Enhancing Bean (Phaseolus vulgaris L.) Resilience: Unveiling the Role of Halopriming against Saltwater Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Determination of Seeds’ Halotolerance
2.2. Plants’ Growth Conditions and Saline Treatments
2.3. Soil Analysis
2.4. Morphological Parameters and Tolerance Index
2.5. Chlorophylls and Soluble Sugars
2.6. Quantification of Intracellular Free Calcium
2.7. Phenolic Compounds and Proline Content
2.7.1. Phenolic Compounds
2.7.2. Proline
2.8. Thiobarbituric Acid Reactive Products
2.9. Antioxidant Activity
2.9.1. 2,2-Diphenyl-1-picryl-hydrazyl-hydrate (DPPH) Free Radical Assay
2.9.2. Potassium Ferricyanide and Ferric Reducing Antioxidant Power (PFRAP and FRAP) Assays
2.10. Enzymatic Activities
2.11. Fruit Characteristics
2.12. Statistical Analysis
3. Results
3.1. Determination of Seeds’ Halotolerance and Selection of Best Priming Agent
3.2. Soil Experiments and Analyses
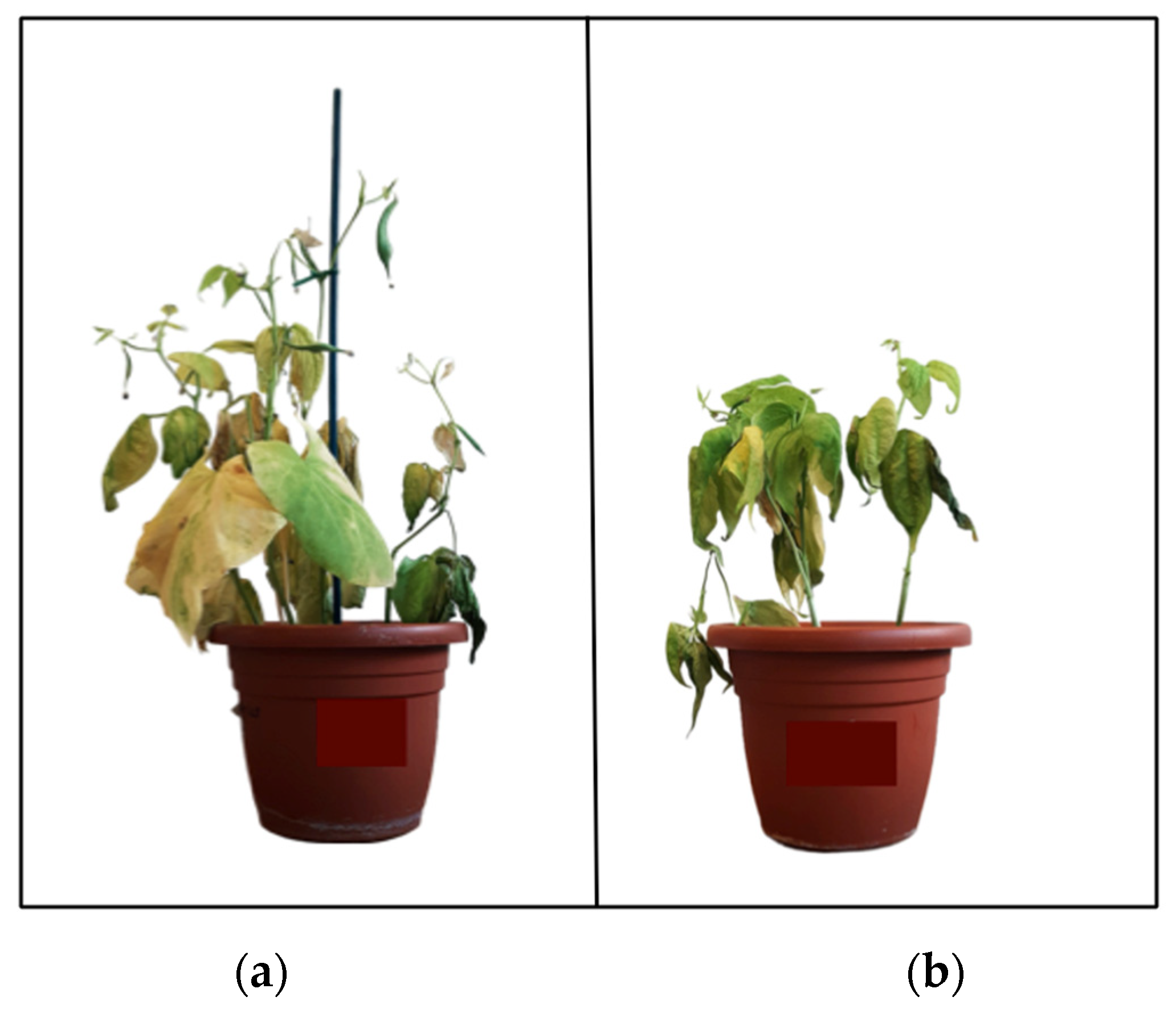
3.3. Effect of Saltwater Stress on Plant Growth
3.4. Effect of Salt Treatment on Chlorophylls and Soluble Sugars
3.5. Calcium Translocation and Activation of the Response to Saltwater Stress
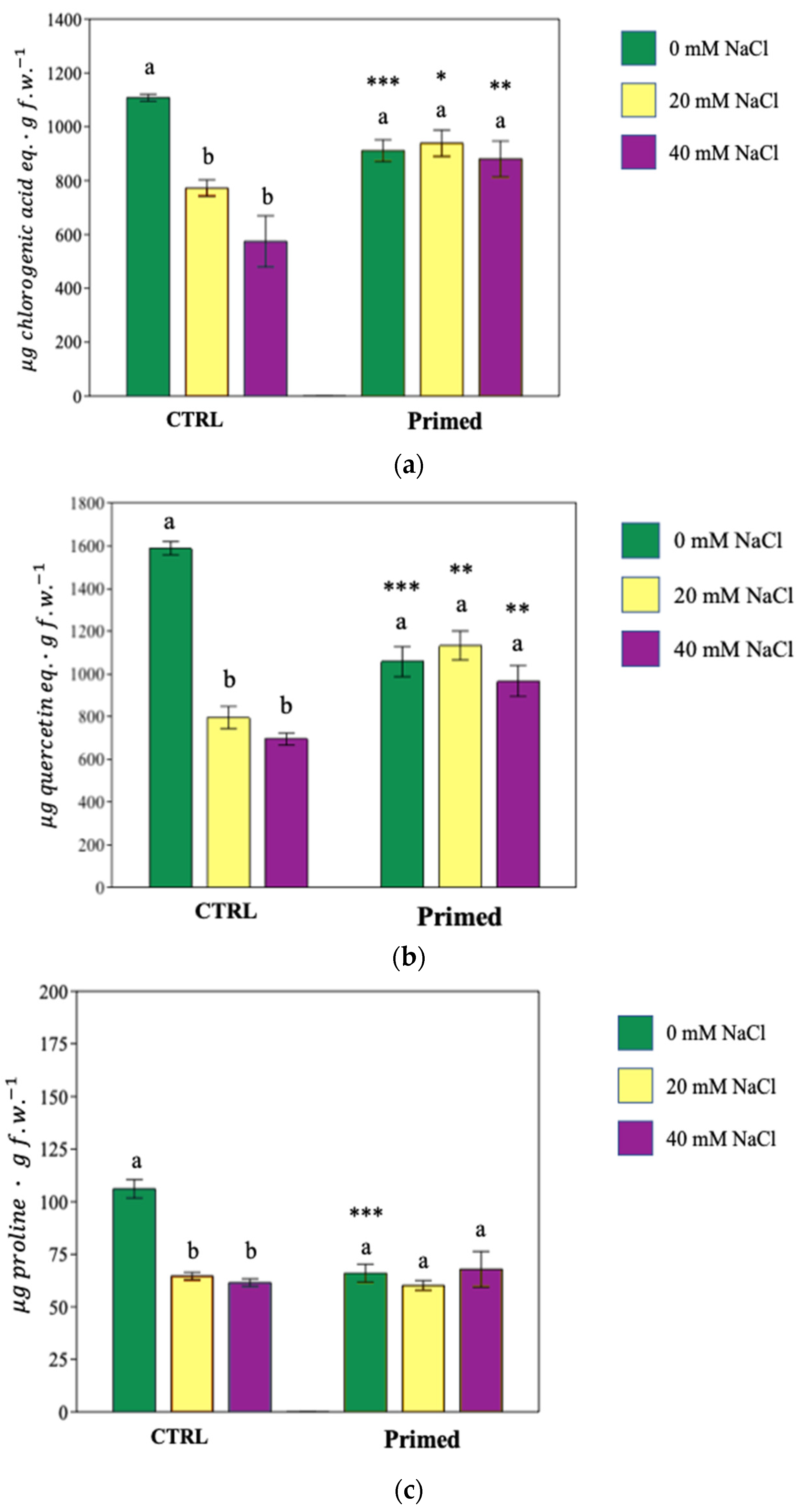
3.6. Changes in Metabolism: Phenolic Compounds and Proline
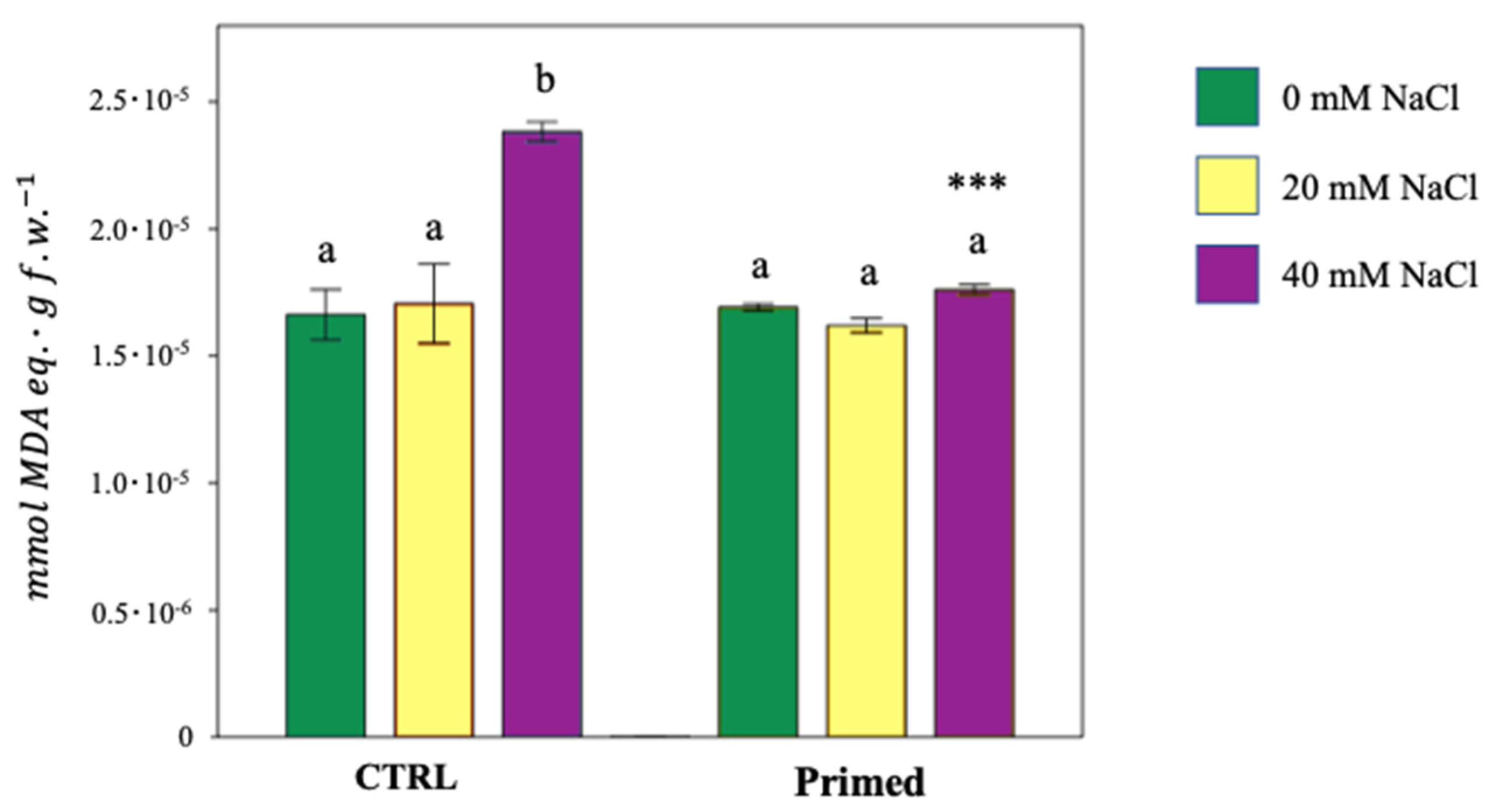
3.7. Lipid Peroxidation Inhibition
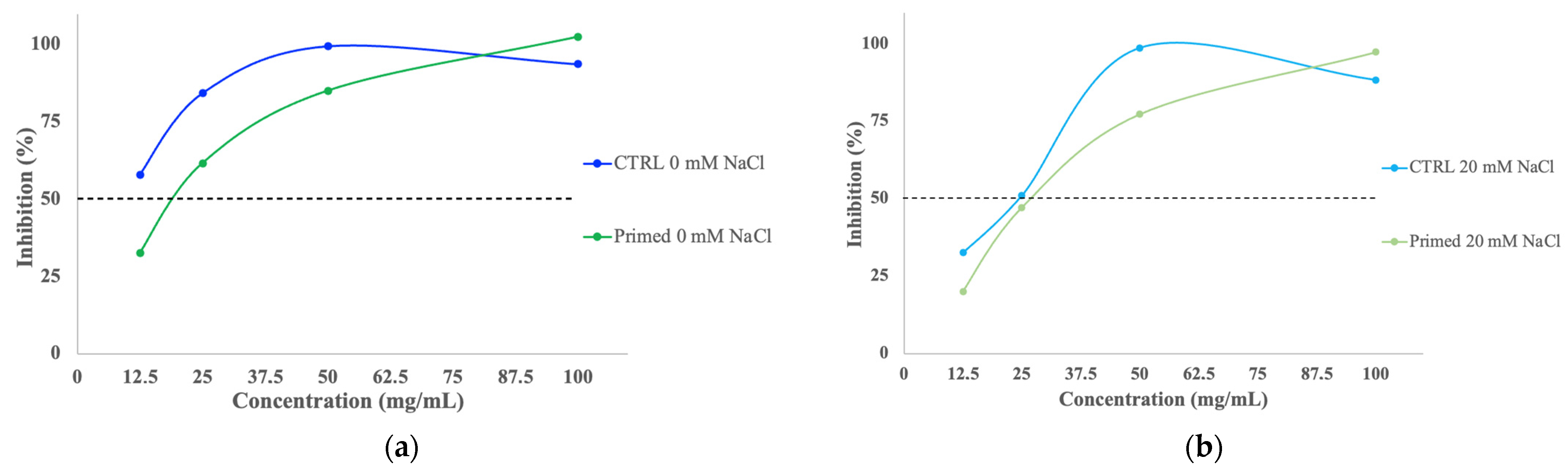
3.8. Total Antioxidant Activity
3.9. Enzymatic Activities
3.10. Effect of Salt Treatment on Pod Production and Seed Analysis
4. Discussion
4.1. Effect of Seed Priming on Bean Germination and Growth
4.2. Relationship between Seed Priming, Photosynthesis, and Sugar Metabolism
4.3. Activation of Signaling and Salt Tolerance Responses
4.4. Seed Priming during Reproductive Phase: Effect on Pod Production and Seed Quality
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Farrow, A.; Muthoni-Andriatsitohaina, R. Atlas of Common Bean Production in Africa: Pan-Africa Bean Research Alliance (PABRA); International Center for Tropical Agriculture (CIAT): Nairobi, Kenya, 2020; p. 242. [Google Scholar] [CrossRef]
- Mullins, A.P.; Arjmandi, B.H. Health Benefits of Plant-Based Nutrition: Focus on Beans in Cardiometabolic Diseases. Nutrients 2021, 13, 519. [Google Scholar] [CrossRef] [PubMed]
- Nchanji, E.B.; Ageyo, O.C. Do Common Beans (Phaseolus vulgaris L.) Promote Good Health in Humans? A Systematic Review and Meta-Analysis of Clinical and Randomized Controlled Trials. Nutrients 2021, 13, 3701. [Google Scholar] [CrossRef] [PubMed]
- Uebersax, M.A.; Cichy, K.A.; Gomez, F.E.; Porch, T.G.; Heitholt, J.; Osorno, J.M.; Kamfwa, K.; Snapp, S.S.; Bales, S. Dry Beans (Phaseolus vulgaris L.) as a Vital Component of Sustainable Agriculture and Food Security-A Review. Legume Sci. 2022, 5, e155. [Google Scholar] [CrossRef]
- Didinger, C.; Foster, M.T.; Bunning, M.; Thompson, H.J. Nutrition and human health benefits of dry beans and other pulses. In Dry Beans and Pulses: Production, Processing, and Nutrition; John Wiley & Sons: Hoboken, NJ, USA, 2022; pp. 481–504. [Google Scholar] [CrossRef]
- Ioniță-Mîndrican, C.B.; Ziani, K.; Mititelu, M.; Oprea, E.; Neacșu, S.M.; Moroșan, E.; Dumitrescu, D.E.; Roșca, A.C.; Drăgănescu, D.; Negrei, C. Therapeutic Benefits and Dietary Restrictions of Fiber Intake: A State of the Art Review. Nutrients 2022, 14, 2641. [Google Scholar] [CrossRef] [PubMed]
- Aranda-Olmedo, I.; Rubio, L.A. Dietary legumes, intestinal microbiota, inflammation and colorectal cancer. J. Funct. Foods 2020, 64, 103707. [Google Scholar] [CrossRef]
- Maereka, E.K.; Nchanji, E.B.; Nyamolo, V.; Cosmas, L.K.; Chataika, B.Y. Women’s visibility and bargaining power in the common bean value chain in Mozambique. CABI Agric. Biosci. 2023, 4, 56. [Google Scholar] [CrossRef]
- Lone, A.A.; Khan, M.N.; Gul, A.; Dar, Z.A.; Iqbal, A.M.; Lone, B.A.; Ahangar, A.; Rasool, F.U.; Ali, G.; Nisar, F.; et al. Common beans and abiotic stress challenges. Curr. J. Appl. Sci. Technol. 2021, 40, 41–53. [Google Scholar] [CrossRef]
- Kopecká, R.; Kameniarová, M.; Černý, M.; Brzobohatý, B.; Novák, J. Abiotic Stress in Crop Production. Int. J. Mol. Sci. 2023, 24, 6603. [Google Scholar] [CrossRef]
- Muhammad, M.; Waheed, A.; Wahab, A.; Majeed, M.; Nazim, M.; Liu, Y.H.; Li, L.; Li, W.J. Soil salinity and drought tolerance: An evaluation of plant growth, productivity, microbial diversity, and amelioration strategies. Plant Stress 2023, 11, 100319. [Google Scholar] [CrossRef]
- Nadeem, M.; Li, J.; Yahya, M.; Wang, M.; Ali, A.; Cheng, A.; Wang, X.; Ma, C. Grain Legumes and Fear of Salt Stress: Focus on Mechanisms and Management Strategies. Int. J. Mol. Sci. 2019, 20, 799. [Google Scholar] [CrossRef]
- Machado, R.M.A.; Serralheiro, R.P. Soil salinity: Effect on vegetable crop growth. Management practices to prevent and mitigate soil salinization. Horticulturae 2017, 3, 30. [Google Scholar] [CrossRef]
- Alharbi, K.; Al-Osaimi, A.A.; Alghamdi, B.A. Sodium chloride (NaCl)-induced physiological alteration and oxidative stress generation in Pisum sativum (L.): A toxicity assessment. ACS Omega 2022, 7, 20819–20832. [Google Scholar] [CrossRef] [PubMed]
- Toscano, S.; Romano, D.; Ferrante, A. Molecular Responses of Vegetable, Ornamental Crops, and Model Plants to Salinity Stress. Int. J. Mol. Sci. 2023, 24, 3190. [Google Scholar] [CrossRef] [PubMed]
- Kesawat, M.S.; Satheesh, N.; Kherawat, B.S.; Kumar, A.; Kim, H.U.; Chung, S.M.; Kumar, M. Regulation of Reactive Oxygen Species during Salt Stress in Plants and Their Crosstalk with Other Signaling Molecules-Current Perspectives and Future Directions. Plants 2023, 12, 864. [Google Scholar] [CrossRef] [PubMed]
- Hnilickova, H.; Kraus, K.; Vachova, P.; Hnilicka, F. Salinity Stress Affects Photosynthesis, Malondialdehyde Formation, and Proline Content in Portulaca oleracea L. Plants 2021, 10, 845. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, Z.; Faizan, S.; Gulzar, B. Salt stress, its impacts on plants and the strategies plants are employing against it: A review. J. Appl. Biol. Biotech. 2020, 8, 81–91. [Google Scholar] [CrossRef]
- Hongqiao, L.; Suyama, A.; Mitani-Ueno, N.; Hell, R.; Maruyama-Nakashita, A. A Low Level of NaCl Stimulates Plant Growth by Improving Carbon and Sulfur Assimilation in Arabidopsis thaliana. Plants 2021, 10, 2138. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez Coca, L.I.; García González, M.T.; Gil Unday, Z.; Jiménez Hernández, J.; Rodríguez Jáuregui, M.M.; Fernández Cancio, Y. Effects of Sodium Salinity on Rice (Oryza sativa L.) Cultivation: A Review. Sustainability 2023, 15, 1804. [Google Scholar] [CrossRef]
- Sade, N.; del Mar Rubio-Wilhelmi, M.; Umnajkitikorn, K.; Blumwald, E. Stress-induced senescence and plant tolerance to abiotic stress. J. Exp. Bot. 2018, 69, 845–853. [Google Scholar] [CrossRef]
- Khan, M.O.; Irfan, M.; Muhammad, A.; Ullah, I.; Nawaz, S.; Khalil, M.K.; Ahmad, M. A practical and economical strategy to mitigate salinity stress through seed priming. Front. Environ. Sci. 2022, 10, 991977. [Google Scholar] [CrossRef]
- Santangeli, M.; Capo, C.; Beninati, S.; Pietrini, F.; Forni, C. Gradual Exposure to Salinity Improves Tolerance to Salt Stress in Rapeseed (Brassica napus L.). Water 2019, 11, 1667. [Google Scholar] [CrossRef]
- Collier, R.J.; Baumgard, L.H.; Zimbelman, R.B.; Xiao, Y. Heat stress: Physiology of acclimation and adaptation. Anim. Front. 2018, 9, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Charng, Y.Y.; Mitra, S.; Yu, S.J. Maintenance of abiotic stress memory in plants: Lessons learned from heat acclimation. Plant Cell 2023, 35, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Kamanga, R.M.; Echigo, K.; Yodoya, K.; Mekawy, A.M.M.; Ueda, A. Salinity acclimation ameliorates salt stress in tomato (Solanum lycopersicum L.) seedlings by triggering a cascade of physiological processes in the leaves. Sci. Hortic. 2020, 270, 109434. [Google Scholar] [CrossRef]
- Lutts, S.; Benincasa, P.; Wojtyla, L.; Kubala, S.S.; Pace, R.; Lechowska, K.; Quinet, M.; Garnczarska, M. Seed Priming: New Comprehensive Approaches for an Old Empirical Technique. In New Challenges in Seed Biology-Basic and Translational Research Driving Seed Technology; IntechOpen: London, UK, 2016; pp. 1–46. [Google Scholar] [CrossRef]
- Louis, N.; Dhankher, O.P.; Puthur, J.T. Seed priming can enhance and retain stress tolerance in ensuing generations by inducing epigenetic changes and trans-generational memory. Physiol. Plant. 2023, 175, e13881. [Google Scholar] [CrossRef] [PubMed]
- Stassinos, P.M.; Rossi, M.; Borromeo, I.; Capo, C.; Beninati, S.; Forni, C. Enhancement of Brassica napus Tolerance to High Saline Conditions by Seed Priming. Plants 2021, 10, 403. [Google Scholar] [CrossRef] [PubMed]
- Sheteiwy, M.; Shen, H.; Xu, J.; Guan, Y.; Song, W.; Hu, J. Seed polyamines metabolism induced by seed priming with spermidine and 5-aminolevulinic acid for chilling tolerance improvement in rice (Oryza sativa L.) seedlings. Environ. Exp. Bot. 2017, 137, 58–72. [Google Scholar] [CrossRef]
- Forni, C.; Borromeo, I. The Utilization of Seed Priming as a Tool to Overcome Salt and Drought Stresses: Is Still a Long Way to Go? Seeds 2023, 2, 406–420. [Google Scholar] [CrossRef]
- Borromeo, I.; Domenici, F.; Del Gallo, M.; Forni, C. Role of Polyamines in the Response to Salt Stress of Tomato. Plants 2023, 12, 1855. [Google Scholar] [CrossRef]
- Sairam, R.K.; Deshmukh, P.S.; Shukla, D.S. Tolerance of drought and temperature stress in relation to increased antioxidant enzyme activity in wheat. J. Agron. Crop Sci. 1997, 178, 171–178. [Google Scholar] [CrossRef]
- Idrees, S.; Shabir, S.; Ilyas, N.; Batool, N.; Kanwal, S. Assessment of cadmium on wheat (Triticum aestivum L.) in hydroponics medium. Agrociencia 2015, 49, 917–929. [Google Scholar]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigment of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar] [CrossRef]
- Chun, Y.; Yin, Z.D. Glycogen assay for diagnosis of female genital Chlamydia trachomatis infection. J. Clin. Microbiol. 1998, 36, 1081–1082. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C.; Yang, M.H.; Wen, H.M.; Chern, J.C. Estimation of total flavonoid content in propolis by two complementary colometric methods. J. Food Drug. Anal. 2002, 10, 3. [Google Scholar] [CrossRef]
- Micheli, L.; Spitoni, S.; Di Cesare Mannelli, L.; Bilia, A.R.; Ghelardini, C.; Pallanti, S. Bacopa monnieri as augmentation therapy in the treatment of anhedonia, preclinical and clinical evaluation. Phytother. Res. 2020, 34, 2331–2340. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Jindal, R. Oxidative stress response in liver, kidney and gills of ctenopharyngodon idellus (cuvier & valenciennes) exposed to chlorpyrifos. MOJ Biol. Med. 2017, 1, 103–112. [Google Scholar] [CrossRef]
- Garcia, E.J.; Oldoni, T.L.; Alencar, S.M.; Reis, A.; Loguercio, A.D.; Grande, R.H. Antioxidant activity by DPPH assay of potential solutions to be applied on bleached teeth. Braz. Dent. J. 2012, 23, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Hue, S.M.; Boyce, A.N.; Somasundram, C. Antioxidant activity, phenolic and flavonoid contents in the leaves of different varieties of sweet potato (‘Ipomoea batatas’). Aust. J. Crop Sci. 2012, 6, 375–380. Available online: http://eprints.um.edu.my/id/eprint/7722 (accessed on 28 October 2023).
- Gohari, A.R.; Hajimehdipoor, H.; Saeidnia, S.; Ajani, Y.; Hadjiakhoondi, A. Antioxidant Activity of some Medicinal Species using FRAP Assay. J. Med. Plants 2011, 10, 54–60. Available online: http://jmp.ir/article-1-233-fa.html (accessed on 28 October 2023).
- Lim, C.S.H.; Lim, S.L. Ferric reducing capacity versus ferric reducing antioxidant power for measuring total antioxidant capacity. Lab. Med. 2013, 44, 51–55. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Orzali, L.; Forni, C.; Riccioni, L. Effect of chitosan seed treatment as elicitor of resistance to Fusarium graminearum in wheat. Seed Sci. Technol. 2014, 42, 132–149. [Google Scholar] [CrossRef]
- Yang, L.; Xi, Y.; Luo, X.Y.; Ni, H.; Liet, H.H. Preparation of peroxidase and phenolics using discarded sweet potato old stems. Sci. Rep. 2019, 9, 3769. [Google Scholar] [CrossRef] [PubMed]
- Iwase, T.; Tajima, A.; Sugimoto, S.; Okuda, K.I.; Hironaka, I.; Kamata, Y.; Takada, K.; Mizunoe, Y. A Simple Assay for Measuring Catalase Activity: A Visual Approach. Sci. Rep. 2013, 3, 3081. [Google Scholar] [CrossRef]
- Forni, C.; Duca, D.; Glick, B.R. Mechanisms of plant response to salt and drought stress and their alteration by rhizobacteria. Plant Soil 2017, 410, 335–356. [Google Scholar] [CrossRef]
- Abbass, K.; Qasim, M.Z.; Song, H.; Murshed, M.; Mahmood, H.; Younis, I. A review of the global climate change impacts, adaptation, and sustainable mitigation measures. Environ. Sci. Pollut. Res. 2022, 29, 42539–42559. [Google Scholar] [CrossRef]
- Raza, A.; Razzaq, A.; Mehmood, S.S.; Zou, X.; Zhang, X.; Lv, Y.; Xu, J. Impact of Climate Change on Crops Adaptation and Strategies to Tackle Its Outcome: A Review. Plants 2019, 8, 34. [Google Scholar] [CrossRef]
- Mladenov, P.; Aziz, S.; Topalova, E.; Renaut, J.; Planchon, S.; Raina, A.; Tomlekova, N. Physiological Responses of Common Bean Genotypes to Drought Stress. Agronomy 2023, 13, 1022. [Google Scholar] [CrossRef]
- Suárez, J.C.; Contreras, A.T.; Anzola, J.A.; Vanegas, J.I.; Rao, I.M. Physiological Characteristics of Cultivated Tepary Bean (Phaseolus acutifolius A. Gray) and Its Wild Relatives Grown at High Temperature and Acid Soil Stress Conditions in the Amazon Region of Colombia. Plants 2022, 11, 116. [Google Scholar] [CrossRef]
- Hummel, M.; Hallahan, B.F.; Brychkova, G.; Ramirez-Villegas, J.; Guwela, V.; Chataika, B.; Curley, E.; McKeown, P.C.; Morrison, L.; Talsma, E.F.; et al. Reduction in nutritional quality and growing area suitability of common bean under climate change induced drought stress in Africa. Sci. Rep. 2018, 8, 16187. [Google Scholar] [CrossRef]
- Majeed, A.; Muhammad, Z. Salinity: A Major Agricultural Problem—Causes, Impacts on Crop Productivity and Management Strategies. In Plant Abiotic Stress Tolerance; Hasanuzzaman, M., Hakeem, K.R., Nahar, K., Alharby, H.F., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 83–99. ISBN 978-3-030-06117-3. [Google Scholar] [CrossRef]
- Al Hassan, M.; Morosan, M.; López-Gresa, M.D.; Prohens, J.; Vicente, O.; Boscaiu, M. Salinity-Induced Variation in Biochemical Markers Provides Insight into the Mechanisms of Salt Tolerance in Common (Phaseolus vulgaris) and Runner (P. coccineus) Beans. Int. J. Mol. Sci. 2016, 17, 1582. [Google Scholar] [CrossRef]
- Labastida, D.; Ingvarsson, P.K.; Rendón-Anaya, M. Dissecting the genetic basis of drought responses in common bean using natural variation. Front. Plant Sci. 2023, 14, 1143873. [Google Scholar] [CrossRef]
- Pinheiro, C.; Nascimento Araújo, H.; de Brito, S.; da Silva Maia, M.; da Silva Viana, J.; Filho, S. Seed Priming and Tolerance to Salt and Water Stress in Divergent Grain Sorghum Genotypes. Am. J. Plant Sci. 2018, 9, 606–616. [Google Scholar] [CrossRef]
- Sarkar, P.K.; Kumar, P.R.; Singh, A.K.; Bhatt, B.P. Effect of priming treatments on seed germination and seedling growth in bamboo [Dendrocalamus strictus (Roxb.) Nees]. Acta Ecol. Sin. 2020, 40, 128–133. [Google Scholar] [CrossRef]
- Monajjem, S.; Soltani, E.M.; Zainali, E.; Esfahani, M.; Ghaderi-Far, F.; Chaleshtori, M.H.; Rezaei, A. Seed Priming Improves Enzymatic and Biochemical Performances of Rice During Seed Germination under Low and High Temperatures. Rice Sci. 2023, 30, 335–347. [Google Scholar] [CrossRef]
- Nakao, Y.; Sone, C.; Sakagami, J.I. Genetic Diversity of Hydro Priming Effects on Rice Seed Emergence and Subsequent Growth under Different Moisture Conditions. Genes 2020, 11, 994. [Google Scholar] [CrossRef]
- Robledo, D.A.R. Effects of halopriming on seed germination and seedling emergence of Capsicum frutescens. J. Bot. Res. 2020, 3, 114–118. [Google Scholar] [CrossRef]
- Akter, L.; Fakir, O.; Alam, M.; Islam, M.; Chakraborti, P.; Alam, M.; Rashid, M.; Begum, M.; Kader, M. Amelioration of Salinity Stress in Maize Seed Germination and Seedling Growth Attributes through Seed Priming. Open J. Soil Sci. 2018, 8, 137–146. [Google Scholar] [CrossRef]
- Cifuentes, L.; González, M.; Pinto-Irish, K.; Álvarez, R.; Coba de la Peña, T.; Ostria-Gallardo, E.; Franck, N.; Fischer, S.; Barros, G.; Castro, C.; et al. Metabolic imprint induced by seed halo-priming promotes a differential physiological performance in two contrasting quinoa ecotypes. Front. Plant Sci. 2023, 13, 1034788. [Google Scholar] [CrossRef]
- Taghvaei, M.; Nasrolahizadehi, A.; Mastinu, A. Effect of Light, Temperature, Salinity, and Halopriming on Seed Germination and Seedling Growth of Hibiscus sabdariffa under Salinity Stress. Agronomy 2022, 12, 2491. [Google Scholar] [CrossRef]
- Benincasa, P.; Pace, R.; Quinet, M.; Lutts, S. Effect of salinity and priming on seedling growth in rapeseed (Brassica napus var oleifera Del.). Acta Sci. Agron. 2013, 35, 479–486. [Google Scholar] [CrossRef]
- Zahir, M.; Farrukh, H.; Rehman, U.; Abdul, M. Effect of halopriming on the induction of NaCl salt tolerance in different wheat genotypes. Pak. J. Bot. 2015, 47, 1613–1620. [Google Scholar]
- Mahmoud, A.M. Tomato halopriming for improving germination and seedling growth under normal and saline conditions. Ann. Agric. Sci. Moshtohor 2015, 53, 233–241. [Google Scholar] [CrossRef]
- Hidayah, A.; Nisak, R.R.; Susanto, F.A.; Nuringtyas, T.R.; Yamaguchi, N.; Purwestri, Y.A. Seed Halopriming Improves Salinity Tolerance of Some Rice Cultivars During Seedling Stage. Bot. Stud. 2022, 63, 24. [Google Scholar] [CrossRef]
- Wang, Y.; Diao, P.; Kong, L.; Yu, R.; Zhang, M.; Zuo, T.; Fan, Y.; Niu, Y.; Yan, F.; Wuriyanghan, H. Ethylene Enhances Seed Germination and Seedling Growth Under Salinity by Reducing Oxidative Stress and Promoting Chlorophyll Content via ETR2 Pathway. Front. Plant Sci. 2020, 11, 1066. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Borromeo, I.; Capo, C.; Glick, B.R.; Del Gallo, M.; Pietrini, F.; Forni, C. PGPB Improve Photosynthetic Activity and Tolerance to Oxidative Stress in Brassica napus Grown on Salinized Soils. Appl. Sci. 2021, 11, 11442. [Google Scholar] [CrossRef]
- Stassinos, P.M.; Rossi, M.; Borromeo, I.; Capo, C.; Beninati, S.; Forni, C. Amelioration of salt stress tolerance in rapeseed (Brassica napus) cultivars by seed inoculation with Arthrobacter globiformis. Plant Biosyst.—Int. J. Deal. All Asp. Plant Biol. 2022, 156, 370–383. [Google Scholar] [CrossRef]
- Lu, C.; Li, L.; Liu, X.; Chen, M.; Wan, S.; Li, G. Salt Stress Inhibits Photosynthesis and Destroys Chloroplast Structure by Downregulating Chloroplast Development–Related Genes in Robinia pseudoacacia Seedlings. Plants 2023, 12, 1283. [Google Scholar] [CrossRef]
- Wang, L.J.; Jiang, W.B.; Liu, H.; Liu, W.Q.; Kang, L.; Hou, X.L. Promotion by 5-aminolevulinic acid of germination of pakchoi (Brassica campestris ssp. chinensis var. communis Tsen et Lee) seeds under salt stress. J. Integr. Plant Biol. 2005, 47, 1084–1091. [Google Scholar] [CrossRef]
- Mbarki, S.; Skalicky, M.; Vachova, P.; Hajihashemi, S.; Jouini, L.; Zivcak, M.; Tlustos, P.; Brestic, M.; Hejnak, V.; Khelil, A.Z. Comparing Salt Tolerance at Seedling and Germination Stages in Local Populations of Medicago ciliaris L. to Medicago intertexta L. and Medicago scutellata L. Plants 2020, 9, 526. [Google Scholar] [CrossRef] [PubMed]
- Amirjani, M.R. Effect of salinity stress on growth, sugar content, pigments and enzyme activity of rice. Int. J. Bot. 2011, 7, 73–81. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Raihan, M.R.H.; Masud, A.A.C.; Rahman, K.; Nowroz, F.; Rahman, M.; Nahar, K.; Fujita, M. Regulation of Reactive Oxygen Species and Antioxidant Defense in Plants under Salinity. Int. J. Mol. Sci. 2021, 22, 9326. [Google Scholar] [CrossRef] [PubMed]
- Salami, R.; Kordi, M.; Delangiz, N.; Lajayer, B.A.; Astatkie, T. Seedling and Seed Priming in Regulating Secondary Metabolite Level for Stress Tolerance. In Biology and Biotechnology of Environmental Stress Tolerance in Plants; Apple Academic Press: Waretown, NJ, USA, 2023; pp. 251–262. [Google Scholar]
- Patanè, C.; Cosentino, S.L.; Romano, D.; Toscano, S. Relative Water Content, Proline, and Antioxidant Enzymes in Leaves of Long Shelf-Life Tomatoes under Drought Stress and Rewatering. Plants 2022, 11, 3045. [Google Scholar] [CrossRef]
- Rachmawati, D.; Aisy, S.P.; Novanursandy, N.B. Effect of seed priming on growth and physiological responses of chili pepper (Capsicum frutescens L.) under salinity stress. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2023; Volume 1165, p. 012016. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, X.; Gao, G.; Ali, I.; Wu, X.; Tang, M.; Chen, L.; Jiang, L.; Liang, T. Effects of various seed priming on morphological, physiological, and biochemical traits of rice under chilling stress. Front. Plant Sci. 2023, 14, 1146285. [Google Scholar] [CrossRef] [PubMed]
- Jisha, K.C.; Puthur, J.T. Halopriming of seeds imparts tolerance to NaCl and PEG induced stress in Vigna radiata (L.) Wilczek varieties. Physiol. Mol. Biol. Plants 2014, 20, 303–312. [Google Scholar] [CrossRef]
- Rouphael, Y.; Petropoulos, S.A.; Cardarelli, M.; Colla, G. Salinity as eustressor for enhancing quality of vegetables. Sci. Hortic. 2018, 234, 361–369. [Google Scholar] [CrossRef]
- Maggio, A.; De Vos, A.; Castanheira, N.; Jung, S.; Zambujo, J.; Mastrorilli, M. EIP-AGRI Focus Group Soil salinisation. In MINIPAPER: Quality Aspects of Plants in Response to Salinity; The European Innovation Partnership: Brussels, Belgium, 2020; Available online: https://ec.europa.eu/eip/agriculture/sites/default/files/fg36_minipaper_salinityquality_2020_en.pdf (accessed on 15 January 2024).


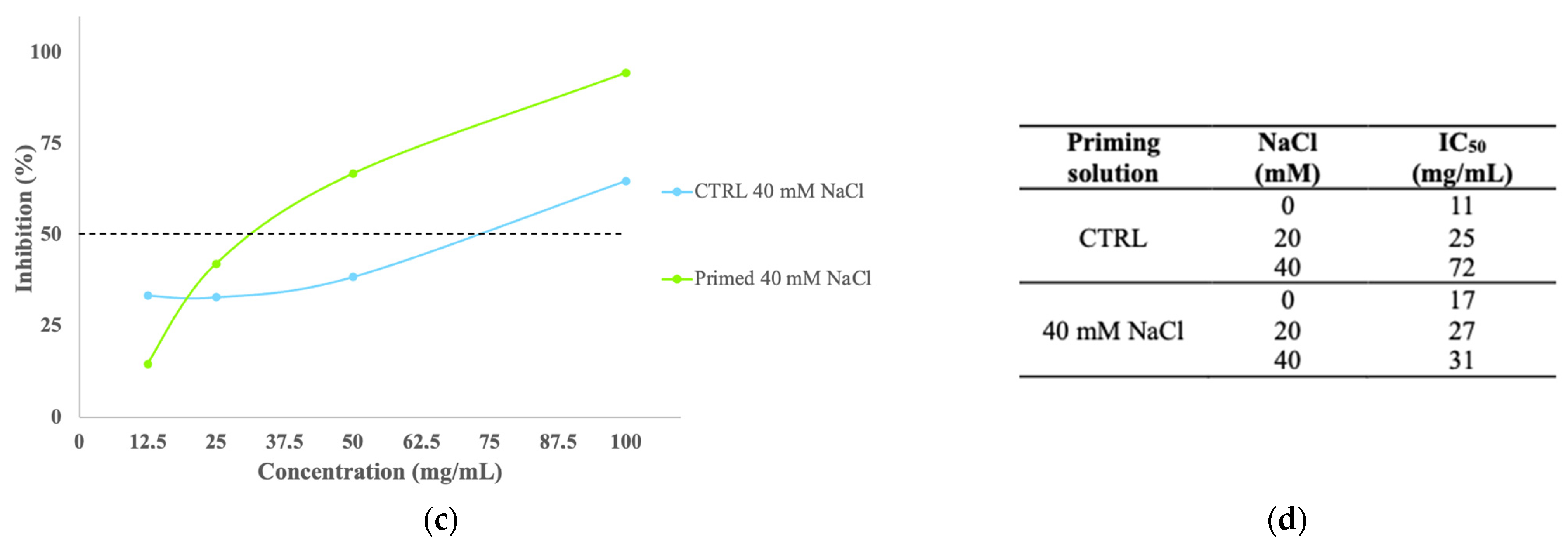
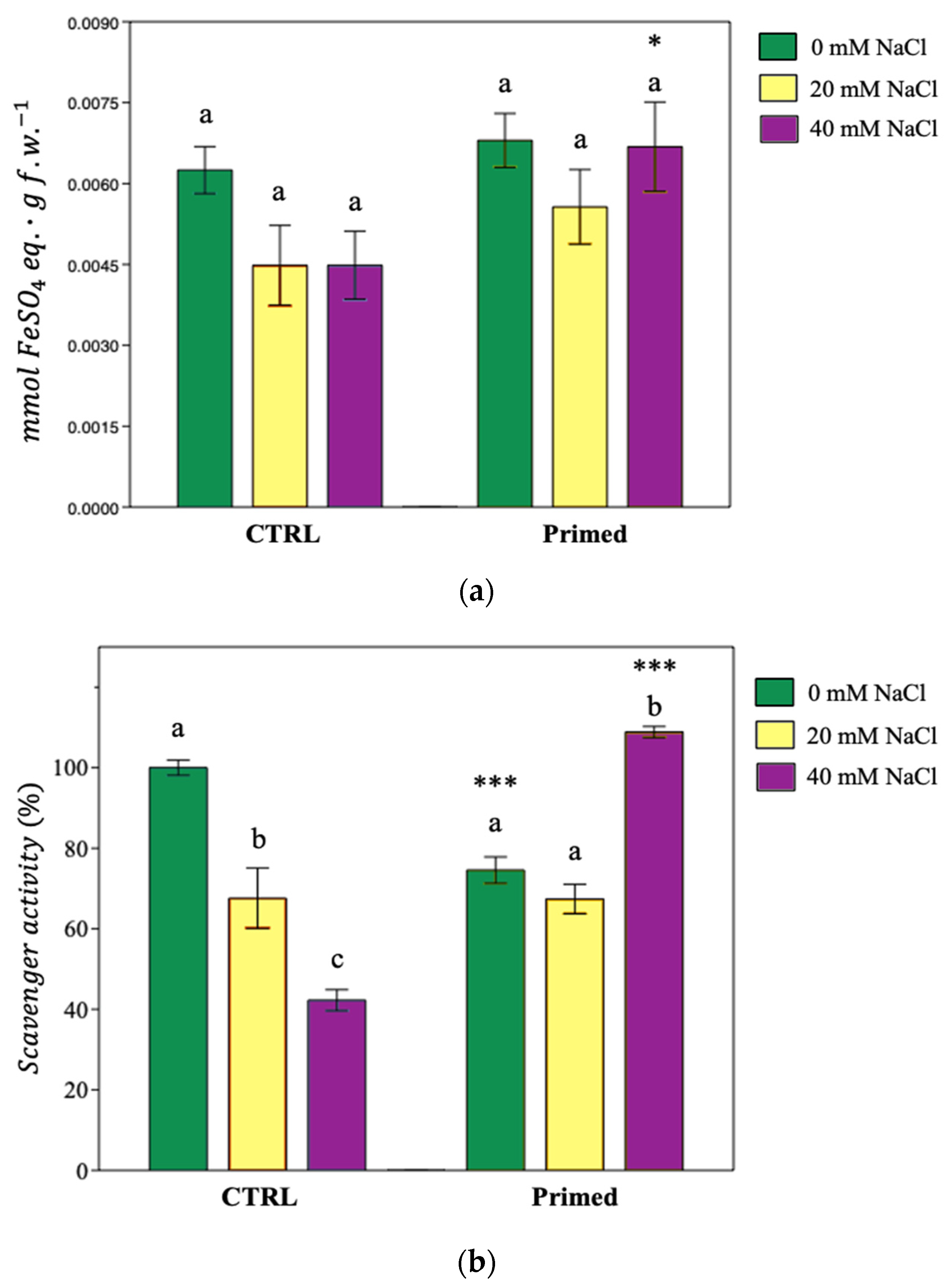

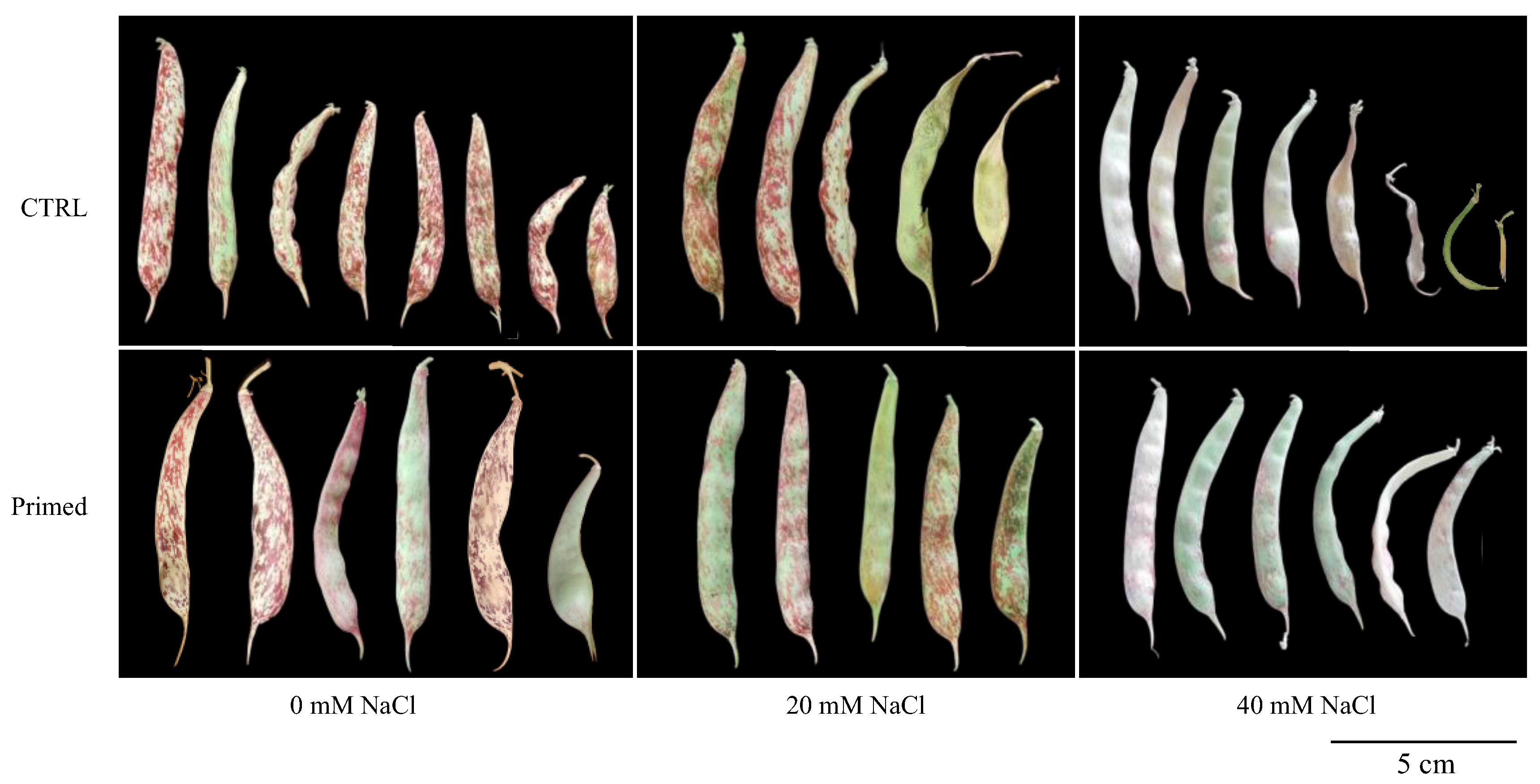

| Cultivar | NaCl (mM) | Non-Primed | Primed |
|---|---|---|---|
| Black Turtle | 0 | 53.2% ± 7.1% a | 63.8% ± 2.1% a |
| 40 | 27.3% ± 3.3% b | 33.2% ± 3.0% b | |
| 80 | 32.1% ± 3.3% b | 31.9% ± 1.9% b | |
| 160 | 29.0% ± 5.4% b | 19.2% ± 2.8% c* | |
| Bola Roja | 0 | 27.4% ± 6.6% a | 33.4% ± 3.3% a |
| 40 | 29.1% ± 4.3% a | 32.7% ± 4.7% a | |
| 80 | 23.4% ± 3.7% a | 32.1% ± 4.1% a | |
| 160 | 28.1% ± 3.4% a | 33.1% ± 3.9% a | |
| Borlotto | 0 | 73.1% ± 3.3% a | 69.3% ± 4.0% a |
| 40 | 67.4% ± 6.8% a | 64.8% ± 2.1% a | |
| 80 | 40.1% ± 6.2% b | 43.1% ± 4.6% b | |
| 160 | 0% c | 3.0% ± 1.3% c | |
| Cargamanto | 0 40 80 160 | 27.7% ± 2.9% a 13.8% ± 3.2% b 11.1% ± 2.2% b 6.2% ± 1.6% c | 27.8% ± 2.2% a 23.1% ± 2.1% ab* 19.2% ± 2.9% b 8.4% ± 3.4% c |
| Priming Solutions | NaCl (mM) | Gravimetric Water Content (%) | Electrical Conductivity (dS/m) |
|---|---|---|---|
| CTRL | 0 20 40 | 1.28 ± 0.51 a 2.56 ± 0.10 a 4.23 ± 0.18 b | 0.246 ± 0.039 a 0.983 ± 0.085 b 1.596 ± 0.077 c |
| 40 mM NaCl | 0 20 40 | 1.16 ± 0.28 a 1.59 ± 0.03 a*** 2.82 ± 0.29 b* | 0.338 ± 0.012 a 0.345 ± 0.020 a*** 1.000 ± 0.073 b*** |
| Priming Solutions | NaCl (mM) | n. Leaves | Shoot Length (cm) | Root Length (cm) | TI (%) |
|---|---|---|---|---|---|
| CTRL | 0 20 40 | 20 ± 1 a 15 ± 2 a 15 ± 2 a | 46 ± 3.3 a 33 ± 3.7 b 38 ± 3.0 ab | 21 ± 1.7 a 19 ± 3.0 a 16 ± 0.8 a | 100 88 78 |
| 40 mM NaCl | 0 20 40 | 26 ± 1 a** 25 ± 2 a** 19 ± 3 a | 63 ± 3.2 a** 64 ± 3.1 a*** 60 ± 3.9 a** | 25 ± 0.4 a 20 ± 1.5 a 20 ± 2.2 a | 119 (+19%) 96 (+8) 95 (+17) |
| Priming Solution | NaCl (mM) | Chl a (μg·g f.w.−1) | Chl b (μg·g f.w.−1) | Total Chl (μg·g f.w.−1) | Soluble Sugars (mg glucose eq.·g f.w.−1) |
|---|---|---|---|---|---|
| CTRL | 0 20 40 | 142.8 ± 5.8 a 96.7 ± 5.8 b 52.6 ± 5.1 c | 85.5 ± 7.2 a 56 ± 6.8 b 39.1 ± 8.9 b | 228.3 ± 4.6 a 152.7 ± 5.3 b 91.7 ± 5.7 c | 0.250 ± 0.008 ab 0.229 ± 0.005 a 0.278 ± 0.010 b |
| 40 mM NaCl | 0 20 40 | 101.9 ± 2.5 a*** 112.2 ± 2.4 a* 155.7 ± 4.4 b*** | 43.7 ± 4.6 a*** 63.7 ± 11.2 ab 71.3 ± 7.0 b* | 145.6 ± 4.1 a*** 175.9 ± 9.4 b* 227 ± 3.3 c*** | 0.203 ± 0.005 a*** 0.203 ± 0.003 a*** 0.238 ± 0.013 b* |
| Priming Solution | NaCl (mM) | Shoot (μg Ca2+·mg f.w.−1) | Root (μg Ca2+·mg f.w.−1) |
|---|---|---|---|
| CTRL | 0 20 40 | 1.31 ± 0.03 a 0.71 ± 0.09 b 0.55 ± 0.06 b | 0.29 ± 0.02 a 0.38 ± 0.03 b 0.31 ± 0.03 ab |
| 40 mM NaCl | 0 20 40 | 1.09 ± 0.05 a** 1.74 ± 0.03 b*** 1.78 ± 0.04 b*** | 0.36 ± 0.03 a 0.29 ± 0.01 b** 0.24 ± 0.02 b* |
| Priming Solution | NaCl (mM) | PPO Activity (E.U.·mg protein−1) | POD Activity (E.U.·mg protein−1) | APX Activity (%) | CAT Activity (%) |
|---|---|---|---|---|---|
| CTRL | 0 20 40 | 0.108 ± 0.007 a 0.120 ± 0.019 a 0.062 ± 0.003 b | 0.186 ± 0.001 a 0.153 ± 0.010 ab 0.112 ± 0.017 b | 100 a 82 ± 6 a 20 ± 11 b | 100 a 98 ± 10 a 70 ± 10 a |
| 40 mM NaCl | 0 20 40 | 0.022 ± 0.001 a** 0.021 ± 0.006 a*** 0.191 ± 0.014 b*** | 0.269 ± 0.070 a 0.433 ± 0.069 a* 1.194 ± 0.176 b*** | 50 ± 7 a** 91 ± 6 b 93 ± 11 b*** | 70 ± 10 a 134 ± 16 b 144 ± 12 b** |
| Priming Solution | NaCl (mM) | n. Pods/Plant | Pod Weight (g) | Pod Length (cm) | n. Beans/Pod | Bean Weight/Pod (g) |
|---|---|---|---|---|---|---|
| CTRL | 0 20 40 | 1.5 ± 0.3 a 1.6 ± 0.3 a 2.1 ± 0.3 a | 3.5 ± 0.3 a 2.5 ± 0.3 a 1.4 ± 0.3 b | 10 ± 0.4 a 9 ± 0.4 a 8 ± 0.3 a | 1.8 ± 0.3 a 1.5 ± 0.2 a 2.1 ± 0.2 a | 0.778 ± 0.052 a 0.092 ± 0.012 b 0.023 ± 0.005 c |
| 40 mM NaCl | 0 20 40 | 1.8 ± 0.3 a 2.9 ± 0.4 b* 2.2 ± 0.2 ab | 5.0 ± 0.4 a* 2.9 ± 0.3 b 2.9 ± 0.3 b* | 10 ± 0.4 a 9 ± 0.5 a 10 ± 0.3 a** | 2.2 ± 0.2 a 1.8 ± 0.3 a 2.5 ± 0.4 a | 0.959 ± 0.067 a 0.254 ± 0.050 b* 0.205 ± 0.037 b** |
| Priming Solutions | NaCl (mM) | Electrical Conductivity of Soils (dS·m−1) | Proteins of Seeds (μg BSA eq.·mg f.w.−1) | Soluble Sugars of Seeds (mg glucose eq.·g f.w.−1) |
|---|---|---|---|---|
| CTRL | 0 20 40 | 0.243 ± 0.018 a 0.573 ± 0.061 b 0.600 ± 0.076 b | 6.31 ± 0.26 a 6.18 ± 0.79 a 1.67 ± 0.13 b | 0.93 ± 0.10 a 0.43 ± 0.12 b 0.08 ± 0.02 c |
| 40 mM NaCl | 0 20 40 | 0.290 ± 0.021 a 0.637 ± 0.072 b 0.773 ± 0.055 b | 7.12 ± 0.08 a* 6.80 ± 0.05 a 7.04 ± 0.21 a*** | 1.19 ± 0.01 a* 0.86 ± 0.02 b** 0.84 ± 0.13 b*** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borromeo, I.; Domenici, F.; Giordani, C.; Del Gallo, M.; Forni, C. Enhancing Bean (Phaseolus vulgaris L.) Resilience: Unveiling the Role of Halopriming against Saltwater Stress. Seeds 2024, 3, 228-250. https://doi.org/10.3390/seeds3020018
Borromeo I, Domenici F, Giordani C, Del Gallo M, Forni C. Enhancing Bean (Phaseolus vulgaris L.) Resilience: Unveiling the Role of Halopriming against Saltwater Stress. Seeds. 2024; 3(2):228-250. https://doi.org/10.3390/seeds3020018
Chicago/Turabian StyleBorromeo, Ilaria, Fabio Domenici, Cristiano Giordani, Maddalena Del Gallo, and Cinzia Forni. 2024. "Enhancing Bean (Phaseolus vulgaris L.) Resilience: Unveiling the Role of Halopriming against Saltwater Stress" Seeds 3, no. 2: 228-250. https://doi.org/10.3390/seeds3020018
APA StyleBorromeo, I., Domenici, F., Giordani, C., Del Gallo, M., & Forni, C. (2024). Enhancing Bean (Phaseolus vulgaris L.) Resilience: Unveiling the Role of Halopriming against Saltwater Stress. Seeds, 3(2), 228-250. https://doi.org/10.3390/seeds3020018










