Abstract
Crops are continually subjected to frequent and extreme changes in climate, such as high temperatures and soil water deficits. Many studies have shown the individual effects of these factors on plants, but their combined effects on reproductive growth and subsequent seed germinability have received little attention. In this study, we used canola (Brassica napus) plants and grew them through their lifecycle under two temperature regimes (20/10 °C and 24/14 °C, 16 h light/8 h dark) in controlled-environment growth chambers. Half of the plants were watered to field capacity (well-watered) and the other half at wilting point (water-stressed). During the reproductive stage, the flower, silique, and seed traits were measured. Higher temperatures decreased the petal width by 1.17 times but increased petal anthocyanins by 1.03 times. The water deficit decreased the silique length and total seed number by 1.21 and 1.32 times, respectively, but increased nectar sugar concentration by 1.28 times. The total volume of nectar was affected by the interaction of temperature and water. The nectar volume was lowest in the water-stressed plants under higher temperatures (2.66 ± 0.29 µL per flower) but highest in the well-watered plants under the same temperature regime (5.73 ± 0.37 µL per flower). In conclusion, the combined effects of temperature and water were less pronounced than the individual effects of these factors on canola reproductive yield.
Keywords:
Brassica napus; canola; crop; plant growth; reproductive yield; temperature; seed germination; watering regime 1. Introduction
An increase in global temperatures is caused by an increase in greenhouse gases, which insulate and prevent heat from escaping into the atmosphere and are, in turn, exacerbated by anthropogenic sources. Not only have temperatures increased in the air and in the sea, but surface temperatures across the globe have increased at a faster rate since 1970 than any 50-year period in the last 2000 years [1]. Global heat stress diminishes water availability worldwide, resulting in decreased food security in areas experiencing such conditions [2].
As temperature increases, evaporation rates subsequently increase, causing greater surface drying, which leads to a long-term limited water condition [3]. Changes in precipitation, such as less rainfall from high temperatures, will lead to lower yields in many crops around the world [4]. The need for the use of freshwater for irrigation increases if seasonal rainfall is expected to decrease over time. A lack of precipitation can propagate into hydrological drought [5], causing freshwater availability issues for irrigation. Irrigated agriculture accounts for 20% of cultivated lands, leading to 40% of the global food output, and is twice as productive as rainfed agriculture [6].
High temperatures can disrupt the flowering time of plants [7], affecting pollinator visitation and egg fertilization [8,9] as well as influencing the development of male and female reproductive organs [10]. High temperatures, as low as 24 °C, can also negatively affect floral structures [11], indicating how sensitive these structures are to environmental conditions. The optimum temperature for floral development can range anywhere from 9 °C to 25 °C [12]; this range shows that there may be severe implications for crop and non-crop species at temperatures that are above the predicated range. High temperatures have also been found to delay flowering [13], affect pollen viability [14], and even increase rates of embryo abortion [15]. When considering floral resources, annual species appear to have a higher sensitivity to high temperatures [16], which may be concerning as the dominant crop species are annuals [17]. High temperatures can also reduce seed mass [18] and silique production [19].
Water deficit affects delicate reproductive stages of plant development, such as flowering and seed and fruit development [20]. Drought stress can reduce the number of flowers produced and the number of open flowers per plant [16], which can ultimately affect plant reproductive success and fitness. During reproductive stages in crops, drought stress negatively affects final yields [21,22,23], thus threatening food security worldwide and reducing economic gains [24].
Seed germination is promoted by various external and internal factors, such as hormone levels, light, temperature, and soil conditions [25]. Germination time may also be affected by the environment in which the parent plant was grown [26]. High temperatures during the seed-filling stages of the parent plant can cause a reduction in subsequent seed germination [27]. Germination rates can also be reduced due to high temperatures [28].
In this study, we selected canola (Brassica napus). It is an annual or biennial plant species with an erect stem that can grow about one and a half meters tall. The canola leaves are waxy with a glabrous underside, and their inflorescences are racemes. The pale-yellow flowers of canola have four sepals, four petals, six stamens (four long and two short), and a superior ovary. Canola fruit is a silique that contains 15 or more seeds. This species reproduces by seeds, and the seedlings grow and form rosettes, which bolt and turn into flowering plants. Canola is a cool-season winter crop, which is temperature- and water-sensitive. For canola growth and development, the optimum temperature is around 20 °C (see Ref. [29]). Both higher temperatures and water deficits negatively affect canola growth and reproductive yield, especially during critical growth stages, such as flowering and seed development [30,31,32]. Although the separate effects of a temperature and water deficit have been studied on canola growth and development [31,33], the combined effects of these environmental factors on the reproductive traits of this crop require further investigation to optimize its yield under varying environmental conditions. Based on earlier studies, we hypothesized that a higher temperature and water deficit in combination would decrease yield-related characteristics of canola. The objective of this study was to determine the individual and interactive effects of a temperature and watering regime on the reproductive yield of canola, including floral and seed traits and germination of seeds matured under these conditions.
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
Canola (Brassica napus L. cv. 6056 CR, BrettYoung Seeds, Winnipeg, MB, Canada) seeds were germinated on one layer of blue filter paper (Anchor Paper Co., St. Paul, MN, USA) in 9 cm Petri dishes (Pyrex, Greencastle, PA, USA) in controlled-environment growth chambers (model PGR15, Conviron, Controlled Environments Ltd., Winnipeg, MB, Canada). The growth chambers were equipped with both incandescent lamps (Lumens 40 W/120 V, Sonepar, Laval, QC, Canada) and cool white fluorescent bulbs (Sylvania Pentron FP39/841/HO/ECO, Redding, CA, USA) with a 16 h photoperiod and light intensity of 600 μmol photons m˗2 s˗1, measured with a LI-250A radiometer/photometer (LICOR Biosciences, Lincoln, NE, USA). Fifty seeds were spaced apart on each Petri dish and given 10 mL of distilled water. When seedlings had full cotyledons and root systems with a minimum of 2 cm, they were planted in a soil mixture of peat moss (Premier Sphagnum Peat Moss, Rivière-du-Loup, QC, Canada), Vermiculite (Medium, Perlite Canada Inc., Lachine, QC, Canada), and Perlite (Professional Horticultural Grade, Saint-Pacôme, QC, Canada) in 2:1:1 ratio (by volume) into twenty-four 1.32 L pots (#6 Grower Pots, Toronto, ON, Canada) with two seedlings per pot, and pellets (~35) of slow-release fertilizer (N-P-K, 14-14-13; Type 100, Chisso-Asahi Fertilizer Co., Tokyo, Japan) were added. Seedlings were thinned after 10 days, leaving the larger one in each pot. Plants were subjected to experimental conditions after one week of growth in control conditions. Approximately 0.52 g of water-soluble fertilizer (N-P-K, 24-8-16; Miracle-Gro All Purpose, Scotts Canada Ltd., Mississauga, ON, Canada) was given to plants every 15 days and water-soluble calcium (~1 tbsp per pot; Gaia Green Organics Bone Meal; Greenstar Plant Products Inc., Langley, BC, Canada) was given during flowering and silique developmental stages as it plays various important roles in plant sexual reproduction, such as pollen tube elongation and gametic interactions [34]. Experimental conditions were based on a split-plot design with two factors: temperature and water. Each factor had two levels. Thus, the experimental conditions were as follows: (1) lower temperatures (20 °C/10 °C, 16 h light/8 h dark) and watering-to-field capacity (well-watered, determined by the excess water drainage), (2) lower temperatures and watering at wilting point (water-stressed, determined by the sign of leaf wilting), (3) higher temperatures (24 °C/14 °C, 16 h light/8 h dark) and well-watered, and (4) higher temperatures and water-stressed. The lower temperature regime is an optimal growth temperature for canola [35], and the higher temperature regime is a simulation of an increase of 4 °C by the end of this century [1]. As global warming leads to drought conditions in some parts of the world [1], plants were watered to field capacity as well as at the wilting point. The lower temperatures and water-to-field capacity were considered as controls because they are suitable conditions for the growth of canola [36]. Experiments were conducted three times (three independent trials) under the same growing conditions of the temperature and watering regime. In each trial, six plants (replications) per treatment were grown to full silique maturity for over three months.
2.2. Measurement of Floral Traits
Floral sizes of the calyx (sepal length and width), corolla (petal length and width), androecium (long and short stamen length), and gynoecium (carpel length) were measured after approximately 65 days of growth by selecting and harvesting three flowers (per plant) of the same maturity, using digital calipers (Mituyo Corp., Kanagawa, Japan). The sepal width was measured at the widest part of the sepal, and the petal width was measured from the middle of the petal. Stamen length was measured from the base of the filament to the top of the anther, and carpel length was measured from the base of the ovary, including the style, to the top of the stigma. Petal flavonoids and anthocyanins were measured using a Dualex ScientificTM handheld optical leafclip meter (Dualex Scientific, Force-A Orsay Cedex, France).
2.3. Measurement of Nectar
Nectar was harvested from the inner (lateral) nectaries, positioned between the bottom of each short stamen and the ovary [37], using a 10 µL capillary micropipette (Drummond® Scientific Company, Broomall, PA, USA) from six flowers of each treatment (six plants, once per treatment) showing the same maturity, as described by Morrant et al. [38]. The sampled flowers exhibited curled anthers on the long stamens that were longer than the carpel. Six flowers from the same experimental treatment were used to harvest nectar between 12:00 and 14:00 h, and the total volume was recorded. The average nectar volume produced per flower was calculated from the amount of nectar harvested and the number of flowers used. Analysis of total nectar sugar concentration was performed using freshly harvested nectar, collected in a capillary micropipette, from flowers of the same maturity using a portable optical refractometer (Aichose® Brix Refractometer with ATC Function, Fuzhou, Fujian, China) by pushing out four drops of nectar from the capillary micropipette along the lens and closing the cover to record readings.
2.4. Measurement of Silique and Seed Traits
Siliques were harvested at maturity to compare silique and seed traits after plants were under experimental conditions for approximately 90 days, after one week of initial growth under control conditions. Harvested mature siliques appeared dry and of a light brown color. Silique length (mm), excluding the beak and pedicel, was measured using digital calipers. Silique width (mm) was measured from the midpoint of the silique. Full silique mass (g; silique husk, sound seeds, and aborted seeds), empty silique mass (g; no seeds), total seed number per silique, sound seed number, aborted seed number, sound seed mass (g), and aborted seed mass (g) were then measured from randomly selected siliques using an analytical balance (model ED224s, Sartorius, Goettingen, Germany). Sound seeds were fully developed, while aborted seeds were not [39].
2.5. Seed Germination Tests
To measure seed germination (total germination percentage) and germination speed (coefficient of germination rate), three replications of 50 sound seeds were removed from randomly selected mature siliques from parent plants of each treatment—lower temperatures (20 °C/10 °C) and well-watered, lower temperatures and water-stressed, higher temperatures (24 °C/14 °C) and well-watered, and higher temperatures and water-stressed. Fifty seeds were placed in each Petri dish, lined with one layer of blue filter paper, and moistened with 10 mL of distilled water; this was repeated twice for each treatment. All seeds were subjected to either a lower temperature (20°C/10 °C) or a higher temperature (24 °C/14 °C) regime to examine seed germination under optimal conditions and simulate the effects of global warming, respectively (see above). Seeds were checked daily for germination, and seedlings with radicles at least 2 mm in length were considered germinated. The experiment was terminated after five consecutive days with no germination (typically after 18 days). Then, the total germination and the coefficient of germination rate (CGR) were calculated. The rate of germination was calculated by using the following equation: CGR = N/∑nidi; N is the total germination, ni is the number of germinated seeds on the day the germinated seeds were counted, and di is the number of days from the beginning of the experiment. The CGR values are between zero (no germination) and one (fastest germination rate), multiplied by 100 to facilitate interpretation [40].
2.6. Data Analysis
Effects of the temperature and watering regime and their interactions were determined on canola growth and reproductive yield, using analysis of variance (ANOVA) for split-plot design. In this analysis, Fisher’s Least Significant Difference (LSD) test was used to determine differences between/among treatments with a 5% confidence level [41]. The temperature was the main plot factor, the watering regime the subplot factor, and the trial was used as the replication. All data are reported as mean ± SEM (standard error of the mean).
3. Result
3.1. Floral Treats
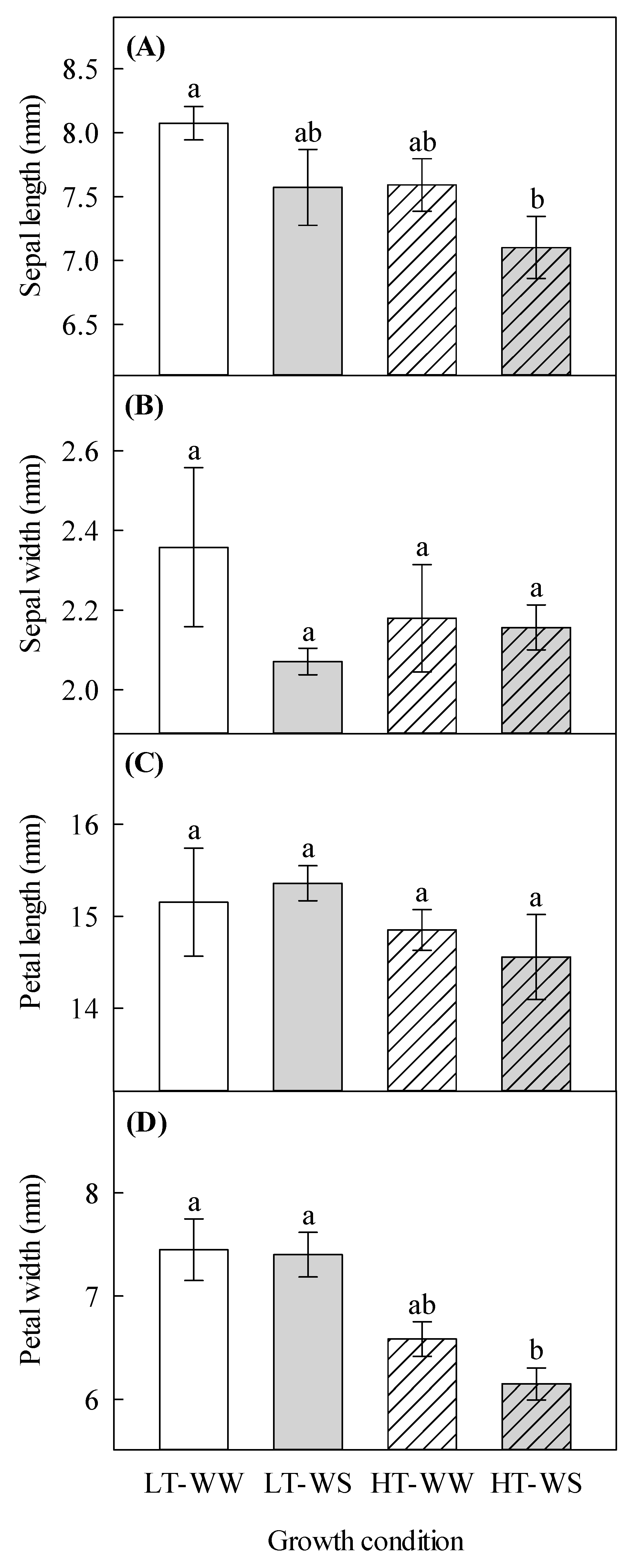
Sepal length was significantly decreased in the water-stressed plants under higher temperatures (7.10 ± 0.24 mm) compared to that in the control plants—the well-watered plants under lower temperatures (8.07 ± 0.13 mm; Table 1, Figure 1A). Petal width was significantly reduced by the higher temperature regime, and it was the smallest in the water-stressed plants under the higher temperature regime (6.15 ± 0.15 mm), compared to that of other treatments, including the control (7.45 ± 0.30 mm; Table 1, Figure 1D). Higher temperatures significantly increased anthocyanins in petals (Table 1 and Table 2).

Table 1.
Summary of split-plot ANOVA (F value) for effects of temperature and watering regime and their interaction on flower characteristics, including petal chemical attributes, of canola (Brassica napus).
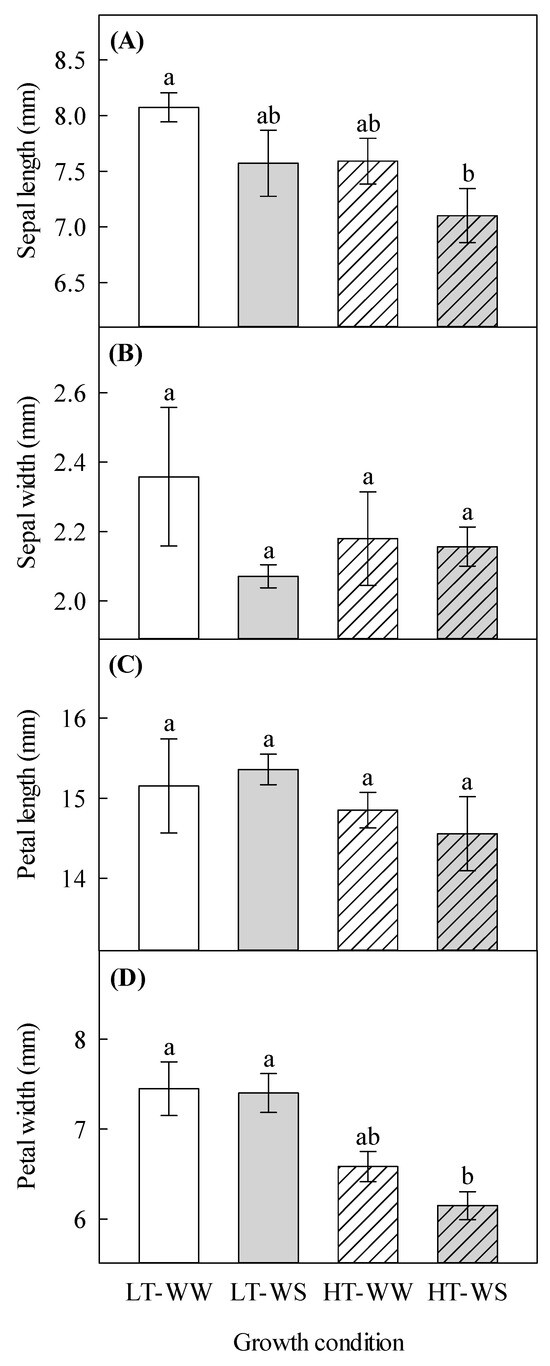
Figure 1.
Effects of temperature and watering regime on flower characteristics of canola (Brassica napus). Plants were grown under lower (LT, 20 °C/10 °C) or higher (HT, 24 °C/14 °C) temperature regime, and either well-watered (WW, watered to field capacity) or water-stressed (WS, watered at wilting point) in controlled-environment growth chambers. Sepal length, sepal width, petal length, and petal width were measured about 65 days after one week of initial growth under controlled conditions (20/10 °C, 16 h light/8 h dark). (A) sepal length, (B) sepal width, (C) petal length, and (D) petal width. Data are means ± SEM (n = 9, from three trials). Bars surmounted by different letters within each panel are significantly different based on Fisher’s LSD test (p < 0.05).

Table 2.
Effects of temperature and watering regime on flower characteristics of canola (Brassica napus).
3.2. Nectar Properties
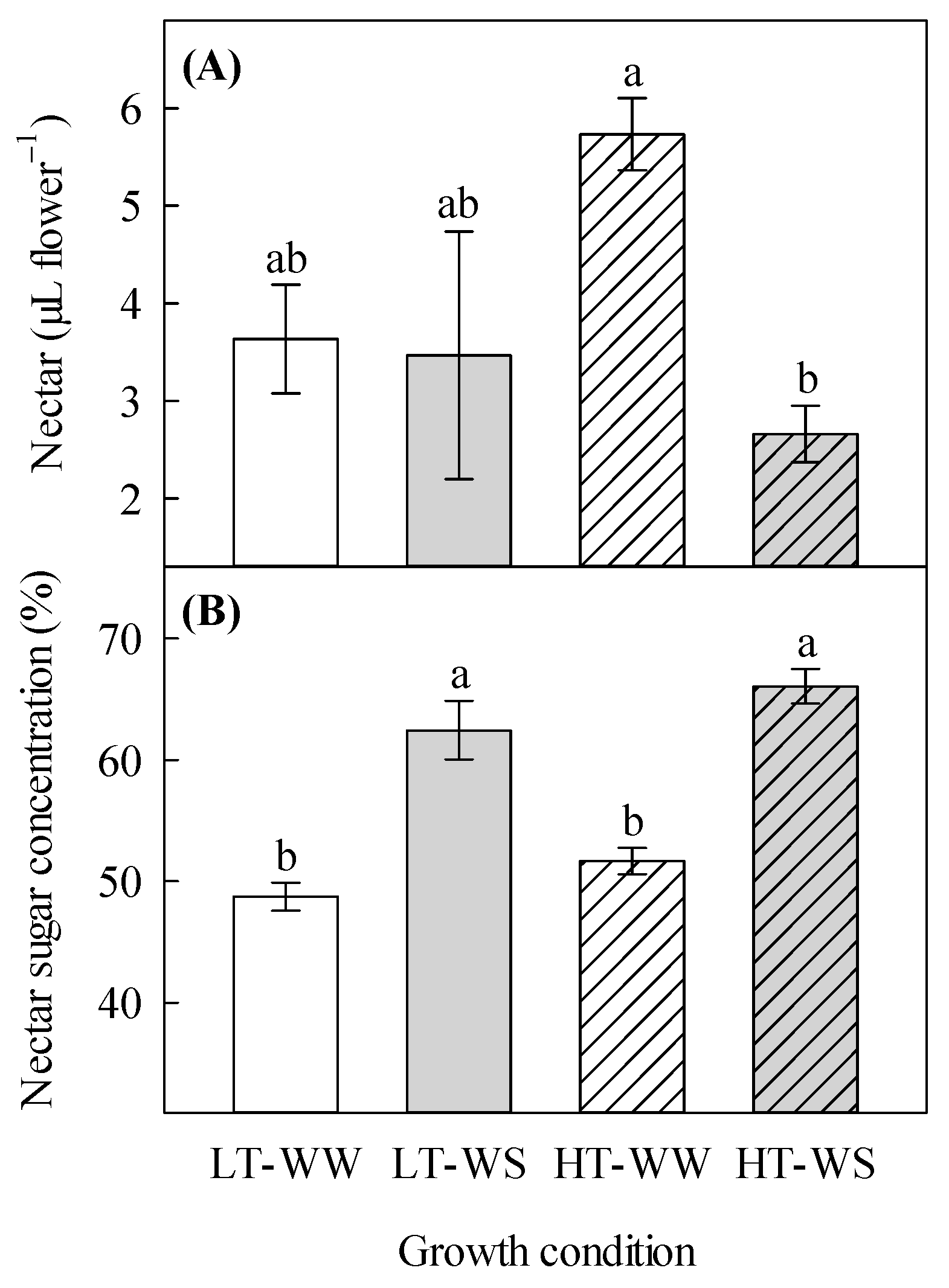
The total volume of nectar was significantly affected by the watering regime and its interaction with temperature (Table 3). The water-stressed plants under higher temperatures produced the lowest volume of nectar (2.66 ± 0.29 µL flower−1), whereas the well-watered plants under the same temperature regime produced the highest volume of nectar (5.73 ± 0.37 µL flower−1; Figure 2A). The nectar sugar concentration was found to be significantly increased in the water-stressed plants, regardless of the temperature regime. It was 1.4 times higher in the water-stressed plants under higher temperatures than in the well-watered plants under lower temperatures (Table 3, Figure 2B).

Table 3.
Summary of split-plot ANOVA (F value) for effects of temperature and watering regime and their interaction on nectar properties of canola (Brassica napus).
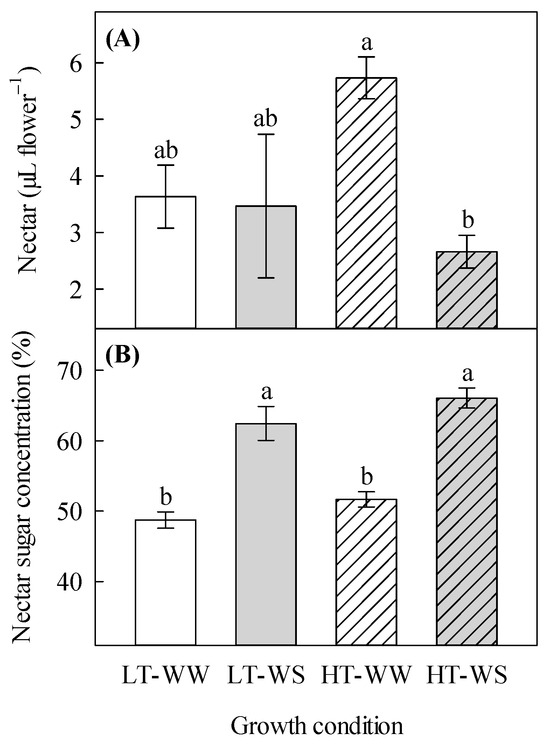
Figure 2.
Effects of temperature and watering regime on nectar properties of canola (Brassica napus). Plants were grown under lower (LT, 20 °C/10 °C) or higher (HT, 24 °C/14 °C) temperature regime, and either well-watered (WW, watered to field capacity) or water-stressed (WS, watered at wilting point) in controlled-environment growth chambers. Nectar sugar concentration and nectar volume per flower were measured about 65 days after one week of initial growth under controlled conditions (20/10 °C, 16 h light/8 h dark). (A) nectar volume per flower, and (B) nectar sugar concentration. Data are means ± SEM (n = 9, from three trials). Bars surmounted by different letters within each panel are significantly different based on Fisher’s LSD test (p < 0.05).
3.3. Silique and Seed Traits
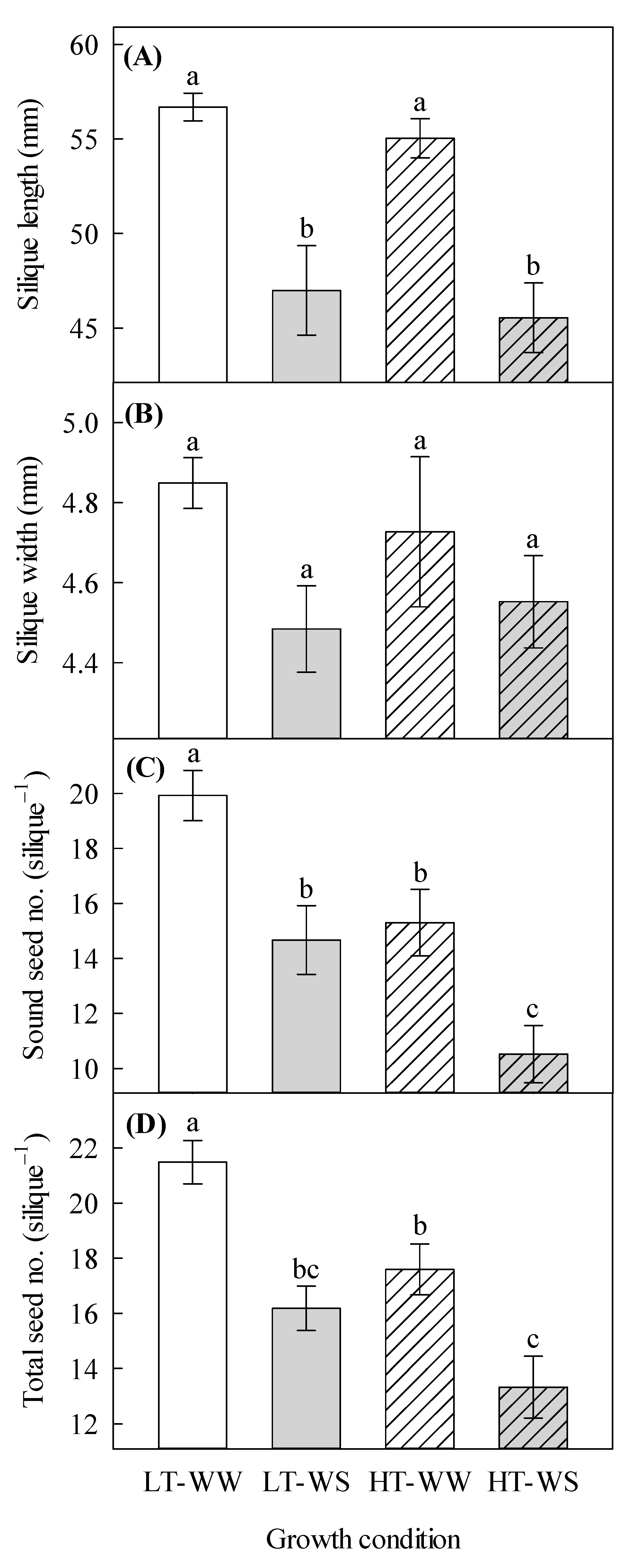
Silique length was significantly decreased by water stress, regardless of the temperature regime (Table 4, Figure 3A). The sound seed number and the total seed number per plant were significantly affected by temperature and watering regime (Table 4). The well-watered plants under lower temperatures had the highest number of sound seeds (19.93 ± 0.91) as well as the total number of seeds per silique (21.48 ± 0.78), whereas the water-stressed plants under higher temperatures had the lowest number of sound seeds (10.52 ± 1.04) and total seed number per silique (13.33 ± 1.11; Figure 3A,D).

Table 4.
Summary of split-plot ANOVA (F value) for effects of temperature and watering regime and their interaction on silique and seed characteristics of canola (Brassica napus).
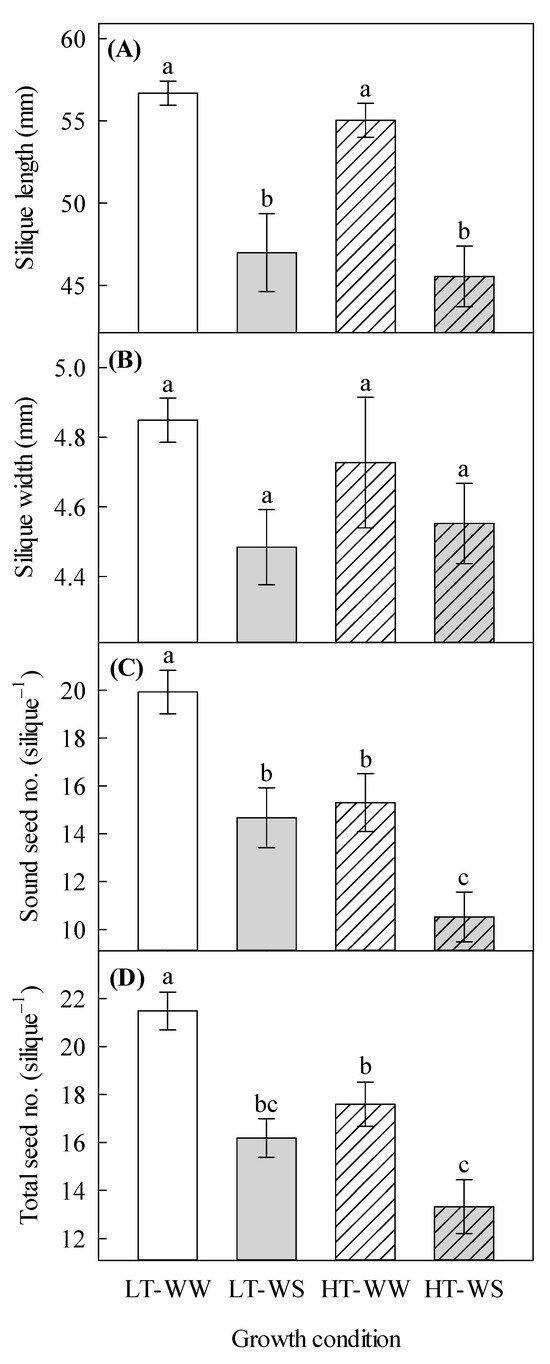
Figure 3.
Effects of temperature and watering regime on silique and seed traits of canola (Brassica napus). Plants were grown under lower (LT, 20 °C/10 °C) or higher (HT, 24 °C/14 °C) temperature regime, and either well-watered (WW, watered to field capacity) or water-stressed (WS, watered at wilting point) in controlled-environment growth chambers. Mature siliques and seeds were harvested from plants that were grown under higher and lower temperature regimes for about 65 days, after one week of initial growth under control conditions (20/10 °C, 16 h light/8 h dark). Silique length, silique width, sound seed number per silique and total seed number per silique were measured. (A) silique length, (B) silique width, (C) sound seed number per silique, and (D) total seed number per silique. Data are means ± SEM (n = 27, from three trials). Bars surmounted by different letters within each panel are significantly different based on Fisher’s LSD test (p < 0.05).
Full silique mass as well as empty silique mass were significantly reduced in the water-stressed plants (Table 4 and Table 5), as shown in Figure 4. Sound seed mass and total seed mass were also significantly decreased by water stress (Table 4 and Table 5, Figure 4A–D).

Table 5.
Effects of temperature and watering regime on silique and seed characteristics of canola (Brassica napus).

Figure 4.
Effects of temperature and watering regime on siliques and seeds of canola (Brassica napus). Plants were grown under lower (LT, 20 °C/10 °C) or higher (HT, 24 °C/14 °C) temperature regime, and either well-watered (WW, watered to field capacity) or water-stressed (WS, watered at wilting point) in controlled-environment growth chambers. Mature siliques and seeds (siliques, upper; sound seeds, middle; and aborted seeds, lower) were harvested from plants that were grown under lower and higher temperature regimes for about 90 days, after one week of initial growth under control conditions (20/10 °C, 16 h light/8 h dark), and their characteristics were measured. (A) LT-WW, (B) LT-WS, (C) HT-WW, and (D) HT-WS. Scale bar = 5 mm.
3.4. Seed Germination Pattern
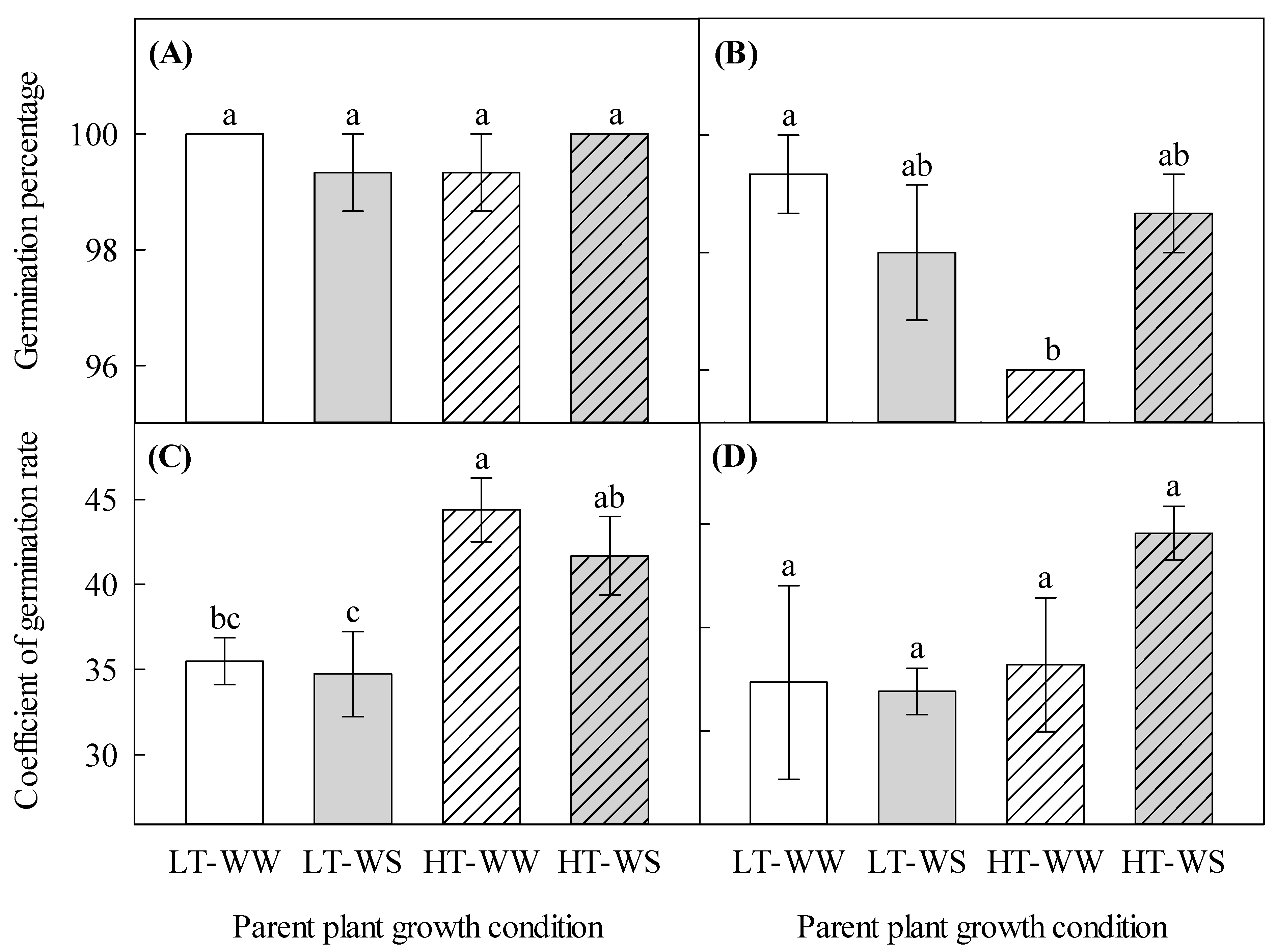
The well-watered plants under higher temperatures produced seeds with a lower total germination percentage (96.00 ± 0.00) than seeds of the well-watered plants that matured under lower temperatures (99.33 ± 0.67) when they were germinated under a higher temperature regime (Figure 5B). The coefficient of germination rate significantly increased in seeds that matured under higher temperatures when they were germinated under a lower temperature regime (Table 6, Figure 5C). Higher maturation temperatures increased the germination rate of seeds in the well-watered and water-stressed plants by 1.3 and 1.2 times, respectively, when they were germinated under lower temperatures. However, the same seeds that were germinated under higher temperatures showed a different pattern of germination rate (Figure 5D).
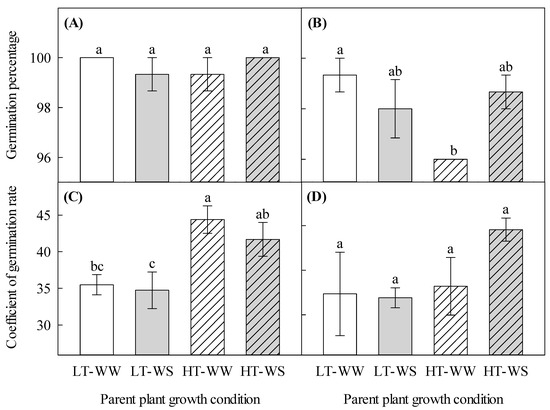
Figure 5.
Effects of temperature and watering regime during seed development on the subsequent germination pattern of canola (Brassica napus). Plants were grown under lower (LT, 20 °C/10 °C) or higher (HT, 24 °C/14 °C) temperature regime, and either well-watered (WW, watered to field capacity) or water-stressed (WS, watered at wilting point) in controlled-environment growth chambers for about 90 days, after one week of initial growth under control conditions (20/10 °C, 16 h light/8 h dark). Mature seeds were harvested, and germination percentage (total germination) and germination speed (coefficient of germination rate) of sound seeds were determined. (A) germination percentage (total germination) of seeds under lower temperatures, (B) germination percentage of seeds under higher temperatures, (C) coefficient of germination rate (germination speed) of seeds under lower temperatures, and (D) coefficient of germination rate of seeds under higher temperatures. Data are means ± SEM (n = 9, from three trials). Bars surmounted by different letters within each panel are significantly different based on Fisher’s LSD test (p < 0.05).

Table 6.
Summary of split-plot ANOVA (F value) for effects of temperature and watering regime and their interaction on seed germination of canola (Brassica napus).
4. Discussion
4.1. Effects of Temperature
In terms of morphological development during reproductive growth, petal width was decreased by a higher temperature regime and water stress (Figure 1D). This finding supports the study by Descamps et al. [42], who found a reduced petal surface area in starflower (Borago officinalis L.) due to higher temperature. There is a positive relationship between flower size and pollinators [43]. Small petals may reduce pollinators’ visits, causing decreased cross-pollination, which may adversely affect plant reproductive fitness and yield [44]. Also, increased anthocyanins in petals of flowers under higher temperatures indicate the protective and attractive roles of these compounds, which have been well documented [45,46]. However, in the petals of snapdragon (Antirrhinum majus L. cv. Floral Shower Deep Rose), high temperature at low light suppressed the biosynthesis of anthocyanins, which was associated with decreased sugar levels [47].
Although temperature did not significantly affect siliques, it did influence seed properties (Table 4), as both seed number and seed mass (although not significant) decreased with higher temperatures (Figure 3C,D). The results support earlier findings. For instance, Pokharel et al. [14] found that silique and seed mass decreased in winter canola when the plants were exposed to high temperatures. Lohani et al. [48] also found a reduction in seed mass and production in canola by exposure to high temperatures. Even though our findings did not show a significant decrease in silique mass under higher temperatures, the data still show a reduction in its mass and could prove that higher temperatures are detrimental to the overall yield of canola.
While lower seed mass may not be indicative of a reduction in germination [18], it is important to note that seed size does affect the early stages of seedling development, such as establishment and survival, and overall fitness [49], which has implications for seeds used for crops instead of being crushed for oil consumption. Lower germination temperatures increased the germination rate of the seeds that were harvested from the parent plants grown under higher temperatures (Figure 5C). Liu et al. [50] have shown that high temperatures increase rice seed germination rate. Khaeim et al. [51] also found that higher temperatures increase the seed germination rate of wheat (Triticum aestivum L.). An increase in seed germination in these crop species could prove to be beneficial in a warming climate, though more studies are needed to detail the outcomes of subsequent seed germination as opposed to not considering the condition in which the parent plant was grown.
Overall, the percentage of germinated seeds was lower under higher temperatures than under lower temperatures (Figure 5A,B). Considering germination within higher temperatures, seeds that matured on the well-watered plants under higher temperatures germinated significantly lower than under lower temperatures (Figure 5B). This indicates that higher temperatures during seed maturation might have induced mechanisms that caused the seeds to be dormant or have reduced germination under the same temperature regime. An earlier study using canola also showed that high temperatures resulted in poor germination compared to lower temperatures [52], which is similar to our findings. Flores et al. [53], using various Chihuahuan Desert species, found that high temperatures result in lower seed germination. Although they did not use seeds from crop species, their results show that high temperatures negatively affect seed germination, indicating that a warming climate poses a threat to seedling establishment and plant productivity. Regardless of the watering regime, seeds that matured under higher temperatures germinated faster than seeds that matured under lower temperatures when they were germinated under a lower temperature regime (Figure 5C). The faster germination of seeds that matured under higher temperatures could be attributed to thinner seed coats and reduced germination inhibitors (e.g., abscisic acid) that increase the vigor of these seeds, facilitating speedy germination [54].
4.2. Effects of Watering Regime
Flower characteristics were mostly unaffected by the watering regime (Table 1 and Table 2); however, sepal length was reduced by water stress, particularly under higher temperatures (Figure 1A). This is a novel finding to fill the gap in our understanding of the effects of climate change on floral morphology, as no earlier studies were found to consider this floral aspect. Our study also showed that nectar volume per flower decreased in the water-stressed plants, particularly under higher temperatures (Table 3, Figure 2A). This supports an earlier finding on the nectar volume from buckwheat (Fagopyrum esculentum L.; [55]). It appears that water is one of the less-studied constituents of nectar because of its variability, which is associated with the plant environment and floral microclimate [56]. The nectar volume can affect the concentration of other solutes found in it because of its interaction with external water availability, as suggested by this and other studies [55]. In this study, the concentration of sugar in nectar was significantly increased by drought stress because of lower water availability (Table 3, Figure 2B), whereas in an earlier study with buckwheat, it was unaffected by the same factor [55]. Descamps et al. [11] also found that, in Himalayan balsam (Impatiens glandulifera Royle), the concentration of sugar in nectar was not affected by drought stress. Nectar is quite chemically complex [56], so the difference between these studies can be attributed to species differences, as the effects of environmental factors on plants are species-specific [57]. The varying effects of drought stress on the nectar volume can be explained by the action of an enzyme known as cell wall invertase, which catalyzes the hydrolysis of sucrose into fructose and glucose [58]. This enzyme can indirectly be affected by drought stress, causing a decrease in its activity [59]. However, conflicting results indicate a need for nectar studies, especially in canola, as it relies on floral rewards for increased genetic diversity, better ripening, and increasing seed mass [60].
Water availability significantly affected many aspects of silique growth and seed development. The water-stressed plants produced siliques that were significantly shorter and lighter than the well-watered plants (Table 4 and Table 5, Figure 3 and Figure 4). Also, the water-stressed plants produced fewer sound seeds, total seeds, and seeds with lighter mass (Table 4 and Table 5, Figure 3 and Figure 4). Shorter siliques that were produced by the water-stressed plants support the results of a previous study in which water stress negatively affected silique length in Arabidopsis thaliana L. [19]. In that study, it was also found that water-stressed plants produced lighter siliques with reduced seed number and mass, which is supported by this study.
4.3. Interactive Effects of Temperature and Watering Regime
In this study, the nectar volume per flower was significantly affected by the interaction of temperature and water (Table 3). Flowers of the water-stressed plants under higher temperatures produced the least amount of nectar, whereas flowers of the well-watered plants under the same temperature regime produced the highest amount of nectar (Figure 2A). This indicates that higher temperature and water deficit, in combination, have profound negative effects on nectar volume. In earlier studies, with Paterson’s Curse (Echium plantagineum L.) and Himalayan balsam (Impatiens glandulifera Royle), high temperature and water stress severely decreased nectar production [11,16]. In our study, the water-stressed plants that were grown under a higher temperature regime produced the fewest seeds per silique and, in turn, had the lowest seed mass (Table 5, Figure 3C,D). It appears that the interactive effects of the temperature and watering regime are less pronounced on the reproductive growth of this canola cultivar than the individual effects of these factors. It is likely that this canola genotype has tolerance to certain levels of these stressors as individual factors. Also, it is possible that higher temperatures reduced the effects of water stress on reproductive growth; such non-synergistic interactions of environmental factors on this species have already been shown [35]. Further studies are required to understand such a phenomenon.
5. Conclusions
This study revealed that higher temperatures can influence canola reproduction by decreasing petal width, reducing aspects of silique and seed growth and development, and subsequent seed germination. Also, the water deficit decreased sepal length, nectar volume per flower, silique length and mass, and sound and total seed number but increased nectar sugar concentration. Interactions between temperature and watering regime affected nectar volume. Although temperature and its interaction with the watering regime did not play a crucial role in the reproductive growth of canola because it was water availability that affected silique and seed development, it is still important to realize the critical effects of temperature on the reproductive success of this agronomically important crop. As our climate continues to be influenced by anthropogenic activities, further studies are required to better understand canola’s responses to multiple co-occurring abiotic stressors in terms of reproductive growth and yield. Moreover, management strategies are required to optimize water use in agricultural systems in the face of global warming.
Author Contributions
M.M.Q. planned and designed the research. A.D.B. performed the experiments. A.D.B. and M.M.Q. analyzed the data and wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by a Discovery grant from the Natural Sciences and Engineering Research Council (NSERC) of Canada awarded to M.M.Q.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be available upon request.
Acknowledgments
We would like to thank NSERC, Canadian Foundation for Innovation, Nova Scotia Research and Innovation Trust, and Mount Saint Vincent University for financial support. We also thank BrettYoung Seeds for supplying canola seeds. We appreciate constructive comments on the manuscript from five anonymous referees.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Intergovernmental Panel on Climate Change (IPCC). Climate Change 2023: Synthesis Report; Contribution of Working Groups I, II, and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2023. [Google Scholar]
- Teixeira, E.I.; Fischer, G.; van Velthuizen, H.; Walter, C.; Ewert, F. Global hot-spots of heat stress on agricultural crops due to climate change. Agric. For. Meteorol. 2013, 170, 206–215. [Google Scholar] [CrossRef]
- Trenberth, K.E. Changes in precipitation with climate change. Clim. Res. 2011, 47, 123–138. [Google Scholar] [CrossRef]
- Arnell, N.W.; Lowe, J.A.; Challinor, A.J.; Osborn, T.J. Global and regional impacts of climate change at different levels of global temperature increase. Clim. Change 2019, 155, 377–391. [Google Scholar] [CrossRef]
- Berg, A.; Sheffield, J. Climate change and drought: The soil moisture perspective. Curr. Clim. Change Rep. 2018, 4, 180–191. [Google Scholar] [CrossRef]
- dos Santos, T.B.; Ribas, A.F.; de Souza, S.G.H.; Budzinski, I.G.F.; Domingues, D.S. Physiological responses to drought, salinity, and heat stress in plants: A review. Stresses 2022, 2, 113–135. [Google Scholar] [CrossRef]
- Tun, W.; Yoon, J.; Jeon, J.-S.; An, G. Influence of climate change on flowering time. J. Plant Biol. 2021, 64, 193–203. [Google Scholar] [CrossRef]
- Hatfield, J.L.; Prueger, J.H. Temperature extremes: Effect on plant growth and development. Weather Clim. Extrem. 2015, 10, 4–10. [Google Scholar] [CrossRef]
- Gérard, M.; Vanderplanck, M.; Wood, T.; Michez, D. Global warming and plant-pollinator mismatches. Emerg. Top. Life Sci. 2020, 4, 77–86. [Google Scholar]
- Hedhly, A. Sensitivity of flowering plant gametophytes to temperature fluctuations. Environ. Exp. Bot. 2011, 74, 9–16. [Google Scholar] [CrossRef]
- Descamps, C.; Boubnan, N.; Jacquemart, A.-L.; Quinet, M. Growing and flowering in a changing climate: Effects of higher temperatures and drought stress on the bee-pollinated species Impatiens glandulifera Royle. Plants 2021, 10, 988. [Google Scholar] [CrossRef]
- Khodorova, N.V.; Boitel-Conti, M. The role of temperature in the growth and flowering of geophytes. Plants 2013, 2, 699–711. [Google Scholar] [CrossRef] [PubMed]
- Nozaki, K.; Fukai, S. Effects of high temperature on floral development and flowering in spray chrysanthemum. J. Appl. Hortic. 2008, 10, 8–14. [Google Scholar] [CrossRef]
- Pokharel, M.; Stamm, M.; Hein, N.T.; Jagadish, K.S.V. Heat stress affects floral morphology, silique set and seed quality in chamber and field grown winter canola. J. Agro Crop Sci. 2021, 207, 465–480. [Google Scholar] [CrossRef]
- Tushabe, D.; Altmann, F.; Koehler, E.; Woods, S.; Rosbakh, S. Negative effects of high-temperature stress on gametophyte performance and their consequences for seed reproduction in wild plants. Environ. Exp. Bot. 2023, 216, 105532. [Google Scholar] [CrossRef]
- Descamps, C.; Marée, S.; Hugon, S.; Quinet, M.; Jacquemart, A.-L. Species-specific responses to combined water stress and increasing temperatures in two bee-pollinated congeners (Echium, Boraginaceae). Ecol. Evol. 2020, 10, 6549–6561. [Google Scholar] [CrossRef]
- Krug, A.S.; Drummond, E.B.M.; Van Tassel, D.L.; Warschefsky, E.J. The next era of crop domestication starts now. Proc. Natl. Acad. Sci. USA 2023, 120, e2205769120. [Google Scholar] [CrossRef]
- Hampton, J.G.; Boelt, B.; Rolston, M.P.; Chastain, T.G. Effects of elevated CO2 and temperature on seed quality. J. Agric. Sci. 2012, 151, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Abo Gamar, M.I.; Dixon, S.L.; Qaderi, M.M. Single and interactive effects of temperature, carbon dioxide and watering regime on plant growth and reproductive yield of two genotypes of Arabidopsis thaliana. Acta Physiol. Plant. 2021, 43, 124. [Google Scholar] [CrossRef]
- Dietz, K.-J.; Zörb, C.; Geilfus, C.-M. Drought and crop yield. Plant Biol. 2021, 23, 881–893. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, S.; Cheng, M.; Jiang, H.; Zhang, X.; Peng, C.; Lu, X.; Zhang, M.; Jin, J. Effect of drought on agronomic traits of rice and wheat: A meta-analysis. Int. J. Environ. Res. Public Health 2018, 15, 839. [Google Scholar] [CrossRef]
- Cohen, I.; Zandalinas, S.; Huck, C.; Fritschi, F.B.; Mittler, R. Meta-analysis of drought and heat stress combination impact on crop yield and yield components. Physiol. Plant. 2020, 171, 66–76. [Google Scholar] [CrossRef]
- Ostmeyer, T.; Parker, N.; Jaenisch, B.; Alkotami, L.; Bustamante, C.; Jagadish, S.V.K. Impacts of heat, drought, and their interaction with nutrients on physiology, grain yield, and quality in field crops. Plant Physiol. Rep. 2020, 25, 549–568. [Google Scholar] [CrossRef]
- Gupta, A.; Rico-Medina, A.; Caño-Delgado, A.I. The physiology of plant responses to drought. Science 2020, 368, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.A.; Ma, W.; Shen, S.; Gu, A. Underlying biochemical and molecular mechanisms for seed germination. Int. J. Mol. Sci. 2022, 23, 8502. [Google Scholar] [CrossRef]
- Klupczyńska, E.A.; Pawłowski, T.A. Regulation of seed dormancy and germination mechanisms in a changing environment. Int. J. Mol. Sci. 2021, 22, 1357. [Google Scholar] [CrossRef] [PubMed]
- Egli, D.B.; TeKrony, D.M.; Heitholt, J.J.; Rupe, J. Air temperature during seed filling and soybean seed germination and vigor. Crop Sci. 2005, 45, 1329–1335. [Google Scholar] [CrossRef]
- Reed, R.C.; Bradford, K.J.; Khanday, I. Seed germination and vigor: Ensuring crop sustainability in a changing climate. Heredity 2022, 128, 450–459. [Google Scholar] [CrossRef]
- Evans, C.C.; Qaderi, M.M. Supplemental nitrogen alleviates the negative effects of higher temperature on the vegetative growth of canola regardless of carbon dioxide concentration. Plant Stress 2024, 13, 100521. [Google Scholar] [CrossRef]
- Jones, H.G. Plants and Microclimate: A Quantitative Approach to Environmental Plant Physiology, 3rd ed.; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Elferjani, R.; Soolanayakanahally, R. Canola responses to drought, heat, and combined stress: Shared and specific effects on carbon assimilation, seed yield, and oil composition. Front. Plant Sci. 2018, 9, 1224. [Google Scholar] [CrossRef]
- McDormand, E.D.; Qaderi, M.M. Individual and interactive effects of temperature and watering regime on canola growth and physiological characteristics. Plant Environ. Interact. 2025, 6, e70030. [Google Scholar] [CrossRef]
- Secchi, M.A.; Fernandez, J.A.; Stamm, M.J.; Durrett, T.; Vara Prasad, P.V.; Messina, C.D.; Ciampitti, I.A. Effects of heat and drought on canola (Brassica napus L.) yield, oil, and protein: A meta-analysis. Field Crops Res. 2023, 293, 108848. [Google Scholar] [CrossRef]
- Ge, L.L.; Tian, H.Q.; Russell, S.D. Calcium function and distribution during fertilization in angiosperms. Am. J. Bot. 2007, 94, 1046–1060. [Google Scholar] [CrossRef] [PubMed]
- Qaderi, M.M.; Kurepin, L.V.; Reid, D.M. Growth and physiological responses of canola (Brassica napus) to three components of global climate change: Temperature, carbon dioxide and drought. Physiol. Plant. 2006, 128, 710–721. [Google Scholar] [CrossRef]
- Canadian Food Inspection Agency (CFIA). The Biology of Brassica napus L. (Canola/Rapeseed); Plant and Biotechnology Risk Assessment Unit, Plant Health Science Division, Canadian Food Inspection Agency: Ottawa, ON, Canada, 2017; Available online: https://inspection.canada.ca/plant-varieties/plants-with-novel-traits/applicants/directive-94-08/biology-documents/brassica-napus-l-/eng/1330729090093/1330729278970#a11 (accessed on 1 April 2024).
- Davis, A.R.; Pylatuik, J.D.; Paradis, J.C.; Low, N.H. Nectar-carbohydrate production and composition vary in relation to nectary anatomy and location within individual flowers of several species of Brassicaceae. Planta 1998, 205, 305–318. [Google Scholar] [CrossRef]
- Morrant, D.S.; Schumann, R.; Petit, S. Field methods for sampling and storing nectar from flowers with low nectar volumes. Ann. Bot. 2009, 103, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Qaderi, M.M.; Reid, D.M.; Yeung, E.C. Morphological and physiological responses of canola (Brassica napus) siliquas and seeds to UVB and CO2 under controlled environment conditions. Environ. Exp. Bot. 2007, 60, 428–437. [Google Scholar] [CrossRef]
- Qaderi, M.M.; Reid, D.M. Combined effects of temperature and carbon dioxide on plant growth and subsequent seed germinability of Silene noctiflora. Int. J. Plant Sci. 2008, 169, 1200–1209. [Google Scholar] [CrossRef]
- SAS Institute. SAS/STAT User’s Guide, Version 9.4; SAS Institute: Cary, NC, USA, 2016. [Google Scholar]
- Descamps, C.; Quinet, M.; Baijot, A.; Jacquemart, A.L. Temperature and water stress affect plant-pollinator interactions in Borago officinalis (Boraginaceae). Ecol. Evol. 2018, 8, 3443–3456. [Google Scholar] [CrossRef]
- Delgado, T.; Leal, L.C.; El Ottra, J.H.L.; Brito, V.L.G.; Nogueira, A. Flower size affects bee species visitation pattern on flowers with poricidal anthers across pollination studies. Flora 2023, 299, 152198. [Google Scholar] [CrossRef]
- Brunet, J.; Thairu, M.W.; Henss, J.M.; Link, R.I.; Kluever, J.A. The effects of flower, floral display, and reward sizes on bumblebee foraging behavior when pollen is the reward and plants are dichogamous. Int. J. Plant Sci. 2015, 176, 811–819. [Google Scholar] [CrossRef]
- Lev-Yadun, S.; Gould, K.S. Role of anthocyanins in plant defence. In Anthocyanins: Biosynthesis, Functions, and Applications; Gould, K., Davies, K., Winefield, C., Eds.; Springer: New York, NY, USA, 2009; pp. 21–48. [Google Scholar]
- Kaur, S.; Tiwari, V.; Kumari, A.; Chaudary, E.; Sharma, A.; Ali, U.; Garg, M. Protective and defensive role of anthocyanins under plant abiotic and biotic stresses: An emerging application in sustainable agriculture. J. Biotechnol. 2023, 361, 12–29. [Google Scholar] [CrossRef]
- Ichimura, K.; Niki, T.; Matoh, M.; Masayoshi, N. High temperature under low light conditions suppresses anthocyanin biosynthesis in snapdragon petals associated with decreased sugar levels. Sci. Hortic. 2021, 290, 110510. [Google Scholar] [CrossRef]
- Lohani, N.; Singh, M.B.; Bhalla, P.L. Short-term heat stress during flowering results in a decline in canola seed productivity. J. Agro Crop Sci. 2021, 208, 486–496. [Google Scholar] [CrossRef]
- Larios, E.; Venable, D.L. Selection for seed size: The unexpected effects of water availability and density. Funct. Ecol. 2018, 32, 2216–2224. [Google Scholar] [CrossRef]
- Liu, J.; Hasanuzzaman, M.; Wen, H.; Zhang, J.; Peng, T.; Sun, H.; Zhao, Q. High temperature and drought stress cause abscisic acid and reactive oxygen species accumulation and suppress seed germination growth in rice. Protoplasma 2019, 256, 1217–1227. [Google Scholar] [CrossRef]
- Khaeim, H.; Kende, Z.; Balla, I.; Gyuricza, C.; Eser, A.; Tarnawa, A. The effect of temperature and water stresses on seed germination and seedling growth of wheat (Triticum aestivum L.). Sustainability 2022, 14, 3887. [Google Scholar] [CrossRef]
- Sghaier, A.H.; Tarnawa, Á.; Kovács, G.P.; Gyuricza, C.; Kende, Z. The effects of temperature and water on the seed germination and seedling development of rapeseed (Brassica napus L.). Plants 2022, 11, 2819. [Google Scholar] [CrossRef] [PubMed]
- Flores, J.; Pérez-Sánchez, R.M.; Jurado, E. The combined effect of water stress and temperature on seed germination of Chihuahuan Desert species. J. Arid. Environ. 2017, 146, 95–98. [Google Scholar] [CrossRef]
- Baskin, C.C.; Baskin, J.M. Seeds: Ecology, Biogeography, and Evolution of Dormancy and Germination, 2nd ed.; Academic Press: San Diego, CA, USA, 2014. [Google Scholar]
- Rering, C.C.; Franco, J.G.; Yeater, K.M.; Mallinger, R.E. Drought stress alters floral volatiles and reduces floral rewards, pollinator activity, and seed set in a global plant. Ecosphere 2020, 11, e03254. [Google Scholar] [CrossRef]
- Nicolson, S.W. Sweet solutions: Nectar chemistry and quality. Phil. Trans. R. Soc. B 2022, 377, 20210163. [Google Scholar] [CrossRef]
- Qaderi, M.M.; Reid, D.M. Crop responses to elevated carbon dioxide and temperature. In Climate Change and Crops; Singh, S.N., Ed.; Springer: New York, NY, USA, 2009; pp. 1–18. [Google Scholar]
- Minami, A.; Kang, X.; Carter, C.J. A cell wall invertase controls nectar volume and sugar composition. Plant J. 2021, 107, 1016–1028. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, X.; Huang, X.; Luo, W.; Long, Y.; Greiner, S.; Rausch, T.; Zhao, H. Functional characterization of a drought-responsive invertase inhibitor from maize (Zea mays L.). Int. J. Mol. Sci. 2019, 20, 4081. [Google Scholar] [CrossRef] [PubMed]
- US Canola Association. Pollinator Health. Available online: https://www.uscanola.com/crop-production/pollinator-health/ (accessed on 8 April 2024).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).