Soil Nutrient, Enzyme Activity, and Microbial Community Characteristics of E. urophylla × E. grandis Plantations in a Chronosequence
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Design of Experiments and Collection of Soil Samples
2.3. Soil Characteristics Analysis
2.4. Extraction of DNA and 16S rRNA Illumina Sequencing
2.5. Statistical and Sequence Analysis
3. Results
3.1. Soil Physicochemical Properties in Eucalyptus Forests
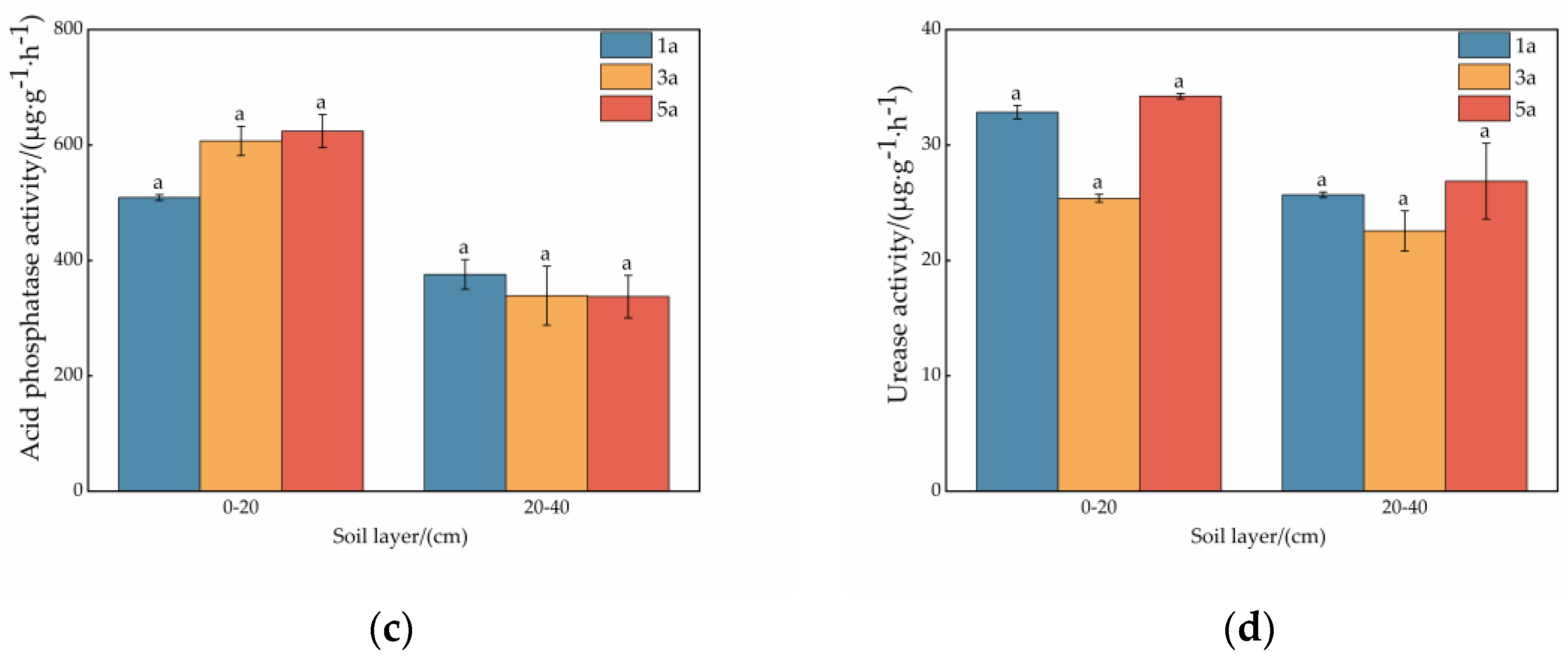
3.2. Enzyme Activity in the Soil at Different Ages of the Plantation
3.3. Soil Microbial Carbon and Nitrogen in Eucalyptus Forests of Different Plantation Ages
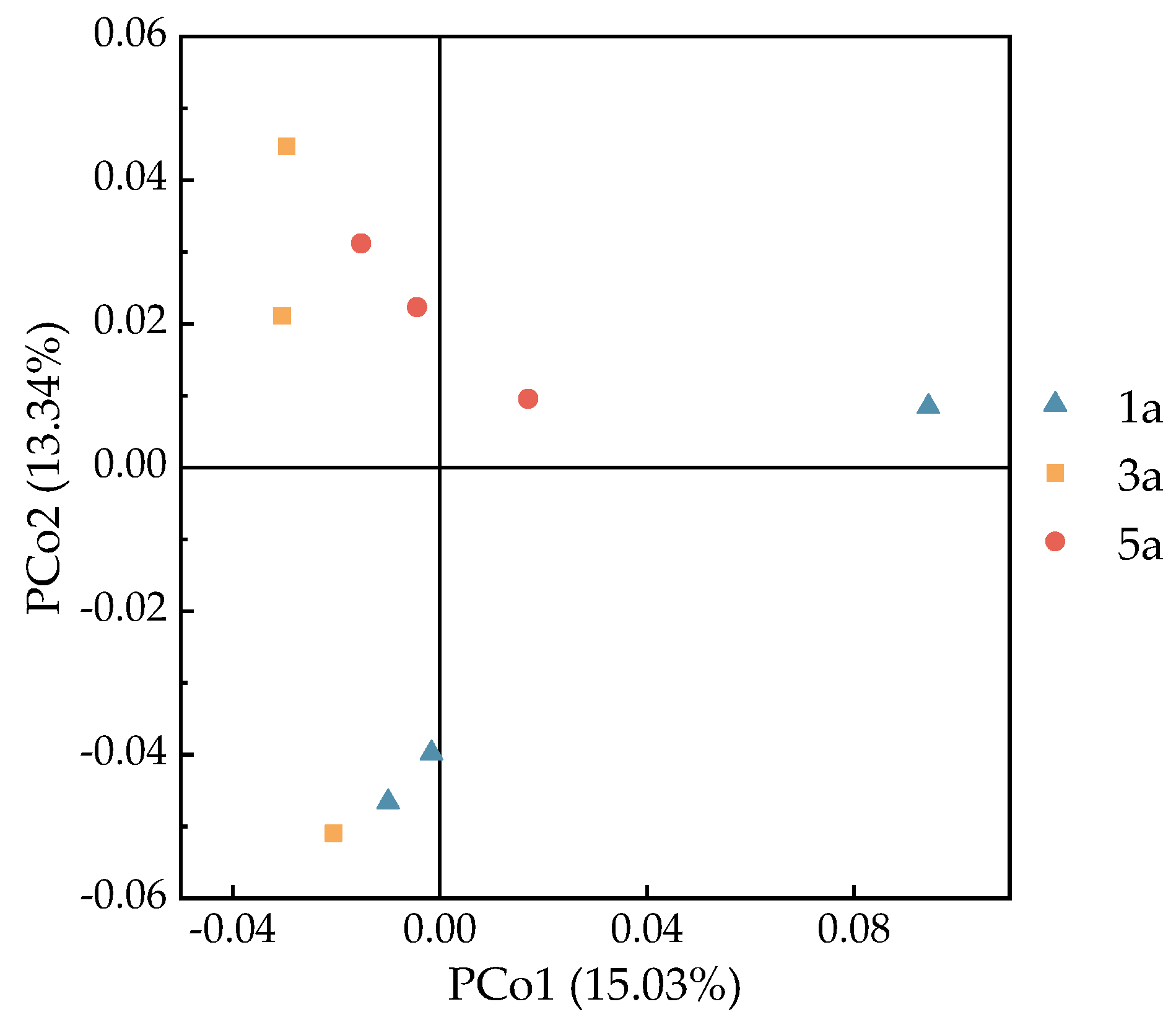
3.4. Soil Microbial Alpha Diversity and Beta Diversity in Eucalyptus Forests of Different Plantation Ages
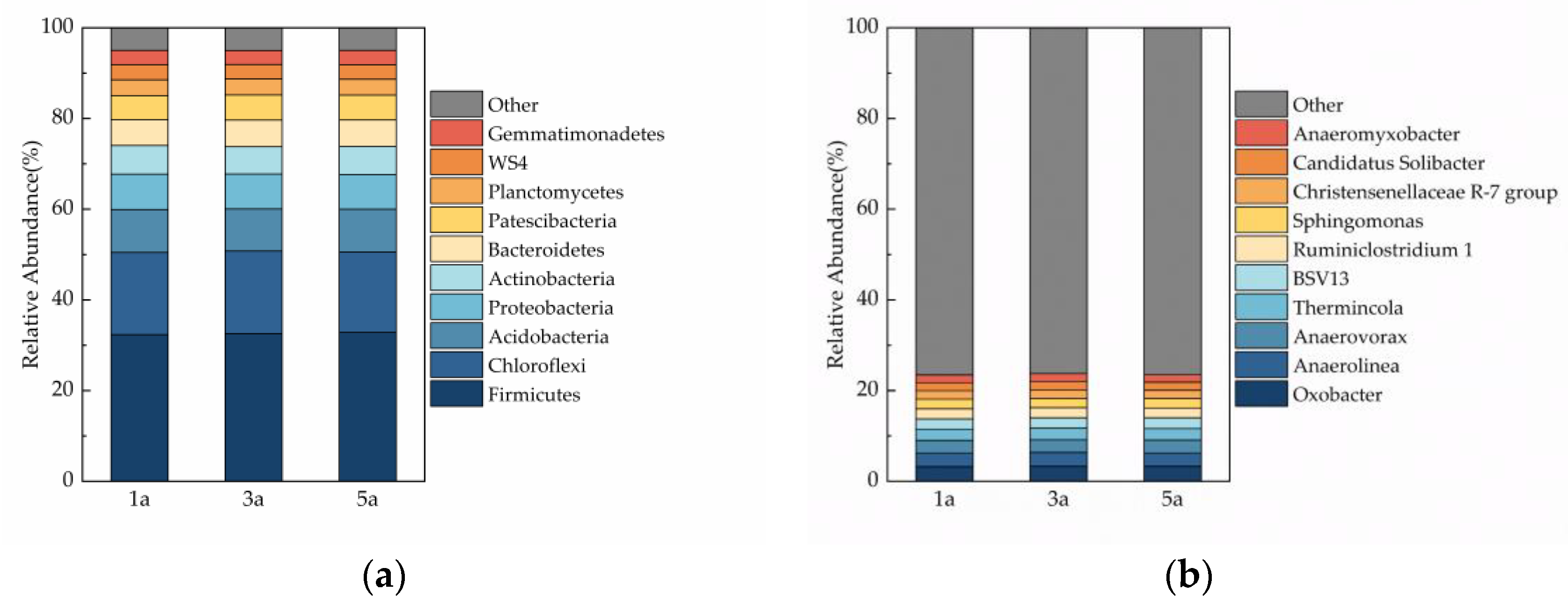
3.5. Soil Microbial Community Composition
4. Discussion
4.1. Influence of Plantation Age on Nutrient Content of Soil
4.2. Effect of Plantation Age on Soil Enzyme Activity
4.3. Influence of Plantation Age on Soil Microbial Biomass and the Diversity of Soil Microbial Community
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sebei, K.; Sakouhi, F.; Herchi, W.; Khouja, M.L.; Boukhchina, S. Chemical Composition and Antibacterial Activities of Seven Eucalyptus Species Essential Oils Leaves. Biol. Res. 2015, 48, 7. [Google Scholar] [CrossRef]
- Zhang, C.; Li, X.; Chen, Y.; Zhao, J.; Wan, S.; Lin, Y.; Fu, S. Effects of Eucalyptus Litter and Roots on the Establishment of Native Tree Species in Eucalyptus Plantations in South China. For. Ecol. Manag. 2016, 375, 76–83. [Google Scholar] [CrossRef]
- Wu, J.; Liu, Z.; Wang, X.; Sun, Y.; Zhou, L.; Lin, Y.; Fu, S. Effects of Understory Removal and Tree Girdling on Soil Microbial Community Composition and Litter Decomposition in Two Eucalyptus Plantations in South China. Funct. Ecol. 2011, 25, 921–931. [Google Scholar] [CrossRef]
- Baohanta, R.; Thioulouse, J.; Ramanankierana, H.; Prin, Y.; Rasolomampianina, R.; Baudoin, E.; Rakotoarimanga, N.; Galiana, A.; Randriambanona, H.; Lebrun, M.; et al. Restoring Native Forest Ecosystems after Exotic Tree Plantation in Madagascar: Combination of the Local Ectotrophic Species Leptolena bojeriana and Uapaca bojeri Mitigates the Negative Influence of the Exotic Species Eucalyptus Camaldulensis and Pinus Patula. Biol. Invasions 2012, 14, 2407–2421. [Google Scholar]
- Zhu, L.; Wang, X.; Chen, F.; Li, C.; Wu, L. Effects of the Successive Planting of Eucalyptus Urophylla on Soil Bacterial and Fungal Community Structure, Diversity, Microbial Biomass, and Enzyme Activity. Land. Degrad. Dev. 2019, 30, 636–646. [Google Scholar] [CrossRef]
- Chen, F.; Zheng, H.; Zhang, K.; Ouyang, Z.; Li, H.; Wu, B.; Shi, Q. Soil Microbial Community Structure and Function Responses to Successive Planting of Eucalyptus. J. Environ. Sci. 2013, 25, 2102–2111. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Wang, Q.-X.; Liu, N.; Li, L.; Zhang, C.-G.; Liu, Z.-B.; Zhang, Y.-Y. Effects of Different Leaf Litters on the Physicochemical Properties and Bacterial Communities in Panax Ginseng-Growing Soil. Appl. Soil. Ecol. 2017, 111, 17–24. [Google Scholar] [CrossRef]
- Xu, J.; Liu, B.; Qu, Z.-L.; Ma, Y.; Sun, H. Age and Species of Eucalyptus Plantations Affect Soil Microbial Biomass and Enzymatic Activities. Microorganisms 2020, 8, 811. [Google Scholar] [CrossRef]
- Li, J.; Lin, J.; Pei, C.; Lai, K.; Jeffries, T.C.; Tang, G. Variation of Soil Bacterial Communities Along a Chronosequence of Eucalyptus Plantation. PEERJ 2018, 6, e5648. [Google Scholar] [CrossRef]
- Hu, Z.; Xiang, W. Inconsistent Responses of Rhizosphere Microbial Community Structure and Extracellular Enzyme Activity to Short-Term Nitrogen and Phosphorus Additions in Chinese Fir (Cunninghamia Lanceolata) Plantations. Forests 2023, 14, 1532. [Google Scholar] [CrossRef]
- Kang, H.; Gao, H.; Yu, W.; Yi, Y.; Wang, Y.; Ning, M. Changes in Soil Microbial Community Structure and Function after Afforestation Depend on Species and Age: Case Study in a Subtropical Alluvial Island. Sci. Total Environ. 2018, 625, 1423–1432. [Google Scholar] [CrossRef] [PubMed]
- Lan, Y.; Wang, S.; Zhang, H.; He, Y.; Jiang, C.; Ye, S. Intercropping and Nitrogen Enhance Eucalyptus Productivity through the Positive Interaction between Soil Fertility Factors and Bacterial Communities Along with the Maintenance of Soil Enzyme Activities. Land. Degrad. Dev. 2023, 34, 2403–2417. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, Z.; Chen, K.; Chen, Z.; Zeng, X.; Yu, H.; Guo, S.; Shangguan, Y.; Chen, Q.; Fan, H.; et al. Changes in Soil Physicochemical Properties and Bacterial Communities at Different Soil Depths after Long-Term Straw Mulching under a No-Till System. Soil 2021, 7, 595–609. [Google Scholar] [CrossRef]
- Xu, Y.; Li, C.; Zhu, W.; Wang, Z.; Wu, L.; Du, A. Effects of Enrichmemt Planting with Native Tree Species on Bacterial Community Structure and Potential Impact on Eucalyptus Plantations in Southern China. J. For. Res. 2022, 33, 1349–1363. [Google Scholar] [CrossRef]
- Sui, X.; Zhang, R.; Frey, B.; Yang, L.; Liu, Y.; Ni, H.; Li, M.-H. Soil Physicochemical Properties Drive the Variation in Soil Microbial Communities Along a Forest Successional Series in a Degraded Wetland in Northeastern China. Ecol. Evol. 2021, 11, 2194–2208. [Google Scholar] [CrossRef]
- Wang, C.Y.; Li, J.; Wu, Y.T.; Song, Y.; Liu, R.Y.; Cao, Z.C.; Cui, Y.S. Shifts of the Nirs and Nirk Denitrifiers in Different Land Use Types and Seasons in the Sanjiang Plain, China. J. Basic Microbiol. 2019, 59, 1040–1048. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.; Zhu, K.; Zhuang, S.; Tang, L. Soil Nutrient Cycling and Bacterial Community Structure in Response to Various Green Manures in a Successive Eucalyptus (Eucalyptus Urophylla × Eucalyptus Grandis) Plantation. Land. Degrad. Dev. 2022, 33, 2809–2821. [Google Scholar] [CrossRef]
- Huang, S.; Yu, J.; Hou, D.; Yue, H.; Zhang, D.; Li, Y.; Lyu, J.; Jin, L.; Jin, N. Response of Soil Microbial Community Diversity to Continuous Cucumber Cropping in Facilities along the Yellow River Irrigation Area. PLoS ONE 2023, 18, e0289772. [Google Scholar] [CrossRef]
- Chen, J.; Du, Y.; Zhu, W.; Pang, X.; Wang, Z. Effects of Organic Materials on Soil Bacterial Community Structure in Long-Term Continuous Cropping of Tomato in Greenhouse. Open Life Sci. 2022, 17, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Chen, J.; Pan, G.; Wang, G.; Liu, X.; Zhang, X.; Li, L.; Bian, R.; Cheng, K.; Zheng, J. A Long-Term Hybrid Poplar Plantation on Cropland Reduces Soil Organic Carbon Mineralization and Shifts Microbial Community Abundance and Composition. Appl. Soil Ecol. 2017, 111, 94–104. [Google Scholar] [CrossRef]
- James, R.S.; Haby, V.A. Simplified Colorimetric Determination of Soil Organic Matter. Soil Sci. 1971, 112, 137–141. [Google Scholar]
- Tsiknia, M.; Vasileios; Tzanakakis, A.; Oikonomidis, D.; Paranychianakis, N.V.; Nikolaidis, N.P. Effects of Olive Mill Wastewater on Soil Carbon and Nitrogen Cycling. Appl. Microbiol. Biotechnol. 2014, 98, 2739–2749. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Y.; Tang, M. Arbuscular Mycorrhizal Fungi Diversity Associated with Two Halophytes Lycium Barbarum L. And Elaeagnus Angustifolia L. In Ningxia, China. Arch. Agron. Soil Sci. 2017, 63, 796–806. [Google Scholar] [CrossRef]
- Yanu, P.; Jakmunee, J. Flow Injection with in-Line Reduction Column and Conductometric Detection for Determination of Total Inorganic Nitrogen in Soil. Talanta 2015, 144, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Kandeler, E.; Gerber, H. Short-Term Assay of Soil Urease Activity Using Colorimetric Determination of Ammonium. Biol. Fertil. Soils 1988, 6, 68–72. [Google Scholar] [CrossRef]
- Yuan, Z.; Liu, H.; Han, J.; Sun, J.; Wu, X.; Yao, J. Monitoring Soil Microbial Activities in Different Cropping Systems Using Combined Methods. Pedosphere 2017, 27, 138–146. [Google Scholar] [CrossRef]
- Tang, Z.; Chen, H.; He, H.; Ma, C. Assays for Alkaline Phosphatase Activity: Progress and Prospects. TrAC Trends Anal. Chem. 2019, 113, 32–43. [Google Scholar] [CrossRef]
- Hadwan, M.H. Simple Spectrophotometric Assay for Measuring Catalase Activity in Biological Tissues. BMC Biochem. 2018, 19, 7. [Google Scholar] [CrossRef]
- Brookes, P.C.; Landman, A.; Pruden, G.; Jenkinson, D.S. Chloroform Fumigation and the Release of Soil Nitrogen: A Rapid Direct Extraction Method to Measure Microbial Biomass Nitrogen in Soil. Soil Biol. Biochem. 1985, 17, 837–842. [Google Scholar] [CrossRef]
- Lu, Z.; Zhou, Y.; Li, Y.; Li, C.; Lu, M.; Sun, X.; Luo, Z.; Zhao, J.; Fan, M. Effects of Partial Substitution of Chemical Fertilizer with Organic Manure on the Activity of Enzyme and Soil Bacterial Communities in the Mountain Red Soil. Front. Microbiol. 2023, 14, 1234904. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Jia, P.; Wu, Y.; Yu, X.; Wu, S.; Yang, L.; Zhang, B.; Wang, F.; Liu, G.; Chen, T.; et al. Diversity and Distribution Characteristics of Culturable Bacteria in Burqin Glacier No. 18, Altay Mountains, China. Diversity 2023, 15, 997. [Google Scholar] [CrossRef]
- Zhao, Q.; Xiong, W.; Xing, Y.; Sun, Y.; Lin, X.; Dong, Y. Long-Term Coffee Monoculture Alters Soil Chemical Properties and Microbial Communities. Sci. Rep. 2018, 8, 6116. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Guo, X.; Guo, Q.; Tan, X.; Wang, Z. Long-Term Chili Monoculture Alters Environmental Variables Affecting the Dominant Microbial Community in Rhizosphere Soil. Front. Microbiol. 2021, 12, 681953. [Google Scholar] [CrossRef]
- Cui, R.; Wang, C.; Cheng, F.; Ma, X.; Cheng, X.; He, B.; Chen, D. Effects of Successive Planting of Eucalyptus on Soil Physicochemical Properties 1–3 Generations after Converting Masson Pine Forests into Eucalyptus Plantations. Pol. J. Environ. Stud. 2023, 32, 4503–4514. [Google Scholar] [CrossRef]
- Ma, G.; Cheng, S.; He, W.; Dong, Y.; Qi, S.; Tu, N.; Tao, W. Effects of Organic and Inorganic Fertilizers on Soil Nutrient Conditions in Rice Fields with Varying Soil Fertility. Land 2023, 12, 1026. [Google Scholar] [CrossRef]
- Fang, X.-M.; Wang, G.G.; Xu, Z.-J.; Zong, Y.-Y.; Zhang, X.-L.; Li, J.-J.; Wang, H.; Chen, F.-S. Litter Addition and Understory Removal Influenced Soil Organic Carbon Quality and Mineral Nitrogen Supply in a Subtropical Plantation Forest. Plant Soil. 2021, 460, 527–540. [Google Scholar] [CrossRef]
- Igalavithana, A.D.; Lee, S.S.; Niazi, N.K.; Lee, Y.-H.; Kim, K.H.; Park, J.-H.; Moon, D.H.; Ok, Y.S. Assessment of Soil Health in Urban Agriculture: Soil Enzymes and Microbial Properties. Sustainability 2017, 9, 310. [Google Scholar] [CrossRef]
- Wang, T.; Dai, Q.; Fu, Y.; Wei, P. Effect of Continuous Planting on Tree Growth Traits and Growth Stress in Plantation Forests of Eucalyptus Urophylla × E. Grandis. Sustainability 2023, 15, 9624. [Google Scholar] [CrossRef]
- Wu, G.; Yu, F.; Yuan, M.; Wang, J.; Liu, C.; He, W.; Ge, Z.; Sun, Y.; Liu, Y. Responses of Rhizosphere Bacterial and Fungal Communities to the Long-Term Continuous Monoculture of Water Oat. Microorganisms 2022, 10, 2174. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhu, L.; Gielen, G.; Wu, Q.; Huang, K.; Wen, J.; Wang, X.; Wang, H.; Lu, S.; Chen, L.; et al. Potential Effects of Soil Chemical and Biological Properties on Wood Volume in Eucalyptus Urophylla × Eucalyptus Grandis Hybrid Plantations and Their Responses to Different Intensity Applications of Inorganic Fertilizer. Environ. Sci. Pollut. Res. 2023, 30, 773–787. [Google Scholar] [CrossRef]
- Hou, S.-L.; Freschet, G.T.; Yang, J.-J.; Zhang, Y.-H.; Yin, J.-X.; Hu, Y.-Y.; Wei, H.-W.; Han, X.-G.; Lü, X.-T. Quantifying the Indirect Effects of Nitrogen Deposition on Grassland Litter Chemical Traits. Biogeochemistry 2018, 139, 261–273. [Google Scholar] [CrossRef]
- Zhang, T.; Luo, Y.; Chen, H.Y.H.; Ruan, H. Responses of Litter Decomposition and Nutrient Release to N Addition: A Meta-Analysis of Terrestrial Ecosystems. Appl. Soil Ecol. 2018, 128, 35–42. [Google Scholar] [CrossRef]
- Wang, T.; Yang, K.; Ma, Q.; Jiang, X.; Zhou, Y.; Kong, D.; Wang, Z.; Parales, R.E.; Li, L.; Zhao, X.; et al. Rhizosphere Microbial Community Diversity and Function Analysis of Cut Chrysanthemum During Continuous Monocropping. Front. Microbiol. 2022, 13, 801546. [Google Scholar] [CrossRef] [PubMed]
- Adetunji, A.T.; Lewu, F.B.; Mulidzi, R.; Ncube, B. The Biological Activities of Β-Glucosidase, Phosphatase and Urease as Soil Quality Indicators: A Review. J. Soil Sci. Plant Nutr. 2017, 17, 794–807. [Google Scholar] [CrossRef]
- Bastida, F.; Eldridge, D.J.; García, C.; Png, G.K.; Bardgett, R.D.; Delgado-Baquerizo, M. Soil Microbial Diversity–Biomass Relationships Are Driven by Soil Carbon Content across Global Biomes. ISME J. 2021, 15, 2081–2091. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Wang, C.; Zhang, J.; Sun, Y.; Xu, X.; Zhu, N.; Cai, Y.; Xu, D.; Wang, X.; Xin, X.; et al. Response of Soil Microbial Biomass C, N, and P and Microbial Quotient to Agriculture and Agricultural Abandonment in a Meadow Steppe of Northeast China. Soil Tillage Res. 2022, 223, 105475. [Google Scholar] [CrossRef]
- Sun, T.; Wang, Y.; Hui, D.; Jing, X.; Feng, W. Soil Properties Rather Than Climate and Ecosystem Type Control the Vertical Variations of Soil Organic Carbon, Microbial Carbon, and Microbial Quotient. Soil Biol. Biochem. 2020, 148, 107905. [Google Scholar] [CrossRef]
- Li, D.; Li, X.; Tao, Y.; Yan, Z.; Ao, Y. Deciphering the Bacterial Microbiome in Response to Long-Term Mercury Contaminated Soil. Ecotoxicol. Environ. Saf. 2022, 229, 113062. [Google Scholar] [CrossRef]
- Kerfahi, D.; Tripathi, B.M.; Dong, K.; Go, R.; Adams, J.M. Rainforest Conversion to Rubber Plantation May Not Result in Lower Soil Diversity of Bacteria, Fungi, and Nematodes. Microb. Ecol. 2016, 72, 359–371. [Google Scholar] [CrossRef]
- Lan, G.; Li, Y.; Wu, Z.; Xie, G. Soil Bacterial Diversity Impacted by Conversion of Secondary Forest to Rubber or Eucalyptus Plantations: A Case Study of Hainan Island, South China. For. Sci. 2016, 63, 87–93. [Google Scholar] [CrossRef]
- Colombo, F.; Macdonald, C.A.; Jeffries, T.C.; Powell, J.R.; Singh, B.K. Impact of Forest Management Practices on Soil Bacterial Diversity and Consequences for Soil Processes. Soil Biol. Biochem. 2016, 94, 200–210. [Google Scholar] [CrossRef]
- Hawke, D.J.; Vallance, J.R. Microbial Carbon Concentration in Samples of Seabird and Non-Seabird Forest Soil: Implications for Leaf Litter Cycling. Pedobiologia 2015, 58, 33–39. [Google Scholar] [CrossRef]
- Muhammad, I.; Wang, J.; Sainju, U.M.; Zhang, S.; Zhao, F.; Khan, A. Cover Cropping Enhances Soil Microbial Biomass and Affects Microbial Community Structure: A Meta-Analysis. Geoderma 2021, 381, 114696. [Google Scholar] [CrossRef]
- Trivedi, P.; Delgado-Baquerizo, M.; Trivedi, C.; Hamonts, K.; Anderson, I.C.; Singh, B.K. Keystone Microbial Taxa Regulate the Invasion of a Fungal Pathogen in Agro-Ecosystems. Soil Biol. Biochem. 2017, 111, 10–14. [Google Scholar] [CrossRef]
- Pérez, C.; Sherlynette; Cleland, E.E.; Wagner, R.; Al Sawad, R.; Lipson, D.A. Soil Microbial Responses to Drought and Exotic Plants Shift Carbon Metabolism. ISME J. 2019, 13, 1776–1787. [Google Scholar] [CrossRef]
- Rampelotto, P.H.; de Siqueira Ferreira, A.; Barboza, A.D.M.; Roesch, L.F.W. Changes in Diversity, Abundance, and Structure of Soil Bacterial Communities in Brazilian Savanna under Different Land Use Systems. Microb. Ecol. 2013, 66, 593–607. [Google Scholar] [CrossRef] [PubMed]
- Mueller, R.C.; Belnap, J.; Kuske, C.R. Soil Bacterial and Fungal Community Responses to Nitrogen Addition across Soil Depth and Microhabitat in an Arid Shrubland. Front. Microbiol. 2015, 6, 157127. [Google Scholar] [CrossRef]
- Fierer, N.; Bradford, M.A.; Jackson, R.B. Toward an Ecological Classification of Soil Bacteria. Ecology 2007, 88, 1354–1364. [Google Scholar] [CrossRef] [PubMed]




| Stand Age/a | Stand Density/N·hm−2 | Average Height/m | Average Diameter at Breast Height/cm |
|---|---|---|---|
| 1 | 1250 | 3.74 | 3.49 |
| 2 | 1250 | 17.08 | 11.58 |
| 3 | 1250 | 21.69 | 14.43 |
| Soil Properties | Soil Layer/cm | Plantation Age/a | ||
|---|---|---|---|---|
| 1 | 3 | 5 | ||
| Bulk density (g·cm−3) | 0–20 cm | 0.983 ± 0.06 a | 1.00 ± 0.10 a | 1.09 ± 0.12 a |
| 20–40 cm | 1.09 ± 0.05 a | 1.21 ± 0.08 a | 1.27 ± 0.17 a | |
| pH | 0–20 cm | 4.86 ± 0.09 a | 4.71 ± 0.04 b | 4.63 ± 0.04 b |
| 20–40 cm | 4.77 ± 0.11 a | 4.68 ± 0.13 b | 4.58 ± 0.09 b | |
| Cation exchange capacity (cmol+·kg−1) | 0–20 cm | 5.06 ± 0.11 a | 3.73 ± 0.18 b | 2.39 ± 0.07 c |
| 20–40 cm | 3.77 ± 0.17 a | 3.67 ± 0.31 b | 2.04 ± 0.38 c | |
| Soil organic matter (g·kg−1) | 0–20 cm | 19.7 ± 0.67 a | 18.5 ± 1.29 a | 14.8 ± 1.16 b |
| 20–40 cm | 17.7 ± 1.55 a | 16.2 ± 0.94 a | 12.6 ± 0.76 b | |
| Alkaline hydrolytic nitrogen (mg·kg−1) | 0–20 cm | 50.4 ± 0.80 c | 65.7 ± 0.89 b | 80.6 ± 2.29 a |
| 20–40 cm | 48.8 ± 1.36 c | 63.3 ± 2.18 b | 73.0 ± 4.84 a | |
| Available phosphorus (mg·kg−1) | 0–20 cm | 32.3 ± 3.04 a | 8.20 ± 0.54 b | 5.63 ± 1.05 b |
| 20–40 cm | 10.2 ± 1.49 a | 3.21 ± 0.53 b | 2.86 ± 0.46 b | |
| Available potassium (mg·kg−1) | 0–20 cm | 100 ± 6.95 a | 79.0 ± 5.67 a | 105 ± 5.18 a |
| 20–40 cm | 54.4 ± 6.89 a | 47.9 ±3.31 a | 57.9 ± 3.67 a | |
| Plantation Age/a | Soil Layer/cm | MBC/(mg·kg−1) | MBN/(mg·kg−1) | MBC:MBN Ratio |
|---|---|---|---|---|
| 1 | 0–20 cm | 242.5 ± 37.43 c | 19.09 ± 1.58 c | 12.7 ± 0.76 a |
| 20–40 cm | 168.1 ± 30.84 c | 16.25 ± 1.54 c | 10.4 ± 0.19 a | |
| 3 | 0–20 cm | 295.8 ± 11.09 b | 67.85 ± 5.39 b | 4.36 ± 0.48 b |
| 20–40 cm | 249.2 ± 10.17 b | 57.53 ± 2.92 b | 4.33 ± 0.46 b | |
| 5 | 0–20 cm | 367.4 ± 18.34 a | 91.51 ± 5.59 a | 4.02 ± 0.55 b |
| 20–40 cm | 331.9 ± 15.74 a | 64.50 ± 5.31 a | 5.15 ± 0.29 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Zhu, K.; Zhuang, S.; Wang, H.; Cao, J. Soil Nutrient, Enzyme Activity, and Microbial Community Characteristics of E. urophylla × E. grandis Plantations in a Chronosequence. Forests 2024, 15, 688. https://doi.org/10.3390/f15040688
Zhang Y, Zhu K, Zhuang S, Wang H, Cao J. Soil Nutrient, Enzyme Activity, and Microbial Community Characteristics of E. urophylla × E. grandis Plantations in a Chronosequence. Forests. 2024; 15(4):688. https://doi.org/10.3390/f15040688
Chicago/Turabian StyleZhang, Yuhe, Kongxin Zhu, Shunyao Zhuang, Huili Wang, and Jizhao Cao. 2024. "Soil Nutrient, Enzyme Activity, and Microbial Community Characteristics of E. urophylla × E. grandis Plantations in a Chronosequence" Forests 15, no. 4: 688. https://doi.org/10.3390/f15040688
APA StyleZhang, Y., Zhu, K., Zhuang, S., Wang, H., & Cao, J. (2024). Soil Nutrient, Enzyme Activity, and Microbial Community Characteristics of E. urophylla × E. grandis Plantations in a Chronosequence. Forests, 15(4), 688. https://doi.org/10.3390/f15040688






