Abstract
Background/Objectives: Directed acyclic graphs (DAGs) inform the epidemiologic statistical modeling confounders to determine close to true causal relationships in a study context. They inform the inclusion of the predictive model variables that affect the causal relationship. Non-small cell lung cancer (NSCLC) is frequently diagnosed, aggressive, and the second leading cause of cancer deaths in the United States. Determining factors affecting both the guideline-concordant treatment receipt and survival outcomes for early-stage lung cancer will help inform future statistical models aiming to achieve a close to true causal relationship. Methods: Peer-reviewed original research published during 2002–2023 was identified through PubMed, Embase, Web of Sciences, Clinical trials registry, and the gray literature. DAGitty version 3.1, an online software program, developed implied DAGs and integrated DAG graphics. The evidence synthesis for constructing directed acyclic graphs (ESC-DAGs) protocol was utilized to guide DAG development. The conceptual models utilized were Andersen and Aday for factors affecting treatment receipt and Shi and Steven for survival outcome factors. Results: A total of 36 studies were included in the DAG synthesis out of 9421 retrieved across databases. Eight studies served in the synthesis of treatment receipt DAG, while 28 studies were used for the survival outcomes DAG. There were 10 causal paths and 13 covariates for treatment receipt and 2 causal pathways and 32 covariates for survival outcomes. Conclusions: There are very few studies reporting on factors affecting early-stage NSCLC guideline-concordant care receipt compared to factors affecting its survival outcomes in the past two decades of original research. Future investigations can utilize data extracted in the current study to develop a meta-analysis informing effect size.
1. Introduction
A long-standing association exists between lung cancer survival and socioeconomic factors in epidemiologic studies on early-stage non-small cell lung cancer (NSCLC) [1]. Several factors contribute to the geographic differences among early-stage NSCLC regarding comorbidity status and carcinogen exposure [2]. Geographic area is a critical factor in treatment utilization and survival for early-stage NSCLC [2]. Treatment modalities for early NSCLC include surgery for medically fit candidates or radiation therapy for medically unfit candidates [3]. Differentiating treatment modalities are associated with survival outcome differences [2,4]. Hence, it is important to establish factors that cause differences in treatment receipt and survival outcomes for this frequently diagnosed, differentially treated, and aggressive cancer to achieve a close to true causal relationship through predictive modeling in cancer epidemiology.
DAG is a simple graphical representation of causal relation assumptions in the study context and multiple factors that must be accounted for to obtain the unconfounded relationship between the exposure and the outcome variable [5]. Conventional statistical models contain several parametric assumptions that may or may not be correct [5]. This is a drawback when identifying assumptions in a study context and model violation [5]. Causal diagrams depicted through DAG represent those study assumptions that can complement statistical models [5]. Causal diagrams illustrate causal relationships without any parametric assumptions, as in the case of conventional statistical models [5]. However, causal diagrams capture the series of causation in the current study context, which a conventional model might not be equipped to perform. Some causal relation assumptions might be untested or unknown, but a causal diagram can capture all possible causal pathways [5]. However, no scientific literature exists that develops directed acyclic graphs (DAGs) through a historic (past 21 years) systematic review regarding factors affecting treatment receipt and survival outcomes among early-stage NSCLC according to a search carried out on PubMed, Embase, Web of Science, and Google.
This study aimed to identify factors associated with non-treatment among TN0M0 NSCLC with the first primary tumor and determine risk factors associated with lung cancer-specific survival after surgery and radiation therapy through a systematic review. What factors affect overall survival (OS) in patients with early-stage primary NSCLC in the United States (U.S.)? What determines the treatment choice? These are the key questions that we aimed to seek through a comprehensive DAG-guided systematic literature review of the topics. We reviewed the existing literature that has sought to measure these factors, especially from the perspective of treatment selection and lung cancer-specific survival among national cancer registry data or clinical trials. To the best of our knowledge, this is the first study to build DAGs informed by the health services research theory conceptual model and to utilize the longest research published year to inculcate evolving medical advances focused on a specific stage of lung cancer. This study will guide future statistical model decision-making by determining the pathways that need to be accounted for to achieve close to true causal relationships. This study will also serve as a foundation for future meta-analysis investigations for early-stage NSCLC in the US.
2. Materials and Methods
A literature search strategy was developed with the assistance of a librarian expert, oncologist, and health economist to ensure that exhaustive literature was included. Three key databases were identified: Embase, PubMed, and Web of Science. For the gray literature, the Connected Papers database [6], manual searching by bibliographically browsing key research articles relevant to the study topic, and the Clinicaltrials.gov [7] database were used. An approach was strategized during the screening phase of the study in an attempt to be consistent across study periods dealing with three different AJCC stages. The hierarchical strategy was informed by the American College of Surgeons (ACS) and the American Cancer Society, which emphasize TNM staging serving as a foundation for defining the overall AJCC staging system. The definition of early-stage from AJCC 6th to 8th moves from broader categorization of T staging to more granular categorization, and for the same reason, the study characteristics Appendix A Table A1 informs about the particular tumor staging each finalized study included, since the definition of stage 1 was relative across AJCC 6th to 8th. Clinical staging informs definitive treatment decision-making and affects survival outcomes, so it is very important to consider studies published after 2001, as the AJCC 6th edition was implemented after that year. No publication year filter was applied for studies found through gray literature searching to capture studies that might be important and relevant to the current topic context. Limited empirical evidence is available to develop a systematic review bibliographic search strategy for healthcare. However, an experiment determined that significant relevant studies were found on Embase, ranking it the second highest of all the pertinent databases in terms of search results [8]. PubMed comprises citations from Medline, another relevant medical literature database, while Web of Science provides only peer-reviewed studies. The decision to use these three databases was made after consultation with a librarian expert and an oncologist. The search strategy across each database is described in the Appendix A. DAGitty version 3.1, an online software program, developed implied DAGs and integrated DAG graphics [9]. Abstrackr, an online free literature screening software, was utilized to maintain objectivity [10]. All the citations from identified databases were imported to Mendley, a reference manager citation software, in which duplicates were removed. The citations were then moved to an Excel sheet in a format in which three researchers (NP, SK, and ME) independently reviewed the first 100 records for title and abstract screening, after which reconciliation was reached through an in-person discussion. Then, independently, two researchers (NP and SK) screened 4646 records for title, abstract, and full-text screening, and in cases in which a consensus was not reached, the third researcher (ME) was reached to resolve the disagreement. The total agreement rate was 89.4%, while the disagreement rate was 10.6%. The data was extracted manually from the final sample by two researchers, NP and SK, independently and later discussed for reconciliation if necessary. The current review complied with PRISMA guidelines, and the PRISMA checklist is described in Appendix B. The current review is not registered.
2.1. Study Inclusion and Exclusion Criteria
Only studies focused on the U.S. were moved toward the final sample from the body screening stage, as the clinical staging and treatment guidelines differ internationally. Additionally, the current study aims to develop causal diagrams to supplement the future statistical model variables utilizing U.S. data. The homogeneity of the included sample in terms of the country was emphasized better to understand the causal relationship within the location context; for the literature database, the publication year was set to 2002–2023 to only capture studies relevant to recent medical advances in this field, as well as clinical staging AJCC 6th and higher. The included studies ranged from AJCC 6th to 8th edition; hence, a strategy of following TN0M0 staging convention first in hierarchy decision was developed to be consistent in the definition of non-metastatic tumors, as mentioned in the protocol section of this paper, in an attempt to avoid the exclusion of studies that do not refer to specific AJCC staging information yet focus on overall stage 1. The title, abstract, and body screening questions were as follows: (1) Is it about stage 1 first primary NSCLC TN0M0? (2) Is the document an article? (3) Is it quantitative research? (4) Is it about assessing the factors affecting treatment receipt? OR is it about assessing the factors affecting survival outcomes?
2.2. Protocol
The protocol for developing the final integrated DAGs (iDAGs) from the shortlisted literature was informed by “Evidence Synthesis for Constructing Directed Acyclic Graphs” (ESC-DAGs) [11]. The protocol comprises three main stages, i.e., mapping, translation, and integration (synthesis and recombination). The current review is divided into two components: (a) factors affecting treatment receipt and (b) factors affecting survival outcomes. The conceptual model utilized in the translation stage of ESC-DAGs for factors affecting treatment receipt is Andersen and Aday’s [12] health services research behavioral model. As for factors affecting survival outcomes, the conceptual model by Shi and Steven [13] for vulnerable populations is utilized. Therefore, the translation stage decisions were guided solely by these conceptual frameworks for each component regarding temporality and construct validity.
3. Results
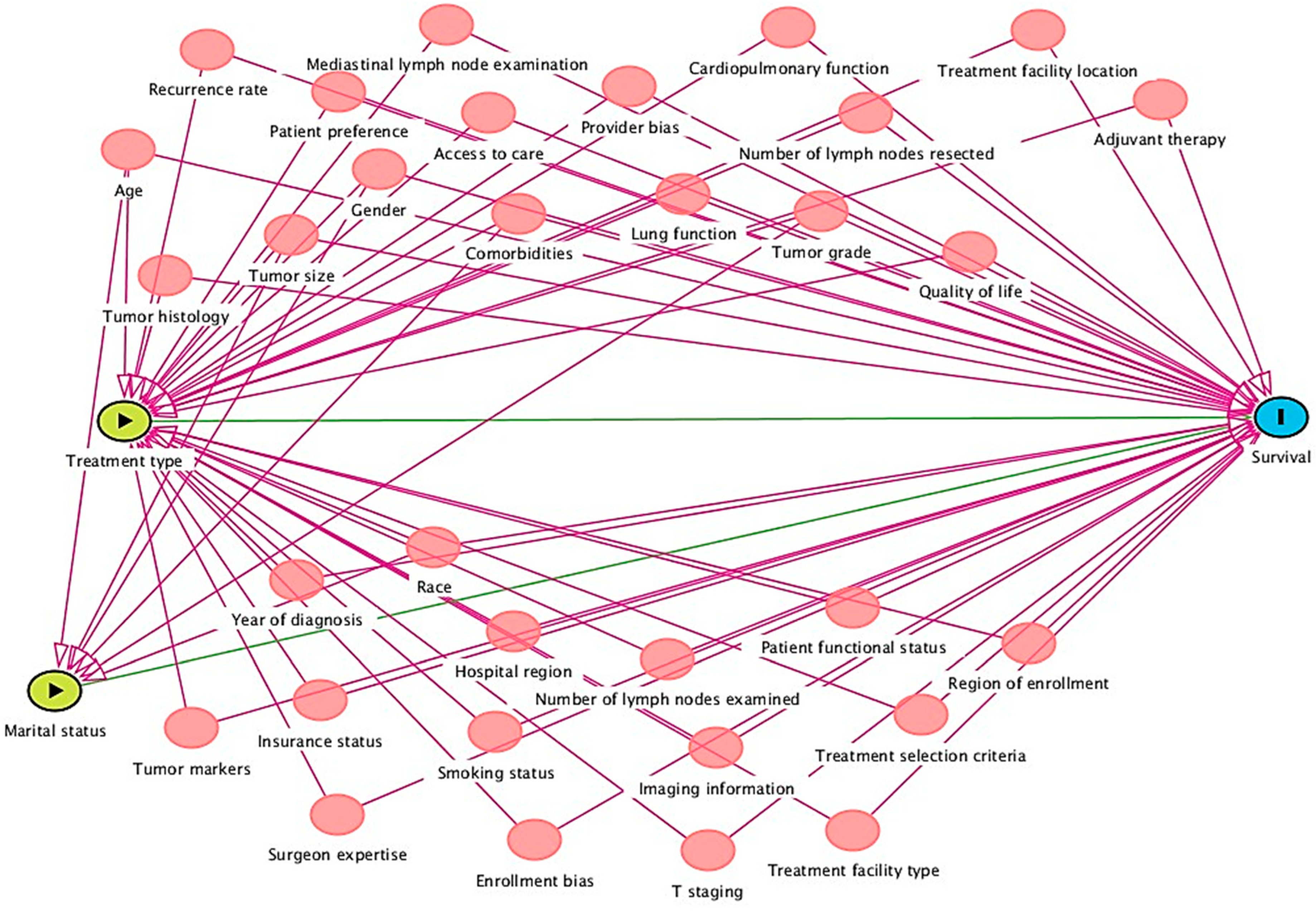
A total of 36 of 9421 studies qualified for final data extraction, as reflected in the PRISMA flowchart in Figure 1. The detailed PRISMA checklist is provided in Appendix B. The baseline characteristics of each study are described in Appendix A, Table A1. It describes the study setting, study period, data registry utilized for the study, age range of the population included, sample size, type of intervention (exposure variable), outcome, AJCC staging version used for study inclusion, and component under which the study falls. The qualified literature study period ranged from 1988 to 2021, and the study designs were both observational and clinical trials. The observational studies utilized the SEER, National Cancer Database (NCDB), SEER-Medicare linked, California Cancer Registry (CCR part of the SEER registry), and primary data collection in clinical trials.
Figure 1.
PRISMA flowchart.
3.1. Mapping Stage
In this stage, each qualified study was used to extract data to develop implied DAGs using DAGitty software separately for each component of the review (Table 1 and Table 2). The studies were read in detail to determine the significant confounders, unobserved/unadjusted confounders, mediators, and colliders. In the implied DAG, the gray bubbles are the identified confounders in each study that were not adjusted in their statistical analysis model. The green bubbles are the study’s exposure variables, and the blue bubble is the study’s outcome variable. Green arrows are the front door causal pathways, while purple indicates the back door pathways that must be closed to achieve a true causal relationship within the study relationship context. In this stage, implied DAGs were developed as determined by the studies, and arrow edges were directed as identified in the study results or conclusions.

Table 1.
Factors affecting treatment receipt mapping stage of ESC-DAG.

Table 2.
Factors affecting survival outcomes mapping stage of ESC-DAG.
3.2. Translation Stage
At this stage, the extracted implied DAGs for each study were utilized to build a DAG edge index (Appendix A, Table A2 and Table A3) to determine the arrow directionality decision-making between an implied set of variables. To reach objective decisions, the proposed theoretical frameworks for each component were utilized to determine whether the arrow directionality was accurate. While deciding to remove or retain the edge, the construct validity and temporality of the edge direction were determined using the theoretical framework. Bi-directionality was determined for each study individually by utilizing their implied graphs to determine if the edge direction of the arrow was bi-directional, given the set of variables in the existing study context. For factors affecting treatment receipt (Appendix A, Table A2), Andersen and Aday’s [12] theoretical framework was used to guide the construct validity and temporality of a particular arrow direction in a given set of variables. Likewise, Shi and Steven’s [13] theoretical framework for vulnerable populations was utilized to identify the factors affecting survival outcomes (Appendix A, Table A3).
3.3. Integration Stage
3.3.1. Factors Affecting Treatment Receipt
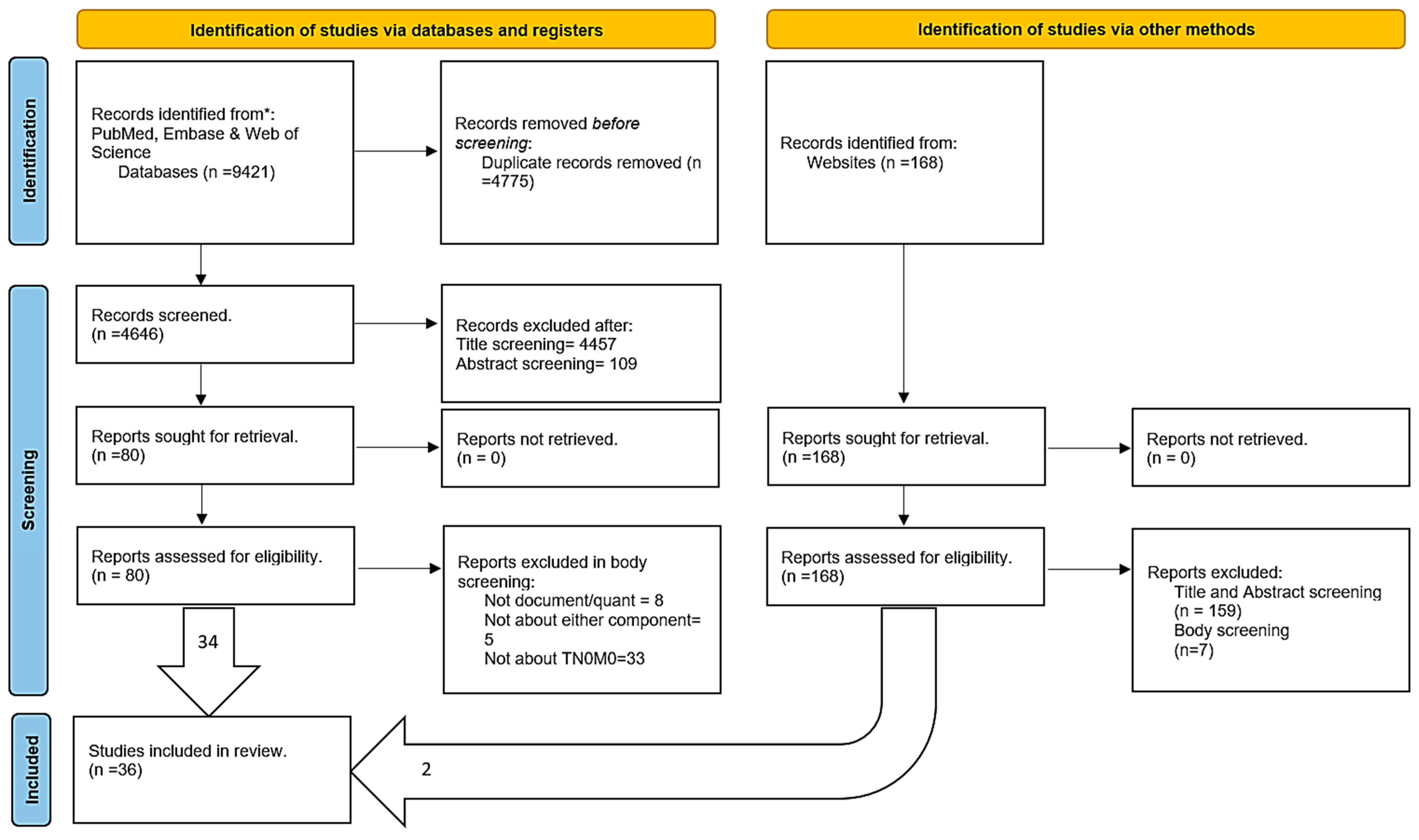
The outcome variable of interest was treatment receipt (Figure 2). Studies with no specific exposure variable were inconclusive regarding the back door causal pathways in iDAGs. There were 10 causal paths and 13 covariates in the iDAG for the exposure variables of interest on the outcome variable. The total effect adjustment for the given effect of interest suggests controlling for only the following necessary variables to close all the back door paths (purple lines): age, chronic obstructive pulmonary disease (COPD), comorbidity score, coronary artery disease, education, sex, health status, income, insurance status, marital status, patient preference, physician preference, and tumor size. The front door paths (green lines) represent the effects of interest in the extracted studies.
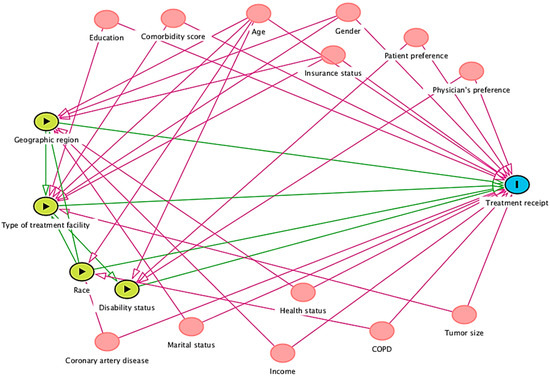
Figure 2.
Integrated DAG for factors affecting treatment receipt.
The following conditional independence testable implications are identified by the iDAG results for total effect adjustment. After adjusting for age and type of treatment facility, the comorbidity score was not related to disability status. In addition, the comorbidity score was unrelated to geographic region, insurance status, marital status, patient preference, physician preference, tumor size, education, sex, income, and race.
Adjusting for age and race, coronary artery disease was unrelated to disability status, geographic region, and type of treatment facility. After adjusting for age and type of treatment facility, coronary artery disease was not related to disability status. It is also unrelated to insurance status, marital status, patient preference, physician preference, tumor size, type of treatment, age, COPD, education, gender, or income.
Adjusting for age, sex, geographic region, insurance, and race, disability status was not related to health status, marital status, or income, while adjusting for age, race, and disability status was unrelated to COPD. Adjusting for age and type of treatment facility, disability status was unrelated to income, race, COPD, education, sex, geographic region, health status, insurance status, tumor size, and marital status.
Hence, future meta-analysis research from the U.S. context attempting to identify factors affecting treatment receipt might benefit by individually investigating each causal relationship between geographic region, type of treatment facility, race, or disability status and treatment receipt. Accounting for individual independent factors in a statistical model, future research must consider differential causal pathways, i.e., while statistically measuring true causal relationship estimate between geographic region and treatment receipt, one must account for significant identified confounders like age, gender, insurance status, marital status, income, and health status. Moreover, a comprehensive approach might include all the independent factors together. However, as identified through integrated DAGs (Figure 2), several confounders, if adjusted together in a statistical model in such an approach, might pose multicollinearity problems.
3.3.2. Factors Affecting Survival Outcomes
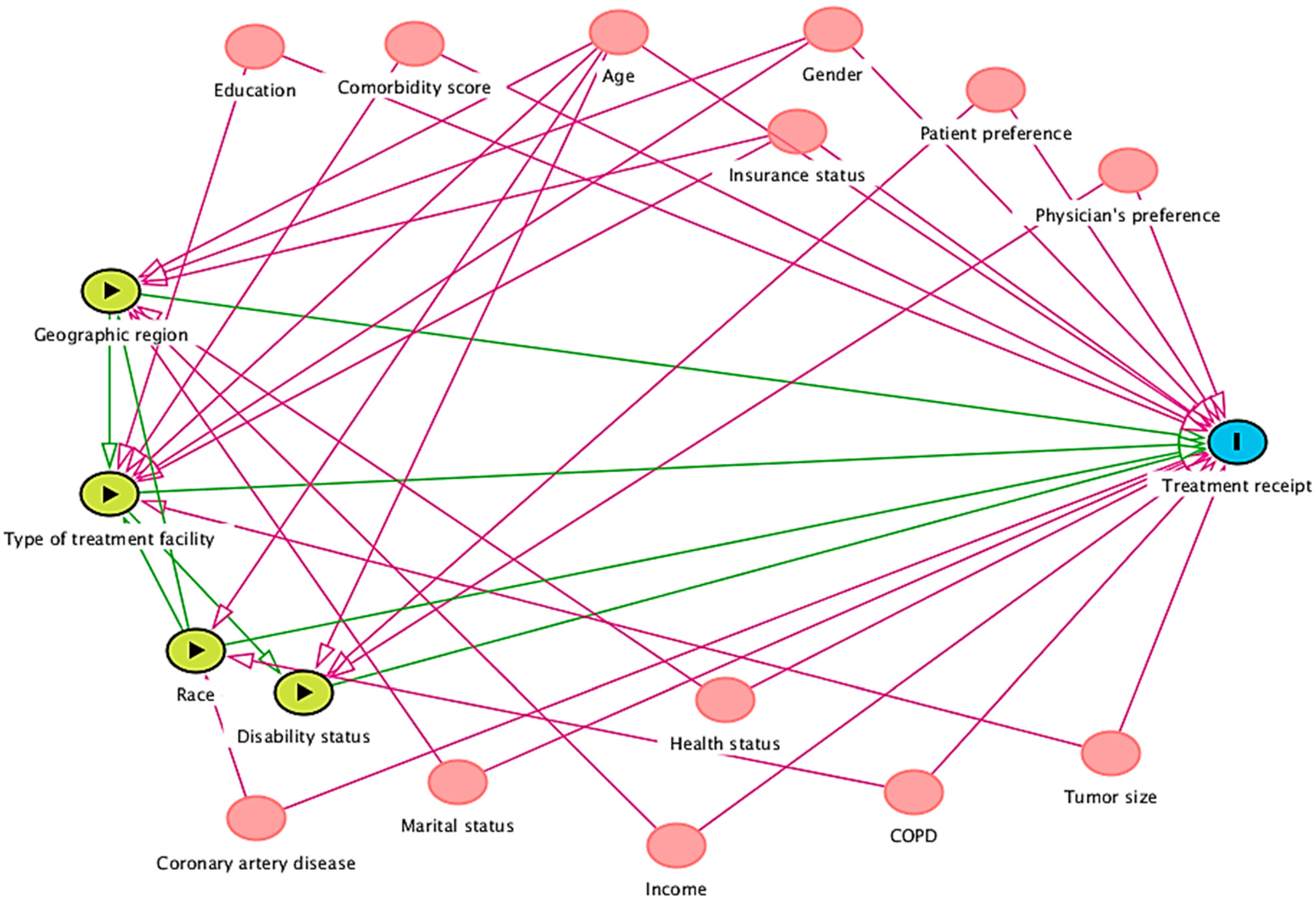
The iDAG (Figure 3) has two causal pathways and 32 covariates for the two exposures of interest, marital status, treatment type, and the outcome variable (survival). The two exposure variables are informed by the extracted studies, and Shi and Steven’s [13] theoretical framework verifies its temporality. The total effect adjustment for the given effect of interest suggests controlling for only the following necessary variables to close all the back door paths (purple lines): access to care, adjuvant therapy, age, cardiopulmonary function, comorbidities, enrollment bias, sex, hospital region, imaging information, insurance status, lung function, mediastinal lymph node examination, number of lymph nodes examined, number of lymph nodes resected, patient functional status, patient preference, provider bias, quality of life, race, recurrence rate, region of enrollment, smoking status, surgeon expertise, T staging, treatment facility location, treatment facility type, treatment selection criteria, tumor grade, tumor histology, tumor markers, tumor size, and year of diagnosis.
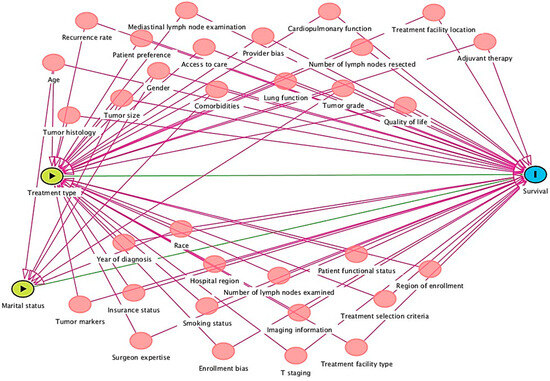
Figure 3.
Integrated DAG for factors affecting survival outcomes.
The following conditional independence testable implications were identified by DAGitty diagnostics for the total effect adjustment of the developed iDAG. Access to care was not related to enrollment bias, imaging information, lung function, marital status, mediastinal lymph node examination, number of lymph nodes examined, number of lymph nodes resected, patient functional status, patient preference, provider bias, quality of life, recurrence rate, region of enrollment, smoking status, surgeon expertise, T staging, treatment facility type, adjuvant therapy, treatment selection criteria, tumor grade, tumor histology, tumor markers, tumor size, year of diagnosis, age, comorbidities, sex, race, and cardiopulmonary function.
Cardiopulmonary function was not related to enrollment bias, hospital region, imaging information, insurance status, lung function, marital status, mediastinal lymph node examination, number of lymph nodes examined, number of lymph nodes resected, patient preference, provider bias, quality of life, recurrence rate, region of enrollment, smoking status, surgeon expertise, T staging, treatment facility location, treatment facility type, tumor grade, tumor histology, tumor markers, tumor size, year of diagnosis, age, comorbidities, sex, and race.
Insurance status was not related to lung function, marital status, mediastinal lymph node examination, number of lymph nodes examined, number of lymph nodes resected, patient functional status, patient preference, provider bias, quality of life, recurrence rate, region of enrollment, smoking status, surgeon expertise, T staging, treatment facility location, treatment facility type, treatment selection criteria, tumor grade, tumor histology, tumor markers, tumor size, year of diagnosis, comorbidities, sex, or race.
The number of lymph nodes resected was not related to patient functional status, patient preference, provider bias, quality of life, recurrence rate, region of enrollment, smoking status, surgeon expertise, T staging, treatment facility location, treatment facility type, treatment selection criteria, tumor grade, tumor histology, tumor markers, tumor size, year of diagnosis, age, comorbidities, sex, and race.
Tumor grade was unrelated to tumor histology, tumor markers, tumor size, year of diagnosis, age, comorbidities, sex, and race. Tumor histology is unrelated to tumor markers, tumor size, year of diagnosis, age, comorbidities, sex, or race. Tumor markers were unrelated to tumor size, year of diagnosis, age, comorbidities, sex, and race. The tumor size was not related to the year of diagnosis, age, comorbidities, sex, or race. The year of diagnosis was not associated with age, comorbidities, sex, or race. Age was not related to comorbidities, sex, or race. Sex is not related to race, and comorbidities are not related to sex or race.
Hence, future meta-analysis research from the U.S. context attempting to identify factors affecting survival outcomes might benefit by individually investigating each causal relationship between marital status or treatment type and treatment receipt. Accounting for individual independent factors in a statistical model, future research must consider differential causal pathways, i.e., while statistically measuring true causal relationship estimate between treatment type and survival, one must account for significant identified confounders like access to care, provider bias, hospital region, tumor grade, quality of life, comorbidities, smoking status, etc. Moreover, a comprehensive approach might include all the independent factors together. However, as identified through integrated DAGs (Figure 3), several confounders, if adjusted together in a statistical model in such an approach, might pose multicollinearity problems.
4. Discussion
To the best of our knowledge and according to searches run on Google, PubMed, Embase, and Web of Science, this is the first study that utilized ESC-DAG for early-stage lung cancer. Hence, it is difficult to provide context with reference to other similar previous literature. However, the results of this study provide commonly identified confounders that corroborate with the final study sample and statements described in their results/conclusion sections. Eight studies provided information on the confounding factors affecting treatment receipt. In comparison, 28 studies provided information on the factors affecting survival outcomes. The confounding factors that affect treatment receipt are age, comorbidity score, education, sex, income, insurance status, marital status, patient preference, physician preference, tumor size, geographic location, and treatment facility type, which aligns with the existing literature. Therefore, adjusting for these confounding factors in a regression model can help improve the prediction abilities of the model in determining close to true causal relationships and the direct effect on the treatment receipt outcome variable.
The confounding factors that affected survival outcomes were access to care, adjuvant therapy, age, cardiopulmonary function, comorbidities, enrollment bias, sex, hospital region, imaging information, insurance status, lung function, mediastinal lymph node examination, number of lymph nodes examined, number of lymph nodes resected, patient functional status, patient preference, provider bias, quality of life, race, recurrence rate, region of enrollment, smoking status, surgeon expertise, T staging, treatment facility location, treatment facility type, treatment selection criteria, tumor grade, tumor histology, tumor markers, tumor size, and year of diagnosis, which can help determine close to true causal relationship and the direct effect of exposure variables on the outcome variable.
The present study has its limitations. Since the final study included a sample that is only focused on the U.S. population, the DAGs might differ when investigated under different study geography conditions, i.e., countries like the UK, Canada, Australia, and others have universal healthcare coverage irrespective of the socioeconomic status, while other countries have a different healthcare services infrastructure. Hence future studies might be able to account for several contextually different confounders, depending on the study setting. Moreover, results from the present study cannot be generalized to other country populations, as several confounders identified in this study, i.e., racial makeup, quality of life, inherent data collection, and country-specific registry limitations, as well as other country-specific health policies might differ that affect the study DAG pathways. Finally, there exists a publication bias, in general, irrespective of the country where original similar studies were conducted, which might affect the included study sample’s unreported factors, thereby affecting the causal pathways. Future studies might want to account for these factors while investigating a causal pathway.
5. Conclusions
The integrated DAGs developed in this study might serve as a supplement to inform statistical modeling decision-making for including confounding covariates in future studies to determine the factors affecting treatment receipt and survival outcomes among patients with stage 1 NSCLC TN0M0. The results of this study are not a substitute for other relevant regression diagnostics, such as correlations or multicollinearity. DAG is a graphical representation that helps identify all possible backdoor pathways to evaluate the total effect of exposures on outcome variables. Several factors, such as sample size, time trend, statistical modeling, composition of the study sample, sample selection bias, age group, type of data, study design, and type of intervention, contribute to the significance of confounders in the study context. Further implication testing can be carried out by future studies through statistical analysis to determine the effect of significance in a given study setting using meta-analysis and regression.
Author Contributions
N.P. conceived the study questions, identified databases, designed the analysis, identified appropriate methods, created DAGitty codes for analysis, performed data analysis, created figures and tables, interpreted results, and drafted the manuscript. S.M.K. co-contributed in design analysis, study results, method selection, and the manuscript. M.E. co-contributed the systematic literature review search strategy and manuscript drafting. B.L. and D.A. co-contributed in developing the results table. All authors participated in reviewing the text and the content of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study is IRB-exempt as it utilized unidentified observational data. The University of Louisville (UofL) Institutional Review Board (IRB) approved this study (IRB number 22.0281). The study is exempt according to 45 CFR 46.101(b) under Category 4: Secondary research, for which consent is not required.
Informed Consent Statement
Not Applicable.
Data Availability Statement
Not Applicable.
Acknowledgments
We would like to thank University of Louisville Library research topic expert Gina Genova for helping us draft the extensive literature search strategy across three databases.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A
Search Strategy across each database
PubMed: n = 753
(“Carcinoma, Non-Small-Cell Lung” [Mesh] OR “non-small-cell lung carcinoma” OR “non-small-cell lung carcinomas” OR “non small cell lung carcinoma” OR “non small cell lung carcinomas” OR “non-small cell lung carcinoma” OR “non-small cell lung carcinomas” OR “non-small cell lung cancer” OR “non small cell lung cancer” OR “non-small-cell lung cancer” OR “Adenocarcinoma of Lung” [Mesh] OR “squamous cell carcinoma of the lung”) AND (“Carcinoma, Non-Small-Cell Lung/surgery” [Mesh] OR “Surgical Procedures, Operative” [Mesh] OR surgery OR “operative procedure” OR “operative procedures” OR “surgical procedure” OR “surgical procedures” OR resection* OR “surgical treatment” OR “Carcinoma, Non-Small-Cell Lung/radiotherapy” [Mesh] OR radiother* OR “radiation therapy” OR “radiation therapies” OR “radiation treatment” OR “radiation treatments” OR irradiation OR Survival [Mesh] OR Mortality [Mesh] OR “Survival Rate” [Mesh] OR outcome* OR mortality OR surviv*) AND (“SEER Program” [Mesh] OR “SEER program” OR SEER OR “Surveillance, Epidemiology, and End Results Program” OR “Surveillance, Epidemiology and End Results Program” OR “National Cancer Registry” OR “ US National Cancer Database”) AND (2002:2023 [pdat])
Embase: n = 1762
(“non small cell lung cancer”/exp OR “bronchial non small cell cancer” OR “bronchial non small cell carcinoma” OR “carcinoma, non-small-cell lung” OR “lung cancer, non small cell” OR “lung non small cell cancer” OR “lung non small cell carcinoma” OR “non small cell bronchial cancer” OR “non small cell cancer, lung” OR “non small cell lung cancer” OR “non small cell lung carcinoma” OR “non small cell pulmonary cancer” OR “non small cell pulmonary carcinoma” OR “non-small-cell lung carcinoma” OR “pulmonary non small cell cancer” OR “pulmonary non small cell carcinoma” OR “adenocarcinoma of lung” OR “squamous cell carcinoma of the lung”) AND (“cancer registry”/exp OR “cdc-npcr” OR “centers for disease control and prevention national program of cancer registries” OR “npcr” OR “national program of cancer registries” OR “seer program” OR “seer programme” OR “united states national program of cancer registries” OR “cancer register” OR “cancer registration” OR “cancer registry”) AND (“surgery”/exp OR “diagnosis, surgical” OR “diagnostic techniques, surgical” OR “operation” OR “operation care” OR “operative intervention” OR “operative repair” OR “operative restoration” OR “operative surgery” OR “operative surgical procedure” OR “operative surgical procedures” OR “operative treatment” OR “research surgery” OR “resection” OR “specialties, surgical” OR “surgery” OR “surgery, operative” OR “surgical care” OR “surgical correction” OR “surgical diagnosis” OR “surgical diagnostic techniques” OR “surgical exposure” OR “surgical intervention” OR “surgical management” OR “surgical operation” OR “surgical practice” OR “surgical procedures, operative” OR “surgical repair” OR “surgical research” OR “surgical restoration” OR “surgical service” OR “surgical speciality” OR “surgical specialties” OR “surgical specialty” OR “surgical therapy” OR “surgical treatment” OR “radiotherapy”/exp OR “bioradiant therapy” OR “bucky irradiation” OR “bucky radiation” OR “bucky radiotherapy” OR “bucky ray” OR “bucky ray radiation” OR “bucky therapy” OR “fractionated radiotherapy” OR “hemibody irradiation” OR “hypophysectomy, radiation” OR “hypophysis irradiation” OR “hypophysis radiation” OR “irradiation therapy” OR “irradiation treatment” OR “irradiation, hypophysis” OR “lymphatic irradiation” OR “pituitary irradiation” OR “radiation beam centration” OR “radiation repair” OR “radiation therapy” OR “radiation treatment” OR “radio therapy” OR “radio treatment” OR “radiohypophysectomy” OR “radiology, therapeutic” OR “radiotherapy” OR “radiotherapy setup errors” OR “radiotreatment” OR “roentgen irradiation, therapeutic” OR “roentgen therapy” OR “roentgen treatment” OR ”rontgen therapy” OR “therapeutic radiology” OR “therapy, irradiation” OR “therapy, radiation” OR “therapy, roentgen” OR “treatment, irradiation” OR “treatment, radiation” OR “treatment, roentgen” OR “x radiotherapy” OR “x ray therapy” OR “x ray treatment” OR “x-ray therapy” OR “survival”/exp OR “survival” OR “mortality”/exp OR “excess mortality” OR “mortality” OR “mortality model” OR “treatment outcome”/exp OR “medical futility” OR “outcome and process assessment (health care)” OR “outcome and process assessment, health care” OR “outcome management” OR “patient outcome” OR “therapeutic outcome” OR “therapy outcome” OR “treatment outcome”) AND (2002:py OR 2003:py OR 2004:py OR 2005:py OR 2006:py OR 2007:py OR 2008:py OR 2009:py OR 2010:py OR 2011:py OR 2012:py OR 2013:py OR 2014:py OR 2015:py OR 2016:py OR 2017:py OR 2018:py OR 2019:py OR 2020:py OR 2021:py OR 2022:py OR 2023:py)
Web of Science: n = 6906
((((((((((((((((( TI = (“non-small-cell lung carcinoma” OR “non-small-cell lung carcinomas” OR “non small cell lung carcinoma” OR “non small cell lung carcinomas” OR “non-small cell lung carcinoma” OR “non-small cell lung carcinomas” OR “non-small cell lung cancer” OR “non small cell lung cancer” OR “non-small-cell lung cancer” OR “adenocarcinoma of lung” OR “squamous cell carcinoma of the lung”)) OR AB = (“non-small-cell lung carcinoma” OR “non-small-cell lung carcinomas” OR “non small cell lung carcinoma” OR “non small cell lung carcinomas” OR “non-small cell lung carcinoma” OR “non-small cell lung carcinomas” OR “non-small cell lung cancer” OR “non small cell lung cancer” OR “non-small-cell lung cancer” “adenocarcinoma of lung” OR “squamous cell carcinoma of the lung”))) AND TI = (surgery OR “operative procedure” OR “operative procedures” OR “surgical procedure” OR “surgical procedures” OR resection* OR “surgical treatment” OR radiother* OR “radiation therapy” OR “radiation therapies” OR “radiation treatment” OR “radiation treatments” OR irradiation)) OR AB = (surgery OR “operative procedure” OR “operative procedures” OR “surgical procedure” OR “surgical procedures” OR resection* OR “surgical treatment” OR radiother* OR “radiation therapy” OR “radiation therapies” OR “radiation treatment” OR “radiation treatments” OR irradiation)) OR TI = (outcome* OR mortality OR surviv*)) OR AB = (outcome* OR mortality OR surviv*)) AND TI = (“SEER program” OR SEER OR “Surveillance, Epidemiology, and End Results Program” OR “Surveillance, Epidemiology and End Results Program” OR “national cancer registry”)) OR AB = (“SEER program” OR SEER OR “Surveillance, Epidemiology, and End Results Program” OR “Surveillance, Epidemiology and End Results Program” OR “national cancer registry”)))))))) AND (PY = =(“2002” OR “2003” OR “2004” OR “2005” OR “2006” OR “2007” OR “2008” OR “2009” OR “2010” OR “2011” OR “2012” OR “2013” OR “2014” OR “2015” OR “2016” OR “2017” OR “2018” OR “2019” OR “2020” OR “2021” OR “2022” OR “2023”) AND SILOID = =(“WOS”) AND CU = =(“USA”) AND LA = =(“ENGLISH”) AND DT = =(“ARTICLE”))))

Table A1.
Study characteristics.
Table A1.
Study characteristics.
| Studies | Study Period | Data Registry | Age (Years) | Sample Size | Intervention | Outcome | AJCC Staging Version | Factor Component (Treatment/Survival) |
|---|---|---|---|---|---|---|---|---|
| Balekian et al. [14] | 2002–2004 | National Lung Cancer Screening Trial (NLST) | 55–74 | 723 | Race | Treatment receipt | 6th | Treatment receipt |
| Berry et al. [15] | 2003–2014 | California Cancer Registry | >=18 | 19,893 | Factors associated with therapy receipt | Treatment receipts | Not mentioned | Treatment receipt |
| Chang et al. [19] | 2015–2020 | STARS trial University of Texas | >=18 | 80 | VATS vs. L-MLND | Survival | 7th | Survival |
| Dai et al. [20] | 2000–2012 | SEER 18 | <= and >65 | 15,760 | Lobectomy vs. Sub-lobectomy | Survival | Not mentioned | Survival |
| Dalwadi et al. [21] | 2002–2012 | SEER 18 | >=60 | 62,213 | Surgery/Radiation/Neither | Survival | 6th | Survival |
| Dalwadi et al. [22] | 2002–2012 | SEER 18 | >=60 | 62,213 | Surgery/Radiation/Neither | Survival | 6th | Survival |
| Dalwadi et al. [1] | 2002–2012 | SEER 18 | >=60 | 62,213 | Rural/Urban/Metropolitan | Treatment receipts | 7th | Treatment receipt |
| Dalwadi et al. [2] | 2004–2012 | SEER 18 | >60 | 62,213 | Rural/Urban/Metropolitan | Treatment receipts | 6th | Treatment receipt |
| Dezube et al. [16] | 2004–2012 | SEER 18 | >=60 | 43,387 | Factors associated with therapy receipt | Treatment receipt | 8th | Treatment receipt |
| Fossum et al. [4] | 2004–2016 | NCDB | >18 | 65,376 | Academic/Community/Comprehensive center Year of diagnosis | Treatment receipt | 6th or 7th | Treatment receipt |
| Ganesh et al. [17] | 2004–2017 | NCDB | Not mentioned | 337,594 | Factors associated with treatment receipt | Treatment receipt | 8th | Treatment receipt |
| Hao et al. [23] | 2004–2013 | SEER | <=69 and >69 | 27,398 | Adenocarcinoma/Squamous cell carcinoma histology | Survival | Not mentioned | Survival |
| Haque et al. [3] | 2004–2012 | SEER 18 | <=50–>=75 | 32,249 | Surgery/Radiation/Neither | Survival | 6th | Survival |
| Huang et al. [24] | 1995–2015 | SEER | <=60–>=75 | 55,207 | Marital Status | Survival | Not mentioned | Survival |
| Li et al. [25] | 2004–2015 | SEER | <=45=>=75 | 5599 | Wedge resection/Segmentectomy | Survival | Not mentioned | Survival |
| Li et al. [26] | 2004–2015 | SEER | <=55–>=75 | 5268 | Radiofrequency ablation/No treatment | Survival | Not mentioned | Survival |
| Li et al. [27] | 2004–2015 | SEER 18 | <=44–>=75 | 6195 | Radiofrequency ablation/Stereotactic body radiotherapy | Survival | Not mentioned | Survival |
| Liang et al. [28] | 2004–2015 | SEER | <=44–>=75 | 6395 | Ablation/Stereotactic body radiotherapy | Survival | Not mentioned | Survival |
| Lin et al. [29] | 2005–2015 | SEER | <=67 and >67 | 1104 | Lobectomy/Sub-lobectomy | Survival | 6th | Survival |
| Ling et al. [30] | 1998–2017 | SEER 18 | 20–80 | 6150 | Lobectomy/Sub-lobectomy | Survival | Not mentioned | Survival |
| Ni et al. [31] | 2012–2017 | SEER 18 | >=80 | 1641 | Surgery/Radiotherapy | Survival | 8th | Survival |
| Razi et al. [32] | 1998–2007 | SEER | >=75 | 1640 | Lobectomy/Sub-lobectomy | Survival | 7th | Survival |
| Wang et al. [33] | 2004–2015 | SEER | <=60 and >=80 | 5783 | Lobectomy/Sub-lobectomy | Survival | 8th | Survival |
| Wang et al. [34] | 1998–2016 | SEER | >=70 | 6197 | Lobectomy/Sub-lobectomy | Survival | 8th | Survival |
| Wu et al. [35] | 2004–2014 | NCDB | Not mentioned | 53,973 | Sub-lobar resection/Ablation/Stereotactic body radiotherapy | Survival | 8th | Survival |
| Wu et al. [36] | 2004–2015 | SEER 18 | <60- and >=75 | 16,511 | Lobectomy/Sub-lobectomy | Survival | Not mentioned | Survival |
| Yendamuri et al. [37] | 2004–2013 | SEER | Not mentioned | 3916 | Wedge/Segmentectomy | Survival | Not mentioned | Survival |
| Yu et al. [38] | 1998–2016 | SEER 18 | >=18 | 9580 | Lobectomy/Sub-lobectomy | Survival | Not mentioned | Survival |
| Zeng et al. [39] | 2004–2014 | SEER | < and >=75 | 4372 | Thermal ablation/Wedge resection | Survival | 8th | Survival |
| Chang et al. [40] | 1988–1997 | SEER | < and >=67 | 10,761 | Lobectomy/Sub-lobectomy | Survival | Not mentioned | Survival |
| Iezzoni et al. [18] | 1988–1999 | SEER11-Medicare | 21–64 | 9500 | Disability status | Treatment receipt | Not mentioned | Treatment receipt |
| Kates et al. [41] | 1988–2005 | SEER | < and >=60 | 2090 | Limited resection/Lobectomy | Survival | Not mentioned | Survival |
| Ludwig et al. [42] | 1990–2000 | SEER | < and >=45 | 16,800 | Number of lymph nodes sampled during surgery | Survival | Not mentioned | Survival |
| Whitson et al. [43] | 1988–2007 | SEER | >=40 | 13,650 | Treatment type | Survival | Not mentioned | Survival |
| STAR trial [44] | 2010–2021 | Clinical trial study | >=18 | 122 | Surgery/Stereotactic Body Radiation Therapy (SBRT) | Survival | Not mentioned | Survival |
| Clinical trial NCT00109876 [45] | 2006–2013 | Clinical trial study | >=18 | 51 | Radiofrequency Ablation | Survival | Not mentioned | Survival |

Table A2.
Directed edge index translation stage for factors affecting treatment receipt.
Table A2.
Directed edge index translation stage for factors affecting treatment receipt.
| Study | Edge Originates From | Edge Terminates at | Bi-Directional | Decision Based on Theory Framework |
|---|---|---|---|---|
| Fossum et al. [4] | Comorbidity score | Treatment receipt | No | Retain |
| Comorbidity score | Type of treatment facility | No | Retain | |
| Geographical area of patient | Treatment receipt | No | Retain | |
| Geographical area of patient | Type of treatment facility | No | Retain | |
| Insurance status | Treatment receipt | No | Retain | |
| Insurance status | Type of treatment facility | No | Retain | |
| Tumor size | Treatment receipt | No | Retain | |
| Tumor size | Type of treatment facility | No | Retain | |
| Type of treatment facility | Treatment receipt | No | Retain | |
| Age | Treatment receipt | No | Retain | |
| Age | Type of treatment facility | No | Retain | |
| Education | Treatment receipt | No | Retain | |
| Education | Type of treatment facility | No | Retain | |
| Gender | Treatment receipt | No | Retain | |
| Gender | Type of treatment facility | No | Retain | |
| Race | Treatment receipt | No | Retain | |
| Dalwadi et al. [1] | Geographic region | Treatment receipt | No | Retain |
| Insurance status | Geographic region | No | Retain | |
| Insurance status | Treatment receipt | No | Retain | |
| Marital status | Geographic region | No | Retain | |
| Marital status | Treatment receipt | No | Retain | |
| Age | Geographic region | No | Retain | |
| Age | Treatment receipt | No | Retain | |
| Gender | Geographic region | No | Retain | |
| Gender | Treatment receipt | No | Retain | |
| Income | Geographic region | No | Retain | |
| Income | Treatment receipt | No | Retain | |
| Race | Geographic region | No | Retain | |
| Race | Treatment receipt | No | Retain | |
| Dalwadi et al. [2] | Geographic region | Treatment receipt | No | Retain |
| Health status | Geographic region | No | Retain | |
| Health status | Treatment receipt | No | Retain | |
| Insurance status | Geographic region | No | Retain | |
| Insurance status | Treatment receipt | No | Retain | |
| Age | Geographic region | No | Retain | |
| Age | Treatment receipt | No | Retain | |
| Gender | Geographic region | No | Retain | |
| Gender | Treatment receipt | No | Retain | |
| Income | Geographic region | No | Retain | |
| Income | Treatment receipt | No | Retain | |
| Balekian et al. [14] | Age at diagnosis | Treatment receipt | No | Retain |
| Age at diagnosis | Race | No | Retain | |
| Coronary artery disease | Treatment receipt | No | Retain | |
| Coronary artery disease | Race | No | Retain | |
| Diagnosis after screening | Treatment receipt | No | Retain | |
| Diagnosis after screening | Race | No | Retain | |
| Smoking status | Treatment receipt | No | Retain | |
| Smoking status | Race | No | Retain | |
| Tumor histology | Treatment receipt | No | Retain | |
| Tumor histology | Race | No | Retain | |
| COPD | Treatment receipt | No | Retain | |
| COPD | Race | No | Retain | |
| Race | Treatment receipt | No | Retain | |
| Berry et al. [15] | Factors associated with Rx receipt | Treatment receipt | No | Retain |
| Insurance type | Factors associated with Rx receipt | No | Remove | |
| Insurance type | Treatment receipt | No | Retain | |
| Marital status | Factors associated with Rx receipt | No | Remove | |
| Marital status | Treatment receipt | No | Retain | |
| NCI hospital designation | Factors associated with Rx receipt | No | Remove | |
| NCI hospital designation | Treatment receipt | No | Retain | |
| SES status | Factors associated with Rx receipt | No | Remove | |
| SES status | Treatment receipt | No | Retain | |
| Tumor size | Factors associated with Rx receipt | No | Remove | |
| Tumor size | Treatment receipt | No | Retain | |
| Age | Factors associated with Rx receipt | No | Remove | |
| Age | Treatment receipt | No | Retain | |
| Gender | Factors associated with Rx receipt | No | Remove | |
| Gender | Treatment receipt | No | Retain | |
| Neighborhood | Factors associated with Rx receipt | No | Remove | |
| Neighborhood | Treatment receipt | No | Retain | |
| Race | Factors associated with Rx receipt | No | Remove | |
| Race | Treatment receipt | No | Retain | |
| Dezube et al. [16] | Factors associated with therapy receipt | Treatment receipt | No | Retain |
| Lower education | Factors associated with therapy receipt | No | Remove | |
| Lower education | Treatment receipt | No | Retain | |
| Lower median income | Factors associated with therapy receipt | No | Remove | |
| Lower median income | Treatment receipt | No | Retain | |
| Specialist availability in area | Factors associated with therapy receipt | No | Remove | |
| Specialist availability in area | Treatment receipt | No | Retain | |
| Age | Factors associated with therapy receipt | No | Remove | |
| Age | Treatment receipt | No | Retain | |
| Comorbidities | Factors associated with therapy receipt | No | Remove | |
| Comorbidities | Treatment receipt | No | Retain | |
| Frailty | Factors associated with therapy receipt | No | Remove | |
| Frailty | Treatment receipt | No | Retain | |
| Race | Factors associated with therapy receipt | No | Remove | |
| Race | Treatment receipt | No | Retain | |
| Ganesh et al. [17] | Comorbidity score | Factors associated with treatment receipt | No | Remove |
| Comorbidity score | Treatment receipt | No | Retain | |
| Factors associated with treatment receipt | Treatment receipt | No | Retain | |
| Geographic region | Factors associated with treatment receipt | No | Remove | |
| Geographic region | Treatment receipt | No | Retain | |
| Insurance status | Factors associated with treatment receipt | No | Remove | |
| Insurance status | Treatment receipt | No | Retain | |
| Rural/Urban region | Factors associated with treatment receipt | No | Remove | |
| Rural/Urban region | Treatment receipt | No | Retain | |
| Type of treatment facility | Factors associated with treatment receipt | No | Remove | |
| Type of treatment facility | Treatment receipt | No | Retain | |
| Gender | Factors associated with treatment receipt | No | Remove | |
| Gender | Treatment receipt | No | Retain | |
| Iezzoni et al. [18] | Disability status | Treatment receipt | No | Retain |
| Patient preference | Disability status | No | Retain | |
| Patient preference | Treatment receipt | No | Retain | |
| Physician preference | Disability status | No | Retain | |
| Physician preference | Treatment receipt | No | Retain | |
| Treatment facility info | Disability status | No | Retain | |
| Treatment facility info | Treatment receipt | No | Retain | |
| Age | Disability status | No | Retain | |
| Age | Treatment receipt | No | Retain |

Table A3.
Directed edge index translation stage for factors affecting survival outcomes.
Table A3.
Directed edge index translation stage for factors affecting survival outcomes.
| Study | Edge Originates From | Edge Terminates at | Bi-Directional | Decision Based on Theory Framework |
|---|---|---|---|---|
| Dai et al. [20] | Histology type | Treatment type | No | Retain |
| Histology type | Survival | No | Retain | |
| Treatment type | Survival | No | Retain | |
| Tumor size | Treatment type | No | Retain | |
| Tumor size | Survival | No | Retain | |
| Age | Treatment type | No | Retain | |
| Age | Survival | No | Retain | |
| Gender | Treatment type | No | Retain | |
| Gender | Survival | No | Retain | |
| Dalwadi et al. [22] | Access to care | Treatment type | No | Retain |
| Access to care | Survival | No | Retain | |
| Histologic type | Treatment type | No | Retain | |
| Histologic type | Survival | No | Retain | |
| Patient preference | Treatment type | No | Retain | |
| Patient preference | Survival | No | Retain | |
| Provider bias | Treatment type | No | Retain | |
| Provider bias | Survival | No | Retain | |
| Treatment type | Survival | No | Retain | |
| Age | Treatment type | No | Retain | |
| Age | Survival | No | Retain | |
| Comorbidities | Treatment type | No | Retain | |
| Comorbidities | Survival | No | Retain | |
| Hao et al. [23] | Histologic type | Treatment type | No | Retain |
| Histologic type | Survival | No | Retain | |
| Lung function | Treatment type | No | Retain | |
| Lung function | Survival | No | Retain | |
| Number of lymph nodes resected | Treatment type | No | Retain | |
| Number of lymph nodes resected | Survival | No | Retain | |
| Treatment type | Survival | No | Retain | |
| Tumor grade | Treatment type | No | Retain | |
| Tumor grade | Survival | No | Retain | |
| Tumor size | Treatment type | No | Retain | |
| Tumor size | Survival | No | Retain | |
| Age | Treatment type | No | Retain | |
| Age | Survival | No | Retain | |
| Comorbidities | Treatment type | No | Retain | |
| Comorbidities | Survival | No | Retain | |
| Gender | Treatment type | No | Retain | |
| Gender | Survival | No | Retain | |
| Haque et al. [3] | Lung function | Treatment type | No | Retain |
| Lung function | Survival | No | Retain | |
| Quality of life | Treatment type | No | Retain | |
| Quality of life | Survival | No | Retain | |
| Treatment type | Survival | No | Retain | |
| Year of Diagnosis | Treatment type | No | Retain | |
| Year of Diagnosis | Survival | No | Retain | |
| Age | Treatment type | No | Retain | |
| Age | Survival | No | Retain | |
| Comorbidities | Treatment type | No | Retain | |
| Comorbidities | Survival | No | Retain | |
| Gender | Treatment type | No | Retain | |
| Gender | Survival | No | Retain | |
| Race | Treatment type | No | Retain | |
| Race | Survival | No | Retain | |
| Huang et al. [24] | Marital status | Survival | No | Retain |
| Tumor grade | Marital status | No | Retain | |
| Tumor grade | Survival | No | Retain | |
| Tumor size | Marital status | No | Retain | |
| Tumor size | Survival | No | Retain | |
| Age | Marital status | No | Retain | |
| Age | Survival | No | Retain | |
| Comorbidities | Marital status | No | Retain | |
| Comorbidities | Survival | No | Retain | |
| Gender | Marital status | No | Retain | |
| Gender | Survival | No | Retain | |
| Race | Marital status | No | Retain | |
| Race | Survival | No | Retain | |
| Kates et al. [41] | Lung function | Treatment type | No | Retain |
| Lung function | Survival | No | Retain | |
| Treatment type | Survival | No | Retain | |
| Comorbidities | Treatment type | No | Retain | |
| Comorbidities | Survival | No | Retain | |
| Li et al. [26] | Hospital region | Treatment type | No | Retain |
| Hospital region | Survival | No | Retain | |
| Treatment type | Survival | No | Retain | |
| Tumor size | Treatment type | No | Retain | |
| Tumor size | Survival | No | Retain | |
| Year of diagnosis | Treatment type | No | Retain | |
| Year of diagnosis | Survival | No | Retain | |
| Liang et al. [28] | Histologic type | Treatment type | No | Retain |
| Histologic type | Survival | No | Retain | |
| Insurance status | Treatment type | No | Retain | |
| Insurance status | Survival | No | Retain | |
| Lung function | Treatment type | No | Retain | |
| Lung function | Survival | No | Retain | |
| Treatment type | Survival | No | Retain | |
| Tumor grade | Treatment type | No | Retain | |
| Tumor grade | Survival | No | Retain | |
| Tumor size | Treatment type | No | Retain | |
| Tumor size | Survival | No | Retain | |
| Age | Treatment type | No | Retain | |
| Age | Survival | No | Retain | |
| Comorbidities | Treatment type | No | Retain | |
| Comorbidities | Survival | No | Retain | |
| Gender | Treatment type | No | Retain | |
| Gender | Survival | No | Retain | |
| Lin et al. [29] | Number of lymph nodes examined | Treatment type | No | Retain |
| Number of lymph nodes examined | Survival | No | Retain | |
| Treatment type | Survival | No | Retain | |
| Gender | Treatment type | No | Retain | |
| Gender | Survival | No | Retain | |
| Ni et al. [31] | Histologic type | Treatment type | No | Retain |
| Histologic type | Survival | No | Retain | |
| Lung function | Treatment type | No | Retain | |
| Lung function | Survival | No | Retain | |
| Number of lymph nodes examined | Treatment type | No | Retain | |
| Number of lymph nodes examined | Survival | No | Retain | |
| Treatment type | Survival | No | Retain | |
| Tumor grade | Treatment type | No | Retain | |
| Tumor grade | Survival | No | Retain | |
| Tumor size | Treatment type | No | Retain | |
| Tumor size | Survival | No | Retain | |
| Year of diagnosis | Treatment type | No | Retain | |
| Year of diagnosis | Survival | No | Retain | |
| Age | Treatment type | No | Retain | |
| Age | Survival | No | Retain | |
| Comorbidities | Treatment type | No | Retain | |
| Comorbidities | Survival | No | Retain | |
| Gender | Treatment type | No | Retain | |
| Gender | Survival | No | Retain | |
| Razi et al. [32] | Lung function | Treatment type | No | Retain |
| Lung function | Survival | No | Retain | |
| Lymph nodes examined status | Treatment type | No | Retain | |
| Lymph nodes examined status | Survival | No | Retain | |
| Treatment type | Survival | No | Retain | |
| Tumor grade | Treatment type | No | Retain | |
| Tumor grade | Survival | No | Retain | |
| Age | Treatment type | No | Retain | |
| Age | Survival | No | Retain | |
| Comorbidities | Treatment type | No | Retain | |
| Comorbidities | Survival | No | Retain | |
| Gender | Treatment type | No | Retain | |
| Gender | Survival | No | Retain | |
| Wang et al. [33] | Imaging information | Treatment type | No | Retain |
| Imaging information | Survival | No | Retain | |
| Patient functional status | Treatment type | No | Retain | |
| Patient functional status | Survival | No | Retain | |
| Smoking status | Treatment type | No | Retain | |
| Smoking status | Survival | No | Retain | |
| Treatment type | Survival | No | Retain | |
| Tumor grade | Treatment type | No | Retain | |
| Tumor grade | Survival | No | Retain | |
| Tumor markers | Treatment type | No | Retain | |
| Tumor markers | Survival | No | Retain | |
| Age | Treatment type | No | Retain | |
| Age | Survival | No | Retain | |
| Wang et al. [34] | Lung function | Treatment type | No | Retain |
| Lung function | Survival | No | Retain | |
| Recurrence rate | Treatment type | No | Retain | |
| Recurrence rate | Survival | No | Retain | |
| Treatment selection criteria | Treatment type | No | Retain | |
| Treatment selection criteria | Survival | No | Retain | |
| Treatment type | Survival | No | Retain | |
| Tumor grade | Treatment type | No | Retain | |
| Tumor grade | Survival | No | Retain | |
| Tumor size | Treatment type | No | Retain | |
| Tumor size | Survival | No | Retain | |
| Age | Treatment type | No | Retain | |
| Age | Survival | No | Retain | |
| Comorbidities | Treatment type | No | Retain | |
| Comorbidities | Survival | No | Retain | |
| Gender | Treatment type | No | Retain | |
| Gender | Survival | No | Retain | |
| Race | Treatment type | No | Retain | |
| Race | Survival | No | Retain | |
| Wu et al. [36] | Cardiopulmonary function | Treatment type | No | Retain |
| Cardiopulmonary function | Survival | No | Retain | |
| Number of lymph nodes dissected | Treatment type | No | Retain | |
| Number of lymph nodes dissected | Survival | No | Retain | |
| Treatment type | Survival | No | Retain | |
| Yendamuri et al. [37] | Histologic type | Treatment type | No | Retain |
| Histologic type | Survival | No | Retain | |
| Lung function | Treatment type | No | Retain | |
| Lung function | Survival | No | Retain | |
| Number of lymph nodes dissected | Treatment type | No | Retain | |
| Number of lymph nodes dissected | Survival | No | Retain | |
| Surgeon expertise | Treatment type | No | Retain | |
| Surgeon expertise | Survival | No | Retain | |
| Treatment type | Survival | No | Retain | |
| Tumor grade | Treatment type | No | Retain | |
| Tumor grade | Survival | No | Retain | |
| Tumor size | Treatment type | No | Retain | |
| Tumor size | Survival | No | Retain | |
| Age | Treatment type | No | Retain | |
| Age | Survival | No | Retain | |
| Comorbidities | Treatment type | No | Retain | |
| Comorbidities | Survival | No | Retain | |
| Gender | Treatment type | No | Retain | |
| Gender | Survival | No | Retain | |
| Yu et al. [38] | Histologic type | Treatment type | No | Retain |
| Histologic type | Survival | No | Retain | |
| Insurance status | Treatment type | No | Retain | |
| Insurance status | Survival | No | Retain | |
| Lung function | Treatment type | No | Retain | |
| Lung function | Survival | No | Retain | |
| Marital status | Treatment type | No | Retain | |
| Marital status | Survival | No | Retain | |
| Number of lymph nodes dissected | Treatment type | No | Retain | |
| Number of lymph nodes dissected | Survival | No | Retain | |
| Quality of life | Treatment type | No | Retain | |
| Quality of life | Survival | No | Retain | |
| Treatment type | Survival | No | Retain | |
| Tumor grade | Treatment type | No | Retain | |
| Tumor grade | Survival | No | Retain | |
| Tumor size | Treatment type | No | Retain | |
| Tumor size | Survival | No | Retain | |
| Year of diagnosis | Treatment type | No | Retain | |
| Year of diagnosis | Survival | No | Retain | |
| Age | Treatment type | No | Retain | |
| Age | Survival | No | Retain | |
| Comorbidities | Treatment type | No | Retain | |
| Comorbidities | Survival | No | Retain | |
| Gender | Treatment type | No | Retain | |
| Gender | Survival | No | Retain | |
| Ling et al. [30] | Histologic type | Treatment type | No | Retain |
| Histologic type | Survival | No | Retain | |
| Number of lymph nodes sampled | Treatment type | No | Retain | |
| Number of lymph nodes sampled | Survival | No | Retain | |
| Treatment type | Survival | No | Retain | |
| Tumor grade | Treatment type | No | Retain | |
| Tumor grade | Survival | No | Retain | |
| Tumor size | Treatment type | No | Retain | |
| Tumor size | Survival | No | Retain | |
| Age | Treatment type | No | Retain | |
| Age | Survival | No | Retain | |
| Gender | Treatment type | No | Retain | |
| Gender | Survival | No | Retain | |
| Clinical trial study NCT # NCT00109876 [45] | Lung function | Treatment type | No | Retain |
| Lung function | Survival | No | Retain | |
| Performance status | Treatment type | No | Retain | |
| Performance status | Survival | No | Retain | |
| Region of enrollment | Treatment type | No | Retain | |
| Region of enrollment | Survival | No | Retain | |
| Treatment type | Survival | No | Retain | |
| Vital capacity | Treatment type | No | Retain | |
| Vital capacity | Survival | No | Retain | |
| Age | Treatment type | No | Retain | |
| Age | Survival | No | Retain | |
| Gender | Treatment type | No | Retain | |
| Gender | Survival | No | Retain | |
| Clinical trial NCT # NCT02357992 [44] | Mediastinal lymph node examination | Treatment type | No | Retain |
| Mediastinal lymph node examination | Survival | No | Retain | |
| Region of enrollment | Treatment type | No | Retain | |
| Region of enrollment | Survival | No | Retain | |
| Treatment type | Survival | No | Retain | |
| Tumor histology | Treatment type | No | Retain | |
| Tumor histology | Survival | No | Retain | |
| Tumor size | Treatment type | No | Retain | |
| Tumor size | Survival | No | Retain | |
| Age | Treatment type | No | Retain | |
| Age | Survival | No | Retain | |
| Gender | Treatment type | No | Retain | |
| Gender | Survival | No | Retain | |
| Race | Treatment type | No | Retain | |
| Race | Survival | No | Retain | |
| Chang et al. [40] | Enrollment bias | Treatment type | No | Retain |
| Enrollment bias | Survival | No | Retain | |
| Patient performance status | Treatment type | No | Retain | |
| Patient performance status | Survival | No | Retain | |
| Treatment type | Survival | No | Retain | |
| Tumor histology | Treatment type | No | Retain | |
| Tumor histology | Survival | No | Retain | |
| Tumor size | Treatment type | No | Retain | |
| Tumor size | Survival | No | Retain | |
| Age | Treatment type | No | Retain | |
| Age | Survival | No | Retain | |
| Dalwadi et al. [21] | Quality of life | Treatment type | No | Retain |
| Quality of life | Survival | No | Retain | |
| T staging | Treatment type | No | Retain | |
| T staging | Survival | No | Retain | |
| Treatment type | Survival | No | Retain | |
| Tumor histology | Treatment type | No | Retain | |
| Tumor histology | Survival | No | Retain | |
| Age | Treatment type | No | Retain | |
| Age | Survival | No | Retain | |
| Gender | Treatment type | No | Retain | |
| Gender | Survival | No | Retain | |
| Li et al. [25] | Adjuvant therapy | Treatment type | No | Retain |
| Adjuvant therapy | Survival | No | Retain | |
| Treatment type | Survival | No | Retain | |
| Tumor grade | Treatment type | No | Retain | |
| Tumor grade | Survival | No | Retain | |
| Tumor size | Treatment type | No | Retain | |
| Tumor size | Survival | No | Retain | |
| Age | Treatment type | No | Retain | |
| Age | Survival | No | Retain | |
| Gender | Treatment type | No | Retain | |
| Gender | Survival | No | Retain | |
| Wang et al. [34] | Lung function | Treatment type | No | Retain |
| Lung function | Survival | No | Retain | |
| Recurrence rate | Treatment type | No | Retain | |
| Recurrence rate | Survival | No | Retain | |
| Treatment selection criteria | Treatment type | No | Retain | |
| Treatment selection criteria | Survival | No | Retain | |
| Treatment type | Survival | No | Retain | |
| Tumor grade | Treatment type | No | Retain | |
| Tumor grade | Survival | No | Retain | |
| Tumor size | Treatment type | No | Retain | |
| Tumor size | Survival | No | Retain | |
| Age | Treatment type | No | Retain | |
| Age | Survival | No | Retain | |
| Comorbidities | Treatment type | No | Retain | |
| Comorbidities | Survival | No | Retain | |
| Gender | Treatment type | No | Retain | |
| Gender | Survival | No | Retain | |
| Race | Treatment type | No | Retain | |
| Race | Survival | No | Retain | |
| Wu et al. [35] | Cardiopulmonary function | Treatment type | No | Retain |
| Cardiopulmonary function | Survival | No | Retain | |
| Comorbidity score | Treatment type | No | Retain | |
| Comorbidity score | Survival | No | Retain | |
| Treatment facility location | Treatment type | No | Retain | |
| Treatment facility location | Survival | No | Retain | |
| Treatment facility type | Treatment type | No | Retain | |
| Treatment facility type | Survival | No | Retain | |
| Treatment type | Survival | No | Retain | |
| Tumor grade | Treatment type | No | Retain | |
| Tumor grade | Survival | No | Retain | |
| Tumor histology | Treatment type | No | Retain | |
| Tumor histology | Survival | No | Retain | |
| Tumor size | Treatment type | No | Retain | |
| Tumor size | Survival | No | Retain | |
| Age | Treatment type | No | Retain | |
| Age | Survival | No | Retain | |
| Gender | Treatment type | No | Retain | |
| Gender | Survival | No | Retain | |
| Race | Treatment type | No | Retain | |
| Race | Survival | No | Retain | |
| Zeng et al. [39] | Cardiopulmonary function | Treatment type | No | Retain |
| Cardiopulmonary function | Survival | No | Retain | |
| Treatment type | Survival | No | Retain | |
| Tumor size | Treatment type | No | Retain | |
| Tumor size | Survival | No | Retain | |
| Comorbidities | Treatment type | No | Retain | |
| Comorbidities | Survival | No | Retain | |
| Race | Treatment type | No | Retain | |
| Race | Survival | No | Retain | |
| Chang et al. [19] | Inaccurate staging | Treatment type | No | Retain |
| Inaccurate staging | Survival | No | Retain | |
| Number of lymph nodes sampled | Treatment type | No | Retain | |
| Number of lymph nodes sampled | Survival | No | Retain | |
| Treatment type | Survival | No | Retain | |
| Tumor size | Treatment type | No | Retain | |
| Tumor size | Survival | No | Retain | |
| Age | Treatment type | No | Retain | |
| Age | Survival | No | Retain | |
| Comorbidities | Treatment type | No | Retain | |
| Comorbidities | Survival | No | Retain | |
| Gender | Treatment type | No | Retain | |
| Gender | Survival | No | Retain | |
| Ludwig et al. [42] | Number of lymph nodes examined | Treatment type | No | Retain |
| Number of lymph nodes examined | Survival | No | Retain | |
| Surgeon experience | Treatment type | No | Retain | |
| Surgeon experience | Survival | No | Retain | |
| Surgeon training | Treatment type | No | Retain | |
| Surgeon training | Survival | No | Retain | |
| Treatment type | Survival | No | Retain | |
| Tumor grade | Treatment type | No | Retain | |
| Tumor grade | Survival | No | Retain | |
| Tumor histology | Treatment type | No | Retain | |
| Tumor histology | Survival | No | Retain | |
| Tumor size | Treatment type | No | Retain | |
| Tumor size | Survival | No | Retain | |
| Whitson et al. [43] | Lung function | Treatment type | No | Retain |
| Lung function | Survival | No | Retain | |
| Number of lymph nodes examined | Treatment type | No | Retain | |
| Number of lymph nodes examined | Survival | No | Retain | |
| Patient function | Treatment type | No | Retain | |
| Patient function | Survival | No | Retain | |
| Pre-treatment staging | Treatment type | No | Retain | |
| Pre-treatment staging | Survival | No | Retain | |
| Surgeon/Hospital volume | Treatment type | No | Retain | |
| Surgeon/Hospital volume | Survival | No | Retain | |
| Surgical approach | Treatment type | No | Retain | |
| Surgical approach | Survival | No | Retain | |
| Treatment type | Survival | No | Retain | |
| Tumor grade | Treatment type | No | Retain | |
| Tumor grade | Survival | No | Retain | |
| Tumor size | Treatment type | No | Retain | |
| Tumor size | Survival | No | Retain | |
| Use of chemotherapy | Treatment type | No | Retain | |
| Use of chemotherapy | Survival | No | Retain | |
| Age | Treatment type | No | Retain | |
| Age | Survival | No | Retain | |
| Gender | Treatment type | No | Retain | |
| Gender | Survival | No | Retain | |
| Histology | Treatment type | No | Retain | |
| Histology | Survival | No | Retain |
#: number.
Appendix B
| Section and Topic | Item # | Checklist Item | Location Where Item Is Reported |
| TITLE | |||
| Title | 1 | Identify the report as a systematic review. | Line 1 |
| ABSTRACT | |||
| Abstract | 2 | See the PRISMA 2020 for abstract checklist. | Lines 12–30 |
| INTRODUCTION | |||
| Rationale | 3 | Describe the rationale for the review in the context of existing knowledge. | Lines 61–75 |
| Objectives | 4 | Provide an explicit statement of the objective(s) or question(s) the review addresses. | Lines 61–75 |
| METHODS | |||
| Eligibility criteria | 5 | Specify the inclusion and exclusion criteria for the review and how studies were grouped for the syntheses. | Lines 127 |
| Information sources | 6 | Specify all databases, registers, websites, organizations, reference lists, and other sources searched or consulted to identify studies. Specify the date when each source was last searched or consulted. | Lines 77–82 |
| Search strategy | 7 | Present the full search strategies for all databases, registers, and websites, including any filters and limits used. | Lines 128–143; Appendix A Search Strategy across each database |
| Selection process | 8 | Specify the methods used to decide whether a study met the inclusion criteria of the review, including how many reviewers screened each record and each report retrieved, whether they worked independently, and, if applicable, details of automation tools used in the process. | Lines 114–124; 139–143 |
| Data collection process | 9 | Specify the methods used to collect data from reports, including how many reviewers collected data from each report, whether they worked independently, any processes for obtaining or confirming data from study investigators, and, if applicable, details of automation tools used in the process. | Lines 118–125 |
| Data items | 10a | List and define all outcomes for which data were sought. Specify whether all results that were compatible with each outcome domain in each study were sought (e.g., for all measures, time points, analyses) and, if not, the methods used to decide which results to collect. | Lines 150–155, results Table 1 and Table 2 |
| 10b | List and define all other variables for which data were sought (e.g., participant and intervention characteristics, funding sources). Describe any assumptions made about any missing or unclear information. | ||
| Study risk of bias assessment | 11 | Specify the methods used to assess the risk of bias in the included studies, including details of the tool(s) used, how many reviewers assessed each study and whether they worked independently, and, if applicable, details of the automation tools used in the process. | Lines 114–125 |
| Effect measures | 12 | Specify for each outcome the effect measure(s) (e.g., risk ratio, mean difference) used in the synthesis or presentation of results. | Results Table 1 and Table 2 |
| Synthesis methods | 13a | Describe the processes used to decide which studies were eligible for each synthesis (e.g., tabulating the study intervention characteristics and comparing against the planned groups for each synthesis (item #5)). | Lines 114–143, results Table 1 and Table 2 |
| 13b | Describe any methods required to prepare the data for presentation or synthesis, such as handling missing summary statistics, or data conversions. | NA | |
| 13c | Describe any methods used to tabulate or visually display results of individual studies and syntheses. | NA | |
| 13d | Describe any methods used to synthesize results and provide a rationale for the choice(s). If a meta-analysis was performed, describe the model(s), method(s) to identify the presence and extent of statistical heterogeneity, and software package(s) used. | NA | |
| 13e | Describe any methods used to explore possible causes of heterogeneity among study results (e.g., subgroup analysis, meta-regression). | NA | |
| 13f | Describe any sensitivity analyses conducted to assess robustness of the synthesized results. | NA | |
| Reporting bias assessment | 14 | Describe any methods used to assess risk of bias due to missing results in a synthesis (arising from reporting biases). | NA |
| Certainty assessment | 15 | Describe any methods used to assess certainty (or confidence) in the body of evidence for an outcome. | Lines 155–157 |
| RESULTS | |||
| Study selection | 16a | Describe the results of the search and selection process, from the number of records identified in the search to the number of studies included in the review, ideally using a flow diagram. | Figure 1 |
| 16b | Cite studies that might appear to meet the inclusion criteria, but which were excluded, and explain why they were excluded. | Figure 1 | |
| Study characteristics | 17 | Cite each included study and present its characteristics. | Appendix A Table A1 |
| Risk of bias in studies | 18 | Present assessments of the risk of bias for each included study. | Results Table 1 and Table 2 |
| Results of individual studies | 19 | For all outcomes, present, for each study: (a) summary statistics for each group (where appropriate) and (b) an effect estimate and its precision (e.g., confidence/credible interval), ideally using structured tables or plots. | Results Table 1 and Table 2 |
| Results of syntheses | 20a | For each synthesis, briefly summarize the characteristics and risk of bias among contributing studies. | Appendix A Table A1 and Results Table 1 and Table 2 |
| 20b | Present results of all statistical syntheses conducted. If a meta-analysis was performed, present for each the summary estimate and its precision (e.g., confidence/credible interval) and measures of statistical heterogeneity. If comparing groups, describe the direction of the effect. | Results Table A1 and Table A2, Appendix A Table A1, Table A2 and Table A3 | |
| 20c | Present the results of all investigations of possible causes of heterogeneity among study results. | Lines 131–135, Appendix A Table A1 | |
| 20d | Present the results of all sensitivity analyses conducted to assess the robustness of the synthesized results. | NA | |
| Reporting biases | 21 | Present assessments of the risk of bias due to missing results (arising from reporting biases) for each synthesis assessed. | NA |
| Certainty of evidence | 22 | Present assessments of certainty (or confidence) in the body of evidence for each outcome assessed. | Results Table 1 and Table 2 |
| DISCUSSION | |||
| Discussion | 23a | Provide a general interpretation of the results in the context of other evidence. | Lines 317–338 |
| 23b | Discuss any limitations of the evidence included in the review. | ||
| 23c | Discuss any limitations of the review processes used. | ||
| 23d | Discuss implications of the results for practice, policy, and future research. | ||
| OTHER INFORMATION | |||
| Registration and protocol | 24a | Provide registration information for the review, including register name and registration number, or state that the review was not registered. | Not registered |
| 24b | Indicate where the review protocol can be accessed, or state that a protocol was not prepared. | ||
| 24c | Describe and explain any amendments to information provided at registration or in the protocol. | ||
| Support | 25 | Describe sources of financial or non-financial support for the review and the role of the funders or sponsors in the review. | NA |
| Competing interests | 26 | Declare any competing interests of review authors. | Line 402 |
| Availability of data, code and other materials | 27 | Report which of the following are publicly available and where they can be found: template data collection forms; data extracted from included studies; data used for all analyses; analytic code; and any other materials used in the review. | in-text |
#: number.
References
- Dalwadi, S.M.; Zhang, J.; Bernicker, E.H.; Butler, E.B.; Teh, B.S.; Farach, A.M. Socioeconomic Factors Associated with Lack of Treatment in Early Stage Non-Small Cell Lung Cancer. Cancer Investig. 2019, 37, 506–511. [Google Scholar] [CrossRef] [PubMed]
- Dalwadi, S.; Teh, B.S.; Bernicker, E.; Butler, E.B.; Farach, A.M. Community-based Disparities in the Treatment and Outcomes of Early-stage Non-small-cell Carcinoma. Cureus 2019, 11, e5889. [Google Scholar] [CrossRef] [PubMed]
- Haque, W.; Szeja, S.; Tann, A.; Kalra, S.; Teh, B.S. Changes in Treatment Patterns and Overall Survival in Patients with Early-Stage Non-Small Cell Lung Cancer in the United States after the Incorporation of Stereotactic Ablative Radiation Therapy. Am. J. Clin. Oncol. Cancer Clin. Trials 2018, 41, 259–266. [Google Scholar] [CrossRef]
- Fossum, C.C.; Ding, L.; Atay, S.M.; David, E.A.; Wightman, S.C.; Kim, A.W.; Ye, J.C. Stereotactic body radiation therapy for early-stage non-small cell lung cancer in the USA: Patterns of adoption and potential healthcare disparities. J. Radiat. Oncol. 2020, 9, 225–234. [Google Scholar] [CrossRef]
- Greenland, S.; Pearl, J.; Robins, J.M. Causal diagrams for epidemiologic research. Epidemiology 1999, 10, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Connected Papers|Find and Explore Academic Papers. Available online: https://www.connectedpapers.com/ (accessed on 11 September 2024).
- Home-ClinicalTrials.gov. Available online: https://clinicaltrials.gov (accessed on 11 September 2024).
- IOM (Institute of Medicine). Finding What Works in Health Care: Standards for Systematic Reviews; Eden, J., Levit, L., Berg, A., Morton, S., Eds.; The National Academies Press: Washington, DC, USA, 2011; Volume 1. Available online: https://www.ncbi.nlm.nih.gov/books/NBK209518/ (accessed on 11 September 2024).
- Textor, J.; Van Der Zander, B.; Gilthorpe, M.S.; Li Skiewicz, M.; Ellison, G.T. Software Application Profile Robust causal inference using directed acyclic graphs: The R package “dagitty”. Int. J. Epidemiol. 2016, 45, 1887–1894. [Google Scholar] [CrossRef]
- Abstrackr: Home. Available online: http://abstrackr.cebm.brown.edu/account/login (accessed on 28 July 2024).
- Ferguson, K.D.; McCann, M.; Katikireddi, S.V.; Thomson, H.; Green, M.J.; Smith, D.J.; Lewsey, J.D. Evidence synthesis for constructing directed acyclic graphs (ESC-DAGs): A novel and systematic method for building directed acyclic graphs. Int. J. Epidemiol. 2020, 49, 322–329. [Google Scholar] [CrossRef]
- Aday, L.; Begley, C.; Lairson, D.; Slater, C. Evaluating the Healthcare System: Effectiveness, Efficiency, and Equity, 3rd ed.; Health Administration Press: Chicago, IL, USA, 2004; ISBN 1567932223. [Google Scholar]
- Shi, L. Health Services Research Methods; Thomson/Delmar Learning: New York, NY, USA, 2008; ISBN 978-1428352292. [Google Scholar]
- Balekian, A.A.; Wisnivesky, J.P.; Gould, M.K. Surgical Disparities Among Patients with Stage I Lung Cancer in the National Lung Screening Trial. Chest 2019, 155, 44–52. [Google Scholar] [CrossRef]
- Berry, M.F.; Canchola, A.J.; Gensheimer, M.F.; Gomez, S.L.; Cheng, I. Factors Associated with Treatment of Clinical Stage I Non–Small-cell Lung Cancer: A Population-based Analysis. Clin. Lung Cancer 2018, 19, e745–e758. [Google Scholar] [CrossRef]
- Dezube, A.R.; Mazzola, E.; Cooper, L.; Deeb, A.L.; De-Leon, L.E.; Singer, L.; Jacobson, F.L.; Jaklitsch, M.T.; Wiener, D. Geographic differences in therapy for stage I non-small-cell lung cancer in older adults. J. Surg. Oncol. 2022, 125, 1053–1060. [Google Scholar] [CrossRef]
- Ganesh, A.; Korpics, M.; Pasquinelli, M.; Feldman, L.; Spiotto, M.; Koshy, M. Increased Utilization of Stereotactic Body Radiotherapy is Associated with Decreased Disparities and Improved Survival for Early-Stage NSCLC. Clin. Lung Cancer 2023, 24, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Iezzoni, L.I.; Ngo, L.H.; Li, D.; Roetzheim, R.G.; Drews, R.E.; McCarthy, E.P. Treatment Disparities for Disabled Medicare Beneficiaries with Stage I Non-Small Cell Lung Cancer. Arch. Phys. Med. Rehabil. 2008, 89, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.Y.; Mehran, R.J.; Feng, L.; Verma, V.; Liao, Z.; Welsh, J.W.; Lin, S.H.; O’Reilly, M.S.; Jeter, M.D.; Balter, P.A.; et al. Stereotactic ablative radiotherapy for operable stage I non-small-cell lung cancer (revised STARS): Long-term results of a single-arm, prospective trial with prespecified comparison to surgery. Lancet Oncol. 2021, 22, 1448–1457. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Shen, J.; Ren, Y.; Zhong, S.; Zheng, H.; He, J.; Xie, D.; Fei, K.; Liang, W.; Jiang, G.; et al. Choice of Surgical Procedure for Patients with Non-Small-Cell Lung Cancer ≤ 1 cm or > 1 to 2 cm Among Lobectomy, Segmentectomy, and Wedge Resection: A Population-Based Study. J. Clin. Oncol. 2016, 34, 3175–3182. [Google Scholar] [CrossRef]
- Dalwadi, S.M.; Lewis, G.D.; Bernicker, E.H.; Butler, E.B.; Teh, B.S.; Farach, A.M. Disparities in the Treatment and Outcome of Stage I Non–Small-Cell Lung Cancer in the 21st Century. Clin. Lung Cancer 2019, 20, 194–200. [Google Scholar] [CrossRef]
- Dalwadi, S.M.; Szeja, S.S.; Bernicker, E.H.; Butler, E.B.; Teh, B.S.; Farach, A.M. Practice Patterns and Outcomes in Elderly Stage I Non–Small-cell Lung Cancer: A 2004 to 2012 SEER Analysis. Clin. Lung Cancer 2018, 19, e269–e276. [Google Scholar] [CrossRef]
- Hao, B.; Li, F.; Wan, X.; Pan, S.; Li, D.; Song, C.; Li, N.; Geng, Q. Squamous cell carcinoma predicts worse prognosis than adenocarcinoma in stage IA lung cancer patients: A population-based propensity score matching analysis. Front. Surg. 2022, 9, 944032. [Google Scholar] [CrossRef]
- Huang, L.; Peng, S.; Sun, C.; Chen, L.; Chu, Q.; Thapa, S.; Chummun, V.; Zhang, L.; Zhang, P.; Chen, E.L.; et al. Impact of marital status on survival in patients with stage 1A NSCLC. Aging 2022, 14, 770–779. [Google Scholar] [CrossRef]
- Li, G.; Xie, S.; Hu, F.; Tan, M.; Fan, L.; Wang, C. Segmentectomy or Wedge Resection in Stage IA Lung Squamous Cell Carcinoma and Adenocarcinoma? J. Cancer 2021, 12, 1708–1714. [Google Scholar] [CrossRef]
- Li, M.; Qin, Y.; Mei, A.; Wang, C.; Fan, L. Effectiveness of radiofrequency ablation therapy for patients with unresected Stage IA non-small cell lung cancer. J. Cancer Res. Ther. 2020, 16, 1007. [Google Scholar] [CrossRef]
- Li, M.; Xu, X.; Qin, Y.; Zhang, P.; Shen, C.; Xia, Q.; Fan, L. Radiofrequency ablation vs. stereotactic body radiotherapy for stage IA non-small cell lung cancer in nonsurgical patients. J. Cancer 2021, 12, 3057–3066. [Google Scholar] [CrossRef]
- Liang, L.; Li, G.; Xie, S.; Sun, G.; Zhang, M.; Sun, F.; Peng, A. Choice of Treatment for Stage IA Non-small Cell Lung Cancer Patients Ineligible for Surgery: Ablation or Stereotactic Body Radiotherapy? J. Cancer 2020, 11, 1634–1640. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.; Liu, H.; Li, J. Lobectomy versus sub-lobar resection in patients with stage IA right middle lobe non-small cell lung cancer: A propensity score matched analysis. J. Thorac. Dis. 2019, 11, 2523–2534. [Google Scholar] [CrossRef] [PubMed]
- Ling, L.; Zheng, H.; Lin, H.; Ge, C.; Li, D.; Wu, L.; Lou, Y.; Jiang, T.; Fu, J. Adequate Number of Lymph Nodes Sampled May Determine Appropriate Surgical Modality for Early-Stage NSCLC: A Population-Based Real-World Study. Clin. Lung Cancer 2022, 24, e141–e151. [Google Scholar] [CrossRef] [PubMed]
- Ni, L.; Lin, G.; Zhang, Z.; Sun, D.; Liu, Z.; Liu, X. Surgery versus radiotherapy in octogenarians with stage Ia non-small cell lung cancer: Propensity score matching analysis of the SEER database. BMC Pulm. Med. 2022, 22, 411. [Google Scholar] [CrossRef] [PubMed]
- Razi, S.S.; John, M.M.; Sainathan, S.; Stavropoulos, C. Sublobar resection is equivalent to lobectomy for T1a non-small cell lung cancer in the elderly: A Surveillance, Epidemiology, and End Results database analysis. J. Surg. Res. 2016, 200, 683–689. [Google Scholar] [CrossRef]
- Wang, L.; Ge, L.; You, S.; Liu, Y.; Ren, Y. Lobectomy versus segmentectomy in patients with stage T (>2 cm and ≤3 cm) N0M0 non-small cell lung cancer: A propensity score matching study. J. Cardiothorac. Surg. 2022, 17, 110. [Google Scholar] [CrossRef]
- Wang, W.; Sun, Y.; Li, H.; Bao, M.; Liu, X.; Jiang, G.; Ye, C.; Hu, Y. Surgical modality for stage IA non-small cell lung cancer among the elderly: Analysis of the surveillance, epidemiology, and end results database. J. Thorac. Dis. 2020, 12, 6731–6742. [Google Scholar] [CrossRef]
- Wu, J.; Bai, H.X.; Chan, L.; Su, C.; Zhang, P.J.; Yang, L.; Zhang, Z. Sublobar resection compared with stereotactic body radiation therapy and ablation for early stage non–small cell lung cancer: A National Cancer Database study. J. Thorac. Cardiovasc. Surg. 2020, 160, 1350–1357.e11. [Google Scholar] [CrossRef]
- Wu, L.; Zhao, W.; Chen, T.; Yang, Y. Surgical choice for patients with stage I non-small-cell lung cancer ≤ 2 cm: An analysis from surveillance, epidemiology, and end results database. J. Cardiothorac. Surg. 2021, 16, 191. [Google Scholar] [CrossRef]
- Yendamuri, S.; Dhillon, S.S.; Groman, A.; Dy, G.; Dexter, E.; Picone, A.; Nwogu, C.; Demmy, T.; Hennon, M. Effect of the number of lymph nodes examined on the survival of patients with stage I non–small cell lung cancer who undergo sublobar resection. J. Thorac. Cardiovasc. Surg. 2018, 156, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Zhang, R.; Zhang, M.; Lin, Y.; Zhang, X.; Wen, Y.; Yang, L.; Huang, Z.; Wang, G.; Zhao, D.; et al. Segmental resection is associated with decreased survival in patients with stage IA non-small cell lung cancer with a tumor size of 21–30 mm. Transl. Lung Cancer Res. 2021, 10, 900–913. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Lu, J.; Tian, Y.; Fu, X. Thermal Ablation Versus Wedge Resection for Stage I Non-small Cell Lung Cancer Based on the Eighth Edition of the TNM Classification: A Population Study of the US SEER Database. Front. Oncol. 2020, 10, 571684. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.Y.; Mentzer, S.J.; Colson, Y.L.; Linden, P.A.; Jaklitsch, M.T.; Lipsitz, S.R.; Sugarbaker, D.J. Factors predicting poor survival after resection of stage IA non–small cell lung cancer. J. Thorac. Cardiovasc. Surg. 2007, 134, 850–856. [Google Scholar] [CrossRef][Green Version]
- Kates, M.; Swanson, S.; Wisnivesky, J.P. Survival Following Lobectomy and Limited Resection for the Treatment of Stage I Non-small Cell Lung Cancer ≤ 1 cm in Size: A Review of SEER Data. Chest 2011, 139, 491–496. [Google Scholar] [CrossRef]
- Ludwig, M.S.; Goodman, M.; Miller, D.L.; Johnstone, P.A.S. Postoperative Survival and the Number of Lymph Nodes Sampled During Resection of Node-Negative Non-Small Cell Lung Cancer. Chest 2005, 128, 1545–1550. [Google Scholar] [CrossRef]
- Whitson, B.A.; Groth, S.S.; Andrade, R.S.; Habermann, E.B.; Maddaus, M.A.; D’Cunha, J. T1/T2 non–small-cell lung cancer treated by lobectomy: Does tumor anatomic location matter? J. Surg. Res. 2012, 177, 185–190. [Google Scholar] [CrossRef]
- Lung Cancer STARS Trial—STARS Revised Clinical Trial Protocol: Stereotactic Ablative Radiotherapy (SABR) in Stage I Non-small Cell Lung Cancer Patients Who Can Undergo Lobectomy—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT02357992?term=NCT02357992&draw=2&rank=1 (accessed on 22 January 2023).
- Radiofrequency Ablation in Treating Patients with Stage I Non-Small Cell Lung Cancer—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/study/NCT00109876?term=NCT00109876&draw=2&rank=1 (accessed on 22 January 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).