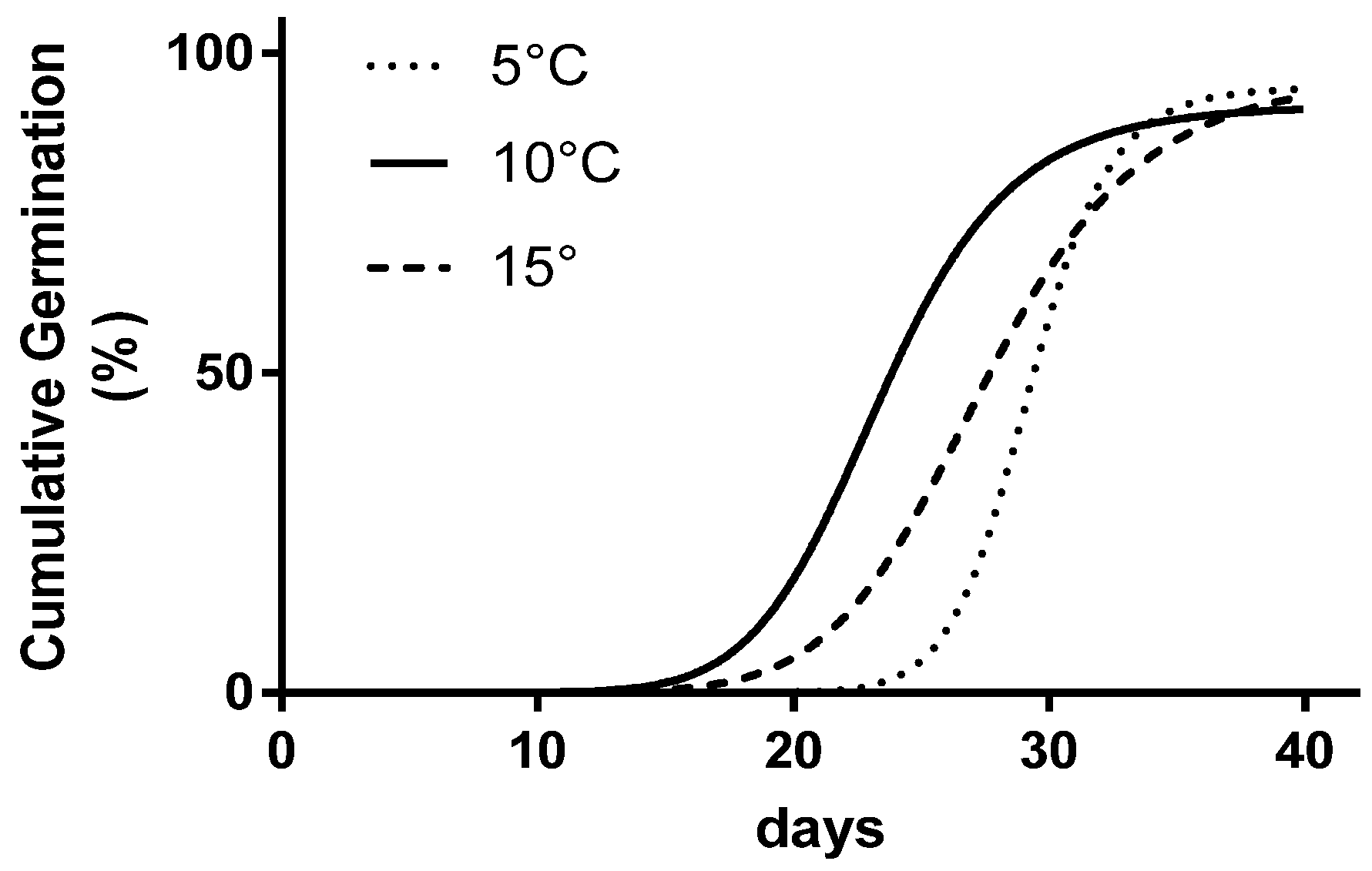
3. Results
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nikolaeva, M.G. Physiology of Deep Dormancy in Seeds; Shapiro, Z., Translator; National Science Foundation: Washington, DC, USA, 1969. (In Russian)
- Al-Yahya, M.A.; Muhammad, I.; Mirza, H.H.; El-Ferali, F.S. Antibacterial constituents from the rhizomes of Ferula communis. Phytother. Res. 1998, 12, 335–339. [Google Scholar] [CrossRef]
- Chen, B.; Teranishi, R.; Kawazoe, K.; Takaishi, Y.; Honda, G.; Itoh, M.; Takeda, Y.; Kodzhimatoy, O.K. Sesquiterpenoides from Ferula kuhistanica. Phytochemistry 2000, 54, 717–722. [Google Scholar] [CrossRef] [PubMed]
- Arnoldi, L.; Ballero, M.; Fuzzati, N.; Maxia, A.; Mercalli, E.; Pagni, L. HPLC-DAD-MS identification of bioactive secondary metabolites from Ferula communis roots. Fitoterapia 2004, 75, 342–354. [Google Scholar] [CrossRef] [PubMed]
- Akaberi, M.; Iranshahy, M.; Iranshahi, M. Review of the traditional uses, phytochemistry, pharmacology and toxicology of giant fennel (Ferula communis L. subsp. communis). Iran. J. Basic Med. Sci. 2015, 18, 1050. [Google Scholar] [PubMed]
- Deniz, G.Y.; Laloglu, E.; Koc, K.; Geyikoglu, F. Hepatoprotective potential of Ferula communis extract for carbon tetrachloride induced hepatotoxicity and oxidative damage in rats. Biotech. Histochem. 2019, 94, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Kavaz, D.; Faraj, R.E. Investigation of composition, antioxidant, antimicrobial and cytotoxic characteristics from Juniperus sabina and Ferula communis extracts. Sci. Rep. 2023, 13, 7193. [Google Scholar] [CrossRef] [PubMed]
- Mamoci, E.; Cavoski, I.; Simeone, V.; Mondelli, D.; Al-Bitar, L.; Caboni, P. Chemical composition and in vitro activity of plant extracts from Ferula communis and Dittrichia viscosa against postharvest fungi. Molecules 2011, 22, 2609–2625. [Google Scholar] [CrossRef] [PubMed]
- Buller, R. The fungus lore of the Greeks and Romans. Trans. Br. Mycol. Soc. 1914, 5, 21–66. [Google Scholar] [CrossRef]
- Zervakis, G.I.; Balis, C. A pluralistic approach in the study of Pleurotus species with emphasis on compatibility and physiology of the European morphotaxa. Mycol. Res. 1996, 6, 717–731. [Google Scholar] [CrossRef]
- Marchi, A.; Appendino, G.; Pirisi, I.; Ballero, M.; Loi, M.C. Genetic differentiation of two distinct chemotypes of Ferula communis (Apiaceae) in Sardinia (Italy). Biochem. Syst. Ecol. 2003, 31, 1397–1408. [Google Scholar] [CrossRef]
- Appendino, G.; Tagliapietra, S.; Gariboldi, P.; Nano, G.M.; Picci, V. w-Oxygenated prenylated coumarins from F. communis. Phytochemistry 1988, 27, 3619–3624. [Google Scholar] [CrossRef]
- Rubiolo, P.; Matteodo, M.; Riccio, G.; Ballero, M.; Christen, P.; Fleury-Souverain, S.; Veuthey, J.L.; Bicchi, C. Analytical discrimination of poisonous and nonpoisonous chemotypes of Giant Fennel (Ferula communis L.) through their biologically active and volatile fractions. J. Agric. Food Chem. 2006, 54, 7556–7563. [Google Scholar] [CrossRef] [PubMed]
- Seki, Y.; Sarikanat, M.; Sever, K.; Durmuşkahya, C. Extraction and properties of Ferula communis (chakshir) fibers as novel reinforcement for composites materials. Compos. B Eng. 2013, 44, 517–523. [Google Scholar] [CrossRef]
- Touil, M.; Rahmoun, O.; Iken, O.; El Harti, K.; Saadani, R.; Rahmoune, M. Exploring the thermal behaviour and thermo-mechanical properties of Ferula communis reinforced plaster and mortar composites: An integrated experimental and numerical approach. Energy Convers. Manag. 2023, 289, 117119. [Google Scholar] [CrossRef]
- Polycarpou, P. Ethanol production from Ferula communis. Biomass Bioenergy 2012, 36, 289–292. [Google Scholar] [CrossRef]
- Zare, A.R.; Solouki, M.; Omidi, M.; Irvani, N.; Abasabadi, A.O.; Nezad, N.M. Effect of various treatments on seed germination and dormancy breaking in Ferula assa foetida L. (Asafetida), a threatened medicinal herb. Trakia J. Sci. 2011, 9, 57–61. [Google Scholar]
- Suran, D.; Bolor, T.; Bayarmaa, G.A. In Vitro seed germination and callus induction of Ferula ferulaeoides (Steud.) Korov. (Apiaceae). Mong. J. Biol. Sci. 2016, 14, 53–58. [Google Scholar]
- Nowruzian, A.; Masoumian, M.; Ebrahimi, A.; Bakhshi Khaniki, G.H. Effect of Breaking Dormancy Treatments on Germination of Ferula assa foetida L. Seeds. Iran. J. Seed Res. 2017, 3, 154–169. [Google Scholar] [CrossRef]
- Salehi Shanjani, P.; Falah Hoseini, L. Germination Responses of Ferula assa-foetida and Ferula gummosa Boiss. Seeds to Continuous Cold Stratification. J. Med. Plants Prod. 2022, 11, 9–16. [Google Scholar]
- ISTA. International rules for seed testing. Seed Sci. Technol. 1993, 21, 160–186. [Google Scholar]
- Baskin, C.C.; Baskin, J.M. Seeds: Ecology, Biogeography, and Evolution Dormancy and Germination, 2nd ed.; Academic: San Diego, CA, USA, 2014. [Google Scholar]
- Cavieres, L.A.; Sierra-Almeida, A. Assessing the importance of cold-stratification for seed germination in alpine plant species of the High-Andes of central Chile. Perspect. Plant Ecol. 2018, 30, 125–131. [Google Scholar] [CrossRef]
- Soltani, E.; Baskin, C.C.; Baskin, J.M. A graphical method for identifying the six types of non-deep physiological dormancy in seeds. Plant Biol. 2017, 19, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Cristaudo, A.; Catara, S.; Mingo, A.; Restuccia, A.; Onofri, A. Temperature and storage time strongly affect the germination success of perennial Euphorbia species in Mediterranean regions. Ecol. Evol. 2019, 9, 10984–10999. [Google Scholar] [CrossRef] [PubMed]
- Carruggio, F.; Onofri, A.; Catara, S.; Impelluso, C.; Castrogiovanni, M.; Lo Cascio, P.; Cristaudo, A. Conditional Seed Dormancy Helps Silene hicesiae Brullo & Signor. Overcome Stressful Mediterranean Summer Conditions. Plants. 2021, 10, 2130. [Google Scholar] [PubMed]
- Baskin, C.C.; Mayer, S.E.; Baskin, J.M. Two type of morphophysiological dormancy in seeds of two genera (Osmorhiza and Erythorium) with an Arcto-Tertiary distribution pattern. Am. J. Bot. 1995, 82, 293–298. [Google Scholar] [CrossRef]
- Walck, J.L.; Hidayati, S.N.; Okagami, N. Seed germination echophysiology of Asian species Osmorhiza aristata (Apiaceae): Comparison with its North American cogenera and implications for evolution of type of dormancy. Am. J. Bot. 2002, 89, 829–835. [Google Scholar] [CrossRef] [PubMed]
- Nadjafi, F.; Bannayan, M.; Tabrizi, L.; Rastgoo, M. Seed germination and dormancy breaking techniques for Ferula gummosa and Teucrium polium. J. Arid. Environ. 2006, 64, 542–547. [Google Scholar] [CrossRef]
- Aghilian, S.; Khajeh-Hosseini, M.; Anvarkhah, S. Evaluation of seed dormancy in forty medicinal plant species. Int. J. Agric. Crop Sci. 2014, 7, 760–768. [Google Scholar]
- Ari, E.; Gürbüz, E.; Tuğrul, A.S. Seed germinations of 20 wild species growing in Antalya (Turkey) with outdoor ornamental plant potential. Fifth International Scientific Agricultural Symposium. Agrosym 2014, 2014, 439–445. [Google Scholar]
- Dettori, C.A.; Loi, M.C.; Brullo, S.; Arguimbau, P.F.; Tamburini, E.; Bacchetta, G. The genetic diversity and structure of the Ferula communis L. complex (Apiaceae) in the Tyrrhenian area. Flora 2016, 223, 138–146. [Google Scholar]
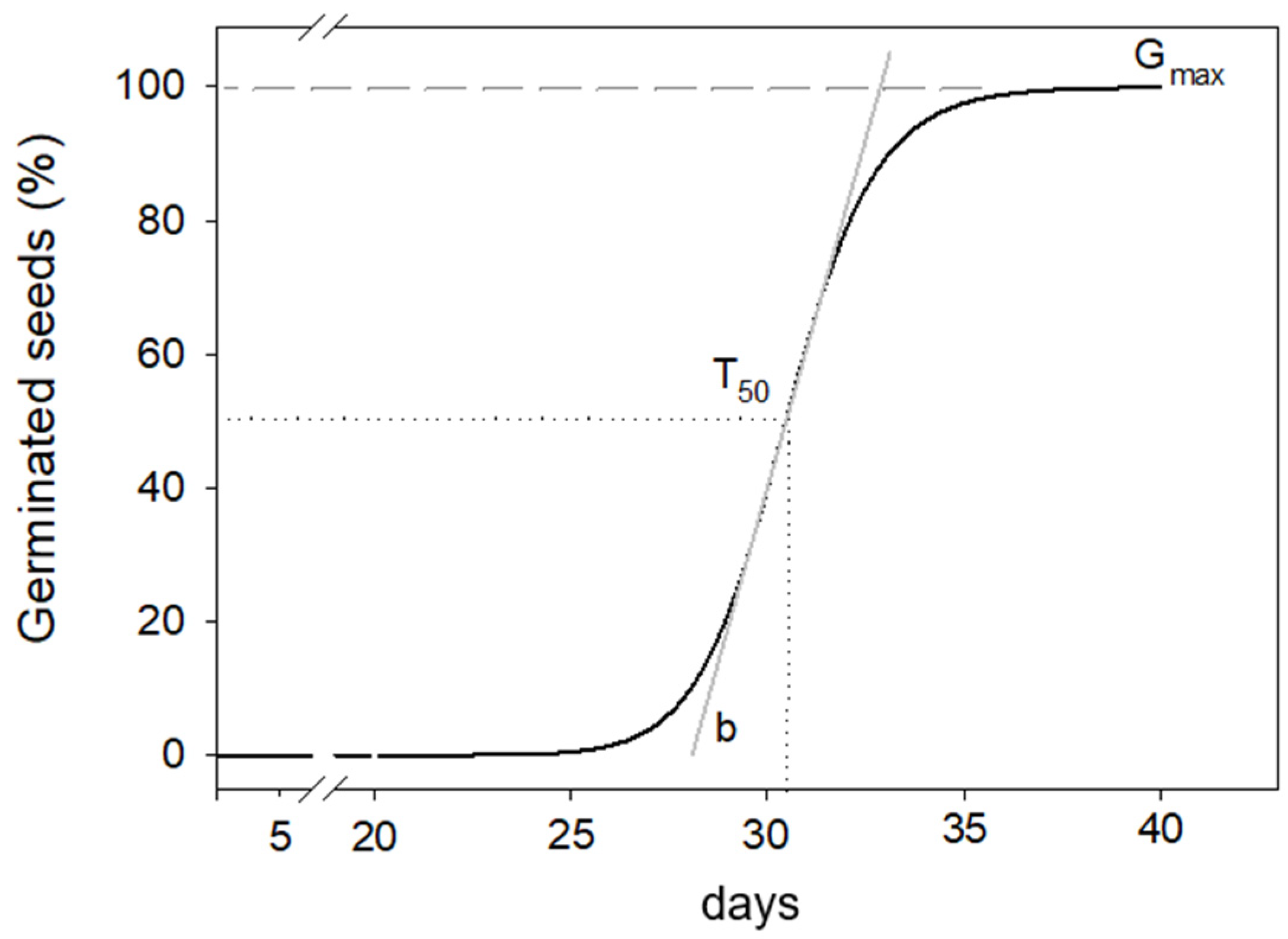
| Source of Variation | FGP | IS-LP | LP-LSG | |||
|---|---|---|---|---|---|---|
| F Values | p | F Values | p | F Values | p | |
| Cold Stratification (CS) | 1.67 | ns | n.d. | n.d. | ||
| Temperature (T) | 113.14 | *** | 7.22 | * | 7.22 | ns |
| Interaction (CS × T) | 0.87 | ns | n.d. | n.d. | ||
| Temperature | FGP | IS-LP | LP-LSG |
|---|---|---|---|
| % | days | days | |
| 5 °C | 96.7 a | 27.0 a | 27.0 |
| 10 °C | 90.0 a | 18.0 b | 18.0 |
| 15 °C | 96.7 a | 20.0 b | 20.0 |
| 20 °C | 10.0 b | -- | -- |
| Temperature | Gmax | T50 | b | |||
|---|---|---|---|---|---|---|
| % | s.e | days | s.e | s.e | ||
| 5 °C | 94.7 | ±1.67 | 29.2 | ±0.15 a | 18.4 | ±1.46 a |
| 10 °C | 91.9 | ±1.98 | 23.4 | ±0.26 b | 9.1 | ±0.80 b |
| 15 °C | 96.6 | ±2.97 | 27.5 | ±0.33 ab | 8.9 | ±0.73 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Distefano, M.; Avola, G.; Berti, S.; Riggi, E. Germination Kinetics of Ferula communis L. Seeds, a Potentially Multipurpose-Use Wild Species. Seeds 2024, 3, 196-202. https://doi.org/10.3390/seeds3020015
Distefano M, Avola G, Berti S, Riggi E. Germination Kinetics of Ferula communis L. Seeds, a Potentially Multipurpose-Use Wild Species. Seeds. 2024; 3(2):196-202. https://doi.org/10.3390/seeds3020015
Chicago/Turabian StyleDistefano, Miriam, Giovanni Avola, Stefano Berti, and Ezio Riggi. 2024. "Germination Kinetics of Ferula communis L. Seeds, a Potentially Multipurpose-Use Wild Species" Seeds 3, no. 2: 196-202. https://doi.org/10.3390/seeds3020015







