Three-Dimensional Printing for Accessible and Personalized Ophthalmic Care: A Review
Abstract
:1. Introduction
2. Brief Review of the Mechanisms of 3D Printing
3. Three-Dimensional Printing in Ophthalmology
3.1. Cornea
3.1.1. Biological Factors for Corneal Transplantation
3.1.2. Bioink in Corneal Applications
3.1.3. Recent Advancements in Bioinks and Fabrication Strategies
3.1.4. Challenges and Future Prospects in Corneal Bioprinting
3.2. Oculoplastics
3.2.1. Ocular Prosthetics
3.2.2. Facial and Orbital Implants
3.2.3. Eyelid Crutches
3.2.4. Dry Eye Syndrome: Lacrimal Gland Regeneration and Punctal Plugs
3.3. Drug Delivery Systems—Glaucoma, Retina, and Uveal Melanoma
3.4. Medical Education
4. Future Directions
4.1. Regulatory Considerations
4.2. Accessibility
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zopf, D.A.; Hollister, S.J.; Nelson, M.E.; Ohye, R.G.; Green, G.E. Bioresorbable Airway Splint Created with a Three-Dimensional Printer. N. Engl. J. Med. 2013, 368, 2043–2045. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Zhang, X. 3D Printing: Print the Future of Ophthalmology. Investig. Ophthalmol. Vis. Sci. 2014, 55, 5380. [Google Scholar] [CrossRef]
- Reinhard, J.; Urban, P.; Bell, S.; Carpenter, D.; Sagoo, M.S. Automatic data-driven design and 3D printing of custom ocular prostheses. Nat. Commun. 2024, 15, 1360. [Google Scholar] [CrossRef] [PubMed]
- Isaacson, A.; Swioklo, S.; Connon, C.J. 3D bioprinting of a corneal stroma equivalent. Exp. Eye Res. 2018, 173, 188–193. [Google Scholar] [CrossRef]
- Lee, L.; Burnett, A.M.; Panos, J.G.; Paudel, P.; Keys, D.; Ansari, H.M.; Yu, M. 3-D printed spectacles: Potential, challenges and the future. Clin. Exp. Optom. 2020, 103, 590–596. [Google Scholar] [CrossRef]
- Tian, Y.; Li, L.; Ball, R. A Qualitative Study for Parametric Designed Custom-Fit Eyewear Frames: Fit Test Evaluation and User Insights. In Design, User Experience, and Usability; In Lecture Notes in Computer Science; Marcus, A., Rosenzweig, E., Soares, M.M., Eds.; Springer Nature Switzerland: Cham, Switzerland, 2024; Volume 14712, pp. 354–370. [Google Scholar] [CrossRef]
- Latip, A.A.A.; Kipli, K.; Kamaruddin, A.M.N.A.; Sapawi, R.; Lias, K.; Jalil, M.A.; Tamrin, K.F.; Tajudin, N.M.A.; Ong, H.Y.; Mahmood, M.H.; et al. Development of 3D-printed universal adapter in enhancing retinal imaging accessibility. 3D Print. Med. 2024, 10, 23. [Google Scholar] [CrossRef]
- Rubegni, G.; Cartocci, A.; Tognetti, L.; Tosi, G.; Salfi, M.; Caruso, A.; Castellino, N.; Orione, M.; Cappellani, F.; Fallico, M.; et al. Design of a new 3D printed all-in-one magnetic smartphone adapter for fundus and anterior segment imaging. Eur. J. Ophthalmol. 2024, 35, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Ruzza, A.; Parekh, M.; Ferrari, S.; Salvalaio, G.; Nahum, Y.; Bovone, C.; Ponzin, D.; Busin, M. Preloaded donor corneal lenticules in a new validated 3D printed smart storage glide for Descemet stripping automated endothelial keratoplasty. Br. J. Ophthalmol. 2015, 99, 1388–1395. [Google Scholar] [CrossRef]
- Chandrakanth, P.; Verghese, S.; Chandrakanth, K.; Basaiawmoit, P.; Joseph, V. The Glowport—Illuminated vitrectomy trocar cannulas. Indian J. Ophthalmol. 2024, 73, S169–S171. [Google Scholar] [CrossRef]
- Navajas, E.V.; Hove, M.T. Three-Dimensional Printing of a Transconjunctival Vitrectomy Trocar-Cannula System. Ophthalmologica 2017, 237, 119–122. [Google Scholar] [CrossRef]
- Gómez-Fernández, H.; Alhakim-Khalak, F.; Ruiz-Alonso, S.; Díaz, A.; Tamayo, J.; Ramalingam, M.; Larra, E.; Pedraz, J.L. Comprehensive review of the state-of-the-art in corneal 3D bioprinting, including regulatory aspects. Int. J. Pharm. 2024, 662, 124510. [Google Scholar] [CrossRef] [PubMed]
- Hu, A.; Damji, K.F. New open source 3-dimensional printed smartphone fundus imaging adaptor. Can. J. Ophthalmol. 2019, 54, 399–400. [Google Scholar] [CrossRef] [PubMed]
- Gain, P.; Jullienne, R.; He, Z.; Aldossary, M.; Acquart, S.; Cognasse, F.; Thuret, G. Global Survey of Corneal Transplantation and Eye Banking. JAMA Ophthalmol. 2016, 134, 167. [Google Scholar] [CrossRef]
- Jeng, B.H.; Ahmad, S. In Pursuit of the Elimination of Corneal Blindness: Is Establishing Eye Banks and Training Surgeons Enough? Ophthalmology 2021, 128, 813–815. [Google Scholar] [CrossRef]
- Balters, L.; Reichl, S. 3D bioprinting of corneal models: A review of the current state and future outlook. J. Tissue Eng. 2023, 14, 20417314231197793. [Google Scholar] [CrossRef] [PubMed]
- Mathews, P.M.; Lindsley, K.; Aldave, A.J.; Akpek, E.K. Etiology of Global Corneal Blindness and Current Practices of Corneal Transplantation: A Focused Review. Cornea 2018, 37, 1198–1203. [Google Scholar] [CrossRef]
- Oliva, M.; Schottman, T.; Gulati, M. Turning the tide of corneal blindness. Indian. J. Ophthalmol. 2012, 60, 423. [Google Scholar] [CrossRef]
- Moraru, E.; Dontu, G.O.; Cananau, S.; Stanescu, V.-A. Approaches and Processing Technologies for Medical Devices: Considerations from Micro- and Macroscale Perspectives. In International Conference on Reliable Systems Engineering (ICoRSE)—2023; In Lecture Notes in Networks and Systems; Cioboată, D.D., Ed.; Springer Nature Switzerland: Cham, Switzerland, 2023; Volume 762, pp. 345–362. [Google Scholar] [CrossRef]
- Mobarak, M.H.; Islam, M.A.; Hossain, N.; Al Mahmud, M.Z.; Rayhan, M.T.; Nishi, N.J.; Chowdhury, M.A. Recent advances of additive manufacturing in implant fabrication—A review. Appl. Surf. Sci. Adv. 2023, 18, 100462. [Google Scholar] [CrossRef]
- Haleem, A.; Javaid, M. Role of CT and MRI in the design and development of orthopaedic model using additive manufacturing. J. Clin. Orthop. Trauma. 2018, 9, 213–217. [Google Scholar] [CrossRef]
- Dong, C.; Petrovic, M.; Davies, I.J. Applications of 3D printing in medicine: A review. Ann. 3D Print. Med. 2024, 14, 100149. [Google Scholar] [CrossRef]
- Lin, N.; Gagnon, M.; Wu, K.Y. The Third Dimension of Eye Care: A Comprehensive Review of 3D Printing in Ophthalmology. Hardware 2024, 2, 1–32. [Google Scholar] [CrossRef]
- Ng, W.L.; An, J.; Chua, C.K. Process, Material, and Regulatory Considerations for 3D Printed Medical Devices and Tissue Constructs. Engineering 2024, 36, 146–166. [Google Scholar] [CrossRef]
- Ganguly, S.; Wulff, D.; Phan, C.-M.; Jones, L.W.; Tang, X.S. Injectable and 3D Extrusion Printable Hydrophilic Silicone-Based Hydrogels for Controlled Ocular Delivery of Ophthalmic Drugs. ACS Appl. Bio Mater. 2024, 7, 6286–6296. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef]
- Aimar, A.; Palermo, A.; Innocenti, B. The Role of 3D Printing in Medical Applications: A State of the Art. J. Healthc. Eng. 2019, 2019, 5340616. [Google Scholar] [CrossRef] [PubMed]
- Ligon, S.C.; Liska, R.; Stampfl, J.; Gurr, M.; Mülhaupt, R. Polymers for 3D Printing and Customized Additive Manufacturing. Chem. Rev. 2017, 117, 10212–10290. [Google Scholar] [CrossRef]
- Nie, X.; Tang, Y.; Wu, T.; Zhao, X.; Xu, Z.; Yang, R.; Sun, Y.; Wu, B.; Han, Q.; Hui, J.; et al. 3D printing sequentially strengthening high-strength natural polymer hydrogel bilayer scaffold for cornea regeneration. Regen. Biomater. 2024, 11, rbae012. [Google Scholar] [CrossRef]
- Sridhar, M. Anatomy of cornea and ocular surface. Indian J. Ophthalmol. 2018, 66, 190. [Google Scholar] [CrossRef]
- Dua, H.S.; Faraj, L.A.; Said, D.G.; Gray, T.; Lowe, J. Human Corneal Anatomy Redefined. Ophthalmology 2013, 120, 1778–1785. [Google Scholar] [CrossRef]
- Downie, L.E.; Bandlitz, S.; Bergmanson, J.P.; Craig, J.P.; Dutta, D.; Maldonado-Codina, C.; Ngo, W.; Siddireddy, J.S.; Wolffsohn, J.S. BCLA CLEAR—Anatomy and physiology of the anterior eye. Contact Lens Anterior Eye 2021, 44, 132–156. [Google Scholar] [CrossRef]
- Ruberti, J.W.; Zieske, J.D. Prelude to corneal tissue engineering—Gaining control of collagen organization. Prog. Retin. Eye Res. 2008, 27, 549–577. [Google Scholar] [CrossRef] [PubMed]
- Muller, L.J. The specific architecture of the anterior stroma accounts for maintenance of corneal curvature. Br. J. Ophthalmol. 2001, 85, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Gungor-Ozkerim, P.S.; Inci, I.; Zhang, Y.S.; Khademhosseini, A.; Dokmeci, M.R. Bioinks for 3D bioprinting: An overview. Biomater. Sci. 2018, 6, 915–946. [Google Scholar] [CrossRef] [PubMed]
- Sorkio, A.; Koch, L.; Koivusalo, L.; Deiwick, A.; Miettinen, S.; Chichkov, B.; Skottman, H. Human stem cell based corneal tissue mimicking structures using laser-assisted 3D bioprinting and functional bioinks. Biomaterials 2018, 171, 57–71. [Google Scholar] [CrossRef]
- Campos, D.F.D.; Zhang, S.; Kreimendahl, F.; Köpf, M.; Fischer, H.; Vogt, M.; Blaeser, A.; Apel, C.; Esteves-Oliveira, M. Hand-held bioprinting for de novo vascular formation applicable to dental pulp regeneration. Connect. Tissue Res. 2020, 61, 205–215. [Google Scholar] [CrossRef]
- Liu, Y.; Ren, L.; Wang, Y. Crosslinked collagen–gelatin–hyaluronic acid biomimetic film for cornea tissue engineering applications. Mater. Sci. Eng. C 2013, 33, 196–201. [Google Scholar] [CrossRef]
- Kutlehria, S.; Dinh, T.C.; Bagde, A.; Patel, N.; Gebeyehu, A.; Singh, M. High-throughput 3D bioprinting of corneal stromal equivalents. J. Biomed. Mater. Res. 2020, 108, 2981–2994. [Google Scholar] [CrossRef]
- Wu, Z.; Su, X.; Xu, Y.; Kong, B.; Sun, W.; Mi, S. Bioprinting three-dimensional cell-laden tissue constructs with controllable degradation. Sci. Rep. 2016, 6, 24474. [Google Scholar] [CrossRef]
- Nikkhah, M.; Akbari, M.; Paul, A.; Memic, A.; Dolatshahi-Pirouz, A.; Khademhosseini, A. Gelatin-Based Biomaterials For Tissue Engineering And Stem Cell Bioengineering. In Biomaterials from Nature for Advanced Devices and Therapies, 1st ed.; Neves, N.M., Reis, R.L., Eds.; Wiley: Hoboken, NJ, USA, 2016; pp. 37–62. [Google Scholar] [CrossRef]
- Tonsomboon, K.; Oyen, M.L. Composite electrospun gelatin fiber-alginate gel scaffolds for mechanically robust tissue engineered cornea. J. Mech. Behav. Biomed. Mater. 2013, 21, 185–194. [Google Scholar] [CrossRef]
- Bektas, C.K.; Hasirci, V. Cell loaded 3D bioprinted GelMA hydrogels for corneal stroma engineering. Biomater. Sci. 2020, 8, 438–449. [Google Scholar] [CrossRef]
- Mahdavi, S.S.; Abdekhodaie, M.J.; Kumar, H.; Mashayekhan, S.; Baradaran-Rafii, A.; Kim, K. Stereolithography 3D Bioprinting Method for Fabrication of Human Corneal Stroma Equivalent. Ann. Biomed. Eng. 2020, 48, 1955–1970. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraghavan, R.; Loganathan, S.; Valapa, R.B. Fabrication of GelMA—Agarose Based 3D Bioprinted Photocurable Hydrogel with In Vitro Cytocompatibility and Cells Mirroring Natural Keratocytes for Corneal Stromal Regeneration. Macromol. Biosci. 2024, 24, 2400136. [Google Scholar] [CrossRef]
- Bhutani, U.; Dey, N.; Chowdhury, S.K.; Waghmare, N.; Das Mahapatra, R.; Selvakumar, K.; Chandru, A.; Bhowmick, T.; Agrawal, P. Biopolymeric corneal lenticules by digital light processing based bioprinting: A dynamic substitute for corneal transplant. Biomed. Mater. 2024, 19, 035017. [Google Scholar] [CrossRef]
- Ulag, S.; Ilhan, E.; Sahin, A.; Yilmaz, B.K.; Kalaskar, D.M.; Ekren, N.; Kilic, O.; Oktar, F.N.; Gunduz, O. 3D printed artificial cornea for corneal stromal transplantation. Eur. Polym. J. 2020, 133, 109744. [Google Scholar] [CrossRef]
- Kim, H.; Park, M.-N.; Kim, J.; Jang, J.; Kim, H.-K.; Cho, D.-W. Characterization of cornea-specific bioink: High transparency, improved in vivo safety. J. Tissue Eng. 2019, 10, 204173141882338. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, F.; Han, D.; Zhang, S.-Y.; Dong, Y.; Li, X.; Ling, L.; Deng, Z.; Cao, X.; Tian, J.; et al. 3D bioprinting of corneal decellularized extracellular matrix: GelMA composite hydrogel for corneal stroma engineering. Int. J. Bioprinting 2023, 9, 774. [Google Scholar] [CrossRef]
- Uyanıklar, M.; Günal, G.; Tevlek, A.; Hosseinian, P.; Aydin, H.M. Hybrid Cornea: Cell Laden Hydrogel Incorporated Decellularized Matrix. ACS Biomater. Sci. Eng. 2020, 6, 122–133. [Google Scholar] [CrossRef] [PubMed]
- Kostenko, A.; Swioklo, S.; Connon, C.J. Alginate in corneal tissue engineering. Biomed. Mater. 2022, 17, 022004. [Google Scholar] [CrossRef]
- Zhang, B.; Xue, Q.; Hu, H.-Y.; Yu, M.-F.; Gao, L.; Luo, Y.-C.; Li, Y.; Li, J.-T.; Ma, L.; Yao, Y.-F.; et al. Integrated 3D bioprinting-based geometry-control strategy for fabricating corneal substitutes. J. Zhejiang Univ. Sci. B 2019, 20, 945–959. [Google Scholar] [CrossRef]
- Sun, M.; Puri, S.; Mutoji, K.N.; Coulson-Thomas, Y.M.; Hascall, V.C.; Jackson, D.G.; Gesteira, T.F.; Coulson-Thomas, V.J. Hyaluronan Derived From the Limbus is a Key Regulator of Corneal Lymphangiogenesis. Investig. Ophthalmol. Vis. Sci. 2019, 60, 1050. [Google Scholar] [CrossRef]
- Mörö, A.; Samanta, S.; Honkamäki, L.; Rangasami, V.K.; Puistola, P.; Kauppila, M.; Narkilahti, S.; Miettinen, S.; Oommen, O.; Skottman, H. Hyaluronic acid based next generation bioink for 3D bioprinting of human stem cell derived corneal stromal model with innervation. Biofabrication 2023, 15, 015020. [Google Scholar] [CrossRef]
- Zhong, Z.; Balayan, A.; Tian, J.; Xiang, Y.; Hwang, H.H.; Wu, X.; Deng, X.; Schimelman, J.; Sun, Y.; Ma, C.; et al. Bioprinting of dual ECM scaffolds encapsulating limbal stem/progenitor cells in active and quiescent statuses. Biofabrication 2021, 13, 044101. [Google Scholar] [CrossRef]
- Singh, M.K. Textiles Functionalization—A Review of Materials, Processes, and Assessment. In Textiles for Functional Applications; Kumar, B., Ed.; IntechOpen: Rijeka, Croatia, 2021. [Google Scholar] [CrossRef]
- Ghosh, A.; Bera, A.K.; Singh, V.; Basu, S.; Pati, F. Bioprinting of anisotropic functional corneal stroma using mechanically robust multi-material bioink based on decellularized cornea matrix. Biomater. Adv. 2024, 165, 214007. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.-Y.; Zhang, M.; Li, X.-Y.; Huang, S.; Han, D.; Chang, L.; Ling, L.; Huo, Y.; Alzogool, M.; Yang, N.; et al. Development of a novel bioartificial cornea using 3D bioprinting based on electrospun micro-nanofibrous decellularized extracellular matrix. Biofabrication 2024, 16, 025039. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Yuan, B.; Chi, M.; Hong, J. Focus on seed cells: Stem cells in 3D bioprinting of corneal grafts. Front. Bioeng. Biotechnol. 2024, 12, 1423864. [Google Scholar] [CrossRef]
- Kong, B.; Chen, Y.; Liu, R.; Liu, X.; Liu, C.; Shao, Z.; Xiong, L.; Liu, X.; Sun, W.; Mi, S. Fiber reinforced GelMA hydrogel to induce the regeneration of corneal stroma. Nat. Commun. 2020, 11, 1435. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Yu, S.; Xu, X.; Amadi, S.M.; Zhang, J.; Wang, Z. Application of artificial intelligence in 3D printing physical organ models. Mater. Today Bio 2023, 23, 100792. [Google Scholar] [CrossRef]
- Grönroos, P.; Mörö, A.; Puistola, P.; Hopia, K.; Huuskonen, M.; Viheriälä, T.; Ilmarinen, T.; Skottman, H. Bioprinting of human pluripotent stem cell derived corneal endothelial cells with hydrazone crosslinked hyaluronic acid bioink. Stem Cell Res. Ther. 2024, 15, 81. [Google Scholar] [CrossRef]
- Kim, K.W.; Lee, S.J.; Park, S.H.; Kim, J.C. Ex Vivo Functionality of 3D Bioprinted Corneal Endothelium Engineered with Ribonuclease 5-Overexpressing Human Corneal Endothelial Cells. Adv. Healthc. Mater. 2018, 7, 1800398. [Google Scholar] [CrossRef]
- Duffy, G.L.; Liang, H.; Williams, R.L.; Wellings, D.A.; Black, K. 3D reactive inkjet printing of poly-ɛ-lysine/gellan gum hydrogels for potential corneal constructs. Mater. Sci. Eng. C 2021, 131, 112476. [Google Scholar] [CrossRef]
- He, B.; Wang, J.; Xie, M.; Xu, M.; Zhang, Y.; Hao, H.; Xing, X.; Lu, W.; Han, Q.; Liu, W. 3D printed biomimetic epithelium/stroma bilayer hydrogel implant for corneal regeneration. Bioact. Mater. 2022, 17, 234–247. [Google Scholar] [CrossRef] [PubMed]
- De Araujo, A.L. Corneal stem cells and tissue engineering: Current advances and future perspectives. World J. Stem Cells 2015, 7, 806. [Google Scholar] [CrossRef]
- Karamichos, D.; Funderburgh, M.L.; Hutcheon, A.E.K.; Zieske, J.D.; Du, Y.; Wu, J.; Funderburgh, J.L. A Role for Topographic Cues in the Organization of Collagenous Matrix by Corneal Fibroblasts and Stem Cells. PLoS ONE 2014, 9, e86260. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, R.M.; Lepert, G.; Gupta, S.; Mohan, R.R.; Paterson, C.; Connon, C.J. Assessment of corneal substrate biomechanics and its effect on epithelial stem cell maintenance and differentiation. Nat. Commun. 2019, 10, 1496. [Google Scholar] [CrossRef]
- Modugno, A.; Mantelli, F.; Sposato, S.; Moretti, C.; Lambiase, A.; Bonini, S. Ocular prostheses in the last century: A retrospective analysis of 8018 patients. Eye 2013, 27, 865–870. [Google Scholar] [CrossRef]
- Groot, A.L.W.; Remmers, J.S.; Hartong, D.T. Three-Dimensional Computer-Aided Design of a Full-Color Ocular Prosthesis with Textured Iris and Sclera Manufactured in One Single Print Job. 3D Print. Addit. Manuf. 2021, 8, 343–348. [Google Scholar] [CrossRef]
- Gunaseelaraj, R.; Karthikeyan, S.; Kumar, M.; Balamurugan, T.; Jagadeeshwaran, A. Custom-made ocular prosthesis. J. Pharm. Bioall Sci. 2012, 4, 177. [Google Scholar] [CrossRef]
- Goiato, M.C.; Bannwart, L.C.; Haddad, M.F.; Santos, D.M.D.; Pesqueira, A.A.; Miyahara, G.I. Fabrication Techniques for Ocular Prostheses—An Overview. Orbit 2014, 33, 229–233. [Google Scholar] [CrossRef]
- van der Stelt, M.; Verhulst, A.C.; Nunes, J.H.V.; Koroma, T.A.R.; Nolet, W.W.E.; Slump, C.H.; Grobusch, M.P.; Maal, T.J.J.; Brouwers, L. Improving Lives in Three Dimensions: The Feasibility of 3D Printing for Creating Personalized Medical Aids in a Rural Area of Sierra Leone. Am. J. Trop. Med. Hyg. 2020, 102, 905–909. [Google Scholar] [CrossRef]
- Sterkenburg, A.; Van der Stelt, M.; Koroma, A.; Gaalen, V.; Van der Pols, M.; Grobusch, M.; Slump, C.; Maal, T.; Brouwers, L. Quality of life of patients with 3D-printed arm prostheses in a rural area of Sierra Leone. Heliyon 2021, 7, e07447. [Google Scholar] [CrossRef]
- Ruiters, S.; Sun, Y.; De Jong, S.; Politis, C.; Mombaerts, I. Computer-aided design and three-dimensional printing in the manufacturing of an ocular prosthesis. Br. J. Ophthalmol. 2016, 100, 879–881. [Google Scholar] [CrossRef] [PubMed]
- Puls, N.; Carluccio, D.; Batstone, M.D.; Novak, J.I. The rise of additive manufacturing for ocular and orbital prostheses: A systematic literature review. Ann. 3D Print. Med. 2021, 4, 100036. [Google Scholar] [CrossRef]
- Kim, B.R.; Kim, S.H.; Ko, J.; Baek, S.W.; Park, Y.K.; Kim, Y.J.; Yoon, J.S. A Pilot Clinical Study of Ocular Prosthesis Fabricated by Three-dimensional Printing and Sublimation Technique. Korean J. Ophthalmol. 2021, 35, 37–43. [Google Scholar] [CrossRef]
- Park, S.-Y.; An, J.-H.; Kwon, H.; Choi, S.-Y.; Lim, K.-Y.; Kwak, H.-H.; Hussein, K.H.; Woo, H.-M.; Park, K.-M. Custom-made artificial eyes using 3D printing for dogs: A preliminary study. PLoS ONE 2020, 15, e0242274. [Google Scholar] [CrossRef]
- Alam, M.S.; Sugavaneswaran, M.; Arumaikkannu, G.; Mukherjee, B. An innovative method of ocular prosthesis fabrication by bio-CAD and rapid 3-D printing technology: A pilot study. Orbit 2017, 36, 223–227. [Google Scholar] [CrossRef]
- Ko, J.; Kim, S.H.; Baek, S.W.; Chae, M.K.; Yoon, J.S. Semi-automated fabrication of customized ocular prosthesis with three–dimensional printing and sublimation transfer printing technology. Sci. Rep. 2019, 9, 2968. [Google Scholar] [CrossRef]
- Kormann, R.B.; Mörschbächer, R.; Moreira, H.; Akaishi, P. A three-dimensional printed photopolymer resin implant for orbital rehabilitation for evisceration. Arq. Bras. Oftalmol. 2019, 82, 471–475. [Google Scholar] [CrossRef]
- ISO 10993-1:2018; Biological Evaluation of Medical Devices—Part 1: Evaluation and Testing Within a Risk Management Process. 5th ed. International Organization for Standardization: Geneva, Switzerland, 2018; 41p. Available online: https://www.iso.org/standard/68936.html (accessed on 2 July 2024).
- Valenti, C.; Federici, M.I.; Masciotti, F.; Marinucci, L.; Xhimitiku, I.; Cianetti, S.; Pagano, S. Mechanical properties of 3D printed prosthetic materials compared with milled and conventional processing: A systematic review and meta-analysis of in vitro studies. J. Prosthet. Dent. 2024, 132, 381–391. [Google Scholar] [CrossRef]
- Tahmawy, Y.A.; Mohamed, F.S.; Elfeki, S.; Abd-ELLAH, M.E. Microbiological evaluation of conjunctival anopthalmic flora after using digital 3D-printed ocular prosthesis compared to conventional one: A randomized clinical trial. BMC Oral Health 2023, 23, 1012. [Google Scholar] [CrossRef]
- Cicinelli, M.; Marmamula, S.; Khanna, R. Comprehensive eye care—Issues, challenges, and way forward. Indian. J. Ophthalmol. 2020, 68, 316. [Google Scholar] [CrossRef]
- Kim, Y.C.; Jeong, W.S.; Park, T.; Choi, J.W.; Koh, K.S.; Oh, T.S. The accuracy of patient specific implant prebented with 3D-printed rapid prototype model for orbital wall reconstruction. J. Cranio-Maxillofac. Surg. 2017, 45, 928–936. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Kwon, J.; Ahn, C.J.; Esmaeli, B.; Kim, G.B.; Kim, N.; Sa, H.-S. Generation of customized orbital implant templates using 3-dimensional printing for orbital wall reconstruction. Eye 2018, 32, 1864–1870. [Google Scholar] [CrossRef]
- Murray-Douglass, A.; Snoswell, C.; Winter, C.; Harris, R. Three-dimensional (3D) printing for post-traumatic orbital reconstruction, a systematic review and meta-analysis. Br. J. Oral Maxillofac. Surg. 2022, 60, 1176–1183. [Google Scholar] [CrossRef]
- Mukai, S.; Tsuge, T.; Akaishi, S.; Ogawa, R.; Kuwahara, H. Utilizing 3D Printing for the Surgical Management of Orbital Floor Fractures. Plast. Reconstr. Surg. Glob. Open 2023, 11, e5433. [Google Scholar] [CrossRef]
- Callahan, A.B.; Campbell, A.A.; Petris, C.; Kazim, M. Low-Cost 3D Printing Orbital Implant Templates in Secondary Orbital Reconstructions. Ophthalmic Plast. Reconstr. Surg. 2017, 33, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Weisson, E.H.; Fittipaldi, M.; Concepcion, C.A.; Pelaez, D.; Grace, L.; Tse, D.T. Automated Noncontact Facial Topography Mapping, 3-Dimensional Printing, and Silicone Casting of Orbital Prosthesis. Am. J. Ophthalmol. 2020, 220, 27–36. [Google Scholar] [CrossRef]
- Oh, T.S.; Jeong, W.S.; Chang, T.J.; Koh, K.S.; Choi, J.-W. Customized Orbital Wall Reconstruction Using Three-Dimensionally Printed Rapid Prototype Model in Patients With Orbital Wall Fracture. J. Craniofacial Surg. 2016, 27, 2020–2024. [Google Scholar] [CrossRef] [PubMed]
- Mourits, D.L.; Wolff, J.; Forouzanfar, T.; Ridwan-Pramana, A.; Moll, A.C.; De Graaf, P.; Remmers, J.S.; Kraal-Biezen, E.; Hartong, D.T. 3D Orbital Reconstruction in a Patient with Microphthalmos and a Large Orbital Cyst—A Case Report. Ophthalmic Genet. 2016, 37, 233–237. [Google Scholar] [CrossRef]
- Vehmeijer, M.; Van Eijnatten, M.; Liberton, N.; Wolff, J. A Novel Method of Orbital Floor Reconstruction Using Virtual Planning, 3-Dimensional Printing, and Autologous Bone. J. Oral Maxillofac. Surg. 2016, 74, 1608–1612. [Google Scholar] [CrossRef]
- Amin, D.; Nguyen, N.; Manhan, A.J.; Kim, J.H.; Roser, S.M.; Bouloux, G.F. Does a Point-of-Care 3-Dimensional Printer Result in a Decreased Length of Surgery for Orbital Fractures? J. Oral Maxillofac. Surg. 2024, 82, 1275–1284. [Google Scholar] [CrossRef]
- Tel, A.; Sembronio, S.; Costa, F.; Stenico, A.S.; Bagatto, D.; D’Agostini, S.; Robiony, M. Endoscopically assisted computer-guided repair of internal orbital floor fractures: An updated protocol for minimally invasive management. J. Cranio-Maxillofac. Surg. 2019, 47, 1943–1951. [Google Scholar] [CrossRef]
- Jamayet, N.B.; Abdullah, Y.J.; Rajion, Z.A.; Husein, A.; Alam, M.K. New Approach to 3D Printing of Facial Prostheses Using Combination of Open Source Software and Conventional Techniques: A Case Report. Bull. Tokyo Dent. Coll. 2017, 58, 117–124. [Google Scholar] [CrossRef]
- Sun, M.G.; Rojdamrongratana, D.; Rosenblatt, M.I.; Aakalu, V.K.; Yu, C.Q. 3D printing for low cost, rapid prototyping of eyelid crutches. Orbit 2019, 38, 342–346. [Google Scholar] [CrossRef]
- Lapid, O. Eyelid Crutches for Ptosis: A Forgotten Solution. Plast. Reconstr. Surg. 2000, 106, 1213–1214. [Google Scholar] [PubMed]
- Wróbel-Dudzińska, D.; Osial, N.; Stępień, P.W.; Gorecka, A.; Żarnowski, T. Prevalence of Dry Eye Symptoms and Associated Risk Factors among University Students in Poland. Int. J. Environ. Res. Public Health 2023, 20, 1313. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.; Ziai, S.; Myageri, V.; Burns, J.G.; Prokopich, C.L. Economic burden and loss of quality of life from dry eye disease in Canada. BMJ Open Ophthalmol. 2021, 6, e000709. [Google Scholar] [CrossRef]
- Lieu, A.C.; Shoji, M.K.; Aakalu, V.K.; Liu, C.Y. Approaches to Restoring Lacrimal Gland Function: From stem Cells to Tissue Engineering. Curr. Ophthalmol. Rep. 2024, 12, 55–62. [Google Scholar] [CrossRef]
- Rodboon, T.; Yodmuang, S.; Chaisuparat, R.; Ferreira, J.N. Development of high-throughput lacrimal gland organoid platforms for drug discovery in dry eye disease. SLAS Discov. 2022, 27, 151–158. [Google Scholar] [CrossRef]
- Adine, C.; Ng, K.K.; Rungarunlert, S.; Souza, G.R.; Ferreira, J.N. Engineering innervated secretory epithelial organoids by magnetic three-dimensional bioprinting for stimulating epithelial growth in salivary glands. Biomaterials 2018, 180, 52–66. [Google Scholar] [CrossRef]
- Ferreira, J.N.; Bhummaphan, N.; Chaisuparat, R.; Van Phan, T.; Oo, Y.; Jaru-Ampornpan, P.; Matangkasombut, O.; Mutirangura, A. Unveiling senescence-associated ocular pathogenesis via lacrimal gland organoid magnetic bioassembly platform and HMGB1-Box A gene therapy. Sci. Rep. 2024, 14, 21784. [Google Scholar] [CrossRef]
- Grumm, L.; Zakour, K.E.W.-B.; Kaya, S.; Groeber-Becker, F.; Geerling, G.; Witt, J. Designing a hybrid hydrogel of lacrimal gland extracellular matrix and alginate for 3D bioprinting. Investig. Ophthalmol. Vis. Sci. 2023, 64, 3291. [Google Scholar]
- Xu, X.; Awwad, S.; Diaz-Gomez, L.; Alvarez-Lorenzo, C.; Brocchini, S.; Gaisford, S.; Goyanes, A.; Basit, A.W. 3D Printed Punctal Plugs for Controlled Ocular Drug Delivery. Pharmaceutics 2021, 13, 1421. [Google Scholar] [CrossRef]
- Khanna, T.; Akkara, J.; Bawa, V.; Sargunam, E. Designing and making an open source, 3D-printed, punctal plug with drug delivery system. Indian. J. Ophthalmol. 2023, 71, 297. [Google Scholar] [CrossRef] [PubMed]
- Marcet, M.M.; Shtein, R.M.; Bradley, E.A.; Deng, S.X.; Meyer, D.R.; Bilyk, J.R.; Yen, M.T.; Lee, W.B.; Mawn, L.A. Safety and Efficacy of Lacrimal Drainage System Plugs for Dry Eye Syndrome. Ophthalmology 2015, 122, 1681–1687. [Google Scholar] [CrossRef]
- Gayton, J. Etiology, prevalence, and treatment of dry eye disease. Clin. Ophthalmol. 2009, 3, 405–412. [Google Scholar] [CrossRef]
- Singh, R.B.; Ichhpujani, P.; Thakur, S.; Jindal, S. Promising therapeutic drug delivery systems for glaucoma: A comprehensive review. Ophthalmol. Eye Dis. 2020, 12, 251584142090574. [Google Scholar] [CrossRef] [PubMed]
- Quaranta, L.; Novella, A.; Tettamanti, M.; Pasina, L.; Weinreb, R.N.; Nobili, A. Adherence and Persistence to Medical Therapy in Glaucoma: An Overview. Ophthalmol. Ther. 2023, 12, 2227–2240. [Google Scholar] [CrossRef] [PubMed]
- Tamrat, L.; Gessesse, G.; Gelaw, Y. Adherence to topical glaucoma medications in Ethiopian patients. Middle East. Afr. J. Ophthalmol. 2015, 22, 59. [Google Scholar] [CrossRef]
- Wagner, F.M.; Schuster, A.K.; Kianusch, K.; Stingl, J.; Pfeiffer, N.; Hoffmann, E.M. Long-term success after trabeculectomy in open-angle glaucoma: Results of a retrospective cohort study. BMJ Open 2023, 13, e068403. [Google Scholar] [CrossRef]
- Gedde, S.J.; Schiffman, J.C.; Feuer, W.J.; Herndon, L.W.; Brandt, J.D.; Budenz, D.L. Treatment Outcomes in the Tube Versus Trabeculectomy (TVT) Study After Five Years of Follow-up. Am. J. Ophthalmol. 2012, 153, 789–803.e2. [Google Scholar] [CrossRef]
- Ioannou, N.; Luo, J.; Qin, M.; Di Luca, M.; Mathew, E.; Tagalakis, A.D.; Lamprou, D.A.; Yu-Wai-Man, C. 3D-printed long-acting 5-fluorouracil implant to prevent conjunctival fibrosis in glaucoma. J. Pharm. Pharmacol. 2023, 75, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Mohamdeen, Y.M.G.; Tabriz, A.G.; Tighsazzadeh, M.; Nandi, U.; Khalaj, R.; Andreadis, I.; Boateng, J.S.; Douroumis, D. Development of 3D printed drug-eluting contact lenses. J. Pharm. Pharmacol. 2022, 74, 1467–1476. [Google Scholar] [CrossRef]
- Alam, F.; Elsherif, M.; AlQattan, B.; Salih, A.; Lee, S.M.; Yetisen, A.K.; Park, S.; Butt, H. 3D Printed Contact Lenses. ACS Biomater. Sci. Eng. 2021, 7, 794–803. [Google Scholar] [CrossRef] [PubMed]
- Hisham, M.; Salih, A.E.; Butt, H. 3D Printing of Multimaterial Contact Lenses. ACS Biomater. Sci. Eng. 2023, 9, 4381–4391. [Google Scholar] [CrossRef]
- Zhao, F.; Wang, J.; Wang, L.; Chen, L. An approach for simulating the fitting of rigid gas-permeable contact lenses using 3D printing technology. Contact Lens Anterior Eye 2019, 42, 165–169. [Google Scholar] [CrossRef]
- Hittini, S.; Salih, A.E.; Alam, F.; Shanti, A.; Lee, S.; Polychronopoulou, K.; AlSafar, H.; Almaskari, F.; Butt, H. Fabrication of 3D-Printed Contact Lenses and Their Potential as Color Blindness Ocular Aids. Macro Mater. Amp. Eng. 2023, 308, 2200601. [Google Scholar] [CrossRef]
- Alam, F.; Elsherif, M.; AlQattan, B.; Ali, M.; Ahmed, I.M.G.; Salih, A.; Antonysamy, D.S.; Yetisen, A.K.; Park, S.; Butt, H. Prospects for Additive Manufacturing in Contact Lens Devices. Adv. Eng. Mater. 2021, 23, 2000941. [Google Scholar] [CrossRef]
- Won, J.Y.; Kim, J.; Gao, G.; Kim, J.; Jang, J.; Park, Y.-H.; Cho, D.-W. 3D printing of drug-loaded multi-shell rods for local delivery of bevacizumab and dexamethasone: A synergetic therapy for retinal vascular diseases. Acta Biomater. 2020, 116, 174–185. [Google Scholar] [CrossRef]
- Lescot, T.; Lebel-Cormier, M.; Seniwal, B.; Gros-Louis, P.; Bellerive, C.; Landreville, S.; Beaulieu, L.; Fortin, M. Tumor Shape-Specific Brachytherapy Implants by 3D-Printing, Precision Radioactivity Painting, and Biomedical Imaging. Adv. Healthc. Mater. 2023, 12, 2300528. [Google Scholar] [CrossRef]
- Łukowiak, M.; Jezierska, K.; Boehlke, M.; Więcko, M.; Łukowiak, A.; Podraza, W.; Lewocki, M.; Masojć, B.; Falco, M. Utilization of a 3D printer to fabricate boluses used for electron therapy of skin lesions of the eye canthi. J. Appl. Clin. Med. Phys. 2017, 18, 76–81. [Google Scholar] [CrossRef]
- Wu, C.; Luo, M.; Liu, Y.; Dai, R.; Zhang, M.; Zhong, Y.; Chen, Y. Application of a 3D-printed eye model for teaching direct ophthalmoscopy to undergraduates. Graefes Arch. Clin. Exp. Ophthalmol. 2022, 260, 2361–2368. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.W.; Paxton, L.; Dawes, K.; Burlak, K.; Quayle, M.; McMenamin, P.G. 3D printed reproductions of orbital dissections: A novel mode of visualizing anatomy for trainees in ophthalmology or optometry. Br. J. Ophthalmol. 2015, 99, 1162–1167. [Google Scholar] [CrossRef] [PubMed]
- Rama, M.; Schlegel, L.; Wisner, D.; Pugliese, R.; Ramesh, S.; Penne, R.; Watson, A. Using three-dimensional printed models for trainee orbital fracture education. BMC Med. Educ. 2023, 23, 467. [Google Scholar] [CrossRef]
- Tsui, J.K.S.; Bell, S.; Cruz, L.D.; Dick, A.D.; Sagoo, M.S. Applications of three-dimensional printing in ophthalmology. Surv. Ophthalmol. 2022, 67, 1287–1310. [Google Scholar] [CrossRef] [PubMed]
- Maloca, P.M.; Tufail, A.; Hasler, P.W.; Rothenbuehler, S.; Egan, C.; de Carvalho, J.E.R.; Spaide, R.F. 3D printing of the choroidal vessels and tumours based on optical coherence tomography. Acta Ophthalmol. 2017, 97, e313–e316. [Google Scholar] [CrossRef]
- Lichtenberger, J.P.; Tatum, P.S.; Gada, S.; Wyn, M.; Ho, V.B.; Liacouras, P. Using 3D Printing (Additive Manufacturing) to Produce Low-Cost Simulation Models for Medical Training. Mil. Med. 2018, 183 (Suppl. S1), 73–77. [Google Scholar] [CrossRef]
- Famery, N.; Abdelmassih, Y.; El-Khoury, S.; Guindolet, D.; Cochereau, I.; Gabison, E.E. Artificial chamber and 3D printed iris: A new wet lab model for teaching Descemet’s membrane endothelial keratoplasty. Acta Ophthalmol. 2019, 97, E179–E183. [Google Scholar] [CrossRef]
- Pugalendhi, A.; Ranganathan, R.; Venkatapathy, N.; Narendran, K.; Shah, P.K. Design and development of model eye for retina laser by using additive manufacturing. Proc. Inst. Mech. Eng. H 2021, 235, 89–98. [Google Scholar] [CrossRef]
- Furdová, A.; Sramka, M.; Thurzo, A.; Furdová, A. Early experiences of planning stereotactic radiosurgery using 3D printed models of eyes with uveal melanomas. Clin. Ophthalmol. 2017, 11, 267–271. [Google Scholar] [CrossRef]
- Dorbandt, D.M.; Joslyn, S.K.; Hamor, R.E. Three-dimensional printing of orbital and peri-orbital masses in three dogs and its potential applications in veterinary ophthalmology. Vet. Ophthalmol. 2017, 20, 58–64. [Google Scholar] [CrossRef]
- Alam, F.; Ali, M.; Elsherif, M.; Salih, A.E.; El-Atab, N.; Butt, H. 3D printed intraocular lens for managing the color blindness. Addit. Manuf. Lett. 2023, 5, 100129. [Google Scholar] [CrossRef]
- Raveendran, R.; Prabakaran, L.; Senthil, R.; Yesudhason, B.V.; Dharmalingam, S.; Sathyaraj, W.V.; Atchudan, R. Current Innovations in Intraocular Pressure Monitoring Biosensors for Diagnosis and Treatment of Glaucoma—Novel Strategies and Future Perspectives. Biosensors 2023, 13, 663. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, K.A.; Drew-Bear, L.E.; Vega-Garces, M.; Betancourt-Belandria, H.; Arevalo, J.F. An update on visual prosthesis. Int. J. Retin. Vitr. 2023, 9, 73. [Google Scholar] [CrossRef]
- Chaurasia, S.; Das, S.; Roy, A. A review of long-term corneal preservation techniques: Relevance and renewed interests in the COVID-19 era. Indian. J. Ophthalmol. 2020, 68, 1357. [Google Scholar] [CrossRef] [PubMed]
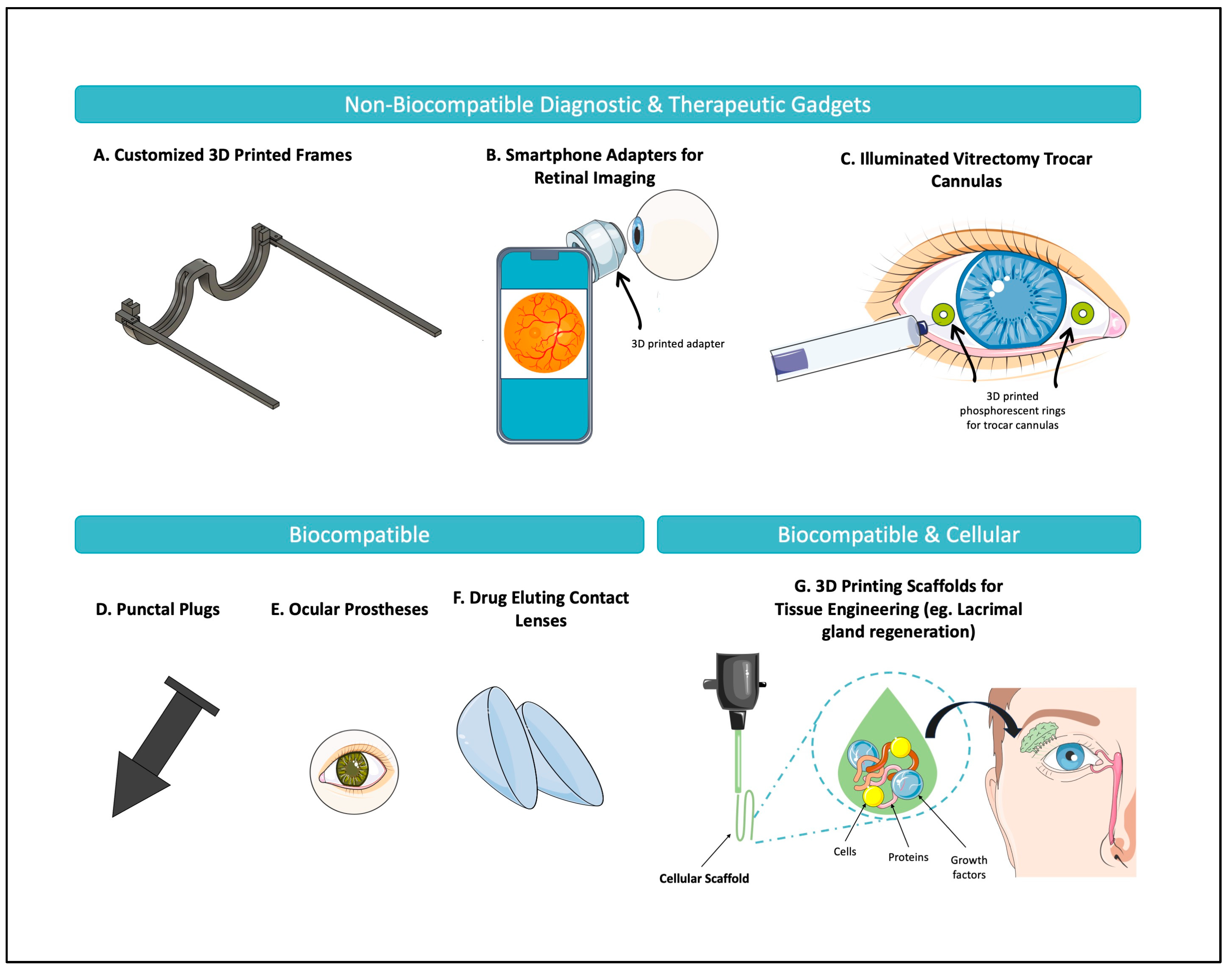
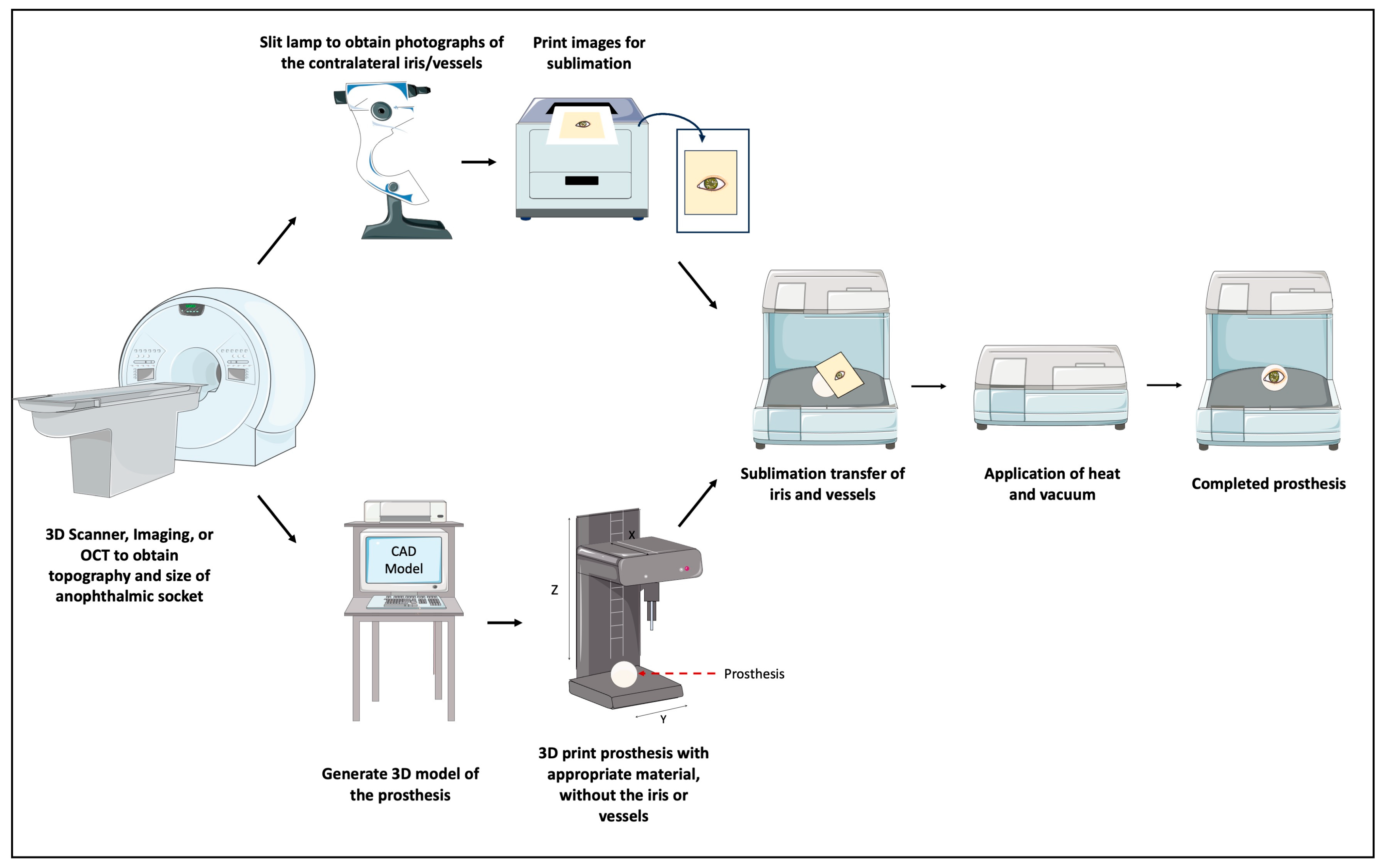
| Bioprinting Method | Material Extrusion | Powder Bed Fusion | Vat Polymerization | Material Jetting |
|---|---|---|---|---|
| Technique | Dispenses material through a nozzle | Uses thermal energy from a laser source to sinter powder | Crosslinks liquid photopolymer resin using a light source | Dispenses inkjet droplets |
| Examples | FDM | SLS | SLA, DLP | MJM |
| Machine Cost [23] | Low–Medium | High | Low–High | Medium–High |
| Material Cost [23] | Low–Medium | High | Medium–High | High |
| Resolution [24] | 200–1200 μm (depending on the size of the nozzle) | 50–100 μm | 20–50 μm | 15–30 μm |
| Advantages | Main 3D printing method for tissue constructs, (53.98% of cases) [25] | Produces porous and dense complex structures with high mechanical strength [22] Material variety [23] | Good surface finish quality [23] Can be used for complex structures with fine details [23] | Allows multi-material and multi-color printing Enables relatively high cell viability (>85% cell viability) [26] |
| Disadvantages | Thermal and shear stresses from the extruder nozzle can impact cell viability (40–80% cell viability) [26] Requires a moderate to high viscosity ink | High cost can limit accessibility Relatively lower speed [27] Limited print sizes [27] | Difficult to print in multi-color, multi-materials [28] The product remains UV sensitive even following curation [23] | Requires low-viscosity inks Requires a completely dense support structure, necessitating more material, and thus, making this method less economical than other methods [28] |
| Biomaterial | Advantages | Disadvantages | References |
|---|---|---|---|
| Collagen |
|
| [33]: Collagen–alginate [36]: Collagen–laminin [37]: Collagen–agarose [38]: Collagen–gelatin–hyaluronic acid [39,40]: Collagen–alginate–gelatin |
| Gelatin |
|
| [42]: Electrospun gelatin nanofibers + infiltrated alginate [43,44]: GelMA [45]: GelMA-agarose [46]: GelMA-HAMA |
| Chitosan |
|
| [47]: Chitosan + PVA |
| dECM |
|
| [48]: dECM [49,50]: dECM + GelMA |
| Alginate |
| [33]: Collagen–alginate [39,40]: Collagen–alginate–gelatin [52]: Alginate–gelatin | |
| HA |
|
| [54]: HA-carbodihydrazide + HA-aldehyde + collagen and HA-carbodihydrazide-dopamine + HA-aldehyde + collagen [55]: HA glycidyl methacrylate + GelMA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mina, M.; Goel, A.K.; Mina, F.; Goubran, D.; Goel, N. Three-Dimensional Printing for Accessible and Personalized Ophthalmic Care: A Review. J. Clin. Transl. Ophthalmol. 2025, 3, 6. https://doi.org/10.3390/jcto3020006
Mina M, Goel AK, Mina F, Goubran D, Goel N. Three-Dimensional Printing for Accessible and Personalized Ophthalmic Care: A Review. Journal of Clinical & Translational Ophthalmology. 2025; 3(2):6. https://doi.org/10.3390/jcto3020006
Chicago/Turabian StyleMina, Mina, Ajay Kumar Goel, Fady Mina, Doris Goubran, and Nand Goel. 2025. "Three-Dimensional Printing for Accessible and Personalized Ophthalmic Care: A Review" Journal of Clinical & Translational Ophthalmology 3, no. 2: 6. https://doi.org/10.3390/jcto3020006
APA StyleMina, M., Goel, A. K., Mina, F., Goubran, D., & Goel, N. (2025). Three-Dimensional Printing for Accessible and Personalized Ophthalmic Care: A Review. Journal of Clinical & Translational Ophthalmology, 3(2), 6. https://doi.org/10.3390/jcto3020006






