A Two-Step Leaching Process Using Thiourea for the Recovery of Precious Metals from Waste Printed Circuit Boards
Abstract
:1. Introduction
2. Materials and Methods
2.1. Initial WPCB Powder
2.2. The First Stage of Leaching
2.3. The Second Stage of Leaching
2.4. Kinetics of Leaching
2.4.1. First Stage of Leaching
2.4.2. Second Stage of Leaching
2.5. Characterization of Residue after the Leaching Process
3. Results and Discussion
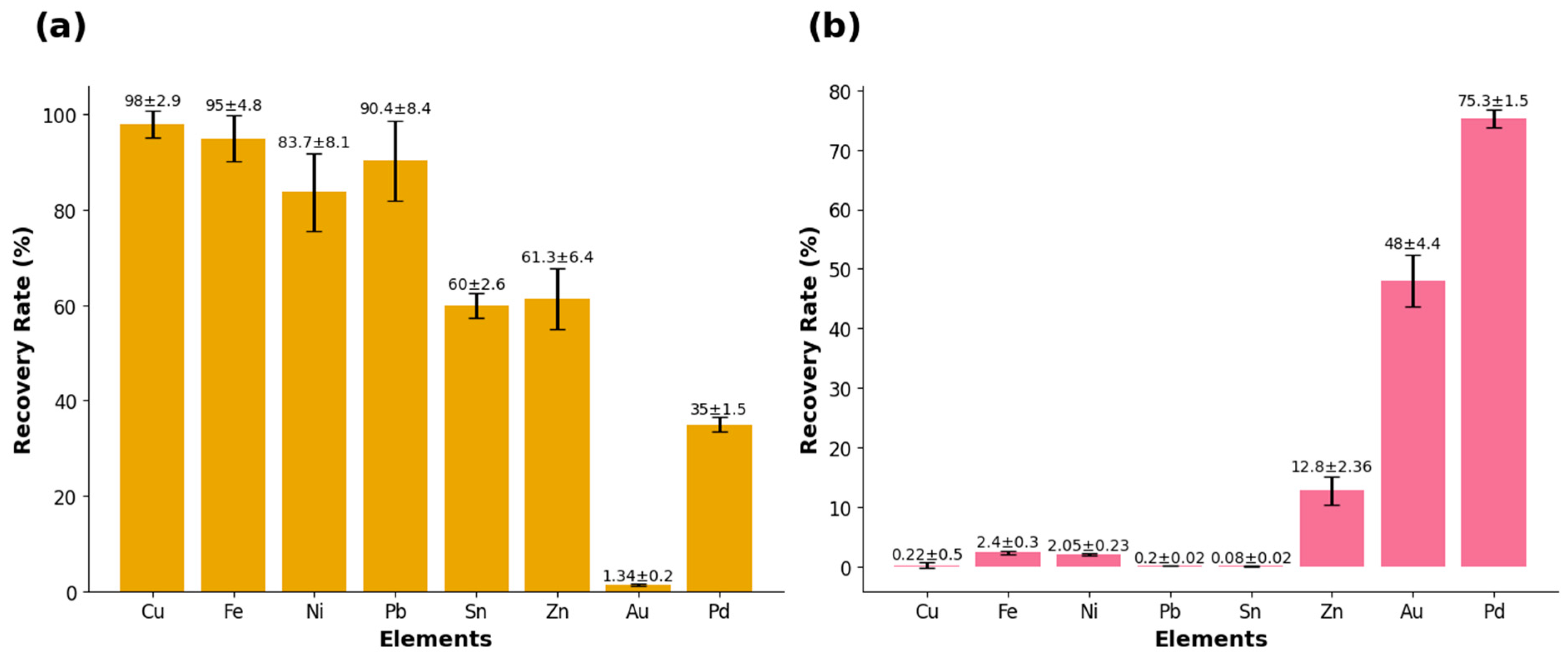
3.1. The First Stage of Leaching
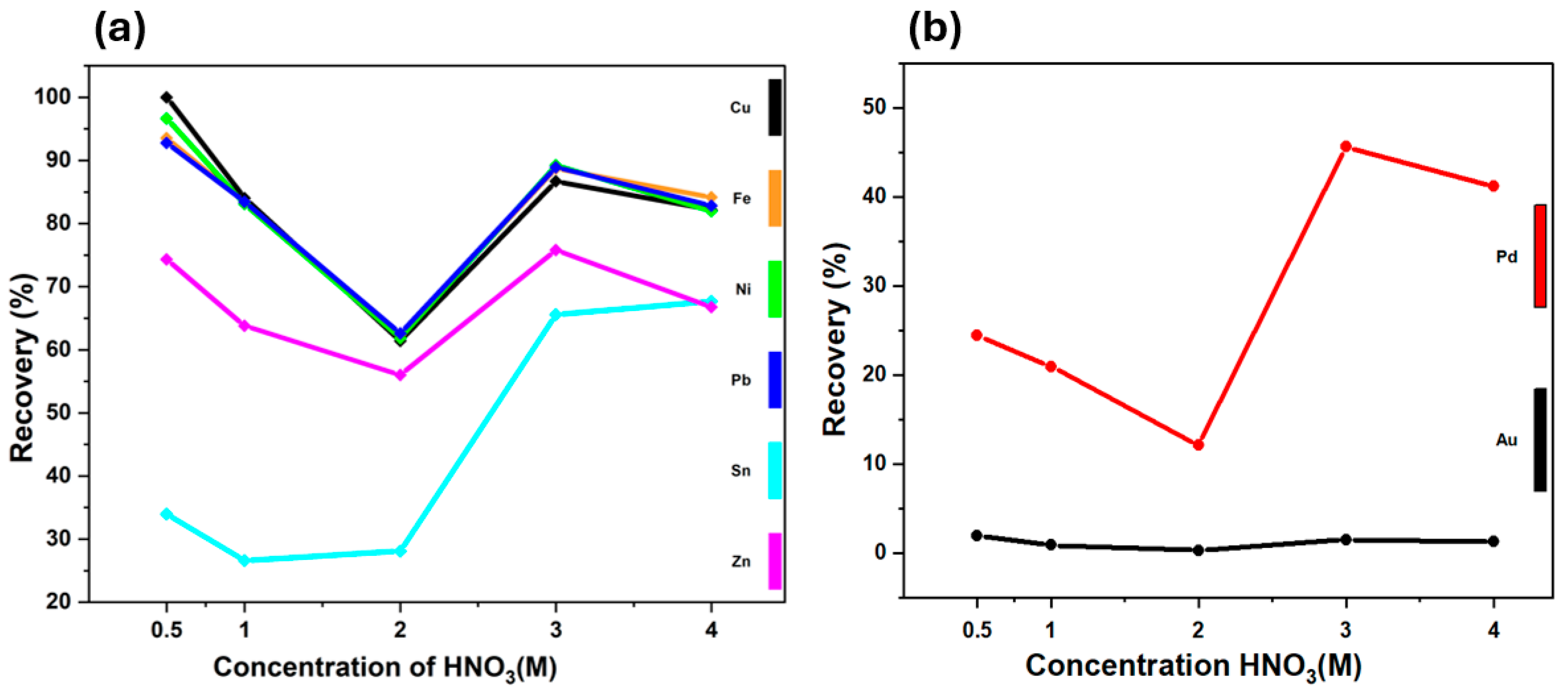
3.1.1. Effects of Acid Concentration
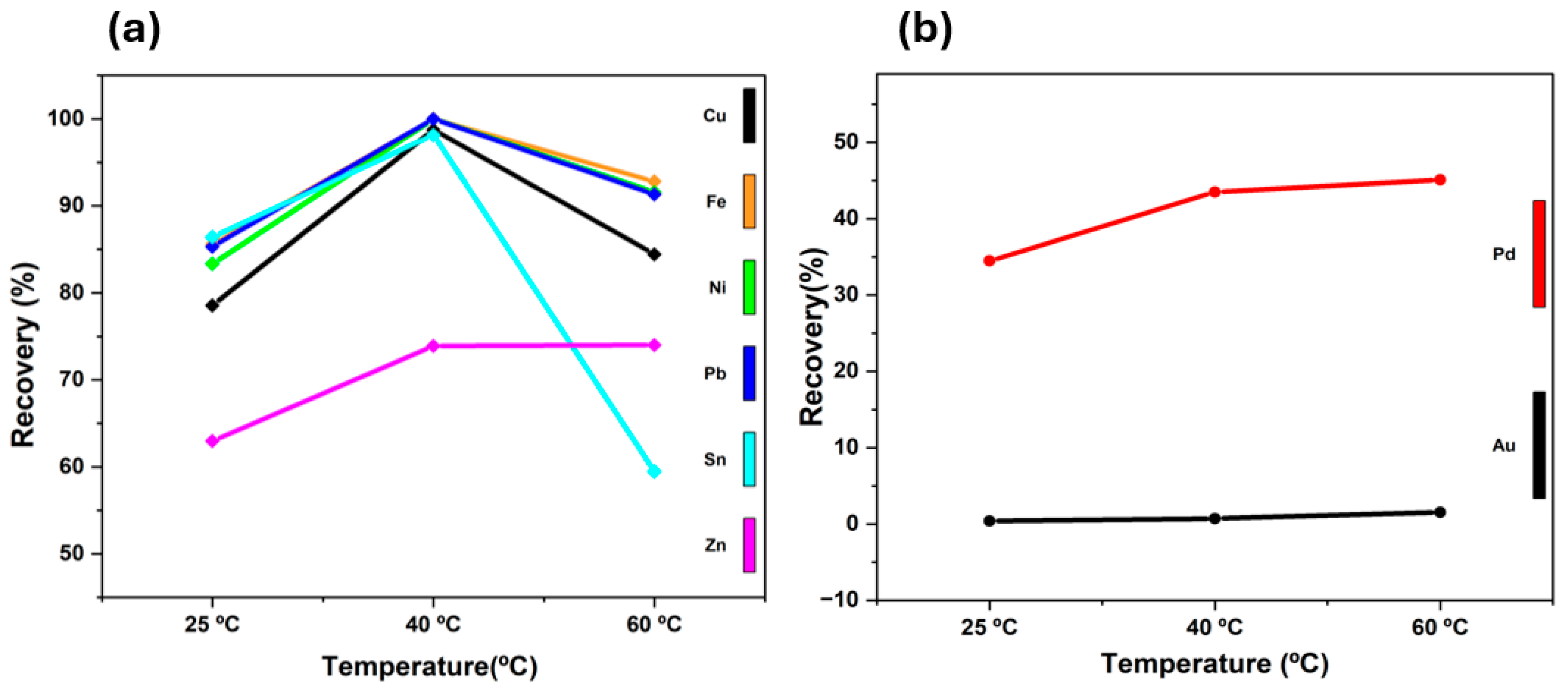
3.1.2. Effect of Temperature
3.2. The Second Stage of Leaching
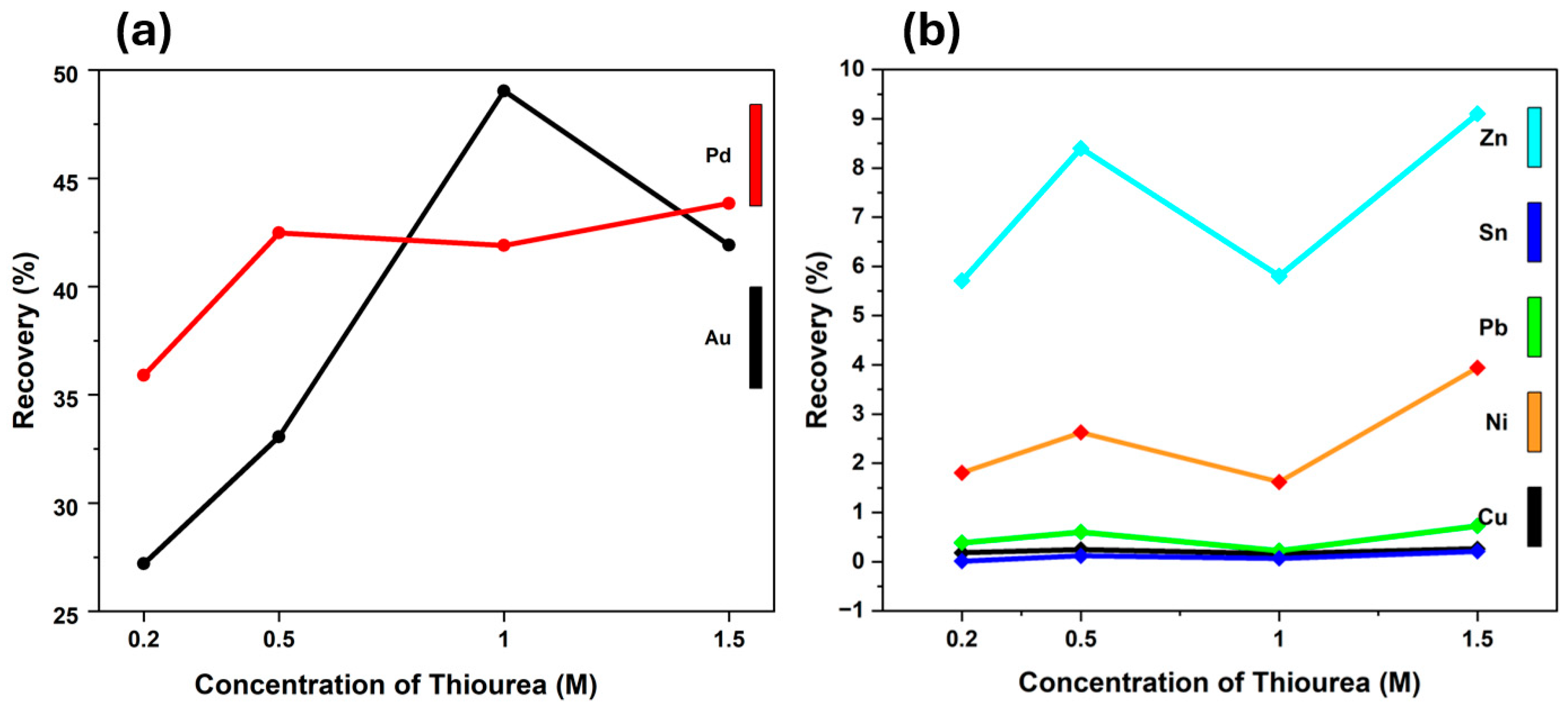
3.2.1. Thiourea Concentration
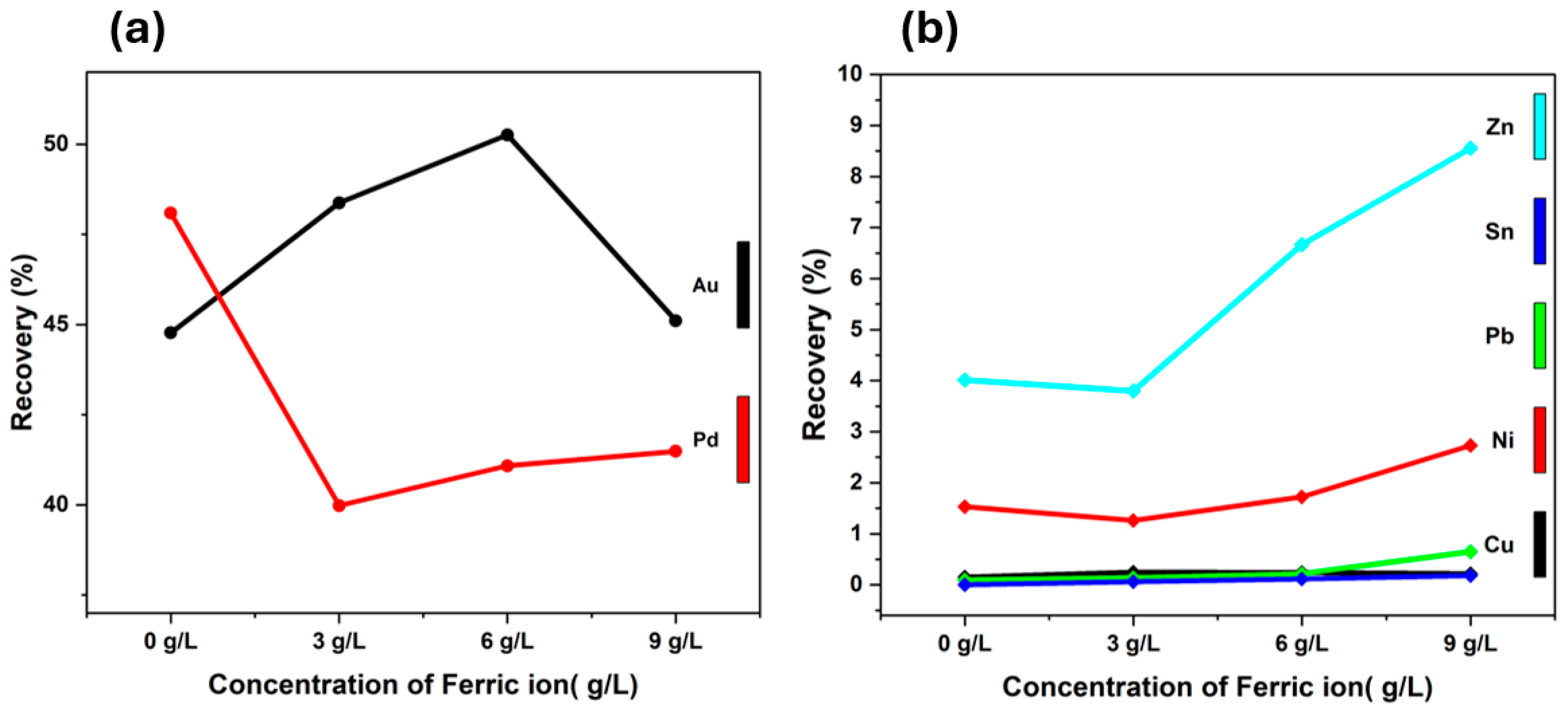
3.2.2. Ferric Ion Concentration
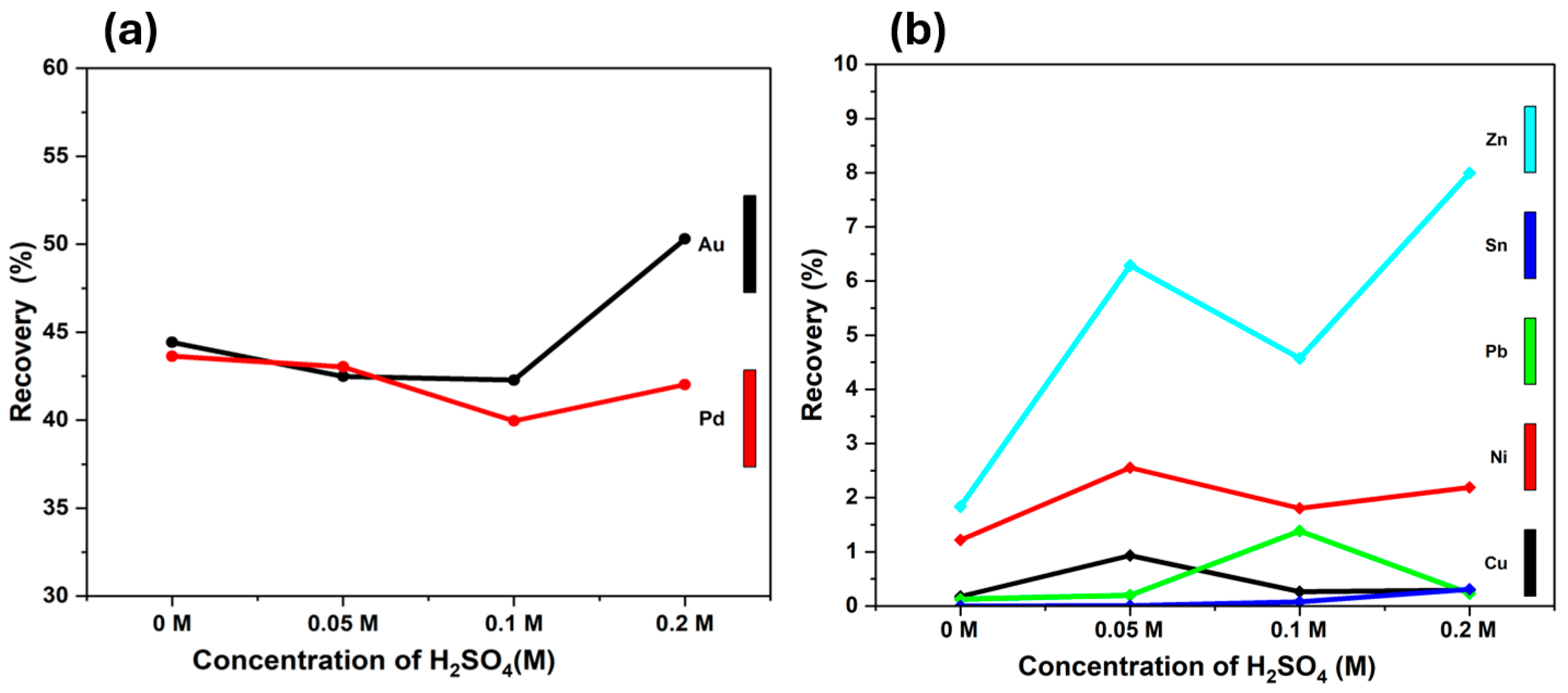
3.2.3. Sulfuric Acid Concentration
3.2.4. Effect of Temperature
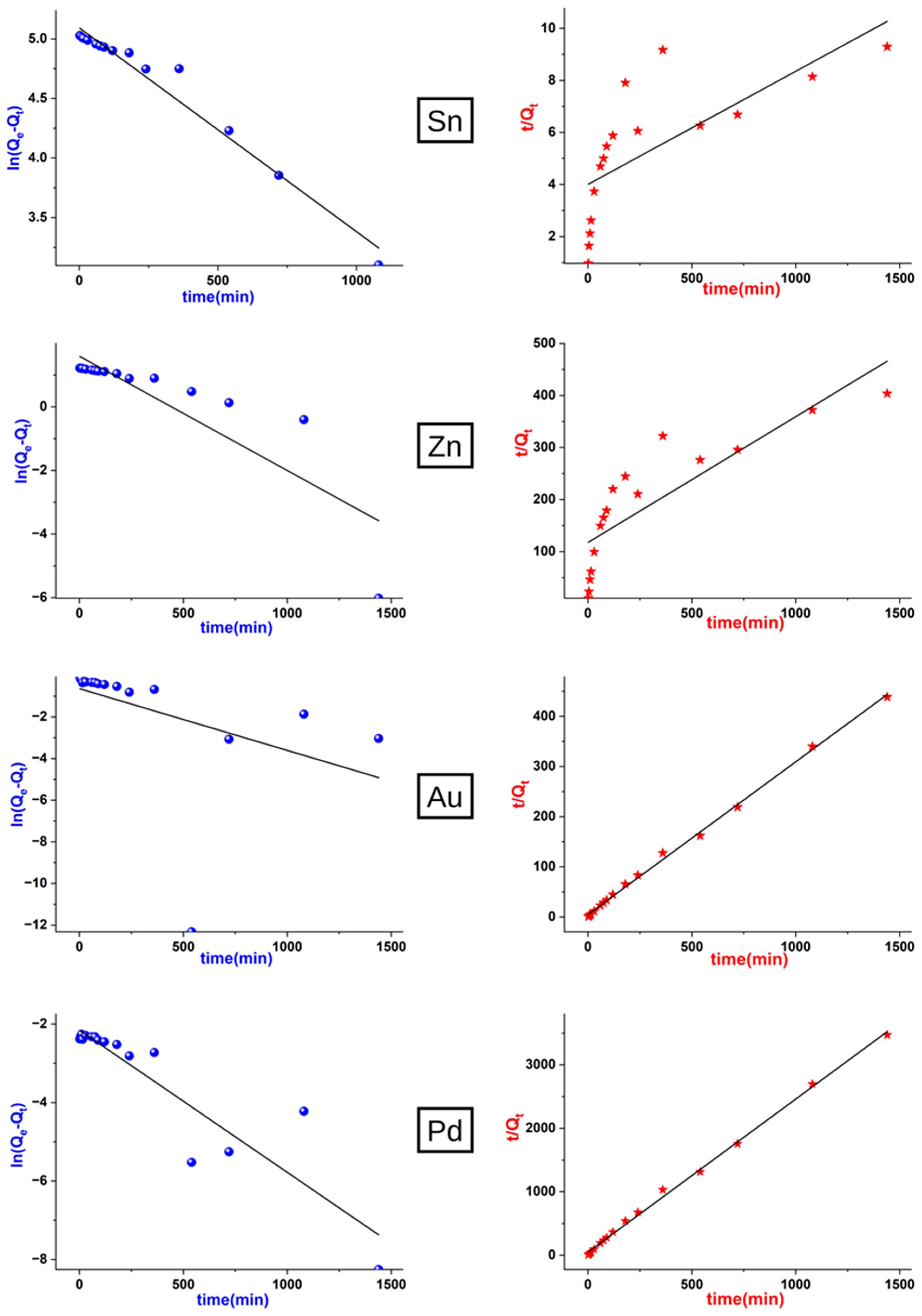
3.3. Kinetics of Leaching Processes
3.4. Morphological Analysis
3.5. Economic Consideration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Batnasan, A.; Haga, K.; Shibayama, A. Recovery of Precious and Base Metals from Waste Printed Circuit Boards Using a Sequential Leaching Procedure. JOM 2018, 70, 124–128. [Google Scholar] [CrossRef]
- Lu, Y.; Xu, Z. Precious metals recovery from waste printed circuit boards: A review for current status and perspective. Resour. Conserv. Recycl. 2016, 113, 28–39. [Google Scholar] [CrossRef]
- Faraji, F.; Golmohammadzadeh, R.; Pickles, C.A. Potential and current practices of recycling waste printed circuit boards: A review of the recent progress in pyrometallurgy. J. Environ. Manag. 2022, 316, 115242. [Google Scholar] [CrossRef] [PubMed]
- Batnasan, A.; Haga, K.; Shibayama, A. Recovery of Valuable Metals from Waste Printed Circuit Boards by Using Iodine-Iodide Leaching and Precipitation; Kim, H., Wesstrom, B., Alam, S., Ouchi, T., Azimi, G., Neelameggham, N.R., Wang, S., Guan, X., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2018; pp. 131–142. [Google Scholar] [CrossRef]
- Huang, K.; Guo, J.; Xu, Z. Recycling of waste printed circuit boards: A review of current technologies and treatment status in China. J. Hazard. Mater. 2009, 164, 399–408. [Google Scholar] [CrossRef]
- Zhang, J.; Shen, S.; Cheng, Y.; Lan, H.; Hu, X.; Wang, F. Dual lixiviant leaching process for extraction and recovery of gold from ores at room temperature. Hydrometallurgy 2014, 144–145, 114–123. [Google Scholar] [CrossRef]
- Cui, J.; Zhang, L. Metallurgical recovery of metals from electronic waste: A review. J. Hazard. Mater. 2008, 158, 228–256. [Google Scholar] [CrossRef]
- Jha, R.; Mishra, G.; Agrawal, M.; Dhanunjaya Rao, M.; Meshram, A.; Singh, K.K. Opportunities for an en-route to polymer inclusion membrane approach from conventional hydrometallurgical recycling of WPCBs: A mini-review. Can. Metall. Q. 2023, 62, 810–824. [Google Scholar] [CrossRef]
- Kavitha, N.; Palanivelu, K. Recovery of copper (II) through polymer inclusion membrane with di (2-ethylhexyl) phosphoric acid as carrier from e-waste. J. Memb. Sci. 2012, 415–416, 663–669. [Google Scholar] [CrossRef]
- Ray, D.A.; Baniasadi, M.; Graves, J.E.; Greenwood, A.; Farnaud, S. Thiourea Leaching: An Update on a Sustainable Approach for Gold Recovery from E-waste. J. Sustain. Metall. 2022, 8, 597–612. [Google Scholar] [CrossRef]
- Ghosh, B.; Ghosh, M.K.; Parhi, P.; Mukherjee, P.S.; Mishra, B.K. Waste Printed Circuit Boards recycling: An extensive assessment of current status. J. Clean. Prod. 2015, 94, 5–19. [Google Scholar] [CrossRef]
- Jing-ying, L.; Xiu-li, X.; Wen-quan, L. Thiourea leaching gold and silver from the printed circuit boards of waste mobile phones. Waste Manag. 2012, 32, 1209–1212. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.H.; Abdul Halim, S.F. Recovery of Precious Metals from Discarded Mobile Phones by Thiourea Leaching. Mater. Sci. Forum 2019, 962, 112–116. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, S.; Xie, H.; Zeng, X.; Li, J. Current Status on Leaching Precious Metals from Waste Printed Circuit Boards. Procedia Environ. Sci. 2012, 16, 560–568. [Google Scholar] [CrossRef]
- Zhang, W.; Ren, J.; Liu, S.; Yuan, Z. Mechanism and Clean Procedure to Extract Gold from Printed Circuit Board. Procedia Environ. Sci. 2016, 31, 171–177. [Google Scholar] [CrossRef]
- Syed, S. Recovery of gold from secondary sources—A review. Hydrometallurgy 2012, 115–116, 30–51. [Google Scholar] [CrossRef]
- Torres, R.; Lapidus, G.T. Copper leaching from electronic waste for the improvement of gold recycling. Waste Manag. 2016, 57, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Behnamfard, A.; Salarirad, M.M.; Veglio, F. Process development for recovery of copper and precious metals from waste printed circuit boards with emphasis on palladium and gold leaching and precipitation. Waste Manag. 2013, 33, 2354–2363. [Google Scholar] [CrossRef] [PubMed]
- Birloaga, I.; Coman, V.; Kopacek, B.; Vegliò, F. An advanced study on the hydrometallurgical processing of waste computer printed circuit boards to extract their valuable content of metals. Waste Manag. 2014, 34, 2581–2586. [Google Scholar] [CrossRef] [PubMed]
- Birloaga, I.; Vegliò, F. Study of multi-step hydrometallurgical methods to extract the valuable content of gold, silver, and copper from waste printed circuit boards. J. Environ. Chem. Eng. 2016, 4, 20–29. [Google Scholar] [CrossRef]
- Nekouei, R.K.; Pahlevani, F.; Rajarao, R.; Golmohammadzadeh, R.; Sahajwalla, V. Two-step pre-processing enrichment of waste printed circuit boards: Mechanical milling and physical separation. J. Clean. Prod. 2018, 184, 1113–1124. [Google Scholar] [CrossRef]
- Noah, N.F.M.; Sulaiman, R.N.R.; Othman, N.; Jusoh, N.; Rosly, M.B. Extractive continuous extractor for chromium recovery: Chromium (VI) reduction to chromium (III) in sustainable emulsion liquid membrane process. J. Clean. Prod. 2020, 247, 119167. [Google Scholar] [CrossRef]
- Panda, R.; Dinkar, O.S.; Jha, M.K.; Pathak, D.D. Hydrometallurgical processing of waste multilayer ceramic capacitors (MLCCs) to recover silver and palladium. Hydrometallurgy 2020, 197, 105476. [Google Scholar] [CrossRef]
- Long Le, H.; Jeong, J.; Lee, J.-C.; Pandey, B.D.; Yoo, J.-M.; Huyunh, T.H. Hydrometallurgical Process for Copper Recovery from Waste Printed Circuit Boards (PCBs). Miner. Process. Extr. Metall. Rev. 2011, 32, 90–104. [Google Scholar] [CrossRef]
- Jeon, S.; Park, I.; Yoo, K.; Ryu, H. The effects of temperature and agitation speed on the leaching behaviors of tin and bismuth from spent lead free solder in nitric acid leach solution. Geosyst. Eng. 2015, 18, 213–218. [Google Scholar] [CrossRef]
- Yoo, K.; Lee, J.; Lee, K.; Kim, B.; Kim, M.; Kim, S.; Pandey, B.D. Recovery of Sn, Ag and Cu from Waste Pb-Free Solder Using Nitric Acid Leaching. Mater. Trans. 2012, 53, 2175–2180. [Google Scholar] [CrossRef]
- Mohammadzadeh, M.; Bagheri, H.; Ghader, S. Study on extraction and separation of Ni and Zn using [bmim][PF6] IL as selective extractant from nitric acid solution obtained from zinc plant residue leaching. Arab. J. Chem. 2020, 13, 5821–5831. [Google Scholar] [CrossRef]
- Mecucci, A.; Scott, K. Leaching and electrochemical recovery of copper, lead and tin from scrap printed circuit boards. J. Chem. Technol. Biotechnol. 2002, 77, 449–457. [Google Scholar] [CrossRef]
- Li, J.; Miller, J.D. A Review of Gold Leaching in Acid Thiourea Solutions. Miner. Process. Extr. Metall. Rev. 2006, 27, 177–214. [Google Scholar] [CrossRef]
- Kai, T.; Hagiwara, T.; Haseba, H.; Takahashi, T. Reduction of Thiourea Consumption in Gold Extraction by Acid Thiourea Solutions. Ind. Eng. Chem. Res. 1997, 36, 2757–2759. [Google Scholar] [CrossRef]
- Camelino, S.; Rao, J.; Padilla, R.L.; Lucci, R. Initial Studies about Gold Leaching from Printed Circuit Boards (PCB’s) of Waste Cell Phones. Procedia Mater. Sci. 2015, 9, 105–112. [Google Scholar] [CrossRef]
- Hiskey, J.B. Thiourea Leaching of Gold and Silver—Technology Update and Additional Applications. Miner. Metall. Process. 1984, 1, 173–179. [Google Scholar] [CrossRef]
- Groenewald, T. The dissolution of gold in acidic solutions of thiourea. Hydrometallurgy 1976, 1, 277–290. [Google Scholar] [CrossRef]
- Mironov, I.V.; Tsvelodub, L.D. Complexation of copper(I) by thiourea in acidic aqueous solution. J. Solut. Chem. 1996, 25, 315–325. [Google Scholar] [CrossRef]
- Chen, C.K.; Lung, T.N.; Wan, C.C. A study of the leaching of gold and silver by acidothioureation. Hydrometallurgy 1980, 5, 207–212. [Google Scholar] [CrossRef]
- Deschênes, G.; Ghali, E. Leaching of gold from a chalcopyrite concentrate by thiourea. Hydrometallurgy 1988, 20, 179–202. [Google Scholar] [CrossRef]
- Arslan, F.; Olgaç Kangal, M.; Bulut, G.; Gül, A. Leaching of Massive Rich Copper Ore with Acidified Ferric Chloride. Miner. Process. Extr. Metall. Rev. 2004, 25, 143–158. [Google Scholar] [CrossRef]
- Pyper, R.A.; Hendrix, J.L. Extraction of Gold from Finely Disseminated Gold Ores by Use of Acidic Thiourea Solution; I.M.M.: London, UK, 1981. [Google Scholar]
- Lee, H.; Molstad, E.; Mishra, B. Recovery of Gold and Silver from Secondary Sources of Electronic Waste Processing by Thiourea Leaching. JOM 2018, 70, 1616–1621. [Google Scholar] [CrossRef]
- Ubaldini, S.; Fornari, P.; Massidda, R.; Abbruzzese, C. An innovative thiourea gold leaching process. Hydrometallurgy 1998, 48, 113–124. [Google Scholar] [CrossRef]
- Zhang, L.; Guo, X.; Tian, Q.; Li, D.; Zhong, S.; Qin, H. Improved thiourea leaching of gold with additives from calcine by mechanical activation and its mechanism. Miner. Eng. 2022, 178, 107403. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Blanchard, G.; Maunaye, M.; Martin, G. Removal of heavy metals from waters by means of natural zeolites. Water Res. 1984, 18, 1501–1507. [Google Scholar] [CrossRef]
- Simonin, J.-P. On the comparison of pseudo-first order and pseudo-second order rate laws in the modeling of adsorption kinetics. Chem. Eng. J. 2016, 300, 254–263. [Google Scholar] [CrossRef]
- Rudzinski, W.; Plazinski, W. Theoretical description of the kinetics of solute adsorption at heterogeneous solid/solution interfaces: On the possibility of distinguishing between the diffusional and the surface reaction kinetics models. Appl. Surf. Sci. 2007, 253, 5827–5840. [Google Scholar] [CrossRef]
- Russo, V.; Tesser, R.; Masiello, D.; Trifuoggi, M.; Di Serio, M. Further verification of adsorption dynamic intraparticle model (ADIM) for fluid–solid adsorption kinetics in batch reactors. Chem. Eng. J. 2016, 283, 1197–1202. [Google Scholar] [CrossRef]
- Ho, Y.-S. Review of second-order models for adsorption systems. J. Hazard. Mater. 2006, 136, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Nekouei, R.K.; Pahlevani, F.; Assefi, M.; Maroufi, S.; Sahajwalla, V. Selective isolation of heavy metals from spent electronic waste solution by macroporous ion-exchange resins. J. Hazard. Mater. 2019, 371, 389–396. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption kinetics and isotherm models of heavy metals by various adsorbents: An overview. Crit. Rev. Environ. Sci. Technol. 2023, 53, 1837–1865. [Google Scholar] [CrossRef]
- Nekouei, R.K.; Tudela, I.; Mofarah, S.S.; Vahidi, E.; Maroufi, S.; Wang, K.; Pahlevani, F.; Sahajwalla, V. Dual functionality of mixed Cu-based two-dimensional (2D) heterostructures derived from electronic waste. Green Chem. 2021, 23, 5511–5523. [Google Scholar] [CrossRef]
- Cui, J.; Forssberg, E. Mechanical recycling of waste electric and electronic equipment: A review. J. Hazard. Mater. 2003, 99, 243–263. [Google Scholar] [CrossRef]
- Estrada-Ruiz, R.H.; Flores-Campos, R.; Gámez-Altamirano, H.A.; Velarde-Sánchez, E.J. Separation of the metallic and non-metallic fraction from printed circuit boards employing green technology. J. Hazard. Mater. 2016, 311, 91–99. [Google Scholar] [CrossRef]
- La Brooy, S.R.; Linge, H.G.; Walker, G.S. Review of gold extraction from ores. Miner. Eng. 1994, 7, 1213–1241. [Google Scholar] [CrossRef]
- Ippolito, N.M.; Medici, F.; Pietrelli, L.; Piga, L. Effect of Acid Leaching Pre-Treatment on Gold Extraction from Printed Circuit Boards of Spent Mobile Phones. Materials 2021, 14, 362. [Google Scholar] [CrossRef] [PubMed]
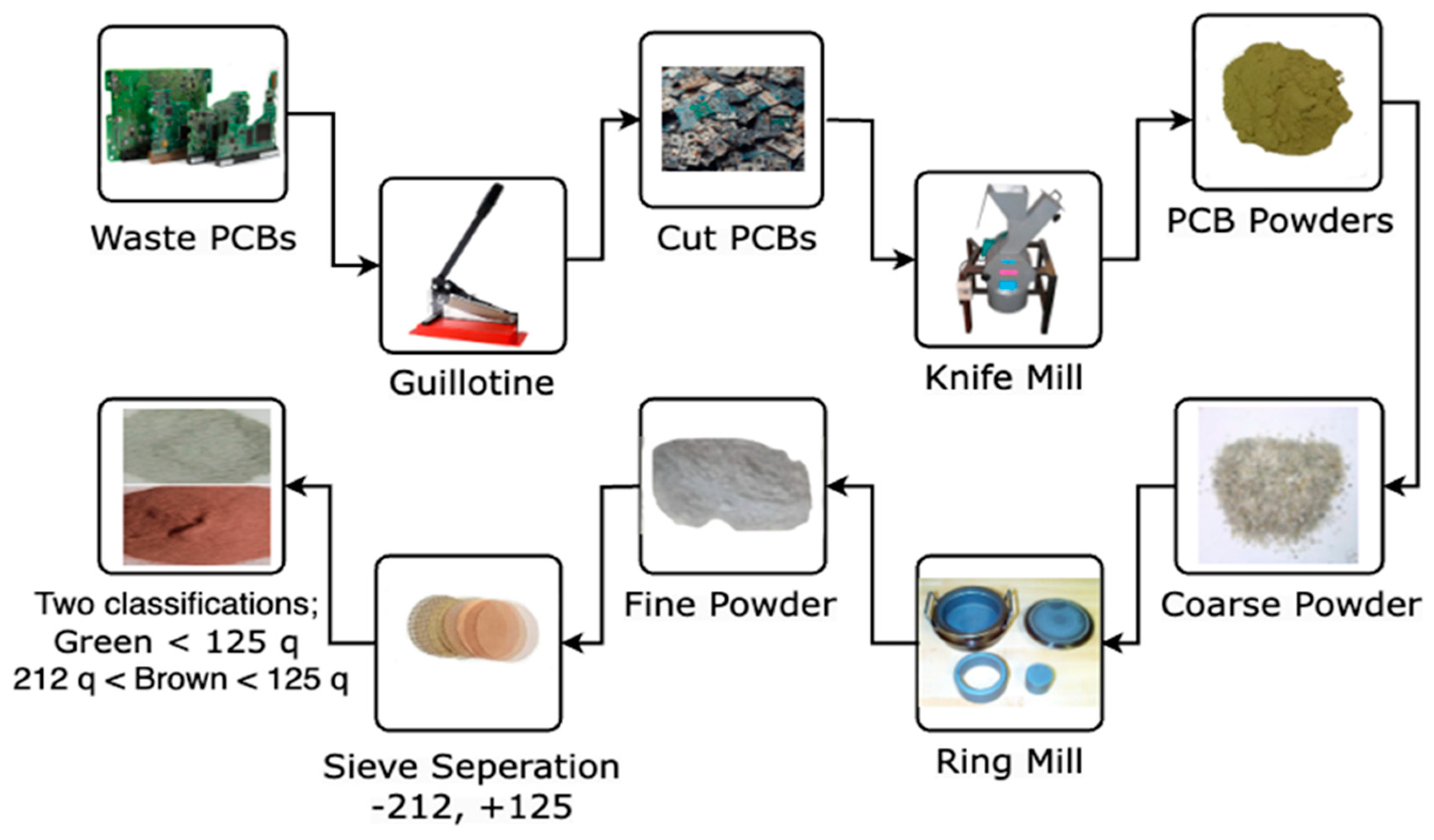




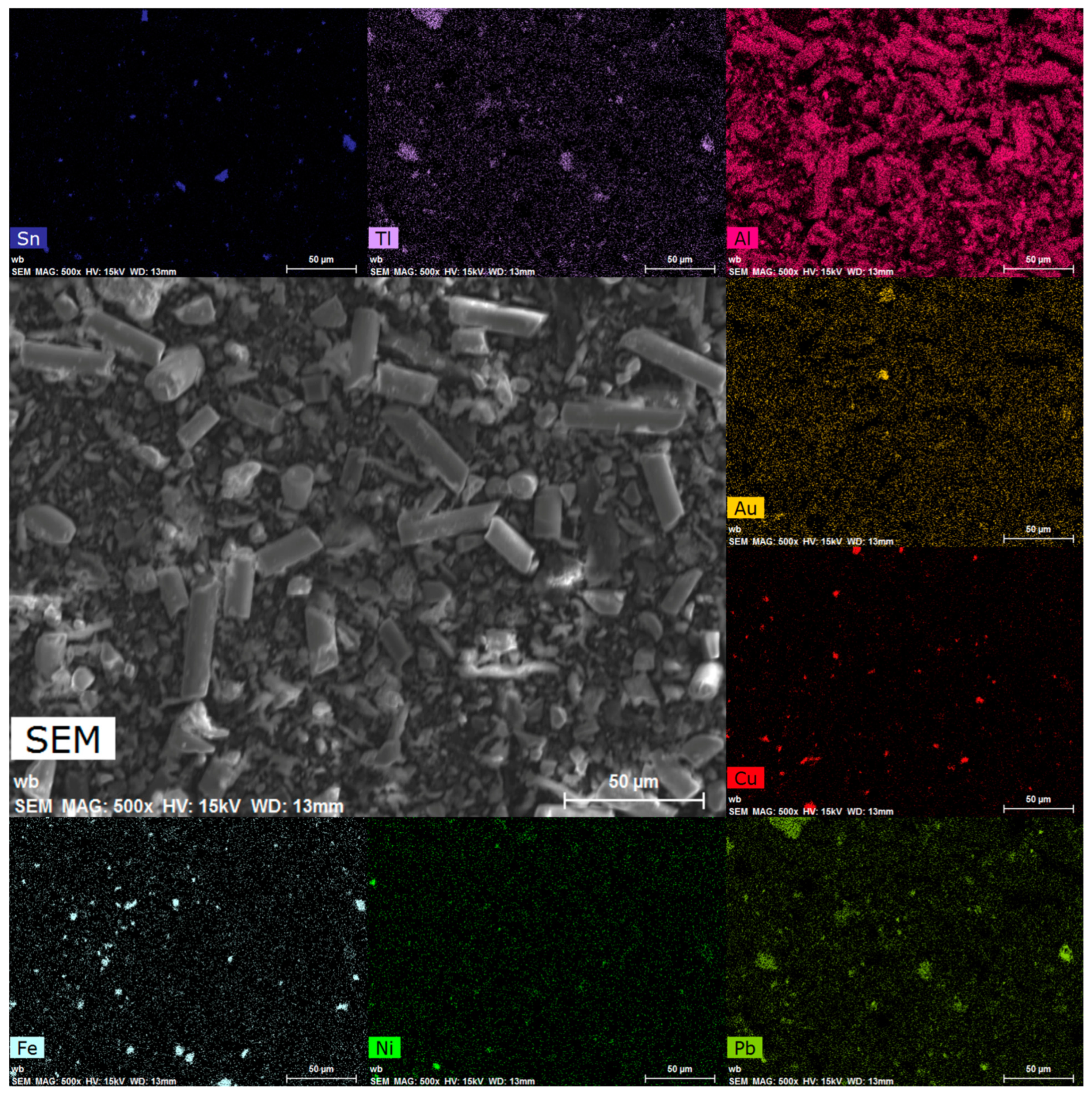
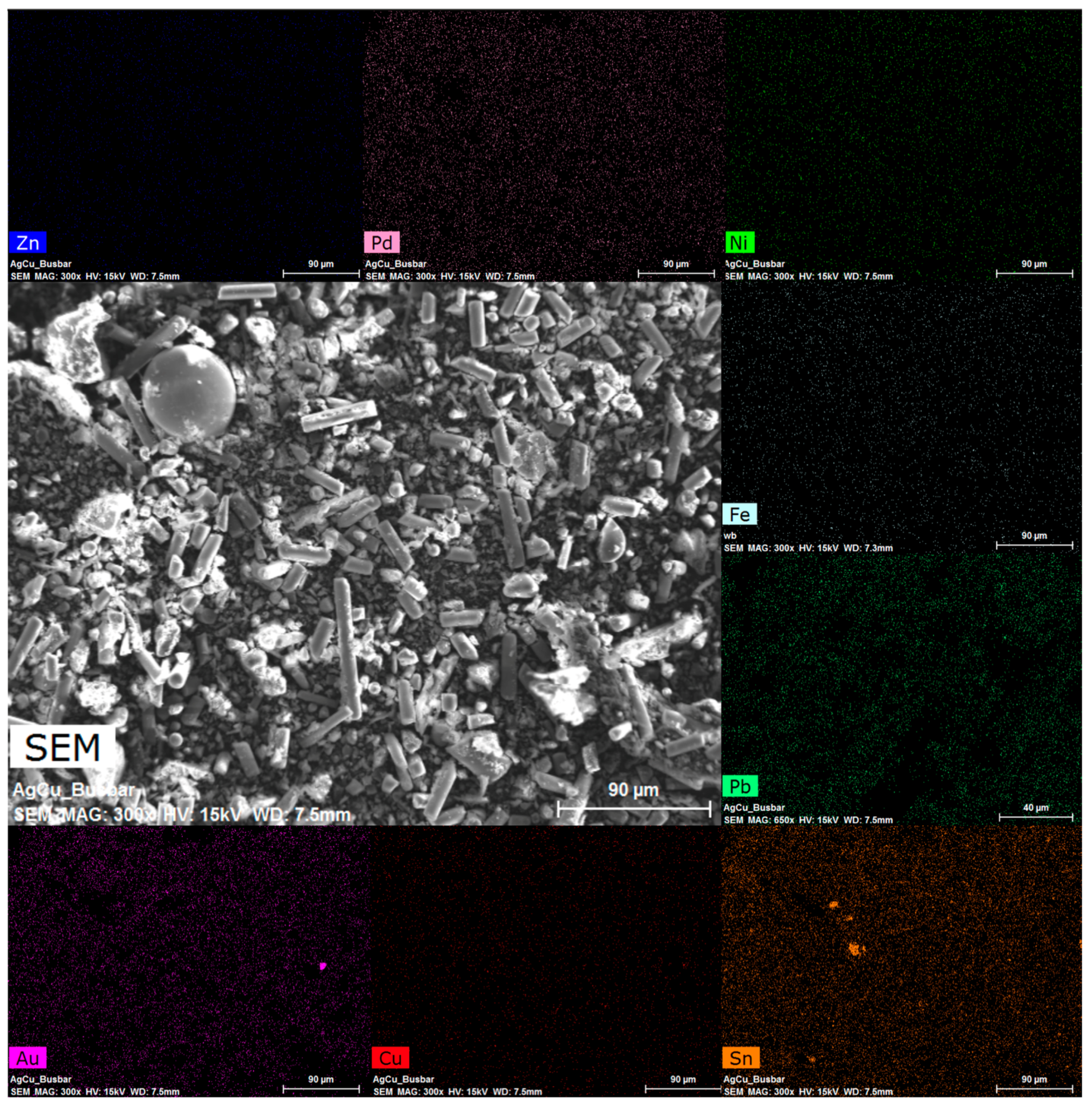
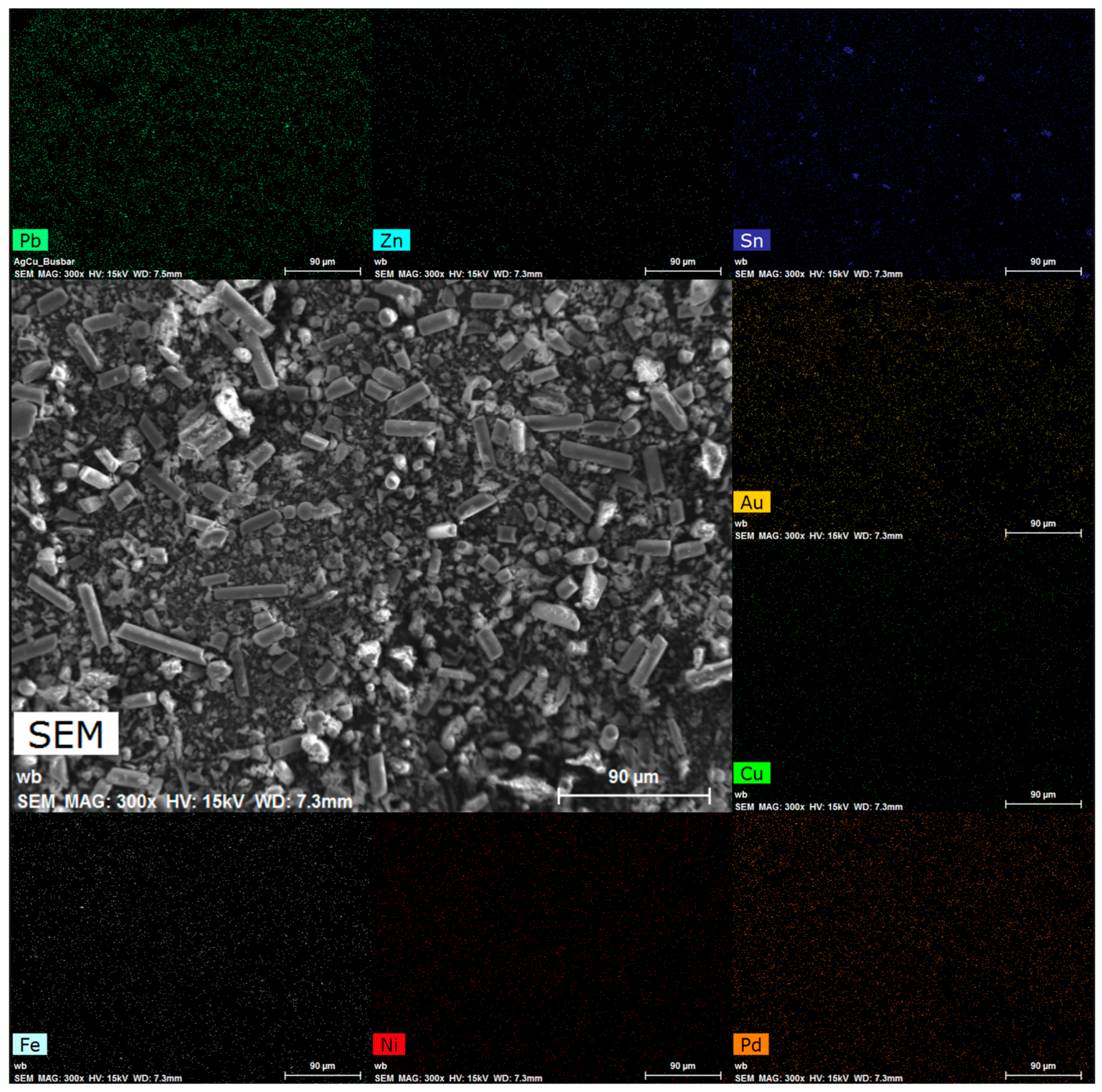
| Elements | Cu (% wt) | Fe (% wt) | Ni (% wt) | Pb (% wt) | Sn (% wt) | Zn (% wt) | Au (mg/kg) | Pd (mg/kg) |
|---|---|---|---|---|---|---|---|---|
| Green Powder | 9.29 | 5.77 | 0.41 | 0.79 | 2.72 | 0.2 | 524 | 19.4 |
| Brown Powder | 42.93 | 1.28 | 0.41 | 1.11 | 6.48 | 2.15 | 44.6 | 34.1 |
| Temperature (°C) | H2SO4 Concentration (M) | Ferric Ion Concentration (g/L) | Thiourea Concentration (M) |
|---|---|---|---|
| 0 | 0 | 0.2 | |
| 25 | 0.05 | 3 | 0.5 |
| 50 | 0.1 | 6 | 1 |
| 70 | 0.2 | 9 | 1.5 |
| First Stage | ||||||||
| Elements Time (min) | Cu (ppm) | Fe (ppm) | Ni (ppm) | Pb (ppm) | Sn (ppm) | Zn (ppm) | Au (ppb) | Pd (ppb) |
| 2 m | 259 | 1008 | 63.1 | 148.7 | 251.6 | 12.9 | 0.65 | 29.5 |
| 5 m | 309 | 1008 | 67.8 | 149.0 | 265.0 | 13.9 | 0.99 | 36.8 |
| 10 m | 343 | 1013 | 70.6 | 149.7 | 269.2 | 14.4 | 1.12 | 42.5 |
| 15 m | 351 | 1011 | 70.0 | 147.4 | 266.8 | 14.4 | 1.18 | 42.7 |
| 20 m | 362 | 1002 | 72.2 | 149.9 | 276.8 | 14.8 | 1.61 | 42.4 |
| 30 m | 366 | 1017 | 69.6 | 152.7 | 265.6 | 14.4 | 1.79 | 41.1 |
| 40 m | 678 | 1032 | 75.0 | 157.2 | 273.8 | 17.6 | 1.98 | 62.7 |
| 50 m | 774 | 1035 | 76.2 | 154.0 | 278.4 | 18.6 | 2.41 | 69.3 |
| 60 m | 842 | 1034 | 75.4 | 158.2 | 274.2 | 18.8 | 2.67 | 80.3 |
| 75 m | 952 | 1040 | 76.1 | 158.8 | 277.8 | 19.4 | 2.92 | 79.9 |
| 90 m | 1040 | 1038 | 77.1 | 157.0 | 281.2 | 20.0 | 3.30 | 85.9 |
| 120 m | 1222 | 1046 | 79.1 | 157.4 | 287.6 | 21.3 | 3.75 | 96.6 |
| 180 m | 1440 | 1053 | 79.6 | 156.2 | 290.4 | 22.8 | 4.63 | 115 |
| 240 m | 1660 | 1052 | 82.0 | 161.3 | 298.2 | 24.6 | 5.61 | 128 |
| 300 m | 1716 | 1102 | 83.2 | 170.7 | 304.2 | 25.3 | 6.53 | 133 |
| 360 m | 1769 | 1104 | 83.6 | 165.6 | 304.8 | 25.6 | 6.57 | 135 |
| Second Stage | ||||||||
| Elements Time (min) | Cu (ppm) | Ni (ppm) | Pb (ppm) | Sn (ppm) | Zn (ppm) | Au (ppb) | Pd (ppb) | |
| 2 m | 1.45 | 0.79 | 0.08 | 2.06 | 0.19 | 2408 | 322 | |
| 5 m | 1.46 | 0.79 | 0.09 | 3.04 | 0.22 | 2494 | 318 | |
| 10 m | 1.52 | 0.80 | 0.09 | 4.72 | 0.22 | 2511 | 310 | |
| 15 m | 1.59 | 0.82 | 0.10 | 5.72 | 0.24 | 2629 | 323 | |
| 30 m | 1.66 | 0.88 | 0.11 | 8.04 | 0.30 | 2602 | 314 | |
| 60 m | 1.72 | 0.89 | 0.13 | 12.8 | 0.40 | 2620 | 317 | |
| 75 m | 1.74 | 0.93 | 0.14 | 15.0 | 0.45 | 2626 | 317 | |
| 90 m | 1.81 | 0.98 | 0.15 | 16.5 | 0.50 | 2665 | 325 | |
| 120 m | 1.80 | 0.98 | 0.16 | 20.4 | 0.55 | 2687 | 329 | |
| 180 m | 1.88 | 1.08 | 0.17 | 22.8 | 0.74 | 2746 | 334 | |
| 240 m | 2.12 | 1.28 | 0.21 | 39.6 | 1.14 | 2889 | 355 | |
| 360 m | 2.11 | 1.27 | 0.21 | 39.3 | 1.12 | 2823 | 349 | |
| 540 m | 2.59 | 1.78 | 0.29 | 86.2 | 1.95 | 3331 | 411 | |
| 720 m | 2.88 | 2.08 | 0.33 | 107.7 | 2.43 | 3284 | 410 | |
| 1080 m | 3.04 | 2.48 | 0.37 | 132.7 | 2.90 | 3177 | 400 | |
| 1440 m | 3.28 | 2.96 | 0.41 | 154.9 | 3.57 | 3283 | 415 | |
| Elements Recovered | (1) Pseudo-First-Order | (2) Pseudo-Second-Order | (3) qmax Experimental (mg/g) | Error (%) between (1) and (3) | Error (%) between (2) and (3) | ||||
|---|---|---|---|---|---|---|---|---|---|
| qmax (mg/g) | k1 (min−1) | Adjusted R-square | qmax (mg/g) | k2 (g/mg·min) | Adjusted R-square | ||||
| Cu | 1755.6 | −0.01102 | 0.98 | 2104.0 | 4.75124 × 101 | 0.93 | 1769.0 | 0.79 | −18.9 |
| Fe | 117.7 | −0.0085 | 0.61 | 1096.4 | 9.12059 × 10−4 | 0.99 | 1104.0 | 89.4 | 0.7 |
| Ni | 16.4 | −0.0108 | 0.93 | 83.8 | 0.01193 | 0.99 | 83.6 | 80.3 | −0.2 |
| Pb | 27.0 | −0.01065 | 0.35 | 166.7 | 0.006 | 0.99 | 170.7 | −5.6 | 0.2 |
| Sn | 68.9 | −0.01643 | 0.81 | 304.9 | 0.00328 | 0.99 | 304.8 | 77.3 | 0.0 |
| Zn | 13.8 | −0.01116 | 0.97 | 26.23 | 0.03813 | 0.99 | 25.6 | 46.2 | −2.4 |
| Au | 0.0098 | −0.01676 | 0.83 | 0.0077 | 129.88199 | 0.911 | 0.0066 | −49.1 | −17.2 |
| Pd | 0.1967 | −0.02061 | 0.74 | 0.1487 | 6.72189 | 0.97 | 0.1354 | −45.3 | −9.82 |
| Elements Recovered | (1) Pseudo-First-Order | (2) Pseudo-Second-Order | (3) qmax Experimental (mg/g) | Error (%) between (1) and (3) | Error (%) between (2) and (3) | ||||
|---|---|---|---|---|---|---|---|---|---|
| qmax (mg/g) | k1 (min−1) | Adjusted R-square | qmax (mg/g) | k2 (g/mg. min) | Adjusted R-square | ||||
| Cu | 1.823 | −0.00189 | 0.97816 | 3.23 | 0.30889 | 0.98004 | 3.28 | 44.40 | 1.52% |
| Fe | 1471 | −0.00213 | 0.66411 | 5224 | 1.91416 × 101 | 0.99723 | 5273 | 72.13 | 0.9% |
| Ni | 2.271 | −0.00134 | 0.97276 | 2.83 | 0.35281 | 0.89712 | 2.96 | 23.31 | 4.39% |
| Pb | 0.3229 | −0.00185 | 0.98894 | 0.4131 | 2.42034 | 0.94234 | 0.41 | 21.24 | 0.14% |
| Sn | 162.846 | −0.00171 | 0.96612 | 229.8 | 0.00435 | 0.47715 | 154.9 | 5.29 | −48.35% |
| Zn | 4.886 | −0.00359 | 0.72107 | 4.13 | 0.24203 | 0.69214 | 3.57 | 36.86 | −15.68% |
| Au | 0.526 | −0.00297 | 0.11806 | 3.28374 | 0.30453 | 0.99809 | 3.28253 | 83.97 | −0.12% |
| Pd | 0.115 | −0.00362 | 0.82173 | 0.414 | 2.41319 | 0.99775 | 0.415 | 31.03 | 0.24% |
| Financial Sustainability | Effect on Environment | Level of Research | |||
|---|---|---|---|---|---|
| Rate of Leaching | Reagent Cost | Corrosive | Toxicity | Reliability | Leaching Technique |
| 3 | 5 | 5 | 0 | 5 | Cyanidation |
| 4 | 4 | 4 | 4 | 4 | Thiourea |
| 2 | 2 | 5 | 4 | 2 | Thiosulfate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ubiç, S.; Nekouei, R.K.; Sahajwalla, V. A Two-Step Leaching Process Using Thiourea for the Recovery of Precious Metals from Waste Printed Circuit Boards. Waste 2024, 2, 312-336. https://doi.org/10.3390/waste2030018
Ubiç S, Nekouei RK, Sahajwalla V. A Two-Step Leaching Process Using Thiourea for the Recovery of Precious Metals from Waste Printed Circuit Boards. Waste. 2024; 2(3):312-336. https://doi.org/10.3390/waste2030018
Chicago/Turabian StyleUbiç, Serap, Rasoul Khayyam Nekouei, and Veena Sahajwalla. 2024. "A Two-Step Leaching Process Using Thiourea for the Recovery of Precious Metals from Waste Printed Circuit Boards" Waste 2, no. 3: 312-336. https://doi.org/10.3390/waste2030018
APA StyleUbiç, S., Nekouei, R. K., & Sahajwalla, V. (2024). A Two-Step Leaching Process Using Thiourea for the Recovery of Precious Metals from Waste Printed Circuit Boards. Waste, 2(3), 312-336. https://doi.org/10.3390/waste2030018







