Abstract
Clostridioides difficile infection (CDI) is the leading cause of nosocomial diarrhea in the United States. Tigecycline has been proposed as a potential treatment for CDI, though limited clinical data exist to support this practice. The objective of this study was to determine if the provision of tigecycline provides a clinically meaningful benefit to inpatients with CDI. This study was a retrospective chart review enrolling inpatients receiving treatment for CDI. Patients were divided into cohorts depending on whether they received a standard antibiotic therapy regimen for CDI or an antibiotic treatment regimen that included tigecycline. The primary outcome was clinical recovery at the time of hospital discharge. A total of 39 and 22 patients were included in the standard antibiotic therapy and tigecycline groups, respectively. ATLAS (Age, Treatment, Leukocyte, Albumin, Serum creatinine) scores at the time of CDI diagnosis were similar between the two groups, though patients in the tigecycline groups were more likely to represent a recurrent episode of CDI. There was no difference in the rate of clinical recovery at the time of hospital discharge between the standard antibiotic therapy and tigecycline groups (38.5% vs. 36.4%, p = 0.8710). These data do not support the routine use of tigecycline for the treatment of CDI, though interpretation is limited due to baseline differences between groups and the retrospective, observational nature of this study.
1. Background
Clostridioides difficile infection (CDI) is the most common cause of nosocomial diarrhea in the United States of America (USA) and represents a major burden to the healthcare system [1]. The hallmark symptom of CDI is diarrhea, with secondary disease complications including volume depletion, sepsis, toxic megacolon, and death. CDI has been estimated to cost the healthcare system approximately USD 5.4 billion in healthcare-related costs [2]. While it has been estimated that the overall incidence of CDI has declined over the last 10 years, this observation has been found alongside a relative increase in the incidence of community-acquired CDI [3,4].
Treatment guidelines for CDI have been issued by the American College of Gastroenterology (ACG) and the Infectious Diseases Society of America (IDSA) [1,5]. Treatment recommendations for CDI include the provision of antibiotics pursuant to a patient’s degree of symptomatology. Patients may be classified as having non-severe CDI in the presence of clinical stability, severe CDI if either a white blood cell count greater than 15,000 cells/mm3 or a serum creatinine value of greater than 1.5 mg/dL is observed, or fulminant CDI in the presence of hypotension, shock, ileus, or toxic megacolon. For patients with an initial episode of either non-severe or severe CDI, either vancomycin 125 mg orally administered four times a day or fidaxomicin 200 mg orally twice daily for 10 days is recommended. Between the two, fidaxomicin is preferred due to lower rates of CDI recurrence, though access may be limited due to medication cost [6]. In cases of fulminant CDI, treatment with vancomycin 500 mg orally four times a day with intravenous administration of metronidazole 500 mg every eight hours is recommended [6]. For patients with recurrences of CDI, pulsed and tapered doses of fidaxomicin with adjunctive bezlotoxumab may be considered [6].
Tigecycline is a tetracycline derivative, broad-spectrum antibiotic with activity against aerobic, anaerobic, Gram-positive, and Gram-negative microbes [7]. Tigecycline is currently approved by the United States Food and Drug Administration for the treatment of pneumonia, infections of the skin and subcutaneous tissues, and intra-abdominal infections. Preclinical studies indicate tigecycline exerts antimicrobial activity against Clostridioides difficile, reduces Clostridioides sporulation, and reduces Clostridioides toxin activity [8,9,10].
A series of non-comparative case reports and case series have detailed both clinical successes and failures with the use of tigecycline in the management of CDI [11,12,13]. Comparative effectiveness data assessing the clinical utility of tigecycline in the treatment of CDI is limited to a small number of case–control and retrospective cohort studies [14,15,16,17,18]. As of the writing of this manuscript, one retrospective comparative study conducted in Hungary reported that tigecycline monotherapy resulted in greater clinical recovery compared to standard antibiotic therapy [14]. However, this clinical benefit has not been identified universally [15,16,17,18]. The remaining studies with standard-of-care active comparators have not identified a clinical benefit of the provision of tigecycline in the treatment of CDI on the basis of mortality or CDI recurrence. Accordingly, both the IDSA and ACG guidelines list the antibiotic tigecycline as a potential agent for the treatment of CDI though refrain from issuing formal recommendations regarding its use [1,5]. The European Society of Clinical Microbiology and Infectious Diseases guidance document on the treatment of CDI suggests that the provision of intravenous tigecycline may be considered in the event of progressive clinical deterioration, fulminant disease, or when oral medication delivery is not possible, though the guidelines offer that this should be considered on a case-by-case basis due to absence of high-quality evidence to support this therapeutic maneuver [19]. The reproducibility of a positive result in a cohort of patients in the USA using a patient-centered outcome, like clinical recovery, could further define tigecycline’s role in the treatment of CDI.
The primary purpose of this study is to identify if the provision of tigecycline is associated with improved clinical outcomes in patients admitted for the treatment of CDI.
2. Results
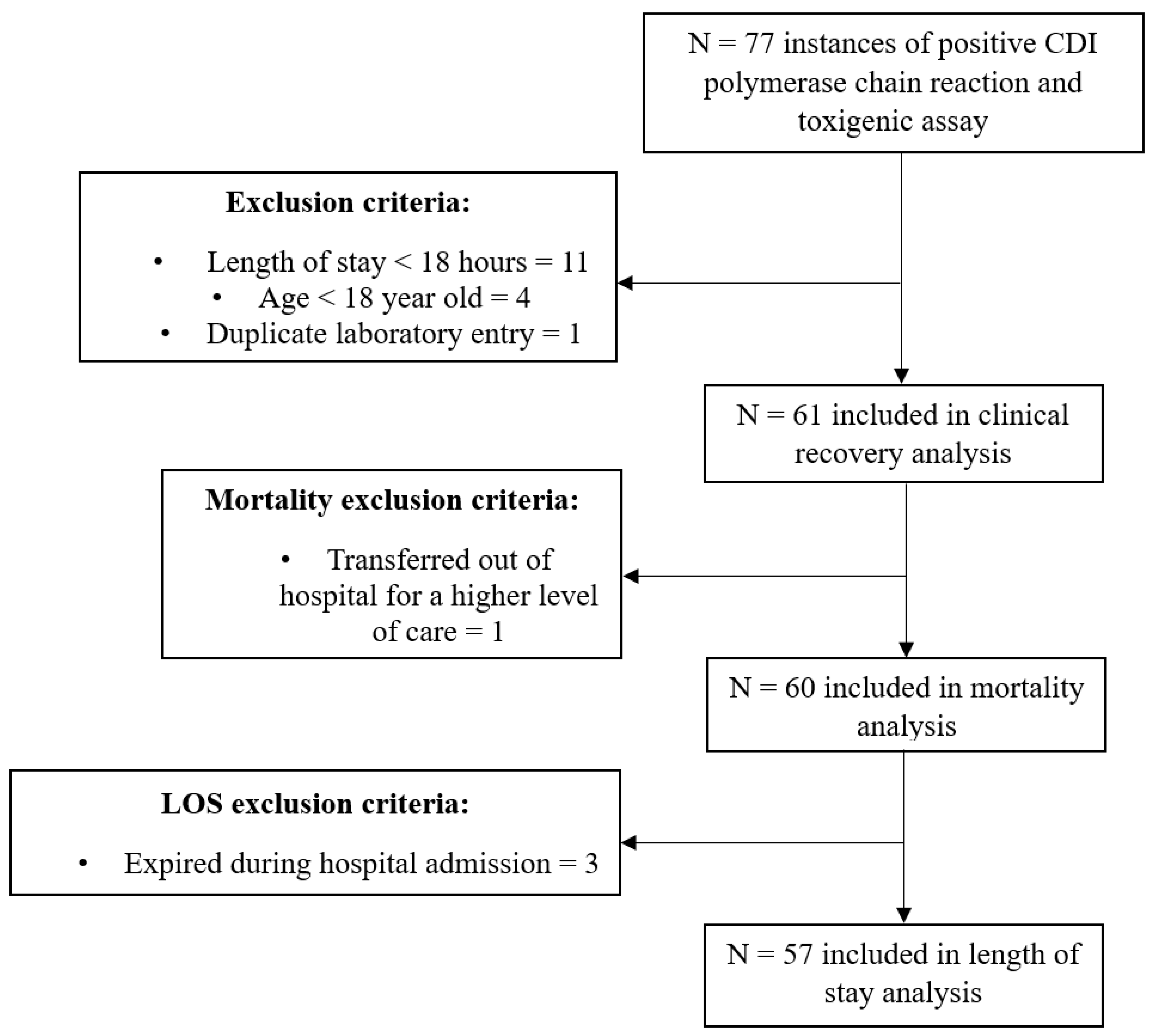
Initial querying of the electronic health record yielded 77 medical records with diagnostically positive CDI labs. After surveillance for exclusion criteria, 61 records were included in the primary outcome analysis. A total of 60 patients were included in our analyses for mortality. Reasons for exclusion are listed in Figure 1.
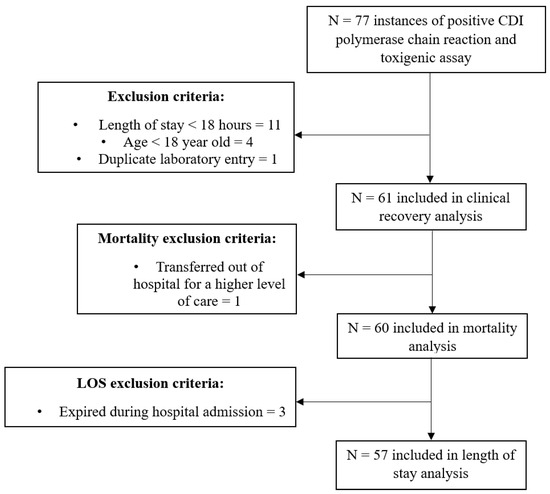
Figure 1.
Participant inclusion. Abbreviation: CDI, Clostridioides difficile infection.
A total of 39 patient records were included in the standard antibiotic therapy (SAT) group and 22 patient records were included in the tigecycline group. While similar in terms of illness severity at the point of CDI diagnosis, patients in the tigecycline group were more likely to experience complicated treatment courses as assessed by IDSA definitions of severity and were more likely for their episode of CDI to be a recurrent episode. The two groups were similar in terms of acute illness severity as assessed by the ATLAS (Age, Treatment, Leukocyte, Albumin, Serum creatinine) score and baseline comorbidity. Metronidazole was used significantly more frequently in the tigecycline group. Additional baseline demographic details may be found in Table 1.

Table 1.
Patient Characteristics.
In the tigecycline group, there were two instances in which tigecycline was initiated prior to collecting Clostridioides difficile diagnostic laboratory tests. In the remaining 20 cases, tigecycline was ordered with a median of 16.7 h (IQR 6.1–27.8 h) after the point in time in which Clostridioides difficile diagnostic laboratory tests were collected. Tigecycline therapy was continued for at least four doses in twelve (54.5%) cases.
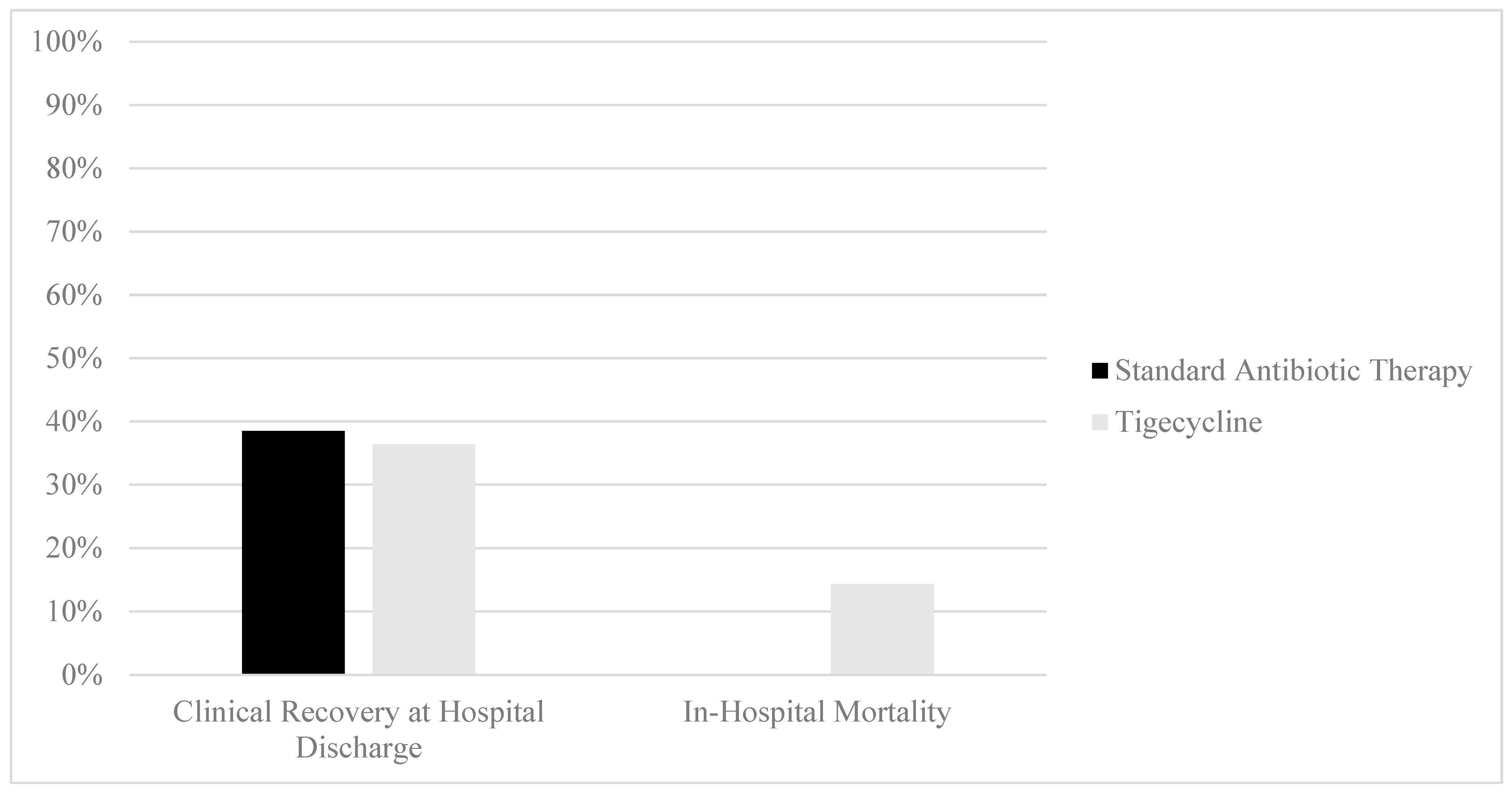
In regard to the primary outcome, no difference in clinical cure attainment at the time of hospital discharge was identified between the SAT and tigecycline groups (38.5% vs. 36.4%, p = 0.8710, Figure 2). In-hospital mortality was observed to be higher in the tigecycline group than in the SAT group (14.3% vs. 0%, p = 0.0154). No difference in hospital length of stay was observed between the SAT and tigecycline groups (4.1 days [2.4–6.4] vs. 4.6 days [2.8–6.6], p = 0.5946). No differences in the rates of 90-day readmission for gastrointestinal complaints were observed between the SAT and tigecycline groups (28.2% vs. 16.7%, p = 0.3469).
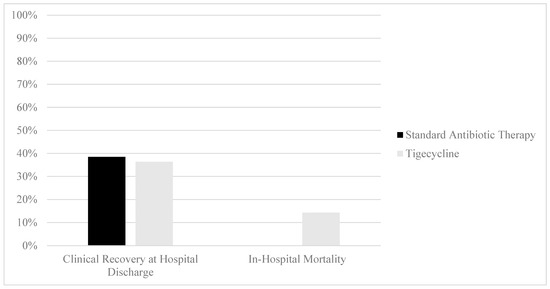
Figure 2.
Clinical outcomes of patients with CDI treatment with standard antibiotic therapy versus adjunctive tigecycline. Clinical recovery comparison: 38.5% (n = 15, standard antibiotic therapy) vs. 36.4% (n = 8, tigecycline), p = 0.8710. Mortality comparison: 0% (n = 0, standard antibiotic therapy) vs. 14.2% (n = 3, tigecycline), p = 0.0154.
3. Discussion
In our study, we did not observe a clinically meaningful benefit in the treatment of CDI when adding adjunctive tigecycline to a standard antibiotic therapy regimen. No difference was observed in rates of clinical cure or hospital length of stay, and mortality was increased in the group of patients receiving tigecycline. There are multiple explanations that may underlie this observation.
One likely reason for this result is the logical probability that patients developing clinical extremis were treated with more advanced therapeutic maneuvers in the context of their care, which may include the provision of tigecycline in the treatment of CDI. Despite the development of antibiotics and treatments that may reduce CDI recurrence, there has been a relative dearth of new treatments that impact acute mortality over the last 15 years [20,21]. Fulminant CDI portends a risk of mortality as high as 30–50% [22,23,24]. The use of an off-label medication that has previously demonstrated in vitro activity against Clostridioides difficile may be an attractive option for managing clinicians when clinical alternatives have been exhausted. This intuitive clinical response is aligned with what we observed within our data set. While similar in terms of clinical acuity at the point of CDI diagnosis, patients in the tigecycline group were more likely to progress to severe or fulminant illness. These data cannot definitively determine whether tigecycline confers no benefit in CDI or whether the declining clinical status led to both the use of tigecycline and the observed increased mortality in our tigecycline comparator group. This progression to fulminant disease may also underlie the observation that patients within the tigecycline group received metronidazole more frequently than those in the standard antibiotic therapy group, as intravenous metronidazole is a first-line therapy in the treatment of fulminant CDI. This practice of reserving tigecycline for patients with declining status would additionally be aligned with the previous literature. A characterization of tigecycline used for CDI in oncology patients demonstrated that in the majority of instances in which tigecycline was used for CDI, treated patients had either severe or severe-complicated disease [25]. Refractory CDI, defined as CDI not responding after three days of vancomycin or metronidazole, was also a common finding in tigecycline-treated patients with CDI. Practice patterns such as this could further explain the higher mortality in the tigecycline cohort of the present study.
A second possible reason for our observation is that when used in acutely ill patients, tigecycline may not exert an impact on clinical outcomes in CDI. The earliest reported clinical uses of tigecycline for CDI were single-patient case reports or non-comparative case series including a small number of patients [26,27,28]. In the time since these reports have been published, a series of observational comparative effectiveness studies have been conducted. The first of these was a multicenter, retrospective, observational study enrolling patients with severe CDI [29]. Comparator groups were defined based on whether patients received either standard antibiotic therapy (n = 46) or adjunctive tigecycline (n = 31) during the course of their CDI treatment. While CDI severity was greater in the adjunctive tigecycline group, 30-day mortality was greater in the tigecycline group (odds ratio [OR] = 2.898, 95% Confidence Interval [CI]= 1.062–7.910). Following this, a series of propensity-matched comparative studies were conducted, providing greater clarity of tigecycline’s effect when confounding variables are minimized [30]. The most recent of these was an observational, retrospective study that enrolled 168 patients hospitalized with CDI in propensity-matched analyses [17]. Divisions between comparator groups were made on the basis of whether patients received a standard antibiotic course for CDI treatment or whether they received two or more doses of tigecycline as a component of CDI treatment. Propensity-matched comparator groups were made on the basis of patient age, gender, markers of clinical acuity, and baseline comorbidities. The use of tigecycline was not associated with an improvement in 30-day mortality (OR = 0.89, 95% CI 0.25–3.12, p = 0.853) and was associated with a longer hospital length of stay. The remaining analyses in this space have included between 44 and 266 patients per study, with enrollment in the tigecycline-using comparator groups ranging from 18 to 66 patients. The majority of these have demonstrated that in acutely ill patients with CDI, tigecycline did not improve in-hospital mortality, CDI recurrence, or the need for colectomy across a range of illness severities [15,16,17,18].
The one positive comparative effectiveness study that assessed tigecycline’s utility in CDI reported that tigecycline monotherapy improved clinical recovery in patients with severe CDI when compared to a standard-of-care antibiotic regimen [14]. In this study, 90 patients hospitalized with severe CDI who received antibiotic therapy for at least 48 h were included. Comparisons between groups were made on the basis of whether patients received either tigecycline or metronidazole and vancomycin for their CDI treatment. Clinical recovery was defined as the resolution of diarrhea, abdominal pain, fever, and leukocytosis. In the final analyses, 45 patients were enrolled in each comparator group. Clinical cure occurred more frequently in the tigecycline group than in the usual care group (n = 34, 75.6% vs. n = 24, 53.3%, p = 0.02). These findings contrast with what we observed in our study. Reasons for these divergent observations could be due to differences in local populations and practice patterns. Our study was a single-center study conducted in the USA, while the study noting a positive finding was a single-center study conducted in Hungary. The interventions in our studies were additionally fundamentally different as the Hungarian study utilized tigecycline monotherapy, whereas tigecycline was used as an adjunctive therapy within our study. The use of tigecycline monotherapy for CDI may be met with resistance in the USA as IDSA guidelines routinely recommend vancomycin, fidaxomicin, or metronidazole in the treatment of CDI. Lastly, differences in the way the outcome of clinical recovery was determined may have been different between our studies. In our study, the subjective symptom of “abdominal pain”, which is a component of the clinical recovery outcome, was assessed using a specific series of physician documentation and nurse-driven charting. This may, in part, explain the difference between the two studies and the relatively low rate of clinical recovery in our study. Strategies to retrospectively collect these data that were different from our own may have yielded different results.
The primary limitations to our study were its retrospective design wherein patients were necessarily studied in the context of their clinical care and a sample size precluding propensity score matching. As noted above, it is possible that higher clinical acuity in the tigecycline group could have pre-selected for higher mortality and worse outcomes in that group. These data cannot conclusively determine whether tigecycline use, patient acuity, or another unidentified confounding factor was the cause for the observed difference in mortality. While ATLAS scores at the point of diagnosis were similar between the two groups, propensity matching may be useful in future studies to account for additional baseline differences. As this study was retrospective, there was no pre-specified protocol with regard to timing or initiation of tigecycline relative to obtaining positive CDI laboratory results. Additionally, there were a variety of antibiotic combinations used within each comparator group. That is to say, a patient may have been treated with vancomycin, fidaxomicin, metronidazole, or all three of the aforementioned antibiotics and still grouped within the SAT comparator group. These differences within and between groups should be considered when interpreting these data.
4. Materials and Methods
4.1. Study Design and Enrollment
This study was a single-center, comparative effectiveness retrospective chart review of inpatients with CDI conducted at a tertiary medical facility in the USA. The study protocol was submitted to the institutional review board and determined to be exempt from review. A Health Insurance Portability and Accountability Act waiver was obtained for this research. Thus, subject consent was not obtained during the data collection process for this research.
Screening for eligible patients was conducted by reviewing records of patient encounters between 1 June 2021 and 31 May 2022 with positive values for both the Clostridioides difficile polymerase chain reaction test and a positive toxigenic assay. Additional inclusion criteria included age of 18 years or older and inpatient antimicrobial treatment for CDI with either metronidazole, vancomycin, fidaxomicin, tigecycline, or a combination thereof. Due to the similarity in clinical management strategies, patients with either nosocomial or community-acquired CDI were included in this study. Exclusion criteria included a hospital length of stay of less than 18 h, as investigators postulated this may have represented an emergency department visit without actual inpatient admission. Patients who were pregnant, prisoners, and patients who transferred in from outside hospitals were also excluded.
This study was a comparative effectiveness study. Patients were divided into a “tigecycline” group or a “standard antibiotic therapy” group on the basis of whether they did or did not receive tigecycline for the treatment of CDI.
4.2. Outcomes and Data Collection
The primary objective of this study was to determine if the provision of tigecycline during the inpatient treatment of CDI was associated with improved rates of clinical recovery. Clinical recovery was defined as a patient meeting all of the following criteria prior to hospital discharge: (1) resolution of fever, (2) resolution of leukocytosis, (3) resolution of abdominal pain, and (4) resolution of diarrhea. Instances of mortality or transfers to a different hospital were deemed clinical failures; that is to say, such a patient encounter could not result in an outcome of clinical recovery. Resolution of fever and leukocytosis were confirmed by a review of the temperature and cell values within institutional reference ranges on the day of discharge. Resolution of abdominal pain was confirmed by reviewing physician discharge notes commenting that abdominal pain had improved or nurse charting omitting complaints of abdominal pain during pain assessments on the day of discharge. Resolution of diarrhea was confirmed by reviewing nursing output charting showing no stool on the day of discharge along with physician discharge notes commenting that diarrhea had improved or nurse output charting showing Bristol type 5 or lower stool on the day of discharge.
The secondary objectives of this study were to determine if the provision of tigecycline during the inpatient treatment of CDI improved in-hospital mortality, hospital length of stay, or hospital readmission for gastrointestinal complaints. Patients were eligible for mortality analyses if they were either discharged as medically stable or expired in the hospital; transfers for higher levels of care were excluded. Patients were eligible for length-of-stay analyses if they were discharged as medically stable. For the length of stay calculations, the time from CDI laboratory positivity to discharge was measured. This was performed instead of using admission time to discharge to more faithfully account for occurrences in which patients developed CDI in the middle of a hospitalization for another reason.
Patient baseline comorbidity was assessed through the calculation of a Charlson Comorbidity Index. Hospital admission and discharge clinical notes were reviewed to gauge the presence or absence of historical diseases for the Charlson Comorbidity Index calculation. Pertinent points of patient assessment in CDI may fluctuate significantly near the point of initial diagnosis [31]. Accordingly, patient acuity was assessed using IDSA definitions of non-severe, severe, and fulminant CDI using both information that was available at the point of initial CDI diagnosis and considering the totality of the individual patient’s clinical course [1,31]. That is to say, if a patient was diagnosed with CDI and, two days into their treatment they developed a laboratory profile consistent with severe CDI, the patient was coded as experiencing severe CDI. ATLAS scores were calculated using clinical information most proximal to the point in time when CDI diagnostic labs were assessed [32].
Data were collected by manual data extraction from individual patient charts within the electronic medical record. Data collection was completed by HJ and LB.
4.3. Data Analysis
Data were analyzed using the Excel statistical package Analyse-it version 5.90 (Leeds, UK). The primary outcome of clinical recovery was assessed using the chi-squared test. Differences between the tigecycline and standard antibiotic therapy cohorts were determined to be statistically significant if the alpha was ≤0.05.
Secondary outcomes of in-hospital mortality and hospital readmission within 90 days for gastrointestinal complaints were assessed using the chi-squared test. A Shapiro–Wilk test was conducted to assess the normality of hospital length of stay. The Mann–Whitney U test was used to determine if significant differences in hospital lengths of stay existed. Differences in baseline clinical features between the tigecycline and standard antibiotic therapy cohorts were assessed using the chi-squared and the Mann–Whitney U test for nominal and continuous variables as appropriate. Comparisons between groups on the basis of CDI severity were conducted using the Mann–Whitney U test using values of one, two, and three for non-severe, severe, and fulminant CDI, respectively. The Mann–Whitney U test was performed preferentially to the chi-squared test as progression in disease severity represents an increase in clinical acuity in a manner that is not absolute, thus lending itself to a ranked analysis. Data distributions are presented as occurrences with percentages and medians with interquartile ranges (IQRs).
5. Conclusions
Adjunctive tigecycline was not associated with improved rates of clinical recovery in the treatment of CDI, though the potential for a confounding relationship between tigecycline use and clinical acuity cannot be excluded due to the retrospective, observational nature of the data presented. Clinicians should consider the relative scarcity of clinical effectiveness data and the risk of adverse reactions and implement collaborative decision making with patients or their families if considering utilizing tigecycline for CDI. Multicenter studies incorporating propensity matching may further guide the medical community in determining if specific phenotypes of CDI patients may benefit from tigecycline.
Author Contributions
H.J.J.: conceptualization, methodology, investigation, formal analysis, writing—original draft, and writing—review and editing. L.B.: conceptualization, investigation, and writing—review and editing. K.K.: conceptualization and writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was declared exempt from institutional review board review.
Informed Consent Statement
Patient consent was waived because a Health Insurance Portability and Accountability Act waiver was obtained for this research.
Data Availability Statement
The data presented in this article are not readily available due to patient privacy protections. Requests to access the datasets should be directed to the principal investigator.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- McDonald, L.C.; Gerding, D.N.; Johnson, S.; Bakken, J.S.; Carroll, K.C.; Coffin, S.E.; Dubberke, E.R.; Garey, K.W.; Gould, C.V.; Kelly, C.; et al. Clinical Practice Guidelines for Clostridium difficile Infection in Adults and Children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin. Infect. Dis. 2018, 66, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Desai, K.; Gupta, S.B.; Dubberke, E.R.; Prabhu, V.S.; Browne, C.; Mast, T.C. Epidemiological and economic burden of Clostridium difficile in the United States: Estimates from a modeling approach. BMC Infect. Dis. 2016, 16, 303. [Google Scholar] [CrossRef] [PubMed]
- Rabatsky-Ehr, T.; Purviance, K.; Mlynarski, D.; Mshar, P.; Hadler, J.; Sosa, L. Surveillance for community-associated Clostridium difficile—Connecticut, 2006. MMWR Morb. Mortal. Wkly. Rep. 2008, 57, 340–343. [Google Scholar]
- Guh, A.Y.; Mu, Y.; Winston, L.G.; Johnston, H.; Olson, D.; Farley, M.M.; Wilson, L.E.; Holzbauer, S.M.; Phipps, E.C.; Dumyati, G.K.; et al. Trends in U.S. Burden of Clostridioides difficile Infection and Outcomes. N. Engl. J. Med. 2020, 382, 1320–1330. [Google Scholar] [CrossRef] [PubMed]
- Kelly, C.R.; Fischer, M.; Allegretti, J.R.; LaPlante, K.; Stewart, D.B.; Limketkai, B.N.; Stollman, N.H. ACG Clinical Guidelines: Prevention, Diagnosis, and Treatment of Clostridioides difficile Infections. Am. J. Gastroenterol. 2021, 116, 1124–1147. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.; Lavergne, V.; Skinner, A.M.; Gonzales-Luna, A.J.; Garey, K.W.; Kelly, C.P.; Wilcox, M.H. Clinical Practice Guideline by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA): 2021 Focused Update Guidelines on Management of Clostridioides difficile Infection in Adults. Clin. Infect. Dis. 2021, 73, 755–757. [Google Scholar] [CrossRef] [PubMed]
- Tygacil (Tigecycline) [Prescribing Information]; Wyeth Pharmaceuticals LLC: Philadelphia, PA, USA, 2021.
- Hecht, D.W.; Galang, M.A.; Sambol, S.P.; Osmolski, J.R.; Johnson, S.; Gerding, D.N. In vitro activities of 15 antimicrobial agents against 110 toxigenic clostridium difficile clinical isolates collected from 1983 to 2004. Antimicrob. Agents Chemother. 2007, 51, 2716–2719. [Google Scholar] [CrossRef]
- Garneau, J.R.; Valiquette, L.; Fortier, L.C. Prevention of Clostridium difficile spore formation by sub-inhibitory concentrations of tigecycline and piperacillin/tazobactam. BMC Infect. Dis. 2014, 14, 29. [Google Scholar] [CrossRef] [PubMed]
- Theriot, C.M.; Schumacher, C.A.; Bassis, C.M.; Seekatz, A.M.; Young, V.B. Effects of tigecycline and vancomycin administration on established Clostridium difficile infection. Antimicrob. Agents Chemother. 2015, 59, 1596–1604. [Google Scholar] [CrossRef] [PubMed]
- Larson, K.C.; Belliveau, P.P.; Spooner, L.M. Tigecycline for the treatment of severe Clostridium difficile infection. Ann. Pharmacother. 2011, 45, 1005–1010. [Google Scholar] [CrossRef]
- Britt, N.S.; Steed, M.E.; Potter, E.M.; Clough, L.A. Tigecycline for the Treatment of Severe and Severe Complicated Clostridium difficile Infection. Infect. Dis. Ther. 2014, 3, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Kopterides, P.; Papageorgiou, C.; Antoniadou, A.; Papadomichelakis, E.; Tsangaris, I.; Dimopoulou, I.; Armaganidis, A. Failure of tigecycline to treat severe Clostridium difficile infection. Anaesth. Intensive Care 2010, 38, 755–758. [Google Scholar] [CrossRef] [PubMed]
- Gergely Szabo, B.; Kadar, B.; Szidonia Lenart, K.; Dezsenyi, B.; Kunovszki, P.; Fried, K.; Kamotsay, K.; Nikolova, R.; Prinz, G. Use of intravenous tigecycline in patients with severe Clostridium difficile infection: A retrospective observational cohort study. Clin. Microbiol. Infect. 2016, 22, 990–995. [Google Scholar] [CrossRef] [PubMed]
- LaSalvia, M.T.; Branch-Elliman, W.; Snyder, G.M.; Mahoney, M.V.; Alonso, C.D.; Gold, H.S.; Wright, S.B. Does Adjunctive Tigecycline Improve Outcomes in Severe-Complicated, Nonoperative Clostridium difficile Infection? Open Forum Infect. Dis. 2017, 4, ofw264. [Google Scholar] [CrossRef] [PubMed]
- Manea, E.; Sojo-Dorado, J.; Jipa, R.E.; Benea, S.N.; Rodríguez-Baño, J.; Hristea, A. The role of tigecycline in the management of Clostridium difficile infection: A retrospective cohort study. Clin. Microbiol. Infect. 2018, 24, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Phillips, E.C.; Warren, C.A.; Ma, J.Z.; Madden, G.R. Impact of Tigecycline on C. difficile Outcomes: Case Series and Propensity-Matched Retrospective Study. Antimicrob. Agents Chemother. 2022, 66, e0000122. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.; Khan, F.; Uddin, N.; Wallace, M.R. Tigecycline for severe Clostridium difficile infection. Int. J. Infect. Dis. 2014, 26, 171–172. [Google Scholar] [CrossRef] [PubMed]
- van Prehn, J.; Reigadas, E.; Vogelzang, E.H.; Bouza, E.; Hristea, A.; Guery, B.; Krutova, M.; Norén, T.; Allerberger, F.; Coia, J.E.; et al. European Society of Clinical Microbiology and Infectious Diseases: 2021 update on the treatment guidance document for Clostridioides difficile infection in adults. Clin. Microbiol. Infect. 2021, 27 (Suppl. S2), S1–S21. [Google Scholar] [CrossRef] [PubMed]
- Louie, T.J.; Miller, M.A.; Mullane, K.M.; Weiss, K.; Lentnek, A.; Golan, Y.; Gorbach, S.; Sears, P.; Shue, Y.K. Fidaxomicin versus vancomycin for Clostridium difficile infection. N. Engl. J. Med. 2011, 364, 422–431. [Google Scholar] [CrossRef]
- Wilcox, M.H.; Gerding, D.N.; Poxton, I.R.; Kelly, C.; Nathan, R.; Birch, T.; Cornely, O.A.; Rahav, G.; Bouza, E.; Lee, C.; et al. Bezlotoxumab for Prevention of Recurrent Clostridium difficile Infection. N. Engl. J. Med. 2017, 376, 305–317. [Google Scholar] [CrossRef]
- Neal, M.D.; Alverdy, J.C.; Hall, D.E.; Simmons, R.L.; Zuckerbraun, B.S. Diverting loop ileostomy and colonic lavage: An alternative to total abdominal colectomy for the treatment of severe, complicated Clostridium difficile associated disease. Ann. Surg. 2011, 254, 423–427, discussion 427–429. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.; Hung, Y.P.; Tsai, B.Y.; Tsai, P.J.; Ko, W.C. Severe Clostridium difficile infections in intensive care units: Diverse clinical presentations. J. Microbiol. Immunol. Infect. 2021, 54, 1111–1117. [Google Scholar] [CrossRef] [PubMed]
- Sailhamer, E.A.; Carson, K.; Chang, Y.; Zacharias, N.; Spaniolas, K.; Tabbara, M.; Alam, H.B.; DeMoya, M.A.; Velmahos, G.C. Fulminant Clostridium difficile colitis: Patterns of care and predictors of mortality. Arch. Surg. 2009, 144, 433–439, discussion 439–440. [Google Scholar] [CrossRef] [PubMed]
- Brinda, B.J.; Pasikhova, Y.; Quilitz, R.E.; Thai, C.M.; Greene, J.N. Use of tigecycline for the management of Clostridium difficile colitis in oncology patients and case series of breakthrough infections. J. Hosp. Infect. 2017, 95, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Herpers, B.L.; Vlaminckx, B.; Burkhardt, O.; Blom, H.; Biemond-Moeniralam, H.S.; Hornef, M.; Welte, T.; Kuijper, E.J. Intravenous tigecycline as adjunctive or alternative therapy for severe refractory Clostridium difficile infection. Clin. Infect. Dis. 2009, 48, 1732–1735. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.L.; Liu, C.Y.; Liao, C.H.; Huang, Y.T.; Wang, H.P.; Hsueh, P.R. Severe and refractory Clostridium difficile infection successfully treated with tigecycline and metronidazole. Int. J. Antimicrob. Agents 2010, 35, 311–312. [Google Scholar] [CrossRef] [PubMed]
- Lao, D., 2nd; Chiang, T.; Gomez, E. Refractory Clostridium difficile Infection Successfully Treated with Tigecycline, Rifaximin, and Vancomycin. Case Rep. Med. 2012, 2012, 702910. [Google Scholar] [CrossRef] [PubMed]
- Marr, C.; Shiley, K. Adjuvant Tigecycline for Severe Clostridium difficile-Associated Diarrhea. Open Forum Infect. Dis. 2015, 2, 1392. [Google Scholar] [CrossRef]
- Kechagias, K.S.; Chorepsima, S.; Triarides, N.A.; Falagas, M.E. Tigecycline for the treatment of patients with Clostridium difficile infection: An update of the clinical evidence. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 1053–1058. [Google Scholar] [CrossRef]
- Bauer, M.P.; Hensgens, M.P.; Miller, M.A.; Gerding, D.N.; Wilcox, M.H.; Dale, A.P.; Fawley, W.N.; Kuijper, E.J.; Gorbach, S.L. Renal failure and leukocytosis are predictors of a complicated course of Clostridium difficile infection if measured on day of diagnosis. Clin. Infect. Dis. 2012, 55 (Suppl. S2), S149–S153. [Google Scholar] [CrossRef]
- Miller, M.A.; Louie, T.; Mullane, K.; Weiss, K.; Lentnek, A.; Golan, Y.; Kean, Y.; Sears, P. Derivation and validation of a simple clinical bedside score (ATLAS) for Clostridium difficile infection which predicts response to therapy. BMC Infect. Dis. 2013, 13, 148. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).