Regeneration of Osteochondral Lesion of the Talus with Retrograde Drilling Technique: An In Vitro Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. OC Sample Harvesting and Surgical Set-Up of OCLT
- Group 1—control untreated (CTR): the defect created was left empty and not treated (empty defect);
- Group 2—treated with ABG: to simulate the clinical surgical procedure, for bone drainage with ABG, the technique developed by Taranow WS et al. was used [20], a method widely validated in the literature which proved to be indicated in the presence of subchondral cystic lesions with vital cartilage [4,14,16]. The created defect was filled with the obtained spongy bone graft, deposited beneath the subchondral plate and adequately packed to prevent it from leaving the defect.
- Group 3—involved a similar retrograde perforation, removing all the subchondral bone and keeping the overlying cartilaginous layer intact, but the defect was treated with the HyaloFast® (Anika Therapeutics, Inc.—Bedford, MA 01730 USA) scaffold: HYAFF® (hyaluronic acid benzyl ester) is a scaffold usually used as a support for the entrapment of mesenchymal stem cells (MSCs) and for the repair of chondral and osteochondral lesions. The created defect was filled with HyaloFast® scaffolds and bone marrow cells harvested from the tibial medullary canal emerging through the tibial holes of the surgical instruments (2 mL) during the preparatory phase for the positioning of the prosthesis.
2.2. Culture Conditions
2.3. Tissue Viability
2.4. Immunoenzymatic Analysis
2.5. Microtomographic Analysis
- -
- Bone density BV/TV (%), expressed as the ratio between the volume of the trabecular bone and the total volume of the VOI;
- -
- Trabecular thickness Tb.Th (in mm), calculated in a model-independent manner described by Hildebrand and Ruegsegger on the entire VOI;
- -
- Trabecular separation Tb.Sp (in mm), calculated as the Tb.Th.
2.6. Histology
2.7. Statistical Analysis
3. Results
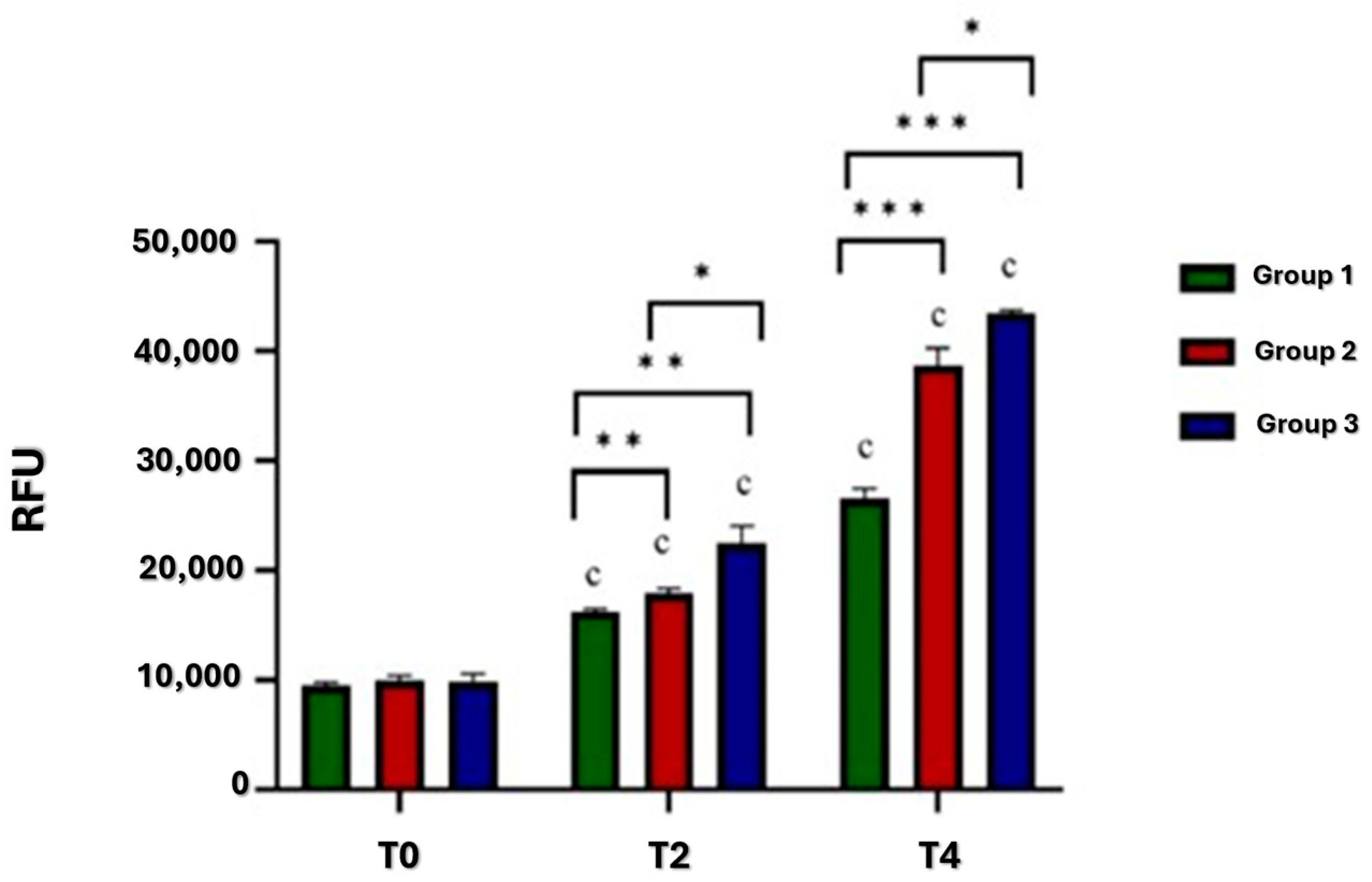
3.1. Viability
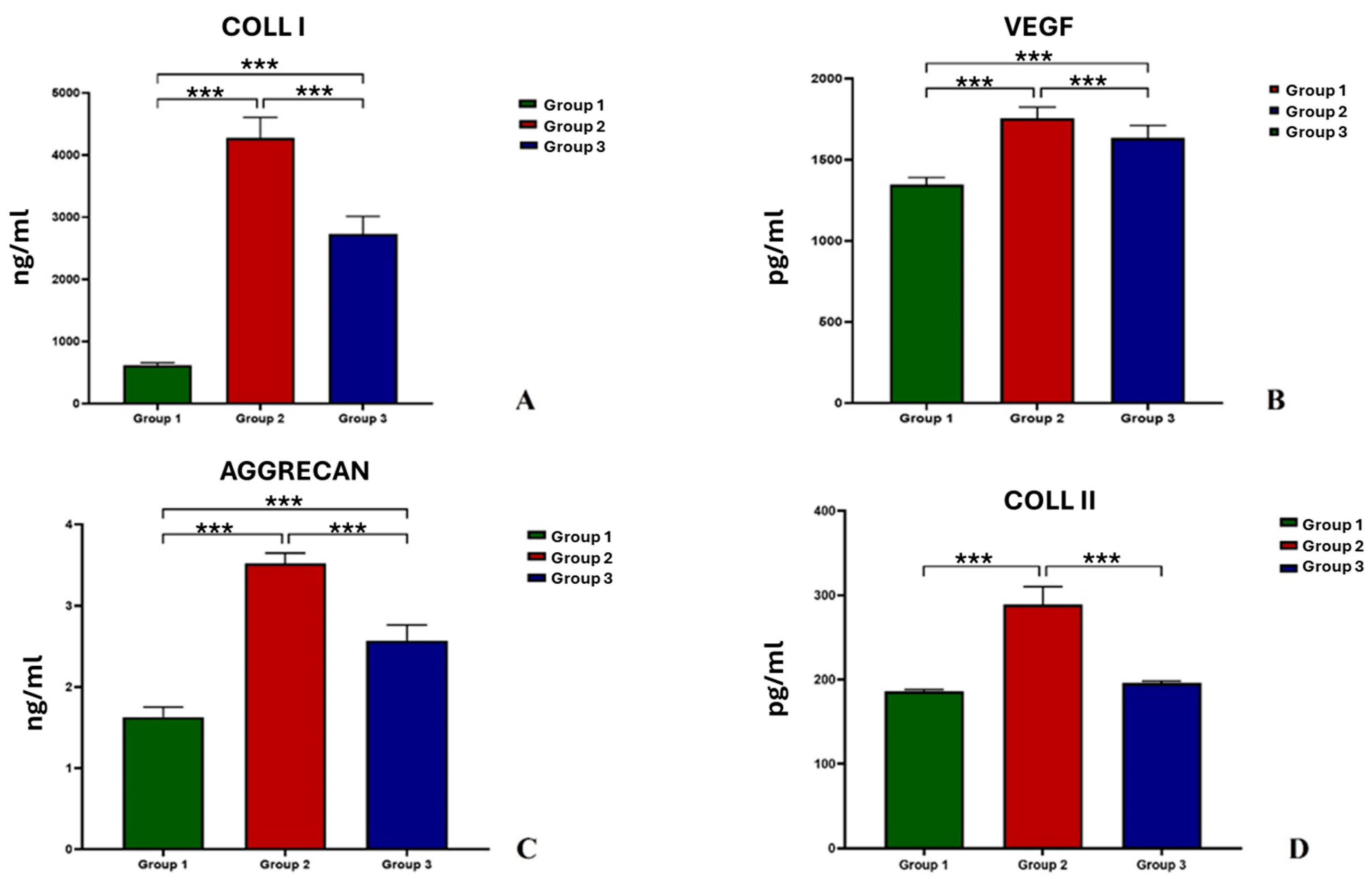
3.2. Immunoenzymatic Analysis
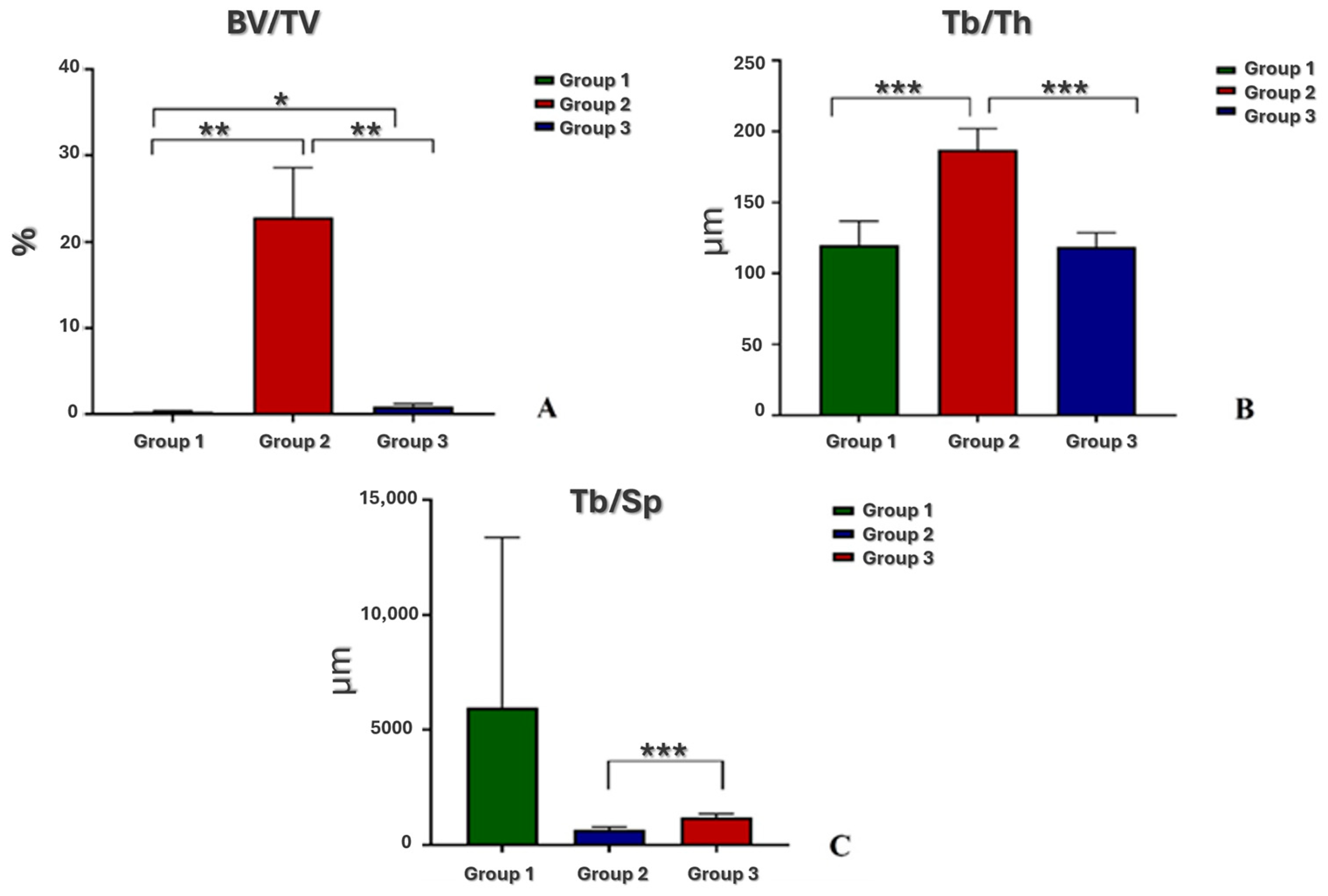
3.3. Microtomographic Analysis
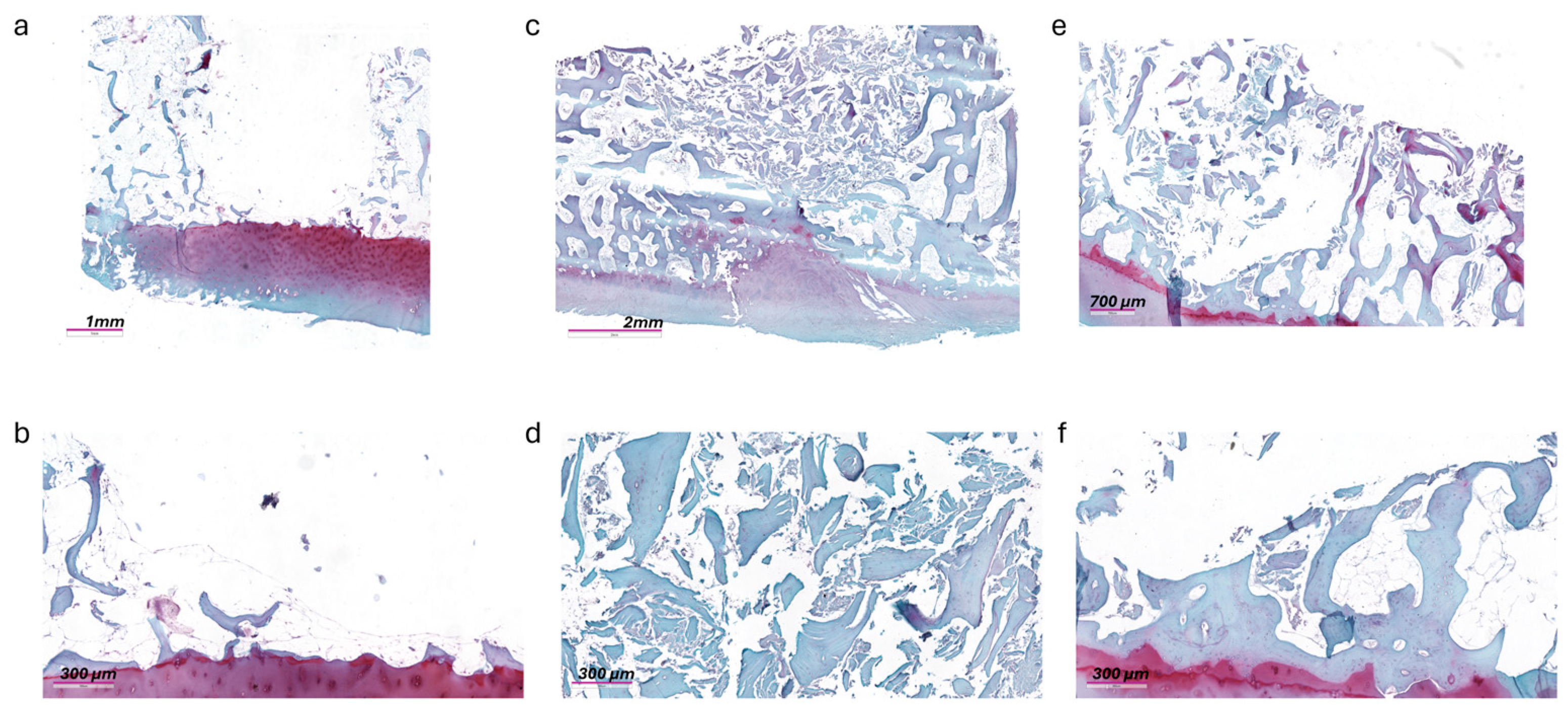
3.4. Histology
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Waterman, B.R.; Belmont, P.J., Jr.; Cameron, K.L.; Deberardino, T.M.; Owens, B.D. Epidemiology of ankle sprain at the United States Military Academy. Am. J. Sports Med. 2010, 38, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Migliorini, F.; Mafulli, N.; Schenker, H.; Eschweiler, J.; Driessen, A.; Knobe, M.; Tingart, M.; Baroncini, A. Surgical management of focal chondral defects of the talus: A Bayesian network meta-analysis. Am. J. Sports Med. 2022, 50, 2853–2859. [Google Scholar] [CrossRef] [PubMed]
- Wodicka, R.; Ferkel, E.; Ferkel, R. Osteochondral lesions of the ankle. Foot Ankle Int. 2016, 37, 1023–1034. [Google Scholar] [CrossRef] [PubMed]
- Anders, S.; Lechler, P.; Rackl, W.; Grifka, J.; Schaumburger, J. Fluoroscopy-guided retrograde core drilling and cancellous bone grafting in osteochondral defects of the talus. Int. Orthop. 2012, 36, 1635–1640. [Google Scholar] [CrossRef] [PubMed]
- Kumai, T.; Takakura, Y.; Higashiyama, I.; Tamai, S. Arthroscopic drilling for the treatment of osteochondral lesions of the talus. J. Bone Jt. Surg. Am. 1999, 81, 1229–1235. [Google Scholar] [CrossRef]
- Vannini, F.; Cavallo, M.; Baldassarri, M.; Castagnini, F.; Olivieri, A.; Ferranti, E.; Buda, R.; Giannini, S. Treatment of juvenile osteochondritis dissecans of the talus: Current concepts review. Joints 2015, 2, 188–191. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.K.; Mercurio, C. Operative treatment of osteochondritis dissecans in situ by retrograde drilling and cancellous bone graft: A preliminary report. Clin. Orthop. Relat. Res. 1981, 158, 129–136. [Google Scholar] [CrossRef]
- Ramponi, L.; Yasui, Y.; Murawski, C.D.; Ferkel, R.D.; DiGiovanni, C.W.; Kerkhoffs, G.M.M.J.; Calder, J.D.F.; Takao, M.; Vannini, F.; Choi, W.J.; et al. Lesion size is a predictor of clinical outcomes after bone marrow stimulation for osteochondral lesions of the talus: A systematic review. Am. J. Sports Med. 2017, 45, 1698–1705. [Google Scholar] [CrossRef]
- Corominas, L.; Sanpera, I., Jr.; Masrouha, K.; Sanpera-Iglesias, J. Retrograde Percutaneous Drilling for Osteochondritis Dissecans of the Head of the Talus: Case Report and Review of the Literature. J. Foot Ankle Surg. 2016, 55, 328–332. [Google Scholar] [CrossRef]
- Dahmen, J.; Lambers, K.T.A.; Reilingh, M.L.; van Bergen, C.J.A.; Stufkens, S.A.S.; Kerkhoffs, G.M.M.J. No superior treatment for primary osteochondral defects of the talus. Knee Surg. Sports Traumatol. Arthrosc. 2018, 26, 2142–2157. [Google Scholar] [CrossRef]
- O’Loughlin, P.F.; Kendoff, D.; Pearle, A.D.; Kennedy, J.G. Arthroscopic-assisted fluoroscopic navigation for retrograde drilling of a talar osteochondral lesion. Foot Ankle Int. 2009, 30, 70–73. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhong, Y.; Wei, S.; Wu, H.; Zheng, B.; Xu, F. Retrograde Drilling and Bone Graft for Hepple Stage V Subchondral Bone Lesion of Talus Using 3D Image-Based Navigation-Assisted Endoscopic Technique. Foot Ankle Int. 2023, 44, 1003–1012. [Google Scholar] [CrossRef] [PubMed]
- Veronesi, F.; Maglio, M.; Brogini, S.; Mazzotti, A.; Artioli, E.; Giavaresi, G. A Systematic Review of the Retrograde Drilling Approach for Osteochondral Lesion of the Talus: Questioning Surgical Approaches, Outcome Evaluation and Gender-Related Differences. J. Clin. Med. 2023, 12, 4523. [Google Scholar] [CrossRef] [PubMed]
- Takao, M.; Innami, K.; Komatsu, F.; Matsushita, T. Retrograde cancellous bone plug transplantation for the treatment of advanced osteochondral lesions with large subchondral lesions of the ankle. Am. J. Sports Med. 2010, 38, 1653–1660. [Google Scholar] [CrossRef] [PubMed]
- Kerimaa, P.; Ojala, R.; Sinikumpu, J.J.; Hyvönen, P.; Korhonen, J.; Markkanen, P.; Tervonen, O.; Sequeiros, R.B. MRI-guided percutaneous retrograde drilling of osteochondritis dissecans of the talus: A feasibility study. Eur. Radiol. 2014, 24, 1572–1576. [Google Scholar] [CrossRef] [PubMed]
- Saxena, A.; Maffulli, N.; Jin, A.; Isa, E.; Jaswal, J.; Allen, R. Outcomes of Talar Osteochondral and Transchondral Lesions Using an Algorithmic Approach Based on Size, Location, and Subchondral Plate Integrity: A 10-Year Study on 204 Lesions. J. Foot Ankle Surg. 2022, 61, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Lu, R.; Zhang, J.; Tao, H.; Hua, Y. Outcomes of arthroscopic bone graft transplantation for Hepple stage V osteochondral lesions of the talus. Ann. Transl. Med. 2021, 9, 884. [Google Scholar] [CrossRef]
- Gorgun, B.; Gamlı, A.; Duran, M.E.; Bayram, B.; Ulku, T.K.; Kocaoglu, B. Collagen Scaffold Application in Arthroscopic Reconstruction of Osteochondral Lesions of the Talus with Autologous Cancellous Bone Grafts. Orthop. J. Sports Med. 2023, 11, 23259671221145733. [Google Scholar] [CrossRef] [PubMed]
- ISO 9001:2015; Quality Management Systems Requirements. ISO: Geneva, Switzerland, 2015.
- Taranow, W.S.; Bisignani, G.A.; Towers, J.D.; Conti, S.F. Retrograde drilling of osteochondral lesions of the medial talar dome. Foot Ankle Int. 1999, 20, 474–480. [Google Scholar] [CrossRef]
- Shimozono, Y.; Yasui, Y.; Ross, A.W.; Miyamoto, W.; Kennedy, J.G. Scaffolds based therapy for osteochondral lesions of the talus: A systematic review. World J. Orthop. 2017, 8, 798–808. [Google Scholar] [CrossRef]
- Ramzan, F.; Salim, A.; Khan, I. Osteochondral Tissue Engineering Dilemma: Scaffolding Trends in Regenerative Medicine. Stem Cell Rev. Rep. 2023, 19, 1615–1634. [Google Scholar] [CrossRef] [PubMed]
- Vannini, F.; Berveglieri, L.; Boffa, A.; Filardo, G.; Viglione, V.; Buda, R.; Giannini, S.; Faldini, C. Hyaluronic scaffold transplantation with bone marrow concentrate for the treatment of osteochondral lesions of the talus: Durable results up to a minimum of 10 years. Knee Surg. Sports Traumatol. Arthrosc. 2023, 31, 4551–4558. [Google Scholar] [CrossRef] [PubMed]
- Dargoush, S.A.; Hanaee-Ahvaz, H.; Irani, S.; Soleimani, M.; Khatami, S.M.; Sohi, A.N. A composite bilayer scaffold functionalized for osteochondral tissue regeneration in rat animal model. J. Tissue Eng. Regen. Med. 2022, 16, 559–574. [Google Scholar] [CrossRef] [PubMed]
- Kilic, A.I.; Hapa, O.; Ozmanevra, R.; Pak, T.; Akokay, P.; Ergur, P.U.; Kosay, M.C. Histomorphological Investigation of Microfracture Location in a Rabbit Osteochondral Defect Model. Am. J. Sports Med. 2023, 51, 3025–3034. [Google Scholar] [CrossRef] [PubMed]
- Basal, O.; Ozmen, O.; Deliormanli, A.M. Effect of polycaprolactone scaffolds containing different weights of graphene on healing in large osteochondral defect model. J. Biomater. Sci. Polym. Ed. 2022, 33, 1123–1139. [Google Scholar] [CrossRef]
- Muñoz-Salamanca, J.A.; Gutierrez, M.; Echevarría-Trujillo, Á. Retrograde “Sandwich” Technique and Implantation of Minced Cartilage in a Hyaluronic Acid Scaffold for Deep Osteochondral Knee Lesions. Arthrosc. Tech. 2023, 12, e395–e400. [Google Scholar] [CrossRef] [PubMed]
- Zavan, B.; Giorgi, C.; Bagnara, G.P.; Vindigni, V.; Abatangelo, G.; Cortivo, R. Osteogenic and chondrogenic differentiation: Comparison of human and rat bone marrow mesenchymal stem cells cultured into polymeric scaffolds. Eur. J. Histochem. 2007, 51 (Suppl. 1), 1–8. [Google Scholar] [PubMed]
- Chen, R.; Pye, J.S.; Li, J.; Little, C.B.; Li, J.J. Multiphasic scaffolds for the repair of osteochondral defects: Outcomes of preclinical studies. Bioact. Mater. 2023, 27, 505–545. [Google Scholar] [CrossRef] [PubMed]
- González Díaz, E.C.; Sinha, S.; Avedian, R.S.; Yang, F. Tissue-engineered 3D models for elucidating primary and metastatic bone cancer progression. Acta Biomater. 2019, 99, 18–32. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Singh, Y.P.; Moses, J.C.; Bhardwaj, N.; Mandal, B.B. Overcoming the Dependence on Animal Models for Osteoarthritis Therapeutics—The Promises and Prospects of In Vitro Models. Adv. Healthc. Mater. 2021, 10, e2100961. [Google Scholar] [CrossRef] [PubMed]
- Veronesi, F.; Torricelli, P.; Martini, L.; Tschon, M.; Giavaresi, G.; Bellini, D.; Casagranda, V.; Alemani, F.; Fini, M. An alternative ex vivo method to evaluate the osseointegration of Ti-6Al-4V alloy also combined with collagen. Biomed. Mater. 2021, 16, 025007. [Google Scholar] [CrossRef] [PubMed]
- Veronesi, F.; Brogini, S.; De Luca, A.; Bellini, D.; Casagranda, V.; Fini, M.; Giavaresi, G. Cell Adhesion and Initial Bone Matrix Deposition on Titanium-Based Implants with Chitosan-Collagen Coatings: An In Vitro Study. Int. J. Mol. Sci. 2023, 24, 4810. [Google Scholar] [CrossRef] [PubMed]


| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Male and female patients suffering from primary or secondary arthritis of the ankle (Kellgren–Lawrence—KL 4) | Presence of active infections at the time of inclusion in the study |
| Thickness of waste osteocartilaginous tissue ≥ 3 mm | State of pregnancy |
| Age ≥ 18 years | Any other pathology or state that makes surgical treatment contraindicated |
| Patients able to provide written informed consent to the study | Patients suffering from autoimmune or rheumatological diseases |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veronesi, F.; Maglio, M.; Brogini, S.; Mazzotti, A.; Artioli, E.; Zielli, S.O.; Faldini, C.; Giavaresi, G. Regeneration of Osteochondral Lesion of the Talus with Retrograde Drilling Technique: An In Vitro Pilot Study. J. Clin. Med. 2024, 13, 4138. https://doi.org/10.3390/jcm13144138
Veronesi F, Maglio M, Brogini S, Mazzotti A, Artioli E, Zielli SO, Faldini C, Giavaresi G. Regeneration of Osteochondral Lesion of the Talus with Retrograde Drilling Technique: An In Vitro Pilot Study. Journal of Clinical Medicine. 2024; 13(14):4138. https://doi.org/10.3390/jcm13144138
Chicago/Turabian StyleVeronesi, Francesca, Melania Maglio, Silvia Brogini, Antonio Mazzotti, Elena Artioli, Simone Ottavio Zielli, Cesare Faldini, and Gianluca Giavaresi. 2024. "Regeneration of Osteochondral Lesion of the Talus with Retrograde Drilling Technique: An In Vitro Pilot Study" Journal of Clinical Medicine 13, no. 14: 4138. https://doi.org/10.3390/jcm13144138






