Journal Description
Pharmacoepidemiology
Pharmacoepidemiology
is an international, peer-reviewed, open access journal on high-quality epidemiological, clinical research across the fields of clinical pharmacology and epidemiology, published quarterly online by MDPI.
- Open Access— free for readers, with article processing charges (APC) paid by authors or their institutions.
- High Visibility: indexed within ESCI (Web of Science), Scopus and other databases.
- Rapid Publication: manuscripts are peer-reviewed and a first decision is provided to authors approximately 21.3 days after submission; acceptance to publication is undertaken in 2.9 days (median values for papers published in this journal in the second half of 2025).
- Recognition of Reviewers: APC discount vouchers, optional signed peer review, and reviewer names published annually in the journal.
- Pharmacoepidemiology is a companion journal of Pharmaceuticals and Journal of Clinical Medicine.
- Journal Clusters-Pharmaceutical Science: Scientia Pharmaceutica, Marine Drugs, Pharmaceuticals, Pharmaceutics, Pharmacy, Future Pharmacology, Pharmacoepidemiology, Drugs and Drug Candidates and Journal of Pharmaceutical and BioTech Industry.
Latest Articles
Pharmacovigilance from the Patient’s Perspective: Self-Reported Adverse Drug Reactions in Kosovo’s Elderly Population
Pharmacoepidemiology 2026, 5(1), 6; https://doi.org/10.3390/pharma5010006 - 30 Jan 2026
Abstract
Background: Pharmacovigilance is a critical component of patient safety, particularly among older adults with chronic diseases who are frequently exposed to polypharmacy. In Kosovo, adverse drug reactions (ADRs) reported by patients remain insufficiently recognized within the healthcare system. Polypharmacy, limited access to pharmaceutical
[...] Read more.
Background: Pharmacovigilance is a critical component of patient safety, particularly among older adults with chronic diseases who are frequently exposed to polypharmacy. In Kosovo, adverse drug reactions (ADRs) reported by patients remain insufficiently recognized within the healthcare system. Polypharmacy, limited access to pharmaceutical counseling, and self-medication practices may contribute to increased medication-related harm. Capturing ADRs directly from patients provides valuable insight into medication safety challenges and communication gaps in clinical care. Objective: To assess the frequency, characteristics, and reporting behavior of adverse drug reactions among adults aged 60–75 years with chronic diseases in Kosovo, and to identify factors associated with awareness and reporting practices. Methods: A multicenter cross-sectional study was conducted between January and September 2025 in four major cities in Kosovo (Prishtina, Prizren, Peja, and Gjilan). A total of 1024 patients receiving continuous therapy for at least one chronic condition were surveyed using a structured questionnaire covering demographic characteristics, drug exposure, ADR experience, and reporting behavior. Statistical analyses included descriptive statistics, chi-square testing, and multivariable logistic regression to identify predictors of ADR reporting. Results: Overall, 47.3% of participants reported experiencing at least one ADR in the preceding 12 months. Among those, 39.5% reported the event to a healthcare professional, whereas 60.5% did not seek professional advice. The most frequently implicated drug classes were antihypertensives (32.8%), analgesics and non-steroidal anti-inflammatory drugs (27.4%), and antirheumatic agents (14.6%), with mainly gastrointestinal (24.1%) and cardiovascular (18.9%) manifestations. Approximately 19.8% of participants reported discontinuing medication due to adverse effects. Female patients were more likely to report ADRs compared to males (p < 0.01). Lack of prior counseling about potential side effects was independently associated with lower reporting (OR = 2.17; 95% CI: 1.41–3.33). Patients using more than six medications had a higher prevalence of ADRs (61.2%). Conclusion: Adverse drug reactions were frequently reported by older patients, while formal reporting to healthcare professionals remained limited. Strengthening patient education, improving patient–provider communication, and integrating clinical pharmacists into primary care may enhance pharmacovigilance practices and medication safety.
Full article
Open AccessSystematic Review
Intraocular Inflammation Following Intravitreal Faricimab: A Systematic Review and Meta-Analysis
by
Jumanah Qedair, Asmaa A. Youssif, Reham Shehada and Hashem Abu Serhan
Pharmacoepidemiology 2026, 5(1), 5; https://doi.org/10.3390/pharma5010005 - 26 Jan 2026
Abstract
►▼
Show Figures
Background/Objectives: To evaluate the incidence, characteristics, and clinical outcomes of intraocular inflammation (IOI) associated with intravitreal faricimab (IVF) in patients with neovascular age-related macular degeneration (nAMD) and diabetic macular edema (DME). Methods: Following PRISMA guidelines, a comprehensive search of PubMed, Web of Science,
[...] Read more.
Background/Objectives: To evaluate the incidence, characteristics, and clinical outcomes of intraocular inflammation (IOI) associated with intravitreal faricimab (IVF) in patients with neovascular age-related macular degeneration (nAMD) and diabetic macular edema (DME). Methods: Following PRISMA guidelines, a comprehensive search of PubMed, Web of Science, Scopus, Embase, and CENTRAL databases was performed from their inception to February 2025. Using the random-effects model, weighted proportions, standardized mean differences, and weighted log odds ratios (OR) were pooled and calculated. A two-tailed p-value of <0.05 was considered statistically significant. The χ2 (z) test and the Higgins I2 test were used to assess studies heterogeneity. Results: We conducted a systematic review and meta-analysis of 24 studies (4761 patients; 5652 eyes). The most common diagnoses were nAMD (n = 4782, 94.6%) and DME (n = 845, 37.1%). The pooled proportion for IOI incidence in eyes receiving IVF was 3.0% (95% CI: 1.0–6.0). The odds of developing IOI did not differ significantly between the DME and nAMD groups (OR: 1.13, p = 0.78). Unspecified IOI was the most common sign (n = 210, 2.9% [95% CI: 1.2–7.3]), followed by anterior uveitis (n = 80, 1.9% [95% CI: 0.1–34.8]), vitritis (n = 63, 2.9% [95% CI: 0.2–32.1]), retinal hemorrhage (n = 27, 0.7% [95% CI: 0.0–15.3]), and endophthalmitis (n = 8, 0.5% [95% CI: 0.3–1.1]). Conclusions: While IVF demonstrates therapeutic efficacy, our findings highlight a clinically relevant risk of IOI. We, therefore, recommend vigilant clinical monitoring in patients receiving this therapy.
Full article

Figure 1
Open AccessArticle
Incidence of Adverse Drug Reactions at the University Hospital Center of Libreville, Gabon: From Data Collection to a Risk Minimization Plan
by
Pierre Constant Ntoutoume Nzoghe, Rim Lakhmiri, Sophie Coniquet, Solange Ntsame, Ihsane Hmamouchi, Yahia Cherrah and Samira Serragui
Pharmacoepidemiology 2026, 5(1), 4; https://doi.org/10.3390/pharma5010004 - 16 Jan 2026
Abstract
►▼
Show Figures
Background: According to the literature, adverse drug reactions (ADRs) account for 5–10% of hospital admissions and affect 25–30% of hospitalized patients, but no data are available for Gabon. Objectives: To estimate the incidence of ADRs among hospitalized patients at the Libreville University Hospital
[...] Read more.
Background: According to the literature, adverse drug reactions (ADRs) account for 5–10% of hospital admissions and affect 25–30% of hospitalized patients, but no data are available for Gabon. Objectives: To estimate the incidence of ADRs among hospitalized patients at the Libreville University Hospital Center (CHUL) and to classify them according to their frequency, severity, mechanism and preventability, while proposing appropriate risk minimization strategies. Patients and Methods: A 14-month, single-center, prospective study included all patients experiencing ADRs, excluding those without ADRs or with intentional overdoses. ADRs were analyzed using the World Health Organization (WHO) causality assessment, the ATC classification, and Rawlins and Thompson criteria. Data were actively collected from patients and hospital records. Results: Among 4999 patients, 105 experienced 177 adverse events (incidence: 3.5%, 95% CI: 1.7–2.5%). Among the identified ADRs, 42% were serious. Nausea and vomiting were the most frequent ADRs, mainly caused by analgesics (nefopam, tramadol) and antibiotics (amoxicillin–clavulanic acid). The gastrointestinal and nervous systems were the most affected. According to the Rawlins and Thompson classification, 90% of ADRs were type A, 8% type B, and 2% type E (withdrawal syndrome). Overall, 90% of ADRs were preventable. Conclusions: This study highlights the importance of pharmacovigilance at CHUL, Gabon, and emphasizes the role of healthcare professionals in ADR reporting and risk minimization.
Full article

Figure 1
Open AccessReview
Clinical Use, Population-Level Impact, and Antimicrobial Resistance Considerations of Probiotics and Microbiome-Based Therapeutics: Review
by
Monthon Lertcanawanichakul, Phuangthip Bhoopong, Husna Madoromae and Tuanhawanti Sahabuddeen
Pharmacoepidemiology 2026, 5(1), 3; https://doi.org/10.3390/pharma5010003 - 15 Jan 2026
Abstract
Probiotics and microbiome-based therapeutics are increasingly used to prevent antibiotic-associated diarrhea (AAD) and support gut microbiota health across children, adults, and elderly populations. Evidence synthesized in this narrative review from randomized controlled trials and meta-analyses (>20,000 participants) suggests that early probiotic administration, particularly
[...] Read more.
Probiotics and microbiome-based therapeutics are increasingly used to prevent antibiotic-associated diarrhea (AAD) and support gut microbiota health across children, adults, and elderly populations. Evidence synthesized in this narrative review from randomized controlled trials and meta-analyses (>20,000 participants) suggests that early probiotic administration, particularly Lactobacillus rhamnosus GG, Bifidobacterium species, multistrain formulations, and Saccharomyces boulardii, is associated with a 30–40% relative reduction in AAD incidence across heterogeneous studies, with absolute risk reductions of approximately 5–12% depending on baseline risk, strain, dose, and timing. Probiotics are generally well tolerated, with mild gastrointestinal adverse effects reported in 3–5% of users and rare serious events mainly in immunocompromised individuals. However, heterogeneity in formulations, populations, and limited long-term real-world data underscores the need for further pharmacoepidemiological studies, microbiome surveillance, and evaluation of antimicrobial resistance implications.
Full article
(This article belongs to the Special Issue Exploring Herbal Medicine: Applying Epidemiology Principles)
Open AccessArticle
Prevalence Rate of Adverse Drug Reactions from Sodium-Glucose Cotransporter-2 Inhibitors: A Retrospective Cohort Study
by
Pichitra Srimaya, Tossapol Warong, Sudarat Kingdang, Titawadee Pradubkham and Wiraphol Phimarn
Pharmacoepidemiology 2026, 5(1), 2; https://doi.org/10.3390/pharma5010002 - 31 Dec 2025
Abstract
►▼
Show Figures
Background/Objectives: Sodium-glucose cotransporter-2 (SGLT2) inhibitors are widely used in type 2 diabetes mellitus for glycemic control and cardiovascular–renal protection, but adverse effects such as acute kidney injury (AKI), urinary tract infection (UTI), euglycemic diabetic ketoacidosis (Eu-DKA), and acute pancreatitis remain concerns. We
[...] Read more.
Background/Objectives: Sodium-glucose cotransporter-2 (SGLT2) inhibitors are widely used in type 2 diabetes mellitus for glycemic control and cardiovascular–renal protection, but adverse effects such as acute kidney injury (AKI), urinary tract infection (UTI), euglycemic diabetic ketoacidosis (Eu-DKA), and acute pancreatitis remain concerns. We aimed to determine the prevalence of adverse drug reactions (ADRs) associated with SGLT2 inhibitor use. Methods: This retrospective study assessed the prevalence of these adverse events and identified factors associated with UTI among SGLT2 inhibitor users at Suddhavej Hospital (1 January 2019–15 August 2023). Data were extracted from the hospital electronic medical record system (BMS-HOSxP). Results: We analyzed 293 patients (59.73% male; mean age 63.08 ± 0.667 years; 62.08% aged >60). Dapagliflozin had the highest prevalence of AKI (11.42%) and UTI (13.40%). No acute pancreatitis cases were reported. Logistic regression identified female sex (odds ratios [OR] 2.31, 95% confidence intervals [CI] 1.08–4.96; p = 0.032), AKI diagnosis (OR 3.31, 95% CI 1.10–9.89; p = 0.032), age ≥ 60 years (OR 2.78, 95% CI 1.09–7.09; p = 0.033), and SGLT2 inhibitor use <6 months (OR 5.78, 95% CI 2.74–14.18; p = 0.017) as significant risk factors for UTI. Conclusions: Dapagliflozin was associated with the highest prevalence of AKI and UTIs. Female sex, AKI diagnosis, age ≥ 60 years, and SGLT2 inhibitor use <6 months were significant risk factors for UTI among SGLT2 inhibitor users.
Full article

Figure 1
Open AccessArticle
Disparity of Prescribed Psychotropics in Alzheimer’s Disease with Neuropsychiatric Symptoms
by
Samuel I. Nathaniel, Maggie Oliver, Thomas I. Nathaniel, Laurie Marie Theriot Roley, Richard L. Goodwin and Adebobola Imeh-Nathaniel
Pharmacoepidemiology 2026, 5(1), 1; https://doi.org/10.3390/pharma5010001 - 22 Dec 2025
Abstract
►▼
Show Figures
Objective: The objective of this study was to determine whether Non-Hispanic Black (NHB) or Non-Hispanic White (NHW) Alzheimer dementia patients with neuropsychiatric symptoms (ADNPS) differ regarding treatment with second-generation antipsychotics (SGAs), central acetylcholinesterase inhibitors (CAIs), and selective serotonin reuptake inhibitors (SSRIs). Methods:
[...] Read more.
Objective: The objective of this study was to determine whether Non-Hispanic Black (NHB) or Non-Hispanic White (NHW) Alzheimer dementia patients with neuropsychiatric symptoms (ADNPS) differ regarding treatment with second-generation antipsychotics (SGAs), central acetylcholinesterase inhibitors (CAIs), and selective serotonin reuptake inhibitors (SSRIs). Methods: Pharmacologic and demographic factors associated with male and female ADNPS were examined using retrospective data collected from a registry from 2016 and 2020 in a regional AD care center. The logistic regression model was developed to generate odds ratios (OR) to determine factors that were associated with male or female ADNPS. Results: A total of 7031 AD patients were identified. Overall, 6237 patients were NHWs, and 794 were NHBs. Among the NHW AD patients, 1909 presented with behavioral disturbances or neuropsychiatric symptoms (NPS), and 168 NHB AD patients presented with NPS. In the adjusted analysis, NHW ADNPS patients were more likely to be treated with galantamine (OR = 1.538, 95% CI, 1.001–2.364, p = 0.049), memantine (OR = 1.222, 95% CI, 1.086–1.375, p < 0.001), olanzapine (OR = 2.323, 95% CI, 1.794–3.009, p < 0.001), risperidone (OR = 4.181, 95% CI, 3.539–4.939, p < 0.001), and escitalopram (OR = 1.401, 95% CI, 1.225–1.602, p < 0.001). In contrast, NHB ADNPS patients were more likely to be treated with memantine (OR = 2.601, 95% CI, 1.746–3.875, p < 0.001) and risperidone (OR = 5.526, 95% CI, 3.411–8.951, p < 0.001). Conclusions: Our findings show the use of memantine and risperidone to treat both NHB and NHW ADNPS patients. NHW ADNPS patients were more likely to be treated with galantamine, memantine, olanzapine, risperidone, and escitalopram. In contrast, NHB patients with ADNPS were more likely to be treated with memantine and risperidone.
Full article

Figure 1
Open AccessArticle
Identifying Myocardial Infarction and Ischemic Stroke Events in China Real-World Data: A Validation Study in Tianjin Regional Healthcare Database
by
Jiamei Liu, Zizhao Zhang, Yin Liu, Liming Zhao, Zhenna Huang, Xuxiao Ye, Jeff L. Lange, Nafeesa Dhalwani, Fan Yang, Kangyin Chen, Hao Zhang and Jifang Zhou
Pharmacoepidemiology 2025, 4(4), 28; https://doi.org/10.3390/pharma4040028 - 15 Dec 2025
Abstract
►▼
Show Figures
Objectives: Real-world evidence that supports decision-making must meet numerous criteria, including validated identification of clinical outcomes. This study aimed to develop and validate a method for identifying new cases of myocardial infarction (MI) and ischemic stroke (IS) within real-world clinical data in China.
[...] Read more.
Objectives: Real-world evidence that supports decision-making must meet numerous criteria, including validated identification of clinical outcomes. This study aimed to develop and validate a method for identifying new cases of myocardial infarction (MI) and ischemic stroke (IS) within real-world clinical data in China. Methods: Algorithms to identify MI and IS events were developed using ICD-10-CM codes and Chinese diagnosis keywords within the Tianjin Regional Healthcare Database. Validation followed predefined criteria: MI required cardiac troponin elevation and ischemic symptoms or cardiac troponin elevation and electrocardiogram changes; IS required clinical symptoms and neuroimaging confirmation of cerebral Magnetic Resonance Imaging (MRI) or Computerized Tomography (CT) reports. Positive predictive value (PPV) with 95% confidence intervals (CI) was calculated for each outcome. Results: Among 304 MI and 302 IS cases randomly selected, approximately half were identified using ICD-10-CM codes and half through Chinese diagnosis keywords. Overall PPV for MI was 69% (95% CI: 63–74%), with similar PPVs across identification methods. PPV increased to 88% for inpatient MI and 97% for primary inpatient MI. For IS, overall PPV was 65% (95% CI: 58–71%), with higher PPV for cases identified by ICD-10-CM codes (76%) compared to keyword-only cases (56%). PPV increased to 76% for inpatient IS and 91% for primary inpatient IS. Conclusions: The use of ICD-10-CM codes and Chinese diagnosis keywords in primary inpatient diagnoses provides a validated approach for the identification of clinical outcomes of MI and IS within real-world clinical data in China.
Full article

Figure 1
Open AccessArticle
Antipsychotic Drugs and Diabetic Ketoacidosis: A Disproportionality Analysis of the FDA Adverse Event Reporting System
by
Nisrine Haddad, Abdallah Alami, Christopher A. Gravel, Derek Tsui, Yue Chen, Franco Momoli, Donald Mattison, Nawal Farhat and Daniel Krewski
Pharmacoepidemiology 2025, 4(4), 27; https://doi.org/10.3390/pharma4040027 - 25 Nov 2025
Abstract
►▼
Show Figures
Objectives: To evaluate reports of diabetic ketoacidosis (DKA) associated with antipsychotic drug (APD) use submitted to the U.S. Food and Drug Administration’s Adverse Event Reporting System (FAERS). Methods: A retrospective pharmacovigilance analysis was conducted using FAERS data from January 2000 to
[...] Read more.
Objectives: To evaluate reports of diabetic ketoacidosis (DKA) associated with antipsychotic drug (APD) use submitted to the U.S. Food and Drug Administration’s Adverse Event Reporting System (FAERS). Methods: A retrospective pharmacovigilance analysis was conducted using FAERS data from January 2000 to December 2022. DKA cases were identified using the MedDRA preferred term “diabetic ketoacidosis” in reports listing antipsychotic drugs as suspect medications. Disproportionality analyses, including the proportional reporting ratio (PRR) and empirical Bayes geometric mean (EBGM), were used to assess reporting patterns. Multiple analyses were performed, including those restricted to primary suspect listed drugs only, expanded to incorporate secondary suspect drugs, and sensitivity analyses excluding reports submitted by legal professionals. Results: Among 19,961 DKA reports in FAERS, 2489 (12.5%) listed atypical antipsychotics as the primary suspect drug, whereas reports involving typical APDs were rare. The majority of reports were submitted by healthcare professionals (74.1%), and nearly half originated from the United States (45.4%). Hospitalization was a frequent outcome, reported in 74.3% of cases. Quetiapine and olanzapine were the most frequently reported atypical APDs, with disproportionality analyses demonstrating strong safety signals when compared to all other drugs in FAERS: olanzapine PRR 13.2 (95% CI: 12.4–14.2) and quetiapine PRR 11.8 (95% CI: 11.1–12.5). The findings remained consistent across multiple sensitivity analyses, including incorporating secondary suspect drugs, when the comparator group was restricted to only psychotropic drugs, and excluding reports submitted by lawyers. Conclusions: This pharmacovigilance analysis highlights a potential safety signal for DKA with atypical antipsychotic drugs, notably quetiapine and olanzapine. While these findings do not establish causality, they underscore the need for further investigation using clinical and epidemiological data.
Full article
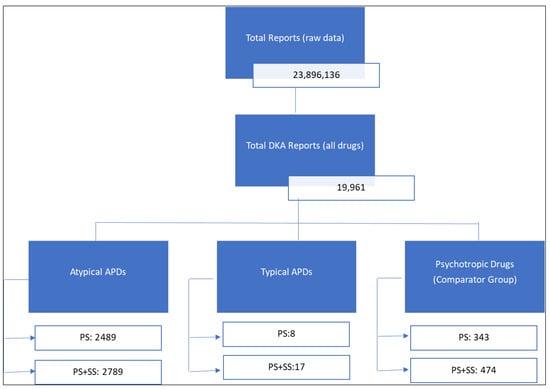
Figure 1
Open AccessReview
Idealized Framework for Assisting Pharmacovigilance Reporting in an Ambulatory Primary Care and Chronic Disease Management Clinic
by
Patrick J. Silva, Sara L. Rogers, Zoya Hassan-Toufique, Jian Tao, Scott A. Bruce, Paula K. Shireman and Kenneth S. Ramos
Pharmacoepidemiology 2025, 4(4), 26; https://doi.org/10.3390/pharma4040026 - 21 Nov 2025
Abstract
►▼
Show Figures
Pharmacovigilance approaches have conventionally focused on the use of epidemiological data to detect emergent adverse drug reactions (ADRs). Recent advances in the use and availability of real-world data have expanded opportunities to detect ADR signals in medical records. We provide a limited review
[...] Read more.
Pharmacovigilance approaches have conventionally focused on the use of epidemiological data to detect emergent adverse drug reactions (ADRs). Recent advances in the use and availability of real-world data have expanded opportunities to detect ADR signals in medical records. We provide a limited review of pharmacovigilance practices and tools we have specifically considered implementing into our comprehensive medication management clinic and associated research programs. Use of pharmacogenomic variants has proven useful only on a limited scale as such data are reliant on low-dimensional approaches matching variants to drugs, often with small effect sizes. As such, most ADRs go unrecognized, undocumented, and unactionable. We posit that an idealized pharmacovigilance framework that relies on artificial-intelligence-assisted reporting with adjudication by pharmacovigilance experts and new models of ambulatory pharmaceutical practice would establish the following attributes: (1) all metadata relating to medication use would be available in the medical record in computable and interoperable data models, (2) digital surveillance tools would detect most ADR events with attributed pharmacological contributions, (3) all events would be characterized using standard adjudication rubrics, and (4) all events would iteratively inform an ADR knowledgebase and improve models to advance detection and prediction of ADR during the course of patient care with a focus on having the necessary tools for clinicians to prevent ADRs. This review provides a limited and focused framework for more systematic documentation of ADRs and tactics to mitigate the idiopathic nature of most ADRs.
Full article
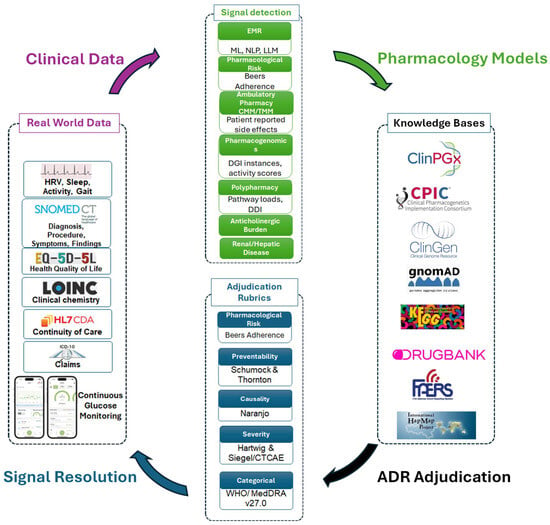
Figure 1
Open AccessArticle
Post-Marketing Pharmacovigilance Study of Darunavir in the United Kingdom: An Analysis of Adverse Drug Reactions Reported to the MHRA
by
Pono Pono, Vicky Cheng, Victoria Skerrett and Alan M. Jones
Pharmacoepidemiology 2025, 4(4), 25; https://doi.org/10.3390/pharma4040025 - 6 Nov 2025
Abstract
Background/Objectives: Human immunodeficiency virus (HIV) continues to be a global public health concern. Several antiretroviral drugs have been approved for the treatment, post-exposure, and pre-exposure prophylaxis of HIV. Darunavir (DRV) is a protease inhibitor (PI) approved for the management of HIV globally.
[...] Read more.
Background/Objectives: Human immunodeficiency virus (HIV) continues to be a global public health concern. Several antiretroviral drugs have been approved for the treatment, post-exposure, and pre-exposure prophylaxis of HIV. Darunavir (DRV) is a protease inhibitor (PI) approved for the management of HIV globally. This study aims to generate safety signals for DRV through data mining and analysis of adverse events (AEs) reported to the United Kingdom (UK) Medicines and Healthcare products Regulatory Agency (MHRA) Yellow Card Scheme. Methods: Disproportionality analysis was conducted using reporting odds ratio (ROR), proportional reporting ratio (PRR), and Bayesian confidence propagation neural network (BCPNN) approaches to identify potential safety signals. Results: The MHRA database contained n = 779 reports (n = 1791 AEs) attributed to DRV. The majority of AEs were reported for males. Positive safety signals were identified at both the system organ class (SOC, n = 5) and preferred term level (PT, n = 95). At SOC level, endocrine disorders emerged as a signal of interest n = 33 cases (ROR: 8.17, 95% CI: 5.78–11.56; PRR:7.96, 95% CI: 5.68–11.15; and IC: 2.85, IC025: 2.51). Among the results, 40 new potential safety signals are not listed on the product labelling in the UK. These include serious AEs such as cerebrovascular accident, brain injury, thrombosis, and pregnancy, puerperium, and perinatal AEs. Conclusions: This study provides additional real-world safety data for DRV in the UK and paves the way for future observational studies to investigate the identified safety signals.
Full article
(This article belongs to the Special Issue Pharmacoepidemiology and Pharmacovigilance in the UK)
Open AccessArticle
Exploratory Signal Detection of Maternal and Perinatal Adverse ART Drug Events in EudraVigilance: Insights from Network and Cluster Analyses
by
Bárbara Costa and Nuno Vale
Pharmacoepidemiology 2025, 4(4), 24; https://doi.org/10.3390/pharma4040024 - 4 Nov 2025
Abstract
►▼
Show Figures
Background: Medication safety in pregnancy, puerperium, and perinatal periods is underexplored because these populations are excluded from clinical trials. EudraVigilance offers post-marketing evidence, but disproportionality analyses focus on isolated drug event pairs and may miss syndromic patterns. We applied a network- and
[...] Read more.
Background: Medication safety in pregnancy, puerperium, and perinatal periods is underexplored because these populations are excluded from clinical trials. EudraVigilance offers post-marketing evidence, but disproportionality analyses focus on isolated drug event pairs and may miss syndromic patterns. We applied a network- and cluster-based framework to EudraVigilance reports on antiviral use in pregnancy to improve surveillance and identify meaningful constellations. Methods: We retrieved all individual case safety reports (ICSRs) from January 2015 to June 2025, including pregnancy, puerperium, or perinatal terms, focusing on suspect antivirals. After parsing terms, disproportionality metrics were computed as a benchmark. A bipartite drug–event network was built and projected to event–event co-occurrence networks; Louvain community detection identified clusters. Clusters were characterized by size, drug mix, seriousness, overlap with disproportionality signals, and stratification across periods. Results: The dataset comprised 106,924 ICSRs and 232,067 unique pairs. Disproportionality yielded 6142 signals, mainly involving antiretrovirals (ritonavir, lamivudine, zidovudine, emtricitabine/tenofovir). Network analysis revealed clusters grouping maternal and fetal/neonatal outcomes (e.g., fetal death, low birth weight), and transplacental transfer, highlighting structures not visible in pairwise analyses. Several clusters combined high-frequency exposures with clinically relevant outcomes, suggesting early-warning potential. Conclusions: Combining disproportionality with network- and cluster-based pharmacovigilance adds value for monitoring pregnancy medication safety. Beyond individual signals, this approach reveals meaningful clusters and “bridge” reactions connecting adverse-event domains, offering a richer framework for perinatal surveillance. Despite spontaneous-reporting limits, findings generate hypotheses for mechanistic and pharmacoepidemiologic follow-up and support network methods as complements to traditional pharmacovigilance.
Full article

Figure 1
Open AccessCase Report
Management of Acute Moderate Iron Poisoning with Oral Chelation and Antioxidant Therapy: A Case Report
by
Mary Isabel Vanegas-Rincón, María A. Barón-Bolívar, Javier A. Aguilar-Mejía, Diana Patricia Amador-Munoz and Luis Carlos Rojas-Rodríguez
Pharmacoepidemiology 2025, 4(4), 23; https://doi.org/10.3390/pharma4040023 - 1 Nov 2025
Abstract
►▼
Show Figures
Introduction: Acute iron poisoning is a potentially life-threatening condition that primarily affects the gastrointestinal, hepatic and cardiovascular systems. While it most often occurs accidentally in children, intentional overdoses in adolescents and adults remain an important clinical concern. Case description: We report
[...] Read more.
Introduction: Acute iron poisoning is a potentially life-threatening condition that primarily affects the gastrointestinal, hepatic and cardiovascular systems. While it most often occurs accidentally in children, intentional overdoses in adolescents and adults remain an important clinical concern. Case description: We report the case of a 14-year-old male patient with a history of depression who intentionally ingested 100 ferrous sulfate tablets (equivalent to 118 mg/kg of elemental iron). The patient was admitted to the emergency department three hours after ingestion. He presented with vomiting tablet remnants, headache, and mild abdominal pain. Supportive measures included intestinal irrigation with polyethylene glycol (PEG), gastric protection, and N-acetylcysteine intravenous administration. The iron chelator therapy with deferoxamine was not possible because the medication was unavailable, so treatment with the oral iron chelator (deferasirox) was initiated. The iron levels gradually decreased, with no evidence of liver or cardiovascular involvement. The patient was discharged on day 20 post-ingestion with outpatient psychiatric follow-up. Discussion: This case highlights the importance of early initiation of gastrointestinal decontamination with PEG to limit systemic iron absorption. The use of deferasirox as an alternative chelating agent in the absence of deferoxamine has been associated with a favorable response. Conclusions: The rational use of oral chelators, gastrointestinal decontamination, and hepatoprotective therapies in acute iron poisoning might prevent major complications and improve prognosis. Alternative therapies can be valuable when an antidote is not immediately available; however, further clinical research is required before making a recommendation.
Full article

Figure 1
Open AccessFeature PaperReview
Pharmacoepidemiological Data on Drug–Herb Interactions: Serotonin Syndrome, Arrhythmias and the Emerging Role of Artificial Intelligence
by
Marios Spanakis, Evangelos Bakaros, Stella-Natalia Papadopoulou, Agapi Fournaraki and Emmanouil K. Symvoulakis
Pharmacoepidemiology 2025, 4(4), 22; https://doi.org/10.3390/pharma4040022 - 9 Oct 2025
Cited by 1
Abstract
Herbal medicinal products are increasingly used alongside conventional medicines, raising the risk of potential interactions such as pharmacodynamic drug–herb interactions (PD-DHIs) that can cause serious adverse drug reactions (ADRs). This review aims to present available pharmacological, clinical and pharmacoepidemiological literature regarding potential DHIs
[...] Read more.
Herbal medicinal products are increasingly used alongside conventional medicines, raising the risk of potential interactions such as pharmacodynamic drug–herb interactions (PD-DHIs) that can cause serious adverse drug reactions (ADRs). This review aims to present available pharmacological, clinical and pharmacoepidemiological literature regarding potential DHIs associated with serotonin syndrome or cardiac arrhythmias. Furthermore, it assesses the current evidence using the Oxford Centre for Evidence-Based Medicine (CEBM) 2009 framework. Serotonin syndrome most often results from combining serotonergic herbs (e.g., St. John’s wort) with antidepressants like serotonin reuptake inhibitors (SSRIs), as supported by repeated case reports and mechanistic plausibility (CEBM Level 3, Grade C). Other herbs such as black cohosh, ginseng, Syrian rue, turmeric, rhodiola, ashwagandha, and L-tryptophan/5-HTP have been linked to serotonin syndrome when used with SSRIs, serotonin-norepinephrine reuptake inhibitors (SNRIs), or monoamine oxidase inhibitors (MAOIs), but evidence is limited (Levels 4–5, Grade D). For cardiac arrhythmias, PD-DHIs arise when herbs interact with drugs that alter cardiac electrophysiology—such as QT-prolonging agents, psychotropics, antiarrhythmics or digoxin—thereby amplifying arrhythmogenic risk. Ephedra with sympathomimetics is strongly associated with arrhythmias (Level 2–3, Grade B). Licorice may potentiate digoxin and QT-prolonging drugs via hypokalemia (Level 4, Grade C). Other related PD-DHIs include aconite with antiarrhythmics, bitter orange or caffeine with QT-prolonging psychotropics, yohimbine with cardiovascular agents, and aloe or senna with digoxin. Overall, the evidence for PD-DHIs varies from moderate to weak but large-scale pharmacoepidemiological data is scarce. Future approaches, including artificial intelligence with explainable machine learning and network pharmacology, may integrate mechanistic, clinical, and real-world data to improve early detection or prediction of PD-DHIs. However, several specific challenges must be addressed. Therefore, it is crucial for healthcare providers in both clinical and community settings to increase their awareness of these interactions and ADRs to ensure the safe use of herbal remedies alongside conventional therapies.
Full article
(This article belongs to the Special Issue Exploring Herbal Medicine: Applying Epidemiology Principles)
►▼
Show Figures

Graphical abstract
Open AccessReview
Antimicrobial Resistance in Immunocompromised Outpatients: A Narrative Review of Current Evidence and Challenges
by
Farhood Sadeghi, Erta Rajabi, Zahra Ghanbari, Sajjad Fattahniya, Reza Samiee, Mandana Akhavan, Mohammadreza Salehi and Maryam Shafaati
Pharmacoepidemiology 2025, 4(4), 21; https://doi.org/10.3390/pharma4040021 - 3 Oct 2025
Abstract
►▼
Show Figures
Immunocompromised outpatients, including people living with HIV/AIDS (PLWH), diabetes, cancer, and organ transplant recipients, are at high risk of antimicrobial resistance (AMR) due to their weakened immune systems and use of immunosuppressive therapies. The high prevalence of prophylactic and therapeutic antibiotic use in
[...] Read more.
Immunocompromised outpatients, including people living with HIV/AIDS (PLWH), diabetes, cancer, and organ transplant recipients, are at high risk of antimicrobial resistance (AMR) due to their weakened immune systems and use of immunosuppressive therapies. The high prevalence of prophylactic and therapeutic antibiotic use in this vulnerable population, coupled with frequent contact with healthcare facilities and limited outpatient antimicrobial resistance surveillance systems, contributes to the increase in antimicrobial resistance. The majority of available data pertains to inpatients, and there is a lack of comprehensive outpatient information on pathogen distribution, resistance patterns, and diagnostic challenges. Moreover, nonspecific clinical presentations, diminished inflammatory responses, and limitations of traditional diagnostic methods complicate infection diagnosis in this population. Increasing resistance surveillance, developing rapid diagnostic tools, and implementing accurate and personalized approaches are key strategies to reduce the burden of disease, mortality, and healthcare costs in the immunocompromised outpatient population. This study was designed as a narrative review based on a comprehensive search of major databases and guidelines. It aims to examine the available evidence and address the challenges associated with AMR in immunocompromised outpatients.
Full article

Graphical abstract
Open AccessArticle
Factors Associated with Suboptimal Adherence to Tyrosine Kinase Inhibitors in Patients with Renal Cell Carcinoma—A Retrospective Cohort Study
by
Fiona Angus, Jingkun Sun, Wan-Chuen Liao, Arfan Khan and Li-Chia Chen
Pharmacoepidemiology 2025, 4(4), 20; https://doi.org/10.3390/pharma4040020 - 3 Oct 2025
Abstract
►▼
Show Figures
Background: Adherence to tyrosine kinase inhibitors (TKIs), the first-line treatment for renal cell carcinoma (RCC), is critical to ensure intended treatment outcomes. However, 75% of patients with RCC have persistency gaps (>7 days) within the first 90 days after initiating TKIs. This
[...] Read more.
Background: Adherence to tyrosine kinase inhibitors (TKIs), the first-line treatment for renal cell carcinoma (RCC), is critical to ensure intended treatment outcomes. However, 75% of patients with RCC have persistency gaps (>7 days) within the first 90 days after initiating TKIs. This study explored factors affecting TKI adherence in RCC patients to inform future interventions. Methods: A retrospective cohort study was conducted at a specialist oncology hospital in Northwest England from October 2020 to October 2022 on patients with RCC treated with TKIs. TKI prescriptions and persistence gaps (>7 days) were identified from electronic dispensing records. Factors associated with persistence gaps were retrieved by reviewing patients’ clinical records. We used descriptive statistics to summarise the results and Kaplan–Meier analysis to assess the probability and the time to the first gap, stratified by adverse drug effect (ADE)-related and non-ADE-related gaps. Results: Among 165 included patients, 611 persistence gaps were identified. ADEs accounted for 59% (n = 464) of 787 recorded factors, with diarrhoea being the most frequent ADE (9.5%). Patients holding leftover TKIs were the primary (15.1%) non-ADE factor for persistency gaps. At least one gap was observed with 82% of patients (n = 135); 19% had ≥5 ADE-related gaps, and 25% had ≥5 non-ADE-related gaps. ADE-related gaps typically occurred within the first three months (50%), while non-ADE-related gaps were not time-dependent. Conclusions: ADEs, including diarrhoea and pain-related reactions, were the most frequently reported issues affecting TKI persistency in patients with RCC. These ADEs are likely to impact patients’ quality of life and adherence. Future qualitative research is warranted to explore patients’ care needs and additional factors such as health literacy and self-efficacy.
Full article

Figure 1
Open AccessArticle
Fact-Finding Survey of Lethal or Fatal Adverse Drug Events in the Japanese Adverse Drug Event Report Database, Fiscal Year 2004–2023 (Adults ≥ 20 Years)
by
Hiroyuki Tanaka and Toshihiro Ishii
Pharmacoepidemiology 2025, 4(4), 19; https://doi.org/10.3390/pharma4040019 - 26 Sep 2025
Abstract
►▼
Show Figures
Background: While adverse drug events (ADEs) are a major public health concern, data on the occurrence of lethal or fatal ADEs in Japan are limited. Therefore, this study aimed to elucidate the characteristics and reporting trends of lethal or fatal ADEs by
[...] Read more.
Background: While adverse drug events (ADEs) are a major public health concern, data on the occurrence of lethal or fatal ADEs in Japan are limited. Therefore, this study aimed to elucidate the characteristics and reporting trends of lethal or fatal ADEs by analyzing the Japanese Adverse Drug Event Report (JADER), a pharmacovigilance database. Methods: Of the individual ADE reports registered in the JADER database between April 2004 and March 2024 (fiscal year (FY) 2004–2023), all data involving individuals aged ≥ 20 years with complete data on sex and age were included in this analysis. Descriptive statistics were used to summarize the results. Results: The number of ADE cases registered in the JADER database increased approximately 2.3-fold from 21,824 in FY 2004 to 50,520 in FY 2023. Lethal or fatal ADE cases increased throughout the study period. In particular, the reporting rate of fatal ADEs reported in JADER appears to have increased in recent years. Lethal or fatal ADEs were reported more frequently among men and individuals aged ≥ 70 years. The recent increase in the reported rates of lethal or fatal ADEs may be largely influenced by the increased number of ADE reports associated with antineoplastic agents. The increase in the number of reports on immune checkpoint inhibitors is particularly notable. Conclusions: This study provides new insights into demographic and drug-related characteristics, as well as time trends associated with lethal or fatal ADEs in Japan. Further studies are needed to confirm these findings.
Full article

Figure 1
Open AccessArticle
Suspected Adverse Drug Reactions Associated with Leukotriene Receptor Antagonists Versus First-Line Asthma Medications: A National Registry–Pharmacology Approach
by
Mohammed Khan, Christine Hirsch and Alan M. Jones
Pharmacoepidemiology 2025, 4(3), 18; https://doi.org/10.3390/pharma4030018 - 19 Sep 2025
Abstract
Background/Objectives: The aim of this study was to determine the suspected adverse drug reaction (ADR) profile of leukotriene receptor antagonists (LTRAs; montelukast and zafirlukast) relative to first-line asthma medications such as short-acting beta agonists (SABAs; salbutamol) and inhaled corticosteroid (ICS; beclomethasone) in
[...] Read more.
Background/Objectives: The aim of this study was to determine the suspected adverse drug reaction (ADR) profile of leukotriene receptor antagonists (LTRAs; montelukast and zafirlukast) relative to first-line asthma medications such as short-acting beta agonists (SABAs; salbutamol) and inhaled corticosteroid (ICS; beclomethasone) in the United Kingdom. to determine the chemical and pharmacological rationale for the suspected ADR signals. Methods: Properties of the asthma medications (pharmacokinetics and pharmacology) were datamined from the chemical database of bioactive molecules with drug-like properties, the European Molecular Biology Laboratory (ChEMBL). Suspected ADR profiles of the asthma medications were curated from the Medicines and Healthcare products Regulatory Authority (MHRA) Yellow Card interactive Drug Analysis Profiles (iDAP) and concatenated to the standardised prescribing levels (using Open Prescribing data) between 2018 and 2023. Results: Total ADRs per 100,000 Rx (p < 0.001) and psychiatric system organ class (SOC) ADRs (p < 0.001) reached statistical significance. Montelukast exhibited the greatest ADR rate at 15.64 per 100,000 Rx. Conclusions: Relative to the controls, montelukast displays a range of suspected system organ class level ADRs. For the credible and previously reported psychiatric ADRs, montelukast is statistically significant (p < 0.001). A mechanistic hypothesis is proposed based on polypharmacological interactions in combination with cerebrospinal fluid (CSF) levels attained. Montelukast had the highest nervous disorder ADR rate at 1.71 per 100,000 Rx, whereas beclomethasone and salbutamol had lower rates (0.43 and 0.14, respectively). These ADRs share a similar background to psychiatric ADRs with CSF penetrability involved and affecting the dopamine axis. This work further supports the monitoring of montelukast for rare but important neuropsychiatric side effects.
Full article
(This article belongs to the Special Issue Pharmacoepidemiology and Pharmacovigilance in the UK)
►▼
Show Figures

Figure 1
Open AccessArticle
Metamizole as the Most Consumed Analgesic in Brazil During the COVID-19 Pandemic: Why Does It Matter?
by
Mayra R. C. de Souza, Alciéllen M. da Silva, Patrícia S. Bazoni, Jéssica B. R. dos Santos and Michael R. R. da Silva
Pharmacoepidemiology 2025, 4(3), 17; https://doi.org/10.3390/pharma4030017 - 30 Aug 2025
Abstract
Background: During the COVID-19 pandemic, analgesic use increased significantly, primarily due to self-medication for symptom relief. In Brazil, metamizole (dipyrone) is widely used despite international restrictions, highlighting the importance of evaluating its consumption patterns. Objective: To assess analgesic use during the COVID-19 pandemic.
[...] Read more.
Background: During the COVID-19 pandemic, analgesic use increased significantly, primarily due to self-medication for symptom relief. In Brazil, metamizole (dipyrone) is widely used despite international restrictions, highlighting the importance of evaluating its consumption patterns. Objective: To assess analgesic use during the COVID-19 pandemic. Methods: This cross-sectional study was conducted via a household survey in Alegre, Espírito Santo, Brazil. Structured questionnaires were used to collect data on sociodemographic characteristics, clinical conditions, and medication use. Descriptive statistics included frequency distributions, medians, and interquartile ranges. Factors associated with analgesic use were analyzed using Poisson regression with robust variance. Results: Among 694 participants, 31.6% reported using analgesics, with metamizole being the most frequently used (87.2%), followed by acetaminophen (paracetamol) (24.7%). Analgesic use was more common among individuals with polypharmacy, lower self-reported quality of life, better self-perceived health, and recent dental appointments. Conclusions: A high prevalence of analgesic use was identified, particularly of metamizole. Given its over-the-counter availability and growing evidence of risks such as liver injury and other adverse events, ongoing monitoring is essential. These findings underscore the need for public health strategies and pharmacist involvement to promote the rational and safe use of analgesics.
Full article
Open AccessArticle
Black Box Warning by the United States Food and Drug Administration: The Impact on the Dispensing Rate of Benzodiazepines
by
Neta Shanwetter Levit, Keren Filosof, Jacob Glazer and Daniel A. Goldstein
Pharmacoepidemiology 2025, 4(3), 16; https://doi.org/10.3390/pharma4030016 - 21 Jul 2025
Abstract
►▼
Show Figures
Background/objectives: In 9/2020, the United States Food and Drug Administration )FDA( posted a black box warning for all benzodiazepines, addressing their association with serious risks of abuse, addiction, physical dependence, and withdrawal reactions. We evaluated changes in benzodiazepine dispensing rate trends after this
[...] Read more.
Background/objectives: In 9/2020, the United States Food and Drug Administration )FDA( posted a black box warning for all benzodiazepines, addressing their association with serious risks of abuse, addiction, physical dependence, and withdrawal reactions. We evaluated changes in benzodiazepine dispensing rate trends after this warning. Methods: The dataset of Clalit Health Services (Israel’s largest insurer, with 5 million members) was used to identify and collect benzodiazepine dispensing data for all patients who were dispensed these drugs at least once during the study period (1/2017–12/2021). The dispensing rate (number of patients who were dispensed benzodiazepines per month divided by the number of patients alive during that month) was calculated for each month in the study period. Linear regression and change point regression were used to review the change in trend before and after the black box warning. New users of benzodiazepines after the black box warning were analyzed by age. Results: A total of 639,515 patients using benzodiazepines were reviewed. The mean benzodiazepine dispensing rate per month was 0.21 and ranged from 0.17 (in 2/2017) to 0.24 (in 3/2020). No significant change in trend was observed before vs. after the black box warning (slopes of 0.00675 percentage points per month and 0.00001 percentage points per month, respectively; p = 0.38). The change point regression analysis identified a change point in 4/2019, which is prior to the black box warning. New users were younger after the black box warning compared to before this warning. Conclusions: The FDA black box warning did not affect the dispensing rate of benzodiazepines.
Full article

Figure 1
Open AccessReview
Prescribing Responsibly: Navigating the Tides of Deprescribing in Proton Pump Inhibitor Stewardship
by
Anna Peyton-Navarrete, Minh Hien Chau Nguyen and Alireza FakhriRavari
Pharmacoepidemiology 2025, 4(3), 15; https://doi.org/10.3390/pharma4030015 - 9 Jul 2025
Cited by 2
Abstract
►▼
Show Figures
Proton pump inhibitors (PPIs) are widely prescribed medications primarily used to treat gastroesophageal reflux disease, peptic ulcer disease, and upper gastrointestinal bleeding. Despite clear therapeutic benefits in appropriate contexts, widespread overprescribing and extended use without clear indications have prompted significant concerns about associated
[...] Read more.
Proton pump inhibitors (PPIs) are widely prescribed medications primarily used to treat gastroesophageal reflux disease, peptic ulcer disease, and upper gastrointestinal bleeding. Despite clear therapeutic benefits in appropriate contexts, widespread overprescribing and extended use without clear indications have prompted significant concerns about associated risks. Accumulating evidence, predominantly from observational studies, suggests that long-term PPI use may lead to complications such as vitamin and mineral deficiencies, increased risks of infections, dysbiosis, renal dysfunction, bone fractures, cardiovascular disease, and certain malignancies. This narrative review not only synthesizes the current evidence surrounding PPI-related harms and existing deprescribing guidelines but also offers a novel perspective on how stewardship principles can be applied to promote responsible PPI prescribing. In particular, we propose a stewardship-oriented deprescribing framework rooted in implementation science, focusing on provider behavior, patient engagement, and health system-level integration. Recognizing these potential harms, evidence-based deprescribing strategies such as tapering, intermittent dosing, and transitions to alternative therapies are critical to mitigate unnecessary patient exposure. Effective implementation of deprescribing requires addressing patient, provider, and institutional barriers through educational initiatives, policy support, and structured monitoring. By promoting judicious PPI prescribing and proactive stewardship practices, clinicians can significantly reduce medication-related harm and improve patient safety.
Full article

Figure 1
Highly Accessed Articles
Latest Books
E-Mail Alert
News
27 January 2026
Meet Us at the 5th Molecules Medicinal Chemistry Symposium, 14–17 May 2026, Beijing, China
Meet Us at the 5th Molecules Medicinal Chemistry Symposium, 14–17 May 2026, Beijing, China

9 December 2025
Meet Us at the 146th Annual Meeting of the Pharmaceutical Society of Japan (Osaka), 26–29 March 2026, Osaka, Japan
Meet Us at the 146th Annual Meeting of the Pharmaceutical Society of Japan (Osaka), 26–29 March 2026, Osaka, Japan

Topics

Conferences
Special Issues
Special Issue in
Pharmacoepidemiology
Recent Advances in the Pharmacoepidemiology of Antirheumatic Medication
Guest Editors: Roberta Foti, Beatrice Maranini, Veronica VenturelliDeadline: 30 June 2026
Special Issue in
Pharmacoepidemiology
Pharmacoepidemiology and Pharmacovigilance in the UK
Guest Editor: Tanja MuellerDeadline: 30 June 2026
Special Issue in
Pharmacoepidemiology
Women’s Special Issue Series: Pharmacoepidemiology
Guest Editors: Carlotta Franchi, Li-Chia Chen, Carlotta LunghiDeadline: 30 June 2026








