Abstract
Neuromyelitis optica spectrum disorder (NMOSD), a demyelinating CNS disorder in which inflammatory cells infiltrate the spinal cord and optic nerve, has been identified as an AQP4-IgG-positive disease. Some of its most common clinical characteristics are optic neuritis, acute myelitis, area postrema syndrome, and brainstem syndrome. However, the relationship between aquaporin-4 (AQP4) and NMOSD appears to be involved in pathologies outside of the CNS due to the fact that autoimmune, muscular, and paraneoplastic syndromes are more common in patients with NMOSD. This perspective presents an analysis of the current literature on neuromyelitis optica in an effort to further understand and compile pathologies that arise outside of the CNS secondary to NMOSD. Recontextualizing neuromyelitis optica as a systemic condition will facilitate greater diagnostic ability and improved treatment approaches.
1. Background
Aquaporins are a family of membrane transport proteins that includes 13 recognized isoforms of fluid transport channels found in the plasma membranes of many different cell types [1]. The main fluid that aquaporin channels transport is water; however, research has also demonstrated their role in transporting substances such as glycerol, urea, and potentially some gases and ions [2,3]. In the central nervous system, AQP1 and AQP4 predominate [4]. Of specific interest in several neurological pathologies is AQP4, which has been principally identified for its role as a water channel found in astrocytes in the CNS, but also plays a role in the epithelial cells of numerous other organs [5]. Knockout studies in mice provide strong evidence that some of the most important roles of AQP4 in the brain include cerebral water balance, astrocyte migration, and neural signal transduction [6]. Therefore, pathologies involving AQP4 often lead to severe neurological symptoms.
The pathogenesis of AQP4 diseases is complex. Within the last decade alone, researchers have identified various ways in which AQP4 expression and distribution change, due to physiologic and pathologic processes. When astrocyte tonicity was altered in rats, it was found that cell surface expression of AQP4 subsequently adapted to compensate [7]. When human astrocytes were cultured at hypothermic and normothermic conditions, ELISA analysis demonstrated that the hypothermic conditions elicited an increase in surface expression of AQP4, suggesting the human body has the capacity to alter AQP4 expression to accommodate various physiologic states [8]. For example, hypoxia induces calmodulin-driven increases in AQP4 cell-surface localization [9].
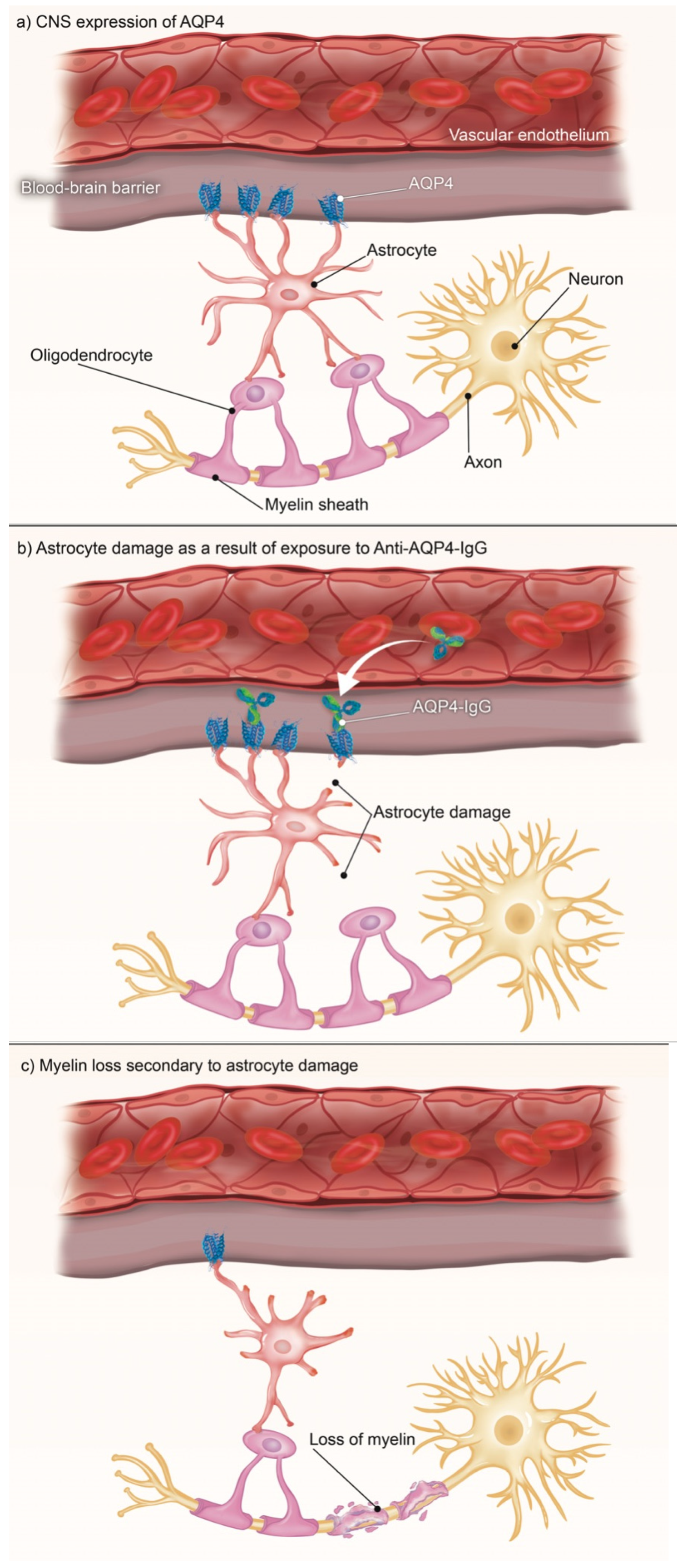
One AQP4-related disease is neuromyelitis optica spectrum disorder (NMOSD) [10]. It was estimated in 2020 that NMOSD has a prevalence among East Asians of 3.5/100,000, among Whites of 1/100,000, and among Blacks ranging from 1.8 to 10/100,000, with a 9:1 female predominance [11]. NMOSD is a demyelinating CNS disorder in which inflammatory cells infiltrate the spinal cord and optic nerve. It has been identified as an AQP4-IgG-positive disease [12]. Recent research has demonstrated that these anti-AQP4 antibodies target primary cortical astrocytes (see Figure 1) and disrupt the ability of the AQP4 channels to redistribute in response to changes in their environment [13], yielding some of the most common clinical characteristics of NMOSD, including optic neuritis, acute myelitis, area postrema syndrome (APS), and brainstem syndrome [14]. See Table 1.
Figure 1.
Anti-AQP4-antibody disease in the CNS occurs when a disruption to the blood–brain barrier allows circulating AQP4-IgG to access the CNS and bind with the AQP4 channels found on astrocytes. These astrocytes can no longer support the oligodendrocytes that myelinate the neurons in the CNS, and this loss of myelination leads to the various symptoms of NMOSD.

Table 1.
Core clinical characteristics frequently seen in NMOSD patients.
Recent advancements in NMOSD treatment have made great strides in improving our knowledge of potential therapeutic modalities to treat patients suffering from this debilitating disease. One example is the transition from immunosuppression to targeted treatments for NMOSD-specific elements of the autoimmune cascade. Another example is the greater emphasis on discovering and utilizing evidence-based therapeutic modalities rather than “off-label” adaptations [20]. Recent advances have also demonstrated that it is possible to target the subcellular relocalization functions of AQP4, such as with the calmodulin-inhibiting antipsychotic drug trifluoperazine [21,22].
Improvements in technology have also facilitated breakthroughs in our understanding of AQP4 diseases. Of note, Computer-aided Drug Design (CADD) [23] and high-throughput screening platforms [24] have enabled researchers to be far more efficient in screening compounds that do or do not have potential applications for their specific projects. Similarly, two novel devices termed “human blood–brain barrier on-a-chip” [25] and “human brain endothelial microvessel-on-a-chip” [26] have allowed greater capacity to test pharmaceuticals in a model resembling the human blood–brain barrier.
However, an area that appears to still be lacking in sufficient research is the relationship between AQP4 and NMOSD outside of the CNS given that autoimmune, muscular, and paraneoplastic syndromes are more common in patients with NMOSD [27]. This is likely due, at least in part, to the fact that AQP4 is found in numerous human tissues.
2. Methods
PubMed and UpToDate databases were searched from March 2022 to August 2022 using the key terms: “autoimmune”, “CNS”, and “systemic”, in conjunction with “aquaporin-4” or “AQP4” and “neuromyelitis optica”, or “NMO”. This search generated 741 results. Results that did not address the nature of NMOSD as an AQP-4 disease or that did not provide examples of symptoms outside of the CNS were excluded. Only articles in English from the last 10 years were included, with the exception of fundamental disease characterizations. This resulted in 62 articles used in this perspective.
Sources were analyzed and categorized by Level of Evidence, following the formulary developed by the U.S. Preventive Services Task Force. Of the 62 articles used, two were considered Level III. The remaining articles were Level I through Level II-3.
- Category I: Evidence from at least one properly randomized controlled trial.
- Category II-1: Evidence from well-designed controlled trials without randomization.
- Category II-2: Evidence from well-designed cohort or case–control analytic studies, preferably from more than one center or research group.
- Category II-3: Evidence from multiple time series with or without intervention, or dramatic results in uncontrolled experiments such as the results of the introduction of penicillin treatment in the 1940s.
- Category III: Opinions of respected authorities, based on clinical experience, descriptive studies and case reports, or reports of expert committees.
3. Results
3.1. CNS
NMOSD is believed to occur due to invasion of anti-AQP4-IgG antibodies through the blood–brain barrier, which then bind the AQP4 channels found on astrocytes. Upon binding, these antibodies cause complement- and cell-mediated damage to the astrocytes, as well as internalization of excitatory amino acid tranporter-2, a glutamate transporter [28]. This leads to astrocyte death, and subsequent oligodendrocyte death. In turn, this causes demyelination of nerves previously supported by these astrocytes and oligodendrocytes, leading to the various NMOSD symptoms previously discussed [14,29].
Emerging research is extracting greater detail regarding the specific neuropathological processes commonly seen in NMOSD. Cognitive impairment in NMOSD patients has a prevalence of approximately 44% [30]. Case studies have also demonstrated a potential for developing acute anosmia and dysphagia [31,32]. Optic neuritis and visual acuity loss have been demonstrated to occur due to physical changes in the cerebrum, specifically decreased gray matter volume and compromised functional connectivity [33]. Acute non-obstructive hydrocephalus has been discovered in patients with NMOSD, most likely due to the loss of AQP4, [34] a loss which mouse-model studies demonstrate cannot be compensated for by AQP1 [35].
The majority of research regarding NMOSD in the past has considered it to be exclusively confined to the CNS. However, given that AQP4 channels are present in various locations throughout the body, emerging evidence is beginning to demonstrate that this is likely not the case, and diagnosticians should use a wider lens when evaluating for, and treating, NMOSD.
3.2. Muscular
For most of its known history, it has been believed that NMOSD had little to no effect on skeletal muscle tissue. However, with greater testing capabilities, evidence is emerging that demonstrates that pathologic changes are, in fact, occurring in the skeletal muscles of NMOSD patients. These include hyperCKemia, loss of AQP4, and deposition of IgG and activated complement products [36]. Muscle biopsy studies have shown that AQP4 expression is significantly reduced in patients with NMOSD, [37] a potentially useful diagnostic tool that may hint at a clinically relevant correlation between NMOSD and potential effects in muscular tissues that is not yet fully understood. This is particularly important in light of the fact that only AQP1 and AQP4 are expressed in skeletal muscle tissue; [38] yet AQP1 does not appear to take on a compensatory role in the event of AQP4 loss [35]. One potential explanation for this discrepancy is that there are supramolecular aggregation differences between CNS and muscular AQP4 [39].
While the evidence does not rise to the level of actionable, a couple of interesting case reports are of note. In 2019, Shang et al. (level III evidence) described the case of a patient who developed rhabdomyolysis and then later developed NMOSD [40]. The authors hypothesized that tissue destruction secondary to rhabdomyolysis may have led to the creation of anti-AQP4-IgG in the muscle, although it is difficult to draw any definitive association between the conditions based on a single case. Nevertheless, the underlying established pathophysiology of NMOSD notes that anti-AQP4-IgG antibodies precipitate the development of NMOSD (See Figure 1). Another case report (level III) described the second known occurrence of myotonic dystrophy type 2 subsequent to an NMOSD diagnosis, though the authors stated the coexistence could be coincidental [41]. While a limited amount of level III evidence exists to connect NMOSD and certain disorders of the musculature, the underlying connection of the conditions through AQP4 lends credence to some of the speculated associations. This molecular link between NMOSD and muscular disorders provides a new perspective and suggests that perhaps the relationship should be re-examined in the light of emerging evidence that demonstrates a poorly understood and under-researched area.
3.3. Cancer
Patients with NMOSD have an increased cancer risk, particularly lung, breast, and genitourinary [42,43]. The relationship underlying this correlation is complex and needs further investigation, but does appear to be due, at least in part, to AQP4 and the distinct AQP4 profiles found in different cancers [44]. Therefore, screening for cancer in patients with NMOSD is encouraged.
A case series from 2021 examined three cases, each with distinct cancer diagnoses but all linked by the underlying diagnosis of NMOSD, demonstrating the paraneoplastic potential of NMOSD [45]. The first NMOSD-positive patient developed grade 2 lymphopenia, normochromic normocytic anemia, and IgG lambda monoclonal gammopathy. The second presented with a lesion extending from C1 to C5, periventricular gliosis, a large uterine mass, and extensive pelvic and bowel invasions. The third developed cerebral white matter cortical lesions and lung adenocarcinoma stage 3b. This reiterates a recommendation to screen NMOSD patients for paraneoplastic conditions.
Various case reports performed within the last five years have demonstrated melanoma, [46] breast cancer, [47] small-bowel neuroendocrine tumor with hepatic metastasis, [48] gastric carcinoid tumor, [49] lung adenocarcinoma, [50] and esophageal squamous cell carcinoma, [51] all occurring secondary to NMOSD. In the majority of cases, the development of the pathologies can be directly linked to NMOSD, given that the paraneoplastic cells are anti-AQP4-IgG positive. Based on the diversity of syndromes and of the patients in which they present, it is reasonable to hypothesize that the above-listed pathologies represent only a portion of the potential paraneoplastic complications that can arise subsequent to developing NMOSD.
3.4. Autoimmune
There is an increasingly distinct connection between development of NMOSD and subsequent development of other autoimmune disorders. Sjogren’s Syndrome, Systemic Lupus Erythematosus (SLE), myasthenia gravis, and autoimmune thyroid diseases are the most reported autoimmune disorders secondary to NMOSD [52,53]. While it is not surprising to find autoimmune diseases secondary to an initiating autoimmune disease, there is an emerging theory to explain this process as a connection between NMOSD and alterations in the gut microbiome.
Assessment of the gut microbiota in patients with NMOSD has shown that the microbiome of NMO is altered when compared to healthy controls and patients with multiple sclerosis [54,55]. An increase in Clostridium perfringens was the most noteworthy change, given that there is ample evidence that Clostridia found in the gastrointestinal tract can affect the equilibrium of regulatory T cells and Th17 cells [56]. This suggests a possible direct link between gut microbiota and the underlying immune responses driving NMOSD development, and at the same time driving development of other autoimmune diseases.
3.5. Other
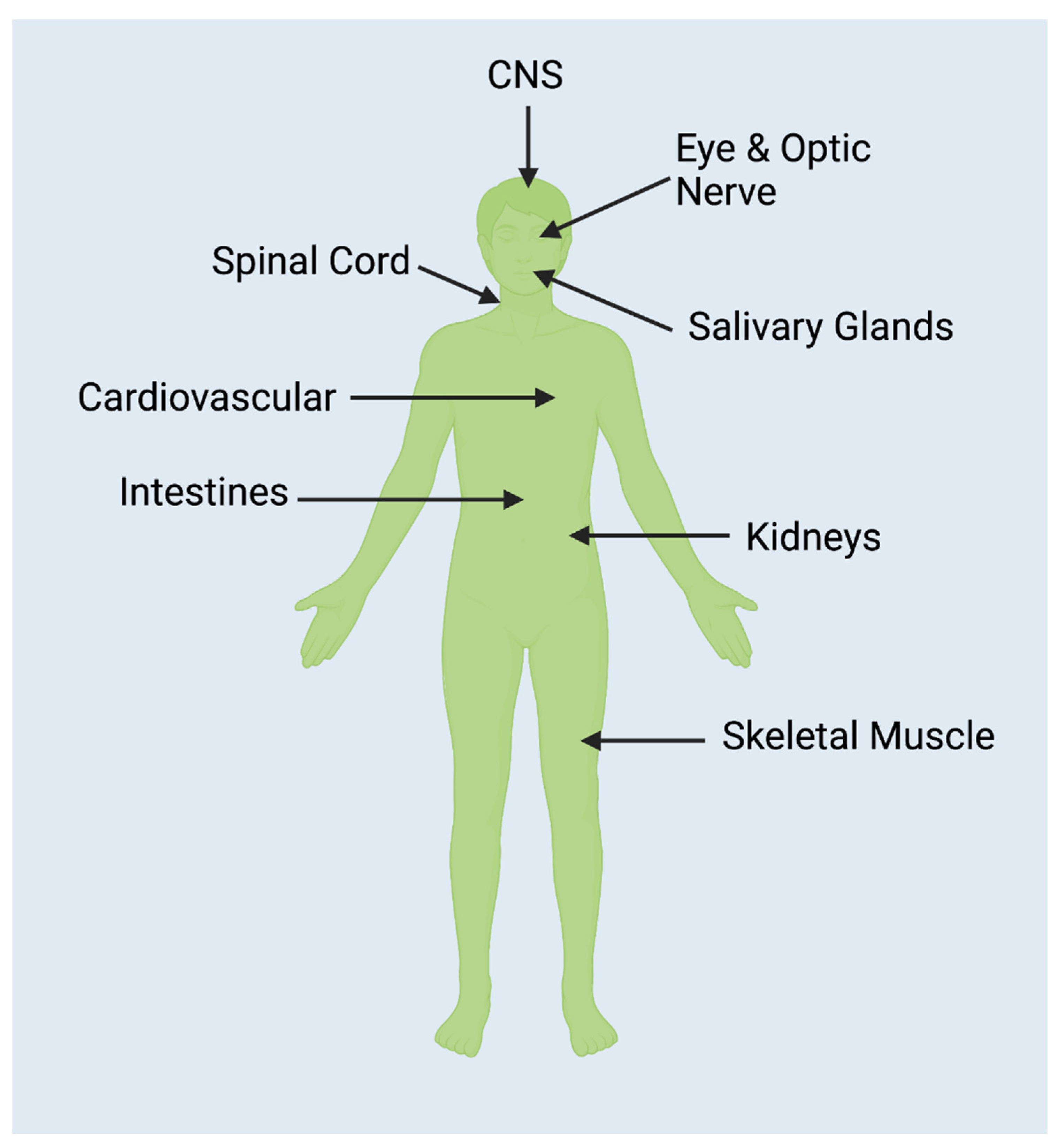
Given that AQP4 is found in numerous and diverse human tissues (see Figure 2), it is unsurprising that case reports have been published which describe symptoms that cannot easily be added to the categories already delineated here. A few notable examples are provided. One study reported that 43.8% of participants developed menstrual irregularities subsequent to developing NMOSD [57]. A 2019 case study investigated a woman who developed posterior reversible encephalopathy syndrome subsequent to being diagnosed with NMOSD [58]. A case report from 2018 detailed the disease course of a pregnant female patient with Hepatitis C who subsequently developed NMOSD. Because of her pregnancy and her positive Hepatitis C status, she was unable to be treated using the typical therapeutic modalities. This resulted in a more severe disease course [59].
Figure 2.
NMOSD is an anti-AQP4 antibody disease. AQP4 is found throughout the body [60], particularly in the regions in the above figure. As the traditional definition of NMOSD as a CNS-exclusive disorder is challenged, further connections are being discovered that demonstrate the possibility of pathologies arising in any body region where AQP4 is expressed.
In another example, a systematic review performed from 2019 to 2021 provided strong evidence to suggest that patients with NMOSD are more susceptible to COVID-19 and have worse outcomes. The authors concluded that the most significant risk factor appeared to be pre-existing treatment of NMOSD with rituximab; therefore, clinicians should bear this in mind as they develop treatment plans for their patients with NMOSD [61].
Interestingly, patients with NMOSD had much higher urine pH and much lower urine specific gravity levels than patients with MS [62]. Additionally, approximately 2% of patients with MS develop pruritus, compared to 21% in patients with NMOSD [62]. This further differentiates NMOSD from MS and supports the view that NMOSD should be considered a systemic condition.
All of these reports, along with the systematic review mentioned, suggest that improvements in NMOSD treatment—particularly for those with comorbidities—should be sought after.
4. Discussion
Given the diverse associations between NMOSD and other pathological processes outside of the CNS, it is highly likely that the anti-AQP4 etiology of NMOSD is not restricted to the CNS (See Table 2). The increased incidence of paraneoplastic disorders secondary to NMOSD diagnosis is of particular note and concern and should be heavily considered by care providers. Screenings for the cancers most commonly associated with NMOSD could be of great benefit, though more research is needed to establish more predictable and universal guidelines. Hemorrhagic conditions and menstrual irregularities are also significant and should be monitored.

Table 2.
A summary of some of the possible presentations and complications discovered in NMOSD patients, as discussed in this work.
Another area that needs further exploration is the minor, but pathologic, skeletal muscle changes and the decrease in AQP4 expression in muscle biopsies of patients with NMOSD. It is not yet understood why this occurs, but further research on this topic could create even more concrete diagnostic criteria for NMOSD.
Also of note are the additional autoimmune conditions that arise with or subsequent to NMOSD. In particular, the potential connection between the gut microbiome and autoimmune disorders should be investigated further.
Fortunately, increasing interest in treating AQP4 diseases has encouraged investigation into therapeutic modalities that may go beyond current conventional treatments [20,21,22,23,24,25,26]. The research is still emerging, and much remains to be understood. Nevertheless, current evidence would dictate that greater resources should be dedicated to researching and reconsidering therapeutic approaches to AQP4 diseases such as NMOSD.
The conditions and associations explored in this article may only graze the surface of the multitude of systemic presentations of NMOSD that warrant both further investigation as well as a more holistic, systemic approach to NMOSD diagnosis, monitoring, and treatment, as opposed to the more prevalent CNS-specific viewpoint.
5. Conclusions
Traditionally, NMOSD has been viewed through the lens of an autoimmune disorder limited almost entirely to the central nervous system. However, emerging research supports NMOSD as a vast systemic disorder, attributed in part to the anti-AQP4 antibodies found in NMOSD patients. Researchers, clinicians, and patients should be aware of the potential development of cancers, other autoimmune disorders, muscular pathology, and more, to guide not only treatment but improved patient outcome and quality of life. Future studies, employing statistical correlational analysis with other comorbidities using national datasets, should be carried out to provide more tangible evidence of observed associations.
Author Contributions
Conceptualization, P.W., B.C.L. and A.E.B.; methodology, P.W.; software, P.W.; validation, P.W., B.C.L. and A.E.B.; formal analysis, P.W.; investigation, P.W. and B.C.L.; resources, P.W. and B.C.L.; data curation, P.W.; writing—original draft preparation, P.W.; writing—review and editing, P.W., B.C.L. and A.E.B.; visualization, P.W.; supervision, A.E.B.; project administration, A.E.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable.
Acknowledgments
Figure 1 was created in collaboration with Parinaz Ghanbari.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Verkman, A. Aquaporins. Curr. Biol. 2013, 23, R52–R55. [Google Scholar] [CrossRef] [PubMed]
- Kitchen, P.; Day, R.E.; Salman, M.M.; Conner, M.T.; Bill, R.M.; Conner, A.C. Beyond water homeostasis: Diverse functional roles of mammalian aquaporins. Biochim. Biophys. Acta 2015, 1850, 2410–2421. [Google Scholar] [CrossRef] [PubMed]
- Hara-Chikuma, M.; Verkman, A.S. Physiological roles of glycerol-transporting aquaporins: The aquaglyceroporins. Cell. Mol. Life Sci. 2006, 63, 1386–1392. [Google Scholar] [CrossRef] [PubMed]
- Filippidis, A.S.; Carozza, R.B.; Rekate, H.L. Aquaporins in brain edema and neuropathological conditions. Int. J. Mol. Sci. 2016, 18, 55. [Google Scholar] [CrossRef]
- Verkman, A.S.; Rossi, A.; Crane, J.M. Live-cell imaging of aquaporin-4 supramolecular assembly and diffusion. Methods Enzymol. 2012, 504, 341–354. [Google Scholar] [CrossRef]
- Verkman, A.S.; Binder, D.K.; Bloch, O.; Auguste, K.; Papadopoulos, M.C. Three distinct roles of aquaporin-4 in brain function revealed by knockout mice. Biochim. Biophys. Acta 2006, 1758, 1085–1093. [Google Scholar] [CrossRef]
- Kitchen, P.; Day, R.E.; Taylor, L.H.; Salman, M.M.; Bill, R.M.; Conner, M.T.; Conner, A.C. Identification and molecular mechanisms of the rapid tonicity-induced relocalization of the aquaporin 4 channel. J. Biol. Chem. 2015, 290, 16873–16881. [Google Scholar] [CrossRef]
- Salman, M.M.; Kitchen, P.; Woodroofe, M.N.; Brown, J.E.; Bill, R.M.; Conner, A.C.; Conner, M.T. Hypothermia increases aquaporin 4 (AQP4) plasma membrane abundance in human primary cortical astrocytes via a calcium/transient receptor potential vanilloid 4 (TRPV4)- and calmodulin-mediated mechanism. Eur. J. Neurosci. 2017, 46, 2542–2547. [Google Scholar] [CrossRef]
- Kitchen, P.; Salman, M.M.; Halsey, A.M.; Clarke-Bland, C.; Macdonald, J.A.; Ishida, H.; Vogel, H.J.; Almutiri, S.; Logan, A.; Kreida, S.; et al. Targeting aquaporin-4 subcellular localization to treat central nervous system edema. Cell 2020, 181, 784–799.e19. [Google Scholar] [CrossRef]
- Papadopoulos, M.C.; Verkman, A.S. Aquaporin 4 and neuromyelitis optica. Lancet Neurol. 2012, 11, 535–544. [Google Scholar] [CrossRef]
- Hor, J.Y.; Asgari, N.; Nakashima, I.; Broadley, S.A.; Leite, M.I.; Kissani, N.; Jacob, A.; Marignier, R.; Weinshenker, B.G.; Paul, F.; et al. Epidemiology of neuromyelitis optica spectrum disorder and its prevalence and incidence worldwide. Front. Neurol. 2020, 11, 501. [Google Scholar] [CrossRef] [PubMed]
- Jarius, S.; Wildemann, B.; Paul, F. Neuromyelitis optica: Clinical features, immunopathogenesis and treatment. Clin. Exp. Immunol. 2014, 176, 149–164. [Google Scholar] [CrossRef]
- Ciappelloni, S.; Bouchet, D.; Dubourdieu, N.; Boué-Grabot, E.; Kellermayer, B.; Manso, C.; Marignier, R.; Oliet, S.H.; Tourdias, T.; Groc, L. Aquaporin-4 surface trafficking regulates astrocytic process motility and synaptic activity in health and autoimmune disease. Cell Rep. 2019, 27, 3860–3872.e4. [Google Scholar] [CrossRef] [PubMed]
- Huda, S.; Whittam, D.; Bhojak, M.; Chamberlain, J.; Noonan, C.; Jacob, A. Neuromyelitis optica spectrum disorders. Clin. Med. 2019, 19, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Wingerchuk, D.M.; Banwell, B.; Bennett, J.L.; Cabre, P.; Carroll, W.; Chitnis, T.; De Seze, J.; Fujihara, K.; Greenberg, B.; Jacob, A.; et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology 2015, 85, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Optic Neuritis. Mayo Clinic. Available online: https://www.mayoclinic.org/diseases-conditions/optic-neuritis/symptoms-causes/syc-20354953?utm_source=Google&utm_medium=abstract&utm_content=Optic-neuritis&utm_campaign=Knowledge-panel (accessed on 12 July 2022).
- Transverse Myelitis Fact Sheet. National Institute of Neurological Disorders and Stroke. Available online: https://www.ninds.nih.gov/health-information/patient-caregiver-education/fact-sheets/transverse-myelitis-fact-sheet. (accessed on 12 July 2022).
- Apetse, K.; Diatewa, J.E.; Tajeuna, J.J.D.; Dansou, Y.M.; Bakoudissa, R.; Waklatsi, K.P.; Kombate, D.; Assogba, K.; Balogou, A.A.K. Case report: An area postrema syndrome revealing a neuromyelitis optica spectrum disorder associated with central nervous system tuberculosis in a young Togolese (black African) woman. BMC Neurol. 2019, 19, 58. [Google Scholar] [CrossRef] [PubMed]
- Nightingale, H.; Witherick, J.; Wilkins, A. Diagnosis of longitudinally extensive transverse myelitis. BMJ Case Rep. 2011, 2011, bcr1020103444. [Google Scholar] [CrossRef]
- Waliszewska-Prosół, M.; Chojdak-Łukasiewicz, J.; Budrewicz, S.; Pokryszko-Dragan, A. Neuromyelitis optica spectrum disorder treatment-current and future prospects. Int. J. Mol. Sci. 2021, 22, 2801. [Google Scholar] [CrossRef]
- Salman, M.M.; Kitchen, P.; Yool, A.J.; Bill, R.M. Recent breakthroughs and future directions in drugging aquaporins. Trends Pharmacol. Sci. 2022, 43, 30–42. [Google Scholar] [CrossRef]
- Sylvain, N.J.; Salman, M.M.; Pushie, M.J.; Hou, H.; Meher, V.; Herlo, R.; Peeling, L.; Kelly, M.E. The effects of trifluoperazine on brain edema, aquaporin-4 expression and metabolic markers during the acute phase of stroke using photothrombotic mouse model. Biochim. Biophys. Acta Biomembr. 2021, 1863, 183573. [Google Scholar] [CrossRef]
- Salman, M.M.; Al-Obaidi, Z.; Kitchen, P.; Loreto, A.; Bill, R.M.; Wade-Martins, R. Advances in applying computer-aided drug design for neurodegenerative diseases. Int. J. Mol. Sci. 2021, 22, 4688. [Google Scholar] [CrossRef] [PubMed]
- Aldewachi, H.; Al-Zidan, R.N.; Conner, M.T.; Salman, M.M. High-throughput screening platforms in the discovery of novel drugs for neurodegenerative diseases. Bioengineering 2021, 8, 30. [Google Scholar] [CrossRef] [PubMed]
- Wevers, N.R.; Kasi, D.G.; Gray, T.; Wilschut, K.J.; Smith, B.; van Vught, R.; Shimizu, F.; Sano, Y.; Kanda, T.; Marsh, G.; et al. A perfused human blood-brain barrier on-a-chip for high-throughput assessment of barrier function and antibody transport. Fluids Barriers CNS 2018, 15, 23. [Google Scholar] [CrossRef] [PubMed]
- Salman, M.M.; Marsh, G.; Kusters, I.; Delincé, M.; Di Caprio, G.; Upadhyayula, S.; de Nola, G.; Hunt, R.; Ohashi, K.G.; Gray, T.; et al. Design and validation of a human brain endothelial microvessel-on-a-chip open microfluidic model enabling advanced optical imaging. Front. Bioeng. Biotechnol. 2020, 8, 573775. [Google Scholar] [CrossRef] [PubMed]
- Zekeridou, A.; Lennon, V.A. Aquaporin-4 autoimmunity. Neurol. Neuroimmunol. Neuroinflamm. 2015, 2, e110. [Google Scholar] [CrossRef]
- Hinson, S.R.; Pittock, S.J.; Lucchinetti, C.F.; Roemer, S.F.; Fryer, J.P.; Kryzer, T.J.; Lennon, V.A. Pathogenic potential of IgG binding to water channel extracellular domain in neuromyelitis optica. Neurology 2007, 69, 2221–2231. [Google Scholar] [CrossRef]
- Jarius, S.; Paul, F.; Franciotta, D.; Waters, P.; Zipp, F.; Hohlfeld, R.; Vincent, A.; Wildemann, B. Mechanisms of disease: Aquaporin-4 antibodies in neuromyelitis optica. Nat. Clin. Pract. Neurol. 2008, 4, 202–214. [Google Scholar] [CrossRef]
- Moghadasi, A.N.; Mirmosayyeb, O.; Mohammadi, A.; Sahraian, M.A.; Ghajarzadeh, M. The prevalence of cognitive impairment in patients with neuromyelitis optica spectrum disorders (NMOSD): A systematic review and meta-analysis. Mult. Scler. Relat. Disord. 2021, 49, 102757. [Google Scholar] [CrossRef]
- Marshall, J.; Kleerekooper, I.; Davagnanam, I.; Trip, S.A. Acute anosmia in neuromyelitis optica spectrum disorder. Mult. Scler. 2020, 26, 1958–1960. [Google Scholar] [CrossRef]
- Cousins, O.; Girelli, E.; Harikrishnan, S. Neuromyelitis optica: An elusive cause of dysphagia. BMJ Case Rep. 2019, 12, bcr-2018-227041. [Google Scholar] [CrossRef]
- Yan, J.; Wang, Y.; Miao, H.; Kwapong, W.R.; Lu, Y.; Ma, Q.; Chen, W.; Tu, Y.; Liu, X. Alterations in the brain structure and functional connectivity in aquaporin-4 antibody-positive neuromyelitis optica spectrum disorder. Front. Neurosci. 2020, 13, 1362. [Google Scholar] [CrossRef]
- Close, L.N.; Zanaty, M.; Kirby, P.; Dlouhy, B.J. Acute hydrocephalus resulting from neuromyelitis optica: A case report and review of the literature. World Neurosurg. 2019, 129, 367–371. [Google Scholar] [CrossRef]
- Trillo-Contreras, J.L.; Ramírez-Lorca, R.; Villadiego, J.; Echevarría, M. Cellular distribution of brain aquaporins and their contribution to cerebrospinal fluid homeostasis and hydrocephalus. Biomolecules 2022, 12, 530. [Google Scholar] [CrossRef]
- He, D.; Li, Y.; Dai, Q.; Zhang, Y.; Xu, Z.; Li, Y.; Cai, G.; Chu, L. Myopathy associated with neuromyelitis optica spectrum disorders. Int. J. Neurosci. 2016, 126, 863–866. [Google Scholar] [CrossRef]
- Shahmohammadi, S.; Doosti, R.; Shahmohammadi, A.; Azimi, A.; Sahraian, M.A.; Fattahi, M.-R.; Moghadasi, A.N. Neuromyelitis optica spectrum disorder (NMOSD) associated with cancer: A systematic review. Mult. Scler. Relat. Disord. 2021, 56, 103227. [Google Scholar] [CrossRef]
- Zhang, W.; Dong, M.; Dong, H.; Wang, W.; Sun, W.; Hao, Y.; Jiao, Y.; Cui, L.; Jiao, J. Reduced sarcolemmal aquaporin 4 expression can support the differential diagnosis of neuromyelitis optica spectrum disorders. J. Neuroimmunol. 2020, 339, 577121. [Google Scholar] [CrossRef]
- Shang, K.; Qin, C.; Bu, B.T.; Tian, D.S. Aquaporin-4 antibody positive neuromyelitis optica spectrum disorder subsequent to rhabdomyolysis: A case report and literature review. Int. J. Neurosci. 2019, 129, 930–932. [Google Scholar] [CrossRef]
- Gelibter, S.; Moiola, L.; Previtali, S.C.; Filippi, M. Neuromyelitis optica and myotonic dystrophy type 2: A rare association with diagnostic implications. J. Neurol. 2020, 267, 2744–2746. [Google Scholar] [CrossRef]
- Frigeri, A.; Nicchia, G.P.; Balena, R.; Nico, B.; Svelto, M. Aquaporins in skeletal muscle: Reassessment of the functional role of aquaporin-4. FASEB J. 2004, 18, 905–907. [Google Scholar] [CrossRef]
- Gibril, M.; Walters, R. Neuromyelitis optica spectrum disorder as a paraneoplastic syndrome: A rare and challenging diagnosis. BMJ Case Rep. 2021, 14, e239389. [Google Scholar] [CrossRef]
- Zou, S.; Lan, Y.-L.; Ren, T.; Li, X.; Zhang, L.; Wang, H.; Wang, X. A bioinformatics analysis of the potential roles of aquaporin 4 in human brain tumors: An immune-related process. Front. Pharmacol. 2021, 12, 692175. [Google Scholar] [CrossRef]
- Virgilio, E.; Vecchio, D.; Vercellino, M.; Naldi, P.; Tesser, F.; Cantello, R.; Cavalla, P.; Comi, C. Paraneoplastic neuromyelitis optica spectrum disorders: A case series. Neurol. Sci. 2021, 42, 2519–2522. [Google Scholar] [CrossRef]
- Morimoto, T.; Hayashida, S.; Yamasaki, K.; Sasahara, Y.; Takaki, T.; Yatera, K. Paraneoplastic neuromyelitis optica spectrum disorder associated with malignant melanoma: A case report. Thorac. Cancer. 2021, 12, 1775–1779. [Google Scholar] [CrossRef]
- Yuan, J.; Jia, Z.; Qin, W.; Hu, W. Paraneoplastic neuromyelitis optica spectrum disorder associated with breast cancer. Clin. Interv. Aging 2019, 14, 1039–1044, Published 6 Jun 2019. [Google Scholar] [CrossRef]
- Figueroa, M.; Guo, Y.; Tselis, A.; Pittock, S.J.; Lennon, V.A.; Lucchinetti, C.F.; Lisak, R.P. Paraneoplastic neuromyelitis optica spectrum disorder associated with metastatic carcinoid expressing aquaporin-4. JAMA Neurol. 2014, 71, 495–498. [Google Scholar] [CrossRef]
- Al-Harbi, T.; Al-Sarawi, A.; Binfalah, M.; Dermime, S. Paraneoplastic Neuromyelitis Optica Spectrum Disorder Associated with Stomach Carcinoid Tumor. Hematology/Oncology and Stem Cell Therapy. Available online: https://www.sciencedirect.com/science/article/pii/S165838761400048X?via%3Dihub (accessed on 18 July 2022).
- Iorio, R.; Rindi, G.; Erra, C.; Damato, V.; Ferilli, M.; Sabatelli, M. Neuromyelitis optica spectrum disorder as a paraneoplastic manifestation of lung adenocarcinoma expressing aquaporin-4. Mult. Scler. 2015, 21, 791–794. [Google Scholar] [CrossRef]
- Kon, T.; Ueno, T.; Suzuki, C.; Nunomura, J.; Igarashi, S.; Sato, T.; Tomiyama, M. Aquaporin-4 antibody positive neuromyelitis optica spectrum disorder associated with esophageal cancer. J. Neuroimmunol. 2017, 309, 38–40. [Google Scholar] [CrossRef]
- Shahmohammadi, S.; Doosti, R.; Shahmohammadi, A.; Mohammadianinejad, S.E.; Sahraian, M.A.; Azimi, A.R.; Harirchian, M.H.; Asgari, N.; Naser Moghadasi, A. Autoimmune diseases associated with neuromyelitis optica spectrum disorders: A literature review. Mult. Scler. Relat. Disord. 2019, 27, 350–363. [Google Scholar] [CrossRef]
- Sangani, V.; Pokal, M.; Balla, M.; Merugu, G.P.; Adapa, S.; Naramala, S.; Konala, V.M. A case of neuromyelitis optica spectrum disorder with coexisting systemic lupus erythematosus. J. Community Hosp. Intern. Med. Perspect. 2021, 11, 531–535. [Google Scholar] [CrossRef]
- Zamvil, S.S.; Spencer, C.M.; Baranzini, S.E.; Cree, B.A.C. The gut microbiome in neuromyelitis optica. Neurotherapeutics 2018, 15, 92–101. [Google Scholar] [CrossRef]
- Cui, C.; Ruan, Y.; Qiu, W. Potential role of the gut microbiota in neuromyelitis optica spectrum disorder: Implication for intervention. J. Clin. Neurosci. 2020, 82, 193–199. [Google Scholar] [CrossRef]
- Ivanov, I.I.; Atarashi, K.; Manel, N.; Brodie, E.L.; Shima, T.; Karaoz, U.; Wei, D.; Goldfarb, K.C.; Santee, C.A.; Lynch, S.V.; et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 2009, 139, 485–498. [Google Scholar] [CrossRef]
- Badihian, S.; Manouchehri, N.; Mirmosayyeb, O.; Ashtari, F.; Shaygannejad, V. Neuromyelitis optica spectrum disorder and menstruation. Rev. Neurol. 2018, 174, 716–721. [Google Scholar] [CrossRef]
- Kamo, H.; Ueno, Y.; Sugiyama, M.; Miyamoto, N.; Yamashiro, K.; Tanaka, R.; Yokoyama, K.; Hattori, N. Pontine hemorrhage accompanied by neuromyelitis optica spectrum disorder. J. Neuroimmunol. 2019, 330, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Obara, K.; Waliszewska-Prosół, M.; Budrewicz, S.; Szewczyk, P.; Ejma, M. Severe course of neuromyelitis optica in a female patient with chronic C hepatitis. Neurol. Neurochir. Pol. 2018, 52, 397–400. [Google Scholar] [CrossRef] [PubMed]
- Barzegar, M.; Mirmosayyeb, O.; Ebrahimi, N.; Bagherieh, S.; Afshari-Safavi, A.; Hosseinabadi, A.M.; Shaygannejad, V.; Asgari, N. COVID-19 susceptibility and outcomes among patients with neuromyelitis optica spectrum disorder (NMOSD): A systematic review and meta-analysis. Mult. Scler. Relat. Disord. 2022, 57, 103359. [Google Scholar] [CrossRef] [PubMed]
- Gleiser, C.; Wagner, A.; Fallier-Becker, P.; Wolburg, H.; Hirt, B.; Mack, A.F. Aquaporin-4 in astroglial cells in the CNS and supporting cells of sensory organs-a comparative perspective. Int. J. Mol. Sci. 2016, 17, 1411. [Google Scholar] [CrossRef]
- Chen, Z.G.; Huang, J.; Fan, R.; Weng, R.H.; Shinohara, R.T.; Landis, J.R.; Chen, Y.; Jiang, Y. Urinalysis in patients with neuromyelitis optica spectrum disorder. Eur. J. Neurol. 2020, 27, 619–625. [Google Scholar] [CrossRef]
- He, M.; Wu, L.; Huang, D.; Yau, V.; Yu, S. Pruritus in neuromyelitis optica spectrum disorders and multiple sclerosis. J. Clin. Neurosci. 2020, 79, 108–112. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).