Exploration of the Muribaculaceae Family in the Gut Microbiota: Diversity, Metabolism, and Function
Abstract
:1. Introduction
2. Materials and Methods
3. Diversity of Muribaculaceae
4. Metabolism of Muribaculaceae
4.1. Polysaccharides
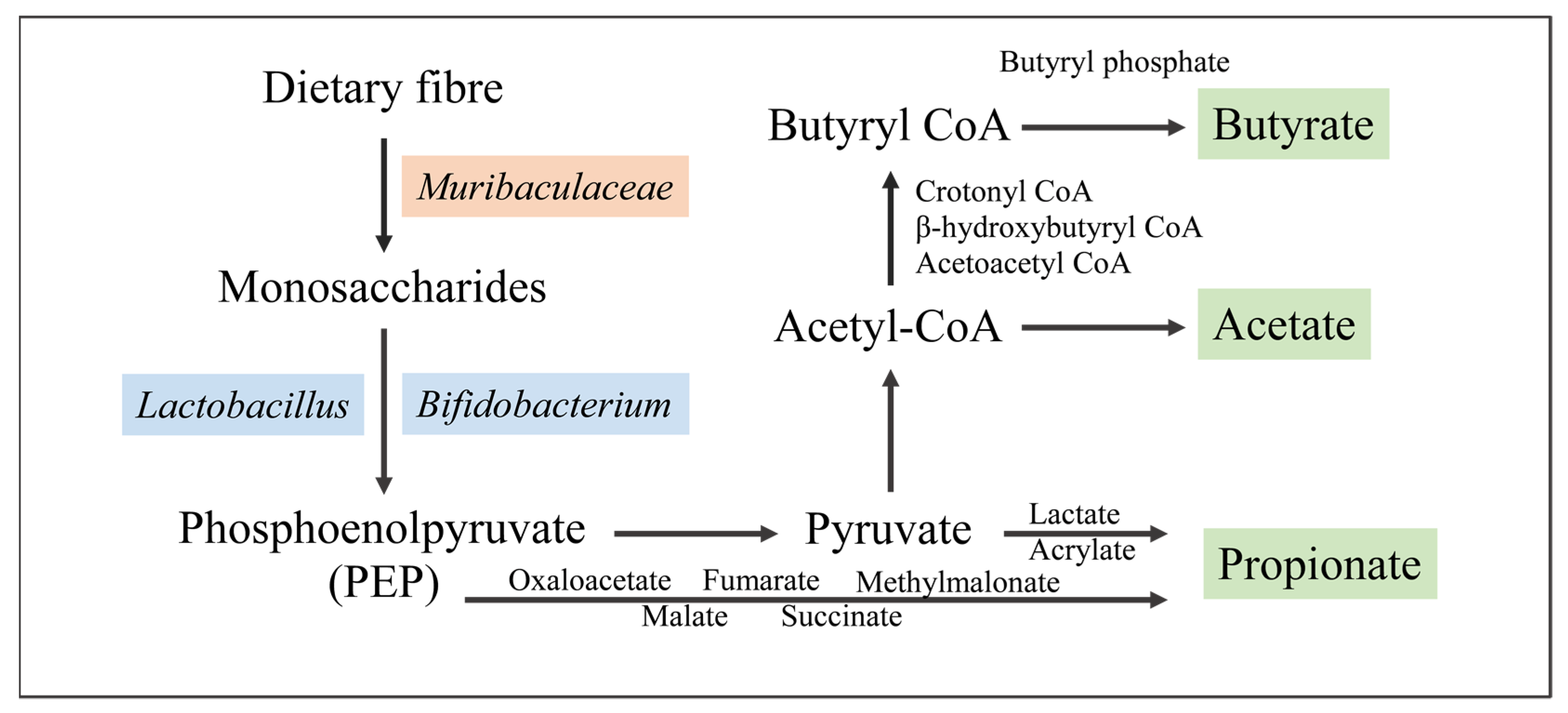
4.2. Short-Chain Fatty Acids
4.3. Probiotics
4.4. Others
5. Functions of Muribaculaceae
5.1. Inflammatory Bowel Disease (IBD)
5.2. Type 2 Diabetes (T2D)
5.3. Obesity
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wexler, A.G.; Goodman, A.L. An insider’s perspective: Bacteroides as a window into the microbiome. Nat. Microbiol. 2017, 2, 17026. [Google Scholar] [CrossRef] [PubMed]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Zhang, Y.; Nan, L.; Zhu, Q.; Wang, S.; Xie, Y.; Dong, X.; Cao, C.; Lin, X.; Lu, Y. Impact of structurally diverse polysaccharides on colonic mucin O-glycosylation and gut microbiota. NPJ Biofilms Microbiomes 2023, 9, 97. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Caulfield, L.E.; Garcia-Larsen, V.; Steffen, L.M.; Coresh, J.; Rebholz, C.M. Plant-based diets are associated with a lower risk of incident cardiovascular disease, cardiovascular disease mortality, and all-cause mortality in a general population of middle-aged adults. J. Am. Heart Assoc. 2019, 8, e012865. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Shen, G.X. Impact of anthocyanin component and metabolite of Saskatoon berry on gut microbiome and relationship with fecal short chain fatty acids in diet-induced insulin resistant mice. J. Nutr. Biochem. 2023, 111, 109201. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-R.; Jia, R.-B.; Wu, J.; Lin, L.; Ou, Z.-R.; Liao, B.; Zhang, L.; Zhang, X.; Song, G.; Zhao, M. Sargassum fusiforme polysaccharide partly replaces acarbose against type 2 diabetes in rats. Int. J. Biol. Macromol. 2021, 170, 447–458. [Google Scholar] [CrossRef]
- Chen, Q.; Luo, Y.; Shen, Y.; Li, X.; Yang, H.; Li, J.; Wang, J.; Xiao, Y. Fructose corn syrup induces inflammatory injury and obesity by altering gut microbiota and gut microbiota-related arachidonic acid metabolism. J. Nutr. Biochem. 2024, 124, 109527. [Google Scholar] [CrossRef] [PubMed]
- Makioka, Y.; Tsukahara, T.; Ijichi, T.; Inoue, R. Oral supplementation of Bifidobacterium longum strain BR-108 alters cecal microbiota by stimulating gut immune system in mice irrespectively of viability. Biosci. Biotechnol. Biochem. 2018, 82, 1180–1187. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Chen, Y.-T.; Li, K.-Y.; Chen, M.-J. Investigating the mechanistic differences of obesity-inducing Lactobacillus kefiranofaciens M1 and anti-obesity Lactobacillus mali APS1 by microbolomics and metabolomics. Front. Microbiol. 2020, 11, 1454. [Google Scholar] [CrossRef]
- Yin, T.; Bayanjargal, S.; Fang, B.; Inaba, C.; Mutoh, M.; Kawahara, T.; Tanaka, S.; Watanabe, J. Lactobacillus plantarum Shinshu N-07 isolated from fermented Brassica rapa L. attenuates visceral fat accumulation induced by high-fat diet in mice. Benef. Microbes 2020, 11, 655–667. [Google Scholar] [CrossRef]
- Bao, C.; Liu, S.; Shang, Z.; Liu, Y.; Wang, J.; Zhang, W.; Dong, B.; Cao, Y. Bacillus amyloliquefaciens TL106 protects mice against enterohaemorrhagic Escherichia coli O157: H7-induced intestinal disease through improving immune response, intestinal barrier function and gut microbiota. J. Appl. Microbiol. 2021, 131, 470–484. [Google Scholar] [CrossRef] [PubMed]
- Ormerod, K.L.; Wood, D.L.; Lachner, N.; Gellatly, S.L.; Daly, J.N.; Parsons, J.D.; Dal’Molin, C.G.; Palfreyman, R.W.; Nielsen, L.K.; Cooper, M.A. Genomic characterization of the uncultured Bacteroidales family S24-7 inhabiting the guts of homeothermic animals. Microbiome 2016, 4, 36. [Google Scholar] [CrossRef]
- Chung, Y.W.; Gwak, H.-J.; Moon, S.; Rho, M.; Ryu, J.-H. Functional dynamics of bacterial species in the mouse gut microbiome revealed by metagenomic and metatranscriptomic analyses. PLoS ONE 2020, 15, e0227886. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.J.; Miller, R.A.; Schmidt, T.M. Muribaculaceae genomes assembled from metagenomes suggest genetic drivers of differential response to acarbose treatment in mice. Msphere 2021, 6, e00851-21. [Google Scholar] [CrossRef] [PubMed]
- Lagkouvardos, I.; Lesker, T.R.; Hitch, T.C.; Gálvez, E.J.; Smit, N.; Neuhaus, K.; Wang, J.; Baines, J.F.; Abt, B.; Stecher, B. Sequence and cultivation study of Muribaculaceae reveals novel species, host preference, and functional potential of this yet undescribed family. Microbiome 2019, 7, 28. [Google Scholar] [CrossRef]
- Miyake, S.; Ding, Y.; Soh, M.; Low, A.; Seedorf, H. Muribaculum gordoncarteri sp. nov., an anaerobic bacterium from the faeces of C57BL/6J mice. Int. J. Syst. Evol. Microbiol. 2020, 70, 4725–4729. [Google Scholar] [CrossRef] [PubMed]
- Miyake, S.; Ding, Y.; Soh, M.; Seedorf, H. Complete genome sequence of Duncaniella muris strain B8, isolated from the feces of C57/BL6 mice. Microbiol. Resour. Announc. 2019, 8, e00566-19. [Google Scholar] [CrossRef]
- Miyake, S.; Ding, Y.; Soh, M.; Low, A.; Seedorf, H. Cultivation and description of Duncaniella dubosii sp. nov., Duncaniella freteri sp. nov. and emended description of the species Duncaniella muris. Int. J. Syst. Evol. Microbiol. 2020, 70, 3105–3110. [Google Scholar] [CrossRef]
- Park, J.K.; Chang, D.-H.; Rhee, M.-S.; Jeong, H.; Song, J.; Ku, B.J.; Kim, S.B.; Lee, M.; Kim, B.-C. Heminiphilus faecis gen. nov., sp. nov., a member of the family Muribaculaceae, isolated from mouse faeces and emended description of the genus Muribaculum. Antonie Van Leeuwenhoek 2021, 114, 275–286. [Google Scholar] [CrossRef]
- Lagkouvardos, I.; Pukall, R.; Abt, B.; Foesel, B.U.; Meier-Kolthoff, J.P.; Kumar, N.; Bresciani, A.; Martínez, I.; Just, S.; Ziegler, C. The Mouse Intestinal Bacterial Collection (miBC) provides host-specific insight into cultured diversity and functional potential of the gut microbiota. Nat. Microbiol. 2016, 1, 16131. [Google Scholar] [CrossRef]
- Schoch, C.L.; Ciufo, S.; Domrachev, M.; Hotton, C.L.; Kannan, S.; Khovanskaya, R.; Leipe, D.; Mcveigh, R.; O’Neill, K.; Robbertse, B. NCBI Taxonomy: A comprehensive update on curation, resources and tools. Database 2020, 2020, baaa062. [Google Scholar] [CrossRef]
- Wylensek, D.; Hitch, T.C.; Riedel, T.; Afrizal, A.; Kumar, N.; Wortmann, E.; Liu, T.; Devendran, S.; Lesker, T.R.; Hernández, S.B. A collection of bacterial isolates from the pig intestine reveals functional and taxonomic diversity. Nat. Commun. 2020, 11, 6389. [Google Scholar] [CrossRef] [PubMed]
- Afrizal, A.; Jennings, S.A.; Hitch, T.C.; Riedel, T.; Basic, M.; Panyot, A.; Treichel, N.; Hager, F.T.; Wong, E.O.-Y.; Wolter, B. Enhanced cultured diversity of the mouse gut microbiota enables custom-made synthetic communities. Cell Host Microbe 2022, 30, 1630–1645.e25. [Google Scholar] [CrossRef] [PubMed]
- Hinsu, A.T.; Pandit, R.J.; Patel, S.H.; Psifidi, A.; Tomley, F.M.; Das, S.K.; Blake, D.P.; Joshi, C.G. Genome reconstruction of a novel carbohydrate digesting bacterium from the chicken caecal microflora. Meta Gene 2019, 20, 100543. [Google Scholar] [CrossRef]
- Gilroy, R.; Ravi, A.; Getino, M.; Pursley, I.; Horton, D.L.; Alikhan, N.-F.; Baker, D.; Gharbi, K.; Hall, N.; Watson, M. Extensive microbial diversity within the chicken gut microbiome revealed by metagenomics and culture. PeerJ 2021, 9, e10941. [Google Scholar] [CrossRef] [PubMed]
- Forster, S.C.; Clare, S.; Beresford-Jones, B.S.; Harcourt, K.; Notley, G.; Stares, M.; Kumar, N.; Soderholm, A.T.; Adoum, A.; Wong, H. Novel gut pathobionts confound results in a widely used mouse model of human inflammatory disease. bioRxiv 2021, 02, 430393. [Google Scholar]
- Wang, Y.; Nan, X.; Zhao, Y.; Jiang, L.; Wang, H.; Zhang, F.; Hua, D.; Liu, J.; Yao, J.; Yang, L. Dietary supplementation of inulin ameliorates subclinical mastitis via regulation of rumen microbial community and metabolites in dairy cows. Microbiol. Spectr. 2021, 9, e00105-21. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Liu, J.; Gao, J.; Wu, X.; Cui, C.; Wei, H.; Zheng, R.; Peng, J. Combined soluble fiber-mediated intestinal microbiota improve insulin sensitivity of obese mice. Nutrients 2020, 12, 351. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Wu, Y.; Pham, Q.; Li, R.W.; Yu, L.; Chen, M.-H.; Boue, S.M.; Yokoyama, W.; Li, B.; Wang, T.T. Effects of differences in resistant starch content of rice on intestinal microbial composition. J. Agric. Food Chem. 2021, 69, 8017–8027. [Google Scholar] [CrossRef]
- Zou, H.; Gao, H.; Liu, Y.; Zhang, Z.; Zhao, J.; Wang, W.; Ren, B.; Tan, X. Dietary inulin alleviated constipation induced depression and anxiety-like behaviors: Involvement of gut microbiota and microbial metabolite short-chain fatty acid. Int. J. Biol. Macromol. 2024, 259, 129420. [Google Scholar] [CrossRef]
- Li, X.; Liang, J.; Gao, W.; Zhang, Y.; Zeng, H.; Zheng, B. Effects of butyrylated lotus seed starch on small intestinal bacteria and short-chain fatty acid production in mice. Food Biosci. 2023, 55, 102931. [Google Scholar] [CrossRef]
- Watanabe, K.; Yamano, M.; Masujima, Y.; Ohue-Kitano, R.; Kimura, I. Curdlan intake changes gut microbial composition, short-chain fatty acid production, and bile acid transformation in mice. Biochem. Biophys. Rep. 2021, 27, 101095. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Zou, X.; Liang, Y.; Zhong, J.; Zhou, K.; Zhang, J.; Zhang, M.; Wang, Z.; Sun, Y.; Li, M. Hypoglycemic effects of different molecular weight konjac glucomannans via intestinal microbiota and SCFAs mediated mechanism. Int. J. Biol. Macromol. 2023, 234, 122941. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Hu, H.; Dai, X.; Che, H.; Zhang, H. Effects of dietary intake of potatoes on body weight gain, satiety-related hormones, and gut microbiota in healthy rats. RSC Adv. 2019, 9, 33290–33301. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Mazcorro, J.F.; Lage, N.N.; Mertens-Talcott, S.; Talcott, S.; Chew, B.; Dowd, S.E.; Kawas, J.R.; Noratto, G.D. Effect of dark sweet cherry powder consumption on the gut microbiota, short-chain fatty acids, and biomarkers of gut health in obese db/db mice. PeerJ 2018, 6, e4195. [Google Scholar] [CrossRef]
- Usui, Y.; Kimura, Y.; Satoh, T.; Takemura, N.; Ouchi, Y.; Ohmiya, H.; Kobayashi, K.; Suzuki, H.; Koyama, S.; Hagiwara, S. Effects of long-term intake of a yogurt fermented with Lactobacillus delbrueckii subsp. bulgaricus 2038 and Streptococcus thermophilus 1131 on mice. Int. Immunol. 2018, 30, 319–331. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, Y.; Ma, F.; Sun, M.; Mu, G.; Tuo, Y. The ameliorative effect of Lactobacillus plantarum Y44 oral administration on inflammation and lipid metabolism in obese mice fed with a high fat diet. Food Funct. 2020, 11, 5024–5039. [Google Scholar] [CrossRef] [PubMed]
- Aindelis, G.; Ypsilantis, P.; Chlichlia, K. Alterations in faecal microbiota and elevated levels of intestinal IgA following oral administration of Lacticaseibacillus casei in mice. Probiotics Antimicrob. Proteins 2021, 15, 524–534. [Google Scholar] [CrossRef]
- Xie, Z.; Li, M.; Qian, M.; Yang, Z.; Han, X. Co-cultures of Lactobacillus acidophilus and Bacillus subtilis enhance mucosal barrier by modulating gut microbiota-derived short-chain fatty acids. Nutrients 2022, 14, 4475. [Google Scholar] [CrossRef]
- Lee, K.S.; Palatinszky, M.; Pereira, F.C.; Nguyen, J.; Fernandez, V.I.; Mueller, A.J.; Menolascina, F.; Daims, H.; Berry, D.; Wagner, M. An automated Raman-based platform for the sorting of live cells by functional properties. Nat. Microbiol. 2019, 4, 1035–1048. [Google Scholar] [CrossRef]
- Paone, P.; Cani, P.D. Mucus barrier, mucins and gut microbiota: The expected slimy partners? Gut 2020, 69, 2232–2243. [Google Scholar] [CrossRef] [PubMed]
- Crouch, L.; Liberato, M.; Urbanowicz, P.; Baslé, A.; Lamb, C.; Stewart, C.; Cooke, K.; Doona, M.; Needham, S.; Brady, R. Prominent members of the human gut microbiota express endo-acting O-glycanases to initiate mucin breakdown. Nat. Commun. 2020, 11, 4017. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Fu, T.; Cheng, H.; Mi, J.; Shang, Q.; Yu, G. Polysaccharide from edible alga Gloiopeltis furcata attenuates intestinal mucosal damage by therapeutically remodeling the interactions between gut microbiota and mucin O-glycans. Carbohydr. Polym. 2022, 278, 118921. [Google Scholar] [CrossRef]
- Pereira, F.C.; Wasmund, K.; Cobankovic, I.; Jehmlich, N.; Herbold, C.W.; Lee, K.S.; Sziranyi, B.; Vesely, C.; Decker, T.; Stocker, R. Rational design of a microbial consortium of mucosal sugar utilizers reduces Clostridiodes difficile colonization. Nat. Commun. 2020, 11, 5104. [Google Scholar] [CrossRef]
- Zhou, K. Strategies to promote abundance of Akkermansia muciniphila, an emerging probiotics in the gut, evidence from dietary intervention studies. J. Funct. Foods 2017, 33, 194–201. [Google Scholar] [CrossRef]
- Pei, L.; Liu, W.; Liu, L.; Wang, X.; Jiang, L.; Chen, Z.; Wang, Q.; Wang, P.; Xu, H. Morel (Morchella spp.) intake alters gut microbial community and short-chain fatty acid profiles in mice. Front. Nutr. 2023, 10, 1237237. [Google Scholar] [CrossRef] [PubMed]
- Moens, F.; Verce, M.; De Vuyst, L. Lactate-and acetate-based cross-feeding interactions between selected strains of lactobacilli, bifidobacteria and colon bacteria in the presence of inulin-type fructans. Int. J. Food Microbiol. 2017, 241, 225–236. [Google Scholar] [CrossRef]
- Coyte, K.Z.; Rakoff-Nahoum, S. Understanding competition and cooperation within the mammalian gut microbiome. Curr. Biol. 2019, 29, R538–R544. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P. Glycan utilisation system in Bacteroides and Bifidobacteria and their roles in gut stability and health. Appl. Microbiol. Biotechnol. 2019, 103, 7287–7315. [Google Scholar] [CrossRef]
- Zeng, Z.; Guo, X.; Zhang, J.; Yuan, Q.; Chen, S. Lactobacillus paracasei modulates the gut microbiota and improves inflammation in type 2 diabetic rats. Food Funct. 2021, 12, 6809–6820. [Google Scholar] [CrossRef]
- Yun, S.-w.; Son, Y.-H.; Lee, D.-Y.; Shin, Y.-J.; Han, M.J.; Kim, D.-H. Lactobacillus plantarum and Bifidobacterium bifidum alleviate dry eye in mice with exorbital lacrimal gland excision by modulating gut inflammation and microbiota. Food Funct. 2021, 12, 2489–2497. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Liu, Y.; Ma, F.; Sun, M.; Song, Y.; Xu, D.; Mu, G.; Tuo, Y. Lactobacillus plantarum Y44 alleviates oxidative stress by regulating gut microbiota and colonic barrier function in Balb/C mice with subcutaneous d-galactose injection. Food Funct. 2021, 12, 373–386. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, S.; Wang, Y.; Bian, H.; Yu, S.; Huang, L.; Ma, W. Complex probiotics alleviate ampicillin-induced antibiotic-associated diarrhea in mice. Front. Microbiol. 2023, 14, 1156058. [Google Scholar] [CrossRef] [PubMed]
- Mu, Z.; Yang, Y.; Xia, Y.; Wang, F.; Sun, Y.; Yang, Y.; Ai, L. Probiotic yeast BR14 ameliorates DSS-induced colitis by restoring the gut barrier and adjusting the intestinal microbiota. Food Funct. 2021, 12, 8386–8398. [Google Scholar] [CrossRef]
- Dong, J.-P.; Zheng, Y.; Wu, T.; He, Q.; Teng, G.-G.; Wang, H.-H. Protective effect of Saccharomyces boulardii on intestinal mucosal barrier of dextran sodium sulfate-induced colitis in mice. Chin. Med. J. 2019, 132, 1951–1958. [Google Scholar] [CrossRef] [PubMed]
- Gryaznova, M.; Burakova, I.; Smirnova, Y.; Morozova, P.; Chirkin, E.; Gureev, A.; Mikhaylov, E.; Korneeva, O.; Syromyatnikov, M. Effect of Probiotic Bacteria on the Gut Microbiome of Mice with Lipopolysaccharide-Induced Inflammation. Microorganisms 2024, 12, 1341. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Lu, W.; Li, L.; Zhang, H.; Lee, Y.k.; Chen, W.; Zhao, J. Both living and dead Faecalibacterium prausnitzii alleviate house dust mite-induced allergic asthma through the modulation of gut microbiota and short-chain fatty acid production. J. Sci. Food Agric. 2021, 101, 5563–5573. [Google Scholar] [CrossRef] [PubMed]
- Pei, L.; Liu, J.; Huang, Z.; Iqbal, M.; Shen, Y. Effects of lactic acid bacteria isolated from equine on salmonella-infected gut mouse model. Probiot. Antimicrob. Proteins 2021, 15, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yang, Z.; Wu, G.; Xu, F.; Zhang, J.; Luo, X.; Ma, Y.; Pang, H.; Duan, Y.; Chen, J. Effects of Probiotic-Fermented Feed on the Growth Profile, Immune Functions, and Intestinal Microbiota of Bamei Piglets. Animals 2024, 14, 647. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, J.; Xie, S.; Zhou, Y.; Wang, T.; Liu, Z.; Li, C.; Gao, L.; Pan, T. Increased abundance of bacteria of the family Muribaculaceae achieved by fecal microbiome transplantation correlates with the inhibition of kidney calcium oxalate stone deposition in experimental rats. Front. Cell. Infect. Microbiol. 2023, 13, 1145196. [Google Scholar] [CrossRef]
- Wang, H.; Wang, G.; Banerjee, N.; Liang, Y.; Du, X.; Boor, P.J.; Hoffman, K.L.; Khan, M.F. Aberrant gut microbiome contributes to intestinal oxidative stress, barrier dysfunction, inflammation and systemic autoimmune responses in MRL/lpr mice. Front. Immunol. 2021, 12, 651191. [Google Scholar] [CrossRef] [PubMed]
- Volk, J.K.; Nyström, E.E.; van der Post, S.; Abad, B.M.; Schroeder, B.O.; Johansson, Å.; Svensson, F.; Jäverfelt, S.; Johansson, M.E.; Hansson, G.C. The Nlrp6 inflammasome is not required for baseline colonic inner mucus layer formation or function. J. Exp. Med. 2019, 216, 2602–2618. [Google Scholar] [CrossRef] [PubMed]
- Li, A.-l.; Ni, W.-w.; Li, Y.; Zhang, X.; Yang, J.-j.; Ma, X.-y.; Jia, X.-d.; Li, C.; Liu, L.-b. Effect of 2′-fucosyllactose supplementation on intestinal flora in mice with intestinal inflammatory diseases. Int. Dairy J. 2020, 110, 104797. [Google Scholar] [CrossRef]
- Luo, J.; Wang, Z.; Fan, B.; Wang, L.; Liu, M.; An, Z.; Zhao, X. A comparative study of the effects of different fucoidans on cefoperazone-induced gut microbiota disturbance and intestinal inflammation. Food Funct. 2021, 12, 9087–9097. [Google Scholar] [CrossRef]
- Li, A.l.; Ni, W.w.; Zhang, Q.m.; Li, Y.; Zhang, X.; Wu, H.y.; Du, P.; Hou, J.c.; Zhang, Y. Effect of cinnamon essential oil on gut microbiota in the mouse model of dextran sodium sulfate-induced colitis. Microbiol. Immunol. 2020, 64, 23–32. [Google Scholar] [CrossRef] [PubMed]
- van der Hee, B.; Wells, J.M. Microbial regulation of host physiology by short-chain fatty acids. Trends Microbiol. 2021, 29, 700–712. [Google Scholar] [CrossRef] [PubMed]
- Alatawi, H.; Mosli, M.; Saadah, O.I.; Annese, V.; Al-Hindi, R.; Alatawy, M.; Al-Amrah, H.; Alshehri, D.; Bahieldin, A.; Edris, S. Attributes of intestinal microbiota composition and their correlation with clinical primary non-response to anti-TNF-α agents in inflammatory bowel disease patients. Bosn. J. Basic Med. Sci. 2022, 22, 412. [Google Scholar]
- Monk, J.M.; Lepp, D.; Zhang, C.P.; Wu, W.; Zarepoor, L.; Lu, J.T.; Pauls, K.P.; Tsao, R.; Wood, G.A.; Robinson, L.E. Diets enriched with cranberry beans alter the microbiota and mitigate colitis severity and associated inflammation. J. Nutr. Biochem. 2016, 28, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Li, Z.; Gao, Y.; Zhao, K.; Lin, M.; Li, Y.; Wang, S.; Liu, Y.; Chen, L. Construction of a “bacteria-metabolites” co-expression network to clarify the anti–ulcerative colitis effect of flavonoids of Sophora flavescens Aiton by regulating the “host–microbe” interaction. Front. Pharmacol. 2021, 12, 710052. [Google Scholar] [CrossRef]
- Shao, X.; Sun, C.; Tang, X.; Zhang, X.; Han, D.; Liang, S.; Qu, R.; Hui, X.; Shan, Y.; Hu, L. Anti-inflammatory and intestinal microbiota modulation properties of Jinxiang garlic (Allium sativum L.) polysaccharides toward dextran sodium sulfate-induced colitis. J. Agric. Food Chem. 2020, 68, 12295–12309. [Google Scholar] [CrossRef]
- Xia, X.; Lin, H.; Luo, F.; Wu, X.; Zhu, L.; Chen, S.; Luo, H.; Ye, F.; Peng, X.; Zhang, Y. Oryzanol ameliorates DSS-stimulated gut barrier damage via targeting the gut microbiota accompanied by the TLR4/NF-κB/NLRP3 cascade response in vivo. J. Agric. Food Chem. 2022, 70, 15747–15762. [Google Scholar] [CrossRef] [PubMed]
- Zhan, F.; Song, W.; Fan, Y.; Wang, F.; Wang, Q. Cucurbitacin E Alleviates Colonic Barrier Function Impairment and Inflammation Response and Improves Microbial Composition on Experimental Colitis Models. J. Inflamm. Res. 2024, 17, 2745–2756. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Geng, W.; Chen, S.; Wang, L.; Rong, X.; Wang, S.; Wang, T.; Xiong, L.; Huang, J.; Pang, X. Ginger alleviates DSS-induced ulcerative colitis severity by improving the diversity and function of gut microbiota. Front. Pharmacol. 2021, 12, 632569. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Sun, M.; Chang, Y.; Chen, H.; Zhang, J.; Liang, X.; Xiao, T. Butyrate ameliorates colorectal cancer through regulating intestinal microecological disorders. Anti-Cancer Drugs 2023, 34, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhou, M.; Yang, M.; Jin, C.; Song, Y.; Chen, J.; Gao, M.; Ai, Z.; Su, D. Pulsatilla chinensis saponins ameliorate inflammation and DSS-induced ulcerative colitis in rats by regulating the composition and diversity of intestinal flora. Front. Cell. Infect. Microbiol. 2021, 11, 728929. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Xu, X.; Maimaiti, A.; Hao, M.; Sang, X.; Shan, Q.; Wu, X.; Cao, G. Gut microbiota disorder caused by diterpenoids extracted from Euphorbia pekinensis aggravates intestinal mucosal damage. Pharmacol. Res. Perspect. 2021, 9, e00765. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Tian, S.; Wang, J.; Zhu, W. Galacto-oligosaccharides improve barrier function and relieve colonic inflammation via modulating mucosa-associated microbiota composition in lipopolysaccharides-challenged piglets. J. Anim. Sci. Biotechnol. 2021, 12, 92. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Xu, K.; Cai, X.; Wang, C.; Cao, Y.; Xiao, J. Rosmarinic acid restores colonic mucus secretion in colitis mice by regulating gut microbiota-derived metabolites and the activation of inflammasomes. J. Agric. Food Chem. 2023, 71, 4571–4585. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Wang, J.; Zhao, Y.; Zhou, Q.; Yang, X.; Gao, Y.; Li, Q.; Bai, M.; Liu, J.; Liang, Y. Study on the mechanism of modified Gegen Qinlian decoction in regulating the intestinal flora-bile acid-TGR5 axis for the treatment of type 2 diabetes mellitus based on macro genome sequencing and targeted metabonomics integration. Phytomedicine 2024, 132, 155329. [Google Scholar] [CrossRef]
- Du, Y.; Li, D.x.; Lu, D.y.; Zhang, R.; Zheng, X.x.; Xu, B.j.; Zhao, Y.l.; Ji, S.; Guo, M.z.; Wang, L. Morus alba L. water extract changes gut microbiota and fecal metabolome in mice induced by high-fat and high-sucrose diet plus low-dose streptozotocin. Phytother. Res. 2022, 36, 1241–1257. [Google Scholar] [CrossRef]
- Li, Z.-R.; Jia, R.-B.; Luo, D.; Lin, L.; Zheng, Q.; Zhao, M. The positive effects and underlying mechanisms of Undaria pinnatifida polysaccharides on type 2 diabetes mellitus in rats. Food Funct. 2021, 12, 11898–11912. [Google Scholar] [CrossRef]
- Zheng, J.; Li, H.; Zhang, X.; Jiang, M.; Luo, C.; Lu, Z.; Xu, Z.; Shi, J. Prebiotic mannan-oligosaccharides augment the hypoglycemic effects of metformin in correlation with modulating gut microbiota. J. Agric. Food Chem. 2018, 66, 5821–5831. [Google Scholar] [CrossRef]
- Tang, Y.; Yan, M.; Fang, Z.; Jin, S.; Xu, T. Effects of metformin, saxagliptin and repaglinide on gut microbiota in high-fat diet/streptozocin-induced type 2 diabetic mice. BMJ Open Diabetes Res. Care 2024, 12, e003837. [Google Scholar] [CrossRef]
- Dong, J.; Liang, Q.; Niu, Y.; Jiang, S.; Zhou, L.; Wang, J.; Ma, C.; Kang, W. Effects of Nigella sativa seed polysaccharides on type 2 diabetic mice and gut microbiota. Int. J. Biol. Macromol. 2020, 159, 725–738. [Google Scholar] [CrossRef]
- Chen, J.; Ding, X.; Wu, R.; Tong, B.; Zhao, L.; Lv, H.; Meng, X.; Liu, Y.; Ren, B.; Li, J. Novel sesquiterpene glycoside from loquat leaf alleviates type 2 diabetes mellitus combined with nonalcoholic fatty liver disease by improving insulin resistance, oxidative stress, inflammation, and gut microbiota composition. J. Agric. Food Chem. 2021, 69, 14176–14191. [Google Scholar] [CrossRef]
- Yu, F.; Han, W.; Zhan, G.; Li, S.; Jiang, X.; Wang, L.; Xiang, S.; Zhu, B.; Yang, L.; Luo, A. Abnormal gut microbiota composition contributes to the development of type 2 diabetes mellitus in db/db mice. Aging 2019, 11, 10454. [Google Scholar] [CrossRef]
- Deng, J.; Zhong, J.; Long, J.; Zou, X.; Wang, D.; Song, Y.; Zhou, K.; Liang, Y.; Huang, R.; Wei, X. Hypoglycemic effects and mechanism of different molecular weights of konjac glucomannans in type 2 diabetic rats. Int. J. Biol. Macromol. 2020, 165, 2231–2243. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, L.; Zhu, M.; Yang, B.; Yang, Y.; Jia, X.; Feng, L. Moutan cortex polysaccharide ameliorates diabetic kidney disease via modulating gut microbiota dynamically in rats. Int. J. Biol. Macromol. 2022, 206, 849–860. [Google Scholar] [CrossRef]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The firmicutes/bacteroidetes ratio: A relevant marker of gut dysbiosis in obese patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef]
- Clemente, J.C.; Pehrsson, E.C.; Blaser, M.J.; Sandhu, K.; Gao, Z.; Wang, B.; Magris, M.; Hidalgo, G.; Contreras, M.; Noya-Alarcón, Ó. The microbiome of uncontacted Amerindians. Sci. Adv. 2015, 1, e1500183. [Google Scholar] [CrossRef]
- Osborne, G.; Wu, F.; Yang, L.; Kelly, D.; Hu, J.; Li, H.; Jasmine, F.; Kibriya, M.G.; Parvez, F.; Shaheen, I. The association between gut microbiome and anthropometric measurements in Bangladesh. Gut Microbes 2020, 11, 63–76. [Google Scholar] [CrossRef]
- Qian, L.; Gao, R.; Hong, L.; Pan, C.; Li, H.; Huang, J.; Qin, H. Association analysis of dietary habits with gut microbiota of a native Chinese community. Exp. Ther. Med. 2018, 16, 856–866. [Google Scholar] [CrossRef]
- Barouei, J.; Bendiks, Z.; Martinic, A.; Mishchuk, D.; Heeney, D.; Hsieh, Y.H.; Kieffer, D.; Zaragoza, J.; Martin, R.; Slupsky, C. Microbiota, metabolome, and immune alterations in obese mice fed a high-fat diet containing type 2 resistant starch. Mol. Nutr. Food Res. 2017, 61, 1700184. [Google Scholar] [CrossRef]
- Muhomah, T.A.; Nishino, N.; Katsumata, E.; Haoming, W.; Tsuruta, T. High-fat diet reduces the level of secretory immunoglobulin A coating of commensal gut microbiota. Biosci. Microbiota Food Health 2019, 38, 55–64. [Google Scholar] [CrossRef]
- Liu, H.; Zhu, H.; Xia, H.; Yang, X.; Yang, L.; Wang, S.; Wen, J.; Sun, G. Different effects of high-fat diets rich in different oils on lipids metabolism, oxidative stress and gut microbiota. Food Res. Int. 2021, 141, 110078. [Google Scholar] [CrossRef]
- Hua, Y.; Fan, R.; Zhao, L.; Tong, C.; Qian, X.; Zhang, M.; Xiao, R.; Ma, W. Trans-fatty acids alter the gut microbiota in high-fat-diet-induced obese rats. Br. J. Nutr. 2020, 124, 1251–1263. [Google Scholar] [CrossRef]
- Júnior, R.E.M.; de Carvalho, L.M.; Pedersen, A.S.B.; Damasceno, S.; Maioli, T.U.; de Faria, A.M.C.; Godard, A.L.B. Interaction between high-fat diet and ethanol intake leads to changes on the fecal microbiome. J. Nutr. Biochem. 2019, 72, 108215. [Google Scholar]
- Ye, J.; Zhao, Y.; Chen, X.; Zhou, H.; Yang, Y.; Zhang, X.; Huang, Y.; Zhang, N.; Lui, E.M.; Xiao, M. Pu-erh tea ameliorates obesity and modulates gut microbiota in high fat diet fed mice. Food Res. Int. 2021, 144, 110360. [Google Scholar] [CrossRef]
- Mu, H.; Zhou, Q.; Yang, R.; Zeng, J.; Li, X.; Zhang, R.; Tang, W.; Li, H.; Wang, S.; Shen, T. Naringin attenuates high fat diet induced non-alcoholic fatty liver disease and gut bacterial dysbiosis in mice. Front. Microbiol. 2020, 11, 585066. [Google Scholar] [CrossRef]
- Loubet Filho, P.S.; Baseggio, A.M.; Vuolo, M.M.; Reguengo, L.M.; Biasoto, A.C.T.; Correa, L.C.; Junior, S.B.; Cagnon, V.H.A.; Cazarin, C.B.B.; Júnior, M.R.M. Gut microbiota modulation by jabuticaba peel and its effect on glucose metabolism via inflammatory signaling. Curr. Res. Food Sci. 2022, 5, 382–391. [Google Scholar] [CrossRef]
- Paone, P.; Suriano, F.; Jian, C.; Korpela, K.; Delzenne, N.M.; Van Hul, M.; Salonen, A.; Cani, P.D. Prebiotic oligofructose protects against high-fat diet-induced obesity by changing the gut microbiota, intestinal mucus production, glycosylation and secretion. Gut Microbes 2022, 14, 2152307. [Google Scholar] [CrossRef]
- Yu, Y.; Zeng, F.; Han, P.; Zhang, L.; Yang, L.; Zhou, F.; Liu, Q.; Ruan, Z. Dietary chlorogenic acid alleviates high-fat diet-induced steatotic liver disease by regulating metabolites and gut microbiota. Int. J. Food Sci. Nutr. 2024, 75, 369–384. [Google Scholar] [CrossRef]
- Yuan, G.; Tan, M.; Chen, X. Punicic acid ameliorates obesity and liver steatosis by regulating gut microbiota composition in mice. Food Funct. 2021, 12, 7897–7908. [Google Scholar] [CrossRef]
- Zhou, C.; Zhou, X.; Wen, Z.; Liu, L.; Yang, Z.; Yang, L.; Li, P.; Guo, X.; Mei, X. Compound Fu brick tea modifies the intestinal microbiome composition in high-fat diet-induced obesity mice. Food Sci. Nutr. 2020, 8, 5508–5520. [Google Scholar] [CrossRef]
- Zhang, M.; Yao, M.; Jia, A.; Shi, Y.; Bai, X.; Liu, X.; Cui, T.; Liu, X.; Liu, C. Hypolipidemic effect of soluble dietary fibers prepared from Asparagus officinalis and their effects on the modulation of intestinal microbiota. Food Sci. Biotechnol. 2021, 30, 1721–1731. [Google Scholar] [CrossRef]
| Bacteria Genus | Strain | Name | Genome Assembly | GenBank | References |
|---|---|---|---|---|---|
| Muribaculum | Muribaculum gordoncarteri | TLL-A4 | ASM480369v1 | GCA_004803695.1 | Miyake et al., 2020 [16] |
| Muribaculum intestinale | YL27 | ASM168884v2 | GCA_001688845.2 | Lagkouvardos et al., 2016 [20] | |
| Muribaculum sp. An287, Muribaculum sp. An289 | An287, An 289 | ASM215958v1 | GCA_002159585.1 | Schoch et al., 2020 [21] | |
| Duncaniella | Duncaniella dubosii, Duncaniella freteri | H5, TLL-A3 | ASM480391v1, ASM476612v1 | GCF_004803915.1, GCF_004766125.1 | Miyake et al., 2020 [18] |
| Duncaniella muris | DSM 103720 | ASM302480v1 | GCA_003024805.1 | Lagkouvardos et al., 2019 [15] | |
| Paramuribaculum | Paramuribaculum intestinale | DSM 100764 | ASM302481v1 | GCA_003024815.1 | Lagkouvardos et al., 2019 [15] |
| Sodaliphilus | Sodaliphilus pleomorphus | Oil-RF-744-WCA-WT-10 | ASM967695v1 | GCA_009676955.1 | Wylensek et al., 2020 [22] |
| Heminiphilus | Heminiphilus faecis | AM35T | ASM872896v1 | GCA_008728965.1 | Schoch et al., 2020 [21] |
| Lepagella | Lepagella muris | NM04_E33 | ASM479397v1 | GCA_004793975.1 | Afrizal et al., 2022 [23] |
| Candidatus amulumruptor | Candidatus amulumruptor caecigallinarius | Hinsu1 | ASM2074201v1 | GCA_020742015.1 | Hinsu et al., 2019 [24] |
| Candidatus merdivivens | Candidatus merdivivens faecigallinarum, Candidatus merdivivens pullicola, Candidatus merdivivens pullistercoris | B3-2255 | ASM1769505v1, ASM1769493v1 | GCA_017695055.1, GCA_017694935.1 | Gilroy et al., 2021 [25] |
| Candidatus homeothermus | Candidatus homeothermus arabinoxylanisolvens | M4 | / | / | Ormerod et al., 2016 [12] |
| Sangeribacter | Sangeribacter muris | A43 | / | / | Forster et al., 2021 [26] |
| Intervention Substance | Subject | Muribaculaceae Abundance | Short-Chain Fatty Acid | References |
|---|---|---|---|---|
| Inulin | Dairy cows | ↑ | Lactic acid, propionic acid, butyric acid | Wang et al., 2021 [27] |
| Soluble fibre | C57BL/6J mice | ↑ | Acetic acid, propionic acid | Xu et al., 2020 [28] |
| Resistant starch | C57BL/6 mice | ↑ | Acetic acid, propionic acid, butyric acid | Wan et al., 2021 [29] |
| Inulin | CD-1 mice | ↑ | Acetic acid, propionic acid, butyric acid | Zou et al., 2024 [30] |
| Resistant starch | BALB/c mice | ↑ | Acetic acid, butyric acid | Li et al., 2023 [31] |
| Corderan gum | C57BL/6J mice | ↑ | Acetic acid, propionic acid, butyric acid | Watanabe et al., 2021 [32] |
| Konjac glucomannans | SD rat | ↑ | Butyric acid | Deng et al., 2023 [33] |
| Potatoes | SD rat | ↑ | Acetic acid, propionic acid, butyric acid | Wu et al., 2019 [34] |
| Black cherry powder | SD rat | ↑ | Acetic acid, propionic acid, butyric acid | Garcia-Mazcorro et al., 2018 [35] |
| Lactobacillus delbrueckii, Streptococcus thermophilus 1131 | ICR mice | ↑ | Propionic acid, butyric acid | Usui et al., 2018 [36] |
| Lactobacillus plantarum Y44 | C57BL/6J mice | ↑ | Acetic acid, propionic acid, butyric acid, valerate acid | Liu et al., 2020 [37] |
| Lacticaseibacillus casei ATCC393 | BALB/c mice | ↑ | Lactic acid, acetic acid | Aindelis et al., 2021 [38] |
| Lactobacillus acidophilus, Bacillus subtilis | Piglets | ↑ | Butyric acid | Xie et al., 2022 [39] |
| Intervention Substance | Subject | Muribaculaceae Abundance | References |
|---|---|---|---|
| Lactobacillus plantarum Shinshu N-07, Lactobacillus curvatus #4G2 | C57BL/6J mice | ↑ | Yin et al., 2020 [10] |
| Lactobacillus kefirnofaciens M1, Lactobacillus mali APS1 | C57BL/6J mice | ↑ | Lin et al., 2020 [9] |
| Lactobacillus paracasei NL41 | SD rat | ↑ | Zeng et al., 2021 [50] |
| Lactobacillus plantarum NK151, Bifidobacterium bifidum NK175 | C57BL/6 mice | ↑ | Yun et al., 2021 [51] |
| Lactobacillus plantarum Y44 | BALB/c mice | ↑ | Gao et al., 2021 [52] |
| Bifidobacterium longum BR-108 | BALB/c mice | ↑ | Makioka et al., 2018 [8] |
| Bifidobacterium lactis XLTG11, Lactobacillus casei Zhang, Lactobacillus plantarum CCFM8661, Lactobacillus rhamnosus Probio-M9 | BALB/c mice | ↑ | Li et al., 2023 [53] |
| Saccharomyces boulardii BR14 | C57BL/6J mice | ↑ | Mu et al., 2021 [54] |
| Saccharomyces boulardii | C57BL/6J mice | ↑ | Dong et al., 2019 [55] |
| Bacillus amyloliquefaciens TL106 | C57BL/6J mice | ↑ | Bao et al., 2021 [11] |
| Lactobacillus plantarum, Weissella confusa | C57BL/6 mice | ↑ | Gryaznova et al., 2024 [56] |
| Faecalibacterium prausnitzii | BALB/c mice | ↑ | Hu et al., 2021 [57] |
| Weissella confuse, Pediococcus acidilactici, Ligilactobacillus equi | KM mice | ↑ | Pei et al., 2021 [58] |
| Lactobacillus plantarum QP28-1, Bacillus subtilis QB8 | Bamei piglets | ↑ | Zhang et al., 2024 [59] |
| Intervention Substance | Subject | Muribaculaceae Abundance | References | |
|---|---|---|---|---|
| Changes before Intervention | Changes after Intervention | |||
| Infliximab and adalimumab | IBD patients | ↓ | / | Alatawi et al., 2021 [67] |
| Recombinant mouse Il18 | C57BL/6 mice | ↓ | / | Volk et al., 2019 [62] |
| N-acetylcysteine | C57BL/6J mice | ↓ | ↑ | Wang et al., 2021 [61] |
| Cranberry beans | C57BL/6 mice | ↓ | ↑ | Monk et al., 2016 [68] |
| Flavones from matrine | C57BL/6 mice | ↓ | ↑ | Shao et al., 2021 [69] |
| Garlic polysaccharide | C57BL/6J mice | ↓ | ↑ | Shao et al., 2020 [70] |
| Oryzanol | C57BL/6J mice | ↓ | ↑ | Xia et al., 2022 [71] |
| 2′-Fucosyllactose | C57BL/6J mice | ↓ | ↑ | Li et al., 2020 [63] |
| Cucurbitacin E | C57BL/6J mice | ↓ | ↑ | Zhan et al., 2024 [72] |
| Cinnamon essential oil | KM mice | ↓ | ↑ | Li et al., 2020 [65] |
| Fresh ginger | BALB/c mice | ↓ | ↑ | Guo et al., 2021 [73] |
| Butyrate | BALB/c mice | ↓ | ↑ | Kang et al., 2023 [74] |
| Pulsatilla saponin | SD rat | ↓ | ↑ | Liu et al., 2021 [75] |
| Fucoidan | C57BL/6J mice | ↓ | ↑ | Luo et al., 2021 [64] |
| Euphorbia total diterpenoids | C57BL/6J mice | ↓ | ↑ | Wang et al., 2021 [76] |
| Galactooligosaccharide | Piglet | ↓ | ↑ | Gao et al., 2021 [77] |
| Rosmarinic acid | ICR mice | ↓ | ↑ | Wang et al., 2023 [78] |
| Intervention Substance | Subject | Muribaculaceae Abundance | References | |
|---|---|---|---|---|
| Changes before Intervention | Changes after Intervention | |||
| Acarbose | C57BL/6J mice | / | ↑ | Smith et al., 2021 [14] |
| Metformin, saxagliptin, and repaglinide | C57BL/6J mice | ↓ | ↑ | Tang et al., 2024 [83] |
| Black seed polysaccharide | KM mice | / | ↑ | Dong et al., 2020 [84] |
| Loquat leaf sesquiterpene | db/db mice | / | ↑ | Chen et al., 2021 [85] |
| Broad-spectrum antibiotics | db/db mice | ↓ | / | Yu et al., 2019 [86] |
| Gegen Qinlian decoction | db/db mice | / | ↑ | Liu et al., 2024 [79] |
| Konjac glucomannan | SD rat | ↓ | ↑ | Deng et al., 2020 [87] |
| Sargassum polysaccharide and acarbose | SD rat | ↓ | ↑ | Li et al., 2021 [6] |
| Wakame polysaccharide | SD rat | ↓ | ↑ | Li et al., 2021 [81] |
| Moutan cortex polysaccharide | SD rat | ↓ | ↑ | Zhang et al., 2022 [88] |
| Mannooligosaccharides | C57BL/6J mice | ↓ | ↑ | Zheng et al., 2021 [82] |
| Morus alba L. water extract | C57BL/6J mice | ↓ | ↑ | Du et al., 2022 [80] |
| Intervention Substance | Subject | Muribaculaceae Abundance | References | |
|---|---|---|---|---|
| Changes before Intervention | Changes after Intervention | |||
| Olive oil, lard oil, soybean oil | C57BL/6J mice | / | ↓ | Liu et al., 2021 [95] |
| Trans-fatty acids | C57BL/6J mice | / | ↓ | Hua et al., 2020 [96] |
| 10% alcohol solution | C57BL/6J mice | / | ↓ | Júnior et al., 2019 [97] |
| Pu-erh tea extract | C57BL/6J mice | ↓ | ↑ | Ye et al., 2021 [98] |
| Resistant starch | C57BL/6J mice | ↓ | ↑ | Barouei et al., 2017 [93] |
| Naringin | C57BL/6J mice | ↓ | ↑ | Mu et al., 2020 [99] |
| Jabuticaba peel | C57BL/6J mice | ↓ | ↑ | Loubet et al., 2022 [100] |
| Saskatoon berry | C57BL/6J mice | ↓ | ↑ | Zhao et al., 2023 [5] |
| Prebiotic oligofructose | C57BL/6J mice | ↓ | ↑ | Paone et al., 2023 [101] |
| Chlorogenic acid | C57BL/6J mice | ↓ | ↑ | Yu et al., 2024 [102] |
| Punicic acid | ICR mice | ↓ | ↑ | Yuan et al., 2020 [103] |
| Fu brick tea | KM mice | ↓ | ↑ | Zhou et al., 2020 [104] |
| Asparagus soluble fibre | KM mice | ↓ | ↑ | Zhang et al., 2021 [105] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Y.; Chen, B.; Zhang, X.; Akbar, M.T.; Wu, T.; Zhang, Y.; Zhi, L.; Shen, Q. Exploration of the Muribaculaceae Family in the Gut Microbiota: Diversity, Metabolism, and Function. Nutrients 2024, 16, 2660. https://doi.org/10.3390/nu16162660
Zhu Y, Chen B, Zhang X, Akbar MT, Wu T, Zhang Y, Zhi L, Shen Q. Exploration of the Muribaculaceae Family in the Gut Microbiota: Diversity, Metabolism, and Function. Nutrients. 2024; 16(16):2660. https://doi.org/10.3390/nu16162660
Chicago/Turabian StyleZhu, Yiqing, Borui Chen, Xinyu Zhang, Muhammad Toheed Akbar, Tong Wu, Yiyun Zhang, Li Zhi, and Qun Shen. 2024. "Exploration of the Muribaculaceae Family in the Gut Microbiota: Diversity, Metabolism, and Function" Nutrients 16, no. 16: 2660. https://doi.org/10.3390/nu16162660
APA StyleZhu, Y., Chen, B., Zhang, X., Akbar, M. T., Wu, T., Zhang, Y., Zhi, L., & Shen, Q. (2024). Exploration of the Muribaculaceae Family in the Gut Microbiota: Diversity, Metabolism, and Function. Nutrients, 16(16), 2660. https://doi.org/10.3390/nu16162660







