Abstract
Trichome is a hair-like structure involved in mechanical and chemical defenses of plants against herbivorous insects. Nicotiana benthamiana, a wild tobacco plant, has well-developed glandular trichomes that secrete sugar esters with potent repellent and insecticidal properties. However, there is a lack of information about the effectiveness of trichome extract in the control of plant-sapping insects. The objective of this study was to investigate the effects of N. benthamiana trichome exudates on Bemisia tabaci MED (Gennadius) (Hemiptera: Aleyrodidae), a highly destructive insect pest that poses significant threats to both vegetable and ornamental plants globally. First, we determined the host preference and mortality of B. tabaci adults using the choice test and feeding assay towards tomato and N. benthamiana plants. B. tabaci preferred tomato over N. benthamiana plants. Second, we extracted N. benthamiana trichome exudates by washing the leaves with either water or ethanol and evaluated their oral toxicities against B. tabaci adults using a parafilm feeding chamber containing 20% sucrose solution. Oral ingestion of both extracts significantly increased mortality in a concentration-dependent manner. Oral ingestion of ethanol-washed 10% trichome extract caused >60% mortality in B. tabaci adults after 36 h. Third, trichome exudates were concentrated by drying to obtain a powder form, which was more potent in killing whiteflies than the liquid form. Oral ingestion of 1% trichome powder was completely lethal to B. tabaci within 36 h. N. benthamiana trichome exudates are highly toxic to B. tabaci through oral ingestion, suggesting that N. benthamiana can be used as a potential natural pesticide for whitefly management.
1. Introduction
The sweetpotato whitefly Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) is a species complex containing at least 40 cryptic species that are morphologically indistinguishable [1,2,3]. Among the species, the Middle East–Asia Minor 1 (also known as MEAM1 and previously known as B biotype) and Mediterranean (MED; previously known as Q biotype) species are responsible for causing severe damage to a wide range of agricultural plants [4]. B. tabaci is highly polyphagous and infests more than 600 plant species, including various agricultural crop plants and weed plants worldwide [5,6]. It is a serious pest that damages plants by direct feeding and by sooty mold fungus due to the excretion of honeydew [7]. Furthermore, B. tabaci transmits more than 100 plant viruses, especially begomoviruses [8,9].
Management of B. tabaci is difficult due to its short generation time, high reproduction rate, wide host range, and significant genetic diversity in global invasion rate and pesticide resistance [10,11,12]. For instance, MEAM1 and MED, which are distributed worldwide, have been found to develop high resistance to pesticides such as imidacloprid, acetamiprid, and thiamethoxam [10,13,14]. Therefore, it is highly necessary to investigate and develop alternate methods for the integrated management of B. tabaci.
In the evolutionary process, plants have developed various physical and chemical defense mechanisms against herbivorous insects [15]. Trichomes are hair-like structures present on the leaves and stems of plants and are used to disturb insect settlement on plants [16]. These hairs primarily act as simple mechanical obstacles; however, some trichomes also secrete sticky substances containing secondary metabolites, such as sugar esters, terpenoids, and flavonoids [16,17]. Glandular trichomes are present in several species of the Solanaceae family, such as the wild species of tomato and tobacco plants [18]. For instance, Lycopersicon pennellii Correll, a species of wild tomato, produces sticky exudates from type IV glandular hairs on its leaves and stems [19]. Moreover, the wild tobacco Nicotiana species are known to produce trichome exudate up to 15% of their dry leaf weight [17].
Nicotiana benthamiana has been used as a model species for investigating the genetics and molecular biology of plants [20]. N. benthamiana leaves possess two main types of glandular trichomes, namely, large swollen-stalk trichomes and small trichomes that include a secretory head consisting of one, two, or four cells [18]. Detached trichomes, a mixture of large and small trichomes, from N. benthamiana are able to synthesize acyl sugars [20]. Glandular trichomes play a crucial role in the production and release of several secondary metabolites, including terpenoids, acyl sugars, phenylpropanoids, and fatty-acid derivatives [21]. Acyl sugars, which are nonvolatile metabolites, are synthesized and accumulated inside glandular trichomes found in certain Solanaceae species, such as Solanum, Nicotiana, and Datura [20]. Acyl sugars have the potential to exert direct toxicity on herbivores while concurrently exhibiting remarkable emulsifying and surfactant properties. Therefore, they may readily adhere to the cuticles of arthropods, leading to the immobilization or suffocation of those arthropods [17,22]. According to Goffreda et al. [23], acyl sugars exhibit contact toxicity against potato aphids and function as a feeding deterrent. Moreover, previous research has shown that acyl sugars have the ability to repel or deter herbivores, including the potato aphid [17]. Furthermore, Puterka et al. [24] observed that the toxic properties of synthetic acyl sugars are influenced by both the sugar backbone and the length of the fatty-acid chain. In addition, various acyl sugars induce mortality in pear psyllids, tobacco aphids, tobacco hornworms, and spider mites [24]. Nevertheless, studies on potential toxicity resulting from the ingestion of trichome exudates are limited. Therefore, this study aimed to investigate the oral toxicity of various forms of trichome extracts of N. benthamiana for controlling B. tabaci. We also determined the host preference of B. tabaci MED between tomato and N. benthamiana plants.
2. Materials and Methods
2.1. Insects and Plants
The B. tabaci MED colony was maintained in tomato plants (Solanum lycopersicum L.) within insect-proof cages (45 cm × 60 cm × 90 cm). Whiteflies were reared under conditions of 25 °C ± 1 °C, 60% ± 5% relative humidity, and a 16:8 h light:dark photoperiodic cycle. Both N. benthamiana and S. lycopersicum plants were grown in a greenhouse under the same conditions.
2.2. Preparation of Trichome Exudate Extracts from N. benthamiana Leaves and the Main Components
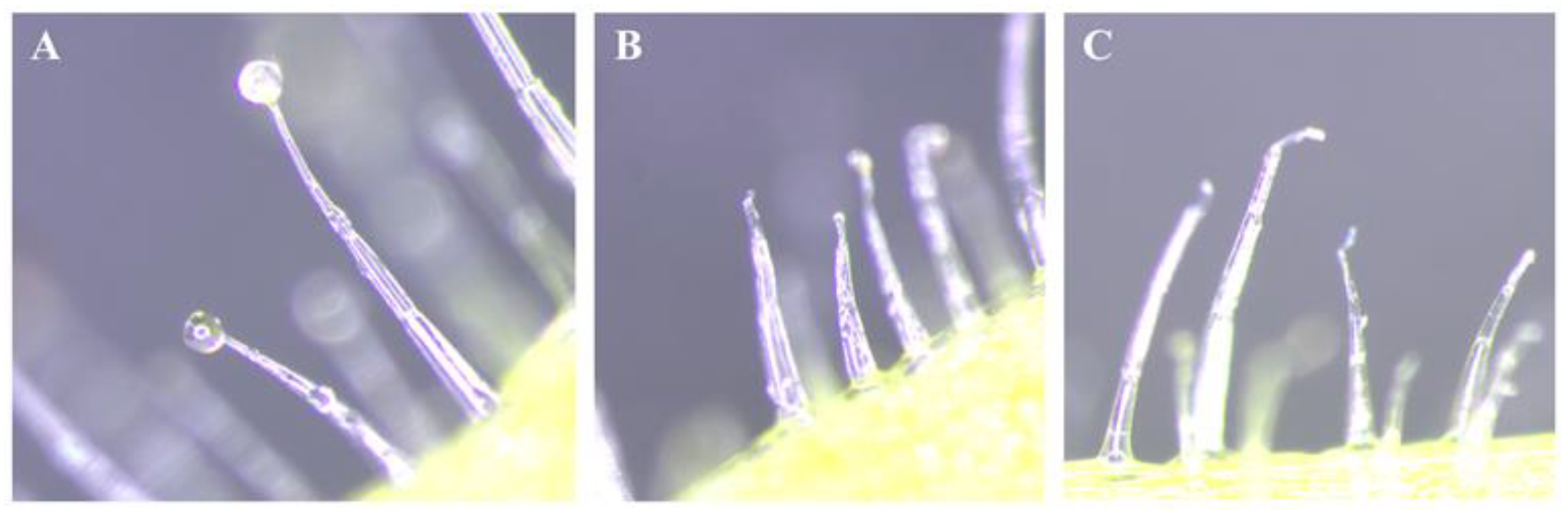
N. benthamiana leaves were collected from plants that had grown to at least 1 m in height (Figure 1A). First, fresh leaves were dipped in either water or absolute ethanol solutions at a ratio of 1:1.5 and shaken using a rotating stirrer for 30 min at room temperature. The solutions were then filtered using Whatman filter paper (Maidstone, UK). These solutions were termed trichome solutions with water (TS-W) or trichome solutions with ethanol (TS-E). Next, the trichome solutions were dried for 12 days at room temperature. These dried materials were termed trichome powder from TS-W (TP-W) or trichome powder from TS-E (TP-E). Both TP-W and TP-E were dissolved in water to prepare different concentrations for use in further experiments. In Figure 2, we show the chemical structure of three secondary metabolites that are produced and released by the glandular tri chomes, according to Wang et al. (licensed under CC BY 4.0) [25].
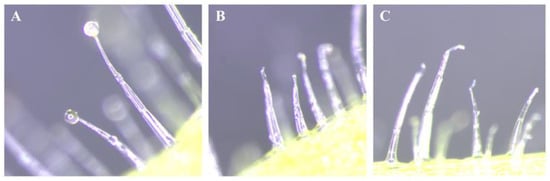
Figure 1.
Collection of glandular trichomes of Nicotiana benthamiana. Glandular trichomes at the abaxial side of N. benthamiana in the fresh leaf (A), and trichomes washed with water (B) and ethanol (C).
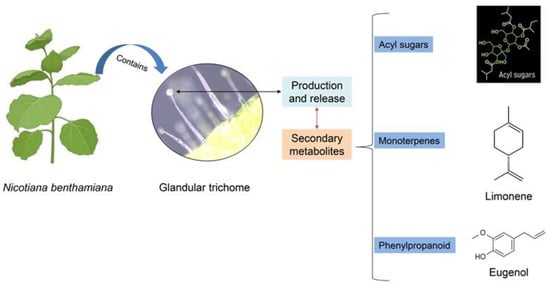
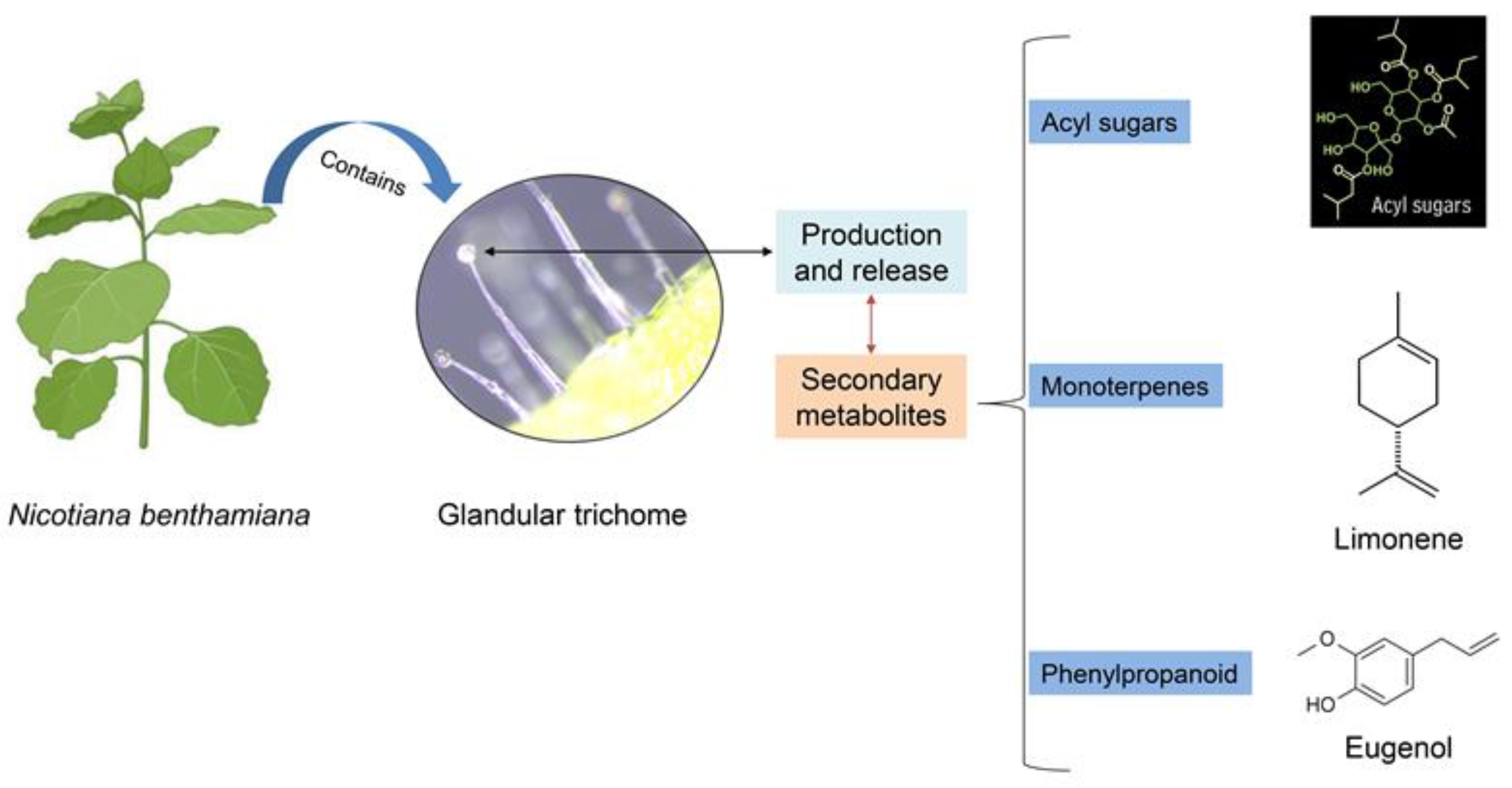
Figure 2.
Two primary forms of glandular trichomes observed in Nicotiana benthamiana leaves: large swollen-stalk trichomes and small trichomes. Here, we showed the chemical structure of three secondary metabolites produced and released by glandular trichomes. Adapted from Wang et al., 2022 [25].
2.3. Host Preferences of Bemisia tabaci between Tomato and Nicotiana benthamiana Plants
To determine the host preference of B. tabaci between tomato and N. benthamiana plants, whiteflies (n = 50) were released into the meshed cage (45 cm × 60 cm × 90 cm) containing these plants placed 50 cm apart from each other. The number of whiteflies attracted to each plant was counted at 24 h intervals up to 72 h. Moreover, the lethal toxicity of tomato and N. benthamiana plants was compared using a glass tube-feeding chamber (length 12 cm × diameter 3 cm). Whiteflies (n = 20) were released into the tube and then covered with a single leaf of either tomato or N. benthamiana. The number of dead whiteflies was counted at 12 h intervals until 60 h.
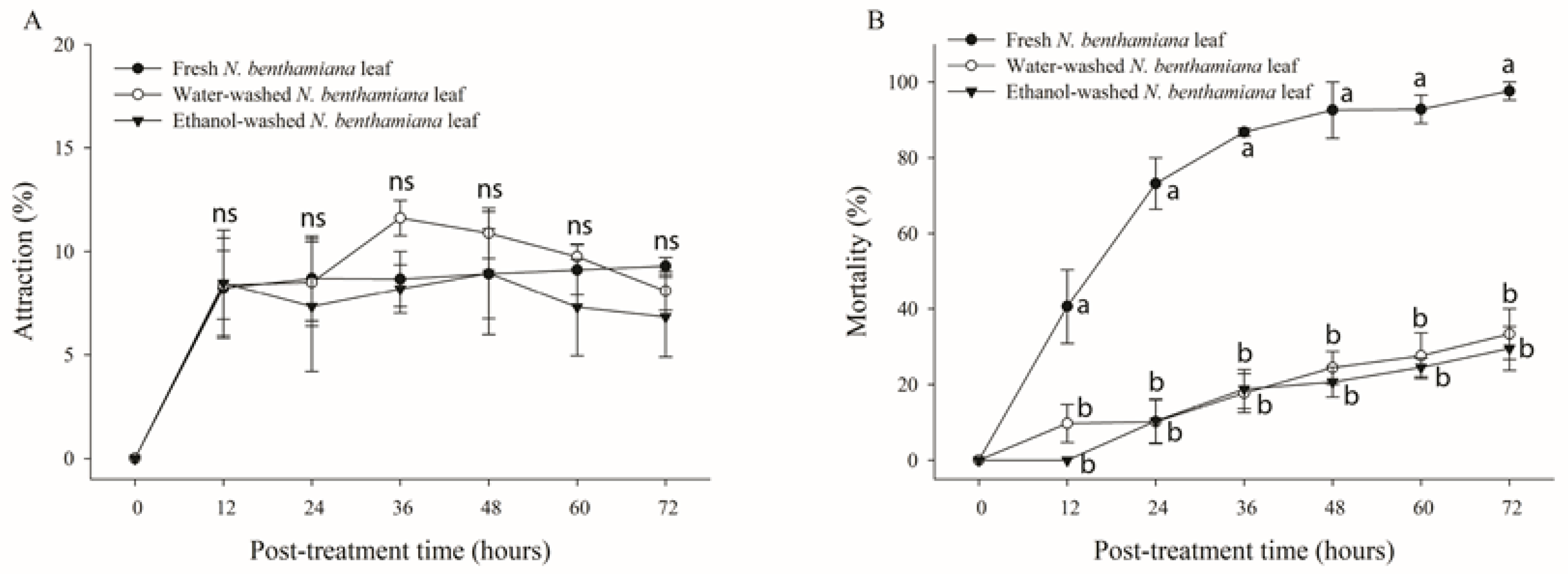
To determine the effect of trichome exudates on the attraction and viability of whiteflies, N. benthamiana leaves were washed with either water or ethanol solutions. Three types of N. benthamiana leaves, i.e., normal, water-washed, and ethanol-washed leaves, were placed into the cage, after which whiteflies (n = 70) were released. The rates of attraction and death were determined at 12 h intervals.
2.4. Oral Toxicity of Trichome Solutions against Bemisia tabaci
To explore the oral toxicity of trichome-washed solutions (TWSs) of N. benthamiana against adult whiteflies, the solutions were diluted in a 20% sucrose solution and used as an artificial diet. Then, B. tabaci adults were allowed to feed in the glass cage (length 12 cm × diameter 3 cm) feeding chamber. One end of the chamber was covered with two layers of parafilm separated by a 20% sucrose solution. The bottom end of the chamber was covered with a thick black paper sheet with a hole at the center. The hole was plugged by a ventilator made from a pipette tip covered with a fine-meshed net. The artificial diet (5 μL) containing various amounts of TWS was placed into the parafilm feeding chamber. Adult whiteflies (n = 20) were released into the chamber and then maintained for 72 h at room temperature. Whiteflies that dropped into the bottom of the feeding chamber were considered dead, and their number was counted over 72 h.
2.5. Effects of Trichome Exudate Powder (TEP) on Bemisia tabaci Adults
To determine the oral toxicity of the TEP of N. benthamiana on adult whiteflies, TEPs concentrated from water-washed or ethanol-washed solutions were diluted in 20% sucrose solution, and B. tabaci were allowed to feed in the glass cage feeding chamber. Adult whiteflies (n = 20) were released into the chamber, and then the mortality of B. tabaci adults was counted over 72 h.
To compare the toxicity of N. benthamiana TEP and the chemical pesticide imidacloprid, both were diluted to various concentrations in 20% sucrose solution. Oral toxicity was determined in the parafilm feeding chamber for 48 h. Whiteflies (n = 10) were released into the cage, and the number of whiteflies on the plants was counted at 12 h intervals up to 48 h.
2.6. Statistical Analysis
One-way analysis of variance (ANOVA), followed by a post hoc Tukey’s HSD test, was used to determine differences in mortality and attraction percentages (p < 0.05). An independent-samples t-test was conducted to identify significant differences between host plants and post-treatment times at p < 0.05. Log–probit regression was used to calculate the lethal median time (LT50) based on mortality due to treatment with different concentrations of N. benthamiana ethanol-washed trichome solutions. All statistical analyses were conducted using the PROC ANOVA, PROC PROBIT, and PROC T-TEST procedures in SAS version 9.4 [26]. The data shown in tables represent the mean ± standard deviation of three replications for all experiments. All graphs were drawn using SigmaPlot 12.5.
3. Results
3.1. Effects of Nicotiana benthamiana on Attraction and Contact Toxicity to Bemisia tabaci Adults
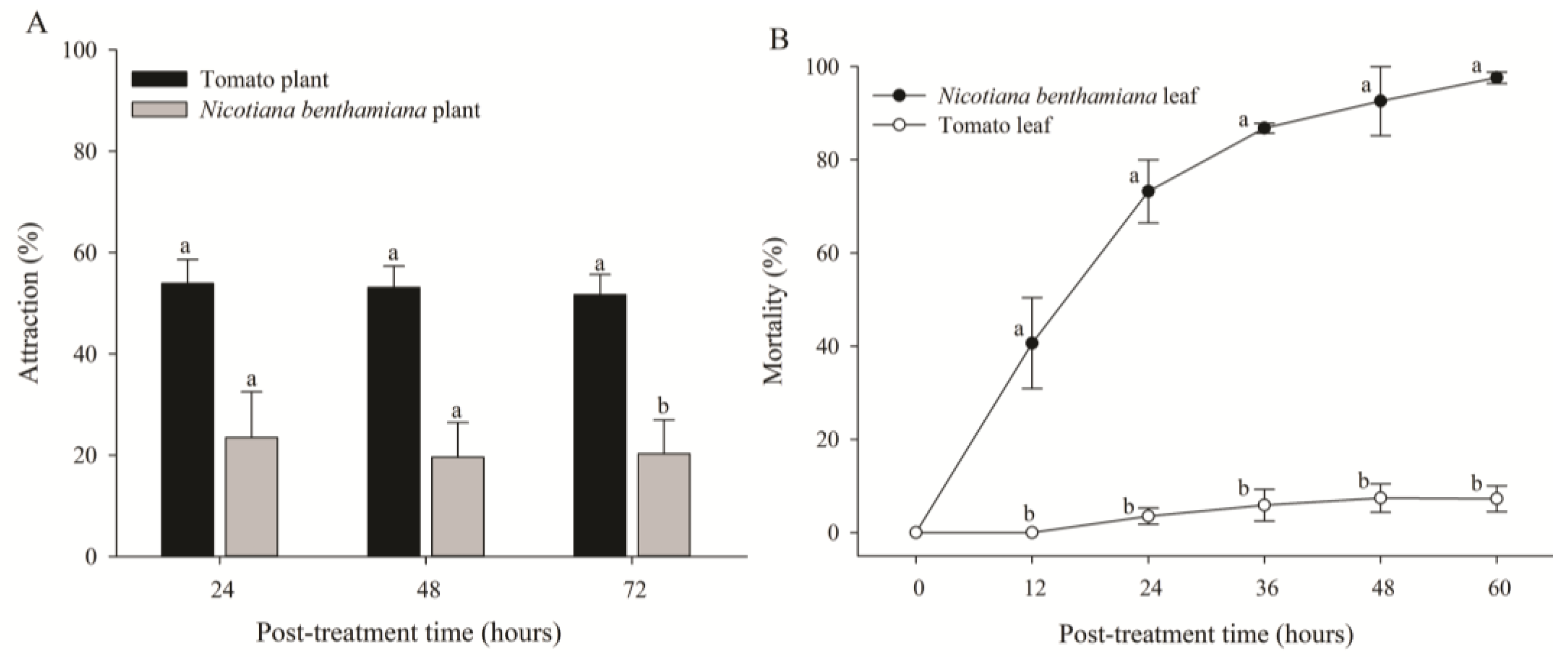
In the host preference test, a significant attraction rate was detected for all three post-treatment times (Figure 3A). The adults of B. tabaci primarily preferred tomato plants over N. benthamiana plants. Their attraction rates to tomato and N. benthamiana plants were 51.7% and 20.3%, respectively, at 72 h after treatment [t(4) = 4.040, p = 0.016] (Figure 3A). However, in the contact toxicity test, the mortality of B. tabaci in N. benthamiana plants gradually increased, with the highest mortality being 97.6% at 60 h after treatment, whereas the mortality of B. tabaci in tomato plants was 7.26% [t(4) = 29.706, p < 0.0001] (Figure 3B). Microscopic observation of N. benthamiana leaves revealed that most whiteflies were trapped in the sticky exudates of trichomes at the abaxial sides of leaves and eventually died.
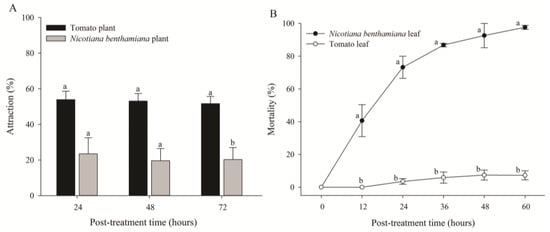
Figure 3.
Attraction rates (A) and mortality (B) of adult whiteflies reared on tomato and Nicotiana benthamiana plants. Attraction rate of whiteflies (n = 50) released into the cage containing both tomato and N. benthamiana plants. Mortality of adult whiteflies (n = 20) reared on a single leaf of different plants in the feeding chamber. Each point of the bars represents the mean ± standard error (SE) of three replications. Different letters at each point indicate statistically significant differences (p < 0.05) between the treatments at each post-treatment time.
3.2. Effects of Nicotiana benthamiana Trichome-Washed Solutions (TWSs) on Oral Toxicity to Bemisia tabaci Adults
To determine the source of the insecticidal toxicity of N. benthamiana, leaves were washed with either water or ethanol. The abaxial sides of washed leaves were observed under a microscope to confirm the absence of trichome exudates (Figure 1). Whiteflies were released into the cage containing fresh and trichome-washed plants. No significant differences were observed in the attraction rate of B. tabaci under the three conditions (nontreated, water-washed, and ethanol-washed) of N. benthamiana leaves over all post-treatment times (p > 0.05) (Figure 4A). However, the mortality rates of whiteflies in nontreated, water-washed, and ethanol-washed N. benthamiana leaves after 72 h of treatment were 97.7%, 33.3%, and 29.5%, respectively (Figure 4B). After a treatment period of 72 h, significant differences in mortality were noted between untreated leaves and leaves of N. benthamiana that had been washed with water or ethanol (F = 52.49; df = 2,6; p = 0.0002) (Figure 4B). Based on the probit analysis, the LT50 value recorded for fresh N. benthamiana leaves was 14.41 h, whereas that for ethanol-washed leaves was 126.11 h. Hence, this result indicates that most of the lethal toxicity of N. benthamiana leaves is retained in the trichome exudate.
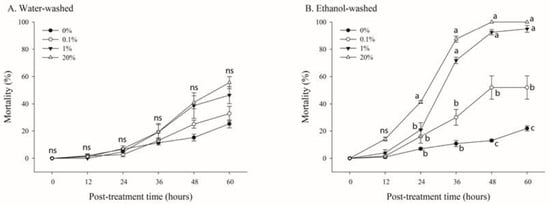
Figure 4.
Attraction and mortality rates of whiteflies on Nicotiana benthamiana leaves treated using different methods. Whiteflies (n = 70) were released into the cage containing fresh, water-, or ethanol-washed leaves of N. benthamiana. Attraction rate (A) and mortality (B) of whiteflies were determined over 72 h after treatment. Each point represents the mean ± standard error (SE) of three replications. Different letters at each point indicate statistically significant differences (p < 0.05) between the treatments at each post-treatment time; ns: not significant (p > 0.05).
To determine the oral toxicity of N. benthamiana extracts, B. tabaci adults were treated with 20% sugar solution including water- or ethanol-washed trichome exudate solutions at various concentrations (0%, 1%, 10%, and 20%) for 60 h in the parafilm feeding glass chamber (Figure 5).
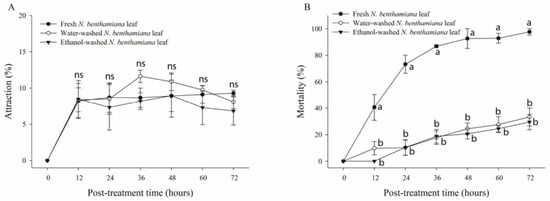
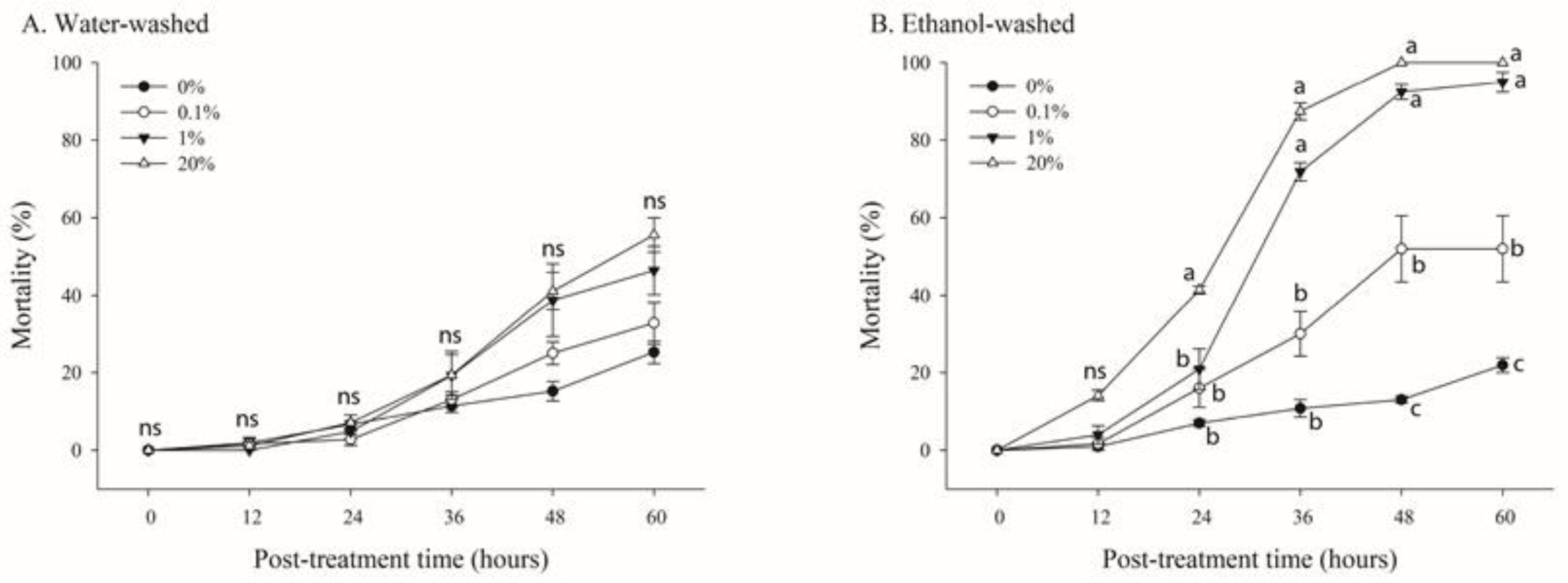
Figure 5.
Effects of various concentrations of trichome extracts from Nicotiana benthamiana leaves on the mortality of adult whiteflies. N. benthamiana leaves were rinsed with either water (A) or ethanol (B). B. tabaci adults (n = 20) were reared in the parafilm feeding chamber containing different concentrations of the extracts. Mortality was determined over 60 h. Each point of the bars represents the mean ± standard error (SE) of three replications. Different letters at each point indicate statistically significant differences (p < 0.05) between the treatments at each post-treatment time; ns: not significant (p > 0.05).
A concentration- and time-dependent mortality was observed under treatment with water- or ethanol-washed trichome exudate solutions (Figure 4). After 60 h of treatment, the mortality rates were 22.4%, 32.8%, 46.3%, and 55.5% at 0%, 1%, 10%, and 20%, respectively, for water-washed trichome exudate solutions (Figure 5A). Furthermore, significant mortality was observed between the treatments after 60 h of treatment (F = 66.87; df = 3,8; p < 0.0001) (Figure 5B). In contrast, the mortality rates of whiteflies in leaves treated with ethanol-washed trichome extracts diluted to 0%, 1%, 10%, and 20% were 22.4%, 52.0%, 94.9%, and 100%, respectively, after 60 h of treatment (Figure 5B). The LT50 values for 0.1%, 1%, 10%, and 20% concentrations of ethanol-washed trichome extracts were 159, 52.2, 29.4, and 22.2 h, respectively (Table 1).

Table 1.
LT50 values for various concentrations of Nicotiana benthamiana trichome ethanol-washed solutions on oral toxicity to Bemisia tabaci adults.
3.3. Effects of Trichome Exudate Powder (TEP) on Bemisia tabaci Adults
Two TEPs were prepared by drying the water- or ethanol-washed solutions. These two powders were diluted to 0.1%, 1%, and 10% concentrations in 20% sugar solution in the parafilm feeding chamber. The TEP of the ethanol-washed solution demonstrated higher oral toxicity than the TEP of the water-washed solution (Table 2). At 36 h of treatment, 1% ethanol-washed TEP caused 100% mortality, whereas 1% water-washed TEP caused 64.3% mortality (Table 1). The highest mortality (%) was recorded under treatment with water-washed TEP at 10% concentration after 48 h of treatment (Table 2).

Table 2.
Comparison of the effects of trichome exudate powder and commercial chemical pesticide following ingestion.
Comparison of the oral toxicity of the TEPs of water- or ethanol-washed solutions and imidacloprid (positive control) revealed no significant differences in mortality between imidacloprid and ethanol-washed TEP at 1% concentration (Table 2).
4. Discussion
This study demonstrated the potential insecticidal and behavioral activity of trichome exudates from N. benthamiana against B. tabaci adults. Our results showed that ethanol-washed trichome exudate exhibited potent insecticidal activity at 1% concentration, and the toxicity level was similar to that of the commercial pesticide imidacloprid.
Host-plant preference of herbivorous insects is dependent on various factors and is affected by the release of volatile substances from plants, such as trichome exudates. We observed that B. tabaci adults were less attracted to N. benthamiana plants than tomato plants, with the attraction rates being 20% and 50%, respectively. This may be caused by the repellent activity of the trichome exudates of N. benthamiana. Extensive research has shown that repellent activity against herbivorous insects occurs in several wild species of tomato and tobacco plants [26]. For instance, the wild tomatoes Solanum pennellii (Correll) D’Arcy and S. habrochaites S. Knapp and D.M. Spooner are preferred less by B. tabaci than cultivated tomato varieties. This difference is related to the production of volatile compounds by the glandular trichomes of wild tomato plants [27]. Similarly, the removal of sticky exudates from L. pennellii increases the feeding activity of the potato aphid Macrosiphum euphorbiae Thomas, whereas transfer of the exudates to L. lycopersicum reduces feeding activity, as measured via electronic feeding monitoring [28].
In the toxicity bioassay, the whiteflies that were attracted to N. benthamiana showed high mortality within a few days. Conversely, the whiteflies that showed attraction towards tomato plants did not exhibit any mortality. Moreover, the mortality significantly decreased in the washed leaves of N. benthamiana, suggesting that the lethal toxicity is caused by trichome exudates. In addition to the contact toxicity of N. benthamiana trichome exudates, this study demonstrated that these exudates exhibited oral toxicity to B. tabaci adults. The feeding experiments conducted utilizing a two-layered parafilm feeding chamber revealed that the trichome extracts, which were washed with either water or ethanol, demonstrated significant oral toxicity against B. tabaci adults. This toxicity was shown to be dependent on the concentration of the extracts. Additionally, it was observed that the oral toxicity was more acute when the trichome extracts were treated in solid powder form compared to the liquid form. This observation might be due to the greater concentration present in the solid form. Acyl sugars are insect-deterrent metabolites produced and exuded from the glandular trichomes of plants [23,28,29]. Acyl sugars can negatively affect the fitness of various insect pests by interfering with behaviors such as feeding and oviposition [30,31]. In a previous study, acyl sugar feeding assay revealed insecticidal effects against Spodoptera litura (Fabricius) larvae from the second to sixth stage and also showed decreased body weight in each larval and pupal stage [25].
Comparison of the toxicities of trichome exudate powders and the chemical pesticide imidacloprid revealed that the exudate powder exerted comparable effects with those of imidacloprid at the same dilutions in our experiment. The major substances of trichome exudates are sugar esters [18,32]. Several species in the Solanaceae family have glandular trichomes that secrete various forms of sugar esters [28]. The toxicity of these sugar esters varies according to the target insect species [18]. The mechanism of contact toxicity of sugar esters involves induction of desiccation by penetrating into the cuticle layer of soft-bodied insects, resulting in death [24]. However, the mechanism underlying the oral toxicity of these exudates remains largely unknown. Further studies are required to determine the internal targets of acyl sugars, which are the major substances of N. benthamiana trichome exudates. Synthetic sugar esters have been investigated for their insecticidal activity for commercial applications in pest control [24]. Recently, Wang et al. [25] revealed that acyl sugar not only killed herbivore insects like S. litura, but it also demonstrated transgenerational fitness losses. Acyl sugar shows efficacy in not only controlling sap-sucking insects but also herbivorous insects.
The use of biologically active natural compounds that possess insect antifeedant, repellent, and toxicant properties has become known as a new strategy in the field of agrochemical development [33]. The functions of plant glandular trichomes are used to avoid feeding damage from herbivores in the field. Glandular trichomes are responsible for the production and release of many secondary metabolites. These include acyl sugars, linalool, limonene (as monoterpenes), as well as eugenol, isoeugenol, and methyl eugenol (as phenylpropanoids). According to research conducted by Johnston et al. [34], it was shown that limonene exhibited a high level of efficacy in preventing whiteflies while also being a cost-effective and easily implementable method for controlling whitefly populations. In a separate investigation, He et al. [35] conducted research that highlighted the potent and persistent repellent properties of eugenol derivatives against red imported fire ants.
Overall, our findings suggest that the use of trichome extracts may have the potential to enhance plant defense mechanisms owing to their pesticidal properties, extensive availability, and low environmental persistence. The powder form of trichome exudates is an important alternative to chemical pesticides. This could represent a complementary and alternative strategy to control populations of insect pests.
5. Conclusions
The use of integrated pest management has been cited as an approach that effectively decreases crop losses while maintaining a low cost of operation. Compared to synthetic pesticides, plant-based insecticides are seen as being more environmentally friendly and cost-effective. Recently, biopesticides (natural products) have emerged as a better alternative for pest control [36,37,38,39], and acyl sugars, one of the products of glandular trichomes that secrete secondary metabolites, are repellent and toxic and can disturb the oviposition and feeding of insect pests. Our research has the potential to contribute to integrated pest management strategies aimed at controlling B. tabaci. Still, more research is needed to find out how trichome extracts at different concentrations might affect B. tabaci adults to stop them from laying eggs and how these effects might be passed down from generation to generation. Further investigation is being suggested to explore the effectiveness of trichome extracts in open greenhouse and field conditions.
Author Contributions
Conceptualization: S.R.S. and K.-Y.L.; methodology: S.R.S. and M.M.M.; software: M.M.M.; formal analysis: M.M.M.; investigation: S.R.S.; resources: K.-Y.L.; data curation: S.R.S. and M.M.M.; writing—original draft preparation: S.R.S. and M.M.M.; writing—review and editing: S.R.S., M.M.M. and K.-Y.L.; supervision: K.-Y.L.; project administration: K.-Y.L.; funding acquisition: K.-Y.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by a research fund (311058-05-3-CG000) from the Ministry for Food, Agriculture, Forestry, and Fisheries, and a research fund (NRF-2016R1A6A1A05011910) from the Basic Science Research Program through the National Research Foundation of Korea (NRF) and funded by the Ministry of Education.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors would like to thank Jae-Kyoung Shim from Kyungpook National University, Daegu, Korea, for providing helpful suggestions on the experimental design.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Boykin, L.M.; Shatters, R.G.; Rosell, R.C.; McKenzie, C.L.; Bagnall, R.A.; De Barro, P.; Frohlich, D.R. Global relationships of Bemisia tabaci (Hemiptera: Aleyrodidae) revealed using Bayesian analysis of mitochondrial COI DNA sequences. Mol. Phylogenet. Evol. 2007, 44, 1306–1319. [Google Scholar] [CrossRef] [PubMed]
- De Barro, P.J.; Liu, S.S.; Boykin, L.M.; Dinsdale, A.B. Bemisia tabaci: A statement of species status. Annu. Rev. Entomol. 2011, 56, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Kanakala, S.; Ghanim, M. Global genetic diversity and geographical distribution of Bemisia tabaci and its bacterial endosymbionts. PLoS ONE 2019, 14, e0213946. [Google Scholar] [CrossRef]
- De Marchi, B.R.; Gama, A.B.; Smith, H.A. Evidence of the association between the Q2 mitochondrial group of Bemisia tabaci MED species (Hemiptera: Aleyrodidae) and low competitive displacement capability. PLoS ONE 2023, 18, e0280002. [Google Scholar] [CrossRef]
- Oliveira, M.R.V.; Henneberry, T.J.; Anderson, P. History, current status, and collaborative research projects for Bemisia tabaci. Crop Prot. 2001, 20, 709–723. [Google Scholar] [CrossRef]
- Gelman, D.B.; Blackburn, M.B.; Hu, J.S. Identification of the molting hormone of the sweet potato (Bemisia tabaci) and greenhouse (Trialeurodes vaporariorum) whitefly. J. Insect Physiol. 2005, 51, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Perring, T.M.; Stansly, P.A.; Liu, T.X.; Smith, H.A.; Andreason, S.A. Whiteflies: Biology, Ecology, and Management. In Sustainable Management of Arthropod Pests of Tomato; Academic Press: Cambridge, MA, USA, 2017; pp. 73–110. ISBN 9780128024416. [Google Scholar]
- Jones, D.R. Plant viruses transmitted by thrips. Eur. J. Plant Pathol. 2005, 113, 119–157. [Google Scholar] [CrossRef]
- Polston, J.E.; De Barro, P.; Boykin, L.M. Transmission specificities of plant viruses with the newly identified species of the Bemisia tabaci species complex. Pest Manag. Sci. 2014, 70, 1547–1552. [Google Scholar] [CrossRef]
- Horowitz, A.R.; Kontsedalov, S.; Khasdan, V.; Ishaaya, I. Biotypes B and Q of Bemisia tabaci and their relevance to neonicotinoid and pyriproxyfen resistance. In Proceedings of the Archives of Insect Biochemistry and Physiology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2005; Volume 58, pp. 216–225. [Google Scholar]
- Watanabe, L.F.M.; Bello, V.H.; De Marchi, B.R.; da Silva, F.B.; Fusco, L.M.; Sartori, M.M.P.; Pavan, M.A.; Krause-Sakate, R. Performance and competitive displacement of Bemisia tabaci MEAM1 and MED cryptic species on different host plants. Crop Prot. 2019, 124, 104860. [Google Scholar] [CrossRef]
- Ally, H.M.; El Hamss, H.; Simiand, C.; Maruthi, M.N.; Colvin, J.; Delatte, H. Genetic diversity, distribution, and structure of Bemisia tabaci whitefly species in potential invasion and hybridization regions of East Africa. PLoS ONE 2023, 18, e0285967. [Google Scholar] [CrossRef]
- Khalid, M.Z.; Ahmed, S.; Al-Ashkar, I.; Sabagh, A.E.L.; Liu, L.; Zhong, G. Evaluation of resistance development in Bemisia tabaci genn. (homoptera: Aleyrodidae) in cotton against different insecticides. Insects 2021, 12, 996. [Google Scholar] [CrossRef] [PubMed]
- Basij, M.; Talebi, K.; Ghadamyari, M.; Hosseininaveh, V.; Salami, S.A. Status of Resistance of Bemisia tabaci (Hemiptera: Aleyrodidae) to Neonicotinoids in Iran and Detoxification by Cytochrome P450-Dependent Monooxygenases. Neotrop. Entomol. 2017, 46, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Johnson, Z.; Kaur, I.; Castillo, F.; Kariyat, R.; Bandyopadhyay, D. Aloe barbadensis rinds employ physical and chemical defense mechanisms against insect herbivores with varying success. Ind. Crops Prod. 2023, 194, 116347. [Google Scholar] [CrossRef]
- Wang, X.; Shen, C.; Meng, P.; Tan, G.; Lv, L. Analysis and review of trichomes in plants. BMC Plant Biol. 2021, 21, 70. [Google Scholar] [CrossRef] [PubMed]
- Wagner, G.J.; Wang, E.; Shepherd, R.W. New approaches for studying and exploiting an old protuberance, the plant trichome. Ann. Bot. 2004, 93, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Slocombe, S.P.; Schauvinhold, I.; McQuinn, R.P.; Besser, K.; Welsby, N.A.; Harper, A.; Aziz, N.; Li, Y.; Larson, T.R.; Giovannoni, J.; et al. Transcriptomic and reverse genetic analyses of branched-chain fatty acid and acyl sugar production in Solanum pennellii and Nicotiana benthamiana. Plant Physiol. 2008, 148, 1830–1846. [Google Scholar] [CrossRef] [PubMed]
- Muigai, S.G.; Schuster, D.J.; Snyder, J.C.; Scott, J.W.; Bassett, M.J.; McAuslane, H.J. Mechanisms of resistance in Lycopersicon germplasm to the whitefly Bemisia argentifolii. Phytoparasitica 2002, 30, 347–360. [Google Scholar] [CrossRef]
- Kroumova, A.B.; Wagner, G.J. Different elongation pathways in the biosynthesis of acyl groups of trichome exudate sugar esters from various solanaceous plants. Planta 2003, 216, 1013–1021. [Google Scholar] [CrossRef]
- Uzelac, B.; Stojičić, D.; Budimir, S. Glandular trichomes on the leaves of Nicotiana tabacum: Morphology, developmental ultrastructure, and secondary metabolites. In Reference Series in Phytochemistry; Springer Science and Business Media B.V.: Cham, Switzerland, 2019; pp. 1–37. [Google Scholar]
- Simmons, A.T.; Gurr, G.M.; McGrath, D.; Martin, P.M.; Nicol, H.I. Entrapment of Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae) on glandular trichomes of Lycopersicon species. Aust. J. Entomol. 2004, 43, 196–200. [Google Scholar] [CrossRef]
- Goffreda, J.C.; Mutschler, M.A.; Tingey, W.M. Feeding behavior of potato aphid affected by glandular trichomes of wild tomato. Entomol. Exp. Appl. 1988, 48, 101–107. [Google Scholar] [CrossRef]
- Puterka, G.J.; Farone, W.; Palmer, T.; Barrington, A. Structure-Function Relationships Affecting the Insecticidal and Miticidal Activity of Sugar Esters. J. Econ. Entomol. 2003, 96, 636–644. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Gao, B.; Zhang, Q.; Zhang, Z.; Li, Y.; Yang, Q.; Zhang, M.; Li, W.; Luo, C. Acylsugar protection of Nicotiana benthamiana confers mortality and transgenerational fitness costs in Spodoptera litura. Front. Plant Sci. 2022, 13, 993279. [Google Scholar] [CrossRef] [PubMed]
- SAS Institute Inc. Base SAS 9.4 Procedures Guide: High-Performance Procedures, 6th ed.; SAS Institute Inc.: Cary, NC, USA, 2016. [Google Scholar]
- Simmons, A.T.; Gurr, G.M. Trichomes of Lycopersicon species and their hybrids: Effects on pests and natural enemies. Agric. For. Entomol. 2005, 7, 265–276. [Google Scholar] [CrossRef]
- Goffreda, J.C.; Mutschler, M.A.; Avé, D.A.; Tingey, W.M.; Steffens, J.C. Aphid deterrence by glucose esters in glandular trichome exudate of the wild tomato, Lycopersicon pennellii. J. Chem. Ecol. 1989, 15, 2135–2147. [Google Scholar] [CrossRef] [PubMed]
- Wagner, G.J. Secreting glandular trichomes: More than just hairs. Plant Physiol. 1991, 96, 675–679. [Google Scholar] [CrossRef] [PubMed]
- Simmons, A.T.; Gurr, G.M.; McGrath, D.; Nicol, H.I.; Martin, P.M. Trichomes of Lycopersicon spp. and their effect on Myzus persicae (Sulzer) (Hemiptera: Aphididae). Aust. J. Entomol. 2003, 42, 373–378. [Google Scholar] [CrossRef]
- Vilela De Resende, J.T.; Maluf, W.R.; Faria, M.V.; Pfann, A.Z.; Rodrigues Do Nascimento, I. Acylsugars in tomato leaflets confer resistance to the South American tomato pinworm, Tuta absoluta Meyr. Sci. Agric. 2006, 63, 20–25. [Google Scholar] [CrossRef]
- Arrendale, R.F.; Severson, R.F.; Sisson, V.A.; Costello, C.E.; Leary, J.A.; Himmelsbach, D.S.; van Halbeek, H.; Sisson, V.A.; Costello, C.E.; Leary, J.A.; et al. Characterization of the Sucrose Ester Fraction from Nicotiana glutinosa. J. Agric. Food Chem. 1990, 38, 75–85. [Google Scholar] [CrossRef]
- Morimoto, M. Chemical defense against insects in Heterotheca subaxillaris and three Orobanchaceae species using exudates from trichomes. Pest Manag. Sci. 2019, 75, 2474–2481. [Google Scholar] [CrossRef]
- Johnston, N.; Paris, T.; Paret, M.L.; Freeman, J.; Martini, X. Repelling whitefly (Bemisia tabaci) using limonene-scented kaolin: A novel pest management strategy. Crop Prot. 2022, 154, 105905. [Google Scholar] [CrossRef]
- He, Y.; Zhang, J.; Shen, L.; Wang, L.; Qian, C.; Lyu, H.; Yi, C.; Cai, J.; Chen, X.; Wen, X.; et al. Eugenol derivatives: Strong and long-lasting repellents against both undisturbed and disturbed red imported fire ants. J. Pest Sci. (2004) 2023, 96, 327–344. [Google Scholar] [CrossRef]
- Mostafiz, M.M.; Jhan, P.K.; Shim, J.-K.; Lee, K.-Y. Methyl benzoate exhibits insecticidal and repellent activities against Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae). PLoS ONE 2018, 13, e0208552. [Google Scholar] [CrossRef] [PubMed]
- Mostafiz, M.M.; Shim, J.-K.; Hwang, H.-S.; Bunch, H.; Lee, K.-Y. Acaricidal effects of methyl benzoate against Tetranychus urticae Koch (Acari: Tetranychidae) on common crop plants. Pest Manag. Sci. 2020, 76, 2347–2354. [Google Scholar] [CrossRef] [PubMed]
- Mostafiz, M.M.; Hassan, E.; Shim, J.K.; Lee, K.Y. Insecticidal efficacy of three benzoate derivatives against Aphis gossypii and its predator Chrysoperla carnea. Ecotoxicol. Environ. Saf. 2019, 184, 109653. [Google Scholar] [CrossRef]
- Mostafiz, M.M.; Hassan, E.; Lee, K.Y. Methyl Benzoate as a Promising, Environmentally Safe Insecticide: Current Status and Future Perspectives. Agriculture 2022, 12, 378. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).