Limb–Girdle Muscular Dystrophy D2 TNPO3-Related: A Quality of Life Study
Abstract
1. Introduction
2. Methods
2.1. Participants
2.2. Procedure
2.3. Sociodemographic Data
2.4. Gait-Scale-Gowers-Chair (GSGC)
2.5. Quality of Life
2.6. Statistical Analysis
3. Results
3.1. Patients
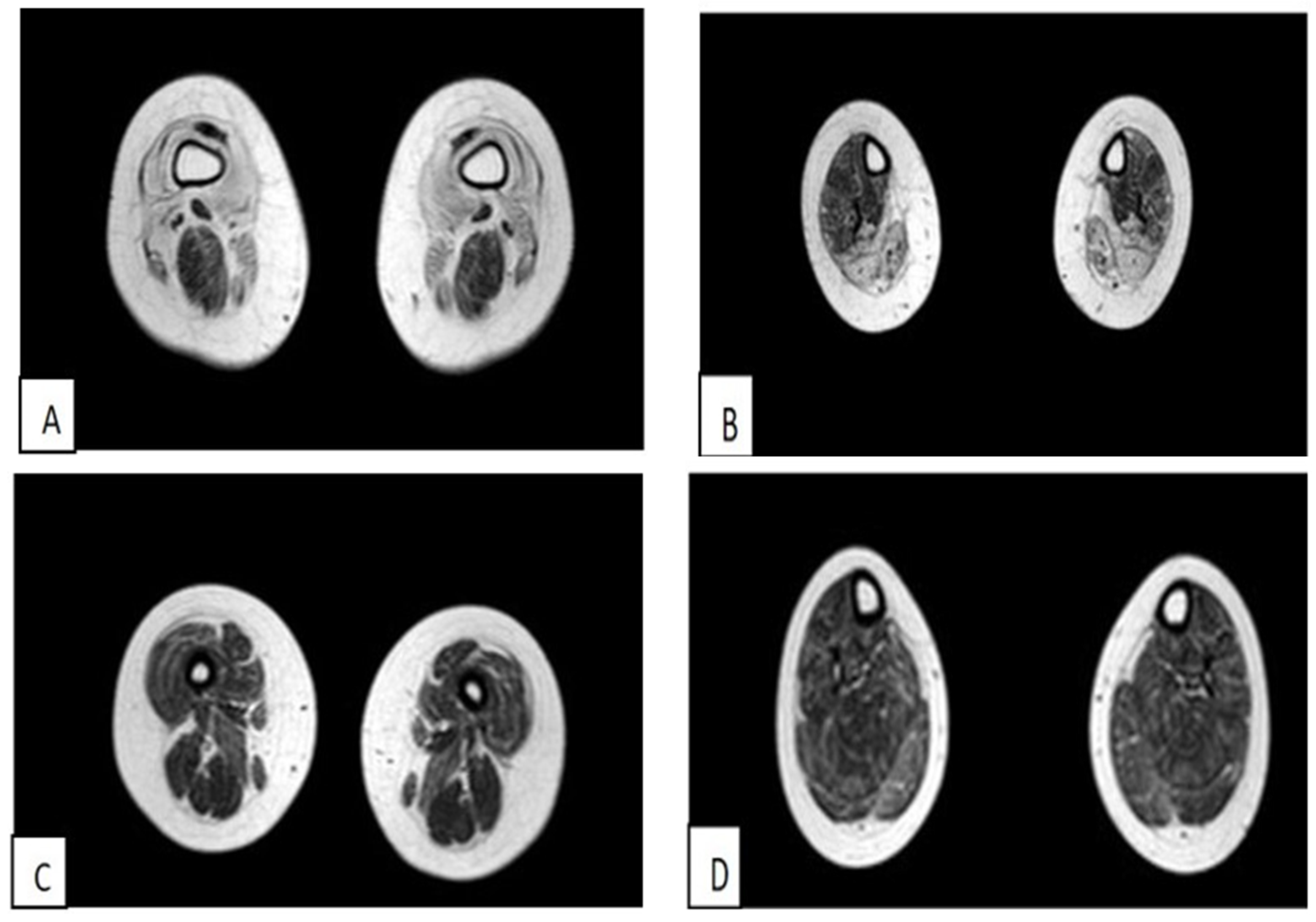
3.1.1. The First Case of Family 1 (Patient 1)
3.1.2. Second Case of Family 1 (Patient 2)
3.1.3. First Clinical Case of Family 2 (Patient 3)
3.1.4. Second Case of Family 2 (Patient 4)
3.1.5. Third Case of Italo-Spanish Family (Patient 5)
3.1.6. Fourth Case of Italo-Spanish Family (Patient 6)
3.2. Analysis of the Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Angelini, C.; Giaretta, L.; Marozzo, R. An update on diagnostic options and considerations in limb-girdle dystrophies. Expert Rev. Neurother. 2018, 18, 693–703. [Google Scholar] [CrossRef] [PubMed]
- Bushby, K.; Norwood, F.; Straub, V. The limb-girdle muscular dystrophies—Diagnostic strategies. Biochim. Biophys. Acta Mol. Basis Dis. 2007, 1772, 238–242. [Google Scholar] [CrossRef]
- Jokela, M.; Lehtinen, S.; Palmio, J.; Saukkonen, A.-M.; Huovinen, S.; Vihola, A.; Udd, B. A novel COL6A2 mutation causing late-onset limb-girdle muscular dystrophy. J. Neurol. 2019, 266, 1649–1654. [Google Scholar] [CrossRef] [PubMed]
- Straub, V.; Murphy, A.; Udd, B.; LGMD Workshop Study Group. 229th ENMC international workshop: Limb girdle muscular dystrophies—Nomenclature and reformed classification Naarden, The Netherlands, 17–19 March 2017. Neuromuscul. Disord. 2018, 28, 702–710. [Google Scholar] [CrossRef] [PubMed]
- Angelini, C. LGMD. Identification, description and classification. Acta Myol. 2020, 39, 207–217. [Google Scholar] [CrossRef]
- Vissing, J. Limb girdle muscular dystrophies: Classification, clinical spectrum and emerging therapies. Curr. Opin. Neurol. 2016, 29, 635–641. [Google Scholar] [CrossRef]
- Zatz, M.; Vainzof, M.; Passos-Bueno, M.R. Limb-girdle muscular dystrophy: One gene with different phenotypes, one phenotype with different genes. Curr. Opin. Neurol. 2000, 13, 511–517. [Google Scholar] [CrossRef]
- Pál, E.; Zima, J.; Hadzsiev, K.; Ito, Y.A.; Hartley, T.; Boycott, K.M.; Melegh, B. A novel pathogenic variant in TNPO3 in a Hungarian family with limb-girdle muscular dystrophy 1F. Eur. J. Med. Genet. 2019, 62, 103662. [Google Scholar] [CrossRef]
- Turan, S.; Farruggio, A.P.; Srifa, W.; Day, J.W.; Calos, M.P. Precise Correction of Disease Mutations in Induced Pluripotent Stem Cells Derived From Patients With Limb Girdle Muscular Dystrophy. Mol. Ther. 2016, 24, 685–696. [Google Scholar] [CrossRef]
- Murphy, A.P.; Straub, V. The Classification, Natural History and Treatment of the Limb Girdle Muscular Dystrophies. J. Neuromuscul. Dis. 2015, 2, S7–S19. [Google Scholar] [CrossRef]
- Costa, R.; Rodia, M.T.; Vianello, S.; Santi, S.; Lattanzi, G.; Angelini, C.; Pegoraro, E.; Cenacchi, G. Transportin 3 (TNPO3) and related proteins in limb girdle muscular dystrophy D2 muscle biopsies: A morphological study and pathogenetic hypothesis. Neuromuscul. Disord. 2020, 30, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Gamez, J.; Navarro, C.; Andreu, A.; Fernandez, J.; Palenzuela, L.; Tejeira, S.; Fernandez-Hojas, R.; Schwartz, S.; Karadimas, C.; DiMauro, S.; et al. Autosomal dominant limb-girdle muscular dystrophy: A large kindred with evidence for anticipation. Neurology 2001, 56, 450–454. Available online: http://www.ncbi.nlm.nih.gov/pubmed/11222786 (accessed on 21 March 2023). [CrossRef] [PubMed]
- Melia, M.J.; Kubota, A.; Ortolano, S.; Vílchez, J.J.; Gamez, J.; Tanji, K.; Bonilla, E.; Palenzuela, L.; Fernández-Cadenas, I.; Přistoupilová, A. Limb-girdle muscular dystrophy 1F is caused by a microdeletion in the transportin 3 gene. Brain 2013, 136, 1508–1517. [Google Scholar] [CrossRef]
- Torella, A.; Fanin, M.; Mutarelli, M.; Peterle, E.; Blanco, F.D.V.; Rispoli, R.; Savarese, M.; Garofalo, A.; Piluso, G.; Morandi, L.; et al. Next-Generation Sequencing Identifies Transportin 3 as the Causative Gene for LGMD1F. PLoS ONE 2013, 8, e63536. [Google Scholar] [CrossRef]
- Thompson, R.; Straub, V. Limb-girdle muscular dystrophies—International collaborations for translational research. Nat. Rev. Neurol. 2016, 12, 294–309. [Google Scholar] [CrossRef]
- Iyadurai, S.J.P.; Kissel, J.T. The Limb-Girdle Muscular Dystrophies and the Dystrophinopathies. Contin. Lifelong Learn. Neurol. 2016, 22, 1954–1977. [Google Scholar] [CrossRef] [PubMed]
- Peric, M.; Peric, S.; Stevanovic, J.; Milovanovic, S.; Basta, I.; Nikolic, A.; Kacar, A.; Rakocevic-Stojanovic, V. Quality of life in adult patients with limb–girdle muscular dystrophies. Acta Neurol. Belg. 2017, 118, 243–250. [Google Scholar] [CrossRef]
- Kovalchick, L.V.; Bates, K.; Statland, J.; Weihl, C.; Kang, P.B.; Lowes, L.P.; Mozaffar, T.; Straub, V.; Wicklund, M.; Heatwole, C.; et al. Patient reported quality of life in limb girdle muscular dystrophy. Neuromuscul. Disord. 2022, 32, 57–64. [Google Scholar] [CrossRef]
- Dany, A.; Rapin, A.; Lavrard, B.; Saoût, V.; Réveillère, C.; Bassez, G.; Tiffreau, V.; Péréon, Y.; Sacconi, S.; Eymard, B.; et al. The quality of life in genetic neuromuscular disease questionnaire: Rasch validation of the French version. Muscle Nerve 2017, 56, 1085–1091. [Google Scholar] [CrossRef]
- Whoqol Group. Development of the World Health Organization WHOQOL-BREF quality of life assessment. Psychol. Med. 1998, 28, 551–558. [Google Scholar] [CrossRef]
- Velarde-Jurado, E.; Avila-Figueroa, C. Evaluación de la calidad de vida. Salud Pública México 2002, 44, 349–361. [Google Scholar] [CrossRef]
- Karimi, M.; Brazier, J. Health, health-related quality of life, and quality of life: What is the difference? Pharmacoeconomics 2016, 34, 645–649. [Google Scholar] [CrossRef] [PubMed]
- Barreto, I.; Lepesqueur Guillen, L.; Asis, S.C. Predictores de calidad de vida en pacientes con enfermedad hepática crónica en Colombia. Rev. Colomb. Gastroenterol. 2015, 30, 390–398. [Google Scholar] [CrossRef][Green Version]
- Rajmil, L.; Perestelo-Pérez, L.; Herdman, M. Quality of life and rare diseases. Rare Dis. Epidemiol. 2010, 686, 251–272. [Google Scholar]
- Pangalila, R.F.; Bos, G.A.v.D.; Bartels, B.; Bergen, M.; Stam, H.J.; Roebroeck, M.E. Prevalence of Fatigue, Pain, and Affective Disorders in Adults With Duchenne Muscular Dystrophy and Their Associations with Quality of Life. Arch. Phys. Med. Rehabil. 2015, 96, 1242–1247. [Google Scholar] [CrossRef]
- Burns, T.M.; Graham, C.D.; Rose, M.R.; Simmons, Z. Quality of life and measures of quality of life in patients with neuromuscular disorders. Muscle Nerve 2011, 46, 9–25. [Google Scholar] [CrossRef]
- Vincent, K.A.; Carr, A.J.; Walburn, J.; Scott, D.L.; Rose, M.R. Construction and validation of a quality of life questionnaire for neuromuscular disease (INQoL). Neurology 2007, 68, 1051–1057. [Google Scholar] [CrossRef]
- Sansone, V.A.; Panzeri, M.; Montanari, M.; Apolone, G.; Gandossini, S.; Rose, M.R.; Politano, L.; Solimene, C.; Siciliano, G.; Volpi, L.; et al. Italian validation of INQoL, a quality of life questionnaire for adults with muscle diseases. Eur. J. Neurol. 2010, 17, 1178–1187. [Google Scholar] [CrossRef]
- Bonanno, S.; Zanin, R.; Bello, L.; Tramacere, I.; Bozzoni, V.; Caumo, L.; Ferraro, M.; Bortolani, S.; Sorarù, G.; Silvestrini, M.; et al. Quality of life assessment in adult spinal muscular atrophy patients treated with nusinersen. J. Neurol. 2022, 269, 3264–3275. [Google Scholar] [CrossRef]
- Richardson, J.C.; Hassell, A.B.; Hay, E.M.; Thomas, E. ‘I’d rather go and know’: Women’s understanding and experience of DEXA scanning for osteoporosis. Health Expect. 2002, 5, 114–126. [Google Scholar] [CrossRef]
- Angelini, C.; Pegoraro, V.; Cenacchi, G. The clinical and molecular spectrum of autosomal dominant limb-girdle muscular dystrophies focusing on transportinopathy. Expert Opin. Orphan Drugs 2019, 7, 223–232. [Google Scholar] [CrossRef]
- Hunter, M.; Heatwole, C.; Wicklund, M.; Weihl, C.C.; Mozaffar, T.; Statland, J.M.; Johnson, N.E. Limb-girdle muscular dystrophy: A perspective from adult patients on what matters most. Muscle Nerve 2019, 60, 419–424. [Google Scholar] [CrossRef]
- Fagoaga, J.; Girabent-Farres, M.; Bagur-Calafat, C. Translation and validation of the Individualised Neuromuscular Quality of Life scale for the Spanish population: Quality of life assessment for persons with neuromuscular diseases. Rev. Neurol. 2017, 64, 194–200. [Google Scholar]
- Rose, M.R.; Sadjadi, R.; Weinman, J.; Akhtar, T.; Pandya, S.; Kissel, J.T.; Jackson, C.E.; the Muscle Study Group. Role of disease severity, illness perceptions, and mood on quality of life in muscle disease. Muscle Nerve 2012, 46, 351–359. [Google Scholar] [CrossRef]
- Bos, I.; Wynia, K.; Almansa, J.; Drost, G.; Kremer, B.; Kuks, J. The prevalence and severity of disease-related disabilities and their impact on quality of life in neuromuscular diseases. Disabil. Rehabil. 2018, 41, 1676–1681. [Google Scholar] [CrossRef] [PubMed]
- Boosman, H.; Visser-Meily, J.M.A.; Meijer, J.-W.G.; Elsinga, A.; Post, M.W.M. Evaluation of change in fatigue, self-efficacy and health-related quality of life, after a group educational intervention programme for persons with neuromuscular diseases or multiple sclerosis: A pilot study. Disabil. Rehabil. 2010, 33, 690–696. [Google Scholar] [CrossRef] [PubMed]
- Hopman, W.M.; Harrison, M.B.; Coo, H.; Friedberg, E.; Buchanan, M.; VanDenKerkhof, E.G. Associations between chronic disease, age and physical and mental health status. Chronic Dis. Can. 2009, 29, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Lakatta, E.G. Age-associated Cardiovascular Changes in Health: Impact on Cardiovascular Disease in Older Persons. Heart Fail. Rev. 2002, 7, 29–49. [Google Scholar] [CrossRef]
- Heilman, K.M.; Barrett, A.M.; Adair, J.C. Possible mechanisms of anosognosia: A defect in self–awareness. Philos. Trans. R. Soc. London. 1998, 353, 1903–1909. [Google Scholar]
- Baldanzi, S.; Bevilacqua, F.; Lorio, R.; Volpi, L.; Simoncini, C.; Petrucci, A.; Cosottini, M.; Massimetti, G.; Tognoni, G.; Ricci, G.; et al. Disease awareness in myotonic dystrophy type 1: An observational cross-sectional study. Orphanet J. Rare Dis. 2016, 11, 34. [Google Scholar] [CrossRef]
| Triceps | Biceps | Deltoid | Wrist Extensor | Wrist Flexor | Neck Flexor Muscles | Quadriceps | Iliopsoas | Semitendinosus Muscles | Semimembranosus Muscles | Tibialis Anterior Muscle | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient 1 | 2/5 | - | 3/5 | 3/5 | - | 3/5 | 3/5 | 3/5 | 3/5 | 3/5 | - |
| Patient 2 | 2/5 | 4/5 | 2/5 | 3/5 | - | - | 3/5 | - | 4/5 | 4/5 | - |
| Patient 3 | 3/3 | 4-/4- | 3+/3+ | 4/4- | 4-/4- | - | 4-/4- | 3+/3- | - | - | 5/5 |
| Patient | Mutation | Age | Sex | GSGC | Muscle Weakness % | Locking % | Muscle Pain % | Fatigue % | Activities % | Independence % | Relationships % | Emotions % | Body Image % | INQoL Total Score % |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient 1 | c.2767delC | 17 | Male | 27 | 47.3 | 0 | 0 | 42.1 | 43.3 | 27.7 | 0 | 13.8 | 22.2 | 21.4 |
| Patient 2 | c.2767delC | 48 | Female | 22 | 52.6 | 68.4 | 36.8 | 47.3 | 26.6 | 5.5 | 16.6 | 8.3 | 22.2 | 15.8 |
| Patient 3 | C.2771delA | 39 | Female | 24 | 68.4 | 0 | 47.3 | 78.9 | 76.6 | 83.3 | 28.3 | 36.1 | 38.8 | 52.6 |
| Patient 4 | C.2771delA | 66 | Female | 21 | 73.6 | 0 | 0 | 68.4 | 73.3 | 83.3 | 45.0 | 52.7 | 66.6 | 64.2 |
| Patient 5 | C.2771delA | 33 | Male | 18 | 42.1 | 36.8 | 10.5 | 52.6 | 30.0 | 22.2 | 10.0 | 22.2 | 22.2 | 21.3 |
| Patient 6 | C.2771delA | 38 | Male | 27 | 57.8 | 42.1 | 21.0 | 31.5 | 33.3 | 55.5 | 15.0 | 13.8 | 38.8 | 31.3 |
| Total (M ± SD) | 40.1 ± 16.2 | 23.1 ± 3.5 | 57.0 ± 12.1 | 24.5 ± 28.9 | 19.2 ± 19.5 | 53.5 ± 17.4 | 47.2 ± 22.2 | 46.2 ± 32.8 | 19.1 ± 15.6 | 24.5 ± 16.8 | 35.1 ± 17.4 | 34.4 ± 19.5 |
| Clinical Variables | Sociodemographic Variables | N | Total (M ± SD) |
|---|---|---|---|
| Muscle weakness | Caregiver | 3 | 72 ± 3 |
| Patient | 3 | 56 ± 11 | |
| Locking | Caregiver | 3 | 44 ± 39 |
| Patient | 3 | 23 ± 39 | |
| Muscle pain | Caregiver | 3 | 23 ± 39 |
| Patient | 3 | 28 ± 25 | |
| Fatigue | Caregiver | 3 | 75 ± 6 |
| Patient | 3 | 56 ± 20 | |
| Activities | Caregiver | 3 | 65 ± 19 |
| Patient | 3 | 49 ± 25 | |
| Independence | Caregiver | 3 | 76 ± 13 |
| Patient | 3 | 39 ± 40 | |
| Relationships | Caregiver | 3 | 39 ± 18 |
| Patient | 3 | 15 ± 14 | |
| Emotions | Caregiver | 3 | 46 ± 7 |
| Patient | 3 | 19 ± 15 | |
| Body image | Caregiver | 3 | 46 ± 19 |
| Patient | 3 | 28 ± 10 | |
| INQoL score | Caregiver | 3 | 56 ± 11 |
| Patient | 3 | 30 ± 20 |
| Patient | Recessive Form | Age | Sex | GSGC | Muscle Weakness % | Locking % | Muscle Pain % | Fatigue % | Activities % | Independence % | Relationships % | Emotions % | Body Image % | INQoL Total Score% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient 1 | LGMD-R2 | 46 | Male | 27 | 57.8 | 57.8 | 0 | 63.1 | 40 | 27.7 | 5 | 11.1 | 33.3 | 23.4 |
| Patient 2 | LGMD-R5 | 47 | Female | 27 | 94.7 | 0 | 36.8 | 68.4 | 66.6 | 88.8 | 33.3 | 38.8 | 33.3 | 52.2 |
| Patient 3 | LGMD-R4 | 42 | Female | 27 | 84.2 | 73.6 | 0 | 68.4 | 53.3 | 88.8 | 8.3 | 19.4 | 44.4 | 42.8 |
| Total (M ± SD) | 45 ± 2.6 | 27 ± 0 | 78.9 ± 18.2 | 43.8 ± 38.8 | 12.2 ± 21.2 | 66.6 ± 3.0 | 53.3 ± 13.3 | 68.5 ± 35.2 | 15.5 ± 15.4 | 69.4 ± 14.2 | 37.0 ± 6.4 | 53.5 ± 14.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez, A.A.; Amayra, I.; García, I.; Angelini, C. Limb–Girdle Muscular Dystrophy D2 TNPO3-Related: A Quality of Life Study. Muscles 2023, 2, 274-285. https://doi.org/10.3390/muscles2030021
Rodríguez AA, Amayra I, García I, Angelini C. Limb–Girdle Muscular Dystrophy D2 TNPO3-Related: A Quality of Life Study. Muscles. 2023; 2(3):274-285. https://doi.org/10.3390/muscles2030021
Chicago/Turabian StyleRodríguez, Alicia Aurora, Imanol Amayra, Irune García, and Corrado Angelini. 2023. "Limb–Girdle Muscular Dystrophy D2 TNPO3-Related: A Quality of Life Study" Muscles 2, no. 3: 274-285. https://doi.org/10.3390/muscles2030021
APA StyleRodríguez, A. A., Amayra, I., García, I., & Angelini, C. (2023). Limb–Girdle Muscular Dystrophy D2 TNPO3-Related: A Quality of Life Study. Muscles, 2(3), 274-285. https://doi.org/10.3390/muscles2030021








