Relaxant Activity of 4H-Pyran and 1,6-Dihydropyridine Derivatives on Isolated Rat Trachea
Abstract
1. Introduction
2. Results and Discussion
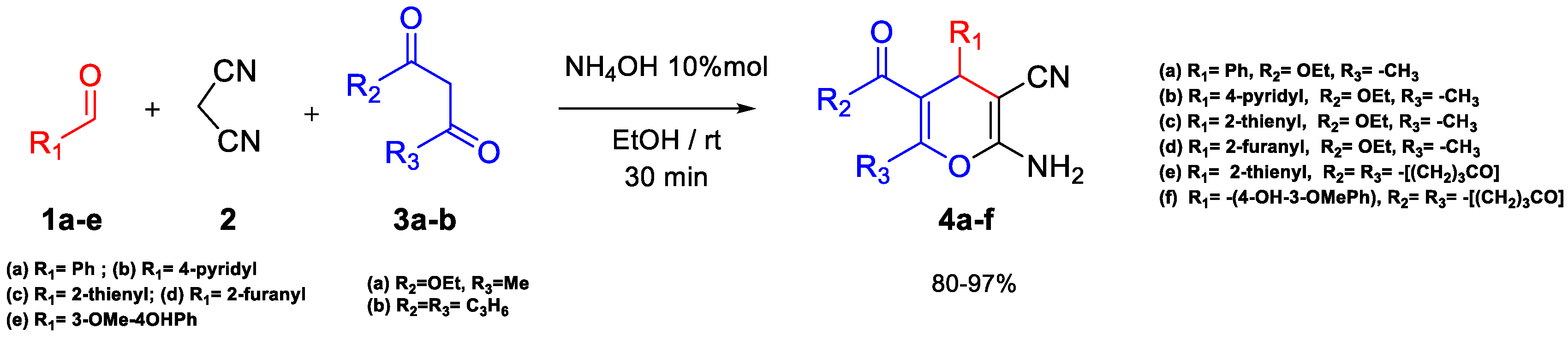
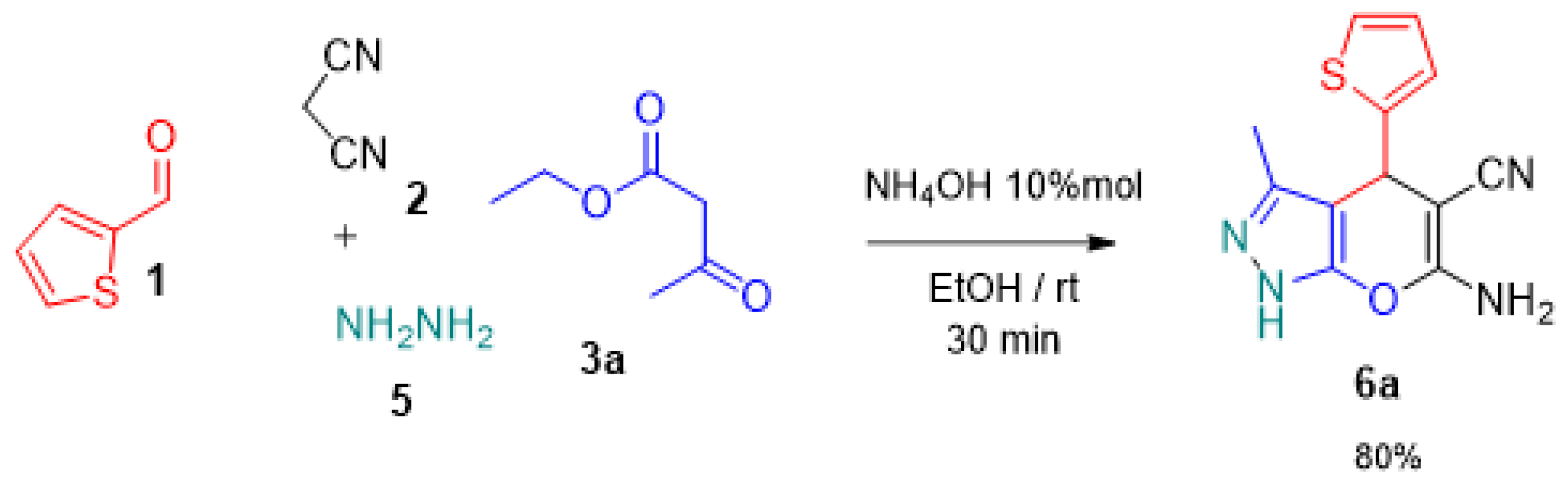
2.1. Synthesis of 4H-Pyran Derivatives 4a–f and 6a
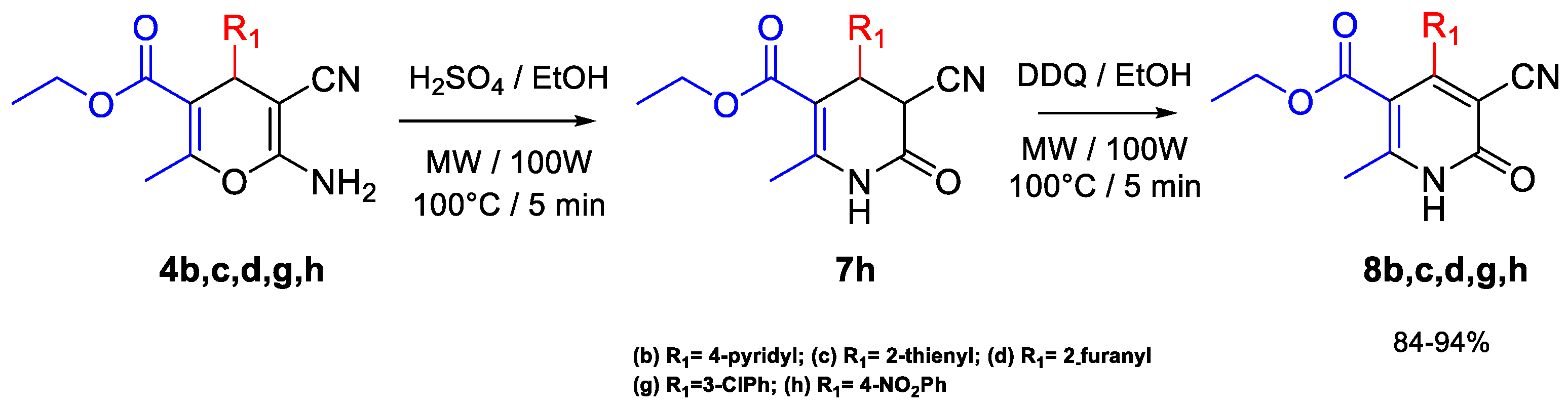
2.2. Synthesis of 1,6-Dihydropyridine Derivatives 8b–d and 8g–h
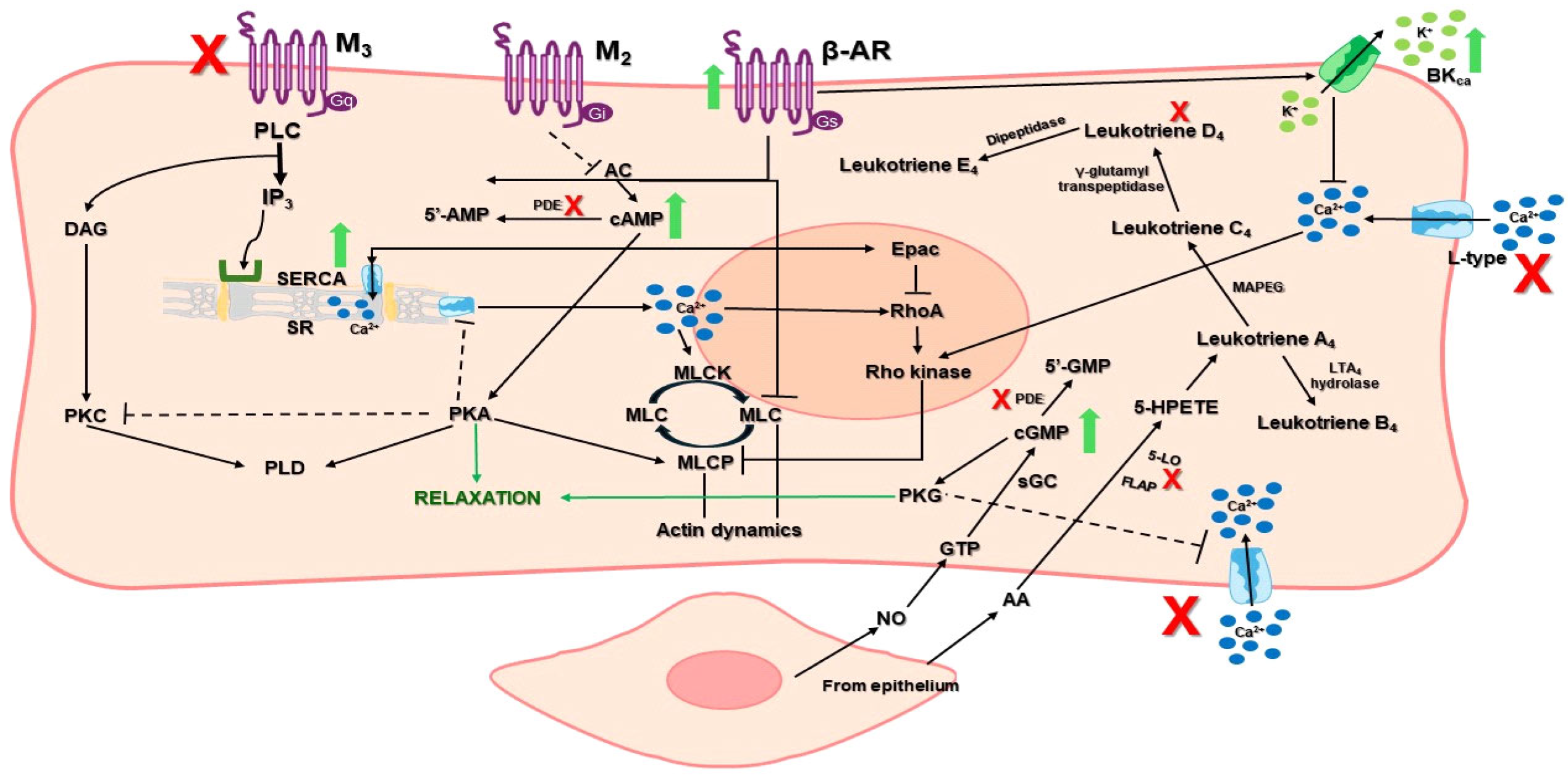
2.3. Relaxant Effect
3. Materials and Methods
3.1. General Procedure for the Synthesis of Compounds 4a–f
3.2. Synthesis of 6-amino-3-methyl-4-(thiophen-2-yl)-1,4-dihydropyrano [2,3-c]pyrazole-5-carbonitrile (6a)
3.3. General Procedure for the Synthesis of Compounds 8b–d and 8g–h
3.4. Pharmacological Evaluation
3.4.1. Rats
3.4.2. Rat Tracheal Ring Assay
3.5. Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hernández, F.; Sánchez, A.; Rendón-Vallejo, P.; Millán-Pacheco, C.; Alcaraz, Y.; Delgado, F.; Vázquez, M.A.; Estrada-Soto, S. Synthesis, ex vivo and in silico studies of 3-cyano-2-piridone derivates with vasorelaxant activity. Eur. J. Med. Chem. 2013, 70, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Farard, J.; Lanceart, G.; Logé, C.; Nourrison, M.R.; Cruzzalegui, F.; Pfeiffer, B.; Duflos, M. Design, synthesis and evaluation of new 6-substituted-5-benzyloxy-4-oxo-4H-pyran-2-carboxamidas as potential Src inhibitors. J. Enzyme Inhib. Med. Chem. 2008, 5, 629–640. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lenaerts, A.J.; Bitting, C.; Woolhiser, L.; Gruppo, V.; Marietta, K.S.; Johnson, C.M.; Orme, I.M. Evaluation of a 2-Pyridone, KRQ- 10018, against Mycobacterium tuberculosis in vitro and in vivo. Antimicrob. Agents Chemother. 2008, 52, 1513–1515. [Google Scholar] [CrossRef] [PubMed]
- Saundane, A.; Vijaykumar, K.; Vaijinath, A.V. Syntesis of novel 2-amino-4-(5′-substituted 2′-phenyl-1H-indol-3′-yl)-6-aryl-4H-pyran-3-cabonitrile derivates as antimicrobial and antioxidant agents. Biorg. Med. Chem. Lett. 2013, 23, 1978–1984. [Google Scholar] [CrossRef] [PubMed]
- Marco, J.L.; de los Ríos, C.; García, A.G.; Villarroya, M.; Carreiras, M.C.; Martins, C.; Eleutério, A.; Morreale, A.; Orozco, M.; Luque, F.J. Synthesis, biological evaluation and molecular modelling of diversely functionalized heterocyclic derivatives as inhibitors of acetylcholinesterase/butyrylcholinesterase and modulators of Ca2+ channels and nicotinic receptors. Bioorg. Med. Chem. 2004, 12, 2199–2218. [Google Scholar] [CrossRef] [PubMed]
- Urbahns, K.; Horváth, E.; Stasch, J.P.; Mauler, F. 4-Phenyl-4H-pyrans as IK(Ca) channel blockers. Bioorg. Med. Chem. Lett. 2003, 13, 2637–2639. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Cooper, G.; Dunne, S.F.; Luan, C.H.; James Surmeier, D.; Silverman, R.B. Antagonism of L-type Ca2+ channels CaV1.3 and CaV1.2 by 1,4-dihydropyrimidines and 4H-pyrans as dihydropyridine mimics. Bioorg. Med. Chem. 2013, 21, 4365–4373. [Google Scholar] [CrossRef] [PubMed]
- Rendón-Vallejo, P.; Estrada-Soto, S.; Vázquez, M.A.; Hernández-Borja, F.; Villalobos-Molina, R.; Ibarra-Barajas, R. Design, Synthesis and ex vivo study of the vasorelaxant activity induced by isosteric derivatives of dihydropyridines (NH→O). Lett. Drug Des. Discov. 2016, 13, 353–359. [Google Scholar] [CrossRef]
- Abhinav Prasoon, M.; Ankit, B.; Awani Kumar, R. 1,4-Dihydropyridine: A dependable heterocyclic ring with the promising and the most anticipable therapeutic Effects. Mini Rev. Med. Chem. 2019, 19, 1219–1254. [Google Scholar]
- Hernandez, J.M.; Janssen, L.J. L-type Ca+2 channels, Ca+2-induced Ca+2 release, and BKca channels in airway stretch-induced contraction. Eur. J. Pharmacol. 2012, 696, 161–165. [Google Scholar] [CrossRef]
- Pelletier, L.; Savignac, M. Ca+2 signaling in T-cell subsets with a focus on the role of Cav1 channels: Possible implications in therapeutics. Front. Immunol. 2013, 4, 150. [Google Scholar] [CrossRef] [PubMed]
- Gomes, B.; Cabral, M.; Gallard, A.; Savignac, M.; Paulet, P.; Druet, P.; Mariamé, M.; Moreau, M.; Leclerc, C.; Guéry, J.; et al. Calcium channel blocker prevents T helper type 2 cell-mediated airway inflamation. Am. J. Respir. Crit. Care Med. 2007, 175, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- O’Byrne, P.M. Global guidelines for asthma management: Summary of the current status and future challenges. Pol. Arch. Med. Wewn. 2010, 120, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Nagai, H. Recent research and developmental strategy of anti-asthma drugs. Pharmacol. Ther. 2012, 133, 70–78. [Google Scholar] [CrossRef]
- Siddiqui, S.; Redhu, N.S.; Ojo, O.O.; Liu, B.; Irechukwu, N.; Billington, C.; Janssen, L.; Moir, L.M. Emerging airway smooth muscle targets to treat asthma. Pulm. Pharmacol. Ther. 2013, 26, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, A.; Hernández, F.; Cruz, P.; Alcaraz, Y.; Tamariz, J.; Delgado, F.; Vázquez, M.A. Infrared irradiation-assisted multicomponent synthesis of 2-amino-3-cyano-4H-pyran derivatives. J. Mex. Chem. Soc. 2012, 56, 121–127. [Google Scholar] [CrossRef]
- Satori, N.A.; Pacini, E.S.A.; Godinho, R.O. Impact of the cAMP efflux and extracellular cAMP-adenosine pathway on airway smooth muscle relaxation induced by formoterol and phosphodiesterase inhibitors. Chem. Biol. Interact. 2023, 382, 110630. [Google Scholar] [CrossRef]
- Uka, A.; Krasniqi, D.; Beretta, G.; Daci, A. Assessment of in vitro airway smooth muscle relaxant activity of Rhus coriaria L. fruit ethanolic extract and its possible mechanisms. J. Med. Food 2023, 26, 820–830. [Google Scholar] [CrossRef]
- Alemán-Pantitlán, S.; Millán-Pacheco, C.; Vázquez, M.A.; Hernández-Borja, F.; Villalobos-Molina, R.; Bazán-Perkins, B.; Estrada-Soto, S. Mechanism of Relaxant action of ethyl 6-amino-5-cyano-2-methyl-4-(pyridin-4-yl)-4H-pyran-3-carboxylate mainly through calcium channel blockade in isolated rat trachea. J. App. Pharm. Sci. 2016, 6, 29–36. [Google Scholar]
- Hughes, J.P.; Rees, S.; Kalindjian, S.B.; Philpott, K.L. Principles of early drug discovery. Br. J. Pharmacol. 2011, 162, 1239–1249. [Google Scholar] [CrossRef]
- Brodniewicz, T.; Grynkiewicz, G. Preclinical drug development. Acta Pol. Pharm. 2010, 67, 578–585. [Google Scholar] [PubMed]


| Compound | Emax (%) | EC50 (µM) |
|---|---|---|
| Theophylline | 102.9 ± 1.6 | 158 ± 3.0 |
| 4a | 77.1 ± 4.9 | 236.8 ± 4.9 |
| 4b | 102.3 ± 4.9 | 96.3 ± 7.5 |
| 4c | 75.9 ± 8.8 | 143.1 ± 8.8 |
| 4d | 74.5 ± 9.4 | 259 ± 9.4 |
| 4e | 64.6 ± 7.1 | 101.9 ± 7.1 |
| 4f | 101.2 ± 1.2 | 25.9 ± 4.5 |
| 6a | 48.63 ± 5.9 | ND |
| 7a | 82.6 ± 2.1 | 98.2 ± 3.1 |
| 8b | 47.1 ± 6.1 | ND |
| 8c | 75.3 ± 4.2 | 152.7 ± 3.6 |
| 8d | 87.2 ± 3.8 | 109.6 ± 7.5 |
| 8g | 46.5 ± 4.6 | ND |
| 8h | 78.4 ± 3.9 | 126.4 ± 6.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Estrada-Soto, S.; Alemán-Pantitlán, S.; Gaona-Tovar, E.; Hernández-Borja, F.; Alcaraz, Y.; Villalobos-Molina, R.; Vázquez, M.A. Relaxant Activity of 4H-Pyran and 1,6-Dihydropyridine Derivatives on Isolated Rat Trachea. Drugs Drug Candidates 2024, 3, 342-352. https://doi.org/10.3390/ddc3020020
Estrada-Soto S, Alemán-Pantitlán S, Gaona-Tovar E, Hernández-Borja F, Alcaraz Y, Villalobos-Molina R, Vázquez MA. Relaxant Activity of 4H-Pyran and 1,6-Dihydropyridine Derivatives on Isolated Rat Trachea. Drugs and Drug Candidates. 2024; 3(2):342-352. https://doi.org/10.3390/ddc3020020
Chicago/Turabian StyleEstrada-Soto, Samuel, Soledad Alemán-Pantitlán, Emmanuel Gaona-Tovar, Fernando Hernández-Borja, Yolanda Alcaraz, Rafael Villalobos-Molina, and Miguel A. Vázquez. 2024. "Relaxant Activity of 4H-Pyran and 1,6-Dihydropyridine Derivatives on Isolated Rat Trachea" Drugs and Drug Candidates 3, no. 2: 342-352. https://doi.org/10.3390/ddc3020020
APA StyleEstrada-Soto, S., Alemán-Pantitlán, S., Gaona-Tovar, E., Hernández-Borja, F., Alcaraz, Y., Villalobos-Molina, R., & Vázquez, M. A. (2024). Relaxant Activity of 4H-Pyran and 1,6-Dihydropyridine Derivatives on Isolated Rat Trachea. Drugs and Drug Candidates, 3(2), 342-352. https://doi.org/10.3390/ddc3020020






