Imaging the Anterior Segment in Spaceflight: Understanding and Preserving Astronaut Ocular Health for Long-Duration Missions
Abstract
:1. Introduction
2. Impacts of Spaceflight on the Anterior Segment of the Eye
2.1. Microgravity
2.2. Radiation
3. Imaging the Anterior Segment of the Eye
3.1. The Critical Role That Imaging Plays in Ocular Health
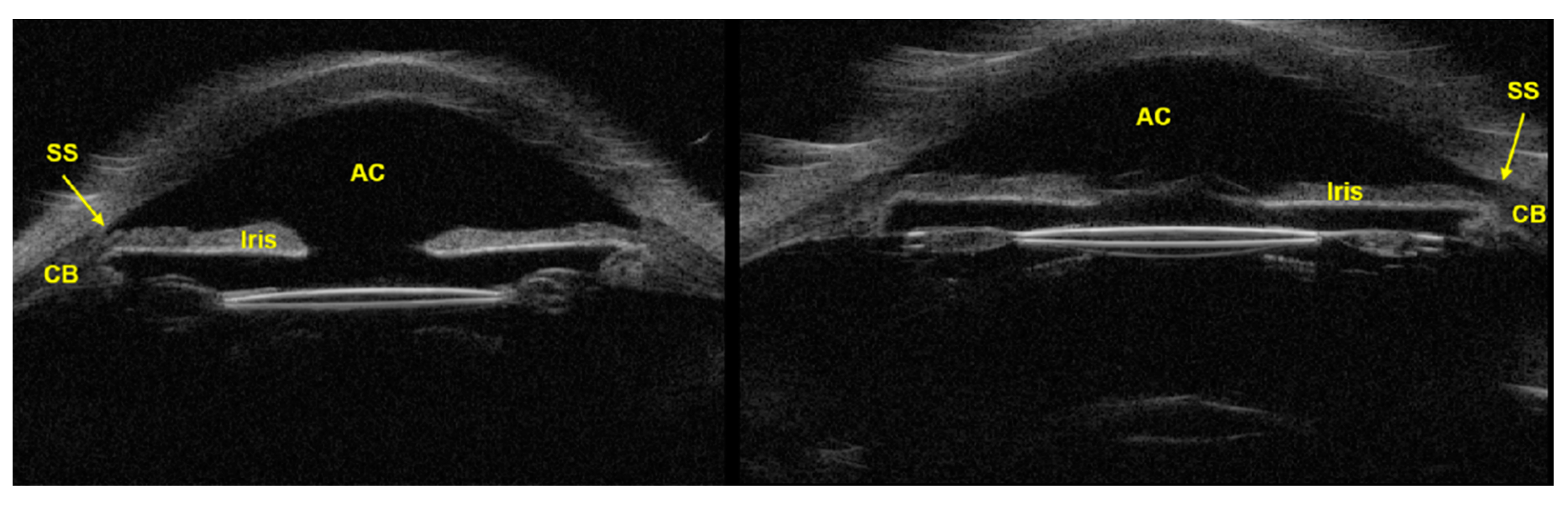
3.2. Ultrasound Biomicroscopy (UBM)
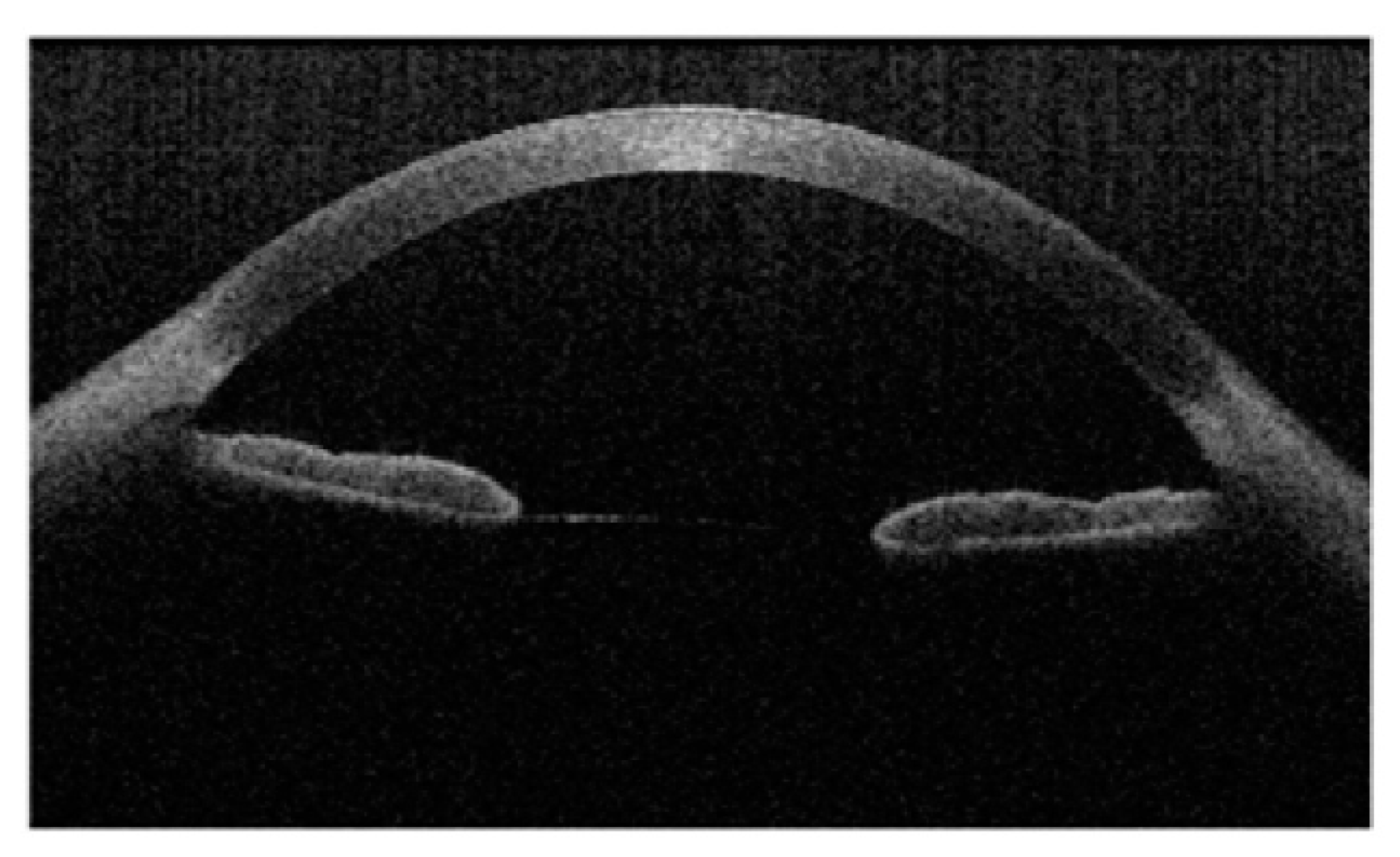
3.3. Anterior Segment Optical Coherence Tomography (AS-OCT)
3.4. Topography, Tomography, and Wavefront Imaging in Space
3.5. Importance, Comparison, and Limitations of Anterior Segment Imaging
- Corneal Morphology: It will be important to capture high-resolution images of the cornea, allowing researchers and clinicians to visualize its structure with exceptional detail. This includes assessing corneal thickness, curvature, and any changes in shape or morphology that may occur during spaceflight. This is especially salient for monitoring conditions like corneal edema, epithelial defects, or changes in the angle structures of the eye.
- Longitudinal Monitoring: By conducting anterior segment imaging before, during, and after spaceflight missions, researchers and clinicians can track the evolution of corneal and anterior segment changes over time. This longitudinal monitoring provides valuable insights into the progression of ocular alterations in response to microgravity and space radiation exposure.
- Evaluation of Interventions: Anterior segment imaging can also be used to assess the efficacy of interventions aimed at mitigating the effects of microgravity on ocular health. These interventions include pharmacological treatments, protective eyewear, and shielding techniques for protection from space radiation. In essence, anterior segment imaging can help space medicine clinicians to evaluate the impact of various interventions on anterior segment anatomy and physiology.
4. Future Directions and Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nelson, E.; Mulugeta, L.; Myers, J. Microgravity-Induced Fluid Shift and Ophthalmic Changes. Life 2014, 4, 621–665. [Google Scholar] [CrossRef]
- McNulty, P.J.; Pease, V.P.; Bond, V.P. Comparison of the Light Flash Phenomena Observed in Space and in Laboratory Experiments; Brookhaven National Lab. (BNL): Upton, NY, USA, 1976; p. 7312082. [Google Scholar] [CrossRef]
- Martin Paez, Y.; Mudie, L.I.; Subramanian, P.S. Spaceflight Associated Neuro-Ocular Syndrome (SANS): A Systematic Review and Future Directions. Eye Brain 2020, 12, 105–117. [Google Scholar] [CrossRef]
- Makarov, I.A.; Voronkov, Y.I.; Aslanjan, M.G. Ophthalmic changes associated with long-term exposure to microgravity. Hum. Physiol. 2017, 43, 105–113. [Google Scholar] [CrossRef]
- Ong, J.; Tarver, W.; Brunstetter, T.; Mader, T.H.; Gibson, C.R.; Mason, S.S.; Lee, A. Spaceflight associated neuro-ocular syndrome: Proposed pathogenesis, terrestrial analogues, and emerging countermeasures. Br. J. Ophthalmol. 2023, 107, 895–900. [Google Scholar] [CrossRef]
- Holly Fischer (WikiMedia Commons). Available online: https://commons.wikimedia.org/wiki/File:Three_Main_Layers_of_the_Eye.png (accessed on 11 November 2024).
- BruceBlaus (WikiMedia Commons). Available online: https://commons.wikimedia.org/wiki/File:Blausen_0390_EyeAnatomy_Sectional.png (accessed on 11 November 2024).
- Ang, M.; Baskaran, M.; Werkmeister, R.M.; Chua, J.; Schmidl, D.; dos Santos, V.A.; Garhöfer, G.; Mehta, J.S.; Schmetterer, L. Anterior segment optical coherence tomography. Prog. Retin. Eye Res. 2018, 66, 132–156. [Google Scholar] [CrossRef]
- Baikoff, G.; Lutun, E.; Ferraz, C.; Wei, J. Static and dynamic analysis of the anterior segment with optical coherence tomography. J. Cataract Refract. Surg. 2004, 30, 1843–1850. [Google Scholar] [CrossRef]
- Mader, T.H.; Gibson, C.R. Early Evidence of Vision Impairment after Long-Duration Spaceflight. In Intracranial Pressure and Its Effect on Vision in Space and on Earth; World Scientific: Singapore, 2017; pp. 5–22. [Google Scholar] [CrossRef]
- Manuel, F.K.; Mader, T.H. Ophthalmologic Concerns. In Principles of Clinical Medicine for Space Flight; Barratt, M.R., Pool, S.L., Eds.; Springer: New York, NY, USA, 2008; pp. 535–544. [Google Scholar] [CrossRef]
- He, M.; Wang, D.; Jiang, Y. Overview of Ultrasound Biomicroscopy. J. Curr. Glaucoma Pract. 2012, 6, 25–53. [Google Scholar] [CrossRef]
- Zanello, S.B.; Theriot, C.A.; Ponce, C.M.P.; Chevez-Barrios, P. Spaceflight Effects and Molecular Responses in the Mouse Eye: Preliminary Observations After Shuttle Mission STS-133. Gravitational Space Res. 2013, 1, 29–46. [Google Scholar] [CrossRef]
- Li, Z.; Rivera, C.A.; Burns, A.R.; Smith, C.W. Hindlimb unloading depresses corneal epithelial wound healing in mice. J. Appl. Physiol. 2004, 97, 641–647. [Google Scholar] [CrossRef]
- Li, X.; Yang, Y.; Li, Q.; Dai, Y.; Wang, C.; Chen, J. Morphologic Characteristics and Proliferation of Rabbit Corneal Stromal Cells Onto Complexes of Collagen–Chitosan–Sodium Hyaluronate Under Simulated Microgravity. Investig. Opthalmol. Vis. Sci. 2013, 54, 6877. [Google Scholar] [CrossRef]
- Schlötzer-Schrehardt, U.; Kruse, F.E. Identification and characterization of limbal stem cells. Exp. Eye Res. 2005, 81, 247–264. [Google Scholar] [CrossRef] [PubMed]
- Grimm, D.; Wehland, M.; Corydon, T.J.; Richter, P.; Prasad, B.; Bauer, J.; Egli, M.; Kopp, S.; Lebert, M.; Krüger, M. The effects of microgravity on differentiation and cell growth in stem cells and cancer stem cells. Stem Cells Transl. Med. 2020, 9, 882–894. [Google Scholar] [CrossRef]
- Delic, N.C.; Lyons, J.G.; Di Girolamo, N.; Halliday, G.M. Damaging Effects of Ultraviolet Radiation on the Cornea. Photochem. Photobiol. 2017, 93, 920–929. [Google Scholar] [CrossRef]
- Horneck, G.; Klaus, D.M.; Mancinelli, R.L. Space Microbiology. Microbiol. Mol. Biol. Rev. 2010, 74, 121–156. [Google Scholar] [CrossRef] [PubMed]
- Meer, E.; Grob, S.; Antonsen, E.L.; Sawyer, A. Ocular conditions and injuries, detection and management in spaceflight. npj Microgravity 2023, 9, 37. [Google Scholar] [CrossRef] [PubMed]
- Cucinotta, F.A.; Durante, M. Cancer risk from exposure to galactic cosmic rays: Implications for space exploration by human beings. Lancet Oncol. 2006, 7, 431–435. [Google Scholar] [CrossRef]
- Meer, E.; Grob, S.R.; Lehnhardt, K.; Sawyer, A. Ocular complaints and diagnoses in spaceflight. npj Microgravity 2024, 10, 1. [Google Scholar] [CrossRef]
- Hessen, M.; Akpek, E.K. Dry eye: An inflammatory ocular disease. J. Ophthalmic. Vis. Res. 2014, 9, 240–250. [Google Scholar]
- Gichuhi, S.; Ohnuma, S.-I.; Sagoo, M.S.; Burton, M.J. Pathophysiology of ocular surface squamous neoplasia. Exp. Eye Res. 2014, 129, 172–182. [Google Scholar] [CrossRef]
- Nuzzi, R.; Trossarello, M.; Bartoncini, S.; Marolo, P.; Franco, P.; Mantovani, C.; Ricardi, U. Ocular Complications After Radiation Therapy: An Observational Study. Clin. Ophthalmol. 2020, 14, 3153–3166. [Google Scholar] [CrossRef]
- Durkin, S.R.; Roos, D.; Higgs, B.; Casson, R.J.; Selva, D. Ophthalmic and adnexal complications of radiotherapy. Acta Ophthalmol. Scand. 2007, 85, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.A.; McCarten, M.; Manuel, K.; Djojonegoro, B.; Murray, J.; Feiversen, A.; Wear, M. Cataract formation mechanisms and risk in aviation and space crews. Aviat. Space Environ. Med. 2007, 78, A56–A66. [Google Scholar]
- Cucinotta, F.A.; Manuel, F.K.; Jones, J.; Iszard, G.; Murrey, J.; Djojonegro, B.; Wear, M. Space Radiation and Cataracts in Astronauts. Radiat. Res. 2001, 156, 460–466. [Google Scholar] [CrossRef]
- Lipman, R.M.; Tripathi, B.J.; Tripathi, R.C. Cataracts induced by microwave and ionizing radiation. Surv. Ophthalmol. 1988, 33, 200–210. [Google Scholar] [CrossRef]
- Blakely, E.A.; Chang, P.Y. Late Effects of Space Radiation: Cataracts. In Handbook of Bioastronautics; Young, L.R., Sutton, J.P., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 277–286. [Google Scholar] [CrossRef]
- Chylack, L.T.; Peterson, L.E.; Feiveson, A.H.; Wear, M.L.; Manuel, F.K.; Tung, W.H.; Hardy, D.S.; Marak, L.J.; Cucinotta, F.A. NASA Study of Cataract in Astronauts (NASCA). Report 1: Cross-Sectional Study of the Relationship of Exposure to Space Radiation and Risk of Lens Opacity. Radiat. Res. 2009, 172, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Rastegar, Z.; Eckart, P.; Mertz, M. Radiation-induced cataract in astronauts and cosmonauts. Graefe’s Arch. Clin. Exp. Ophthalmol. 2002, 240, 543–547. [Google Scholar] [CrossRef]
- Li, J.; Zhang, H.; Wang, X.; Wang, H.; Hao, J.; Bai, G. Inpainting Saturation Artifact in Anterior Segment Optical Coherence Tomography. Sensors 2023, 23, 9439. [Google Scholar] [CrossRef] [PubMed]
- Dalal, S.R.; Ramachandran, V.; Khalid, R.; Keith Manuel, F.; Knowles, J.R.; Jones, J.A. Increased Intraocular Pressure in Glaucomatous, Ocular Hypertensive, and Normotensive Space Shuttle Crew. Aerosp. Med. Hum. Perform. 2021, 92, 728–733. [Google Scholar] [CrossRef]
- Huang, A.S.; Stenger, M.B.; Macias, B.R. Gravitational Influence on Intraocular Pressure: Implications for Spaceflight and Disease. J. Glaucoma 2019, 28, 756–764. [Google Scholar] [CrossRef]
- De Paula, R.P.; Edwards, C.D.; Flamini, E. Evolution of the communications systems and technology for Mars exploration. Acta Astronaut. 2002, 51, 207–212. [Google Scholar] [CrossRef]
- Isasi, E.; Isasi, M.E.; Van Loon, J.J.W.A. The application of artificial gravity in medicine and space. Front. Physiol. 2022, 13, 952723. [Google Scholar] [CrossRef] [PubMed]
- NASA. 001 MEDICAL KIT-CONTENTS AND REFERENCE. 2015. Available online: https://www.nasa.gov/wp-content/uploads/2015/03/medical_kit_checklist_-_full_release.pdf (accessed on 1 November 2024).
- Kapoor, R.; Parameswarappa, D.C.; Dhurandhar, D.; Peguda, H.K.; Rani, P.K. Peering into the eye: A comprehensive look at ultrasound biomicroscopy (UBM) and its diagnostic value in anterior segment disorders. Indian J. Ophthalmol. 2023, 71, 3118. [Google Scholar] [CrossRef]
- Patel, A.S.; Akkara, J.D.; DelMonte, D.W.; Morkin, M.; Murchison, A.; Alexander, J.L.; Giaconi, J.A.; Desai, M.; Sheybani, A.; Sollenberger, E.L. Ultrasound Biomicroscopy. Available online: https://eyewiki.org/Ultrasound_Biomicroscopy (accessed on 14 September 2024).
- Helms, R.W.; Minhaz, A.T.; Wilson, D.L.; Örge, F.H. Clinical 3D Imaging of the Anterior Segment with Ultrasound Biomicroscopy. Trans. Vis. Sci. Technol. 2021, 10, 11. [Google Scholar] [CrossRef] [PubMed]
- Shan, J.; DeBoer, C.; Xu, B.Y. Anterior Segment Optical Coherence Tomography: Applications for Clinical Care and Scientific Research. Asia Pac. J. Ophthalmol. 2019, 8, 146–157. [Google Scholar] [CrossRef]
- Wang, S.B.; Cornish, E.E.; Grigg, J.R.; Mccluskey, P.J. Anterior segment optical coherence tomography and its clinical applications. Clin. Exp. Optom. 2019, 102, 195–207. [Google Scholar] [CrossRef]
- Macias, B.R.; Patel, N.B.; Gibson, C.R.; Samuels, B.C.; Laurie, S.S.; Otto, C.; Ferguson, C.R.; Lee, S.M.C.; Ploutz-Snyder, R.; Kramer, L.A.; et al. Association of Long-Duration Spaceflight with Anterior and Posterior Ocular Structure Changes in Astronauts and Their Recovery. JAMA Ophthalmol. 2020, 138, 553–559. [Google Scholar] [CrossRef]
- Muscat, S.; McKay, N.; Parks, S.; Kemp, E.; Keating, D. Repeatability and reproducibility of corneal thickness measurements by optical coherence tomography. Investig. Ophthalmol. Vis. Sci. 2002, 43, 1791–1795. [Google Scholar]
- Jancevski, M.; Foster, C.S. Anterior Segment Optical Coherence Tomography. Semin. Ophthalmol. 2010, 25, 317–323. [Google Scholar] [CrossRef]
- Mansouri, K.; Sommerhalder, J.; Shaarawy, T. Prospective comparison of ultrasound biomicroscopy and anterior segment optical coherence tomography for evaluation of anterior chamber dimensions in European eyes with primary angle closure. Eye 2010, 24, 233–239. [Google Scholar] [CrossRef]
- Konstantopoulos, A.; Hossain, P.; Anderson, D.F. Recent advances in ophthalmic anterior segment imaging: A new era for ophthalmic diagnosis? Br. J. Ophthalmol. 2007, 91, 551–557. [Google Scholar] [CrossRef]
- Liu, B.; Kang, C.; Fang, F. Biometric Measurement of Anterior Segment: A Review. Sensors 2020, 20, 4285. [Google Scholar] [CrossRef] [PubMed]
- Martin, R. Cornea and anterior eye assessment with placido-disc keratoscopy, slit scanning evaluation topography and scheimpflug imaging tomography. Indian J. Ophthalmol. 2018, 66, 360–366. [Google Scholar] [CrossRef]
- Belin, M.W.; Ambrósio, R. Scheimpflug imaging for keratoconus and ectatic disease. Indian J. Ophthalmol. 2013, 61, 401–406. [Google Scholar] [CrossRef]
- Fan, R.; Chan, T.C.; Prakash, G.; Jhanji, V. Applications of corneal topography and tomography: A review. Clin. Exp. Ophthalmol. 2018, 46, 133–146. [Google Scholar] [CrossRef]
- Crawford, A.Z.; Patel, D.V.; McGhee, C.N.J. Comparison and Repeatability of Keratometric and Corneal Power Measurements Obtained by Orbscan II, Pentacam, and Galilei Corneal Tomography Systems. Am. J. Ophthalmol. 2013, 156, 53–60. [Google Scholar] [CrossRef]
- Motlagh, M.N.; Moshirfar, M.; Murri, M.S.; Skanchy, D.F.; Momeni-Moghaddam, H.; Ronquillo, Y.C.; Hoopes, P.C. Pentacam® Corneal Tomography for Screening of Refractive Surgery Candidates: A Review of the Literature, Part I. Med. Hypothesis Discov. Innov. Ophthalmol. 2019, 8, 177–203. [Google Scholar]
- Moshirfar, M.; Tenney, S.; McCabe, S.; Schmid, G. Repeatability and reproducibility of the galilei G6 and its agreement with the pentacam® AXL in optical biometry and corneal tomography. Expert Rev. Med. Devices 2022, 19, 375–383. [Google Scholar] [CrossRef]
- Maeda, N. Clinical applications of wavefront aberrometry—A review. Clin. Exp. Ophthalmol. 2009, 37, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Charman, W.N. Wavefront technology: Past, present and future. Contact Lens Anterior Eye 2005, 28, 75–92. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, M.; Belloni, G.; Chang, Y.-C.; Durkee, H.; Masetti, E.; Cabot, F.; Yoo, S.H.; Ho, A.; Parel, J.-M.; Manns, F. Combined anterior segment OCT and wavefront-based autorefractor using a shared beam. Biomed. Opt. Express 2021, 12, 6746–6761. [Google Scholar] [CrossRef]
- Dada, T.; Sihota, R.; Gadia, R.; Aggarwal, A.; Mandal, S.; Gupta, V. Comparison of anterior segment optical coherence tomography and ultrasound biomicroscopy for assessment of the anterior segment. J. Cataract Refract. Surg. 2007, 33, 837–840. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, S. Comparison of Optical Coherence Tomography and Ultrasound Biomicroscopy for Detection of Narrow Anterior Chamber Angles. Arch. Ophthalmol. 2005, 123, 1053. [Google Scholar] [CrossRef]
- Vodapalli, H.; Murthy, S.; Jalali, S.; Ali, M.; Rani, P. Comparison of immersion ultrasonography, ultrasound biomicroscopy and anterior segment optical coherence tomography in the evaluation of traumatic phacoceles. Indian J. Ophthalmol. 2012, 60, 63. [Google Scholar] [CrossRef] [PubMed]
- Bianciotto, C.; Shields, C.L.; Guzman, J.M.; Romanelli-Gobbi, M.; Mazzuca, D.; Green, W.R.; Shields, J.A. Assessment of anterior segment tumors with ultrasound biomicroscopy versus anterior segment optical coherence tomography in 200 cases. Ophthalmology 2011, 118, 1297–1302. [Google Scholar] [CrossRef]
- Krema, H.; Santiago, R.A.; Gonzalez, J.E.; Pavlin, C.J. Spectral-Domain Optical Coherence Tomography Versus Ultrasound Biomicroscopy for Imaging of Nonpigmented Iris Tumors. Am. J. Ophthalmol. 2013, 156, 806–812.e1. [Google Scholar] [CrossRef]
- Ramos, J.L.B.; Li, Y.; Huang, D. Clinical and research applications of anterior segment optical coherence tomography—A review. Clin. Exp. Ophthalmol. 2009, 37, 81–89. [Google Scholar] [CrossRef]
- Heidelberg Engineering’s SPECTRALIS OCT2 Module Headed to the International Space Station|Heidelberg Engineering Inc. 22 May 2018. Available online: https://www.heidelbergengineering.com/us/press-releases/heidelberg-engineerings-spectralis-oct2-module-headed-to-the-international-space-station/ (accessed on 5 November 2024).


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ong, J.; Sampige, R.; Lee, R.; Memon, H.; Panzo, N.; Kadipasaoglu, C.M.; Guo, Y.; Sandhur, B.S.; Soares, B.; Osteicoechea, D.; et al. Imaging the Anterior Segment in Spaceflight: Understanding and Preserving Astronaut Ocular Health for Long-Duration Missions. J. Clin. Transl. Ophthalmol. 2025, 3, 5. https://doi.org/10.3390/jcto3010005
Ong J, Sampige R, Lee R, Memon H, Panzo N, Kadipasaoglu CM, Guo Y, Sandhur BS, Soares B, Osteicoechea D, et al. Imaging the Anterior Segment in Spaceflight: Understanding and Preserving Astronaut Ocular Health for Long-Duration Missions. Journal of Clinical & Translational Ophthalmology. 2025; 3(1):5. https://doi.org/10.3390/jcto3010005
Chicago/Turabian StyleOng, Joshua, Ritu Sampige, Ryung Lee, Hamza Memon, Nicholas Panzo, Cihan Mehmet Kadipasaoglu, Yannie Guo, Baltaj S. Sandhur, Benjamin Soares, Daniela Osteicoechea, and et al. 2025. "Imaging the Anterior Segment in Spaceflight: Understanding and Preserving Astronaut Ocular Health for Long-Duration Missions" Journal of Clinical & Translational Ophthalmology 3, no. 1: 5. https://doi.org/10.3390/jcto3010005
APA StyleOng, J., Sampige, R., Lee, R., Memon, H., Panzo, N., Kadipasaoglu, C. M., Guo, Y., Sandhur, B. S., Soares, B., Osteicoechea, D., Waisberg, E., Suh, A., Nguyen, T., Masalkhi, M., Sarker, P., Zaman, N., Tavakkoli, A., Berdahl, J., Chévez-Barrios, P., & Lee, A. G. (2025). Imaging the Anterior Segment in Spaceflight: Understanding and Preserving Astronaut Ocular Health for Long-Duration Missions. Journal of Clinical & Translational Ophthalmology, 3(1), 5. https://doi.org/10.3390/jcto3010005






