Effects of Marquandomyces marquandii SGSF043 on Maize Growth Promotion and Soil Enzyme Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Strain
2.2. Media Formulation and Fermentation Cultivation
2.3. Experimental Design
2.4. Effect of Soaking Seeds in Fermentation Solution on Germination of Maize Seeds
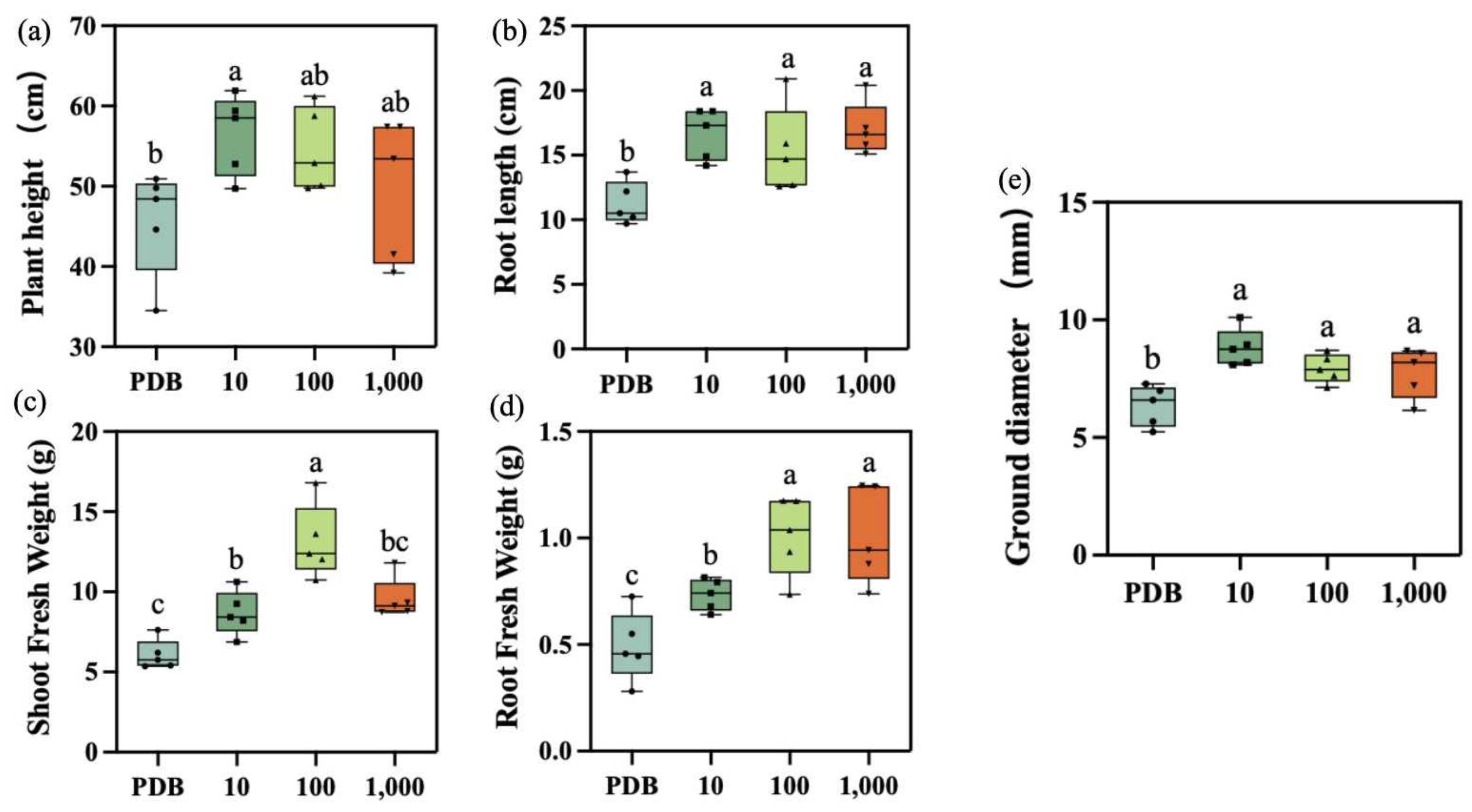
2.5. Determination of Biomass in Maize Seedlings
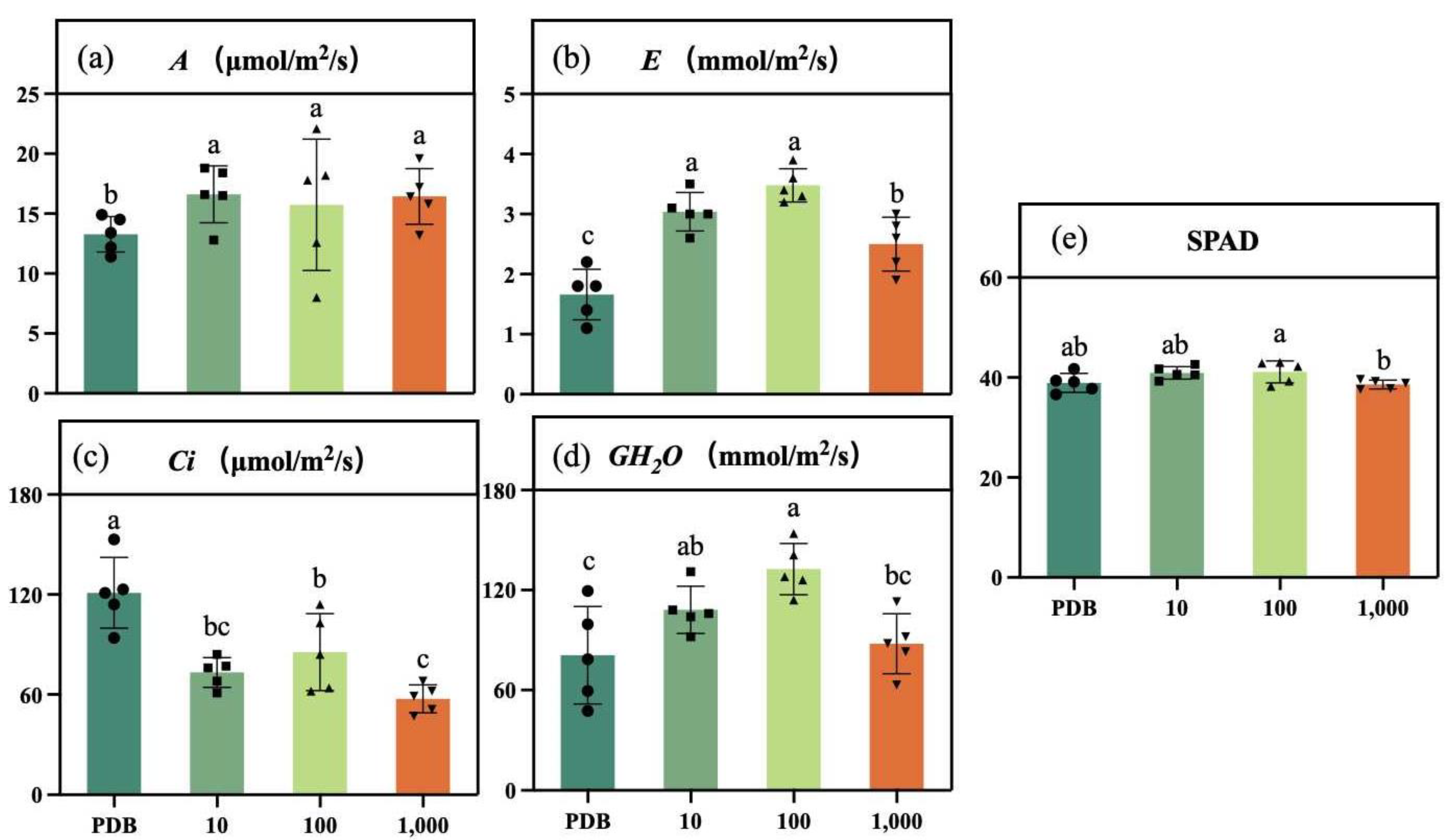
2.6. Determination of Photosynthetic Indexes and Chlorophyll SPAD Content in Maize Seedlings
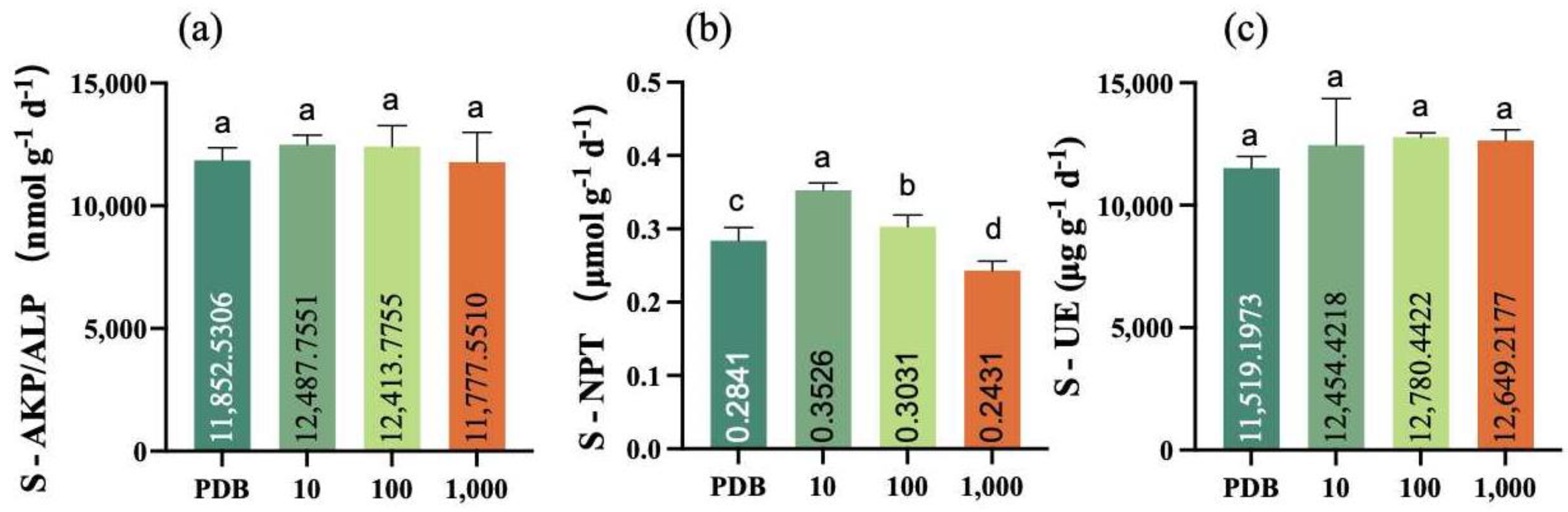
2.7. Determination of Inter-Root Soil Enzyme Activities in Maize Seedlings
2.8. Statistical Analysis
3. Results
3.1. Effect of Fermentation Solution Soaking on Germination of Maize Seeds
3.2. Effect on Maize Seedling Biomass
3.3. Effect on Photosynthetic Indexes and Chlorophyll SPAD in Maize Seedlings
3.4. Inter-Root Soil Enzyme Activities in Maize Seedlings
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, L.; Xu, Y.; Chang, J.; Li, M.; Zhang, Y.; Dang, Y.; Wang, M.; Cheng, Y.; Zhang, B. Screening, resistance and growth-promoting effect of endophytic bacteria with ACC deaminase activity isolated from soybean nodules. Acta Microbiol. Sin. 2016, 56, 1009–1021. [Google Scholar]
- Xu, J.; Sun, T.; Li, S. Application of microbial fertilizers in agricultural production of China. Crops 2016, 1, 1–6. [Google Scholar]
- Grasswitz, T.R. Integrated pest management (IPM) for small-scale farms in developed economies: Challenges and opportunities. Insects 2019, 10, 179. [Google Scholar] [CrossRef]
- Ab Rahman, S.F.S.; Singh, E.; Pieterse, C.M.; Schenk, P.M. Emerging microbial biocontrol strategies for plant pathogens. Plant Sci. 2018, 267, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Ghorbanpour, M.; Omidvari, M.; Abbaszadeh-Dahaji, P.; Omidvar, R.; Kariman, K. Mechanisms underlying the protective effects of beneficial fungi against plant diseases. Biol. Control Theory Appl. Pest Manag. 2018, 117, 147–157. [Google Scholar] [CrossRef]
- Raad, M.; Glare, T.R.; Brochero, H.L.; Müller, C.; Rostás, M. Transcriptional Reprogramming of Arabidopsis thaliana Defence Pathways by the Entomopathogen Beauveria bassiana Correlates with Resistance against a Fungal Pathogen but Not Against Insects. Front. Microbiol. 2019, 10, 438959. [Google Scholar] [CrossRef]
- Rai, R.; Srinivasamurthy, R.; Dash, P.K.; Gupta, P. Isolation, characterization and evaluation of the biocontrol potential of Pseudomonas protegens RS-9 against Ralstonia solanacearum in Tomato. Indian J. Exp. Biol. 2017, 55, 595–603. [Google Scholar]
- Abd-Elsalam, K.A.; Murugan, K. (Eds.) Index. In Bio-Based Nanoemulsions for Agri-Food Applications; Elsevier: Amsterdam, The Netherlands, 2022; pp. 451–458. [Google Scholar]
- Behie, S.W.; Zelisko, P.M.; Bidochka, M.J. Endophytic Insect-Parasitic Fungi Translocate Nitrogen Directly from Insects to Plants. Science 2012, 336, 1576–1577. [Google Scholar] [CrossRef]
- Vega, F.E.; Goettel, M.S.; Blackwell, M.; Chandler, D.; Jackson, M.A.; Keller, S.; Koike, M.; Maniania, N.K.; Monzon, A.; Ownley, B.H.; et al. Fungal entomopathogens: New insights on their ecology. Fungal Ecol. 2009, 2, 149–159. [Google Scholar] [CrossRef]
- Sorokin, N. Plant parasites of man and animals as causes of infectious diseases. J. Mil. Med. 1883, 2, 268–291. [Google Scholar]
- Sasan, R.K.; Bidochka, M.J. The insect-pathogenic fungus Metarhizium robertsii (Clavicipitaceae) is also an endophyte that stimulates plant root development. Am. J. Bot. 2012, 99, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Diniz, K.A.; Silva, P.d.A.; Oliveira, J.A.; Emiliorelli Evangelista, J.R. Sweet pepper seed responses to inoculation with microorganisms and coating with micronutrients, aminoacids and plant growth regulators. Sci. Agric. 2009, 66, 293–297. [Google Scholar] [CrossRef]
- Pérez, E.G.; Amaro, M.A.O.; Elihú, B.; Pablo, D.S.; Francisco, J.B.J. The entomopathogenic fungus Metarhizium anisopliae enhances Arabidopsis, tomato, and maize plant growth. Plant Physiol. Biochem. 2022, 176, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Baron, N.C.; Pollo, A.d.S.; Rigobelo, E.C. Purpureocillium lilacinum and Metarhizium marquandii as plant growth-promoting fungi. PeerJ 2020, 8, e9005. [Google Scholar] [CrossRef]
- Elena, G.J.; Beatriz, P.J.; Alejandro, P.; Lecuona, R. Metarhizium anisopliae (Metschnikoff) Sorokin promotes growth and has endophytic activity in tomato plants. Adv. Biol. Res. 2011, 5, 22–27. [Google Scholar]
- Liao, X.G.; O’Brien, T.R.; Fang, W.G.; St Leger, R.J. The plant beneficial effects of Metarhizium species correlate with their association with roots. Appl. Microbiol. Biotechnol. 2014, 98, 7089–7096. [Google Scholar] [CrossRef]
- Bangari, M.P.S.; Nataraja, K.N. Can endophytes minimize photosynthetic limitation? Trends Plant Sci. 2023, 29, 403–405. [Google Scholar] [CrossRef]
- Shagufta; Noor-un-Nisa; Jamali, F.A.; Ahmad, W.; Ul-Allah, S.; Wahocho, N.A.; Jamali, M.F.; Shah, S.A. Comparative Effect of Varieties and Types of Containers on Seed Germination and Seedling Growth of Geranium (Palergonium graveolens). Seeds 2023, 2, 165–176. [Google Scholar] [CrossRef]
- Barelli, L.; Moonjely, S.; Behie, S.W.; Bidochka, M.J. Fungi with multifunctional lifestyles: Endophytic insect pathogenic fungi. Plant Mol. Biol. 2016, 90, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Behie, S.W.; Moreira, C.C.; Sementchoukova, I.; Barelli, L.; Zelisko, P.M.; Bidochka, M.J. Carbon translocation from a plant to an insect-pathogenic endophytic fungus. Nat. Commun. 2017, 8, 14245. [Google Scholar] [CrossRef]
- Filipello Marchisio, V.; Curetti, D.; Cassinelli, C.; Bordese, C. Keratinolytic and keratinophilic fungi in the soils of Papua New Guinea. Mycopathologia 1991, 115, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Mongkolsamrit, S.; Khonsanit, A.; Thanakitpipattana, D.; Tasanathai, K.; Luangsa-Ard, J. Revisiting Metarhizium and the description of new species from Thailand. Stud. Mycol. 2020, 95, 171–251. [Google Scholar] [CrossRef] [PubMed]
- Bais, H.P.; Vepachedu, R.; Gilroy, S.; Callaway, R.M.; Vivanco, J.M. Allelopathy and exotic plant invasion: From molecules and genes to species interactions. Science 2003, 5638, 301. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Xi, X.; Huang, S.; Liang, G.; Sun, J.; Zhou, W.; Wang, X. Short-Term Responses of Soil Respiration and C-Cycle Enzyme Activities to Additions of Biochar and Urea in a Calcareous Soil. PLoS ONE 2016, 11, e0161694. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Hockaday, W.C.; Masiello, C.A.; Alvarez, P.J.J. Earthworm avoidance of biochar can be mitigated by wetting. Soil Biol. Biochem. 2011, 43, 1732–1737. [Google Scholar] [CrossRef]
- Oleszczuk, P.; Jośko, I.; Futa, B.; Pasieczna-Patkowska, S.; Pałys, E.; Kraska, P. Effect of pesticides on microorganisms, enzymatic activity and plant in biochar-amended soil. Geoderma Int. J. Soil Sci. 2014, 214–215, 10–18. [Google Scholar] [CrossRef]
- Xiao-Guang, J.; Chong-Sheng, G.; Guo-Hong, L. Effect of Long-Term Fertilization on Soil Enzyme Activities under Different Hydrothermal Conditions in Northeast China. Agric. Sci. China 2011, 10, 11. [Google Scholar]
- Szewczyk, R.; Sobon, A.; Slaba, M.; Dlugonski, J. Mechanism study of alachlor biodegradation by Paecilomyces marquandii with proteomic and metabolomic methods. J. Hazard. Mater. 2015, 291, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Dash, P.K.; Gupta, P.; Panwar, B.S.; Rai, R. Isolation, cloning and characterization of phlB gene from an Indian strain of Gram negative soil bacteria Pseudomonas fluorescens. Indian J. Exp. Biol. 2020, 58, 412–419. [Google Scholar]
- Wang, L.X.; Chen, M.X.; Lam, P.Y.; Dini-Andreote, F.; Dai, L.; Wei, Z. Multifaceted roles of flavonoids mediating plant-microbe interactions. Microbiome 2022, 10, 233. [Google Scholar] [CrossRef]
- Tatematsu, K.; Kumagai, S.; Muto, H.; Sato, A.; Watahiki, M.K.; Harper, R.M.; Liscum, E.; Yamamoto, K.T. MASSUGU2 Encodes Aux/IAA19, an Auxin-Regulated Protein That Functions Together with the Transcriptional Activator NPH4/ARF7 to Regulate Differential Growth Responses of Hypocotyl and Formation of Lateral Roots in Arabidopsis thaliana. Plant Cell Online 2004, 16, 379–393. [Google Scholar] [CrossRef]
- Oldroyd, G.E.D. Speak, friend, and enter: Signalling systems that promote beneficial symbiotic associations in plants. Nat. Rev. Microbiol. 2013, 11, 252–263. [Google Scholar] [CrossRef] [PubMed]
- Vitale, S.; Di Pietro, A.; Turra, D. Autocrine pheromone signalling regulates community behaviour in the fungal pathogen Fusarium oxysporum. Nat. Microbiol. 2019, 4, 1443–1449. [Google Scholar] [CrossRef]
- Ma, Y.; Cao, M.; Shi, X.; Li, Z.; Luo, Y. Function of plant growth-promoting bacteria and its application in sustainable agriculture. Acta Pedol. Sin. 2023, 606, 1555–1568. [Google Scholar]
- Eisenhardt, K.M.; Reuscher, C.M.; Klug, G. PcrX, an sRNA derived from the 3′–UTR of the Rhodobacter sphaeroides puf operon modulates expression of puf genes encoding proteins of the bacterial photosynthetic apparatus. Mol. Microbiol. 2018, 110, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Pitman, M.G.; Läuchli, A. Global impact of salinity and agricultural ecosystems. Salin. Environ.-Plants-Mol. 2002, 3, 20. [Google Scholar]
- Tu, C.M. Effect of Four Experimental Insecticides on Enzyme Activities and Levels of Adenosine Triphosphate in Mineral and Organic Soils. J. Environ. Sci. Health Part B 1990, 25, 787–800. [Google Scholar] [CrossRef]
- Wachendorf, C.; Lampe, C.; Taube, F.; Dittert, K. Nitrous oxide emissions and dynamics of soil nitrogen under 15N-labeled cow urine and dung patches on a sandy grassland soil. J. Plant Nutr. Soil Sci. 2008, 171, 171–180. [Google Scholar] [CrossRef]
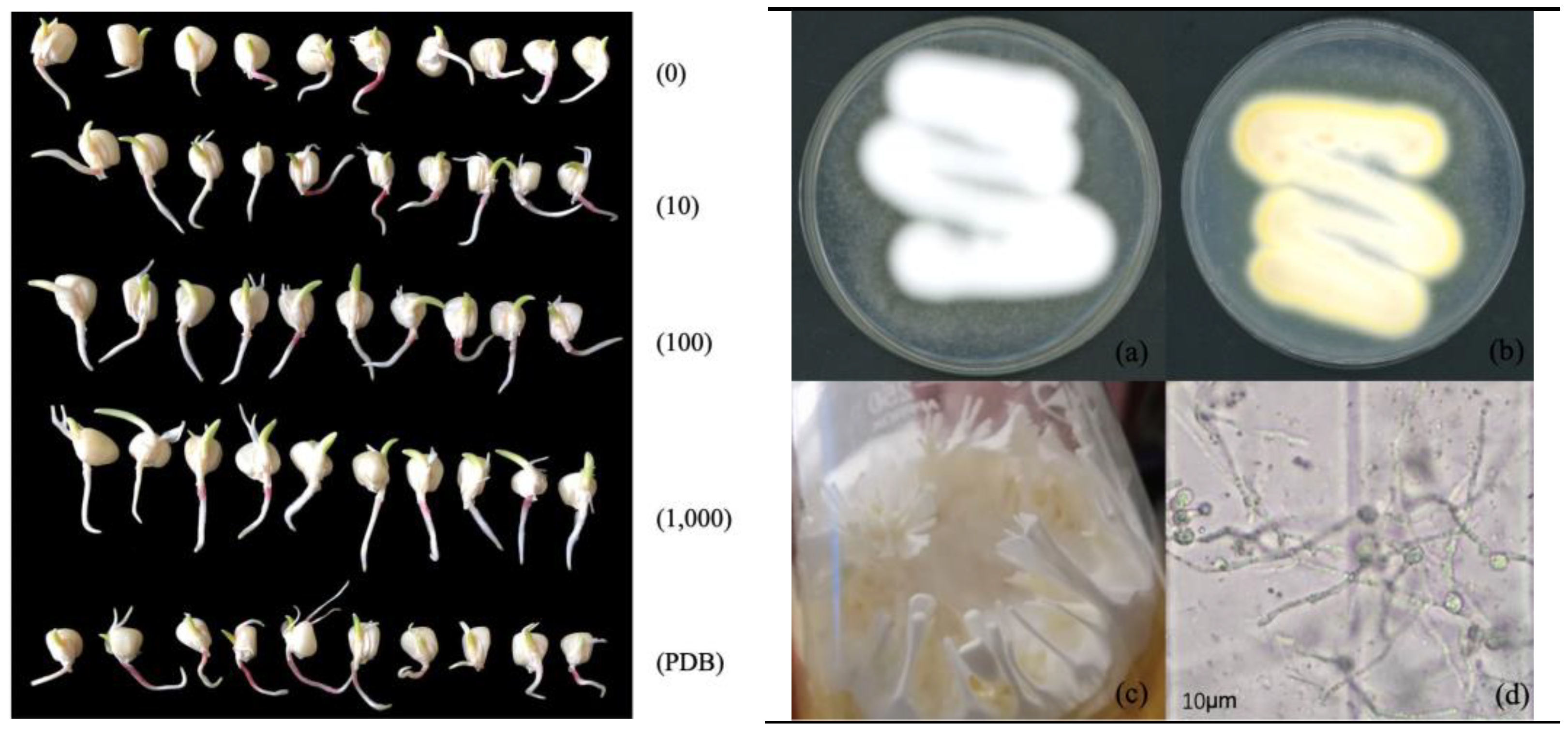
| Treatment | Dilution Times | Germination Days/d | Germinating Energy/% | Germination Percentage/% | Germination Index |
|---|---|---|---|---|---|
| M. Marquandii SGSF043 | 0 | 2 | 37 ± 0.10 c | 15 ± 1.90 c | 5.50 ± 0.10 c |
| 10 | 1 | 97 ± 0.10 a | 97 ± 0.69 a | 14.53 ± 0.06 a | |
| 100 | 2 | 94 ± 0.24 b | 94 ± 1.39 b | 14.00 ± 0.00 b | |
| 1000 | 2 | 97 ± 0.10 a | 94 ± 0.84 b | 14.50 ± 0.10 a | |
| Control (PDB) | - | 1 | 97 ± 0.15 a | 94 ± 2.12 b | 14.47 ± 0.06 a |
| Treatment | Dilution Times | Radicle Length (cm) | Radicle Coarse (cm) | Plumule Length (cm) | Plumule Coarse (cm) | Hypocotyl Length (cm) |
|---|---|---|---|---|---|---|
| M. Marquandii SGSF043 | 0 | 0.46 ± 1.41 c | 0.19 ± 0.56 c | 0.98 ± 1.46 d | 0.54 ± 0.76 c | 0.42 ± 1.10 c |
| 10 | 12.16 ± 3.48 a | 1.68 ± 0.64 ab | 5.17 ± 1.81 ab | 1.83 ± 0.64 ab | 3.10 ± 1.24 b | |
| 100 | 8.17 ± 4.36 b | 1.37 ± 0.78 b | 4.58 ± 2.76 bc | 1.59 ± 0.77 b | 3.13 ± 1.71 b | |
| 1000 | 11.38 ± 4.51 a | 1.74 ± 0.66 a | 5.56 ± 1.73 a | 2.02 ± 0.48 a | 4.07 ± 1.24 a | |
| Control (PDB) | — | 11.37 ± 1.11 a | 1.52 ± 0.41 ab | 4.20 ± 1.39 c | 1.83 ± 0.43 ab | 3.2 ± 0.75 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, X.; Zhang, B.; Shi, F.; Chen, Y.; Zhao, X. Effects of Marquandomyces marquandii SGSF043 on Maize Growth Promotion and Soil Enzyme Activity. Seeds 2024, 3, 203-215. https://doi.org/10.3390/seeds3020016
Zheng X, Zhang B, Shi F, Chen Y, Zhao X. Effects of Marquandomyces marquandii SGSF043 on Maize Growth Promotion and Soil Enzyme Activity. Seeds. 2024; 3(2):203-215. https://doi.org/10.3390/seeds3020016
Chicago/Turabian StyleZheng, Xu, Bo Zhang, Feng Shi, Yuanlong Chen, and Xiumei Zhao. 2024. "Effects of Marquandomyces marquandii SGSF043 on Maize Growth Promotion and Soil Enzyme Activity" Seeds 3, no. 2: 203-215. https://doi.org/10.3390/seeds3020016
APA StyleZheng, X., Zhang, B., Shi, F., Chen, Y., & Zhao, X. (2024). Effects of Marquandomyces marquandii SGSF043 on Maize Growth Promotion and Soil Enzyme Activity. Seeds, 3(2), 203-215. https://doi.org/10.3390/seeds3020016







