Advancements in Multiple Myeloma Therapies: A Comprehensive Review by Disease Stage
Abstract
:1. Introduction
2. Early-Stage Myeloma
2.1. Smoldering Multiple Myeloma
2.2. Low-Risk SMM
2.3. High-Risk SMM
2.4. Non-Secretory Multiple Myeloma
3. Advanced-Stage Myeloma
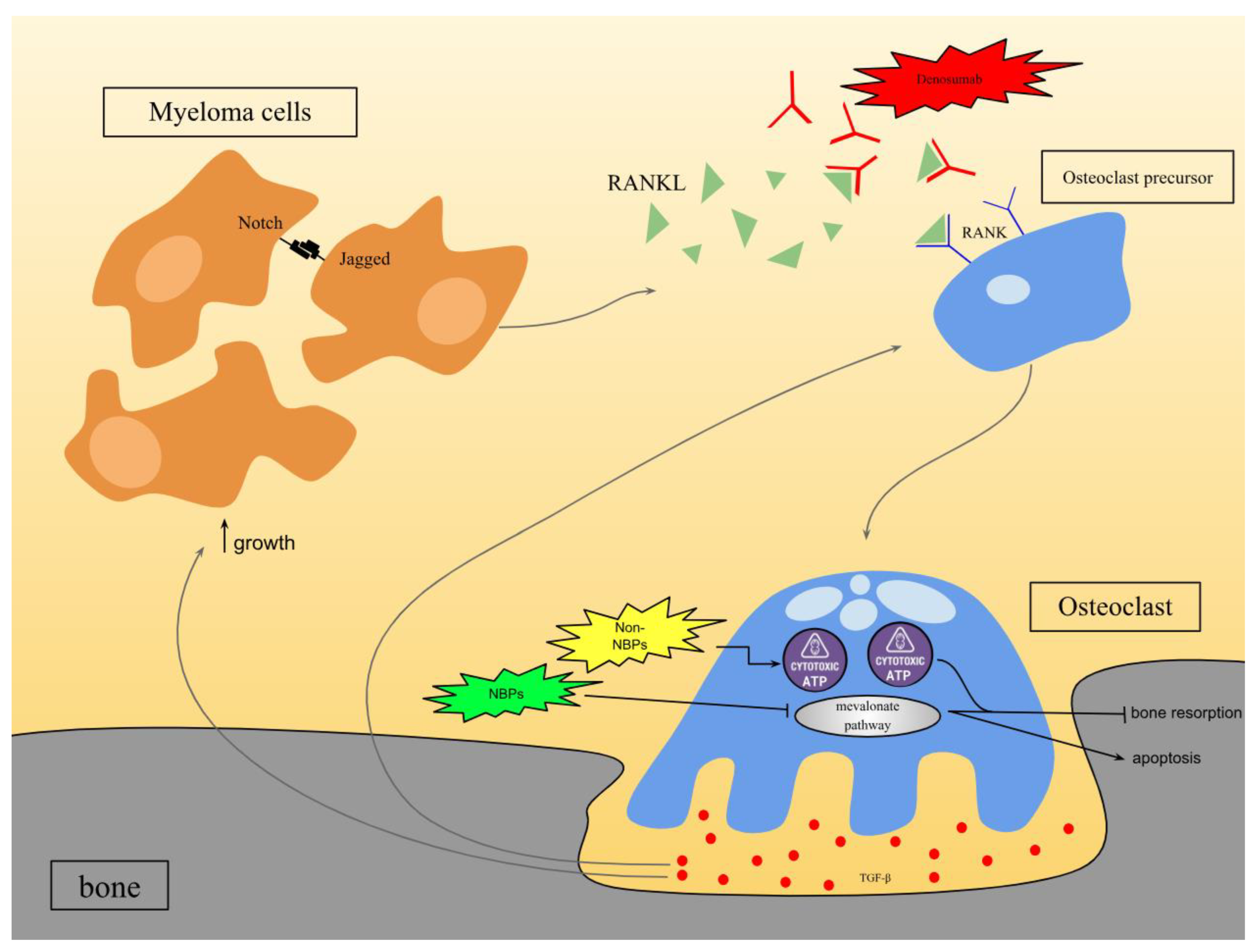
3.1. Bone-Modifying Drugs
3.1.1. Bisphosphonates
3.1.2. Denosumab
3.2. Targeted Therapy
3.2.1. Proteasome Inhibitors
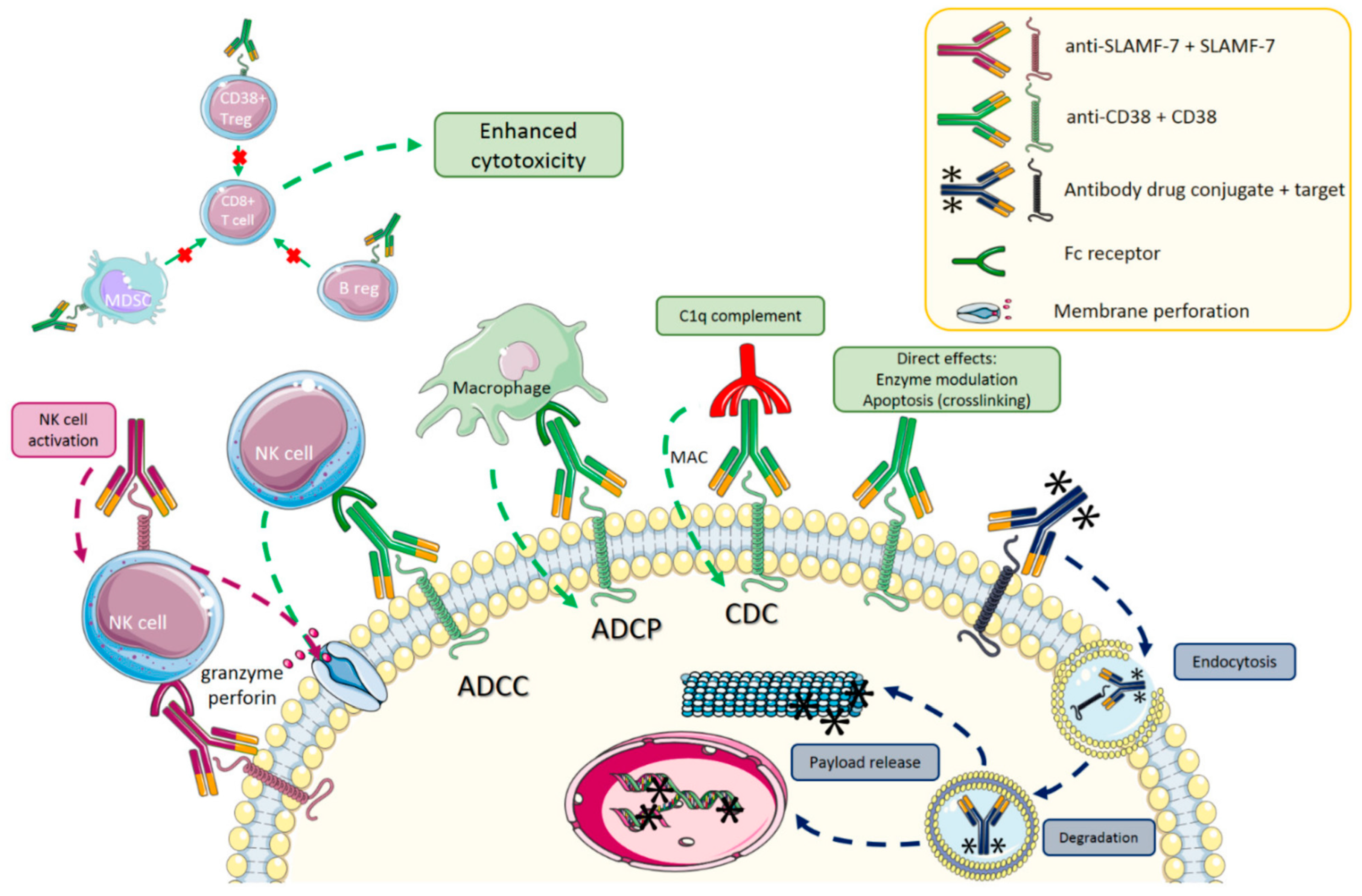
3.2.2. Monoclonal Antibodies
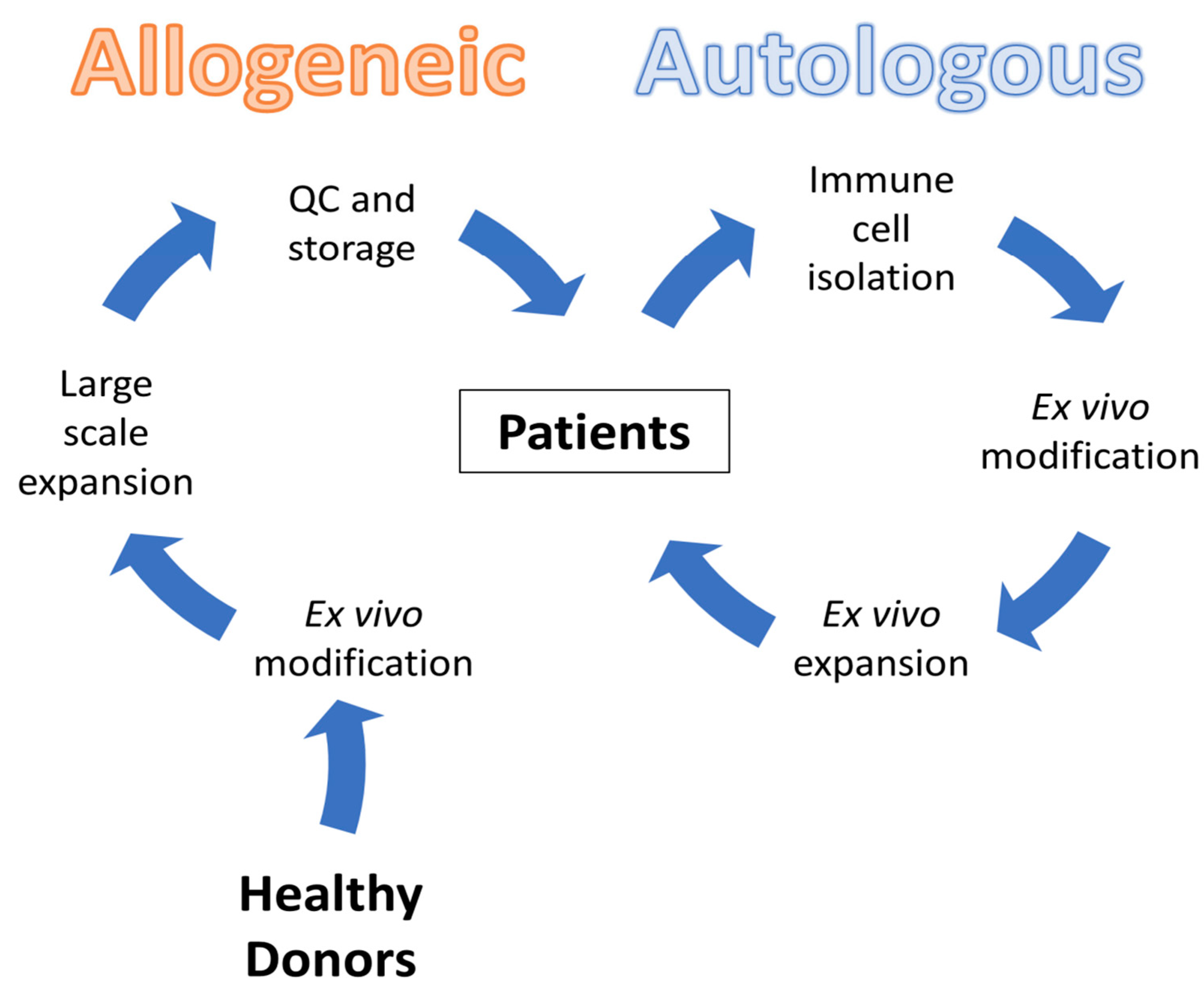
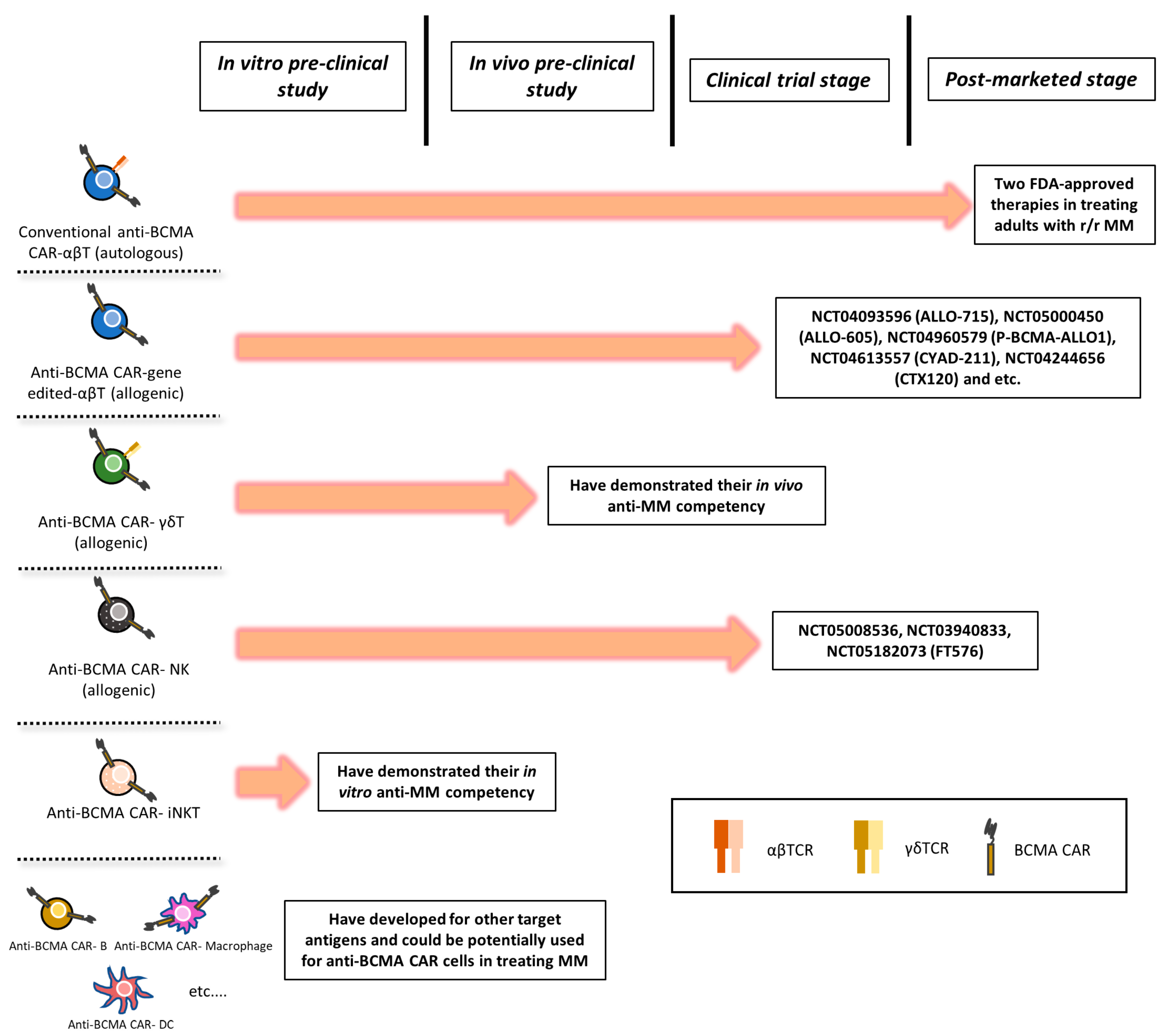
3.3. CAR-T Therapy
3.3.1. ALLO-715 Therapy
3.3.2. Limitations and Challenges
4. Relapse
4.1. Novel Therapies in Relapsed MM
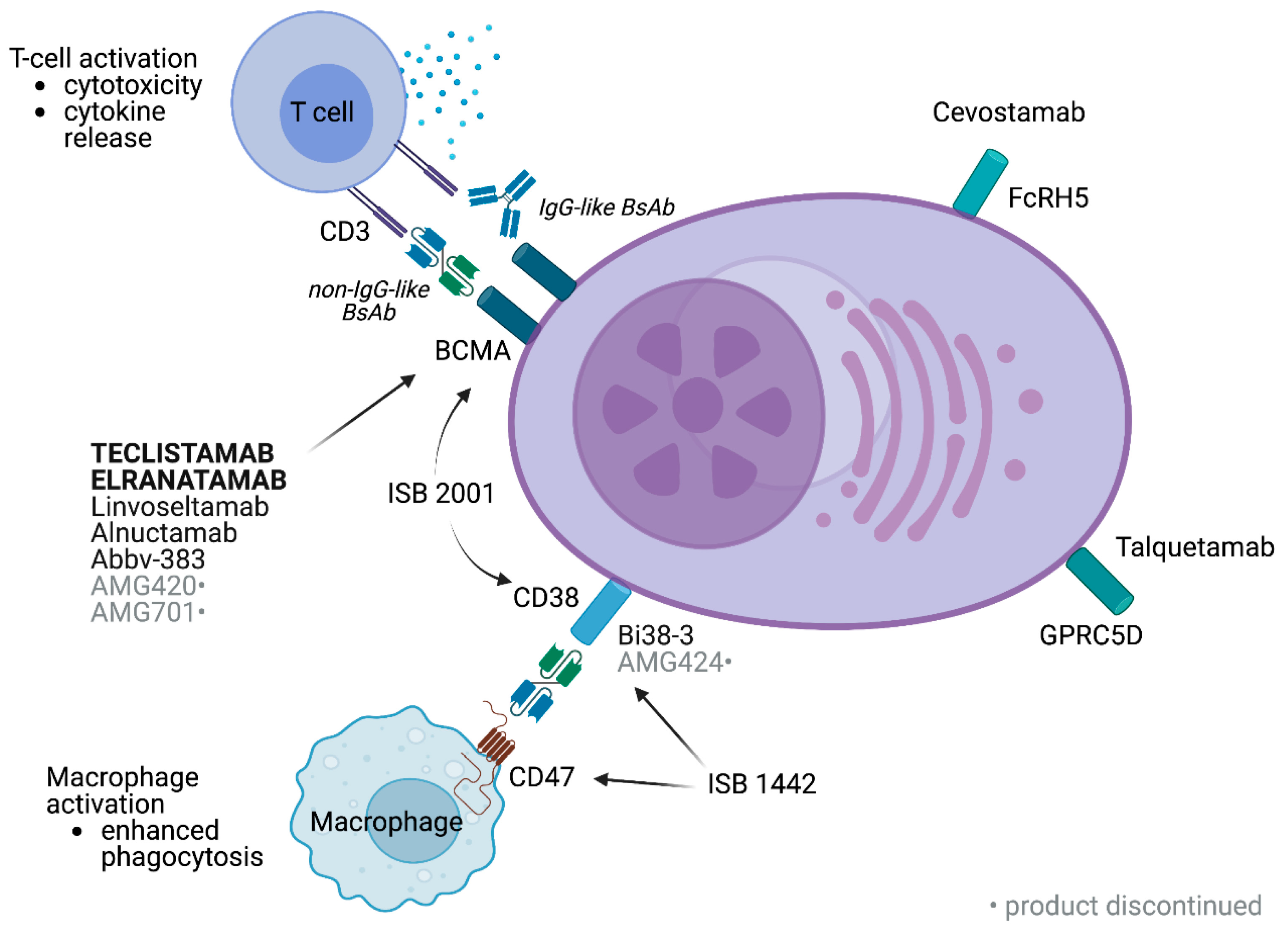
4.1.1. Bispecific Antibodies (BsAbs)
4.1.2. Immunocytokine Therapy
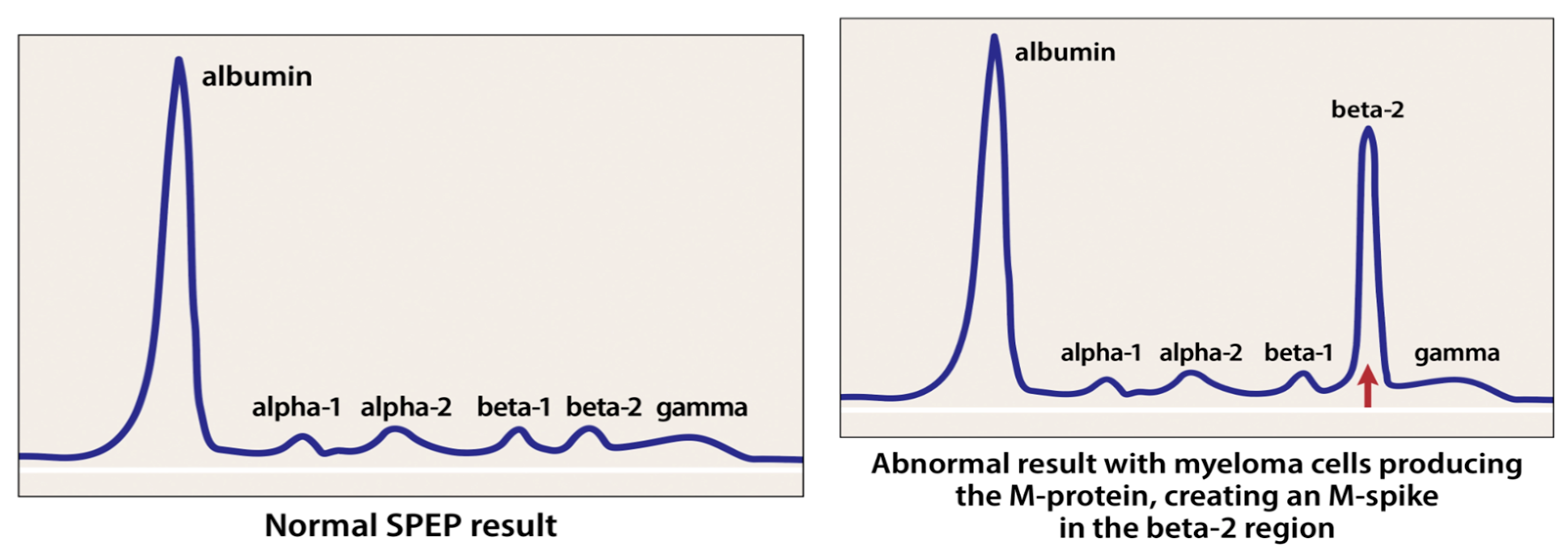
4.2. Mass Spectrometry in Detecting M-Proteins
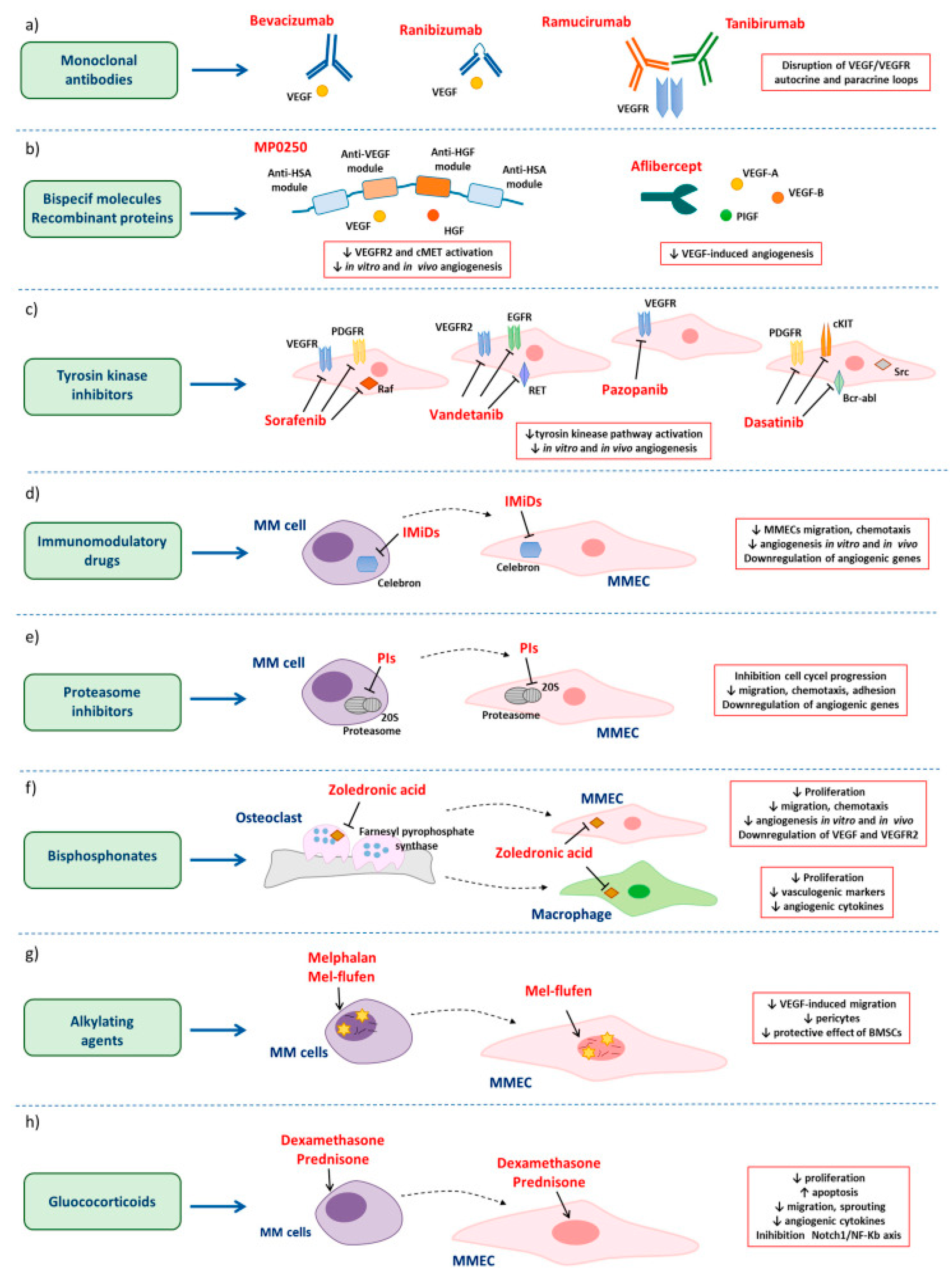
4.3. Angiogenesis and Its Effect on MM Progression
5. Comparative Discussion on the Safety Profiles of the Novel Therapies
Funding
Conflicts of Interest
References
- Rajkumar, S.V.; Kumar, S. Multiple myeloma current treatment algorithms. Blood Cancer J. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Gerecke, C.; Fuhrmann, S.; Strifler, S.; Schmidt-Hieber, M.; Einsele, H.; Knop, S. The Diagnosis and Treatment of Multiple Myeloma. Dtsch. Aerzteblatt Online 2016, 113, 470–476. [Google Scholar] [CrossRef]
- Hatjiharissi, P. The progress in multiple myeloma. Hell. J. Nucl. Med. 2023, 26, 30–35. [Google Scholar] [PubMed]
- Giuliani, N.; Storti, P.; Bolzoni, M.; Palma, B.D.; Bonomini, S. Angiogenesis and Multiple Myeloma. Cancer Microenviron. 2011, 4, 325. [Google Scholar] [CrossRef] [PubMed]
- San Miguel, J. Multiple myeloma: A model for scientific and clinical progress. Hematology 2014, 2014, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Killeen, R.B. Relapsed and Refractory Multiple Myeloma. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK592405/ (accessed on 8 June 2023).
- Dadzie, T.G.; Green, A.C. The role of the bone microenvironment in regulating myeloma residual disease and treatment. Front. Oncol. 2022, 12, 999939. [Google Scholar] [CrossRef]
- Kaseb, H.; Annamaraju, P.; Babiker, H.M. Monoclonal Gammopathy of Undetermined Significance (MGUS); PubMed; StatPearls Publishing: Treasure Island, FL, USA, 2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK507880/ (accessed on 10 July 2022).
- Fonseca, R.; Gonzalez-Velez, M. Treatment of Smoldering Multiple Myeloma: Expectant Observation Should Still Be the Standard. Am. Soc. Clin. Oncol. Educ. Book/Educ. Book 2020, 40, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Mateos, M.-V.; Hernandez, M.T.; Giraldo, P.; de la Rubia, J.; de Arriba, F.; Corral, L.L.; Rosiñol, L.; Paiva, B.; Palomera, L.; Bargay, J.; et al. Lenalidomide plus dexamethasone versus observation in patients with high-risk smouldering multiple myeloma (QuiRedex): Long-term follow-up of a randomised, controlled, phase 3 trial. Lancet Oncol. 2016, 17, 1127–1136. [Google Scholar] [CrossRef]
- Rajkumar, S.V.; Mesa, R.A.; Fonseca, R.; Schroeder, G.; Plevak, M.F.; Dispenzieri, A.; Lacy, M.Q.; Lust, J.A.; Witzig, T.E.; Gertz, M.A.; et al. Bone Marrow Angiogenesis in 400 Patients with Monoclonal Gammopathy of Undetermined Significance, Multiple Myeloma, and Primary Amyloidosis1. Clin. Cancer Res. 2002, 8, 2210–2216. [Google Scholar]
- Rajkumar, S.V.; Dimopoulos, M.A.; Palumbo, A.; Blade, J.; Merlini, G.; Mateos, M.-V.; Kumar, S.; Hillengass, J.; Kastritis, E.; Richardson, P.; et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014, 15, e538–e548. [Google Scholar] [CrossRef]
- Kapoor, P.; Rajkumar, S.V. Smoldering Multiple Myeloma. Cancer J. 2019, 25, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Hillengass, J.; Fechtner, K.; Weber, M.-A.; Bäuerle, T.; Ayyaz, S.; Heiss, C.; Hielscher, T.; Moehler, T.M.; Egerer, G.; Neben, K.; et al. Prognostic Significance of Focal Lesions in Whole-Body Magnetic Resonance Imaging in Patients with Asymptomatic Multiple Myeloma. J. Clin. Oncol 2010, 28, 1606–1610. [Google Scholar] [CrossRef]
- Merz, M.; Hielscher, T.; Wagner, B.; Sauer, S.; Shah, S.; Raab, M.S.; Jauch, A.; Neben, K.; Hose, D.; Egerer, G.; et al. Predictive value of longitudinal whole-body magnetic resonance imaging in patients with smoldering multiple myeloma. Leukemia 2014, 28, 1902–1908. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, S.V.; Kumar, S.; Lonial, S.; Mateos, M.V. Smoldering multiple myeloma current treatment algorithms. Blood Cancer J. 2022, 8, 2210–2216. [Google Scholar] [CrossRef]
- Mateos, M.-V.; Hernández, M.-T.; Giraldo, P.; de la Rubia, J.; de Arriba, F.; López Corral, L.; Rosiñol, L.; Paiva, B.; Palomera, L.; Bargay, J.; et al. Lenalidomide plus dexamethasone for high-risk smoldering multiple myeloma. N. Engl. J. Med. 2013, 369, 438–447. [Google Scholar] [CrossRef] [PubMed]
- D’Arena, G.; Gobbi, P.G.; Broglia, C.; Sacchi, S.; Quarta, G.; Baldini, L.; Iannitto, E.; Falcone, A.; Guariglia, R.; Pietrantuono, G.; et al. Pamidronate versus observation in asymptomatic myeloma: Final results with long-term follow-up of a randomized study. Leuk. Lymphoma 2011, 52, 771–775. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, S.V. Multiple myeloma: 2020 update on diagnosis, risk-stratification and management. Am. J. Hematol. 2020, 95, 548–567. [Google Scholar] [CrossRef] [PubMed]
- Korde, N.; Roschewski, M.; Zingone, A.; Kwok, M.; Manasanch, E.E.; Bhutani, M.; Tageja, N.; Kazandjian, D.; Mailankody, S.; Wu, P.; et al. Treatment with Carfilzomib-Lenalidomide-Dexamethasone With Lenalidomide Extension in Patients With Smoldering or Newly Diagnosed Multiple Myeloma. JAMA Oncol. 2015, 1, 746. [Google Scholar] [CrossRef] [PubMed]
- Charliński, G.; Jurczyszyn, A. Non-secretory multiple myeloma: Diagnosis and management. Adv. Clin. Exp. Med. 2021, 31, 95–100. [Google Scholar] [CrossRef]
- Dupuis, M.M.; Tuchman, S.A. Non-secretory multiple myeloma: From biology to clinical management. OncoTargets Ther. 2016, 9, 7583–7590. [Google Scholar] [CrossRef]
- Hill, E.; Dew, A.; Kazandjian, D. State of the science in smoldering myeloma: Should we be treating in the clinic? Semin. Oncol. 2019, 46, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Boyce, B.; Yao, Z.; Xing, L. Osteoclasts have multiple roles in bone in addition to Bone Resorption. Crit. Rev. Eukaryot. Gene Expr. 2009, 19, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Mundy, G.R.; Yoneda, T.; Hiraga, T. Preclinical studies with zoledronic acid and other bisphosphonates: Impact on the bone microenvironment. Semin. Oncol. 2001, 28, 35–44. [Google Scholar] [CrossRef]
- Hussain, M.; Khan, F.; Al Hadidi, S. The use of bone-modifying agents in multiple myeloma. Blood Rev. 2023, 57, 100999. [Google Scholar] [CrossRef] [PubMed]
- Terpos, E.; Ntanasis-Stathopoulos, I.; Dimopoulos, M.A. Myeloma bone disease: From biology findings to treatment approaches. Blood 2019, 133, 1534–1539. [Google Scholar] [CrossRef]
- Dunford, J.E.; Thompson, K.; Coxon, F.P.; Luckman, S.P.; Hahn, F.M.; Poulter, C.D.; Ebetino, F.H.; Rogers, M.J. Structure-activity relationships for inhibition of farnesyl diphosphate synthase in vitro and inhibition of bone resorption in vivo by nitrogen-containing bisphosphonates. J. Pharmacol. Exp. Ther. 2001, 296, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Chu, X.; Qiu, Y.; Wu, L.; Qiao, Y.; Wu, J.; Li, D. Discovery of potent inhibitor for farnesyl pyrophosphate synthase in the mevalonate pathway. Chem. Commun. 2010, 46, 5340. [Google Scholar] [CrossRef] [PubMed]
- Kanakis, I.; Kousidou, O.C.; Karamanos, N.K. In vitro and in vivo antiresorptive effects of bisphosphonates in metastatic bone disease. In Vivo 2005, 19, 311–318. [Google Scholar]
- Mhaskar, R.; Redzepovic, J.; Wheatley, K.; Clark, O.A.; Miladinovic, B.; Glasmacher, A.; Kumar, A.; Djulbegovic, B. Bisphosphonates in multiple myeloma: A network meta-analysis. Cochrane Database Syst. Rev. 2012. [Google Scholar] [CrossRef]
- Shipman, C.M.; Rogers, M.J.; Apperley, J.F.; Russell, R.G.; Croucher, P.I. Bisphosphonates induce apoptosis in human myeloma cell lines: A novel anti-tumour activity. Br. J. Haematol. 1997, 98, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Noriega Aldave, A.P.; Jaiswal, S. Severe resistant hypocalcemia in multiple myeloma after zoledronic acid administration: A case report. J. Med Case Rep. 2014, 8, 353. [Google Scholar] [CrossRef] [PubMed]
- Duh, M.; Weide, R.; Koppler, H.; Antras, L.; Smith, M.; Eva Chang, M.; Green, J.; Wintfeld, N.; Neary, M. Renal toxicity in patients with multiple myeloma receiving zoledronic acid vs, ibandronate: A Retrospective Medical Records Review. J. Cancer Res. Ther. 2010, 6, 31. [Google Scholar] [CrossRef] [PubMed]
- Reiriz, A.B.; de Moraes De Zorzi, P.; Lovat, C.P. Bisphosphonates and Osteonecrosis of the jaw: A case report. Clinics 2008, 63, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Raje, N.; Terpos, E.; Willenbacher, W.; Shimizu, K.; García-Sanz, R.; Durie, B.; Legieć, W.; Krejčí, M.; Laribi, K.; Zhu, L.; et al. Denosumab versus zoledronic acid in bone disease treatment of newly diagnosed multiple myeloma: An international, double-blind, double-dummy, randomised, controlled, phase 3 study. Lancet Oncol. 2018, 19, 370–381. [Google Scholar] [CrossRef]
- West, H. Denosumab for prevention of skeletal-related events in patients with bone metastases from solid tumors: Incremental benefit, debatable value. J. Clin. Oncol. 2011, 29, 1095–1098. [Google Scholar] [CrossRef]
- Body, J.-J.; Facon, T.; Coleman, R.E.; Lipton, A.; Geurs, F.; Fan, M.; Holloway, D.; Peterson, M.C.; Bekker, P.J. A study of the biological receptor activator of nuclear factor-kappab ligand inhibitor, denosumab, in patients with multiple myeloma or bone metastases from breast cancer. Clin. Cancer Res. 2006, 12, 1221–1228. [Google Scholar] [CrossRef]
- Wudhikarn, K.; Wills, B.; Lesokhin, A.M. Monoclonal antibodies in multiple myeloma: Current and emerging targets and mechanisms of action. Best Pract. Amp; Res. Clin. Haematol. 2020, 33, 101143. [Google Scholar] [CrossRef]
- Adams, J. The Proteasome: A suitable antineoplastic target. Nat. Rev. Cancer 2004, 4, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Crawford, L.J.; Irvine, A.E. Targeting the ubiquitin proteasome system in haematological malignancies. Blood Rev. 2013, 27, 297–304. [Google Scholar] [CrossRef]
- Jayaweera, S.P.; Wanigasinghe Kanakanamge, S.P.; Rajalingam, D.; Silva, G.N. Carfilzomib: A promising proteasome inhibitor for the treatment of relapsed and refractory multiple myeloma. Front. Oncol. 2021, 11. [Google Scholar] [CrossRef]
- Ito, S. Proteasome inhibitors for the treatment of multiple myeloma. Cancers 2020, 12, 265. [Google Scholar] [CrossRef]
- Niewerth, D.; Jansen, G.; Assaraf, Y.G.; Zweegman, S.; Kaspers, G.J.L.; Cloos, J. Molecular basis of resistance to proteasome inhibitors in hematological malignancies. Drug Resist. Updates 2015, 18, 18–35. [Google Scholar] [CrossRef] [PubMed]
- Parlati, F.; Lee, S.J.; Aujay, M.; Suzuki, E.; Levitsky, K.; Lorens, J.B.; Micklem, D.R.; Ruurs, P.; Sylvain, C.; Lu, Y.; et al. Carfilzomib can induce tumor cell death through selective inhibition of the chymotrypsin-like activity of the proteasome. Blood 2009, 114, 3439–3447. [Google Scholar] [CrossRef] [PubMed]
- Demo, S.D.; Kirk, C.J.; Aujay, M.A.; Buchholz, T.J.; Dajee, M.; Ho, M.N.; Jiang, J.; Laidig, G.J.; Lewis, E.R.; Parlati, F.; et al. Antitumor activity of PR-171, a novel irreversible inhibitor of the Proteasome. Cancer Res. 2007, 67, 6383–6391. [Google Scholar] [CrossRef] [PubMed]
- Usmani, S.Z.; Weiss, B.M.; Plesner, T.; Bahlis, N.J.; Belch, A.; Lonial, S.; Lokhorst, H.M.; Voorhees, P.M.; Richardson, P.G.; Chari, A.; et al. Clinical efficacy of daratumumab monotherapy in patients with heavily pretreated relapsed or refractory multiple myeloma. Blood 2016, 128, 37–44. [Google Scholar] [CrossRef]
- Mateos, M.V.; Dimopoulos, M.A.; Cavo, M.; Suzuki, K.; Jakubowiak, A.; Knop, S.; Doyen, C.; Lúcio, P.; Nagy, Z.; Kaplan, P.; et al. Daratumumab plus bortezomib, melphalan, and prednisone for untreated myeloma. N. Engl. J. Med. 2018, 378, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Radocha, J.; van de Donk, N.W.; Weisel, K. Monoclonal antibodies and antibody drug conjugates in multiple myeloma. Cancers 2021, 13, 1571. [Google Scholar] [CrossRef] [PubMed]
- Collins, S.M.; Bakan, C.E.; Swartzel, G.D.; Hofmeister, C.C.; Efebera, Y.A.; Kwon, H.; Starling, G.C.; Ciarlariello, D.; Bhaskar, S.; Briercheck, E.L.; et al. Elotuzumab directly enhances NK cell cytotoxicity against myeloma via CS1 ligation: Evidence for augmented NK cell function complementing ADCC. Cancer Immunol. Immunother. 2013, 62, 1841–1849. [Google Scholar] [CrossRef] [PubMed]
- Leow, C.C.-Y.; Low, M.S. Targeted therapies for multiple myeloma. J. Pers. Med. 2021, 11, 334. [Google Scholar] [CrossRef] [PubMed]
- Chames, P.; Van Regenmortel, M.; Weiss, E.; Baty, D. Therapeutic antibodies: Successes, limitations and hopes for the future. Br. J. Pharmacol. 2009, 157, 220–233. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Yan, X.; Puchalski, T.; Lonial, S.; Lokhorst, H.M.; Voorhees, P.; Plesner, T.; Liu, K.; Khan, I.; Jansson, R.; et al. Clinical Implications of Complex Pharmacokinetics for Daratumumab Dose Regimen in Patients With Relapsed/Refractory Multiple Myeloma. Clin. Pharmacol. Ther./Clin. Pharmacol. Ther. 2017, 101, 721–724. [Google Scholar] [CrossRef] [PubMed]
- Michels, T.C.; Petersen, K.E. Multiple Myeloma: Diagnosis and Treatment. Am. Fam. Physician 2017, 95, 373–383. [Google Scholar]
- Palumbo, A.; Anderson, K. Multiple Myeloma. N. Engl. J. Med. 2011, 364, 1046–1060. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Zhu, S.; Zhang, X.; Gong, Z.; Wang, S. Non-Conventional Allogeneic Anti-BCMA Chimeric Antigen Receptor-Based Immune Cell Therapies for Multiple Myeloma Treatment. Cancers 2023, 15, 567. [Google Scholar] [CrossRef] [PubMed]
- Mailankody, S.; Matous, J.V.; Chhabra, S.; Liedtke, M.; Sidana, S.; Oluwole, O.O.; Malik, S.; Nath, R.; Anwer, F.; Cruz, J.C.; et al. Allogeneic BCMA-targeting CAR T cells in relapsed/refractory multiple myeloma: Phase 1 UNIVERSAL trial interim results. Nat. Med. 2023, 29, 422–429. [Google Scholar] [CrossRef] [PubMed]
- International Myeloma Foundation. What Is Relapse? Available online: https://www.myeloma.org/treatment/relapse-definition (accessed on 19 July 2021).
- Rajkumar, S.V.; Harousseau, J.; Durie, B.; Anderson, K.C.; Dimopoulos, M.; Kyle, R.; Blade, J.; Richardson, P.; Orlowski, R.; Siegel, D.; et al. Consensus recommendations for the uniform reporting of clinical trials: Report of the International Myeloma Workshop Consensus Panel 1. Blood 2011, 117, 4691–4695. [Google Scholar] [CrossRef]
- Sonneveld, P. Management of multiple myeloma in the relapsed/refractory patient. Hematology 2017, 508–517. [Google Scholar] [CrossRef]
- Alwahsh, M.; Farhat, J.; Talhouni, S.; Hamadneh, L.; Hergenröder, R. Bortezomib advanced mechanisms of action in multiple myeloma, solid and liquid tumors along with its novel therapeutic applications. PubMed 2023, 22, 146–168. [Google Scholar] [CrossRef]
- Bird, S.A.; Pawlyn, C. IMID resistance in Multiple myeloma: Current understanding of the underpinning biology and clinical impact. Blood 2023, 142, 131–140. [Google Scholar] [CrossRef]
- Rivellese, F.; Manou-Stathopoulou, S.; Mauro, D.; Goldmann, K.; Pyne, D.; Rajakariar, R.; Gordon, P.; Schafer, P.; Bombardieri, M.; Pitzalis, C.; et al. Effects of targeting the transcription factors Ikaros and Aiolos on B cell activation and differentiation in systemic lupus erythematosus. Lupus Sci. Med. 2021, 8, e000445. [Google Scholar] [CrossRef]
- Frere, G.A.; De Araujo, E.D.; Gunning, P.T. Emerging mechanisms of targeted protein degradation by molecular glues. In Methods in Cell Biology; Academic Press: Cambridge, MA, USA, 2022; pp. 1–26. [Google Scholar] [CrossRef]
- Chanan-Khan, A.A.; Swaika, A.; Paulus, A.; Kumar, S.K.; Mikhael, J.R.; Rajkumar, S.V.; Dispenzieri, A.; Lacy, M.Q. Pomalidomide: The new immunomodulatory agent for the treatment of multiple myeloma. Blood Cancer J. 2013, 3, e143. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, G.; Cliff, E.R.S.; Mohyuddin, G.R.; Popat, R.; Midha, S.; Hing, M.N.L.; Harrison, S.J.; Kesselheim, A.S.; Teh, B.W. Infections following bispecific antibodies in myeloma: A systematic review and meta-analysis. Blood Adv. 2023, 7, 5898–5903. [Google Scholar] [CrossRef]
- Xia, J.; Li, H.; Yan, Z.; Zhou, D.; Wang, Y.; Qi, Y.; Cao, J.; Li, D.; Cheng, H.; Sang, W.; et al. Anti–G Protein–Coupled Receptor, Class C Group 5 member D chimeric antigen receptor T cells in patients with relapsed or refractory multiple myeloma: A Single-Arm, Phase II trial. J. Clin. Oncol. 2023, 41, 2583–2593. [Google Scholar] [CrossRef]
- Elkins, K.; Zheng, B.; Go, M.; Slaga, D.; Du, C.; Scales, S.J.; Yu, S.; McBride, J.; De Tute, R.; Rawstron, A.; et al. FCRL5 as a target of Antibody–Drug conjugates for the treatment of multiple myeloma. Mol. Cancer Ther. 2012, 11, 2222–2232. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Otero, P.; van de Donk, N.W.C.J.; Pillarisetti, K.; Cornax, I.; Vishwamitra, D.; Gray, K.; Hilder, B.; Tolbert, J.; Renaud, T.; Masterson, T.; et al. GPRC5D as a novel target for the treatment of multiple myeloma: A narrative review. Blood Cancer J. 2024, 14, 1–13. [Google Scholar] [CrossRef]
- Swan, D.; Murphy, P.; Glavey, S.; Quinn, J. Bispecific Antibodies in multiple myeloma: Opportunities to enhance Efficacy and improve safety. Cancers 2023, 15, 1819. [Google Scholar] [CrossRef]
- Runbeck, E.; Crescioli, S.; Karagiannis, S.N.; Papa, S. Utilizing immunocytokines for cancer therapy. Antibodies 2021, 10, 10. [Google Scholar] [CrossRef] [PubMed]
- International Myeloma Foundation. Tests to Assess Monoclonal Protein. Available online: https://www.myeloma.org/monoclonal-protein-tests (accessed on 19 July 2021).
- Turner, K.A.; Frinack, J.L.; Ettore, M.W.; Tate, J.R.; Graziani, M.S.; Jacobs, J.F.; Booth, R.A.; McCudden, C.R.; Keren, D.F.; Delgado, J.C.; et al. An international multi-center serum protein electrophoresis accuracy and M-protein isotyping study, Part I: Factors impacting limit of quantitation of serum protein electrophoresis. Clin. Chem. Lab. Med. (CCLM) 2020, 58, 533–546. [Google Scholar] [CrossRef]
- Murray, D.L.; Puig, N.; Kristinsson, S.; Usmani, S.Z.; Dispenzieri, A.; Bianchi, G.; Kumar, S.; Chng, W.J.; Hajek, R.; Paiva, B.; et al. Mass spectrometry for the evaluation of monoclonal proteins in multiple myeloma and related disorders: An International Myeloma Working Group Mass Spectrometry Committee Report. Blood Cancer J. 2021, 11, 1–6. [Google Scholar] [CrossRef]
- Banerjee, R.; Biru, Y.; Cole, C.E.; Faiman, B.; Midha, S.; Ailawadhi, S. Disparities in relapsed or refractory multiple myeloma: Recommendations from an interprofessional consensus panel. Blood Cancer J. 2024, 14, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Vacca, A. New Insights in Anti-Angiogenesis in Multiple Myeloma. Int. J. Mol. Sci. 2018, 19, 2031. [Google Scholar] [CrossRef] [PubMed]
- Saltarella, I.; Altamura, C.; Campanale, C.; Laghetti, P.; Vacca, A.; Frassanito, M.A.; Desaphy, F. Anti-Angiogenic Activity of Drugs in Multiple Myeloma. Cancers 2023, 15, 1990. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.H.; Liu, N.; Klimek, V.; Hassoun, H.; Mazumder, A.; Nimer, S.D.; Jagannath, S.; Dhodapkar, M.V. Enhancement of ligand-dependent activation of human natural killer T cells by lenalidomide: Therapeutic implications. Blood 2006, 108, 618–621. [Google Scholar] [CrossRef]
- Dredge, K.; Horsfall, R.; Robinson, S.P.; Zhang, L.; Lu, L.; Tang, Y.; Shirley, M.A.; Muller, G.; Schafer, P.; Stirling, D.; et al. Orally administered lenalidomide (CC-5013) is anti-angiogenic in vivo and inhibits endothelial cell migration and Akt phosphorylation in vitro. Microvasc. Res. 2005, 69, 56–63. [Google Scholar] [CrossRef]
- Lu, L.; Payvandi, F.; Wu, L.; Zhang, L.; Hariri, R.J.; Man, H.; Chen, R.S.; Muller, G.W.; Hughes, C.C.; Stirling, D.I.; et al. The anti-cancer drug lenalidomide inhibits angiogenesis metastasis via multiple inhibitory effects on endothelial cell, f.u.n.ction in normoxic and hypoxic conditions. Microvasc. Res. 2009, 77, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Gorgun, G.; Calabrese, E.; Soydan, E.; Hideshima, T.; Perrone, G.; Bandi, M.; Cirstea, D.; Santo, L.; Hu, Y.; Tai, Y.T.; et al. Immunomodulatory effects of lenalidomide and pomalidomide on interaction of tumor and bone marrow accessory cells in multiple myeloma. Blood 2010, 116, 3227–3237. [Google Scholar] [CrossRef]
- Hideshima, T.; Mitsiades, C.; Tonon, G.; Richardson, P.G.; Anderson, K.C. Understanding multiple myeloma pathogenesis in the bone marrow to identify new therapeutic targets. Nat. Rev. Cancer 2007, 7, 585–598. [Google Scholar] [CrossRef]
- Ria, R.; Melaccio, A.; Racanelli, V.; Vacca, A. Anti-VEGF Drugs in the Treatment of Multiple Myeloma Patients. J. Clin. Med. 2020, 9, 1765. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, P.; Kloock, C.; Comenzo, R. Relapsed/Refractory Multiple Myeloma: A review of available therapies and clinical scenarios encountered in myeloma relapse. Curr. Oncol. 2023, 30, 2322–2347. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Baizer, L.; Callander, N.S.; Giralt, S.A.; Hillengass, J.; Freidlin, B.; Hoering, A.; Richardson, P.G.; Schwartz, E.I.; Reiman, A.; et al. Gaps and opportunities in the treatment of relapsed-refractory multiple myeloma: Consensus recommendations of the NCI Multiple Myeloma Steering Committee. Blood Cancer J. 2022, 12, 98. [Google Scholar] [CrossRef] [PubMed]
- Bringhen, S.; De Wit, E.; Dimopoulos, M.-A. New Agents in Multiple Myeloma: An Examination of Safety Profiles. Clin. Lymphoma Myeloma Leuk. 2017, 17, 391–407.e5. [Google Scholar] [CrossRef] [PubMed]
- Lancman, G.; Sastow, D.L.; Cho, H.J.; Jagannath, S.; Madduri, D.; Parekh, S.S.; Richard, S.; Richter, J.; Sanchez, L.; Chari, A. Bispecific antibodies in Multiple myeloma: Present and future. Blood Cancer Discov. 2021, 2, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Devasia, A.J.; Chari, A.; Lancman, G. Bispecific antibodies in the treatment of multiple myeloma. Blood Cancer J. 2024, 14, 158. [Google Scholar] [CrossRef]
- Lesokhin, A.M.; Tomasson, M.H.; Arnulf, B.; Bahlis, N.J.; Miles Prince, H.; Niesvizky, R.; Koehne, G.; Touzeau, C.; Jethava, Y.; Quach, H.; et al. Elranatamab in relapsed or refractory multiple myeloma: Phase 2 MagnetisMM-3 trial results. Nat. Med. 2023, 29, 2259–2267. [Google Scholar] [CrossRef] [PubMed]
- Albertini, M.R.; Yang, R.K.; Ranheim, E.A.; Hank, J.A.; Zuleger, C.L.; Weber, S.; Neuman, H.; Hartig, G.; Weigel, T.; Mahvi, D.; et al. Pilot trial of the hu14.18-IL2 immunocytokine in patients with completely resectable recurrent stage III or stage IV melanoma. Cancer Immunol. Immunother. 2018, 67, 1647–1658. [Google Scholar] [CrossRef]


| Monoclonal Protein | Clonal Plasma Cells | Myeloma-Defining Event |
|---|---|---|
| Serum Monoclonal Protein ≥3 g/dL | not necessary | None |
| Urinary Monoclonal Protein ≥ 500 mg/24 h | 10–60% bone marrow plasma cell infiltration | None |
| not necessary | 10–60% bone marrow plasma cell infiltration | None |
| Criteria | MGUS | SMM | High-Risk SMM | Multiple Myeloma |
|---|---|---|---|---|
| Serum M Protein | <3 g/dL | ≥3 g/dL | ≥3 g/dL | Any |
| Bone Marrow Monoclonal Plasma Cells | <10% | ≥10% | ≥10% Aberrant Cells ≥ 95% | ≥10% |
| Symptoms | Absent | Absent | Absent | CRAB Criteria |
| Other | sFLCR ≥ 8 Immunoparesis | BM Plasmacytosis ≥ 60% sFLCR ≥ 100 Focal BM lesions on MRI |
| Anti-Angiogenic Factors | Type | Effects |
|---|---|---|
| Thalidomide |
First-generation immunomodulatory drug (IMIDs) | |
| Lenalidomide | Second-generation IMiD |
|
| Therapy | Common Side Effects | Key Toxicities | Recommendations for Monitoring |
|---|---|---|---|
| Monoclonal Antibodies (Daratumumab and Elotuzumab) [86] | Infusion-related reactions (IRRs) |
| Premedication to prevent IRRs; Prophylaxis and Monitor infection signs; Regular CBC monitoring. |
| CAR-T and ALLO-715 [56] | Cytokine Release Syndrome (CRS) | CRS (fever, hypotension, and organ dysfunction) | Regular monitoring for early signs of CRS (fever and hypotension); assess for neurotoxicity symptoms like confusion or seizures; frequent blood counts for cytopenias |
| CAR-T [56] | Neurotoxicity (ICANS) |
| Close neurological monitoring, especially for signs of confusion, seizures, or altered mental status. Corticosteroids if symptoms worsen |
| Infections | Immune suppression | Prophylactic antimicrobials, regular screening for infections, and continuous monitoring for fever or other infection signs | |
| ALLO-715 [56] | Graft-versus-Host Disease (GvHD) | Genetic engineering of T cells reduce alloreactivity | Monitor for GvHD symptoms, such as rash, diarrhea, and liver dysfunction |
| Prolonged Cytopenias | Decreased white blood cell count, hemoglobin, platelet count due to immune suppression | Regular blood counts to monitor for cytopenias; support with transfusions if needed |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Khatib, H.H.; Abdulla, K.; Nassar, L.K.; Ellabban, M.G.; Kakarougkas, A. Advancements in Multiple Myeloma Therapies: A Comprehensive Review by Disease Stage. Lymphatics 2025, 3, 2. https://doi.org/10.3390/lymphatics3010002
El Khatib HH, Abdulla K, Nassar LK, Ellabban MG, Kakarougkas A. Advancements in Multiple Myeloma Therapies: A Comprehensive Review by Disease Stage. Lymphatics. 2025; 3(1):2. https://doi.org/10.3390/lymphatics3010002
Chicago/Turabian StyleEl Khatib, Hager Hisham, Kanz Abdulla, Layla Khaled Nassar, Mariam Gouda Ellabban, and Andreas Kakarougkas. 2025. "Advancements in Multiple Myeloma Therapies: A Comprehensive Review by Disease Stage" Lymphatics 3, no. 1: 2. https://doi.org/10.3390/lymphatics3010002
APA StyleEl Khatib, H. H., Abdulla, K., Nassar, L. K., Ellabban, M. G., & Kakarougkas, A. (2025). Advancements in Multiple Myeloma Therapies: A Comprehensive Review by Disease Stage. Lymphatics, 3(1), 2. https://doi.org/10.3390/lymphatics3010002







