Anaerobic Treatment of Food Waste with Biogas Recirculation under Psychrophilic Temperature
Abstract
:1. Introduction
2. Experimental Materials and Methodology
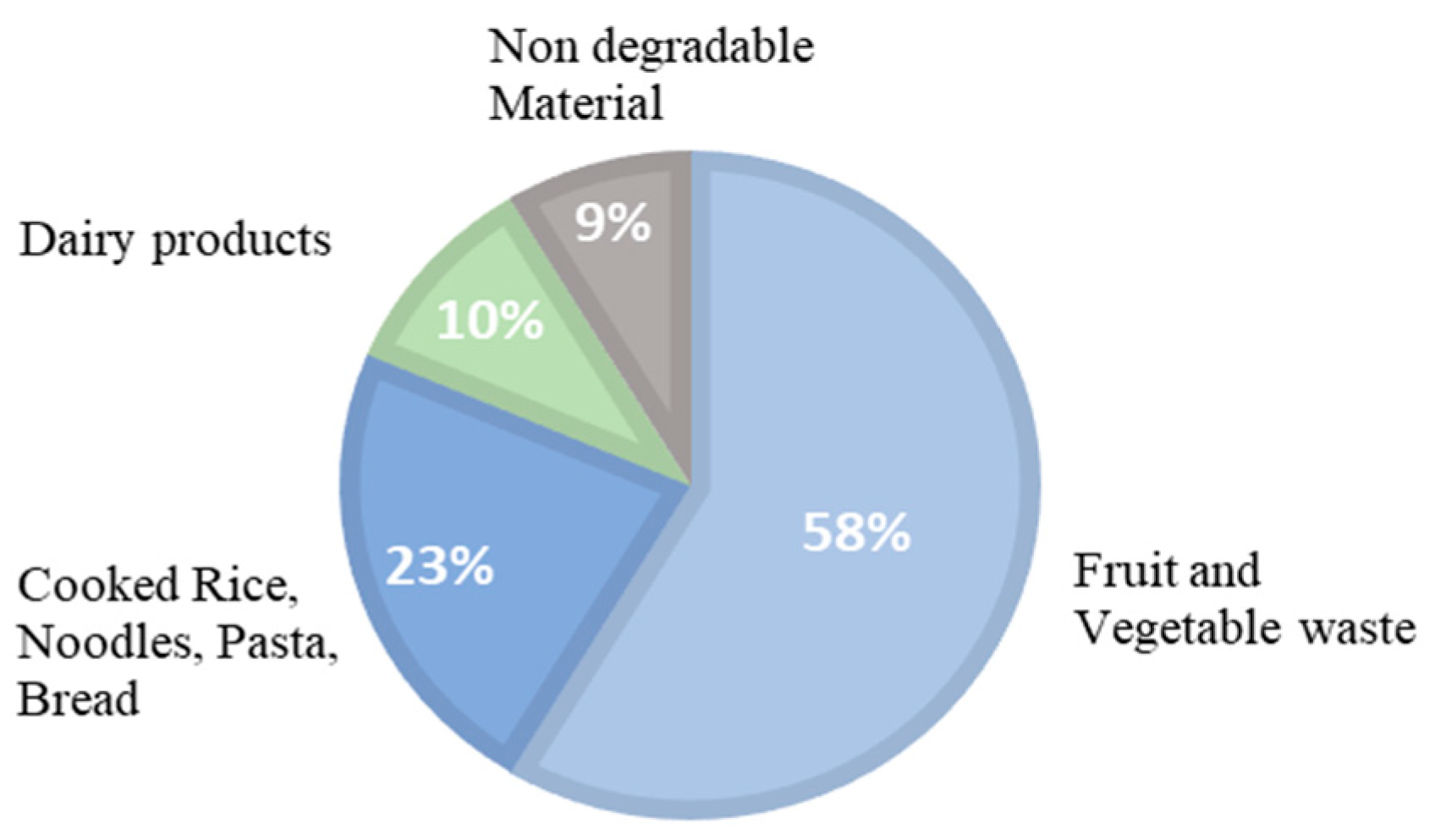
2.1. Characteristics of Food Waste and Inoculum
Inoculum
2.2. Experimental Setup and Operation
2.3. Biogas Withdrawal and Analysis
2.4. Analytical Parameters
- Chemical Oxygen Demand (COD) Measurement
- Alkalinity Analysis
- Total Nitrogen (TN) Measurement
- Volatile Fatty Acids (VFAs) Measurement
- Microbial Parameters (TS, VS) Analysis
- TS: total solid concentration; VS: volatile solid concentration
- Wd: dried sample weight, g
- Ws: fresh sample weight, g
- Wc: blank crucible weight, g
- Wash: ash weight, g.
3. Results and Discussion
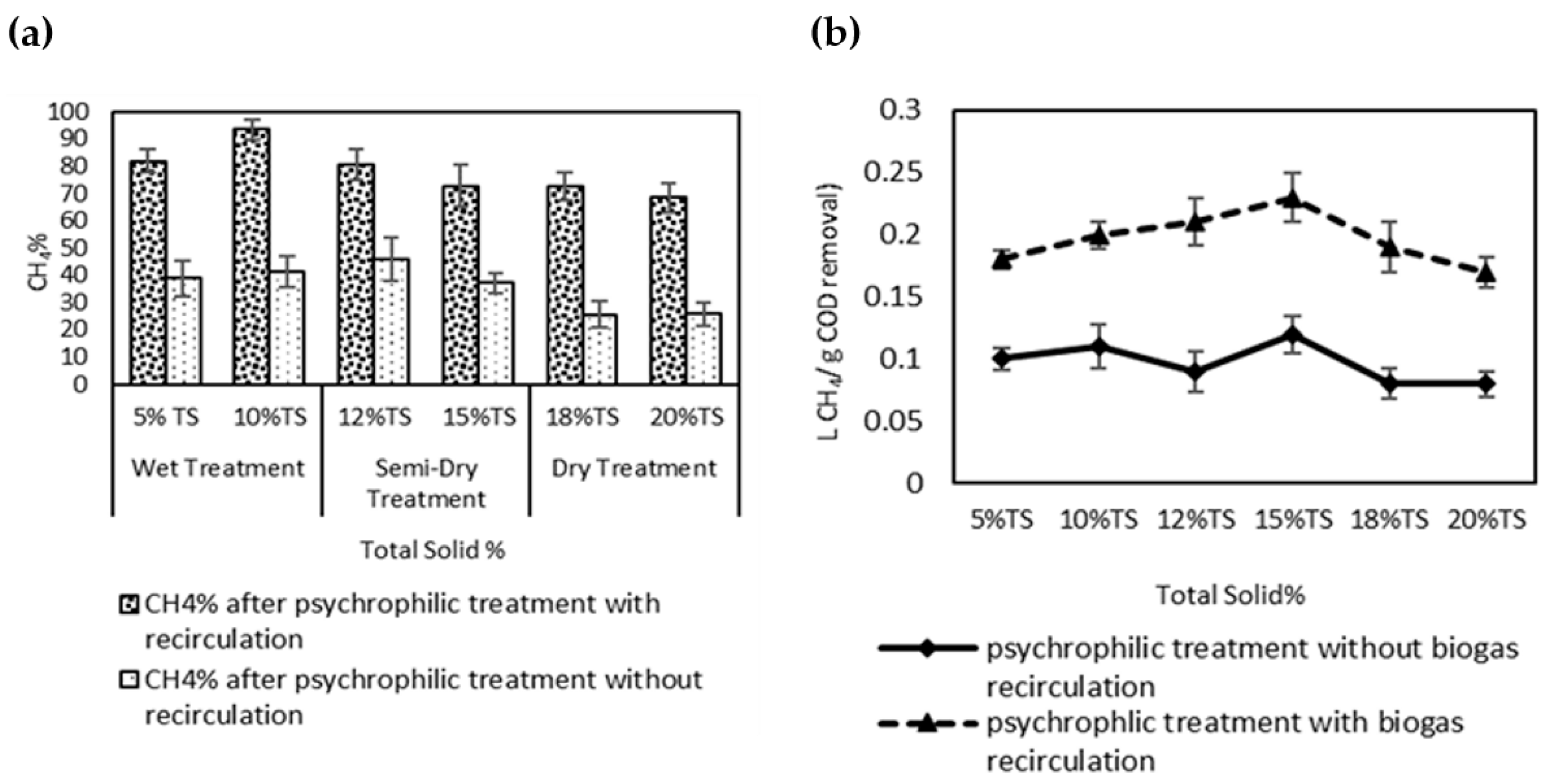
3.1. Biogas Composition during Psychrophilic Anaerobic Treatment with or without Recirculation
3.2. Methane Concentration and Methane Yield at Different Total Solid Concentrations
3.3. Removal Efficiency of VS and COD at Different Total Solid Contents after Anaerobic Treatment of Food Waste
3.4. Temperature Profile during Anaerobic Treatment of Food Waste
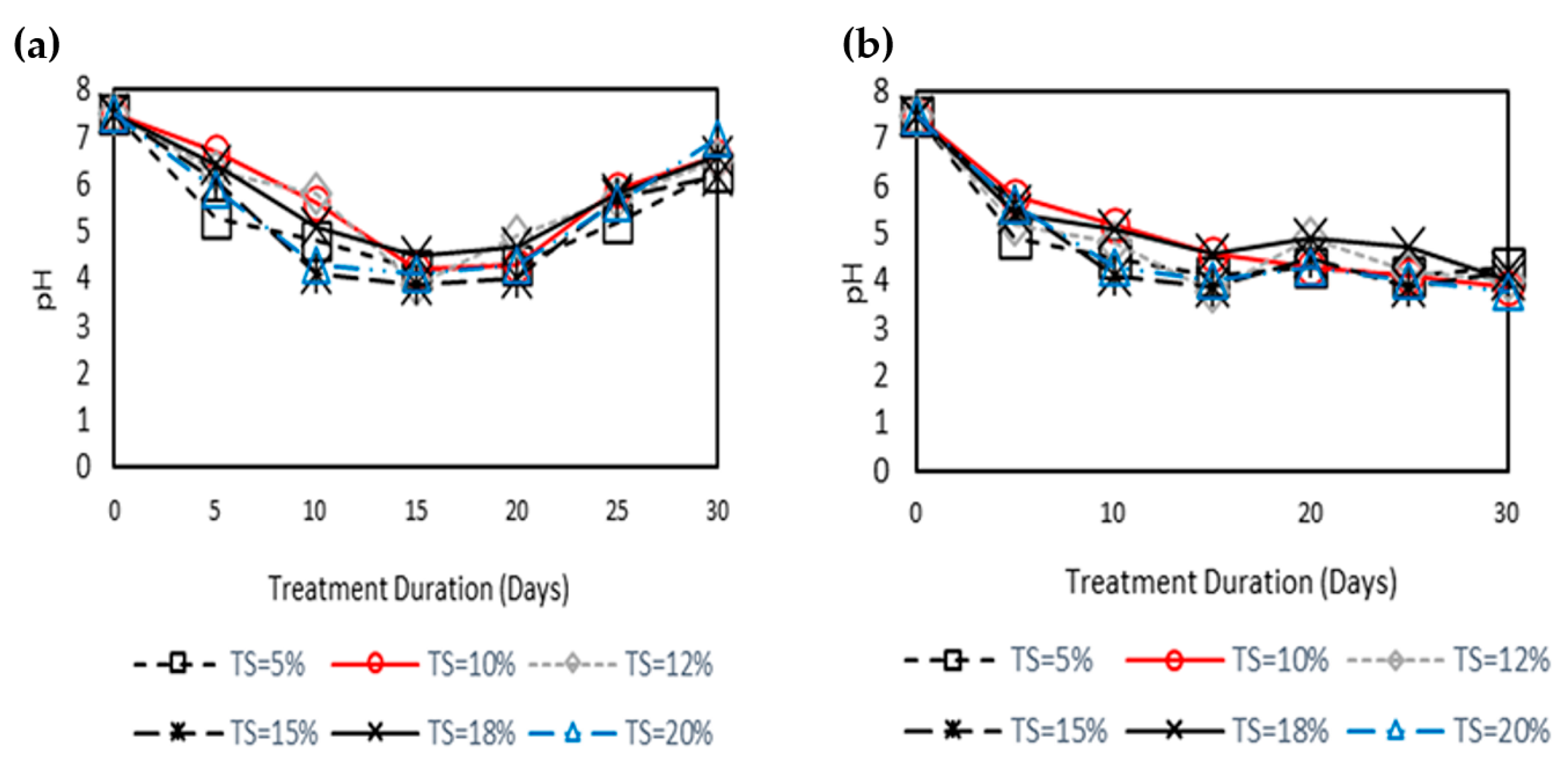
3.5. pH Profile during Psychrophilic Treatment with or without Recirculation Technique
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ma, Y.; Liu, Y. Turning food waste to energy and resources towards a great environmental and economic sustainability: An innovative integrated biological approach. Biotechnol. Adv. Manag. 2019, 34, 2627–2633. [Google Scholar] [CrossRef]
- Slorach, P.C.; Jeswani, H.K.; Cuéllar-Franca, R.; Azapagic, A. Environmental sustainability of anaerobic digestion of household food waste. J. Environ. Manag. 2019, 236, 798814. [Google Scholar] [CrossRef]
- Lin, C.S.K.; Pfaltzgraff, L.A.; Herrero-Davila, L.; Mubofu, E.B.; Abderrahim, S.; Clark, J.H.; Luque, R. Food waste as a valuable resource for the production of chemicals, materials, and fuels. Current situation and global perspective. Energy Environ. Sci. 2013, 6, 426–464. [Google Scholar] [CrossRef]
- Zamanzadeh, M.; Hagen, L.H.; Svensson, K.; Linjordet, R.; Horn, S.J. Biogas production from food waste via co-digestion and digestion-effects on performance and microbial ecology. Sci. Rep. 2017, 7, 17664. [Google Scholar] [CrossRef]
- U.S. EPA. Municipal Solid Waste Generation, Recycling, and Disposal in the United States: 2014. Available online: https://www.epa.gov/sites/default/files/2015-09/documents/2012_msw_fs.pdf (accessed on 22 January 2024).
- Clercq, D.D.; Wen, Z.; Gottfried, O.; Schmidt, F.; Fei, F. A review of global strategies promoting the conversion of food waste to bioenergy via anaerobic digestion. Renew. Sustain. Energy Rev. 2017, 79, 204–221. [Google Scholar] [CrossRef]
- Papargyropoulou, E.; Lozano, R.; Steinberger, K.; Wright, J.; Ujang, N.; Bin, Z. The food waste hierarchy as a framework for the management of food surplus and food waste. J. Clean. Prod. 2014, 76, 106–115. [Google Scholar] [CrossRef]
- Gokarn, S.; Kuthambalayan, T.S. Analysis of challenges inhibiting the reduction of waste in food supply chain. J. Clean. Prod. 2017, 168, 595–604. [Google Scholar] [CrossRef]
- Ariunbaatar, J.; Panico, A.; Esposito, G.; Pirozzi, F.; Lens, P.N.L. Pre-treatment methods to enhance anaerobic digestion of organic solid waste. Appl. Energy 2014, 123, 143–156. [Google Scholar] [CrossRef]
- Morales-polo, C.; Cledera, M.; Moratilla Soria, B.Y. Reviewing the Anaerobic Digestion of Food Waste: From Waste Generation and Anaerobic Process to Its Perspectives. Appl. Sci. 2018, 8, 1804. [Google Scholar] [CrossRef]
- Posmanik, R.; Labatut, R.A.; Kim, A.H.; Usack, J.G.; Tester, J.W.; Angenent, L.T. Coupling hydrothermal liquefaction and anaerobic digestion for energy valorization from model biomass feedstocks. Bioresour. Technol. 2017, 233, 134143. [Google Scholar] [CrossRef]
- Bachmaier, J.; Effenberger, M.; Gronauer, A. Greenhouse gas balance and resource demand of biogas plants in agriculture. Eng. Life Sci. 2010, 10, 560–569. [Google Scholar] [CrossRef]
- Ratanatamskul, C.; Onnum, G.; Yamamoto, K. A prototype single-stage anaerobic digester for co-digestion of food waste and sewage sludge from high rise building for on-site biogas production. Int. Biodeterior. Biodegrad. 2014, 95, 176–180. [Google Scholar] [CrossRef]
- Cirne, D.G.; Paloumet, X.; Björnsson, L.; Alves, M.M.; Mattiasson, B. Anaerobic digestion of lipid-rich waste—Effects of lipid concentration. Renew. Energy 2007, 32, 965–975. [Google Scholar] [CrossRef]
- Moriarty, K. Feasibility Study of Anaerobic Digestion of Food Waste in St. Bernard, Louisiana: A study prepared in partnership with the Environmental Protection Agency for the RE-Powering America’s Land Initiative: Siting Renewable Energy on potentially Contaminated land and Mine Sites. 2013. Available online: https://www.nrel.gov/docs/fy13osti/57082.pdf (accessed on 22 January 2024).
- Mulligan, C.N. Environmental Biotreatment Technologies for Air, Water, Soil, and Wastes; ABS Consulting, Government Institutes: Rockville, MD, USA, 2002. [Google Scholar]
- Rivas-García, P.; Botello-Álvarez, J.; Miramontes-Martínez, L.; Cano-Gómez, J.; Rico Martínez, R. New model of hydro lysis in the anaerobic co-digestion of bovine manure with vegetable waste: Modification of anaerobic digestion model No. 1. Rev. Mex. Ing. Química 2020, 19, 109–122. [Google Scholar] [CrossRef]
- Zhang, C.; Su, H.; Baeyens, J.; Tan, T. Reviewing the anaerobic digestion of food waste for biogas production. Renew. Sustain. Energy Rev. 2014, 38, 383–392. [Google Scholar] [CrossRef]
- Dhaked, R.K.; Singh, P.; Singh, L. Bio methanation under psychrophilic conditions. Waste Manag. 2010, 30, 2490–2496. [Google Scholar] [CrossRef]
- Lettinga, G.; Rebac, S.; Zeeman, G. Challenge of psychrophilic anaerobic wastewater treatment. Trends Biotechnol. 2001, 19, 363–370. [Google Scholar] [CrossRef]
- Rusín, J.; Chamrádová, K.; Basinas, P. Two-stage psychrophilic anaerobic digestion of food waste: Comparison to conventional single-stage mesophilic process. Waste Manag. J. 2021, 119, 172–182. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association, American Water Works Association, Water Environment Federation: Washington, DC, USA, 2005. [Google Scholar]
- Hach Company. Hach Procedure Manual; Hach Company: Loveland, CO, USA, 2009. [Google Scholar]
- Clesceri, L.S.; Greenberg, A.E.; Eaton, A.D. (Eds.) Standard Methods for the Examination of Water and Wastewater; United Book Press: Baltimore, MD, USA, 1998. [Google Scholar]
- U.S. EPA. Wastewater Technology Fact Sheet. Chemical Precipitation; Office of Water: Washington, DC, USA, 2000.
- Environmental and Energy Study Institute (EESI). Biogas: Converting Waste to Energy. 2017. Available online: http://www.eesi.org/papers (accessed on 1 October 2017).
- Costa, C.; Rodríguez, J.; Márquez, M. A simplified dynamic model for the activated sludge process with high strength wastewaters. Environ. Model Assess 2009, 14, 739–747. [Google Scholar] [CrossRef]
- Michaud, S.; Bernet, N.; Buere, P.; Roustan, M.; Moletta, R. Methane yield as a monitoring parameter for the start-up of anaerobic fixed film reactors. Water Res. 2002, 36, 1385–1391. [Google Scholar] [CrossRef]
- Tiwari, B.R.; Rouissi, T.; Brar, S.K.; Surampalli, R.Y. Critical insights into psychrophilic anaerobic digestion: Novel strategies for improving biogas production. Waste Manag. 2021, 131, 513–526. [Google Scholar] [CrossRef]
- Kayhanian, M. Biodegradability of the organic fraction of municipal solid waste in a high-solids anaerobic digester. Waste Manag. Res. 1995, 13, 123–136. [Google Scholar] [CrossRef]
- Minale, M.; Worku, T. Anaerobic co-digestion of sanitary wastewater and kitchen solid waste for biogas and fertilizer production under ambient temperature: Waste generated from condominium house. Int. J. Environ. Sci. Technol. 2014, 11, 509–516. [Google Scholar] [CrossRef]
- Rajagopal, R.; Bellavance, D.; Rahman, S. Psychrophilic anaerobic digestion of semi-dry mixed municipal food waste: ForNorth American context. Process Saf. Environ. Prot. 2017, 105, 101–108. [Google Scholar] [CrossRef]
- Zupančič, G.D.; Grilc, V. Anaerobic Treatment and Biogas Production from Organic Waste, Management of Organic Waste; Kumar, S., Ed.; InTech: Berlin, Germany, 2012; ISBN 978-953-307-925-7. [Google Scholar]
- Maurus, K.; Kremmeter, N.; Ahmed, S.; Kazda, M. Biomass Conversion and Biorefinery High-resolution monitoring of VFA dynamics reveals process failure and exponential decrease of biogas production. Biomass Convers. Biorefinery 2023, 13, 10653–10663. [Google Scholar] [CrossRef]
- Amani, T.; Nosrati, M.; Sreekrishnan, T.R. Anaerobic digestion from the viewpoint of microbiological, chemical, and operational aspects—A review. Environ. Rev. 2010, 18, 225–278. [Google Scholar] [CrossRef]
- Wheatley, A. Anaerobic Digestion: A Waste Treatment Technology for Developing Countries, 2nd ed.; Elsevier Science Publisher Ltd.: London, UK, 1991. [Google Scholar]
- Kondusamy, D.; Kalamdhad, A.S. Pre-treatment and anaerobic digestion of food waste for high-rate methane production—A review. J. Environ. Chem. Eng. 2012, 2, 1821–1830. [Google Scholar] [CrossRef]
- Anderson, G.K.; Yang, G. pH control in anaerobic treatment of industrial wastewater. J. Environ. Eng. 1992, 118, 551–567. [Google Scholar] [CrossRef]
- Rao, M.S.; Singh, S.P.; Singh, A.K.; Sodha, M.S. Bioenergy conversion studies of the organic fraction of MSW: Assessment of ultimate bioenergy production potential of municipal garbage. Appl. Energy 2000, 66, 75–78. [Google Scholar] [CrossRef]
- Gunaseelan, V.N. Biochemical methane potential of fruit and vegetable solid waste feedstocks. Biomass Bioenergy 2004, 26, 389–399. [Google Scholar] [CrossRef]



| Attribute | Value |
|---|---|
| Total Solid% | 28.2 ± 1.3 |
| Volatile Solid% | 27.13 ± 0.94 |
| pH | 5.93 ± 0.24 |
| VFA, Volatile Fatty Acid (g/L) | 9.73 ± 0.65 |
| (VS/TS) % | 97.8 ± 0.39 |
| Total Nitrogen (mg/L) | 1026 ± 6.5 |
| Moisture Content (%) | 72.3 ± 1.4 |
| Total Carbon (%TS) | 53.35 ± 0.77 |
| Alkalinity (g/L CaCO3) | 1.231 ± 0.0164 |
| C/N ratio | 26.07 ± 0.66 |
| Total COD (TCOD) (mg/L) | 180,362.5 ± 962.5 |
| Soluble COD (SCOD) (mg/L) | 123,203 ± 6253 |
| Parameter | Value |
|---|---|
| Total Solid% | 0.75 ± 0.25 |
| Volatile Solid% | 98 ± 1.1 |
| pH | 6.1 ± 0.2 |
| VFA (Volatile Fatty Acids) (g/L) | 0.46 ± 0.04 |
| TN (Total Nitrogen) (mg/L) | 340 ± 2.5 |
| TCOD (Total COD) (mg/L) | 5250 ± 250 |
| Moisture Content (%) | 99.25 ± 0.25 |
| Alkalinity (g/L CaCO3) | 0.861 ± 0.0164 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torsha, T.; Mulligan, C.N. Anaerobic Treatment of Food Waste with Biogas Recirculation under Psychrophilic Temperature. Waste 2024, 2, 58-71. https://doi.org/10.3390/waste2010003
Torsha T, Mulligan CN. Anaerobic Treatment of Food Waste with Biogas Recirculation under Psychrophilic Temperature. Waste. 2024; 2(1):58-71. https://doi.org/10.3390/waste2010003
Chicago/Turabian StyleTorsha, Tafannum, and Catherine N. Mulligan. 2024. "Anaerobic Treatment of Food Waste with Biogas Recirculation under Psychrophilic Temperature" Waste 2, no. 1: 58-71. https://doi.org/10.3390/waste2010003
APA StyleTorsha, T., & Mulligan, C. N. (2024). Anaerobic Treatment of Food Waste with Biogas Recirculation under Psychrophilic Temperature. Waste, 2(1), 58-71. https://doi.org/10.3390/waste2010003








