Role of the Bone Microenvironment in the Development of Painful Complications of Skeletal Metastases
Abstract
:1. Introduction
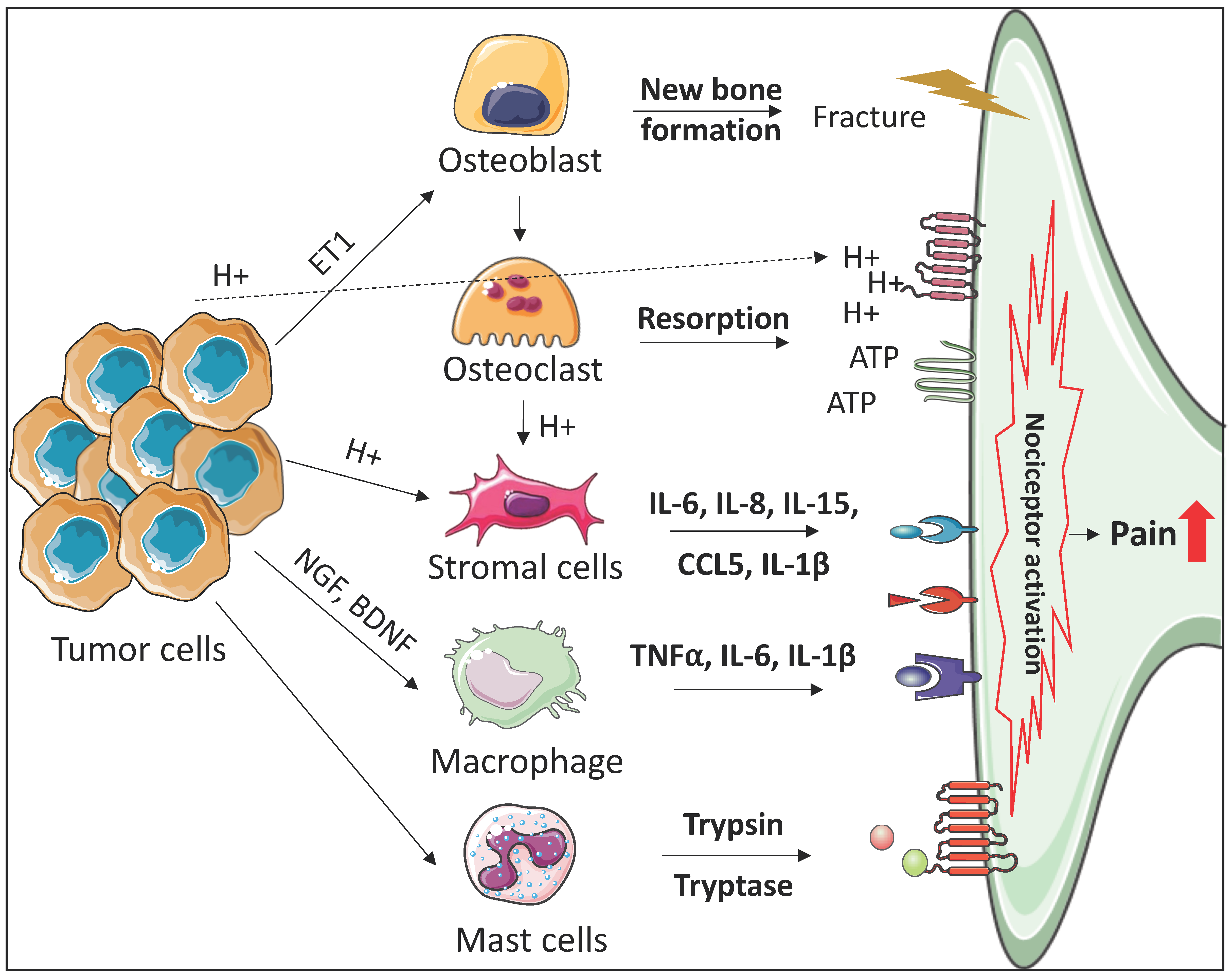
2. The Roles of the Bone Marrow Microenvironment in the Development of Cancer-Induced Bone Pain
2.1. Osteoclasts
2.2. Osteoblasts
2.3. Immune Cells
2.4. Stromal Cells
3. Discussion
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Tsuzuki, S.; Park, S.H.; Eber, M.R.; Peters, C.M.; Shiozawa, Y. Skeletal complications in cancer patients with bone metastases. Int. J. Urol. 2016, 23, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Meuser, T.; Pietruck, C.; Radbruch, L.; Stute, P.; Lehmann, K.A.; Grond, S. Symptoms during cancer pain treatment following who-guidelines: A longitudinal follow-up study of symptom prevalence, severity and etiology. Pain 2001, 93, 247–257. [Google Scholar] [CrossRef]
- Berruti, A.; Dogliotti, L.; Bitossi, R.; Fasolis, G.; Gorzegno, G.; Bellina, M.; Torta, M.; Porpiglia, F.; Fontana, D.; Angeli, A. Incidence of skeletal complications in patients with bone metastatic prostate cancer and hormone refractory disease: Predictive role of bone resorption and formation markers evaluated at baseline. J. Urol. 2000, 164, 1248–1253. [Google Scholar] [CrossRef]
- Laird, B.J.; Walley, J.; Murray, G.D.; Clausen, E.; Colvin, L.A.; Fallon, M.T. Characterization of cancer-induced bone pain: An exploratory study. Support Care Cancer 2011, 19, 1393–1401. [Google Scholar] [CrossRef] [PubMed]
- Benyamin, R.; Trescot, A.M.; Datta, S.; Buenaventura, R.; Adlaka, R.; Sehgal, N.; Glaser, S.E.; Vallejo, R. Opioid complications and side effects. Pain Phys. 2008, 11, S105–S120. [Google Scholar]
- Pergolizzi, J.; Boger, R.H.; Budd, K.; Dahan, A.; Erdine, S.; Hans, G.; Kress, H.G.; Langford, R.; Likar, R.; Raffa, R.B.; et al. Opioids and the management of chronic severe pain in the elderly: Consensus statement of an international expert panel with focus on the six clinically most often used world health organization step III opioids (buprenorphine, fentanyl, hydromorphone, methadone, morphine, oxycodone). Pain Pract. 2008, 8, 287–313. [Google Scholar] [PubMed]
- Mercadante, S. The use of anti-inflammatory drugs in cancer pain. Cancer Treat. Rev. 2001, 27, 51–61. [Google Scholar] [CrossRef] [PubMed]
- De Felice, F.; Piccioli, A.; Musio, D.; Tombolini, V. The role of radiation therapy in bone metastases management. Oncotarget 2017, 8, 25691–25699. [Google Scholar] [CrossRef] [PubMed]
- Stopeck, A.T.; Lipton, A.; Body, J.J.; Steger, G.G.; Tonkin, K.; de Boer, R.H.; Lichinitser, M.; Fujiwara, Y.; Yardley, D.A.; Viniegra, M.; et al. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: A randomized, double-blind study. J. Clin. Oncol. 2010, 28, 5132–5139. [Google Scholar] [CrossRef] [PubMed]
- Fizazi, K.; Carducci, M.; Smith, M.; Damiao, R.; Brown, J.; Karsh, L.; Milecki, P.; Shore, N.; Rader, M.; Wang, H.; et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: A randomised, double-blind study. Lancet 2011, 377, 813–822. [Google Scholar] [CrossRef]
- Badrising, S.K.; van der Noort, V.; Hamberg, P.; Coenen, J.L.; Aarts, M.J.; van Oort, I.M.; van den Eertwegh, A.J.; Los, M.; van den Berg, H.P.; Gelderblom, H.; et al. Enzalutamide as a fourth- or fifth-line treatment option for metastatic castration-resistant prostate cancer. Oncology 2016, 91, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Vignani, F.; Bertaglia, V.; Buttigliero, C.; Tucci, M.; Scagliotti, G.V.; Di Maio, M. Skeletal metastases and impact of anticancer and bone-targeted agents in patients with castration-resistant prostate cancer. Cancer Treat. Rev. 2016, 44, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Abou, D.S.; Ulmert, D.; Doucet, M.; Hobbs, R.F.; Riddle, R.C.; Thorek, D.L. Whole-body and microenvironmental localization of radium-223 in naive and mouse models of prostate cancer metastasis. J. Natl. Cancer Inst. 2016, 108, djv380. [Google Scholar] [CrossRef] [PubMed]
- Delaney, A.; Fleetwood-Walker, S.M.; Colvin, L.A.; Fallon, M. Translational medicine: Cancer pain mechanisms and management. Br. J. Anaesth. 2008, 101, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Ondoua, A.N.; Symons-Liguori, A.M.; Vanderah, T.W. Cancer-induced bone pain: Mechanisms and models. Neurosci. Lett. 2013, 557 Pt A, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Rowe, M.J.; Tracey, D.J.; Mahns, D.A.; Sahai, V.; Ivanusic, J.J. Mechanosensory perception: Are there contributions from bone-associated receptors? Clin. Exp. Pharmacol. Physiol. 2005, 32, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Nieder, C.; Pawinski, A.; Dalhaug, A. Continuous controversy about radiation oncologists’ choice of treatment regimens for bone metastases: Should we blame doctors, cancer-related features, or design of previous clinical studies? Radiat. Oncol. 2013, 8, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrafiello, G.; Lagana, D.; Pellegrino, C.; Mangini, M.; Fontana, F.; Piacentino, F.; Recaldini, C.; Rovera, F.; Dionigi, G.; Boni, L.; et al. Ablation of painful metastatic bone tumors: A systematic review. Int. J. Surg. 2008, 6 (Suppl. 1), S47–S52. [Google Scholar] [CrossRef] [PubMed]
- Mantyh, P.W.; Clohisy, D.R.; Koltzenburg, M.; Hunt, S.P. Molecular mechanisms of cancer pain. Nat. Rev. Cancer 2002, 2, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Peters, C.M.; Lindsay, T.H.; Pomonis, J.D.; Luger, N.M.; Ghilardi, J.R.; Sevcik, M.A.; Mantyh, P.W. Endothelin and the tumorigenic component of bone cancer pain. Neuroscience 2004, 126, 1043–1052. [Google Scholar] [CrossRef] [PubMed]
- Sevcik, M.A.; Ghilardi, J.R.; Peters, C.M.; Lindsay, T.H.; Halvorson, K.G.; Jonas, B.M.; Kubota, K.; Kuskowski, M.A.; Boustany, L.; Shelton, D.L.; et al. Anti-NGF therapy profoundly reduces bone cancer pain and the accompanying increase in markers of peripheral and central sensitization. Pain 2005, 115, 128–141. [Google Scholar] [CrossRef] [PubMed]
- Ghilardi, J.R.; Rohrich, H.; Lindsay, T.H.; Sevcik, M.A.; Schwei, M.J.; Kubota, K.; Halvorson, K.G.; Poblete, J.; Chaplan, S.R.; Dubin, A.E.; et al. Selective blockade of the capsaicin receptor TRPV1 attenuates bone cancer pain. J. Neurosci. 2005, 25, 3126–3131. [Google Scholar] [CrossRef] [PubMed]
- Yoneda, T.; Hata, K.; Nakanishi, M.; Nagae, M.; Nagayama, T.; Wakabayashi, H.; Nishisho, T.; Sakurai, T.; Hiraga, T. Involvement of acidic microenvironment in the pathophysiology of cancer-associated bone pain. Bone 2011, 48, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.; Trumpp, A. Bone-marrow haematopoietic-stem-cell niches. Nat. Rev. Immunol. 2006, 6, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Yin, T.; Li, L. The stem cell niches in bone. J. Clin. Investig. 2006, 116, 1195–1201. [Google Scholar] [CrossRef] [PubMed]
- Shiozawa, Y.; Eber, M.R.; Berry, J.E.; Taichman, R.S. Bone marrow as a metastatic niche for disseminated tumor cells from solid tumors. Bonekey Rep. 2015, 4, 689. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zhou, H.; Dunstan, C.R.; Sutherland, R.L.; Seibel, M.J. The role of the bone microenvironment in skeletal metastasis. J. Bone Oncol. 2013, 2, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Buenrostro, D.; Park, S.I.; Sterling, J.A. Dissecting the role of bone marrow stromal cells on bone metastases. BioMed Res. Int. 2014, 2014, 875305. [Google Scholar] [CrossRef] [PubMed]
- Chirgwin, J.M.; Guise, T.A. Molecular mechanisms of tumor-bone interactions in osteolytic metastases. Crit. Rev. Eukaryot. Gene Expr. 2000, 10, 159–178. [Google Scholar] [CrossRef] [PubMed]
- Guise, T.A.; Kozlow, W.M.; Heras-Herzig, A.; Padalecki, S.S.; Yin, J.J.; Chirgwin, J.M. Molecular mechanisms of breast cancer metastases to bone. Clin. Breast Cancer 2005, 5 (Suppl. 2), S46–S53. [Google Scholar] [CrossRef] [PubMed]
- Guise, T.A.; Mohammad, K.S.; Clines, G.; Stebbins, E.G.; Wong, D.H.; Higgins, L.S.; Vessella, R.; Corey, E.; Padalecki, S.; Suva, L.; et al. Basic mechanisms responsible for osteolytic and osteoblastic bone metastases. Clin. Cancer Res. 2006, 12, 6213s–6216s. [Google Scholar] [CrossRef] [PubMed]
- Kakonen, S.M.; Mundy, G.R. Mechanisms of osteolytic bone metastases in breast carcinoma. Cancer 2003, 97, 834–839. [Google Scholar] [CrossRef] [PubMed]
- Kingsley, L.A.; Fournier, P.G.; Chirgwin, J.M.; Guise, T.A. Molecular biology of bone metastasis. Mol. Cancer Ther. 2007, 6, 2609–2617. [Google Scholar] [CrossRef] [PubMed]
- Mundy, G.R. Mechanisms of bone metastasis. Cancer 1997, 80, 1546–1556. [Google Scholar] [CrossRef]
- Honore, P.; Luger, N.M.; Sabino, M.A.; Schwei, M.J.; Rogers, S.D.; Mach, D.B.; O’Keefe P, F.; Ramnaraine, M.L.; Clohisy, D.R.; Mantyh, P.W. Osteoprotegerin blocks bone cancer-induced skeletal destruction, skeletal pain and pain-related neurochemical reorganization of the spinal cord. Nat. Med. 2000, 6, 521–528. [Google Scholar] [PubMed]
- Luger, N.M.; Honore, P.; Sabino, M.A.; Schwei, M.J.; Rogers, S.D.; Mach, D.B.; Clohisy, D.R.; Mantyh, P.W. Osteoprotegerin diminishes advanced bone cancer pain. Cancer Res. 2001, 61, 4038–4047. [Google Scholar] [PubMed]
- Schwei, M.J.; Honore, P.; Rogers, S.D.; Salak-Johnson, J.L.; Finke, M.P.; Ramnaraine, M.L.; Clohisy, D.R.; Mantyh, P.W. Neurochemical and cellular reorganization of the spinal cord in a murine model of bone cancer pain. J. Neurosci. 1999, 19, 10886–10897. [Google Scholar] [CrossRef] [PubMed]
- Qin, A.; Cheng, T.S.; Pavlos, N.J.; Lin, Z.; Dai, K.R.; Zheng, M.H. V-atpases in osteoclasts: Structure, function and potential inhibitors of bone resorption. Int. J. Biochem. Cell Biol. 2012, 44, 1422–1435. [Google Scholar] [CrossRef] [PubMed]
- Lingueglia, E. Acid-sensing ion channels in sensory perception. J. Biol. Chem. 2007, 282, 17325–17329. [Google Scholar] [CrossRef] [PubMed]
- Holzer, P. Acid-sensitive ion channels and receptors. Handb. Exp. Pharmacol. 2009, 283–332. [Google Scholar]
- Li, Y.; Cai, J.; Han, Y.; Xiao, X.; Meng, X.L.; Su, L.; Liu, F.Y.; Xing, G.G.; Wan, Y. Enhanced function of TRPV1 via up-regulation by insulin-like growth factor-1 in a rat model of bone cancer pain. Eur. J. Pain 2014, 18, 774–784. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Zhang, X.M.; Duan, K.Z.; Gu, X.Y.; Han, M.; Liu, B.L.; Zhao, Z.Q.; Zhang, Y.Q. Peripheral TGF-beta1 signaling is a critical event in bone cancer-induced hyperalgesia in rodents. J. Neurosci. 2013, 33, 19099–19111. [Google Scholar] [CrossRef] [PubMed]
- Kadenbach, B.; Huttemann, M.; Arnold, S.; Lee, I.; Bender, E. Mitochondrial energy metabolism is regulated via nuclear-coded subunits of cytochrome C oxidase. Free Radic. Biol. Med. 2000, 29, 211–221. [Google Scholar] [CrossRef]
- Brandao-Burch, A.; Key, M.L.; Patel, J.J.; Arnett, T.R.; Orriss, I.R. The P2X7 receptor is an important regulator of extracellular ATP levels. Front. Endocrinol. (Lausanne) 2012, 3, 41. [Google Scholar] [CrossRef] [PubMed]
- Reyes, J.P.; Sims, S.M.; Dixon, S.J. P2 receptor expression, signaling and function in osteoclasts. Front. Biosci. (Schol. Ed.) 2011, 3, 1101–1118. [Google Scholar] [CrossRef] [PubMed]
- North, R.A. P2X3 receptors and peripheral pain mechanisms. J. Physiol. 2004, 554, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Wirkner, K.; Sperlagh, B.; Illes, P. P2X3 receptor involvement in pain states. Mol. Neurobiol. 2007, 36, 165–183. [Google Scholar] [CrossRef] [PubMed]
- Kaan, T.K.; Yip, P.K.; Patel, S.; Davies, M.; Marchand, F.; Cockayne, D.A.; Nunn, P.A.; Dickenson, A.H.; Ford, A.P.; Zhong, Y.; et al. Systemic blockade of P2X3 and P2X2/3 receptors attenuates bone cancer pain behaviour in rats. Brain 2010, 133, 2549–2564. [Google Scholar] [CrossRef] [PubMed]
- Burnstock, G. P2X receptors in sensory neurones. Br. J. Anaesth. 2000, 84, 476–488. [Google Scholar] [CrossRef] [PubMed]
- Hansen, R.R.; Nasser, A.; Falk, S.; Baldvinsson, S.B.; Ohlsson, P.H.; Bahl, J.M.; Jarvis, M.F.; Ding, M.; Heegaard, A.M. Chronic administration of the selective P2X3, P2X2/3 receptor antagonist, A-317491, transiently attenuates cancer-induced bone pain in mice. Eur. J. Pharmacol. 2012, 688, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.X.; Xu, M.Y.; Miao, X.R.; Lu, Z.J.; Yuan, X.M.; Li, X.Q.; Yu, W.F. Functional up-regulation of p2X3 receptors in dorsal root ganglion in a rat model of bone cancer pain. Eur. J. Pain 2012, 16, 1378–1388. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Rodriguez, S.; Pevida, M.; Roques, B.P.; Fournie-Zaluski, M.C.; Hidalgo, A.; Menendez, L.; Baamonde, A. Involvement of enkephalins in the inhibition of osteosarcoma-induced thermal hyperalgesia evoked by the blockade of peripheral P2X3 receptors. Neurosci. Lett. 2009, 465, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Epstein, T.; Gatenby, R.A.; Brown, J.S. The warburg effect as an adaptation of cancer cells to rapid fluctuations in energy demand. PLoS ONE 2017, 12, e0185085. [Google Scholar] [CrossRef] [PubMed]
- Grygorczyk, R.; Furuya, K.; Sokabe, M. Imaging and characterization of stretch-induced ATP release from alveolar a549 cells. J. Physiol. 2013, 591, 1195–1215. [Google Scholar] [CrossRef] [PubMed]
- Hoebertz, A.; Townsend-Nicholson, A.; Glass, R.; Burnstock, G.; Arnett, T.R. Expression of P2 receptors in bone and cultured bone cells. Bone 2000, 27, 503–510. [Google Scholar] [CrossRef]
- Morrison, M.S.; Turin, L.; King, B.F.; Burnstock, G.; Arnett, T.R. ATP is a potent stimulator of the activation and formation of rodent osteoclasts. J. Physiol. 1998, 511 Pt 2, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Li, X.; Koh, A.J.; Berry, J.E.; Thudi, N.; Rosol, T.J.; Pienta, K.J.; McCauley, L.K. Tumor expressed PTHRP facilitates prostate cancer-induced osteoblastic lesions. Int. J. Cancer 2008, 123, 2267–2278. [Google Scholar] [CrossRef] [PubMed]
- Achbarou, A.; Kaiser, S.; Tremblay, G.; Ste-Marie, L.G.; Brodt, P.; Goltzman, D.; Rabbani, S.A. Urokinase overproduction results in increased skeletal metastasis by prostate cancer cells in vivo. Cancer Res. 1994, 54, 2372–2377. [Google Scholar] [PubMed]
- Killian, C.S.; Corral, D.A.; Kawinski, E.; Constantine, R.I. Mitogenic response of osteoblast cells to prostate-specific antigen suggests an activation of latent TGF-beta and a proteolytic modulation of cell adhesion receptors. Biochem. Biophys. Res. Commun. 1993, 192, 940–947. [Google Scholar] [CrossRef] [PubMed]
- Kitano, Y.; Kurihara, H.; Kurihara, Y.; Maemura, K.; Ryo, Y.; Yazaki, Y.; Harii, K. Gene expression of bone matrix proteins and endothelin receptors in endothelin-1-deficient mice revealed by in situ hybridization. J. Bone Miner. Res. 1998, 13, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Kasperk, C.H.; Borcsok, I.; Schairer, H.U.; Schneider, U.; Nawroth, P.P.; Niethard, F.U.; Ziegler, R. Endothelin-1 is a potent regulator of human bone cell metabolism in vitro. Calcif. Tissue Int. 1997, 60, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.J.; Mohammad, K.S.; Kakonen, S.M.; Harris, S.; Wu-Wong, J.R.; Wessale, J.L.; Padley, R.J.; Garrett, I.R.; Chirgwin, J.M.; Guise, T.A. A causal role for endothelin-1 in the pathogenesis of osteoblastic bone metastases. Proc. Natl. Acad. Sci. USA 2003, 100, 10954–10959. [Google Scholar] [CrossRef] [PubMed]
- Pomonis, J.D.; Rogers, S.D.; Peters, C.M.; Ghilardi, J.R.; Mantyh, P.W. Expression and localization of endothelin receptors: Implications for the involvement of peripheral GLIA in nociception. J. Neurosci. 2001, 21, 999–1006. [Google Scholar] [CrossRef] [PubMed]
- Nelson, J.B.; Nabulsi, A.A.; Vogelzang, N.J.; Breul, J.; Zonnenberg, B.A.; Daliani, D.D.; Schulman, C.C.; Carducci, M.A. Suppression of prostate cancer induced bone remodeling by the endothelin receptor a antagonist atrasentan. J. Urol. 2003, 169, 1143–1149. [Google Scholar] [CrossRef] [PubMed]
- Carducci, M.A.; Saad, F.; Abrahamsson, P.A.; Dearnaley, D.P.; Schulman, C.C.; North, S.A.; Sleep, D.J.; Isaacson, J.D.; Nelson, J.B.; Atrasentan Phase, I.I.I.S.G.I. A phase 3 randomized controlled trial of the efficacy and safety of Atrasentan in men with metastatic hormone-refractory prostate cancer. Cancer 2007, 110, 1959–1966. [Google Scholar] [CrossRef] [PubMed]
- Wacnik, P.W.; Eikmeier, L.J.; Ruggles, T.R.; Ramnaraine, M.L.; Walcheck, B.K.; Beitz, A.J.; Wilcox, G.L. Functional interactions between tumor and peripheral nerve: Morphology, algogen identification, and behavioral characterization of a new murine model of cancer pain. J. Neurosci. 2001, 21, 9355–9366. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Liang, Y.; Li, N.; Hu, X.; Luo, D.; Gu, J.; Lu, Y.; Zheng, Q. Endothelin-A receptor antagonists in prostate cancer treatment-a meta-analysis. Int. J. Clin. Exp. Med. 2015, 8, 3465–3473. [Google Scholar] [PubMed]
- Weiner, S.; Wagner, H.D. The material bone: Structure-mechanical function relations. Annu. Rev. Mater. Sci. 1998, 28, 271–298. [Google Scholar]
- Prondvai, E.; Stein, K.H.W.; de Ricqlès, A.; Cubo, J. Development-based revision of bone tissue classification: The importance of semantics for science. Biol. J. Linn. Soc. 2014, 112, 799–816. [Google Scholar] [CrossRef]
- Halvorson, K.G.; Sevcik, M.A.; Ghilardi, J.R.; Rosol, T.J.; Mantyh, P.W. Similarities and differences in tumor growth, skeletal remodeling and pain in an osteolytic and osteoblastic model of bone cancer. Clin. J. Pain 2006, 22, 587–600. [Google Scholar] [CrossRef] [PubMed]
- Raoof, R.; Willemen, H.; Eijkelkamp, N. Divergent roles of immune cells and their mediators in pain. Rheumatology (Oxford) 2018, 57, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Hiraoka, K.; Zenmyo, M.; Watari, K.; Iguchi, H.; Fotovati, A.; Kimura, Y.N.; Hosoi, F.; Shoda, T.; Nagata, K.; Osada, H.; et al. Inhibition of bone and muscle metastases of lung cancer cells by a decrease in the number of monocytes/macrophages. Cancer Sci. 2008, 99, 1595–1602. [Google Scholar] [CrossRef] [PubMed]
- Sawa-Wejksza, K.; Kandefer-Szerszen, M. Tumor-associated macrophages as target for antitumor therapy. Arch. Immunol. Ther. Exp. 2018, 66, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Zelenka, M.; Schafers, M.; Sommer, C. Intraneural injection of interleukin-1beta and tumor necrosis factor-alpha into rat sciatic nerve at physiological doses induces signs of neuropathic pain. Pain 2005, 116, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Adriaenssens, E.; Vanhecke, E.; Saule, P.; Mougel, A.; Page, A.; Romon, R.; Nurcombe, V.; Le Bourhis, X.; Hondermarck, H. Nerve growth factor is a potential therapeutic target in breast cancer. Cancer Res. 2008, 68, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Hondermarck, H. Neurotrophins and their receptors in breast cancer. Cytokine Growth Factor Rev. 2012, 23, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.S.; Killebrew, D.A.; Clary, G.P.; Seawell, J.A.; Meeker, R.B. Differential regulation of macrophage phenotype by mature and pro-nerve growth factor. J. Neuroimmunol. 2015, 285, 76–93. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.C.; Kainz, V.; Burstein, R.; Levy, D. Tumor necrosis factor-alpha induces sensitization of meningeal nociceptors mediated via local COX and P38 map kinase actions. Pain 2011, 152, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Binshtok, A.M.; Wang, H.; Zimmermann, K.; Amaya, F.; Vardeh, D.; Shi, L.; Brenner, G.J.; Ji, R.R.; Bean, B.P.; Woolf, C.J.; et al. Nociceptors are interleukin-1beta sensors. J. Neurosci. 2008, 28, 14062–14073. [Google Scholar] [CrossRef] [PubMed]
- Huang, E.J.; Reichardt, L.F. Neurotrophins: Roles in neuronal development and function. Annu. Rev. Neurosci. 2001, 24, 677–736. [Google Scholar] [CrossRef] [PubMed]
- Barrios-Rodiles, M.; Chadee, K. Novel regulation of cyclooxygenase-2 expression and prostaglandin e2 production by IFN-gamma in human macrophages. J. Immunol. 1998, 161, 2441–2448. [Google Scholar] [PubMed]
- Sabino, M.C.; Ghilardi, J.R.; Feia, K.J.; Jongen, J.L.; Keyser, C.P.; Luger, N.M.; Mach, D.B.; Peters, C.M.; Rogers, S.D.; Schwei, M.J.; et al. The involvement of prostaglandins in tumorigenesis, tumor-induced osteolysis and bone cancer pain. J. Musculoskelet. Neuronal Interact. 2002, 2, 561–562. [Google Scholar] [PubMed]
- Fox, A.; Medhurst, S.; Courade, J.P.; Glatt, M.; Dawson, J.; Urban, L.; Bevan, S.; Gonzalez, I. Anti-hyperalgesic activity of the COX-2 inhibitor lumiracoxib in a model of bone cancer pain in the rat. Pain 2004, 107, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Sabino, M.A.; Ghilardi, J.R.; Jongen, J.L.; Keyser, C.P.; Luger, N.M.; Mach, D.B.; Peters, C.M.; Rogers, S.D.; Schwei, M.J.; de Felipe, C.; et al. Simultaneous reduction in cancer pain, bone destruction, and tumor growth by selective inhibition of cyclooxygenase-2. Cancer Res. 2002, 62, 7343–7349. [Google Scholar] [PubMed]
- Bottner, F.; Roedl, R.; Wortler, K.; Grethen, C.; Winkelmann, W.; Lindner, N. Cyclooxygenase-2 inhibitor for pain management in osteoid osteoma. Clin. Orthop. Relat. Res. 2001, 258–263. [Google Scholar] [CrossRef]
- Carpintero-Benitez, P.; Aguirre, M.A.; Serrano, J.A.; Lluch, M. Effect of rofecoxib on pain caused by osteoid osteoma. Orthopedics 2004, 27, 1188–1191. [Google Scholar] [PubMed]
- Vane, J.R. Introduction: Mechanism of action of NSAIDs. Br. J. Rheumatol. 1996, 35 (Suppl. 1), 1–3. [Google Scholar] [CrossRef] [PubMed]
- Laneuville, O.; Breuer, D.K.; Dewitt, D.L.; Hla, T.; Funk, C.D.; Smith, W.L. Differential inhibition of human prostaglandin endoperoxide h synthases-1 and -2 by nonsteroidal anti-inflammatory drugs. J. Pharmacol. Exp. Ther. 1994, 271, 927–934. [Google Scholar] [PubMed]
- Antman, E.M.; Bennett, J.S.; Daugherty, A.; Furberg, C.; Roberts, H.; Taubert, K.A. Use of nonsteroidal antiinflammatory drugs. Update Clin. Sci. Statement Am. Heart Assoc. 2007, 115, 1634–1642. [Google Scholar]
- Bombardier, C.; Laine, L.; Reicin, A.; Shapiro, D.; Burgos-Vargas, R.; Davis, B.; Day, R.; Ferraz, M.B.; Hawkey, C.J.; Hochberg, M.C.; et al. Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. Vigor study group. N. Engl. J. Med. 2000, 343, 1520–1528. [Google Scholar] [CrossRef] [PubMed]
- Isono, M.; Suzuki, T.; Hosono, K.; Hayashi, I.; Sakagami, H.; Uematsu, S.; Akira, S.; DeClerck, Y.A.; Okamoto, H.; Majima, M. Microsomal prostaglandin e synthase-1 enhances bone cancer growth and bone cancer-related pain behaviors in mice. Life Sci. 2011, 88, 693–700. [Google Scholar] [CrossRef] [PubMed]
- McCaffrey, G.; Thompson, M.L.; Majuta, L.; Fealk, M.N.; Chartier, S.; Longo, G.; Mantyh, P.W. Ngf blockade at early times during bone cancer development attenuates bone destruction and increases limb use. Cancer Res. 2014, 74, 7014–7023. [Google Scholar] [CrossRef] [PubMed]
- Halvorson, K.G.; Kubota, K.; Sevcik, M.A.; Lindsay, T.H.; Sotillo, J.E.; Ghilardi, J.R.; Rosol, T.J.; Boustany, L.; Shelton, D.L.; Mantyh, P.W. A blocking antibody to nerve growth factor attenuates skeletal pain induced by prostate tumor cells growing in bone. Cancer Res. 2005, 65, 9426–9435. [Google Scholar] [CrossRef] [PubMed]
- Bloom, A.P.; Jimenez-Andrade, J.M.; Taylor, R.N.; Castaneda-Corral, G.; Kaczmarska, M.J.; Freeman, K.T.; Coughlin, K.A.; Ghilardi, J.R.; Kuskowski, M.A.; Mantyh, P.W. Breast cancer-induced bone remodeling, skeletal pain, and sprouting of sensory nerve fibers. J. Pain 2011, 12, 698–711. [Google Scholar] [CrossRef] [PubMed]
- Lane, N.E.; Schnitzer, T.J.; Birbara, C.A.; Mokhtarani, M.; Shelton, D.L.; Smith, M.D.; Brown, M.T. Tanezumab for the treatment of pain from osteoarthritis of the knee. N. Engl. J. Med. 2010, 363, 1521–1531. [Google Scholar] [CrossRef] [PubMed]
- Schnitzer, T.J.; Ekman, E.F.; Spierings, E.L.; Greenberg, H.S.; Smith, M.D.; Brown, M.T.; West, C.R.; Verburg, K.M. Efficacy and safety of tanezumab monotherapy or combined with non-steroidal anti-inflammatory drugs in the treatment of knee or hip osteoarthritis pain. Ann. Rheum. Dis. 2015, 74, 1202–1211. [Google Scholar] [CrossRef] [PubMed]
- Katz, N.; Borenstein, D.G.; Birbara, C.; Bramson, C.; Nemeth, M.A.; Smith, M.D.; Brown, M.T. Efficacy and safety of tanezumab in the treatment of chronic low back pain. Pain 2011, 152, 2248–2258. [Google Scholar] [CrossRef] [PubMed]
- Bramson, C.; Herrmann, D.N.; Carey, W.; Keller, D.; Brown, M.T.; West, C.R.; Verburg, K.M.; Dyck, P.J. Exploring the role of tanezumab as a novel treatment for the relief of neuropathic pain. Pain Med. 2015, 16, 1163–1176. [Google Scholar] [CrossRef] [PubMed]
- Sopata, M.; Katz, N.; Carey, W.; Smith, M.D.; Keller, D.; Verburg, K.M.; West, C.R.; Wolfram, G.; Brown, M.T. Efficacy and safety of tanezumab in the treatment of pain from bone metastases. Pain 2015, 156, 1703–1713. [Google Scholar] [CrossRef] [PubMed]
- Bunnett, N.W. Protease-activated receptors: How proteases signal to cells to cause inflammation and pain. Semin. Thromb. Hemost. 2006, 32 (Suppl. 1), 39–48. [Google Scholar] [CrossRef] [PubMed]
- Vergnolle, N.; Bunnett, N.W.; Sharkey, K.A.; Brussee, V.; Compton, S.J.; Grady, E.F.; Cirino, G.; Gerard, N.; Basbaum, A.I.; Andrade-Gordon, P.; et al. Proteinase-activated receptor-2 and hyperalgesia: A novel pain pathway. Nat. Med. 2001, 7, 821–826. [Google Scholar] [CrossRef] [PubMed]
- Mrozkova, P.; Palecek, J.; Spicarova, D. The role of protease-activated receptor type 2 in nociceptive signaling and pain. Physiol. Res. 2016, 65, 357–367. [Google Scholar] [PubMed]
- Reed, D.E.; Barajas-Lopez, C.; Cottrell, G.; Velazquez-Rocha, S.; Dery, O.; Grady, E.F.; Bunnett, N.W.; Vanner, S.J. Mast cell tryptase and proteinase-activated receptor 2 induce hyperexcitability of guinea-pig submucosal neurons. J. Physiol. 2003, 547, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Molino, M.; Barnathan, E.S.; Numerof, R.; Clark, J.; Dreyer, M.; Cumashi, A.; Hoxie, J.A.; Schechter, N.; Woolkalis, M.; Brass, L.F. Interactions of mast cell tryptase with thrombin receptors and par-2. J. Biol. Chem. 1997, 272, 4043–4049. [Google Scholar] [CrossRef] [PubMed]
- Nystedt, S.; Emilsson, K.; Wahlestedt, C.; Sundelin, J. Molecular cloning of a potential proteinase activated receptor. Proc. Natl. Acad. Sci. USA 1994, 91, 9208–9212. [Google Scholar] [CrossRef] [PubMed]
- Kleij, H.P.; Bienenstock, J. Significance of conversation between mast cells and nerves. Allergy Asthma Clin. Immunol. 2005, 1, 65–80. [Google Scholar] [CrossRef] [PubMed]
- Barbara, G.; Stanghellini, V.; De Giorgio, R.; Cremon, C.; Cottrell, G.S.; Santini, D.; Pasquinelli, G.; Morselli-Labate, A.M.; Grady, E.F.; Bunnett, N.W.; et al. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology 2004, 126, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Demir, I.E.; Schorn, S.; Schremmer-Danninger, E.; Wang, K.; Kehl, T.; Giese, N.A.; Algul, H.; Friess, H.; Ceyhan, G.O. Perineural mast cells are specifically enriched in pancreatic neuritis and neuropathic pain in pancreatic cancer and chronic pancreatitis. PLoS ONE 2013, 8, e60529. [Google Scholar] [CrossRef] [PubMed]
- Lam, D.K.; Schmidt, B.L. Serine proteases and protease-activated receptor 2-dependent allodynia: A novel cancer pain pathway. Pain 2010, 149, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Mantyh, P.W. The neurobiology of skeletal pain. Eur. J. Neurosci. 2014, 39, 508–519. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.; Byers, M.R.; Oswald, R.J. Dexamethasone treatment reduces sensory neuropeptides and nerve sprouting reactions in injured teeth. Pain 1993, 55, 171–181. [Google Scholar] [CrossRef]
- Ghilardi, J.R.; Freeman, K.T.; Jimenez-Andrade, J.M.; Coughlin, K.A.; Kaczmarska, M.J.; Castaneda-Corral, G.; Bloom, A.P.; Kuskowski, M.A.; Mantyh, P.W. Neuroplasticity of sensory and sympathetic nerve fibers in a mouse model of a painful arthritic joint. Arthritis Rheum 2012, 64, 2223–2232. [Google Scholar] [CrossRef] [PubMed]
- Chartier, S.R.; Thompson, M.L.; Longo, G.; Fealk, M.N.; Majuta, L.A.; Mantyh, P.W. Exuberant sprouting of sensory and sympathetic nerve fibers in nonhealed bone fractures and the generation and maintenance of chronic skeletal pain. Pain 2014, 155, 2323–2336. [Google Scholar] [CrossRef] [PubMed]
- Riesco, N.; Cernuda-Morollon, E.; Pascual, J. Neuropeptides as a marker for chronic headache. Curr. Pain Headache Rep. 2017, 21, 18. [Google Scholar] [CrossRef] [PubMed]
- Schou, W.S.; Ashina, S.; Amin, F.M.; Goadsby, P.J.; Ashina, M. Calcitonin gene-related peptide and pain: A systematic review. J. Headache Pain 2017, 18, 34. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liu, Y.P.; Yue, D.M.; Liu, G.J. Protease-activated receptor 2 in dorsal root ganglion contributes to peripheral sensitization of bone cancer pain. Eur. J. Pain 2014, 18, 326–337. [Google Scholar] [CrossRef] [PubMed]
- Leporini, C.; Ammendola, M.; Marech, I.; Sammarco, G.; Sacco, R.; Gadaleta, C.D.; Oakley, C.; Russo, E.; De Sarro, G.; Ranieri, G. Targeting mast cells in gastric cancer with special reference to bone metastases. World J. Gastroenterol. 2015, 21, 10493–10501. [Google Scholar] [CrossRef] [PubMed]
- Ammendola, M.; Marech, I.; Sammarco, G.; Zuccala, V.; Luposella, M.; Zizzo, N.; Patruno, R.; Crovace, A.; Ruggieri, E.; Zito, A.F.; et al. Infiltrating mast cells correlate with angiogenesis in bone metastases from gastric cancer patients. Int. J. Mol. Sci. 2015, 16, 3237–3250. [Google Scholar] [CrossRef] [PubMed]
- Tondevold, E.; Eriksen, J.; Jansen, E. Observations on long bone medullary pressure in relation to mean arterial blood pressure in the anaesthetized dog. Acta Orthop. Scand. 1979, 50, 527–531. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Van Valckenborgh, E.; Menu, E.; De Bruyne, E.; Vanderkerken, K. Understanding the hypoxic niche of multiple myeloma: Therapeutic implications and contributions of mouse models. Dis. Models Mech. 2012, 5, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wan, C.; Deng, L.; Liu, X.; Cao, X.; Gilbert, S.R.; Bouxsein, M.L.; Faugere, M.C.; Guldberg, R.E.; Gerstenfeld, L.C.; et al. The hypoxia-inducible factor alpha pathway couples angiogenesis to osteogenesis during skeletal development. J. Clin. Investig. 2007, 117, 1616–1626. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.; Gilbert, S.R.; Wang, Y.; Cao, X.; Shen, X.; Ramaswamy, G.; Jacobsen, K.A.; Alaql, Z.S.; Eberhardt, A.W.; Gerstenfeld, L.C.; et al. Activation of the hypoxia-inducible factor-1alpha pathway accelerates bone regeneration. Proc. Natl. Acad. Sci. USA 2008, 105, 686–691. [Google Scholar] [CrossRef] [PubMed]
- Shomento, S.H.; Wan, C.; Cao, X.; Faugere, M.C.; Bouxsein, M.L.; Clemens, T.L.; Riddle, R.C. Hypoxia-inducible factors 1alpha and 2alpha exert both distinct and overlapping functions in long bone development. J. Cell. Biochem. 2010, 109, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Knowles, H.J.; Athanasou, N.A. Acute hypoxia and osteoclast activity: A balance between enhanced resorption and increased apoptosis. J. Pathol. 2009, 218, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Rankin, E.B.; Giaccia, A.J.; Schipani, E. A central role for hypoxic signaling in cartilage, bone, and hematopoiesis. Curr. Osteoporos. Rep. 2011, 9, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the warburg effect: The metabolic requirements of cell proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef] [PubMed]
- Peppicelli, S.; Bianchini, F.; Toti, A.; Laurenzana, A.; Fibbi, G.; Calorini, L. Extracellular acidity strengthens mesenchymal stem cells to promote melanoma progression. Cell Cycle 2015, 14, 3088–3100. [Google Scholar] [CrossRef] [PubMed]
- Di Pompo, G.; Lemma, S.; Canti, L.; Rucci, N.; Ponzetti, M.; Errani, C.; Donati, D.M.; Russell, S.; Gillies, R.; Chano, T.; et al. Intratumoral acidosis fosters cancer-induced bone pain through the activation of the mesenchymal tumor-associated stroma in bone metastasis from breast carcinoma. Oncotarget 2017, 8, 54478–54496. [Google Scholar] [CrossRef] [PubMed]
- Montazeri, A. Quality of life data as prognostic indicators of survival in cancer patients: An overview of the literature from 1982 to 2008. Health Qual. Life Outcomes 2009, 7, 102. [Google Scholar] [CrossRef] [PubMed]
- Halabi, S.; Vogelzang, N.J.; Kornblith, A.B.; Ou, S.S.; Kantoff, P.W.; Dawson, N.A.; Small, E.J. Pain predicts overall survival in men with metastatic castration-refractory prostate cancer. J. Clin. Oncol. 2008, 26, 2544–2549. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, M.; Yoshimoto, M.; Kasumi, F.; Iwase, T.; Ogata, E. Post-operative breast cancer patients diagnosed with skeletal metastasis without bone pain had fewer skeletal-related events and deaths than those with bone pain. BMC Cancer 2010, 10, 423. [Google Scholar] [CrossRef] [PubMed]
- Fizazi, K.; Massard, C.; Smith, M.; Rader, M.; Brown, J.; Milecki, P.; Shore, N.; Oudard, S.; Karsh, L.; Carducci, M.; et al. Bone-related parameters are the main prognostic factors for overall survival in men with bone metastases from castration-resistant prostate cancer. Eur. Urol. 2015, 68, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Niikura, N.; Liu, J.; Hayashi, N.; Palla, S.L.; Tokuda, Y.; Hortobagyi, G.N.; Ueno, N.T.; Theriault, R.L. Treatment outcome and prognostic factors for patients with bone-only metastases of breast cancer: A single-institution retrospective analysis. Oncologist 2011, 16, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Parker, C.; Nilsson, S.; Heinrich, D.; Helle, S.I.; O’Sullivan, J.M.; Fossa, S.D.; Chodacki, A.; Wiechno, P.; Logue, J.; Seke, M.; et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N. Engl. J. Med. 2013, 369, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Boilly, B.; Faulkner, S.; Jobling, P.; Hondermarck, H. Nerve dependence: From regeneration to cancer. Cancer Cell 2017, 31, 342–354. [Google Scholar] [CrossRef] [PubMed]
- Magnon, C.; Hall, S.J.; Lin, J.; Xue, X.; Gerber, L.; Freedland, S.J.; Frenette, P.S. Autonomic nerve development contributes to prostate cancer progression. Science 2013, 341, 1236361. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, Y.; Sakitani, K.; Konishi, M.; Asfaha, S.; Niikura, R.; Tomita, H.; Renz, B.W.; Tailor, Y.; Macchini, M.; Middelhoff, M.; et al. Nerve growth factor promotes gastric tumorigenesis through aberrant cholinergic signaling. Cancer Cell 2017, 31, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.M.; Hayakawa, Y.; Kodama, Y.; Muthupalani, S.; Westphalen, C.B.; Andersen, G.T.; Flatberg, A.; Johannessen, H.; Friedman, R.A.; Renz, B.W.; et al. Denervation suppresses gastric tumorigenesis. Sci. Transl. Med. 2014, 6, 250ra115. [Google Scholar] [CrossRef] [PubMed]
- Saloman, J.L.; Albers, K.M.; Li, D.; Hartman, D.J.; Crawford, H.C.; Muha, E.A.; Rhim, A.D.; Davis, B.M. Ablation of sensory neurons in a genetic model of pancreatic ductal adenocarcinoma slows initiation and progression of cancer. Proc. Natl. Acad. Sci. USA 2016, 113, 3078–3083. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Hou, W.; Yang, L.; Kong, X.; Du, M.; Zheng, H.; Gao, Y.; Hua, B. Protease-activated receptor 2 antagonist potentiates analgesic effects of systemic morphine in a rat model of bone cancer pain. Reg. Anesth. Pain Med. 2015, 40, 158–165. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.H.; Eber, M.R.; Widner, D.B.; Shiozawa, Y. Role of the Bone Microenvironment in the Development of Painful Complications of Skeletal Metastases. Cancers 2018, 10, 141. https://doi.org/10.3390/cancers10050141
Park SH, Eber MR, Widner DB, Shiozawa Y. Role of the Bone Microenvironment in the Development of Painful Complications of Skeletal Metastases. Cancers. 2018; 10(5):141. https://doi.org/10.3390/cancers10050141
Chicago/Turabian StylePark, Sun H., Matthew R. Eber, D. Brooke Widner, and Yusuke Shiozawa. 2018. "Role of the Bone Microenvironment in the Development of Painful Complications of Skeletal Metastases" Cancers 10, no. 5: 141. https://doi.org/10.3390/cancers10050141
APA StylePark, S. H., Eber, M. R., Widner, D. B., & Shiozawa, Y. (2018). Role of the Bone Microenvironment in the Development of Painful Complications of Skeletal Metastases. Cancers, 10(5), 141. https://doi.org/10.3390/cancers10050141





