Scanning Electron Microscopy Analysis of Lymphatic Regeneration in a Secondary Lymphedema Mouse Model: A Preliminary Study
Abstract
:1. Introduction
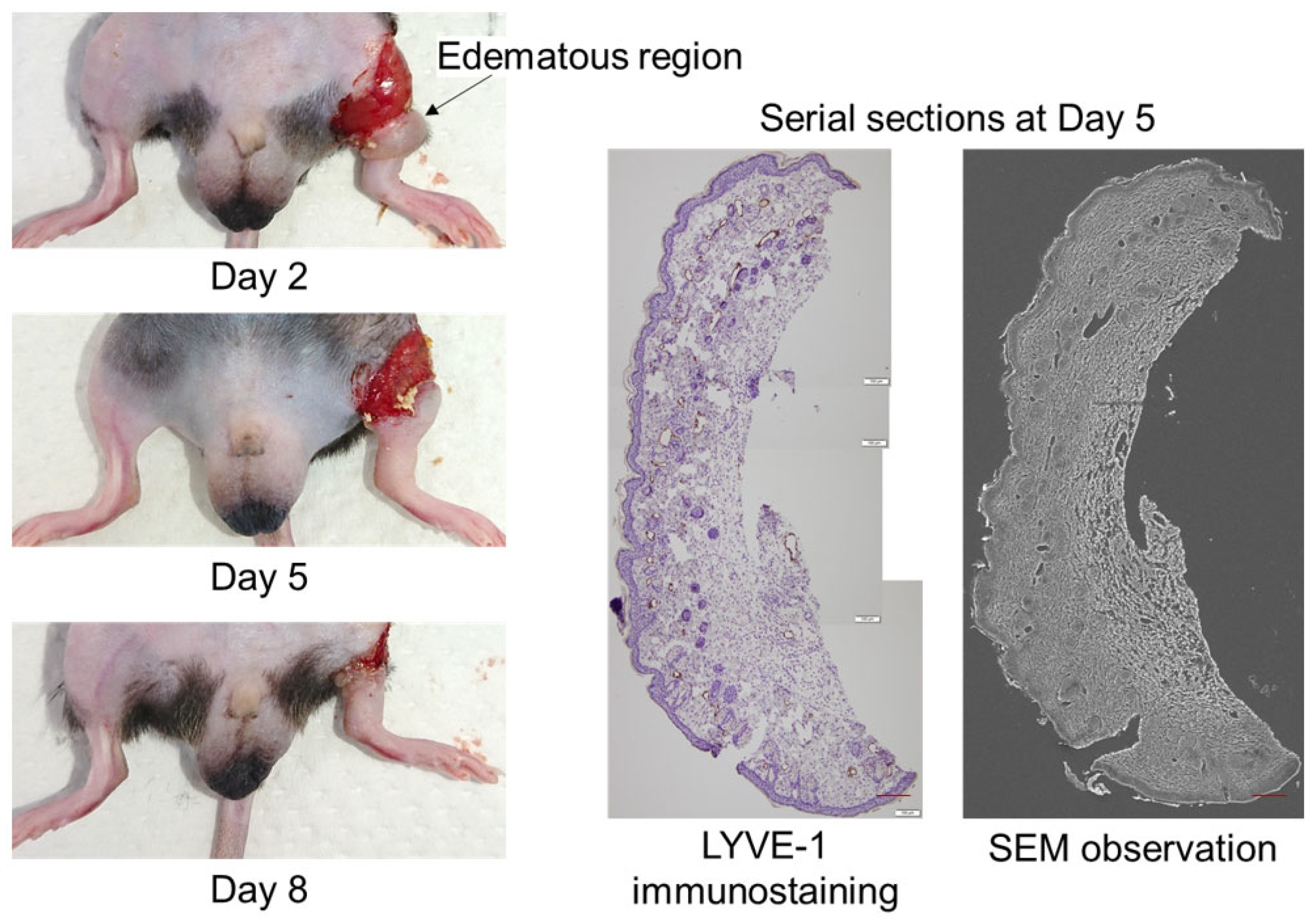
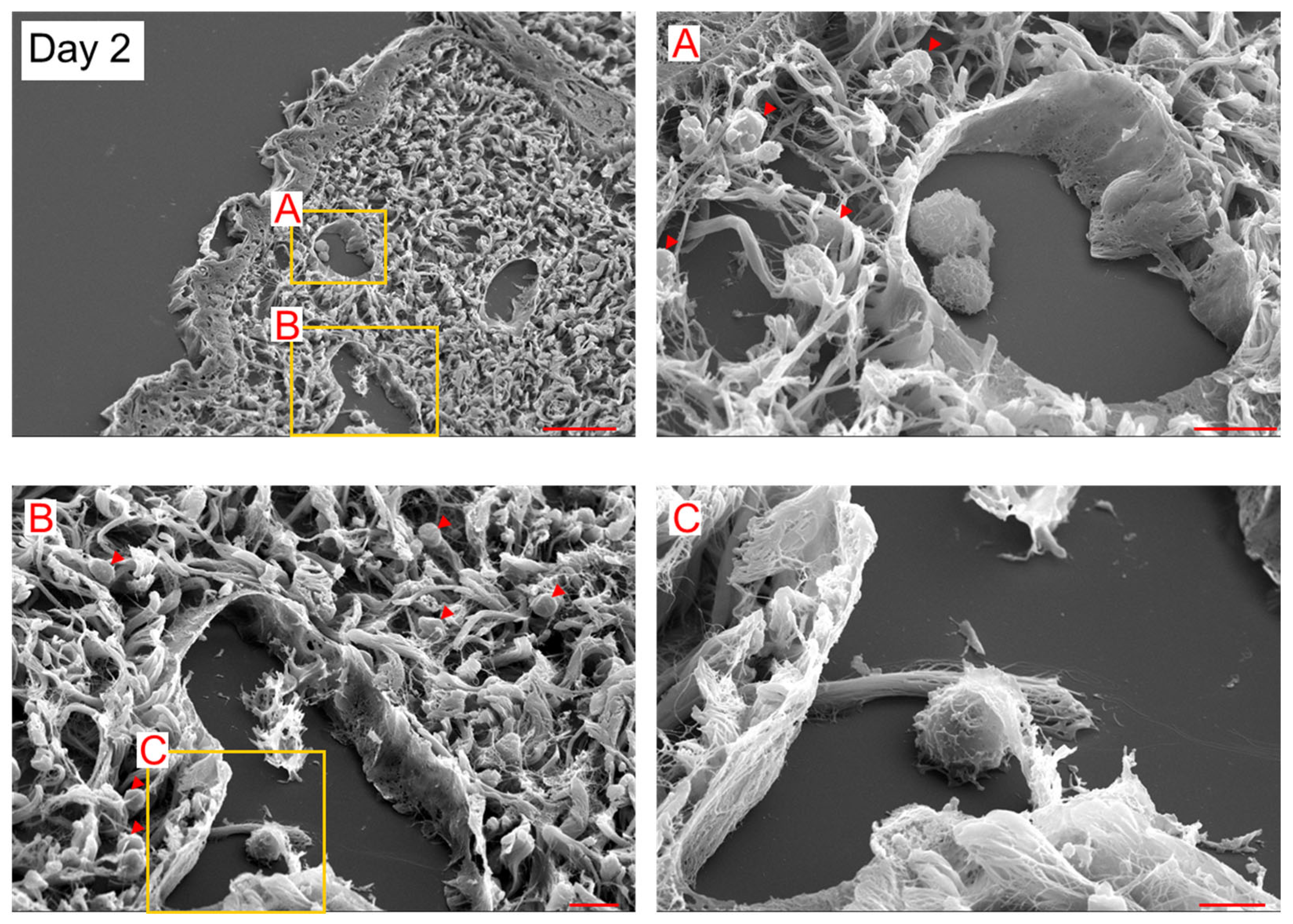
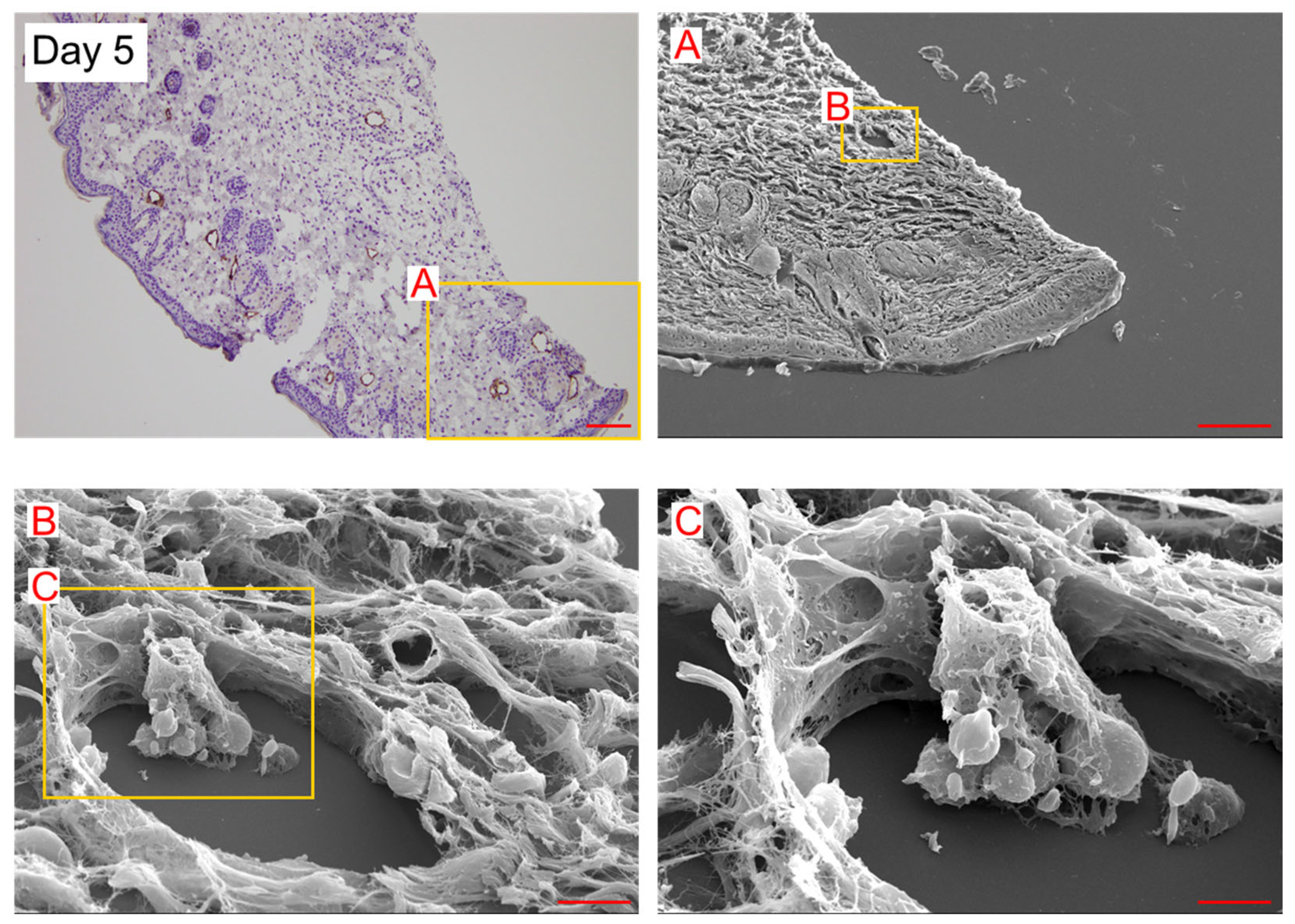
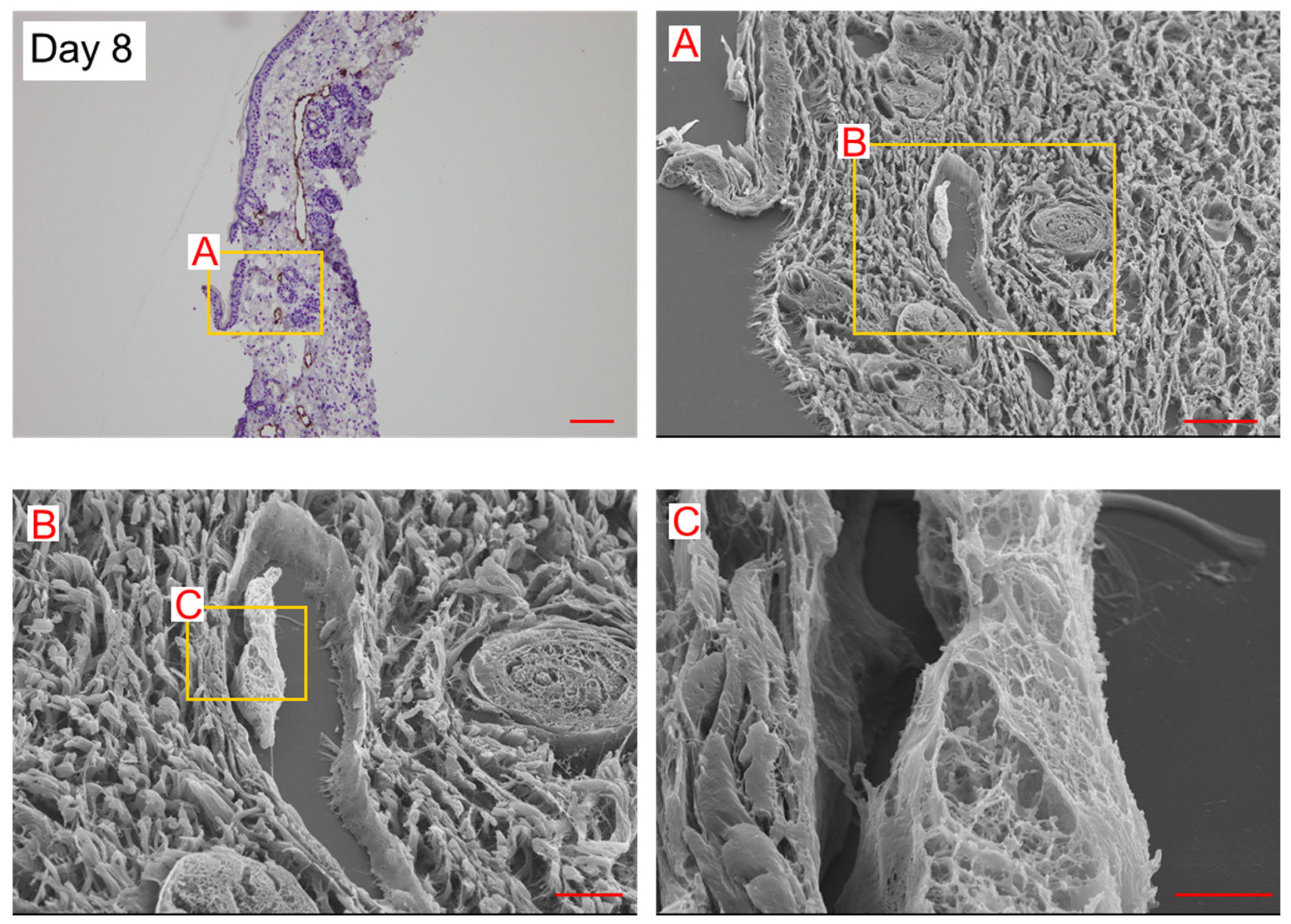
2. Results
3. Discussion
4. Materials and Methods
4.1. Surgical Procedure
4.2. Observation of Lymphangiogenesis Using a Combination Method of SEM and LYVE-1 Immunoreactivity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahmadzadeh, N.; Robering, J.W.; Kengelbach-Weigand, A.; Al-Abboodi, M.; Beier, J.P.; Horch, R.E.; Boos, A.M. Human adipose-derived stem cells support lymphangiogenesis in vitro by secretion of lymphangiogenic factors. Exp. Cell Res. 2020, 388, 111816. [Google Scholar] [CrossRef]
- Tammela, T.; Alitalo, K. Lymphangiogenesis: Molecular mechanisms and future promise. Cell 2010, 140, 460–476. [Google Scholar] [CrossRef]
- Volk-Draper, L.D.; Hall, K.L.; Wilber, A.C.; Ran, S. Lymphatic endothelial progenitors originate from plastic myeloid cells activated by toll-like receptor-4. PLoS ONE 2017, 12, e0179257. [Google Scholar] [CrossRef]
- Ji, R.C. Macrophages are important mediators of either tumor- or inflammation-induced lymphangiogenesis. Cell. Mol. Life Sci. 2012, 69, 897–914. [Google Scholar] [CrossRef]
- Ding, M.; Fu, X.; Tan, H.; Wang, R.; Chen, Z.; Ding, S. The effect of vascular endothelial growth factor C expression in tumor-associated macrophages on lymphangiogenesis and lymphatic metastasis in breast cancer. Mol. Med. Rep. 2012, 6, 1023–1029. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, K.; Ii, M.; Cursiefen, C.; Jackson, D.G.; Keino, H.; Tomita, M.; Van Rooijen, N.; Takenaka, H.; D’Amore, P.A.; Stein-Streilein, J.; et al. Inflammation-induced lymphangiogenesis in the cornea arises from CD11b-positive macrophages. J. Clin. Investig. 2005, 115, 2363–2372. [Google Scholar] [CrossRef] [PubMed]
- Zumsteg, A.; Baeriswyl, V.; Imaizumi, N.; Schwendener, R.; Rüegg, C.; Christofori, G. Myeloid cells contribute to tumor lymphangiogenesis. PLoS ONE 2009, 4, e7067. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Park, C.; Cho, Y.P.; Lee, E.; Kim, H.; Kim, P.; Yun, S.H.; Yoon, Y.S. Podoplanin-expressing cells derived from bone marrow play a crucial role in postnatal lymphatic neovascularization. Circulation 2010, 122, 1413–1425. [Google Scholar] [CrossRef] [PubMed]
- Kerjaschki, D.; Huttary, N.; Raab, I.; Regele, H.; Bojarski-Nagy, K.; Bartel, G.; Kröber, S.M.; Greinix, H.; Rosenmaier, A.; Karlhofer, F.; et al. Lymphatic endothelial progenitor cells contribute to de novo lymphangiogenesis in human renal transplants. Nat. Med. 2006, 12, 230–234. [Google Scholar] [CrossRef]
- Religa, P.; Cao, R.; Bjorndahl, M.; Zhou, Z.; Zhu, Z.; Cao, Y. Presence of bone marrow-derived circulating progenitor endothelial cells in the newly formed lymphatic vessels. Blood 2005, 106, 4184–4190. [Google Scholar] [CrossRef]
- Hall, K.L.; Volk-Draper, L.D.; Flister, M.J.; Ran, S. New model of macrophage acquisition of the lymphatic endothelial phenotype. PLoS ONE 2012, 7, e31794. [Google Scholar] [CrossRef]
- Espinosa Gonzalez, M.; Volk-Draper, L.; Bhattarai, N.; Wilber, A.; Ran, S. Th2 cytokines IL-4, IL-13, and IL-10 promote differentiation of pro-lymphatic progenitors derived from bone marrow myeloid precursors. Stem Cells Dev. 2022, 31, 322–333. [Google Scholar] [CrossRef]
- Ogino, R.; Hayashida, K.; Yamakawa, S.; Morita, E. Adipose-derived stem cells promote intussusceptive lymphangiogenesis by restricting dermal fibrosis in irradiated tissue of mice. Int. J. Mol. Sci. 2020, 21, 3885. [Google Scholar] [CrossRef]
- Patan, S.; Tanda, S.; Roberge, S.; Jones, R.C.; Jain, R.K.; Munn, L.L. Vascular morphogenesis and remodeling in a human tumor xenograft: Blood vessel formation and growth after ovariectomy and tumor implantation. Circ. Res. 2001, 89, 732–739. [Google Scholar] [CrossRef] [PubMed]
- Rossi-Schneider, T.R.; Verli, F.D.; Marinho, S.A.; Yurgel, L.S.; De Souza, M.A. Study of intussusceptive angiogenesis in inflammatory regional lymph nodes by scanning electron microscopy. Microsc. Res. Tech. 2010, 73, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Flores, L.; Gutiérrez, R.; Gayoso, S.; García, M.P.; González-Gómez, M.; Díaz-Flores, L., Jr.; Sánchez, R.; Carrasco, J.L.; Madrid, J.F. Intussusceptive angiogenesis and its counterpart intussusceptive lymphangiogenesis. Histol. Histopathol. 2020, 35, 1083–1103. [Google Scholar] [PubMed]
- Burri, P.H.; Hlushchuk, R.; Djonov, V. Intussusceptive angiogenesis: Its emergence, its characteristics, and its significance. Dev. Dyn. 2004, 231, 474–488. [Google Scholar] [CrossRef] [PubMed]
- Hayashida, K.; Yoshida, S.; Yoshimoto, H.; Fujioka, M.; Saijo, H.; Migita, K.; Kumaya, M.; Akita, S. Adipose-derived stem cells and vascularized lymph node transfers successfully treat mouse hindlimb secondary lymphedema by early reconnection of the lymphatic system and lymphangiogenesis. Plast. Reconstr. Surg. 2017, 139, 639–651. [Google Scholar] [CrossRef] [PubMed]
- Ogino, R.; Yokooji, T.; Hayashida, M.; Suda, S.; Yamakawa, S.; Hayashida, K. Emerging anti-inflammatory pharmacotherapy and cell-based therapy for lymphedema. Int. J. Mol. Sci. 2022, 23, 7614. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hayashida, K.; Ogino, R.; Suda, S.; Yamakawa, S. Scanning Electron Microscopy Analysis of Lymphatic Regeneration in a Secondary Lymphedema Mouse Model: A Preliminary Study. Lymphatics 2023, 1, 237-243. https://doi.org/10.3390/lymphatics1030014
Hayashida K, Ogino R, Suda S, Yamakawa S. Scanning Electron Microscopy Analysis of Lymphatic Regeneration in a Secondary Lymphedema Mouse Model: A Preliminary Study. Lymphatics. 2023; 1(3):237-243. https://doi.org/10.3390/lymphatics1030014
Chicago/Turabian StyleHayashida, Kenji, Ryohei Ogino, Shota Suda, and Sho Yamakawa. 2023. "Scanning Electron Microscopy Analysis of Lymphatic Regeneration in a Secondary Lymphedema Mouse Model: A Preliminary Study" Lymphatics 1, no. 3: 237-243. https://doi.org/10.3390/lymphatics1030014
APA StyleHayashida, K., Ogino, R., Suda, S., & Yamakawa, S. (2023). Scanning Electron Microscopy Analysis of Lymphatic Regeneration in a Secondary Lymphedema Mouse Model: A Preliminary Study. Lymphatics, 1(3), 237-243. https://doi.org/10.3390/lymphatics1030014





