Blood Pressure Control in the DIAbetes and LifEstyle Cohort Twente (DIALECT): The Role of Patient Adherence and Physician’s Follow-Up Action
Abstract
:1. Introduction
2. Results
2.1. Patient Inclusion
2.2. Descriptive Data
2.3. Adherence and Baseline BP
2.4. Adherence to Individual Antihypertensive Drug Classes
2.5. Intensification of Antihypertensive Drug Therapy and BP Course
2.6. Uncontrolled BP and Role of Adherence and Clinical Inertia
3. Discussion
3.1. Strengths and Limitations
3.2. Clinical Implications
4. Materials and Methods
4.1. Study Design and Setting
4.2. Participants
4.3. Measurement of Adherence to Antihypertensive Drugs
4.4. Intensification of Antihypertensive Drug Therapy
4.5. BP Targets
4.6. Baseline BP Measurements
4.7. Follow-Up BP Measurements
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Total Population | BP-OT | BP-NOT | p | |
|---|---|---|---|---|
| Patients with albuminuria * | 108 | 35 (32.4) | 73 (67.6) | <0.001 |
| Adherent (MPR ≥ 80%) | 102 (94.4) | 34 (97.1) | 68 (93.2) | ** |
References
- VizHub—GBD Results. Available online: https://vizhub.healthdata.org/gbd-results/ (accessed on 9 January 2023).
- Caussy, C.; Aubin, A.; Loomba, R. The Relationship Between Type 2 Diabetes, NAFLD, and Cardiovascular Risk. Curr. Diabetes Rep. 2021, 21, 15. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; McAlister, F.A.; Walker, R.L.; Hemmelgarn, B.R.; Campbell, N.R.C. Cardiovascular Outcomes in Framingham Participants With Diabetes. Hypertension 2011, 57, 891–897. [Google Scholar] [CrossRef] [PubMed]
- Gant, C.M.; Binnenmars, S.H.; van den Berg, E.; Bakker, S.J.L.; Navis, G.; Laverman, G.D. Integrated Assessment of Pharmacological and Nutritional Cardiovascular Risk Management: Blood Pressure Control in the DIAbetes and LifEstyle Cohort Twente (DIALECT). Nutrients 2017, 9, 709. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.F.; Zhang, Z.Y.; Huang, Q.F.; Yang, W.Y.; Staessen, J.A. Resistant Hypertension. Kardiol. Pol. 2018, 76, 1031–1042. [Google Scholar] [CrossRef]
- Phillips, L.S.; Branch, J.; Cook, C.B.; Doyle, J.P.; El-Kebbi, I.M.; Gallina, D.L.; Miller, C.D.; Ziemer, D.C.; Barnes, C.S. Clinical Inertia. Ann. Intern. Med. 2001, 135, 825–834. [Google Scholar] [CrossRef]
- Milman, T.; Joundi, R.A.; Alotaibi, N.M.; Saposnik, G. Clinical Inertia in the Pharmacological Management of Hypertension: A Systematic Review and Meta-Analysis. Medicine 2018, 97, e11121. [Google Scholar] [CrossRef]
- Burnier, M.; Egan, B.M. Adherence in Hypertension. Circ. Res. 2019, 124, 1124–1140. [Google Scholar] [CrossRef]
- Hill, M.N.; Miller, N.H.; DeGeest, S. ASH Position Paper: Adherence and Persistence with Taking Medication to Control High Blood Pressure. J. Clin. Hypertens. 2010, 12, 757–764. [Google Scholar] [CrossRef]
- Vrijens, B.; Vincze, G.; Kristanto, P.; Urquhart, J.; Burnier, M. Adherence to Prescribed Antihypertensive Drug Treatments: Longitudinal Study of Electronically Compiled Dosing Histories. BMJ 2008, 336, 1114–1117. [Google Scholar] [CrossRef]
- Beernink, J.M.; Oosterwijk, M.M.; van Boven, J.F.M.; Heerspink, H.J.L.; Bakker, S.J.L.; Navis, G.; Nijboer, R.M.; Gant, C.M.; Haverkate, H.; Kruik-Kollöffel, W.J.; et al. Adherence to Statin Therapy and Attainment of LDL Cholesterol Targets in an Outpatient Population of Type 2 Diabetes Patients: Analysis in the DIAbetes and LifEstyle Cohort Twente (DIALECT). Front. Pharmacol. 2022, 13, 888110. [Google Scholar] [CrossRef]
- Hope, H.F.; Binkley, G.M.; Fenton, S.; Kitas, G.D.; Verstappen, S.M.M.; Symmons, D.P.M. Systematic Review of the Predictors of Statin Adherence for the Primary Prevention of Cardiovascular Disease. PLoS ONE 2019, 14, e0201196. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Kwon, O.D.; Han, E.B.; Lee, C.M.; Oh, S.W.; Joh, H.K.; Oh, B.; Kwon, H.; Cho, B.; Choi, H.C. Impact of Number of Medications and Age on Adherence to Antihypertensive Medications: A Nationwide Population-Based Study. Medicine 2019, 98, e17825. [Google Scholar] [CrossRef] [PubMed]
- Uchmanowicz, B.; Jankowska, E.A.; Uchmanowicz, I.; Morisky, D.E. Self-Reported Medication Adherence Measured With Morisky Medication Adherence Scales and Its Determinants in Hypertensive Patients Aged ≥60 Years: A Systematic Review and Meta-Analysis. Front. Pharmacol. 2019, 10, 168. [Google Scholar] [CrossRef] [PubMed]
- Alencar de Pinho, N.; Levin, A.; Fukagawa, M.; Hoy, W.E.; Pecoits-Filho, R.; Reichel, H.; Robinson, B.; Kitiyakara, C.; Wang, J.; Eckardt, K.U.; et al. Considerable International Variation Exists in Blood Pressure Control and Antihypertensive Prescription Patterns in Chronic Kidney Disease. Kidney Int. 2019, 96, 983–994. [Google Scholar] [CrossRef] [PubMed]
- Gyberg, V.; Bacquer, D.; Backer, G.; Jennings, C.; Kotseva, K.; Mellbin, L.; Schnell, O.; Tuomilehto, J.; Wood, D.; Rydén, L.; et al. On behalf of the EUROASPIRE Investigators. Patients with Coronary Artery Disease and Diabetes Need Improved Management: A Report from the EUROASPIRE IV Survey: A Registry from the EuroObservational Research Programme of the European Society of Cardiology. Cardiovasc. Diabetol. 2015, 14, 1–11. [Google Scholar] [CrossRef]
- Naderi, S.H.; Bestwick, J.P.; Wald, D.S. Adherence to Drugs That Prevent Cardiovascular Disease: Meta-Analysis on 376,162 Patients. Am. J. Med. 2012, 125, 882–887.e1. [Google Scholar] [CrossRef]
- Elliott, W.J.; Plauschinat, C.A.; Skrepnek, G.H.; Gause, D. Persistence, Adherence, and Risk of Discontinuation Associated with Commonly Prescribed Antihypertensive Drug Monotherapies. J. Am. Board Fam. Med. 2007, 20, 72–80. [Google Scholar] [CrossRef]
- Jankowska-Polańska, B.; Karniej, P.; Polański, J.; Seń, M.; Świątoniowska-Lonc, N.; Grochans, E. Diabetes Mellitus Versus Hypertension—Does Disease Affect Pharmacological Adherence? Front. Pharmacol. 2020, 11, 1157. [Google Scholar] [CrossRef]
- Lee, C.Y.; Huang, C.C.; Shih, H.C.; Huang, K.H. Factors Influencing Antihypertensive Medication Compliance in Taiwan: A Nationwide Population-Based Study. Eur. J. Prev. Cardiol. 2013, 20, 930–937. [Google Scholar] [CrossRef]
- Kronish, I.M.; Woodward, M.; Sergie, Z.; Ogedegbe, G.; Falzon, L.; Mann, D.M. Meta-Analysis: Impact of Drug Class on Adherence to Antihypertensives. Circulation 2011, 123, 1611–1621. [Google Scholar] [CrossRef]
- Brown, M.J.; Palmer, C.R.; Castaigne, A.; De Leeuw, P.W.; Mancia, G.; Rosenthal, T.; Ruilope, L.M. Morbidity and Mortality in Patients Randomised to Double-Blind Treatment with a Long-Acting Calcium-Channel Blocker or Diuretic in the International Nifedipine GITS Study: Intervention as a Goal in Hypertension Treatment (INSIGHT). Lancet 2000, 356, 366–372. [Google Scholar] [CrossRef]
- Dahlöf, B.; Devereux, R.B.; Kjeldsen, S.E.; Julius, S.; Beevers, G.; De Faire, U.; Fyhrquist, F.; Ibsen, H.; Kristiansson, K.; Lederballe-Pedersen, O.; et al. Cardiovascular Morbidity and Mortality in the Losartan Intervention For Endpoint Reduction in Hypertension Study (LIFE): A Randomised Trial against Atenolol. Lancet 2002, 359, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Josiah Willock, R.; Miller, J.B.; Mohyi, M.; Abuzaanona, A.; Muminovic, M.; Levy, P.D. Therapeutic Inertia and Treatment Intensification. Curr. Hypertens. Rep. 2018, 20, 4. [Google Scholar] [CrossRef] [PubMed]
- Almigbal, T.H.; Alzarah, S.A.; Aljanoubi, F.A.; Alhafez, N.A.; Aldawsari, M.R.; Alghadeer, Z.Y.; Alrasheed, A.A. Clinical Inertia in the Management of Type 2 Diabetes Mellitus: A Systematic Review. Medicina 2023, 59, 182. [Google Scholar] [CrossRef] [PubMed]
- Ali, D.H.; Kiliç, B.; Hart, H.E.; Bots, M.L.; Biermans, M.C.J.; Spiering, W.; Rutten, F.H.; Hollander, M. Therapeutic Inertia in the Management of Hypertension in Primary Care. J. Hypertens. 2021, 39, 1238–1245. [Google Scholar] [CrossRef] [PubMed]
- Shah, B.R.; Hux, J.E.; Laupacis, A.; Zinman, B.; Van Walraven, C. Clinical Inertia in Response to Inadequate Glycemic Control: Do Specialists Differ from Primary Care Physicians? Diabetes Care 2005, 28, 600–606. [Google Scholar] [CrossRef]
- Schutte, A.E.; Kollias, A.; Stergiou, G.S. Blood Pressure and Its Variability: Classic and Novel Measurement Techniques. Nat. Rev. Cardiol. 2022, 19, 643–654. [Google Scholar] [CrossRef]
- Steiner, J.F.; Prochazka, A.V. The Assessment of Refill Compliance Using Pharmacy Records: Methods, Validity, and Applications. J. Clin. Epidemiol. 1997, 50, 105–116. [Google Scholar] [CrossRef]
- Anghel, L.A.; Farcas, A.M.; Oprean, R.N. An Overview of the Common Methods Used to Measure Treatment Adherence. Med. Pharm. Rep. 2019, 92, 117. [Google Scholar] [CrossRef]
- Beernink, J.M.; Oosterwijk, M.M.; Khunti, K.; Gupta, P.; Patel, P.; van Boven, J.F.M.; Lambers Heerspink, H.J.; Bakker, S.J.L.; Navis, G.; Nijboer, R.M.; et al. Biochemical Urine Testing of Medication Adherence and Its Association with Clinical Markers in an Outpatient Population of Type 2 Diabetes Patients: Analysis in the DIAbetes and LifEstyle Cohort Twente (DIALECT). Diabetes Care 2021, 44, 1419. [Google Scholar] [CrossRef]
- Cheung, A.K.; Chang, T.I.; Cushman, W.C.; Furth, S.L.; Hou, F.F.; Ix, J.H.; Knoll, G.A.; Muntner, P.; Pecoits-Filho, R.; Sarnak, M.J.; et al. KDIGO 2021 Clinical Practice Guideline for the Management of Blood Pressure in Chronic Kidney Disease. Kidney Int. 2021, 99, S1–S87. [Google Scholar] [CrossRef] [PubMed]
- Cosentino, F.; Grant, P.J.; Aboyans, V.; Bailey, C.J.; Ceriello, A.; Delgado, V.; Federici, M.; Filippatos, G.; Grobbee, D.E.; Hansen, T.B.; et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: The Task Force for diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and the European Association for the Study of Diabetes (EASD). Eur. Heart J. 2020, 41, 255–323. [Google Scholar] [CrossRef] [PubMed]
- Becker, G.J.; Wheeler, D.C.; De Zeeuw, D.; Fujita, T.; Furth, S.L.; Holdaas, H.; Mendis, S.; Oparil, S.; Perkovic, V.; Saad Rodrigues, C.I.; et al. Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group. KDIGO Clinical Practice Guideline for the Management of Blood Pressure in Chronic Kidney Disease. Kidney Int. Suppl. 2012, 2, 337–414. [Google Scholar] [CrossRef]
- Rydén, L.; Grant, P.J.; Anker, S.D.; Berne, C.; Cosentino, F.; Danchin, N.; Deaton, C.; Escaned, J.; Hammes, H.P.; Huikuri, H.; et al. ESC Guidelines on Diabetes, Pre-Diabetes, and Cardiovascular Diseases Developed in Collaboration with the EASD: The Task Force on Diabetes, Pre-Diabetes, and Cardiovascular Diseases of the European Society of Cardiology (ESC) and Developed in Collaboration with the European Association for the Study of Diabetes (EASD). Eur. Heart J. 2013, 34, 3035–3087. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.S.; Choi, H.S.; Bae, E.H.; Kim, S.W.; Ma, S.K. Optimal Blood Pressure Target and Measurement in Patients with Chronic Kidney Disease. Korean J. Intern. Med. 2019, 34, 1181–1187. [Google Scholar] [CrossRef]
- Hebert, S.A.; Ibrahim, H.N. Hypertension Management in Patients with Chronic Kidney Disease. Methodist DeBakey Cardiovasc. J. 2022, 18, 41. [Google Scholar] [CrossRef]
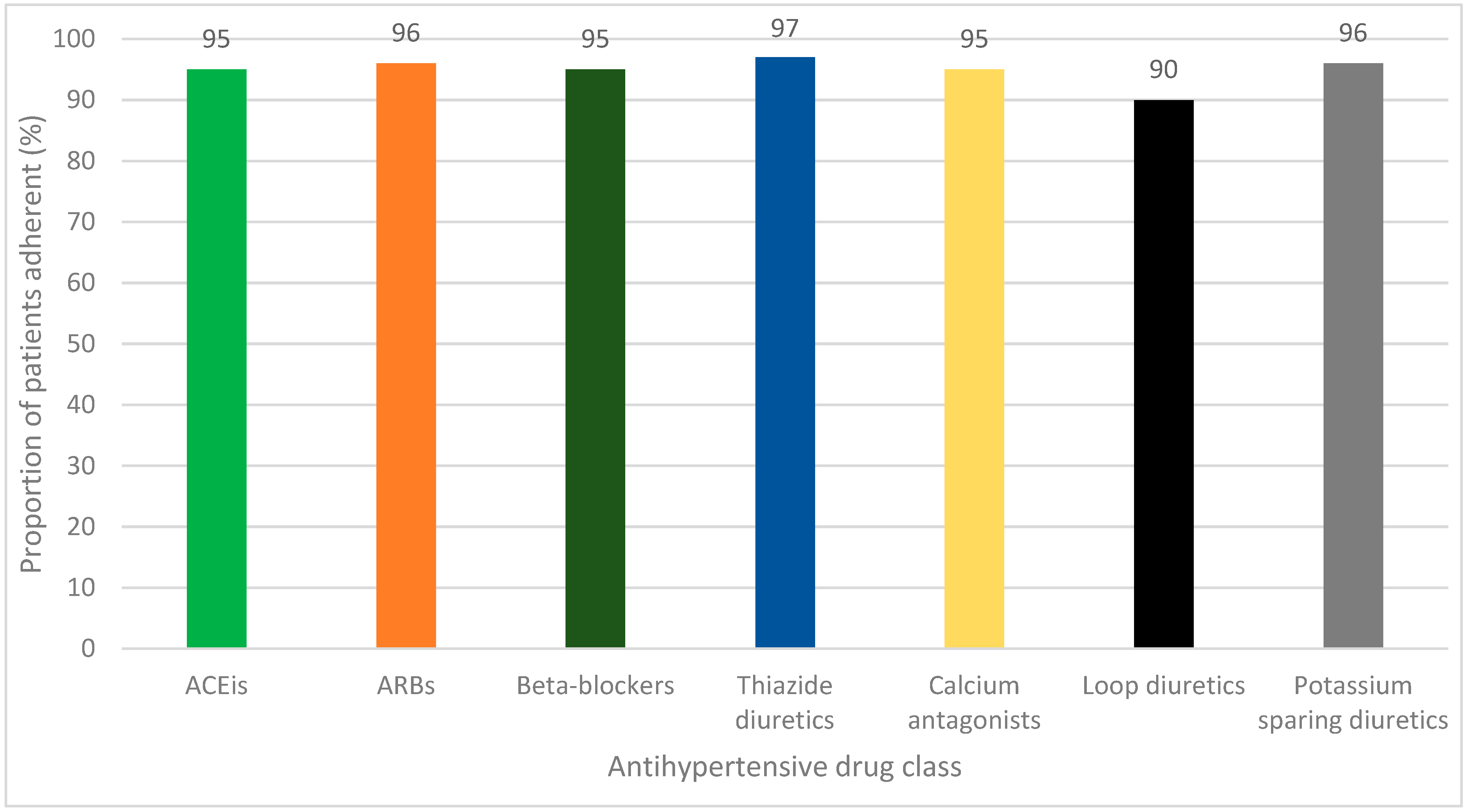


| Total Population | BP-OT | BP-NOT | p | |
|---|---|---|---|---|
| Patients | 385 | 216 (56.1) | 169 (43.9) | |
| Age, years | 63 ± 9 | 63 ± 9 | 63 ± 9 | 0.31 |
| Male sex | 224 (58.2) | 118 (54.6) | 106 (62.7) | 0.11 |
| Years of diabetes | 13 ± 9 | 12 ± 9 | 13 ± 8 | 0.31 |
| HbA1c, mmol/mol | 57 ± 11 | 56 ± 11 | 58 ± 12 | 0.12 |
| Total number of chronic drugs | 7.0 ± 2.7 | 6.9 ± 2.6 | 7.0 ± 2.9 | 0.78 |
| Automated medication dispensing system | 28 (7.3) | 12 (5.6) | 16 (9.5) | 0.14 |
| BMI ≥ 30 kg/m2 | 242 (67.8) | 136 (67.7) | 106 (67.9) | 0.95 |
| eGFR < 60 mL/min | 93 (24.2) | 45 (20.8) | 48 (28.4) | 0.09 |
| BP target | <0.001 | |||
| BP < 140/85 mmHg | 226 (58.7) | 144 (66.7) | 82 (48.5) | |
| BP ≤ 140/90 mmHg | 45 (11.7) | 35 (16.2) | 10 (5.9) | |
| BP ≤ 130/80 mmHg | 114 (29.6) | 37 (17.1) | 77 (45.6) | |
| Systolic BP, mmHg | 136 ± 15 | 126 ± 9 | 148 ± 12 | <0.001 |
| Diastolic BP, mmHg | 74 ± 9 | 70 ± 7 | 80 ± 8 | <0.001 |
| MAP, mmHg | 95 ± 10 | 89 ± 7 | 102 ± 8 | <0.001 |
| Antihypertensive drug use | 324 (84.2) | 184 (85.2) | 140 (82.8) | 0.53 |
| ACEis | 113 (29.4) | 64 (29.6) | 49 (29.0) | 0.89 |
| ARBs | 163 (42.4) | 88 (40.7) | 75 (44.6) | 0.44 |
| Beta-blockers | 189 (49.1) | 111 (51.4) | 78 (46.2) | 0.31 |
| Thiazide diuretics | 139 (36.1) | 70 (32.4) | 69 (40.8) | 0.09 |
| Calcium antagonists | 90 (23.4) | 42 (19.4) | 48 (28.4) | 0.04 |
| Loop diuretics | 69 (17.9) | 41 (19.0) | 28 (16.6) | 0.54 |
| Potassium sparing diuretics 1 | 40 (10.4) | 21 (9.7) | 19 (11.2) | 0.63 |
| Other antihypertensive drugs 2 | 32 (8.3) | 17 (7.9) | 15 (8.9) | 0.72 |
| Number of antihypertensive drugs | 2.1 ± 1.4 | 2.1 ± 1.4 | 2.2 ± 1.5 | 0.46 |
| No antihypertensive therapy | 61 (15.8) | 32 (14.8) | 29 (17.2) | 0.53 |
| 1 drug | 80 (20.8) | 47 (21.8) | 33 (19.5) | |
| 2 drugs | 88 (22.9) | 55 (25.5) | 33 (19.5) | |
| 3 drugs | 84 (21.8) | 47 (21.8) | 37 (21.9) | |
| 4 drugs | 55 (14.3) | 27 (12.5) | 28 (16.6) | |
| 5 or more drugs | 17 (4.4) | 8 (3.7) | 9 (5.3) |
| Primary Analysis: Baseline BP Measurements | ||||
|---|---|---|---|---|
| Total Population | BP-OT | BP-NOT | p | |
| Patients | 334 | 186 (55.7) | 148 (44.3) | |
| Adherent (MPR ≥ 80%) | 320 (95.8) | 178 (95.7) | 142 (95.9) | 0.91 |
| MPR > 100% | 150 (44.9) | 76 (40.9) | 74 (50.0) | 0.10 |
| Sensitivity analysis: excluding patients with an automated dispensing system | ||||
| Patients | 308 | 175 (56.8) | 133 (43.2) | |
| Adherent (MPR ≥ 80%) | 294 (95.5) | 167 (95.4) | 127 (95.5) | 0.98 |
| Secondary analysis: follow-up BP measurements | ||||
| Patients | 176 | 61 (34.7) | 115 (65.3) | |
| Adherent (MPR ≥ 80%) | 167 (94.9) | 58 (95.1) | 109 (94.8) | * |
| Total Population | BP-OT | BP-NOT | p | |
|---|---|---|---|---|
| Patients | 202 | 76 (37.6) | 126 (62.4) | |
| Any intensification | 62 (30.7) | 16 (21.1) | 46 (36.5) | 0.02 |
| Start of a new drug | 44 (21.8) | 13 (17.1) | 31 (24.6) | 0.21 |
| Increase in dosage | 31 (15.3) | 6 (7.9) | 25 (19.8) | 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dam, S.L.; Masselink-Haverkate, H.M.; Gant, C.M.; Bakker, S.J.L.; Nijboer, R.M.; Kruik-Kollöffel, W.J.; Laverman, G.D. Blood Pressure Control in the DIAbetes and LifEstyle Cohort Twente (DIALECT): The Role of Patient Adherence and Physician’s Follow-Up Action. Pharmacoepidemiology 2023, 2, 307-319. https://doi.org/10.3390/pharma2040026
Dam SL, Masselink-Haverkate HM, Gant CM, Bakker SJL, Nijboer RM, Kruik-Kollöffel WJ, Laverman GD. Blood Pressure Control in the DIAbetes and LifEstyle Cohort Twente (DIALECT): The Role of Patient Adherence and Physician’s Follow-Up Action. Pharmacoepidemiology. 2023; 2(4):307-319. https://doi.org/10.3390/pharma2040026
Chicago/Turabian StyleDam, Simone L., Heleen M. Masselink-Haverkate, Christina M. Gant, Stephan J. L. Bakker, Roos M. Nijboer, Willemien J. Kruik-Kollöffel, and Gozewijn D. Laverman. 2023. "Blood Pressure Control in the DIAbetes and LifEstyle Cohort Twente (DIALECT): The Role of Patient Adherence and Physician’s Follow-Up Action" Pharmacoepidemiology 2, no. 4: 307-319. https://doi.org/10.3390/pharma2040026
APA StyleDam, S. L., Masselink-Haverkate, H. M., Gant, C. M., Bakker, S. J. L., Nijboer, R. M., Kruik-Kollöffel, W. J., & Laverman, G. D. (2023). Blood Pressure Control in the DIAbetes and LifEstyle Cohort Twente (DIALECT): The Role of Patient Adherence and Physician’s Follow-Up Action. Pharmacoepidemiology, 2(4), 307-319. https://doi.org/10.3390/pharma2040026






