Factors Associated with Primary Liver Cancer Survival in a Southern Italian Setting in a Changing Epidemiological Scenario
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Allemani, C.; Matsuda, T.; Di Carlo, V.; Harewood, R.; Matz, M.; Nikšić, M.; Bonaventure, A.; Valkov, M.; Johnson, C.J.; Estève, J.; et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): Analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018, 391, 1023–1075. [Google Scholar] [CrossRef] [PubMed]
- Lepage, C.; Capocaccia, R.; Hackl, M.; Lemmens, V.; Molina, E.; Pierannunzio, D.; Sant, M.; Trama, A.; Faivre, J. Survival in patients with primary liver cancer, gallbladder and extrahepatic biliary tract cancer and pancreatic cancer in Europe 1999–2007: Results of EUROCARE-5. Eur. J. Cancer 2015, 51, 2169–2178. [Google Scholar] [CrossRef] [PubMed]
- Bannon, F.; Di Carlo, V.; Harewood, R.; Engholm, G.; Ferretti, S.; Johnson, C.J.; Aitken, J.F.; Marcos-Gragera, R.; Bonaventure, A.; Gavin, A.; et al. Survival trends for primary liver cancer, 1995–2009: Analysis of individual data for 578,740 patients from 187 population-based registries in 36 countries (CONCORD-2). Ann. Cancer Epidemiol. 2019, 2019, 6-1–6-25. [Google Scholar] [CrossRef]
- Wallace, M.C.; Preen, D.; Jeffrey, G.P.; Adams, L.A. The evolving epidemiology of hepatocellular carcinoma: A global perspective. Expert Rev. Gastroenterol. Hepatol. 2015, 9, 765–779. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics: GLOBOCAN, Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Schütte, K.; Balbisi, F.; Malfertheiner, P. Prevention of hepatocellular carcinoma. Gastrointest. Tumors 2016, 3, 37–43. [Google Scholar] [CrossRef] [PubMed]
- El-Serag, H.B. Current concepts Hepatocellular carcinoma. N. Engl. J. Med. 2011, 365, 1118–1127. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Primers 2021, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Di Bisceglie, A.M. Hepatitis C and hepatocellular carcinoma. Hepatology 1997, 26 (Suppl. S1), 34S–38S. [Google Scholar] [CrossRef] [PubMed]
- Lyratzopoulos, G.; Saunders, C.L.; Abel, G.A. Are emergency diagnoses of cancer avoidable? A proposed taxonomy to motivate study design and support service improvement. Future Oncol. 2014, 10, 1329–1333. [Google Scholar] [CrossRef] [PubMed]
- Quaglia, A.; Lillini, R.; Mamo, C.; Ivaldi, E.; Vercelli, M. Socio-economic inequalities: A review of methodological issues and the relationships with cancer survival. Crit. Rev. Oncol./Hematol. 2013, 85, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Field, K.S.; Briggs, D.J. Socio-economic and locational determinants of accessibility and utilization of primary health-care. Health Soc. Care Community 2001, 9, 294–308. [Google Scholar] [CrossRef] [PubMed]
- Yun, E.H.; Lim, M.K.; Oh, J.K.; Park, J.H.; Shin, A.; Sung, J.; Park, E.C. Combined effect of socioeconomic status, viral hepatitis, and lifestyles on hepatocelluar carcinoma risk in Korea. Br. J. Cancer 2010, 103, 741–746. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rahman, O. Treatment choices and outcomes of non-metastatic hepatocellular carcinoma patients in relationship to neighborhood socioeconomic status: A population-based study. Int. J. Clin. Oncol. 2020, 25, 861–866. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, S.; Montella, M.; Polesel, J.; La Vecchia, C.; Crispo, A.; Maso, L.D.; Casarin, P.; Izzo, F.; Tommasi, L.G.; Chemin, I.; et al. Hepatitis viruses, alcohol, and tobacco in the etiology of hepatocellular carcinoma in Italy. Cancer Epidemiol. Biomark. Prev. 2006, 15, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Haynes, R.; Gale, S. Deprivation and poor health in rural areas: Inequalities hidden by averages. Health Place 2000, 6, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Gilthorpe, M.S.; Wilson, R.C. Rural/urban differences in the association between deprivation and healthcare utilisation. Soc. Sci. Med. 2003, 57, 2055–2063. [Google Scholar] [CrossRef]
- Fusco, M.; Girardi, E.; Piselli, P.; Palombino, R.; Polesel, J.; Maione, C.; Scognamiglio, P.; Pisanti, F.A.; Solmone, M.; Di Cicco, P.; et al. Epidemiology of viral hepatitis infections in an area of southern Italy with high incidence rates of liver cancer. Eur. J. Cancer 2008, 44, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Colombet, M.; Mery, L.; Pin, M.; Znaor, A.; Zanetti, R.; Ferlay, J.; IACR; IARC-WHO. Cancer Incidence in Five Continents—Volume XI; IARC Scientific Publications No. 166; IARC Press: Lyon, France, 2021. [Google Scholar]
- Bréchot, C.; Jaffredo, F.; Lagorce, D.; Gerken, G.; Büschenfelde, K.M.Z.; Papakonstontinou, A.; Hadziyannis, S.; Romeo, R.; Colombo, M.; Rodes, J.; et al. Impact of HBV, HCV and GBV-C/HGV on hepatocellular carcinomas in Europe: Results of a European concerted action. J. Hepatol. 1998, 29, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Bucci, L.; Garuti, F.; Lenzi, B.; Pecorelli, A.; Farinati, F.; Giannini, E.G.; Granito, A.; Ciccarese, F.; Rapaccini, G.L.; Di Marco, M.; et al. The evolutionary scenario of hepatocellular carcinoma in Italy: An update. Liver Int. 2017, 37, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Mancini, S.; Bucchi, L.; Zamagni, F.; Guzzinati, S.; Dal Maso, L.; Rugge, M.; Bisceglia, L.; Serraino, D.; Casella, C.; Caldarella, A.; et al. Trends in Liver Cancer Incidence and Survival in Italy by Histologic Type, 2003–2017. Cancers 2022, 14, 6162. [Google Scholar] [CrossRef] [PubMed]
- Mazzucco, W.; Vitale, F.; Mazzola, S.; Amodio, R.; Zarcone, M.; Alba, D.; Marotta, C.; Cusimano, R.; Allemani, C. Does access to care play a role in liver cancer survival? The ten-year (2006–2015) experience from a population-based cancer registry in Southern Italy. BMC Cancer 2021, 21, 307. [Google Scholar] [CrossRef] [PubMed]
- World Cancer Research Fund/American Institute for Cancer. Diet, Nutrition, Physical Activity and Cancer: A Global Perspective. Continuous Update Project Expert Report 2018. Available online: https://www.wcrf.org/diet-activity-and-cancer/ (accessed on 31 March 2024).
- Lauria, L.; Spinelli, A.; Buoncristiano, M.; Nardone, P. Decline of childhood overweight and obesity in Italy from 2008 to 2016: Results from 5 rounds of the population-based surveillance system. BMC Public Health 2019, 19, 618. [Google Scholar] [CrossRef] [PubMed]
- Fritz, A.; Percy, C.; Jack, A.; Shanmugaratnam, K.; Sobin, L.; Parkin, D.M.W.S. International Classification of Diseases for Oncology (ICD-O), 3rd ed.; 1st Revision; World Health Organization: Geneva, Switzerland, 2000. [Google Scholar]
- Tyczynski, J.E.; Démaret, E.; Parkin, D.M. (Eds.) Standards and Guidelines for Cancer Registration in Europe. The ENCR Reccomandation; IARC Tech Pubblication no. 40; IARC Press: Lyon, France, 2003. [Google Scholar]
- Variabili Censuarie-Censimento della Popolazione e delle Abitazioni. 2001. Available online: https://www.istat.it/it/archivio/104317 (accessed on 31 March 2024).
- Caranci, N.; Biggeri, A.; Grisotto, L.; Pacelli, B.; Spadea, T.; Costa, G. The Italian deprivation index at census block level: Definition, description and association with general mortality. Epidemiol. Prev. 2010, 34, 167–176. [Google Scholar] [PubMed]
- Rosano, A.; Pacelli, B.; Zengarini, N.; Costa, G.; Cislaghi, C.; Caranci, N. Update and review of the 2011 Italian deprivation index calculated at the census section level. Epidemiol. Prev. 2020, 44, 162–170. [Google Scholar] [PubMed]
- Austin, P.C. A comparison of 12 algorithms for matching on the propensity score. Stat. Med. 2014, 33, 1057–1069. [Google Scholar] [CrossRef]
- R Studio Team. RStudio: Integrated Development for R; RStudio, Inc.: Boston, MA, USA, 2024. [Google Scholar]
- Pohar Perme, M.; Pavlic, K. Nonparametric relative survival analysis with the R Package relsurv. J. Stat. Softw. 2018, 87, 1–27. [Google Scholar] [CrossRef]
- Charvat, H.; Belot, A. mexhaz: An R Package for Fitting Flexible Hazard-Based Regression Models for Overall and Excess Mortality with a Random Effect. J. Stat. Softw. 2021, 98, 1–36. [Google Scholar] [CrossRef]
- Ho, D.E.; Imai, K.; King, G.; Stuart, E.A. MatchIt: Nonparametric Preprocessing for Parametric Causal Inference 2007. Available online: https://cran.r-project.org/web/packages/MatchIt/index.html (accessed on 31 March 2024).
- McGlynn, K.A.; Petrick, J.L.; El-Serag, H.B. Epidemiology of Hepatocellular Carcinoma. Hepatology 2021, 73 (Suppl. S1), 4–13. [Google Scholar] [CrossRef] [PubMed]
- Morrisa, M.; Landon, S.; Reguilon, I.; Butlerb, J.; McKee, M.; Nolte, E. Understanding the link between health systems and cancer survival: A novel T methodological approach using a system-level conceptual model. J. Cancer Policy 2020, 25, 100233. [Google Scholar] [CrossRef]
- Murage, P.; Bachmann, M.O.; Crawford, S.M.; McPhail, S.; Jones, A. Geographical access to GPs and modes of cancer diagnosis in England: A cross-sectional study. Fam. Pract. 2019, 36, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Hiscock, R.; Pearce, J.; Blakely, T.; Witten, K. Is neighborhood access to health care provision associated with individual-level utilization and satisfaction? Health Serv. Res. 2008, 43, 2183–2200. [Google Scholar] [CrossRef] [PubMed]
- Cucchetti, A.; Gramenzi, A.; Johnson, P.; Giannini, E.G.; Tovoli, F.; Rapaccini, G.L.; Marra, F.; Cabibbo, G.; Caturelli, E.; Gasbarrini, A.; et al. Material deprivation affects the management and clinical outcome of hepatocellular carcinoma in a high-resource environment. Eur. J. Cancer 2021, 158, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Abel, G.A.; Shelton, J.; Johnson, S.; Elliss-Brookes, L.; Lyratzopoulos, G. Cancer-specific variation in emergency presentation by sex, age and deprivation across 27 common and rarer cancers. Br. J. Cancer 2015, 112, S129–S136. [Google Scholar] [CrossRef]
- Curran, C.; Stanley, A.J.; Barclay, S.T.; Priest, M.; Graham, J. The association between deprivation and the incidence and survival of patients with hepatocellular carcinoma in the West of Scotland. Expert Rev. Gastroenterol. Hepatol. 2021, 15, 1427–1433. [Google Scholar] [CrossRef] [PubMed]
- Garuti, F.; Neri, A.; Avanzato, F.; Gramenzi, A.; Rampoldi, D.; Rucci, P.; Farinati, F.; Giannini, E.G.; Piscaglia, F.; Rapaccini, G.L.; et al. The changing scenario of hepatocellular carcinoma in Italy: An update. Liver Int. Off. J. Int. Assoc. Study Liver 2021, 41, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Jembere, N.; Campitelli, M.A.; Sherman, M.; Feld, J.J.; Lou, W.; Peacock, S.; Yoshida, E.; Krahn, M.D.; Earle, C.; Thein, H.H. Influence of socioeconomic status on survival of hepatocellular carcinoma in the Ontario population; a population-based study, 1990–2009. PLoS ONE 2012, 7, e40917. [Google Scholar] [CrossRef] [PubMed]
- Sellers, C.M.; Uhlig, J.; Ludwig, J.M.; Taddei, T.; Stein, S.M.; Lim, J.K.; Kim, H.S. The impact of socioeconomic status on outcomes in hepatocellular carcinoma: Inferences from primary insurance. Cancer Med. 2019, 8, 5948–5958. [Google Scholar] [CrossRef] [PubMed]
- Richards, M.; Anderson, M.; Carter, P.; Ebert, B.L.; Mossialos, E. The impact of the COVID-19 pandemic on cancer care. Nat. Cancer 2020, 1, 565–567. [Google Scholar] [CrossRef] [PubMed]
- Angelini, M.; Teglia, F.; Astolfi, L.; Casolari, G.; Boffetta, P. Decrease of cancer diagnosis during COVID-19 pandemic: A systematic review and meta-analysis. Eur. J. Epidemiol. 2023, 38, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Pennisi, F.; Odelli, S.; Borlini, S.; Morani, F.; Signorelli, C.; Renzi, C. Impact of the Covid pandemic on timely cancer diagnosis across European healthcare settings: A scoping review. Ann. Ig. Med. Prev. Comunita 2024, 36, 194–214. [Google Scholar]
- Nardone, P.; Lauria, L.; Buoncristiano, M.; Pizzi, E.; Galeone, D.; Spinelli, A.; Gruppo OKkio alla SALUTE 2008/9-2014. Dietary behaviour of children attending primary school in Italy found by the surveillance system “OKkio alla salute”. Epidemiol. Prev. 2015, 39, 380–385. [Google Scholar] [PubMed]
- Piano Nazionale della Prevenzione 2020–2025, Ministero della Salute. Available online: https://www.salute.gov.it/imgs/C_17_pubblicazioni_2955_allegato.pdf (accessed on 31 March 2024).
- Piano Oncologico Nazionale 2023–2027. Available online: https://www.salute.gov.it/portale/tumori/dettaglioContenutiTumori.jsp?lingua=italiano&id=6012&area=tumori&menu=vuoto (accessed on 31 March 2024).
- SINTESI: SIcilian Network for Therapy, Epidemiology and Screening in Hepatology. Available online: http://www.sintesihepatology.org (accessed on 31 March 2024).
- DigitAl lifelong pRevEntion (DARE) Project. Directorial decree, Italian Ministry of University and Research Direttoriale June 6 2022, n. 931. Italian Complementary National Plan PNC-I.1 “Research Initiatives for Innovative Technologies and Pathways in the Health and Welfare Sector”. Available online: https://www.fondazionedare.it/project.html (accessed on 31 March 2024).
- Parigi, T.L.; Aghemo, A. HCV screening: Moving from theory to practice. Liver Int. Off. J. Int. Assoc. Study Liver 2020, 40, 1538–1540. [Google Scholar] [CrossRef]
- Friend, S.H.; Ginsburg, G.S.; Picard, R.W. Wearable Digital Health Technology. N. Engl. J. Med. 2023, 389, 2100–2101. [Google Scholar] [CrossRef] [PubMed]
- Mazzucco, W.; Stracci, F.; Gatta, G.; D’Argenzio, A.; Bidoli, E.; Carone, S.; Vitarelli, S.; Castelli, M.; Fruscione, S.; Vitale, F. Cancer registries and data protection in the age of health digital interoperability in Europe: The perspective of the Italian Network of Cancer Registries (AIRTUM). Front. Oncol. 2022, 12, 1052057. [Google Scholar] [CrossRef] [PubMed]
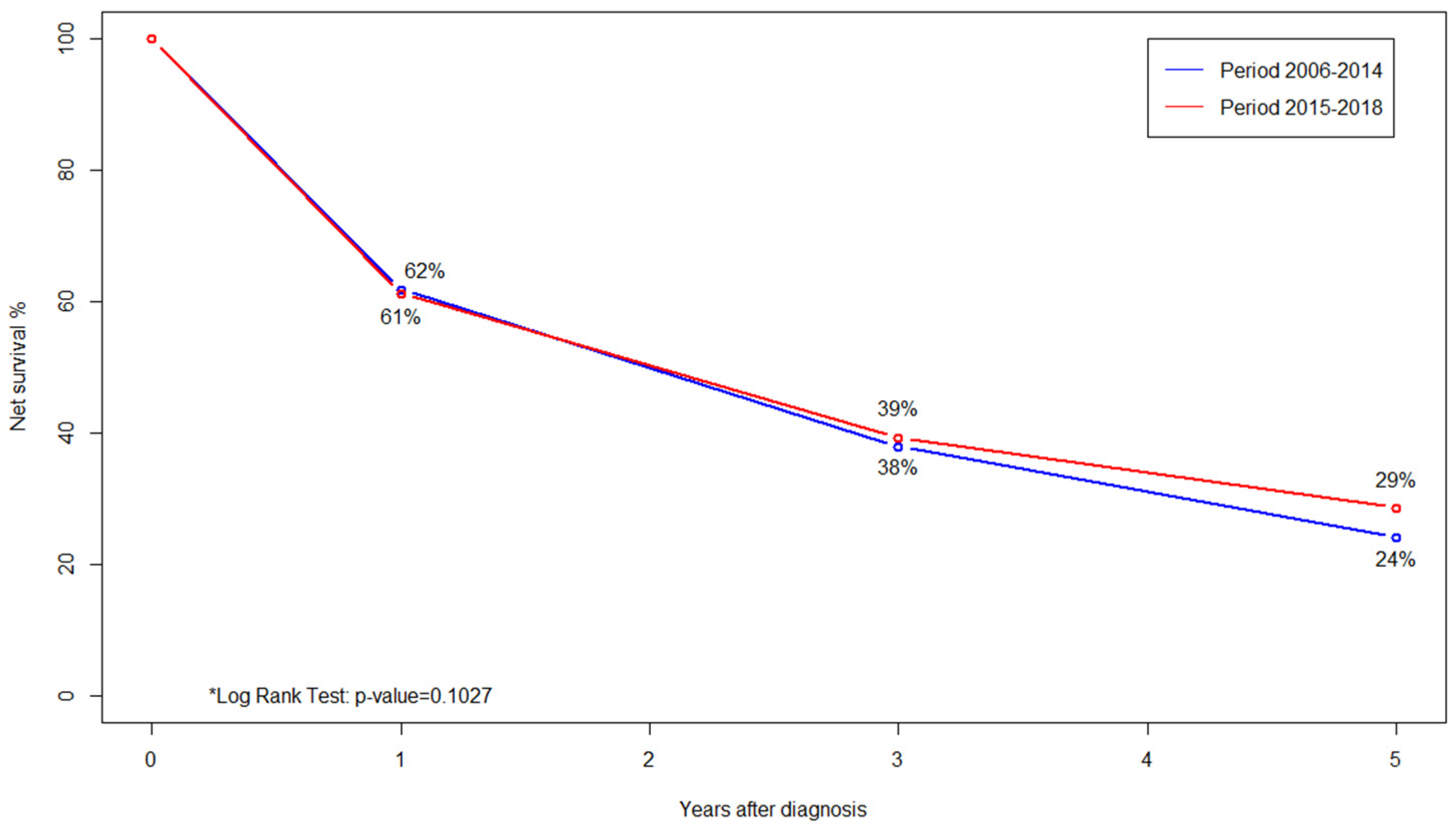
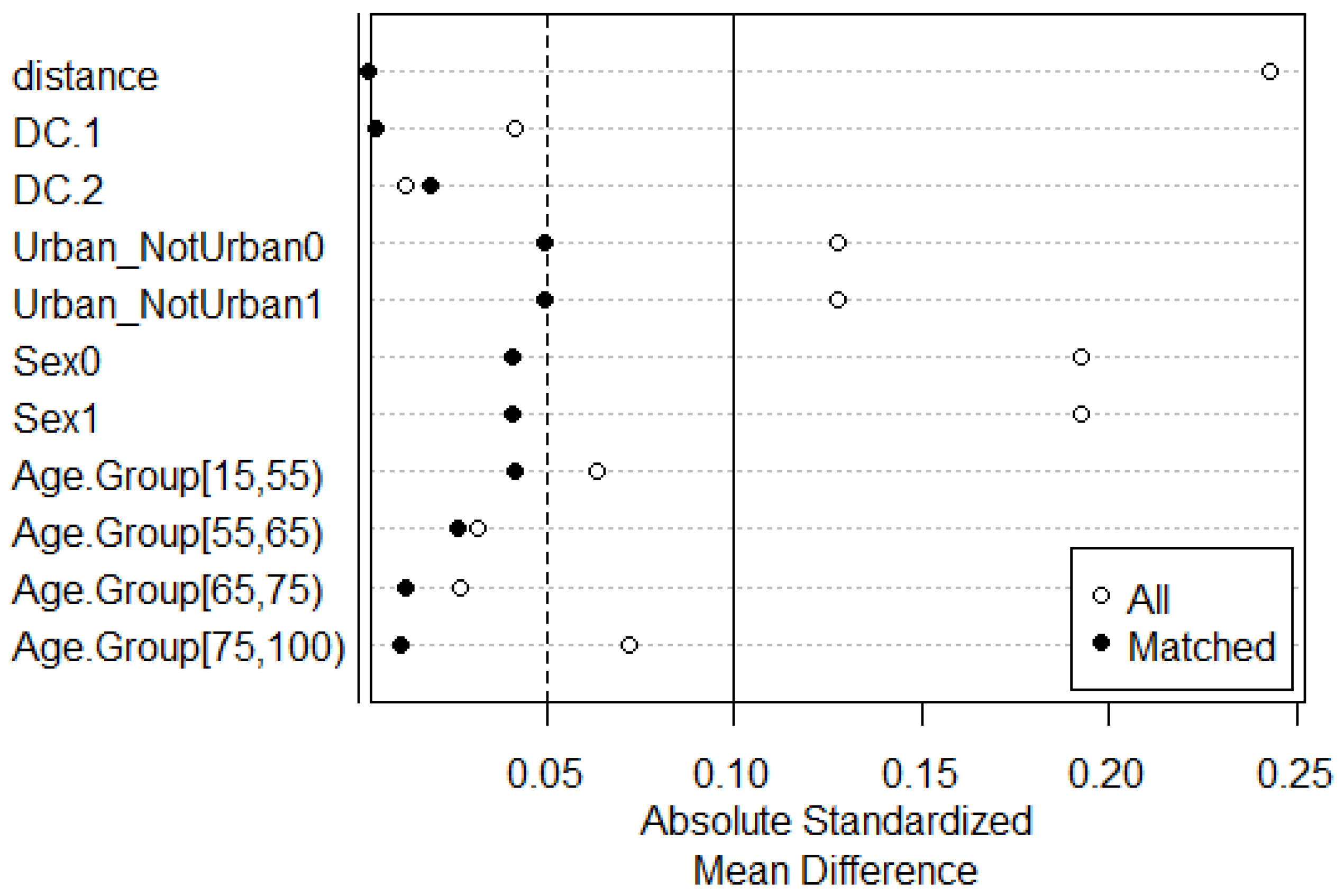
| Characteristics | Total (%) | Access to Care | |||
|---|---|---|---|---|---|
| Easy (%) | Poor (%) | Poorest * (%) | p-Value | ||
| 2687 (100) | 1744 (64.9) | 700 (26.1) | 243 (9.0) | ||
| Gender | |||||
| Male (%) | 1754 (65.3%) | 1213 (69.6) | 421 (60.0) | 120 (49.4) | <0.00001 |
| Females (%) | 933 (34.7%) | 531 (30.4) | 279 (40.0) | 123 (50.6) | |
| Age at diagnosis (in years) | |||||
| Mean | 71 | 69 | 72 | 79 | <0.0001 |
| 15–44 (%) | 43 (1.6%) | 33 (2.0) | 6 (0.9) | 4 (1.7) | <0.00001 |
| 45–54 (%) | 167 (6.2%) | 128 (7.3) | 36 (5.1) | 3 (1.2) | |
| 55–64 (%) | 446 (16.6%) | 321 (18.4) | 107 (15.3) | 18 (7.4) | |
| 65–74 (%) | 953 (35.5%) | 687 (39.3) | 228 (36.2) | 38 (15.6) | |
| ≥75 (%) | 1078 (40.1%) | 575 (33.0) | 323 (46.1) | 180 (74.1) | |
| Residence | |||||
| Urban area (%) | 1676 (62%) | 1142 (65.5) | 415 (59.0) | 119 (49.0) | <0.0001 |
| Non-urban area (%) | 1011 (38%) | 602 (34.5) | 285 (41.0) | 124 (51.0) | |
| Variables | OR | 95%CIs | p-Value |
|---|---|---|---|
| Deprivation component 1 (DC.1 > 0) | 1.41 | 1.07–1.87 | <0.05 |
| Deprivation component 2 (DC.2 > 0) | 1.12 | 0.84–1.51 | 0.41 |
| Gender (female) | 1.68 | 1.27–2.22 | <0.0001 |
| Age group (55–64) | 1.17 | 0.50–3.07 | 0.72 |
| Age group (65–74) | 1.11 | 0.52–2.76 | 0.79 |
| Age group (≥75) | 5.08 | 2.51–12.16 | <0.0001 |
| Residence (Urban versus Non-urban area) | 1.97 | 1.45–2.67 | <0.0001 |
| Variables | Before Matching | After Matching | ||||
|---|---|---|---|---|---|---|
| HR | 95%CIs | p-Value | HR | 95%CIs | p-Value | |
| Deprivation component 1 (DC.1 > 0) | 1.29 | 1.12–1.48 | <0.001 | 1.38 | 1.12–1.70 | 0.002 |
| Deprivation component 2 (DC.2 > 0) | 1.07 | 0.96–1.20 | 0.22 | 0.90 | 0.76–1.06 | 0.20 |
| Access to care (poor to care) | 2.08 | 1.84–2.36 | <0.0001 | 2.25 | 1.91–2.66 | <0.0001 |
| Gender (female) | 1.03 | 0.93–1.15 | 0.57 | 0.96 | 0.82–1.11 | 0.56 |
| Age group (65–75) | 1.29 | 1.06–1.57 | <0.05 | 1.17 | 0.87–1.58 | 0.29 |
| Age group (≥75) | 1.83 | 1.50–2.23 | <0.0001 | 1.79 | 1.33–2.42 | <0.0001 |
| Municipality of residence (Non-urban area) | 1.32 | 1.13–1.54 | <0.001 | 1.47 | 1.17–1.84 | 0.0008 |
| Interaction between DC.1 > 0 and residence in a Non-urban area | 0.81 | 0.65–1.01 | 0.06 | 0.69 | 0.50–0.95 | 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazzola, S.; Vittorietti, M.; Fruscione, S.; De Bella, D.D.; Savatteri, A.; Belluzzo, M.; Ginevra, D.; Gioia, A.; Costanza, D.; Castellone, M.D.; et al. Factors Associated with Primary Liver Cancer Survival in a Southern Italian Setting in a Changing Epidemiological Scenario. Cancers 2024, 16, 2046. https://doi.org/10.3390/cancers16112046
Mazzola S, Vittorietti M, Fruscione S, De Bella DD, Savatteri A, Belluzzo M, Ginevra D, Gioia A, Costanza D, Castellone MD, et al. Factors Associated with Primary Liver Cancer Survival in a Southern Italian Setting in a Changing Epidemiological Scenario. Cancers. 2024; 16(11):2046. https://doi.org/10.3390/cancers16112046
Chicago/Turabian StyleMazzola, Sergio, Martina Vittorietti, Santo Fruscione, Daniele Domenico De Bella, Alessandra Savatteri, Miriam Belluzzo, Daniela Ginevra, Alice Gioia, Davide Costanza, Maria Domenica Castellone, and et al. 2024. "Factors Associated with Primary Liver Cancer Survival in a Southern Italian Setting in a Changing Epidemiological Scenario" Cancers 16, no. 11: 2046. https://doi.org/10.3390/cancers16112046








