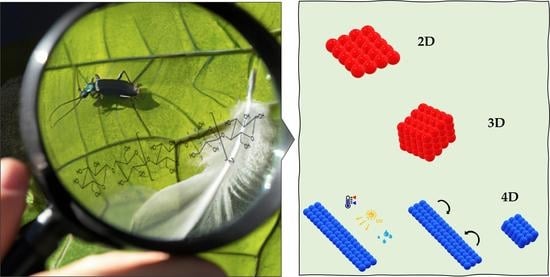
Nature-Inspired Cellulose-Based Active Materials: From 2D to 4D
Abstract
:1. Introduction
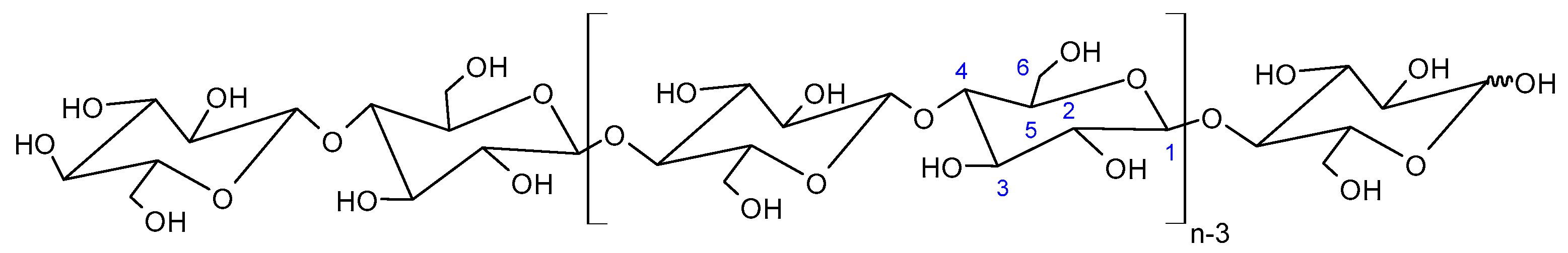
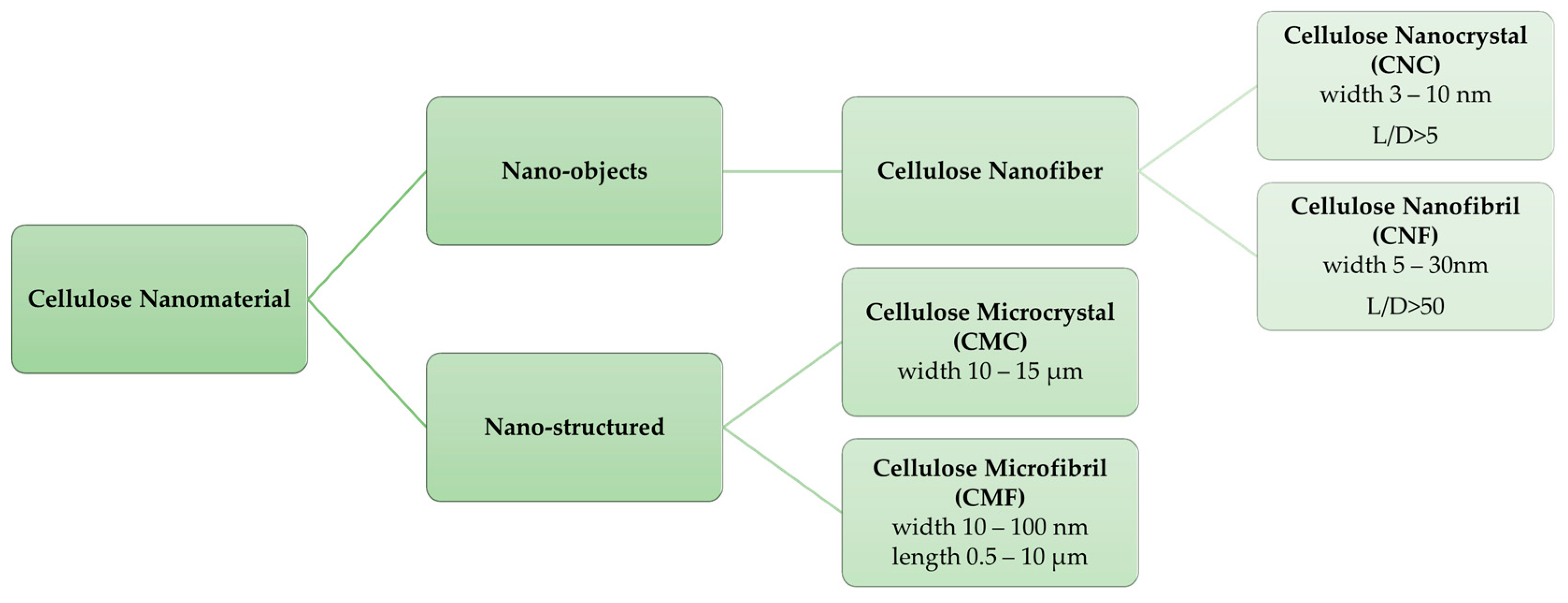
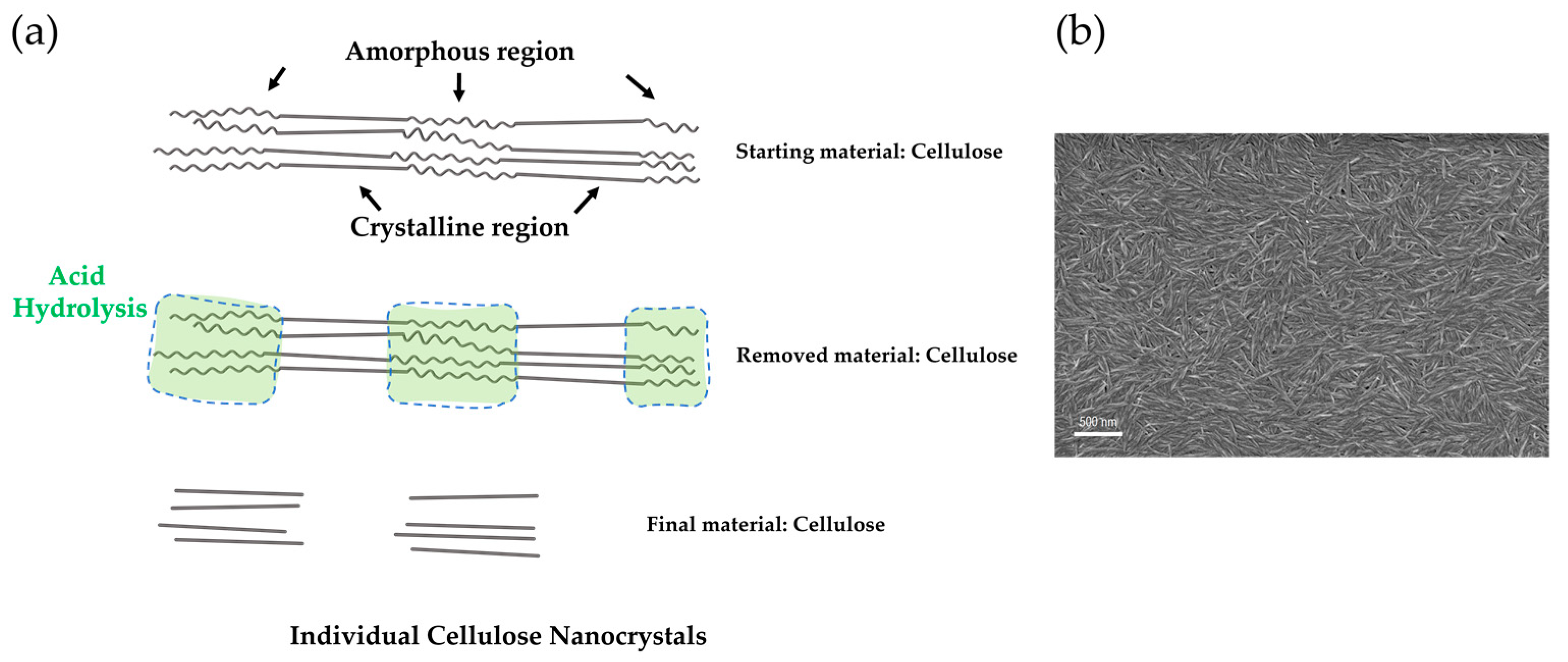
Cellulose Micro-/Nano- and Macroscale
2. Nature-Designed Materials

3. Cellulose Biomimetic Fiber for Developing Functional Materials
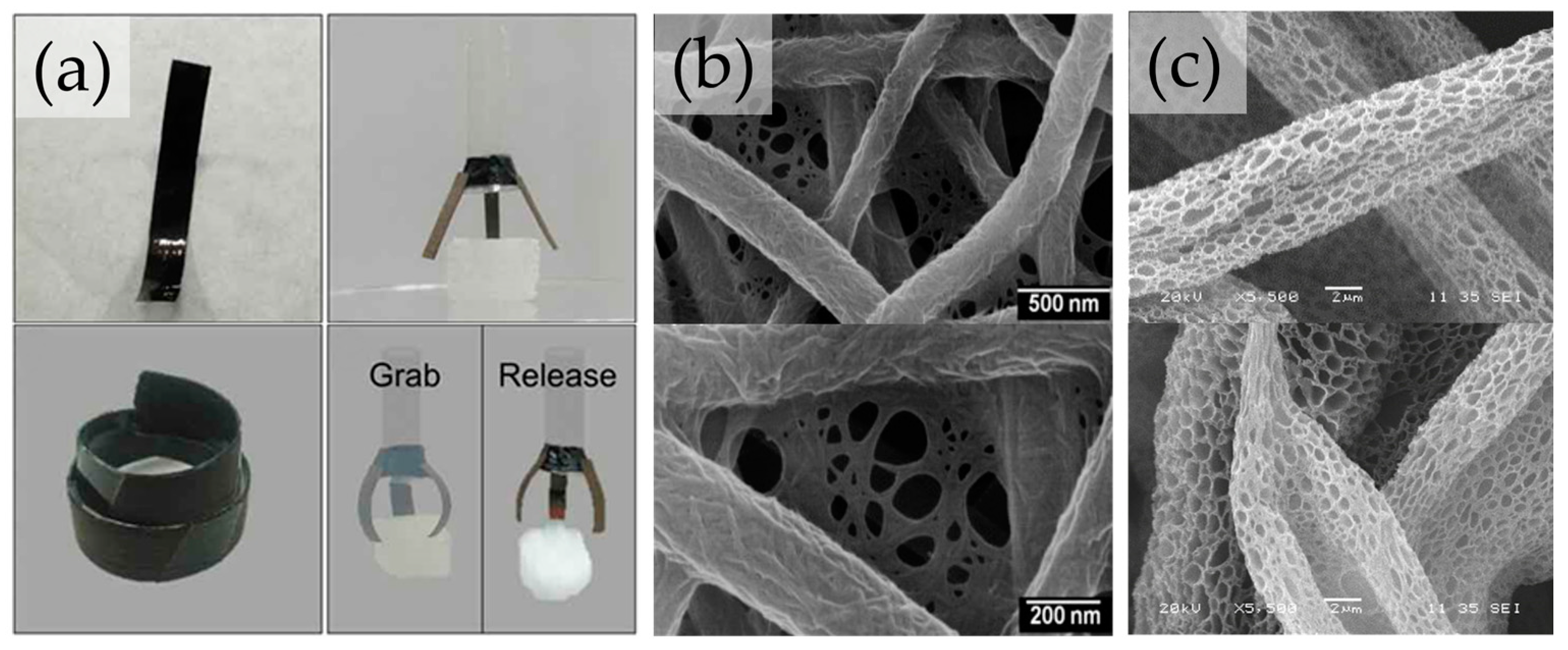
4. Biomimetic Cellulosic Active Films
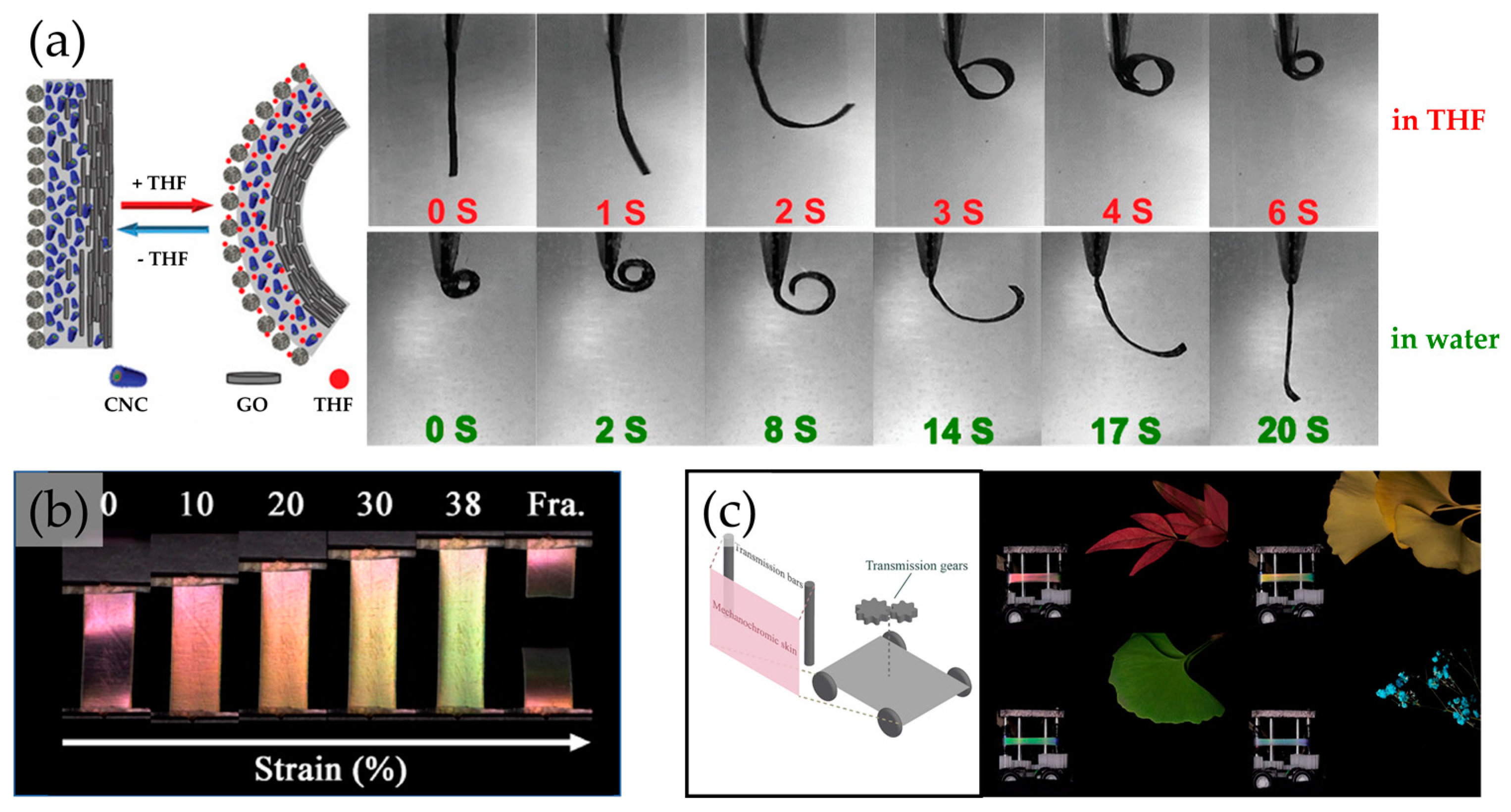
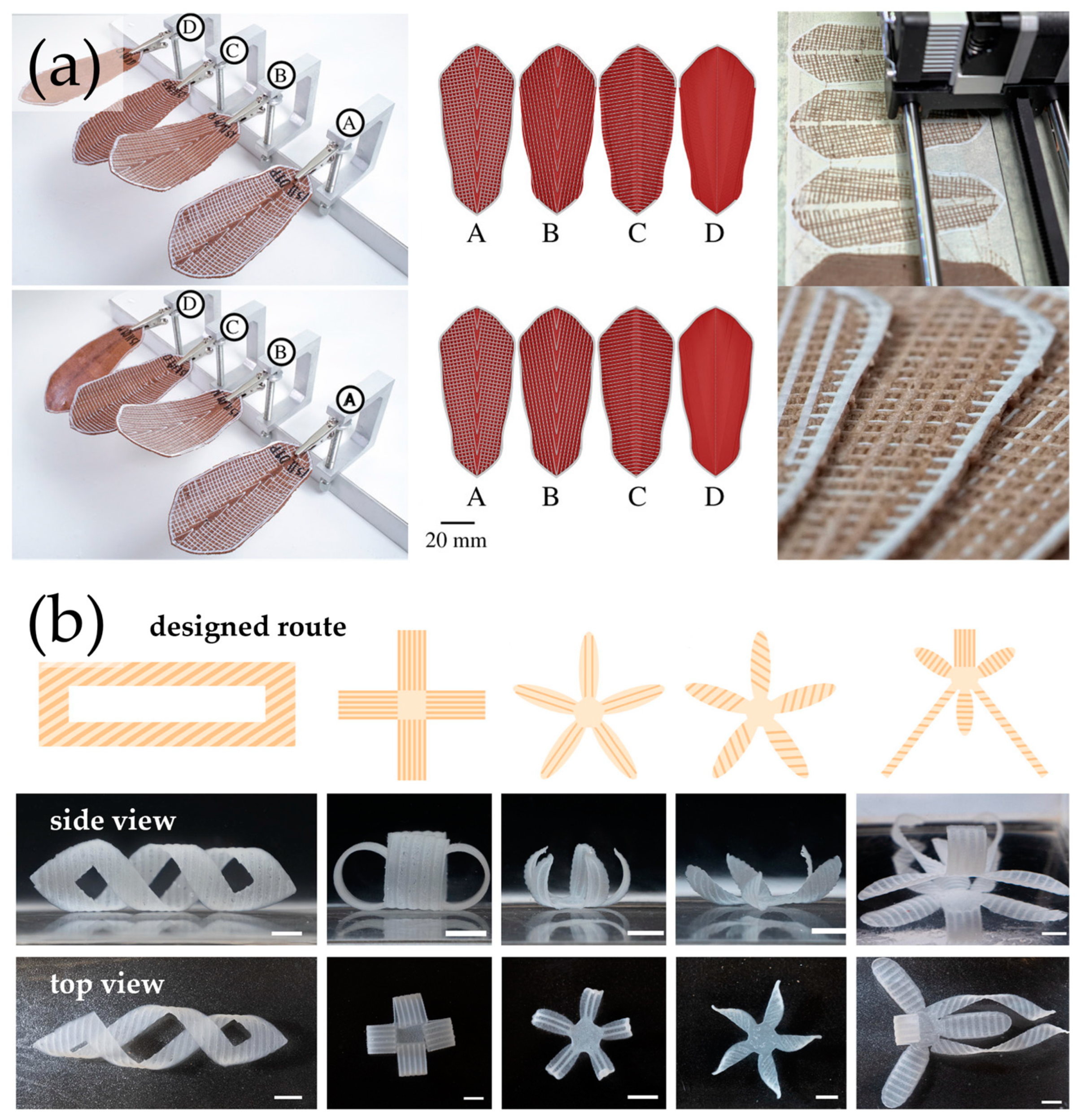
5. Printed 3D Structures
6. 4D-Printed Cellulosic Responsive Materials
7. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Payen, A. Mémoire sur la composition du tissu propre des plantes et du ligneux. J. Comptes Rendus 1838, 7, 1052–1056. [Google Scholar]
- Fisher, C.H. Anselm Payen Pioneer in Natural Polymers and Industrial Chemistry. In Pioneers in Polymer Science; Seymour, R.B., Ed.; Springer: Dordrecht, The Netherlands, 1989; pp. 47–61. [Google Scholar] [CrossRef]
- Brown, A.J. XIX.—The chemical action of pure cultivations of bacterium aceti. J. Chem. Soc. Trans. 1886, 49, 172–187. [Google Scholar] [CrossRef] [Green Version]
- Ohad, I.; Danon, D.; Hestrin, S. Synthesis of Cellulose by Acetobacter Xylinum: V. Ultrastructure of Polymer. J. Cell Biol. 1962, 12, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Portela, R.; Leal, C.R.; Almeida, P.L.; Sobral, R.G. Bacterial cellulose: A versatile biopolymer for wound dressing applications. Microb. Biotechnol. 2019, 12, 586–610. [Google Scholar] [CrossRef] [PubMed]
- Hol, H.R.; Jehli, J. The presence of cellulose microfibrils in the proteinaceous slime track of Dictyostelium discoideum. Arch. Für Mikrobiol. 1973, 92, 179–187. [Google Scholar] [CrossRef]
- De Souza Lima, M.M.; Borsali, R. Static and Dynamic Light Scattering from Polyelectrolyte Microcrystal Cellulose†. Langmuir 2002, 18, 992–996. [Google Scholar] [CrossRef]
- Dunlop, M.J.; Acharya, B.; Bissessur, R. Isolation of nanocrystalline cellulose from tunicates. J. Environ. Chem. Eng. 2018, 6, 4408–4412. [Google Scholar] [CrossRef]
- Staudinger, H. Über Polymerisation. Ber. Dtsch. Chem. Ges. 1920, 53, 1073–1085. [Google Scholar] [CrossRef]
- O’Sullivan, A.C. Cellulose: The structure slowly unravels. Cellulose 1997, 4, 173–207. [Google Scholar] [CrossRef]
- Kamide, K. Characterization of Molecular Structure of Cellulose Derivatives. In Cellulose and Cellulose Derivatives; Kamide, K., Ed.; Elsevier: Amsterdam, The Netherlands, 2005; pp. 25–188. [Google Scholar] [CrossRef]
- Klemm, D.; Heublein, B.; Fink, H.-P.; Bohn, A. Cellulose: Fascinating Biopolymer and Sustainable Raw Material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef]
- Ioyelovich, M.Y. Supermolecular structure of native and isolated cellulose. Polym. Sci. USSR 1991, 33, 1670–1676. [Google Scholar] [CrossRef]
- Bergenstråhle, M.; Wohlert, J.; Himmel, M.E.; Brady, J.W. Simulation studies of the insolubility of cellulose. Carbohydr. Res. 2010, 345, 2060–2066. [Google Scholar] [CrossRef]
- French, A.D.; Concha, M.; Dowd, M.K.; Stevens, E.D. Electron (charge) density studies of cellulose models. Cellulose 2014, 21, 1051–1063. [Google Scholar] [CrossRef]
- French, A.D.; Pérez, S.; Bulone, V.; Rosenau, T.; Gray, D. Cellulose. In Encyclopedia of Polymer Science and Technology; John Wiley & Sons: Hoboken, NJ, USA, 2018; pp. 1–69. [Google Scholar] [CrossRef]
- Klemm, D.; Kramer, F.; Moritz, S.; Lindström, T.; Ankerfors, M.; Gray, D.; Dorris, A. Nanocelluloses: A New Family of Nature-Based Materials. Angew. Chem. Int. Ed. 2011, 50, 5438–5466. [Google Scholar] [CrossRef]
- Mariano, M.; El Kissi, N.; Dufresne, A. Cellulose nanocrystals and related nanocomposites: Review of some properties and challenges. J. Polym. Sci. Pol. Phys. 2014, 52, 791–806. [Google Scholar] [CrossRef]
- Turbak, A.F.; Snyder, F.W.; Sandberg, K.R. Microfibrillated cellulose, a new cellulose product: Properties, uses, and commercial potential. J. Appl. Polym. Sci. Appl. Polym. Symp. 1983, 37, 815–827. [Google Scholar]
- Pääkkö, M.; Ankerfors, M.; Kosonen, H.; Nykänen, A.; Ahola, S.; Österberg, M.; Ruokolainen, J.; Laine, J.; Larsson, P.T.; Ikkala, O.; et al. Enzymatic Hydrolysis Combined with Mechanical Shearing and High-Pressure Homogenization for Nanoscale Cellulose Fibrils and Strong Gels. Biomacromolecules 2007, 8, 1934–1941. [Google Scholar] [CrossRef]
- Ganapathy, V.; Muthukumaran, G.; Sudhagar, P.E.; Rashedi, A.; Norrrahim, M.N.F.; Ilyas, R.A.; Goh, K.L.; Jawaid, M.; Naveen, J. Mechanical properties of cellulose-based multiscale composites: A review. Polym. Compos. 2023, 44, 734–756. [Google Scholar] [CrossRef]
- Ciolacu, D.E.; Darie, R.N. Nanocomposites Based on Cellulose, Hemicelluloses, and Lignin. In Nanomaterials and Nanocomposites; John Wiley & Sons: Hoboken, NJ, USA, 2016; pp. 391–424. [Google Scholar] [CrossRef]
- Rojas, O.J. Cellulose Chemistry and Properties: Fibers, Nanocelluloses and Advanced Materials; Springer International Publishing: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Visakh, P.M. Introduction for Nanomaterials and Nanocomposites: State of Art, New Challenges, and Opportunities. In Nanomaterials and Nanocomposites; Wiley: Hoboken, NJ, USA, 2016; pp. 1–20. [Google Scholar] [CrossRef]
- Bangar, S.P.; Harussani, M.M.; Ilyas, R.A.; Ashogbon, A.O.; Singh, A.; Trif, M.; Jafari, S.M. Surface modifications of cellulose nanocrystals: Processes, properties, and applications. Food Hydrocoll. 2022, 130, 107689. [Google Scholar] [CrossRef]
- Mishra, R.K.; Sabu, A.; Tiwari, S.K. Materials chemistry and the futurist eco-friendly applications of nanocellulose: Status and prospect. J. Saudi Chem. Soc. 2018, 22, 949–978. [Google Scholar] [CrossRef]
- Almeida, A.P.C.; Oliveira, J.; Fernandes, S.N.; Godinho, M.H.; Canejo, J.P. All-cellulose composite membranes for oil microdroplet collection. Cellulose 2020, 27, 4665–4677. [Google Scholar] [CrossRef]
- Trache, D.; Tarchoun, A.F.; Derradji, M.; Hamidon, T.S.; Masruchin, N.; Brosse, N.; Hussin, M.H. Nanocellulose: From Fundamentals to Advanced Applications. Front. Chem. 2020, 8, 392. [Google Scholar] [CrossRef] [PubMed]
- Beck-Candanedo, S.; Roman, M.; Gray, D.G. Effect of Reaction Conditions on the Properties and Behavior of Wood Cellulose Nanocrystal Suspensions. Biomacromolecules 2005, 6, 1048–1054. [Google Scholar] [CrossRef] [PubMed]
- Barja, F. Bacterial nanocellulose production and biomedical applications. J. Biomed. Res. 2021, 35, 310–317. [Google Scholar] [CrossRef]
- Maturavongsadit, P.; Paravyan, G.; Shrivastava, R.; Benhabbour, S.R. Thermo-/pH-responsive chitosan-cellulose nanocrystals based hydrogel with tunable mechanical properties for tissue regeneration applications. Materialia 2020, 12, 100681. [Google Scholar] [CrossRef]
- Kamel, R.; El-Wakil, N.A.; Abdelkhalek, A.A.; Elkasabgy, N.A. Topical cellulose nanocrystals-stabilized nanoemulgel loaded with ciprofloxacin HCl with enhanced antibacterial activity and tissue regenerative properties. J. Drug Deliv. Sci. Technol. 2021, 64, 102553. [Google Scholar] [CrossRef]
- Yang, W.; Zheng, Y.; Chen, J.; Zhu, Q.; Feng, L.; Lan, Y.; Zhu, P.; Tang, S.; Guo, R. Preparation and characterization of the collagen/cellulose nanocrystals/USPIO scaffolds loaded kartogenin for cartilage regeneration. Mater. Sci. Eng. C 2019, 99, 1362–1373. [Google Scholar] [CrossRef]
- Patel, D.K.; Dutta, S.D.; Ganguly, K.; Lim, K.-T. Multifunctional bioactive chitosan/cellulose nanocrystal scaffolds eradicate bacterial growth and sustain drug delivery. Int. J. Biol. Macromol. 2021, 170, 178–188. [Google Scholar] [CrossRef]
- Dutta, S.D.; Hexiu, J.; Patel, D.K.; Ganguly, K.; Lim, K.-T. 3D-printed bioactive and biodegradable hydrogel scaffolds of alginate/gelatin/cellulose nanocrystals for tissue engineering. Int. J. Biol. Macromol. 2021, 167, 644–658. [Google Scholar] [CrossRef]
- Patel, D.K.; Dutta, S.D.; Hexiu, J.; Ganguly, K.; Lim, K.-T. Bioactive electrospun nanocomposite scaffolds of poly(lactic acid)/cellulose nanocrystals for bone tissue engineering. Int. J. Biol. Macromol. 2020, 162, 1429–1441. [Google Scholar] [CrossRef]
- Shaheen, T.I.; Montaser, A.S.; Li, S. Effect of cellulose nanocrystals on scaffolds comprising chitosan, alginate and hydroxyapatite for bone tissue engineering. Int. J. Biol. Macromol. 2019, 121, 814–821. [Google Scholar] [CrossRef]
- Luo, W.; Cheng, L.; Yuan, C.; Wu, Z.; Yuan, G.; Hou, M.; Chen, J.Y.; Luo, C.; Li, W. Preparation, characterization and evaluation of cellulose nanocrystal/poly(lactic acid) in situ nanocomposite scaffolds for tissue engineering. Int. J. Biol. Macromol. 2019, 134, 469–479. [Google Scholar] [CrossRef]
- Yusefi, M.; Soon, M.L.-K.; Teow, S.-Y.; Monchouguy, E.I.; Neerooa, B.N.H.M.; Izadiyan, Z.; Jahangirian, H.; Rafiee-Moghaddam, R.; Webster, T.J.; Shameli, K. Fabrication of cellulose nanocrystals as potential anticancer drug delivery systems for colorectal cancer treatment. Int. J. Biol. Macromol. 2022, 199, 372–385. [Google Scholar] [CrossRef]
- Long, W.; Ouyang, H.; Zhou, C.; Wan, W.; Yu, S.; Qian, K.; Liu, M.; Zhang, X.; Feng, Y.; Wei, Y. Simultaneous surface functionalization and drug loading: A novel method for fabrication of cellulose nanocrystals-based pH responsive drug delivery system. Int. J. Biol. Macromol. 2021, 182, 2066–2075. [Google Scholar] [CrossRef]
- Kumari, P.; Seth, R.; Meena, A.; Sharma, D. Enzymatic synthesis of cellulose nanocrystals from lemongrass and its application in improving anti-cancer drug release, uptake and efficacy. Ind. Crops Prod. 2023, 192, 115933. [Google Scholar] [CrossRef]
- Kumar, R.; Chauhan, S. Cellulose nanocrystals based delivery vehicles for anticancer agent curcumin. Int. J. Biol. Macromol. 2022, 221, 842–864. [Google Scholar] [CrossRef]
- Gabriel, T.; Belete, A.; Hause, G.; Neubert, R.H.H.; Gebre-Mariam, T. Nanocellulose-based nanogels for sustained drug delivery: Preparation, characterization and in vitro evaluation. J. Drug Deliv. Sci. Technol. 2022, 75, 103665. [Google Scholar] [CrossRef]
- Patiño Vidal, C.; Velásquez, E.; Galotto, M.J.; López de Dicastillo, C. Development of an antibacterial coaxial bionanocomposite based on electrospun core/shell fibers loaded with ethyl lauroyl arginate and cellulose nanocrystals for active food packaging. Food Packag. Shelf Life 2022, 31, 100802. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, W.; Farag, M.A.; Shao, P. Functionalized cellulose nanocrystal embedded into citrus pectin coating improves its barrier, antioxidant properties and potential application in food. Food Chem. 2023, 401, 134079. [Google Scholar] [CrossRef]
- Halloub, A.; Raji, M.; Essabir, H.; Chakchak, H.; Boussen, R.; Bensalah, M.-o.; Bouhfid, R.; Qaiss, A.e.k. Intelligent food packaging film containing lignin and cellulose nanocrystals for shelf life extension of food. Carbohydr. Polym. 2022, 296, 119972. [Google Scholar] [CrossRef]
- Lei, Y.; Yao, Q.; Jin, Z.; Wang, Y.-C. Intelligent films based on pectin, sodium alginate, cellulose nanocrystals, and anthocyanins for monitoring food freshness. Food Chem. 2023, 404, 134528. [Google Scholar] [CrossRef] [PubMed]
- Mu, R.; Hong, X.; Ni, Y.; Li, Y.; Pang, J.; Wang, Q.; Xiao, J.; Zheng, Y. Recent trends and applications of cellulose nanocrystals in food industry. Trends Food Sci. Technol. 2019, 93, 136–144. [Google Scholar] [CrossRef]
- Dong, D.; Chen, R.; Jia, J.; Zhao, C.; Chen, Z.; Lu, Q.; Sun, Y.; Huang, W.; Wang, C.; Li, Y.; et al. Tailoring and application of a multi-responsive cellulose nanofibre-based 3D nanonetwork wound dressing. Carbohydr. Polym. 2023, 305, 120542. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Chen, Z.; He, Y.; Lu, Q.; Chen, R.; Zhao, C.; Dong, D.; Sun, Y.; He, H. Dual light-responsive cellulose nanofibril-based in situ hydrogel for drug-resistant bacteria infected wound healing. Carbohydr. Polym. 2022, 297, 120042. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhu, J.; Jin, S.; Zheng, Y.; Gao, W.; Wu, D.; Yu, J.; Dai, Z. Cellulose-nanofibril-reinforced hydrogels with pH sensitivity and mechanical stability for wound healing. Mater. Lett. 2022, 323, 132596. [Google Scholar] [CrossRef]
- Liu, J.; Cheng, F.; Grénman, H.; Spoljaric, S.; Seppälä, J.; Eriksson, J.E.; Willför, S.; Xu, C. Development of nanocellulose scaffolds with tunable structures to support 3D cell culture. Carbohydr. Polym. 2016, 148, 259–271. [Google Scholar] [CrossRef]
- Li, Q.; Hatakeyama, M.; Kitaoka, T. Bioadaptive Porous 3D Scaffolds Comprising Cellulose and Chitosan Nanofibers Constructed by Pickering Emulsion Templating. Adv. Funct. Mater. 2022, 32, 2200249. [Google Scholar] [CrossRef]
- Kim, H.J.; Oh, D.X.; Choy, S.; Nguyen, H.-L.; Cha, H.J.; Hwang, D.S. 3D cellulose nanofiber scaffold with homogeneous cell population and long-term proliferation. Cellulose 2018, 25, 7299–7314. [Google Scholar] [CrossRef]
- Orasugh, J.T.; Saha, N.R.; Rana, D.; Sarkar, G.; Mollick, M.M.R.; Chattoapadhyay, A.; Mitra, B.C.; Mondal, D.; Ghosh, S.K.; Chattopadhyay, D. Jute cellulose nano-fibrils/hydroxypropylmethylcellulose nanocomposite: A novel material with potential for application in packaging and transdermal drug delivery system. Ind. Crops Prod. 2018, 112, 633–643. [Google Scholar] [CrossRef]
- Zhao, J.; Lu, C.; He, X.; Zhang, X.; Zhang, W.; Zhang, X. Polyethylenimine-Grafted Cellulose Nanofibril Aerogels as Versatile Vehicles for Drug Delivery. ACS Appl. Mater. Interfaces 2015, 7, 2607–2615. [Google Scholar] [CrossRef]
- Löbmann, K.; Svagan, A.J. Cellulose nanofibers as excipient for the delivery of poorly soluble drugs. Int. J. Pharm. 2017, 533, 285–297. [Google Scholar] [CrossRef]
- Bhandari, J.; Mishra, H.; Mishra, P.K.; Wimmer, R.; Ahmad, F.J.; Talegaonkar, S. Cellulose nanofiber aerogel as a promising biomaterial for customized oral drug delivery. Int. J. Nanomed. 2017, 12, 2021–2031. [Google Scholar] [CrossRef] [Green Version]
- Yu, Z.; Wang, W.; Kong, F.; Lin, M.; Mustapha, A. Cellulose nanofibril/silver nanoparticle composite as an active food packaging system and its toxicity to human colon cells. Int. J. Biol. Macromol. 2019, 129, 887–894. [Google Scholar] [CrossRef]
- Yu, Z.; Dhital, R.; Wang, W.; Sun, L.; Zeng, W.; Mustapha, A.; Lin, M. Development of multifunctional nanocomposites containing cellulose nanofibrils and soy proteins as food packaging materials. Food Packag. Shelf Life 2019, 21, 100366. [Google Scholar] [CrossRef]
- Kowalska-Ludwicka, K.; Cala, J.; Grobelski, B.; Sygut, D.; Jesionek-Kupnicka, D.; Kolodziejczyk, M.; Bielecki, S.; Pasieka, Z. Special paper—New method Modified bacterial cellulose tubes for regeneration of damaged peripheral nerves. Arch. Med. Sci. 2013, 9, 527–534. [Google Scholar] [CrossRef]
- Pértile, R.; Moreira, S.; Andrade, F.; Domingues, L.; Gama, M. Bacterial cellulose modified using recombinant proteins to improve neuronal and mesenchymal cell adhesion. Biotechnol. Prog. 2012, 28, 526–532. [Google Scholar] [CrossRef] [Green Version]
- Hwang, D.W.; Park, J.B.; Sung, D.; Park, S.; Min, K.-A.; Kim, K.W.; Choi, Y.; Kim, H.Y.; Lee, E.; Kim, H.S.; et al. 3D graphene-cellulose nanofiber hybrid scaffolds for cortical reconstruction in brain injuries. 2D Mater. 2019, 6, 045043. [Google Scholar] [CrossRef]
- Fey, C.; Betz, J.; Rosenbaum, C.; Kralisch, D.; Vielreicher, M.; Friedrich, O.; Metzger, M.; Zdzieblo, D. Bacterial nanocellulose as novel carrier for intestinal epithelial cells in drug delivery studies. Mater. Sci. Eng. C 2020, 109, 110613. [Google Scholar] [CrossRef]
- Jaberifard, F.; Ghorbani, M.; Arsalani, N.; Mostafavi, H. A novel insoluble film based on crosslinked-starch with gelatin containing ZnO-loaded halloysite nanotube and bacterial nanocellulose for wound healing applications. Appl. Clay Sci. 2022, 230, 106667. [Google Scholar] [CrossRef]
- Li, Q.; Gao, R.; Wang, L.; Xu, M.; Yuan, Y.; Ma, L.; Wan, Z.; Yang, X. Nanocomposites of Bacterial Cellulose Nanofibrils and Zein Nanoparticles for Food Packaging. ACS Appl. Nano Mater. 2020, 3, 2899–2910. [Google Scholar] [CrossRef]
- Cazón, P.; Vázquez, M. Bacterial cellulose as a biodegradable food packaging material: A review. Food Hydrocoll. 2021, 113, 106530. [Google Scholar] [CrossRef]
- Kamal, T.; Ul-Islam, M.; Fatima, A.; Ullah, M.W.; Manan, S. Cost-Effective Synthesis of Bacterial Cellulose and Its Applications in the Food and Environmental Sectors. Gels 2022, 8, 552. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.P.C.; Saraiva, J.N.; Cavaco, G.; Portela, R.P.; Leal, C.R.; Sobral, R.G.; Almeida, P.L. Crosslinked bacterial cellulose hydrogels for biomedical applications. Eur. Polym. J. 2022, 177, 111438. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, Z.; Hao, J.; Ding, F.; Li, Z.; Ren, X. Hollow nanosphere-doped bacterial cellulose and polypropylene wound dressings: Biomimetic nanocatalyst mediated antibacterial therapy. Chem. Eng. J. 2022, 432, 134309. [Google Scholar] [CrossRef]
- Resch, A.; Staud, C.; Radtke, C. Nanocellulose-based wound dressing for conservative wound management in children with second-degree burns. Int. Wound J. 2021, 18, 478–486. [Google Scholar] [CrossRef]
- Jantarat, C.; Muenraya, P.; Srivaro, S.; Nawakitrangsan, A.; Promsornpason, K. Comparison of drug release behavior of bacterial cellulose loaded with ibuprofen and propranolol hydrochloride. RSC Adv. 2021, 11, 37354–37365. [Google Scholar] [CrossRef]
- Beekmann, U.; Schmölz, L.; Lorkowski, S.; Werz, O.; Thamm, J.; Fischer, D.; Kralisch, D. Process control and scale-up of modified bacterial cellulose production for tailor-made anti-inflammatory drug delivery systems. Carbohydr. Polym. 2020, 236, 116062. [Google Scholar] [CrossRef]
- Meneguin, A.B.; da Silva Barud, H.; Sábio, R.M.; de Sousa, P.Z.; Manieri, K.F.; de Freitas, L.A.P.; Pacheco, G.; Alonso, J.D.; Chorilli, M. Spray-dried bacterial cellulose nanofibers: A new generation of pharmaceutical excipient intended for intestinal drug delivery. Carbohydr. Polym. 2020, 249, 116838. [Google Scholar] [CrossRef]
- Park, D.; Kim, J.W.; Shin, K.; Kim, J.W. Bacterial cellulose nanofibrils-reinforced composite hydrogels for mechanical compression-responsive on-demand drug release. Carbohydr. Polym. 2021, 272, 118459. [Google Scholar] [CrossRef]
- Barthlott, W.; Neinhuis, C. Purity of the sacred lotus, or escape from contamination in biological surfaces. Planta 1997, 202, 1–8. [Google Scholar] [CrossRef]
- Oliver, K.; Seddon, A.; Trask, R.S. Morphing in nature and beyond: A review of natural and synthetic shape-changing materials and mechanisms. J. Mater. Sci. 2016, 51, 10663–10689. [Google Scholar] [CrossRef] [Green Version]
- Ball, P. Botanists’ blues. Nature 2007, 449, 982. [Google Scholar] [CrossRef] [Green Version]
- Lee, D. Nature’s Palette: The Science of Plant Color; University of Chicago Press: Chicago, IL, USA, 2010. [Google Scholar]
- Vukusic, P.; Sambles, J.R. Photonic structures in biology. Nature 2003, 424, 852–855. [Google Scholar] [CrossRef]
- Doucet, S.M.; Meadows, M.G. Iridescence: A functional perspective. J. R. Soc. Interface 2009, 6 (Suppl. S2), S115–S132. [Google Scholar] [CrossRef] [Green Version]
- Gruson, H.; Andraud, C.; Marcillac, W.D.d.; Berthier, S.; Elias, M.; Gomez, D. Quantitative characterization of iridescent colours in biological studies: A novel method using optical theory. Interface Focus 2019, 9, 20180049. [Google Scholar] [CrossRef] [Green Version]
- Morris, R.B. Iridescence from diffraction structures in the wing scales of Callophrys rubi, the Green Hairstreak. J. Entomol. Ser. A Gen. Entomol. 1975, 49, 149–154. [Google Scholar] [CrossRef]
- Whitney, H.M.; Kolle, M.; Andrew, P.; Chittka, L.; Steiner, U.; Glover, B.J. Floral iridescence, produced by diffractive optics, acts as a cue for animal pollinators. Science 2009, 323, 130–133. [Google Scholar] [CrossRef] [Green Version]
- Kevan, P.G.; Lane, M.A. Flower petal microtexture is a tactile cue for bees. Proc. Natl. Acad. Sci. USA 1985, 82, 4750–4752. [Google Scholar] [CrossRef] [Green Version]
- Vignolini, S.; Rudall, P.J.; Rowland, A.V.; Reed, A.; Moyroud, E.; Faden, R.B.; Baumberg, J.J.; Glover, B.J.; Steiner, U. Pointillist structural color in Pollia fruit. Proc. Natl. Acad. Sci. USA 2012, 109, 15712–15715. [Google Scholar] [CrossRef] [Green Version]
- Vignolini, S.; Gregory, T.; Kolle, M.; Lethbridge, A.; Moyroud, E.; Steiner, U.; Glover, B.J.; Vukusic, P.; Rudall, P.J. Structural colour from helicoidal cell-wall architecture in fruits of Margaritaria nobilis. J. R. Soc. Interface 2016, 13, 20160645. [Google Scholar] [CrossRef] [Green Version]
- Almeida, A.P.C.; Canejo, J.P.; Fernandes, S.N.; Echeverria, C.; Almeida, P.L.; Godinho, M.H. Cellulose-Based Biomimetics and Their Applications. Adv. Mater. 2018, 30, 1703655. [Google Scholar] [CrossRef]
- Ligon, R.A.; McGraw, K.J. Chameleons communicate with complex colour changes during contests: Different body regions convey different information. Biol. Lett. 2013, 9, 20130892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darwin, C. The Movements and Habits of Climbing Plants; John Murray: London, UK, 1875. [Google Scholar]
- Almeida, A.P.; Canejo, J.; Mur, U.; Čopar, S.; Almeida, P.L.; Žumer, S.; Godinho, M.H. Spotting plants’ microfilament morphologies and nanostructures. Proc. Natl. Acad. Sci. USA 2019, 116, 13188–13193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murugesan, Y.K.; Rey, A.D. Modeling Textural Processes during Self-Assembly of Plant-Based Chiral-Nematic Liquid Crystals. Polymers 2010, 2, 766–785. [Google Scholar] [CrossRef] [Green Version]
- Tan, T.; Ribbans, B. A bioinspired study on the compressive resistance of helicoidal fibre structures. Proc. R. Soc. A Math. Phys. Eng. Sci. 2017, 473, 20170538. [Google Scholar] [CrossRef] [Green Version]
- Fratzl, P.; Elbaum, R.; Burgert, I. Cellulose fibrils direct plant organ movements. Faraday Discuss. 2008, 139, 275–282. [Google Scholar] [CrossRef]
- Elbaum, R.; Abraham, Y. Insights into the microstructures of hygroscopic movement in plant seed dispersal. Plant Sci. 2014, 223, 124–133. [Google Scholar] [CrossRef]
- Lacey, E.P.; Kaufman, P.B.; Dayanandan, P. The Anatomical Basis for Hygroscopic Movement in Primary Rays of Daucus carota Ssp. carota (Apiaceae). Bot. Gaz. 1983, 144, 371–375. [Google Scholar] [CrossRef] [Green Version]
- Bertinetti, L.; Fischer, F.D.; Fratzl, P. Physicochemical Basis for Water-Actuated Movement and Stress Generation in Nonliving Plant Tissues. Phys. Rev. Lett. 2013, 111, 238001. [Google Scholar] [CrossRef]
- Friedman, J.; Gunderman, N.; Ellis, M. Water response of the hygrochastic skeletons of the true rose of Jericho (Anastatica hierochuntica L.). Oecologia 1978, 32, 289–301. [Google Scholar] [CrossRef]
- Hegazy, A.K.; Barakat, H.N.; Kabiel, H.F. Anatomical significance of the hygrochastic movement in Anastatica hierochuntica. Ann. Bot. 2006, 97, 47–55. [Google Scholar] [CrossRef] [Green Version]
- Lebkuecher, J.G.; Eickmeier, W.G. Reduced photoinhibition with stem curling in the resurrection plant Selaginella lepidophylla. Oecologia 1991, 88, 597–604. [Google Scholar] [CrossRef]
- Le Duigou, A.; Castro, M. Evaluation of force generation mechanisms in natural, passive hydraulic actuators. Sci. Rep. 2016, 6, 18105. [Google Scholar] [CrossRef] [Green Version]
- Dawson, C.; Vincent, J.F.V.; Rocca, A.-M. How pine cones open. Nature 1997, 390, 668. [Google Scholar] [CrossRef]
- Song, K.; Yeom, E.; Seo, S.-J.; Kim, K.; Kim, H.; Lim, J.-H.; Joon Lee, S. Journey of water in pine cones. Sci. Rep. 2015, 5, 9963. [Google Scholar] [CrossRef] [Green Version]
- Song, K.; Chang, S.-S.; Lee, S.J. How the pine seeds attach to/detach from the pine cone scale? Front. Life Sci. 2017, 10, 38–47. [Google Scholar] [CrossRef] [Green Version]
- Poppinga, S.; Nestle, N.; Šandor, A.; Reible, B.; Masselter, T.; Bruchmann, B.; Speck, T. Hygroscopic motions of fossil conifer cones. Sci. Rep. 2017, 7, 40302. [Google Scholar] [CrossRef] [Green Version]
- Newcombe, F.C.J.B.G. Spore-dissemination of Equisetum. Bot. Gaz. 1888, 13, 173–178. [Google Scholar] [CrossRef] [Green Version]
- Katifori, E.; Alben, S.; Cerda, E.; Nelson, D.R.; Dumais, J. Foldable structures and the natural design of pollen grains. Proc. Natl. Acad. Sci. USA 2010, 107, 7635–7639. [Google Scholar] [CrossRef] [Green Version]
- Noblin, X.; Rojas, N.O.; Westbrook, J.; Llorens, C.; Argentina, M.; Dumais, J. The Fern Sporangium: A Unique Catapult. Science 2012, 335, 1322. [Google Scholar] [CrossRef] [Green Version]
- Marmottant, P.; Ponomarenko, A.; Bienaimé, D. The walk and jump of Equisetum spores. Proc. R. Soc. B Biol. Sci. 2013, 280, 20131465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bar-On, B.; Sui, X.; Livanov, K.; Achrai, B.; Kalfon-Cohen, E.; Wiesel, E.; Wagner, H.D. Structural origins of morphing in plant tissues. Appl. Phys. Lett. 2014, 105, 033703. [Google Scholar] [CrossRef]
- Ghafouri, R.; Bruinsma, R. Helicoid to Spiral Ribbon Transition. Phys. Rev. Lett. 2005, 94, 138101. [Google Scholar] [CrossRef] [PubMed]
- Armon, S.; Efrati, E.; Kupferman, R.; Sharon, E. Geometry and mechanics in the opening of chiral seed pods. Science 2011, 333, 1726–1730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abraham, Y.; Elbaum, R. Hygroscopic movements in Geraniaceae: The structural variations that are responsible for coiling or bending. New Phytol. 2013, 199, 584–594. [Google Scholar] [CrossRef]
- Almeida, A.P.C.; Querciagrossa, L.; Silva, P.E.S.; Gonçalves, F.; Canejo, J.P.; Almeida, P.L.; Godinho, M.H.; Zannoni, C. Reversible water driven chirality inversion in cellulose-based helices isolated from Erodium awns. Soft Matter 2019, 15, 2838–2847. [Google Scholar] [CrossRef]
- Jung, W.; Kim, W.; Kim, H.Y. Self-burial mechanics of hygroscopically responsive awns. Integr. Comp. Biol. 2014, 54, 1034–1042. [Google Scholar] [CrossRef] [Green Version]
- Zhao, C.; Liu, Q.; Ren, L.; Song, Z.; Wang, J. A 3D micromechanical study of hygroscopic coiling deformation in Pelargonium seed: From material and mechanics perspective. J. Mater. Sci. 2017, 52, 415–430. [Google Scholar] [CrossRef]
- Stamp, N.E. Self-Burial Behaviour of Erodium Cicutarium Seeds. J. Ecol. 1984, 72, 611–620. [Google Scholar] [CrossRef]
- Elbaum, R.; Gorb, S.; Fratzl, P. Structures in the cell wall that enable hygroscopic movement of wheat awns. J. Struct. Biol. 2008, 164, 101–107. [Google Scholar] [CrossRef]
- Rafsanjani, A.; Brulé, V.; Western, T.L.; Pasini, D. Hydro-Responsive Curling of the Resurrection Plant Selaginella lepidophylla. Sci. Rep. 2015, 5, 8064. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X.; Tian, B.; Xuan, X.; Zhou, W.; Zhou, J.; Chen, Y.; Lu, Y.; Zhang, Z. Cellulose membranes as moisture-driven actuators with predetermined deformations and high load uptake. Int. J. Smart Nano Mater. 2021, 12, 146–156. [Google Scholar] [CrossRef]
- Cao, X.; Wang, X.; Ding, B.; Yu, J.; Sun, G. Novel spider-web-like nanoporous networks based on jute cellulose nanowhiskers. Carbohydr. Polym. 2013, 92, 2041–2047. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, D.; Cheng, W.; Huang, J.; Cao, M.; Niu, Z.; Zhao, Y.; Yue, Y.; Han, G. Spider-web-inspired membrane reinforced with sulfhydryl-functionalized cellulose nanocrystals for oil/water separation. Carbohydr. Polym. 2022, 282, 119049. [Google Scholar] [CrossRef]
- Laboy-López, S.; Méndez Fernández, P.O.; Padilla-Zayas, J.G.; Nicolau, E. Bioactive Cellulose Acetate Electrospun Mats as Scaffolds for Bone Tissue Regeneration. Int. J. Biomater. 2022, 2022, 3255039. [Google Scholar] [CrossRef]
- Shigezawa, N.; Ito, F.; Murakami, Y.; Yamanaka, S.; Morikawa, H. Development of combination textile of thin and thick fiber for fog collection bioinspired by Burkheya purpurea. J. Text. Inst. 2016, 107, 1014–1021. [Google Scholar] [CrossRef]
- Han, D.; Gouma, P.-I. Electrospun bioscaffolds that mimic the topology of extracellular matrix. Nanomed. Nanotechnol. Biol. Med. 2006, 2, 37–41. [Google Scholar] [CrossRef]
- Gruppuso, M.; Turco, G.; Marsich, E.; Porrelli, D. Polymeric wound dressings, an insight into polysaccharide-based electrospun membranes. Appl. Mater. Today 2021, 24, 101148. [Google Scholar] [CrossRef]
- Vatankhah, E.; Prabhakaran, M.P.; Jin, G.; Mobarakeh, L.G.; Ramakrishna, S. Development of nanofibrous cellulose acetate/gelatin skin substitutes for variety wound treatment applications. J. Biomater. Appl. 2014, 28, 909–921. [Google Scholar] [CrossRef]
- Liao, N.; Unnithan, A.R.; Joshi, M.K.; Tiwari, A.P.; Hong, S.T.; Park, C.-H.; Kim, C.S. Electrospun bioactive poly (ε-caprolactone)–cellulose acetate–dextran antibacterial composite mats for wound dressing applications. Colloids Surf. A Physicochem. Eng. Asp. 2015, 469, 194–201. [Google Scholar] [CrossRef]
- Gaydhane, M.K.; Kanuganti, J.S.; Sharma, C.S. Honey and curcumin loaded multilayered polyvinylalcohol/cellulose acetate electrospun nanofibrous mat for wound healing. J. Mater. Res. 2020, 35, 600–609. [Google Scholar] [CrossRef]
- Liu, F.; Li, X.; Wang, L.; Yan, X.; Ma, D.; Liu, Z.; Liu, X. Sesamol incorporated cellulose acetate-zein composite nanofiber membrane: An efficient strategy to accelerate diabetic wound healing. Int. J. Biol. Macromol. 2020, 149, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, E.; Eslami-Arshaghi, T.; Hosseinzadeh, S.; Elahirad, E.; Jamalpoor, Z.; Hatamie, S.; Soleimani, M. The biomedical potential of cellulose acetate/polyurethane nanofibrous mats containing reduced graphene oxide/silver nanocomposites and curcumin: Antimicrobial performance and cutaneous wound healing. Int. J. Biol. Macromol. 2020, 152, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Beikzadeh, S.; Akbarinejad, A.; Swift, S.; Perera, J.; Kilmartin, P.A.; Travas-Sejdic, J. Cellulose acetate electrospun nanofibers encapsulating Lemon Myrtle essential oil as active agent with potent and sustainable antimicrobial activity. React. Funct. Polym. 2020, 157, 104769. [Google Scholar] [CrossRef]
- Wang, X.; Huang, Z.; Miao, D.; Zhao, J.; Yu, J.; Ding, B. Biomimetic Fibrous Murray Membranes with Ultrafast Water Transport and Evaporation for Smart Moisture-Wicking Fabrics. ACS Nano 2019, 13, 1060–1070. [Google Scholar] [CrossRef]
- Murray, C.D. The Physiological Principle of Minimum Work: I. The Vascular System and the Cost of Blood Volume. Proc. Natl. Acad. Sci. USA 1926, 12, 207–214. [Google Scholar] [CrossRef] [Green Version]
- Guan, Q.-F.; Han, Z.-M.; Zhu, Y.; Xu, W.-L.; Yang, H.-B.; Ling, Z.-C.; Yan, B.-B.; Yang, K.-P.; Yin, C.-H.; Wu, H.; et al. Bio-Inspired Lotus-Fiber-like Spiral Hydrogel Bacterial Cellulose Fibers. Nano Lett. 2021, 21, 952–958. [Google Scholar] [CrossRef]
- Liu, H.; Pang, B.; Tang, Q.; Müller, M.; Zhang, H.; Dervişoğlu, R.; Zhang, K. Self-Assembly of Surface-Acylated Cellulose Nanowhiskers and Graphene Oxide for Multiresponsive Janus-Like Films with Time-Dependent Dry-State Structures. Small 2020, 16, 2004922. [Google Scholar] [CrossRef]
- Gevorkian, A.; Morozova, S.M.; Kheiri, S.; Khuu, N.; Chen, H.; Young, E.; Yan, N.; Kumacheva, E. Actuation of Three-Dimensional-Printed Nanocolloidal Hydrogel with Structural Anisotropy. Adv. Funct. Mater. 2021, 31, 2010743. [Google Scholar] [CrossRef]
- Wang, M.; Tian, X.; Ras, R.H.A.; Ikkala, O. Sensitive Humidity-Driven Reversible and Bidirectional Bending of Nanocellulose Thin Films as Bio-Inspired Actuation. Adv. Mater. Interfaces 2015, 2, 1500080. [Google Scholar] [CrossRef]
- Zhu, Q.; Jin, Y.; Wang, W.; Sun, G.; Wang, D. Bioinspired Smart Moisture Actuators Based on Nanoscale Cellulose Materials and Porous, Hydrophilic EVOH Nanofibrous Membranes. ACS Appl. Mater. Interfaces 2019, 11, 1440–1448. [Google Scholar] [CrossRef]
- Duan, R.; Lu, M.; Tang, R.; Guo, Y.; Zhao, D. Structural Color Controllable Humidity Response Chiral Nematic Cellulose Nanocrystalline Film. Biosensors 2022, 12, 707. [Google Scholar] [CrossRef]
- Wu, T.; Li, J.; Li, J.; Ye, S.; Wei, J.; Guo, J. A bio-inspired cellulose nanocrystal-based nanocomposite photonic film with hyper-reflection and humidity-responsive actuator properties. J. Mater. Chem. C 2016, 4, 9687–9696. [Google Scholar] [CrossRef]
- Zhang, Z.-L.; Dong, X.; Fan, Y.-N.; Yang, L.-M.; He, L.; Song, F.; Wang, X.-L.; Wang, Y.-Z. Chameleon-Inspired Variable Coloration Enabled by a Highly Flexible Photonic Cellulose Film. ACS Appl. Mater. Interfaces 2020, 12, 46710–46718. [Google Scholar] [CrossRef]
- Zhang, Z.-L.; Dong, X.; Zhao, Y.-Y.; Song, F.; Wang, X.-L.; Wang, Y.-Z. Bioinspired Optical Flexible Cellulose Nanocrystal Films with Strain-Adaptive Structural Coloration. Biomacromolecules 2022, 23, 4110–4117. [Google Scholar] [CrossRef]
- Lin, F.; Wang, Z.; Shen, Y.; Tang, L.; Zhang, P.; Wang, Y.; Chen, Y.; Huang, B.; Lu, B. Natural skin-inspired versatile cellulose biomimetic hydrogels. J. Mater. Chem. A 2019, 7, 26442–26455. [Google Scholar] [CrossRef]
- Hou, K.; Nie, Y.; Tendo Mugaanire, I.; Guo, Y.; Zhu, M. A novel leaf inspired hydrogel film based on fiber reinforcement as rapid steam sensor. Chem. Eng. J. 2020, 382, 122948. [Google Scholar] [CrossRef]
- Spadaccini, C.M. Additive manufacturing and processing of architected materials. Mater. Res. Soc. Bull. 2019, 44, 782–788. [Google Scholar] [CrossRef]
- Wang, D.; Chen, D.; Chen, Z. Recent Progress in 3D Printing of Bioinspired Structures. Front. Mater. 2020, 7, 286. [Google Scholar] [CrossRef]
- Alexander, A.E.; Wake, N.; Chepelev, L.; Brantner, P.; Ryan, J.; Wang, K.C. A guideline for 3D printing terminology in biomedical research utilizing ISO/ASTM standards. 3D Print. Med. 2021, 7, 8. [Google Scholar] [CrossRef]
- ISO/ASTM52900-21; Additive Manufacturing—General Principles—Fundamentals and Vocabulary. West ASTM International: Conshohocken, PA, USA, 2022; p. 14. [CrossRef]
- Kam, D.; Chasnitsky, M.; Nowogrodski, C.; Braslavsky, I.; Abitbol, T.; Magdassi, S.; Shoseyov, O. Direct Cryo Writing of Aerogels via 3D Printing of Aligned Cellulose Nanocrystals Inspired by the Plant Cell Wall. Colloid Interfac 2019, 3, 46. [Google Scholar] [CrossRef] [Green Version]
- Mendes, B.B.; Gómez-Florit, M.; Hamilton, A.G.; Detamore, M.S.; Domingues, R.M.A.; Reis, R.L.; Gomes, M.E. Human platelet lysate-based nanocomposite bioink for bioprinting hierarchical fibrillar structures. Biofabrication 2020, 12, 015012. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Oguzlu, H.; Jiang, F. 3D printing of lightweight, super-strong yet flexible all-cellulose structure. Chem. Eng. J. 2021, 405, 126668. [Google Scholar] [CrossRef]
- Guo, J.; Li, Q.; Zhang, R.; Li, B.; Zhang, J.; Yao, L.; Lin, Z.; Zhang, L.; Cao, X.; Duan, B. Loose Pre-Cross-Linking Mediating Cellulose Self-Assembly for 3D Printing Strong and Tough Biomimetic Scaffolds. Biomacromolecules 2022, 23, 877–888. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Bhat, M.P.; Kim, C.S.; Kim, J.; Lee, K.-H. Improved 3D-Printability of Cellulose Acetate to Mimic Water Absorption in Plant Roots through Nanoporous Networks. Macromolecules 2022, 55, 1855–1865. [Google Scholar] [CrossRef]
- Esmaeili, M.; Norouzi, S.; George, K.; Rezvan, G.; Taheri-Qazvini, N.; Sadati, M. 3D Printing-Assisted Self-Assembly to Bio-Inspired Bouligand Nanostructures. Small 2023, 2023, 2206847. [Google Scholar] [CrossRef]
- Raviv, D.; Zhao, W.; McKnelly, C.; Papadopoulou, A.; Kadambi, A.; Shi, B.; Hirsch, S.; Dikovsky, D.; Zyracki, M.; Olguin, C.; et al. Active Printed Materials for Complex Self-Evolving Deformations. Sci. Rep. 2014, 4, 7422. [Google Scholar] [CrossRef] [Green Version]
- Tibbits, S. The emergence of “4D printing”. In Proceedings of TED Conferences. Available online: https://youtu.be/0gMCZFHv9v8 (accessed on 20 December 2022).
- Tibbits, S.; McKnelly, C.; Olguin, C.; Dikovsky, D.; Hirsch, S. 4D printing and universal transformation. In Proceedings of the 34th Annual Conference of the Association for Computer Aided Design in Architecture, Los Angeles, CA, USA, 23–25 October 2014; pp. 539–548. [Google Scholar]
- Momeni, F.; Hassani.N, S.M.; Liu, X.; Ni, J. A review of 4D printing. Mater. Des. 2017, 122, 42–79. [Google Scholar] [CrossRef]
- 4D Printing Market, by Application (Aerospace and Defense, Healthcare, Automotive, Construction, Clothing, Utility, Others.), by Region-Forecast to 2030. 2023, p. 100. Available online: https://www.precedenceresearch.com/4d-printing-market (accessed on 4 January 2023).
- Gladman, A.S.; Matsumoto, E.A.; Nuzzo, R.G.; Mahadevan, L.; Lewis, J.A. Biomimetic 4D printing. Nat. Mater. 2016, 15, 413–418. [Google Scholar] [CrossRef]
- Siqueira, G.; Kokkinis, D.; Libanori, R.; Hausmann, M.K.; Gladman, A.S.; Neels, A.; Tingaut, P.; Zimmermann, T.; Lewis, J.A.; Studart, A.R. Cellulose Nanocrystal Inks for 3D Printing of Textured Cellular Architectures. Adv. Funct. Mater. 2017, 27, 1604619. [Google Scholar] [CrossRef]
- Zhao, X.; Tekinalp, H.; Meng, X.; Ker, D.; Benson, B.; Pu, Y.; Ragauskas, A.J.; Wang, Y.; Li, K.; Webb, E.; et al. Poplar as Biofiber Reinforcement in Composites for Large-Scale 3D Printing. ACS Appl. Bio Mater. 2019, 2, 4557–4570. [Google Scholar] [CrossRef]
- Correa, D.; Papadopoulou, A.; Guberan, C.; Jhaveri, N.; Reichert, S.; Menges, A.; Tibbits, S. 3D-Printed Wood: Programming Hygroscopic Material Transformations. 3D Print Addit. Manuf. 2015, 2, 106–116. [Google Scholar] [CrossRef]
- Le Duigou, A.; Correa, D.; Ueda, M.; Matsuzaki, R.; Castro, M. A review of 3D and 4D printing of natural fibre biocomposites. Mater. Des. 2020, 194, 108911. [Google Scholar] [CrossRef]
- Correa, D.; Poppinga, S.; Mylo, M.D.; Westermeier, A.S.; Bruchmann, B.; Menges, A.; Speck, T. 4D pine scale: Biomimetic 4D printed autonomous scale and flap structures capable of multi-phase movement. Philos. Trans. A Math. Phys. Eng. Sci. 2020, 378, 20190445. [Google Scholar] [CrossRef]
- Mulakkal, M.C.; Trask, R.S.; Ting, V.P.; Seddon, A.M. Responsive cellulose-hydrogel composite ink for 4D printing. Mater. Des. 2018, 160, 108–118. [Google Scholar] [CrossRef]
- Wang, W.; Yao, L.N.; Zhang, T.; Cheng, C.Y.; Levine, D.; Ishii, H. Transformative Appetite: Shape-Changing Food Transforms from 2D to 3D by Water Interaction through Cooking. In Proceedings of the 2017 ACM Sigchi Conference on Human Factors in Computing Systems (Chi’17), Denver, CO, USA, 6–11 May 2017; pp. 6123–6132. [Google Scholar] [CrossRef]
- Teng, X.; Zhang, M.; Mujumdar, A.S. 4D printing: Recent advances and proposals in the food sector. Trends Food Sci. Technol. 2021, 110, 349–363. [Google Scholar] [CrossRef]
- Navaf, M.; Sunooj, K.V.; Aaliya, B.; Akhila, P.P.; Sudheesh, C.; Mir, S.A.; George, J. 4D printing: A new approach for food printing; effect of various stimuli on 4D printed food properties. A comprehensive review. Appl. Food Res. 2022, 2, 100150. [Google Scholar] [CrossRef]
- Lai, J.; Ye, X.; Liu, J.; Wang, C.; Li, J.; Wang, X.; Ma, M.; Wang, M. 4D printing of highly printable and shape morphing hydrogels composed of alginate and methylcellulose. Mater. Des. 2021, 205, 109699. [Google Scholar] [CrossRef]
| Cellulose Nanomaterial | Field of Application | Ref |
|---|---|---|
| Cellulose Nanocrystal (CNC) | Tissue regeneration | [31,32,33] |
| Scaffolds | [34,35,36,37,38] | |
| Drug delivery systems | [39,40,41,42,43] | |
| Food industry | [44,45,46,47,48] | |
| Cellulose Nanofibril (CNF) | Wound healing | [49,50,51] |
| 3D cell culture | [52,53,54] | |
| Drug delivery systems | [55,56,57,58] | |
| Food industry | [59,60] | |
| Bacterial Nanocellulose (BNC) | Tissue regeneration | [61,62,63] |
| Scaffolds | [64,65] | |
| Food packing | [66,67,68] | |
| Wound dressing | [69,70,71] | |
| Drug delivery systems | [72,73,74,75] |
| Standard Term | Commercial Term | Description |
|---|---|---|
| PBF (Powder bed fusion molding) | SLS—Selective Laser Sintering | Powder media is deposited on a build platform and subsequently bonded together through a heating process. |
| SLM—Selective Laser Melting | ||
| DMP—Direct Metal Printing | ||
| DMLS—Direct Metal Laser Sintering | ||
| EBM—Electron Beam Melting | ||
| MJF—Multi Jet Fusion | ||
| ME (Material extrusion molding) | FDM—Fused Deposition Modeling | Material is dispensed, usually through a heated nozzle, onto a build platform. |
| FFF—Fused Filament Fabrication | ||
| BJ (Binder injection molding) | CJP—ProJet Color Jet Printing | Liquid agents are selectively dropped onto powder media. Subsequent infiltration or heating may be required. |
| Photopolymerization curing | SLA—Stereolithography apparatus | Liquid photopolymer is selectively exposed to a light source facilitating layer-by-layer curing. |
| DLP—Direct Light Processing | ||
| CLIP—Continuous liquid interface production | ||
| MJ (Material inject forming) | NPJ—Nanoparticle Jetting | A print head dispenses droplets of media, usually a photopolymer, onto a build platform where each layer is solidified or cured. |
| DOD—Drop-on-Demand | ||
| PolyJet | ||
| MJP—PolyJet Multijet Printing | ||
| DED (Direct energy deposition) | LENS—Laser Engineered Net Shape | Focused application of energy and material selectively melted and fused on a build platform or part. |
| EBAM—Electron Beam Additive Manufacture | ||
| SL (Sheet lamination) | LOM—Laminated Object Manufacturing | Discrete layers of material are fused or glued together to form a 3D object. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magalhães, M.I.; Almeida, A.P.C. Nature-Inspired Cellulose-Based Active Materials: From 2D to 4D. Appl. Biosci. 2023, 2, 94-114. https://doi.org/10.3390/applbiosci2010009
Magalhães MI, Almeida APC. Nature-Inspired Cellulose-Based Active Materials: From 2D to 4D. Applied Biosciences. 2023; 2(1):94-114. https://doi.org/10.3390/applbiosci2010009
Chicago/Turabian StyleMagalhães, Marta I., and Ana P. C. Almeida. 2023. "Nature-Inspired Cellulose-Based Active Materials: From 2D to 4D" Applied Biosciences 2, no. 1: 94-114. https://doi.org/10.3390/applbiosci2010009
APA StyleMagalhães, M. I., & Almeida, A. P. C. (2023). Nature-Inspired Cellulose-Based Active Materials: From 2D to 4D. Applied Biosciences, 2(1), 94-114. https://doi.org/10.3390/applbiosci2010009