Rapamycin’s Impact on Age-Related Macular Degeneration—A Systematic Review and Hormesis Perspective
Abstract
:1. Introduction
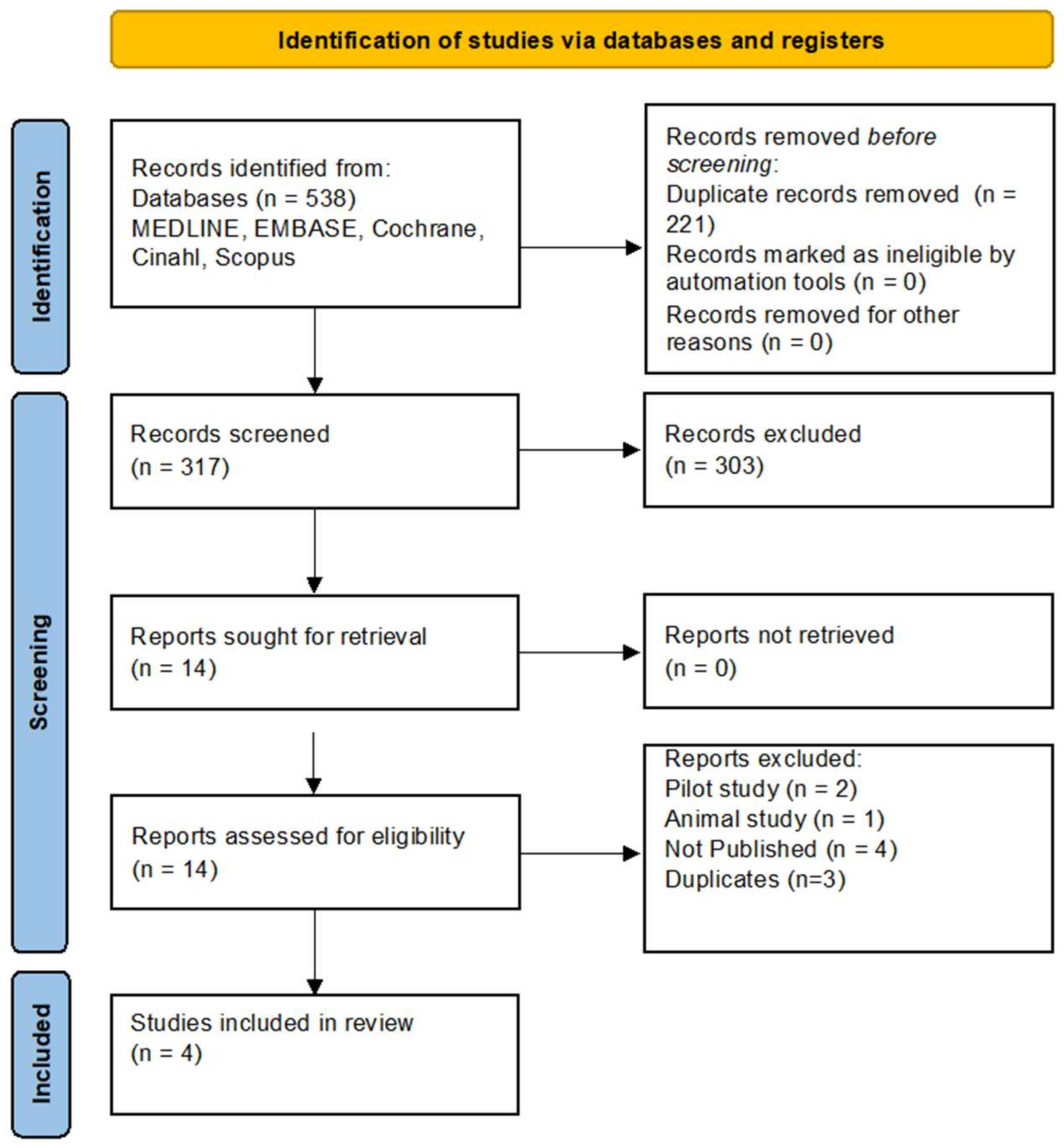
2. Materials and Methods
3. Results
| Author (et al.) | Year | Study Design | Dosing | Sample Size (Eyes) | Intervention | GRADE | Level (OCEBM) |
|---|---|---|---|---|---|---|---|
| Gensler [12] | 2018 | Randomized | 440 µg monthly IVT | 27 | Intravitreal Sirolimus® | Moderate | 2 |
| Petrou [13] | 2014 | Randomized | 440 µg bimonthly IVT | 5 | Intravitreal Sirolimus® | Moderate | 2 |
| Wong [14] | 2013 | Randomized | 440 µg trimonthly SCJ | 8 | Subconjunctival Sirolimus® | Moderate | 2 |
| Dugel [15] | 2012 | Randomized | Various doses ** | 50 | IVT and SCJ Sirolimus® * | Moderate | 2 |
| Mean Change in BCVA (No. letters (SD)) | Mean Change in Subfoveal Thickness µm (SD) | Mean Absolute Change in Drusen Area (MPS DA (SD)) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author | Year | No. Months | Intervention for Sirolimus | N (Sirolimus, Control) | IVT Sirolimus | Sham | Untreated Eye | SCJ Sirolimus | N (Sirolimus, Control) | IVT Sirolimus | Sham | SCJ Sirolimus | N (Sirolimus, Control) | IVT Sirolimus | Untreated Eye | SCJ Sirolimus |
| Gensler et al. [12] | 2018 | 12 | IVT | 22, 20 | −3.7 (8.7) | −7.3 (16.8) | - | - | 20, 20 | −16.7 (20.3) | −12.8 (20.5) | - | - | - | - | - |
| Gensler et al. [12] | 2018 | 24 | IVT | 7, 5 | −3.7 (8.6) | −10.6 (16.2) | - | - | 11, 10 | −31.8 (32.8) | −25 (25.6) | - | - | - | - | - |
| Petrou et al. [13] | 2015 | 12 | IVT | 5, 5 | −15.6 (7.23) | - | 0 (13.47) | - | - | - | - | - | 3, 3 | 0.02 (0.19) | 0.29 (0.78) | - |
| Wong et al. [14] | 2013 | 24 | SCJ | 8, 8 | - | - | −3 (8.1) * | −21 (21.5) * | - | - | - | - | 8, 8 | - | 0.08 (0.36) | 0.04 (0.58) |
| Dugel et al. [15] | 2012 | 3 | IVT | 25, 0 | 1.3 (7.1) | - | - | - | 25, 0 | −71.8 (60.4) | - | - | - | - | - | - |
| Dugel et al. [15] | 2012 | 3 | SCJ | 25, 0 | - | - | - | 4.0 (7.6) | 25, 0 | - | - | -11.1 (118.6) | - | - | - | - |
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Database | Number of Retrieved References |
|---|---|
| MEDLINE (Ovid) | 96 |
| Embase | 153 |
| Cochrane | 15 |
| CINAHL | 12 |
| Scopus | 262 |
| Number of references before deduplication | 538 |
| Number of references after deduplication | 317 |
| Ovid Databases | |||||
|---|---|---|---|---|---|
| exp/ | Exploded index term | ||||
| / | After an index term indicates a subject heading was selected. | ||||
| .ti,ab,kf. | Search for a term in the title, abstract, and author keywords. | ||||
| .kw. | =keyword heading | ||||
| * | At the end of a term indicates that this term has been truncated, e.g., diet* retrieves both diet, diets, dietary. | ||||
| Adj3 | Search for two terms next to each other, in any order, up to 3 words in between. | ||||
| Cochrane Library | |||||
| ti,ab,kw | Search for a word in the title, abstract, or keyword. | ||||
| NEAR/3 | Search for two terms next to each other, in any order, up to 3 words in between. | ||||
| # | Searches | Results |
|---|---|---|
| 1 | Sirolimus/ | 20,663 |
| 2 | (rapamune or rapamycin or sirolimus or w36zg6ft64 or abi009 or ay22989 or de109 or drgt182 or fyarro or hyftor or nabrapamycin or nabsirolimus or npc12 or opsiria or pascomer or perceiva or ptx001 or rapalimus or rapammune).tw,kw,kf. | 43,895 |
| 3 | 1 or 2 | 48,752 |
| 4 | exp Macular Degeneration/ | 29,853 |
| 5 | (tay adj3 choroiditis).tw,kw,kf. | 1 |
| 6 | (age adj3 related adj3 (maculopathies or maculopathy)).tw,kw,kf. | 894 |
| 7 | (age adj3 related adj3 (macular or macula) adj3 (degeneration or degenerations or dystrophies or dystrophy)).tw,kw,kf. | 22,546 |
| 8 | (senile adj3 (macula or macular) adj3 degeneration).tw,kw,kf. | 388 |
| 9 | (central adj3 areolar adj3 choroidal adj3 (atrophy or sclerosis)).tw,kw,kf. | 9 |
| 10 | or/4–9 | 38,382 |
| 11 | 3 and 10 | 96 |
| # | Searches | Results |
|---|---|---|
| 1 | sirolimus/ or rapamycin/ | 5022 |
| 2 | (rapamune or rapamycin or sirolimus or w36zg6ft64 or abi009 or ay22989 or de109 or drgt182 or fyarro or hyftor or nabrapamycin or nabsirolimus or npc12 or opsiria or pascomer or perceiva or ptx001 or rapalimus or rapammune).tw,kw,kf. | 65,065 |
| 3 | 1 or 2 | 67,301 |
| 4 | exp age related macular degeneration/ | 15,071 |
| 5 | (tay adj3 choroiditis).tw,kw,kf. | 1 |
| 6 | (age adj3 related adj3 (maculopathies or maculopathy)).tw,kw,kf. | 1091 |
| 7 | (age adj3 related adj3 (macular or macula) adj3 (degeneration or degenerations or dystrophies or dystrophy)).tw,kw,kf. | 31,821 |
| 8 | (senile adj3 (macula or macular) adj3 degeneration).tw,kw,kf. | 586 |
| 9 | (central adj3 areolar adj3 choroidal adj3 (atrophy or sclerosis)).tw,kw,kf. | 12 |
| 10 | or/4–9 | 35,712 |
| 11 | 3 and 10 | 153 |
| # | Searches | Results |
|---|---|---|
| 1 | (rapamune or rapamycin or sirolimus or w36zg6ft64 or abi009 or ay22989 or de109 or drgt182 or fyarro or hyftor or nabrapamycin or nabsirolimus or npc12 or opsiria or pascomer or perceiva or ptx001 or rapalimus or rapammune):ti,ab,kw | 5094 |
| 2 | (age) NEAR/3 (related) NEAR/3 (maculopathies OR maculopathy):ti,ab,kw | 112 |
| 3 | (senile) NEAR/3 (macula OR macular) NEAR/3 (degeneration):ti,ab,kw | 47 |
| 4 | senile macula degeneration OR senile macular degeneration OR central areolar choroidal atrophy OR central areolar choroidal sclerosis or age related macular degeneration | 3574 |
| 5 | #2 or #3 or #4 | 3603 |
| 6 | #1 AND #5 | 15 |
| # | Searches | Results |
|---|---|---|
| 1 | (MH ‘Sirolimus’) OR (rapamune or rapamycin or sirolimus or w36zg6ft64 or abi009 or ay22989 or de109 or drgt182 or fyarro or hyftor or nabrapamycin or nabsirolimus or npc12 or opsiria or pascomer or perceiva or ptx001 or rapalimus or rapammune) | 6841 |
| 2 | age related macular degeneration OR ((age) N3 (related) N3 (maculopathies OR maculopathy)) OR ((age) N3 (related) N3 (macular OR macula) N3 (degeneration OR degenerations OR dystrophies OR dystrophy)) OR ((senile) N3 (macula OR macular) N3 (degeneration)) OR ((central) N3 (areolar) N3 (choroidal) N3 (atrophy OR sclerosis)) | 6862 |
| 3 | S1 AND S2 | 12 |
| # | Searches | Results |
|---|---|---|
| 1 | rapamune OR rapamycin OR sirolimus OR w36zg6ft64 OR abi009 OR ay22989 OR de109 OR drgt182 OR fyarro OR hyftor OR nabrapamycin OR nabsirolimus OR npc12 OR opsiria OR pascomer OR perceiva OR ptx001 OR rapalimus OR rapammune | 112,807 |
| 2 | ‘age related macular degeneration’ OR ‘tay choroiditis’ OR ‘age related maculopathies’ OR ‘age related maculopathy’ OR ‘age related macular degeneration’ OR‘age related macular degenerations’ OR ‘age related macula degeneration’ OR ‘age related macular dystrophies’ OR ‘age related macula dystrophy’ | 30,407 |
| 3 | 1 AND 2 | 262 |
References
- Rein, D.B.; Wittenborn, J.S.; Burke-Conte, Z.; Gulia, R.; Robalik, T.; Ehrlich, J.R.; Lundeen, E.A.; Flaxman, A.D. Prevalence of Age-Related Macular Degeneration in the US in 2019. JAMA Ophthalmol. 2022, 140, 1202–1208. [Google Scholar] [CrossRef] [PubMed]
- Colijn, J.M.; Buitendijk, G.H.S.; Prokofyeva, E.; Alves, D.; Cachulo, M.L.; Khawaja, A.P.; Cougnard-Gregoire, A.; Merle, B.M.J.; Korb, C.; Erke, M.G.; et al. European Eye Epidemiology, Prevalence of Age-Related Macular Degeneration in Europe: The Past and the Future. Ophthalmology 2017, 124, 1753–1763. [Google Scholar] [CrossRef] [PubMed]
- Gehrs, K.M.; Anderson, D.H.; Johnson, L.V.; Hageman, G.S. Age-related macular degeneration--emerging pathogenetic and therapeutic concepts. Ann. Med. 2006, 38, 450–471. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Moreno, J.M.; Arias, L.; Abraldes, M.J.; Montero, J.; Udaondo, P.; RAMDEBURS Study Group. Economic burden of age-related macular degeneration in routine clinical practice: The RAMDEBURS study. Int. Ophthalmol. 2021, 41, 3427–3436. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Gomez, Y.; Alba-Molina, D.; Blanco-Blanco, M.; Perez-Fajardo, L.; Reyes-Ortega, F.; Ortega-Llamas, L.; Villalba-Gonzalez, M.; Fernandez-Choquet de Isla, I.; Pugliese, F.; Stoikow, I.; et al. Novel Treatments for Age-Related Macular Degeneration: A Review of Clinical Advances in Sustained Drug Delivery Systems. Pharmaceutics 2022, 14, 1473. [Google Scholar] [CrossRef]
- Hobbs, S.D.; Pierce, K. Wet Age-Related Macular Degeneration (Wet AMD). In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Gheorghe, A.; Mahdi, L.; Musat, O. Age-Related Macular Degeneration. Rom. J. Ophthalmol. 2015, 59, 74–77. [Google Scholar]
- Kaarniranta, K.; Tokarz, P.; Koskela, A.; Paterno, J.; Blasiak, J. Autophagy regulates death of retinal pigment epithelium cells in age-related macular degeneration. Cell Biol. Toxicol. 2017, 33, 113–128. [Google Scholar] [CrossRef]
- Mitter, S.K.; Song, C.; Qi, X.; Mao, H.; Rao, H.; Akin, D.; Lewin, A.; Grant, M.; Dunn, W., Jr.; Ding, J.; et al. Dysregulated autophagy in the RPE is associated with increased susceptibility to oxidative stress and AMD. Autophagy 2014, 10, 1989–2005. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Durieux, N.; Vandenput, S.; Pasleau, F. OCEBM levels of evidence system. Rev. Med. Liege 2013, 68, 644–649. [Google Scholar]
- Gensler, G.; Clemons, T.E.; Domalpally, A.; Danis, R.P.; Blodi, B.; Wells, J., 3rd; Rauser, M.; Hoskins, J.; Hubbard, G.B.; Elman, M.J.; et al. Treatment of Geographic Atrophy with Intravitreal Sirolimus: The Age-Related Eye Disease Study 2 Ancillary Study. Ophthalmol. Retin. 2018, 2, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Petrou, P.A.; Cunningham, D.; Shimel, K.; Harrington, M.; Hammel, K.; Cukras, C.A.; Ferris, F.L.; Chew, E.Y.; Wong, W.T. Intravitreal sirolimus for the treatment of geographic atrophy: Results of a phase I/II clinical trial. Investig. Ophthalmol. Vis. Sci. 2014, 56, 330–338. [Google Scholar] [CrossRef]
- Wong, W.T.; Dresner, S.; Forooghian, F.; Glaser, T.; Doss, L.; Zhou, M.; Cunningham, D.; Shimel, K.; Harrington, M.; Hammel, K.; et al. Treatment of geographic atrophy with subconjunctival sirolimus: Results of a phase I/II clinical trial. Investig. Ophthalmol. Vis. Sci. 2013, 54, 2941–2950. [Google Scholar] [CrossRef]
- Dugel, P.U.; Blumenkranz, M.S.; Haller, J.A.; Williams, G.A.; Solley, W.A.; Kleinman, D.M.; Naor, J. A randomized, dose-escalation study of subconjunctival and intravitreal injections of sirolimus in patients with diabetic macular edema. Ophthalmology 2012, 119, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.M.; Trenor, C.C., 3rd; Hammill, A.M.; Vinks, A.A.; Patel, M.N.; Chaudry, G.; Wentzel, M.S.; Mobberley-Schuman, P.S.; Campbell, L.M.; Brookbank, C.; et al. Efficacy and Safety of Sirolimus in the Treatment of Complicated Vascular Anomalies. Pediatrics 2016, 137, e20153257. [Google Scholar] [CrossRef]
- Li, M.; Zhou, Y.; Chen, C.; Yang, T.; Zhou, S.; Chen, S.; Wu, Y.; Cui, Y. Efficacy and safety of mTOR inhibitors (rapamycin and its analogues) for tuberous sclerosis complex: A meta-analysis. Orphanet J. Rare Dis. 2019, 14, 39. [Google Scholar] [CrossRef]
- Boers-Doets, C.B.; Raber-Durlacher, J.E.; Treister, N.S.; Epstein, J.B.; Arends, A.B.; Wiersma, D.R.; Lalla, R.V.; Logan, R.M.; van Erp, N.P.; Gelderblom, H. Mammalian target of rapamycin inhibitor-associated stomatitis. Future Oncol. 2013, 9, 1883–1892. [Google Scholar] [CrossRef]
- Bruss, Z.; Farook, S.; Shah, S.; Baker Sheikh, A. Sirolimus-Induced Rash in a Kidney Transplant Patient. Eur. J. Case Rep. Intern. Med. 2022, 9, 003565. [Google Scholar] [CrossRef]
- Nguyen, Q.D.; Merrill, P.T.; Sepah, Y.J.; Ibrahim, M.A.; Banker, A.; Leonardi, A.; Chernock, M.; Mudumba, S.; Do, D.V. Intravitreal Sirolimus for the Treatment of Noninfectious Uveitis: Evolution through Preclinical and Clinical Studies. Ophthalmology 2018, 125, 1984–1993. [Google Scholar] [CrossRef]
- Lin, H.; Salech, F.; Lim, A.; Vogrin, S.; Duque, G. The effect of rapamycin and its analogues on age-related musculoskeletal diseases: A systematic review. Aging Clin. Exp. Res. 2022, 34, 2317–2333. [Google Scholar] [CrossRef]
- Kolosova, N.G.; Muraleva, N.A.; Zhdankina, A.A.; Stefanova, N.A.; Fursova, A.Z.; Blagosklonny, M.V. Prevention of age-related macular degeneration-like retinopathy by rapamycin in rats. Am. J. Pathol. 2012, 181, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Salas, A.; Badia, A.; Fontrodona, L.; Zapata, M.; Garcia-Arumi, J.; Duarri, A. Neovascular Progression and Retinal Dysfunction in the Laser-Induced Choroidal Neovascularization Mouse Model. Biomedicines 2023, 11, 2445. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J.; Mattson, M.P. How does hormesis impact biology, toxicology, and medicine? NPJ Aging Mech. Dis. 2017, 3, 13. [Google Scholar] [CrossRef]
- Li, X.; Yang, T.; Sun, Z. Hormesis in Health and Chronic Diseases. Trends Endocrinol. Metab. 2019, 30, 944–958. [Google Scholar] [CrossRef] [PubMed]
- Rattan, S.I. Hormesis in aging. Ageing Res. Rev. 2008, 7, 63–78. [Google Scholar] [CrossRef]
- Kyriazis, M.; Swas, L.; Orlova, T. The Impact of Hormesis, Neuronal Stress Response, and Reproduction, upon Clinical Aging: A Narrative Review. J. Clin. Med. 2023, 12, 5433. [Google Scholar] [CrossRef]
- Gaya, A.; Akle, C.A.; Mudan, S.; Grange, J. The Concept of Hormesis in Cancer Therapy-Is Less More? Cureus 2015, 7, e261. [Google Scholar] [CrossRef]
- Panfoli, I.; Puddu, A.; Bertola, N.; Ravera, S.; Maggi, D. The Hormetic Effect of Metformin: “Less Is More”? Int. J. Mol. Sci. 2021, 22, 6297. [Google Scholar] [CrossRef]
- Pravin, M.; Dugel, U. Sirolimus in the Treatment of Retinal Diseases: mTOR Inhibitors: A New Class of Therapeutics. 2009. Available online: https://retinatoday.com/articles/2009-oct/1009_07-php (accessed on 22 March 2024).
- Asani, B.; Siedlecki, J.; Wertheimer, C.; Liegl, R.; Wolf, A.; Ohlmann, A.; Priglinger, S.; Priglinger, C. Anti-angiogenic properties of rapamycin on human retinal pericytes in an in vitro model of neovascular AMD via inhibition of the mTOR pathway. BMC Ophthalmol. 2022, 22, 138. [Google Scholar] [CrossRef]
- Seront, E.; Van Damme, A.; Legrand, C.; Bisdorff-Bresson, A.; Orcel, P.; Funck-Brentano, T.; Sevestre, M.A.; Dompmartin, A.; Quere, I.; Brouillard, P.; et al. Preliminary results of the European multicentric phase III trial regarding sirolimus in slow-flow vascular malformations. JCI Insight 2023, 8, e173095. [Google Scholar] [CrossRef]
- Keogh, A.; Richardson, M.; Ruygrok, P.; Spratt, P.; Galbraith, A.; O’Driscoll, G.; Macdonald, P.; Esmore, D.; Muller, D.; Faddy, S. Sirolimus in de novo heart transplant recipients reduces acute rejection and prevents coronary artery disease at 2 years: A randomized clinical trial. Circulation 2004, 110, 2694–2700. [Google Scholar] [CrossRef] [PubMed]
- Weiss, B.; Widemann, B.C.; Wolters, P.; Dombi, E.; Vinks, A.; Cantor, A.; Perentesis, J.; Schorry, E.; Ullrich, N.; Gutmann, D.H.; et al. Sirolimus for progressive neurofibromatosis type 1-associated plexiform neurofibromas: A neurofibromatosis Clinical Trials Consortium phase II study. Neuro Oncol. 2015, 17, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Minturn, R.J.; Bracha, P.; Klein, M.J.; Chhablani, J.; Harless, A.M.; Maturi, R.K. Intravitreal sirolimus for persistent, exudative age-related macular degeneration: A Pilot Study. Int. J. Retin. Vitr. 2021, 7, 11. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wigestrand, K.S.; Gupta, S.; Sharma, K.; Petrovski, G. Rapamycin’s Impact on Age-Related Macular Degeneration—A Systematic Review and Hormesis Perspective. J. Clin. Transl. Ophthalmol. 2024, 2, 99-112. https://doi.org/10.3390/jcto2030009
Wigestrand KS, Gupta S, Sharma K, Petrovski G. Rapamycin’s Impact on Age-Related Macular Degeneration—A Systematic Review and Hormesis Perspective. Journal of Clinical & Translational Ophthalmology. 2024; 2(3):99-112. https://doi.org/10.3390/jcto2030009
Chicago/Turabian StyleWigestrand, Knut Sandok, Santosh Gupta, Kulbhushan Sharma, and Goran Petrovski. 2024. "Rapamycin’s Impact on Age-Related Macular Degeneration—A Systematic Review and Hormesis Perspective" Journal of Clinical & Translational Ophthalmology 2, no. 3: 99-112. https://doi.org/10.3390/jcto2030009
APA StyleWigestrand, K. S., Gupta, S., Sharma, K., & Petrovski, G. (2024). Rapamycin’s Impact on Age-Related Macular Degeneration—A Systematic Review and Hormesis Perspective. Journal of Clinical & Translational Ophthalmology, 2(3), 99-112. https://doi.org/10.3390/jcto2030009







