Abstract
The effects of toxic and non-toxic Microcystis aeruginosa on the Ceratophyllum demersum–Scenedesmus obliquus system were simulated in the laboratory, and some parameters in relation to these organisms were measured. In this experiment, C. demersum increased the biomass of S. obliquus, and both toxic and non-toxic M. aeruginosa significantly inhibited the colony formation of S. obliquus and inhibited the promotion of S. obliquus biomass. On the 14th day, the soluble polysaccharide content of C. demersum decreased when it was coexisted with S. obliquus, but it rose again because of M. aeruginosa, which significantly increased the protein content of C. demersum. The species composition and diversity of epiphytic microorganisms also vary with different treatments. Proteobacteria is dominant in all the groups, especially in the Toxic_SMC group. In addition, bacteria that can degrade organic pollutants are more abundant in Toxic_SMC group. This study focuses on the defense response of S. obliquus induced by C. demersum under the pressure of toxic or non-toxic M. aeruginosa and evaluates the changes to C. demersum and its epiphytic microorganisms, which provides insights for the study of aquatic plant–algae integrated action systems in eutrophic or cyanobacterial blooms.
1. Introduction
Aquatic macrophytes play a crucial role in aquatic ecosystems, as they function as primary producers. They not only contribute to the purification of water quality and the improvement of water transparency, but also provide shelter for various organisms and impact the spatial distribution pattern of the plankton communities in water bodies [1,2]. Studies have demonstrated that certain submerged macrophytes have the ability to effectively restrain the growth of cyanobacteria, particularly Microcystis aeruginosa. Among the various methods available for controlling cyanobacteria blooms, biological approaches are considered environmentally friendly, simple to implement, and less likely to cause secondary pollution [3,4,5]. Currently, the widely accepted biological method primarily relies on the root absorption and allelopathy of aquatic macrophytes [6,7]. Allelopathy refers to the ability of submerged macrophytes to release chemically active substances [8], such as polyphenols, fatty acids, terpenoids, alkaloids, and more, which inhibit the growth of neighboring organisms [9,10].
In water bodies with abundant submerged macrophytes, green algae are typically the dominant species. These beneficial algae serve as primary producers in freshwater ecosystems, providing natural prey for aquatic animals and contributing to energy transmission and ecological stability [11]. Researchers have discovered that allelopathic substances secreted by submerged plants not only impact the biomass of green algae but also significantly influence their morphology and distribution [12]. For example, during periods when submerged macrophytes are prevalent in China’s Dianchi Lake, the dominant green algae species are Scenedesmus sp., Pediastrum sp., and Coelastrum sp. [13]. It has been reported that certain submerged macrophytes, such as Stratiotes aloides and Ceratophyllum demersum, induce the formation of colonies in Chlorella vulgaris and S. obliquus through co-cultivation, exclusion, and extraction experiments [14,15,16]. Studies have also demonstrated that Acorus calamus, Pontederia cordata, Azolla pinnata, and Nymphaea sp. promote or inhibit the growth of green algae at low or high doses [17]. The induction of colony formation in green algae by macrophytes may explain the dominance of green algae in the presence of macrophytes [15,16].
Furthermore, the multicellular communities formed by aggregated green algae often exceed the size range of zooplankton, effectively reducing their risk of being consumed. This plays a vital role in maintaining the biomass and dominance of green algae in water [18]. Currently, two widely recognized mechanisms for the formation of green algae colonies exist: one involves daughter cells remaining attached to the mother cells after reproduction [19], whereas the other involves the aggregation of single green algae cells [20]. It has been observed that increased exopolysaccharides contribute to the transition from single-cell to multi-cell morphology in green algae, as they increase the surface viscosity of the cells [15,21,22]. Despite advancements in understanding, research on the chemically active substances that induce the colony formation of green algae by submerged macrophytes remains scarce. The specific mechanisms and active components involved are still not well understood. Besides, it was observed that the ability of green algae to form colonies was not always stable. In instances in which external disturbance reduced the pressure of interspecific competition, the colonial algal cells would revert back to their unicellular forms [15,23]. The unicellular form of green algae is better suited to the size range of zooplankton and increases their feeding sensitivity, which has a significant impact on the structure of the food web and the community.
In recent years, there have been studies reporting that the colony formation of green algae induced by zooplankton or macrophytes has been destroyed due to the increased use of dissolved antibiotics or surface active agents [24,25]. The consequences of this destruction include a reduction in the dominance of green algae and changes in the structure of phytoplankton. It has also been found that heavy metals such as Cu2+ and Zn2+ can interfere with the transfer of allelochemicals along the food chain, leading to disruptions in the induced colony formation of green algae [26,27].
Cyanobacteria are a type of algae with a long history of evolution that can photosynthesize and produce oxygen. Cyanobacteria have different effects because of the variety of species. Some cyanobacteria are driven by the imbalance of nitrogen and phosphorus in water to form red tides or blooms [28,29], which seriously endangers water resources and fishery resources. Some cyanobacteria, such as Microcystis sp., release toxins into the environment that can poison human organs [30,31]. There are also some economically valuable cyanobacteria, such as Nostoc Sphaeroides and Spirulina sp., with high nutritional value [32,33]. In water bodies, many kinds of microorganisms coexist with cyanobacteria, but compared with other microorganisms, cyanobacteria are more dominant and competitive [34]. Therefore, the structure of the microbial network is relatively simple during the outbreak of water blooms [35], but the microbial network may be more complex and stable during the decline of water blooms than during their outbreak. At present, compared with some mature physical, chemical, and biological methods, the technology of using water bacteria to reduce cyanobacteria toxins is not mature [36].
Submerged macrophytes have the ability to inhibit the growth of cyanobacteria in eutrophic water, thereby maintaining the biomass and colonies of green algae. This regulation of the algal phase helps to shift the dominant species from cyanobacteria to green algae. However, during the degradation of M. aeruginosa induced by allelopathic submerged macrophytes, it has been found that the macrophytes release several times or even tens of times the normal dose of microcystins (MCs) into the water [37,38]. Additionally, phenolic acids, found in many allelochemicals, promote the expression of toxic synthesis genes (mcyB and mcyD) in toxic M. aeruginosa, resulting in an increase in the content of microcystins in the water [39,40]. Through co-cultivation of Scenedesmus, Zhu et al. (2017) [41] discovered that the presence of Microcystis aeruginosa. resulted in a decrease in the colonial cell number of Scenedesmus sp. Furthermore, as the density of Microcystis sp. increased, the average colonial cell number of Scenedesmus sp. decreased even further. However, it remains unclear whether the active substances secreted by toxic Microcystis can interfere with the colony formation of green algae induced by submerged macrophytes during freshwater restoration. The phenotypic plasticity of green algae and the stress response of microorganisms to cyanobacteria toxins are not clear enough, and the bloom environment of cyanobacteria may change the morphological response of green algae induced by aquatic plants. Based on this, this study explored the changes in the growth and colony formation of S. obliquus induced by the submerged plant C. demersum in the presence of M. aeruginosa, preliminarily explored the algal phase transformation process induced by sinking water plants in eutrophic water and predicted the algal community structure. Most importantly, it provides a scientific reference for microalgae biotechnology and the biological restoration of eutrophic water bodies.
2. Materials and Methods
2.1. Acclimation of Submerged Plants and Algae
The toxic strain of M. aeruginosa (FACHB-905), non-toxic strain of M. aeruginosa (FACHB-1005), and S. obliquus (FACHB-417) used in the experiment were obtained from the Freshwater Algae Collection of the Institute of Hydrobiology, Chinese Academy of Sciences, Wuhan, China. They were then acclimated in modified BG11 medium (1/10 BG11) [42]. The cultivation conditions were 25 °C with a light intensity of 25 μmol photons s−1 m−2 and a photoperiod of 12 h: 12 h [43,44].
The C. demersum used in this study was obtained from the aquaculture base of Henan Normal University (35° 19′ 38.363″ N, 113° 54′ 09.482″ E). All sampled macrophytes were thoroughly rinsed to remove surface contaminants and periphyton [45]. The robust apical section of the plants (10 cm in length) was obtained and acclimatized in the 1/10 BG11 medium. The cultivation conditions were the same as for the algae mentioned above.
2.2. Experimental Design
In this experiment, in order to detect the influences of toxic and non-toxic M. aeruginosa on the interaction between C. demersum and S. obliquus, five groups were set, as follows: (1) C. demersum alone (C); (2) S. obliquus + plastic grass (S), to remove the shading effects of the submerged macrophytes; (3) S. obliquus + C. demersum (SC); (4) S. obliquus + C. demersum + FACHB-905 (Toxic_SMC); (5) S. obliquus + C. demersum + FACHB-1005 (SMC). Each group was set with three parallels, and the initial biomass of C. demersum was 5 g/L, with the initial optical density (OD665) of M. aeruginosa at 0.1 and the initial OD680 of S. obliquus at 0.1. At the beginning of the experiment, the initial algal density of M. aeruginosa was 5.71 × 105 cells/L, and that of S. obliquus was 5.95 × 104 cells/L. The experimental system was cultured in 1/10 BG11 medium.
2.3. Parameter Measurement
2.3.1. Growth of S. obliquus and MC-LR Concentration in M. aeruginosa
On days 0, 1, 7, and 14 of the experiment, 1 mL algal samples were obtained and counted under an optical microscope. The specific rate of growth, colony proportion (cell number ≥ 3), and average cell number per colony of S. obliquus in each treatment were calculated using the following formulas:
where the following apply: Nt1, number of cells at t1; Nt2, number of cells at t2; N2, the cell density of S. obliquus with colonial morphology; N, total cell density; W, the average cell numbers of the colonial S. obliquus; Q, the colony numbers of S. obliquus.
Specific rate of growth (SGR) = (lnNt2 − lnNt1)/(t2 − t1)
Colony proportion (PC) = N2/N × 100%
Average cells per colony (A) = ΣW × Q/ΣQ
2.3.2. Determination of Soluble Proteins and Polysaccharides in C. demersum
A total of 0.1 g of C. demersum was sampled into a 1.5 mL centrifuge tube at the beginning (0 d) and end of the experiment (14 d). Next, 1 mL of 0.1 M PBS was added to homogenize the tissue. After centrifugation at 4 °C with a speed of 10,000 rpm for 10 min, the crude soluble proteins were obtained and measured using the Coomassie Brilliant Blue-G250 method [46].
For the determination of soluble polysaccharides, a sample of 0.1 g of C. demersum was taken and homogenized with 0.1 M PBS. The sample was then subjected to a water bath at 100 °C for 10 min and cooled to 30 °C to stop the reaction. After centrifugation, the soluble intracellular polysaccharides (IPS) were obtained and determined using the Anthrone colorimetric method [46].
2.3.3. Analysis of the Epiphytic Microorganisms of C. demersum
At the end of the experimentation, the fresh plant tissues in each treatment were set into a sterile centrifuge, to which 10 mL of 0.1 M PBS were added, and then ultrasonically washed for 1 min. This was repeated three times [47]. The washing liquid from the three washings was collected and filtered using a 0.22 μm acetate fiber filter membrane. The membrane was stored at −80 °C in a sterile 10 mL centrifuge tube for subsequent microbial diversity detection and analysis [48]. DNA extraction, high-throughput sequencing, and analysis were performed by Shanghai Majorbio Bio-Pharm Technology Co., Ltd., Shanghai, China.
2.4. Statistical Analysis
All the data were obtained in triplicate. Microsoft Excel 2019 software was utilized for calculating, GraphPad Prism 10.1.2 and Origin 2021 software were used to analyze dates and plot the results, and all the data were expressed as mean ± SD. One-way analysis of variance (one-way ANOVA) was used to determine significant differences among each group. Statistical significance was set at p < 0.05. The microbial community diversity in the C. demersum biofilm was described and analyzed by Majorbio online analysis software (http://www.majorbio.com/), accessed on 18 December 2023.
3. Result
3.1. S. obliquus Abundance and Colony
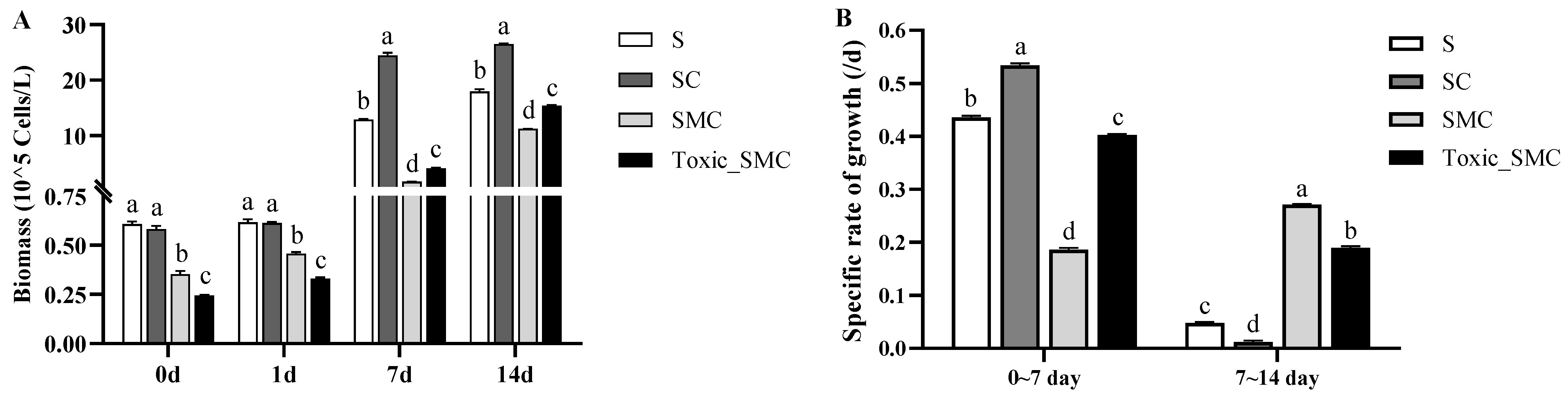
During the experiment, the biomass of the C group (presence of C. demersum) became gradually higher than that of the S group (S. obliquus alone) (Figure 1A). The specific cell growth rate of S. obliquus was significantly higher in the presence of C. demersum (SC group) compared to the control with S. obliquus alone (S group) from the 0th to the 7th day. However, from the 7th to the 14th day, the growth rate was significantly lower in the SC group than in the other three groups. Similarly, the specific growth rate of S. obliquus in the presence of toxic M. aeruginosa (Toxic_SMC group) was higher from the 0th to the 7th day but lower than that with the non-toxic M. aeruginosa (SMC group) from the 7th to the 14th day (p < 0.05) (Figure 1B).
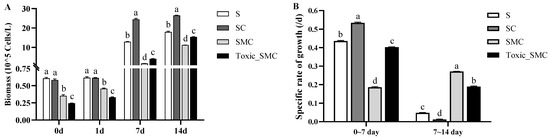
Figure 1.
Biomass of different groups of S. obliquus at different times, and specific rates of growth of different groups of S. obliquus in different time periods. Mean values and standard deviations were calculated for the different replicates (n = 3); different letters represent significant differences detected (p < 0.05).
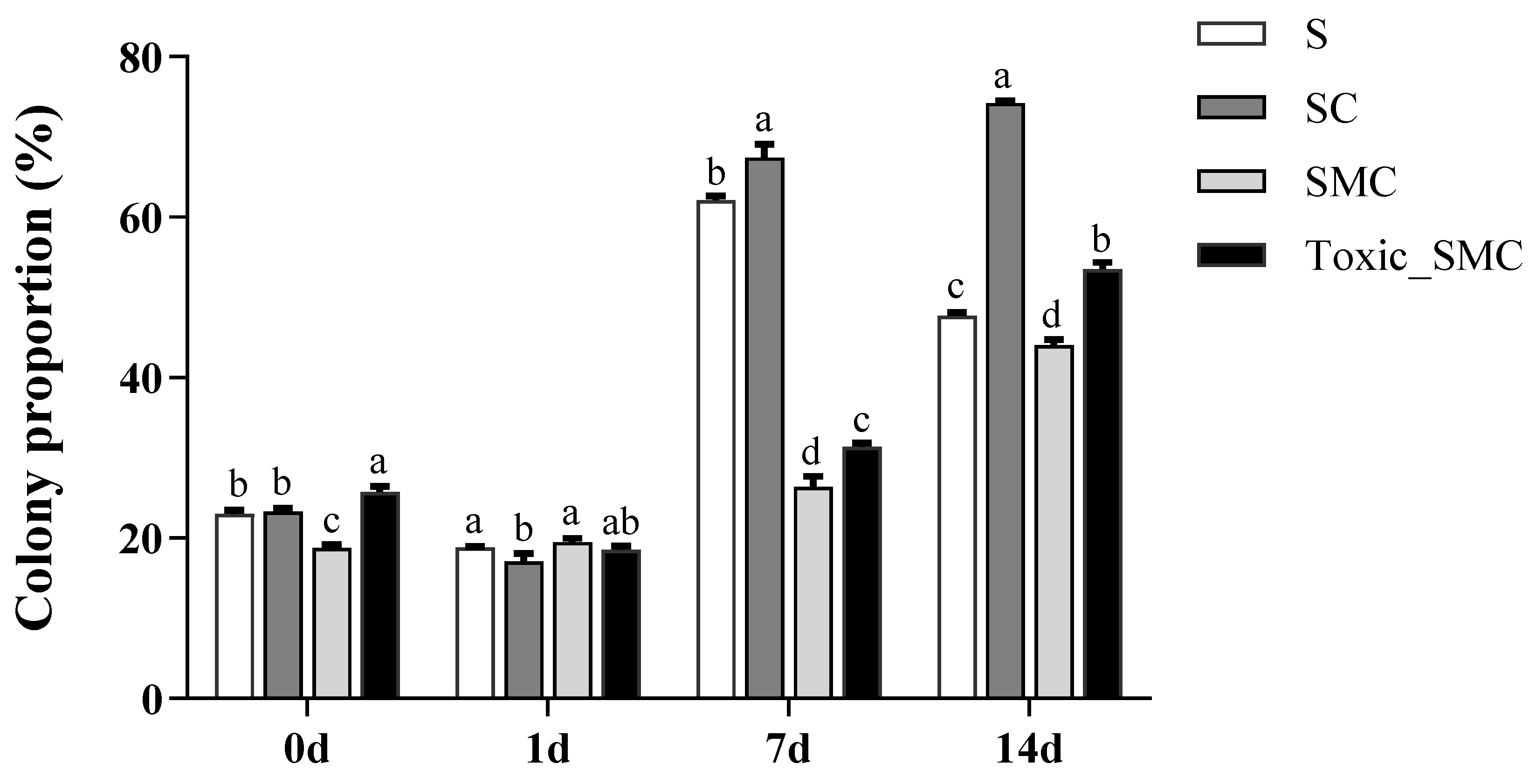
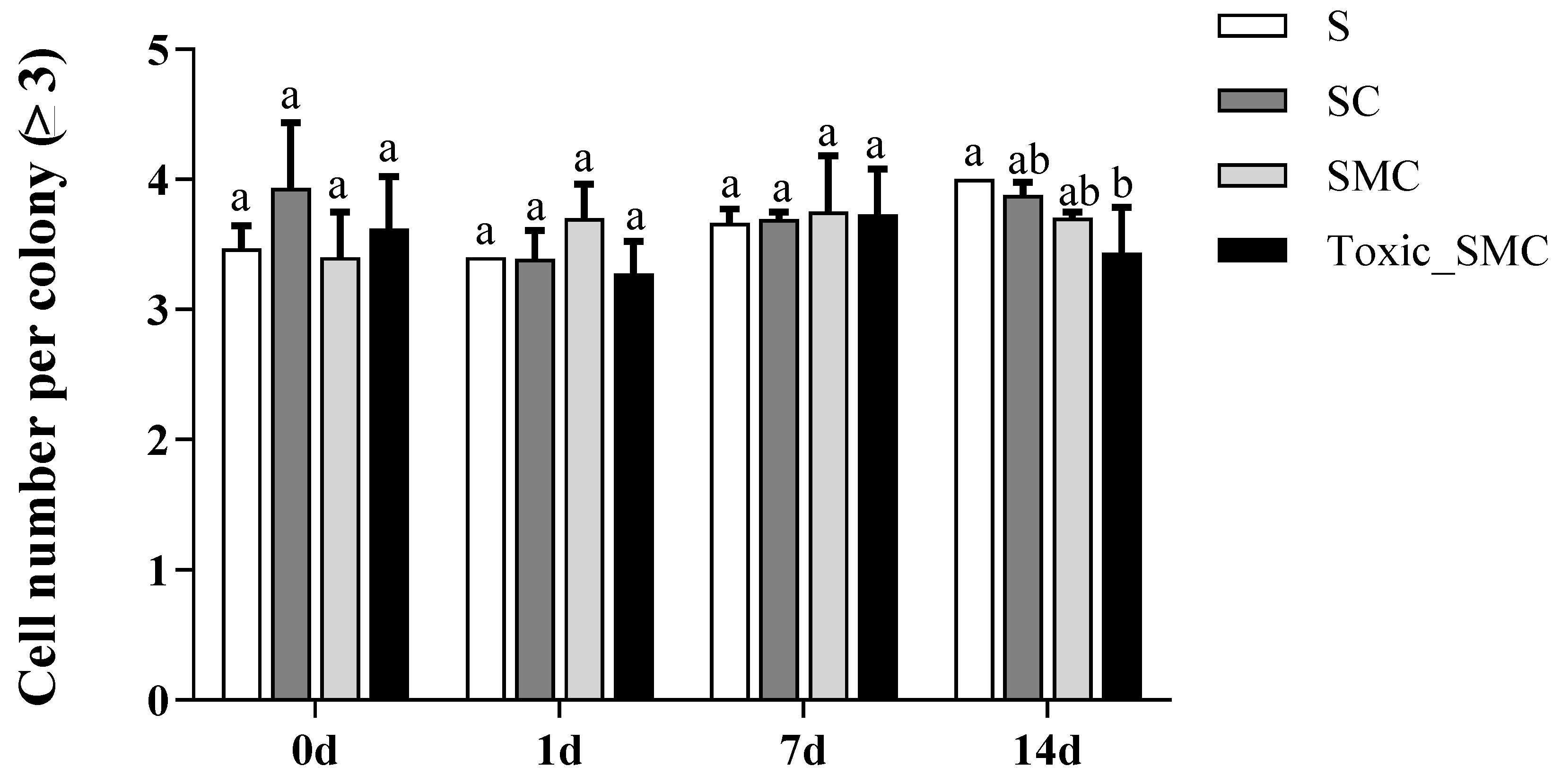
Furthermore, the presence of C. demersum significantly promoted the colony formation of S. obliquus, whereas the presence of both toxic and non-toxic M. aeruginosa significantly reduced colony induction. This reduction was observed as a decrease in the colony proportion of S. obliquus in the SMC and Toxic_SMC groups. It should be noted that the colony proportion was lower in the SMC group compared to the Toxic_SMC group (p < 0.05) (Figure 2). However, in the present experiment, there was no significant difference in the cell number per colony among the experimental groups in different periods (p > 0.05), except that the Toxic_SMC group had significantly fewer cells per colony than the S group on the 14th day (p < 0.05) (Figure 3). The ANOVA table also showed that the experimental treatment and date had significant effects on the biomass and colony proportion of S. obliquus, but for the average cell number per colony, the experimental conditions made almost no difference (Table 1).
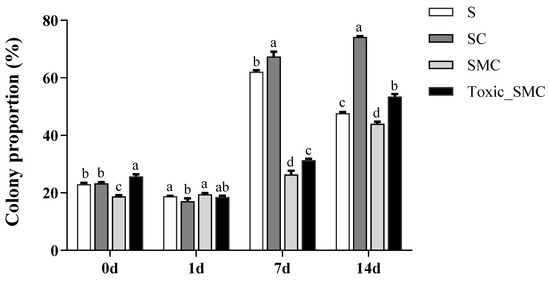
Figure 2.
Colony proportion of different groups of S. obliquus in different time periods. Mean values and standard deviations were calculated for the different replicates (n = 3); different letters represent significant differences detected (p < 0.05).
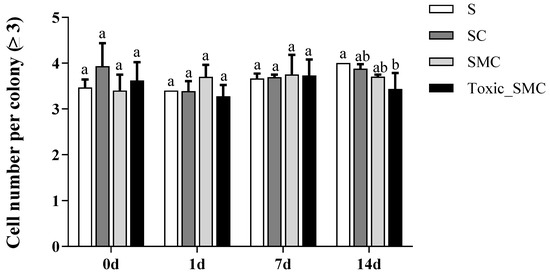
Figure 3.
Cell number per colony (≥3) of different groups of S. obliquus in different time periods. Mean values and standard deviations were calculated for the different replicates (n = 3); different letters represent significant differences detected (p < 0.05).

Table 1.
Repeated measurement analysis of variance comparing algal biomass, colony proportion, and cell number per colony under different experiment conditions during the experimental period.
3.2. Soluble Proteins and Polysaccharides of C. demersum
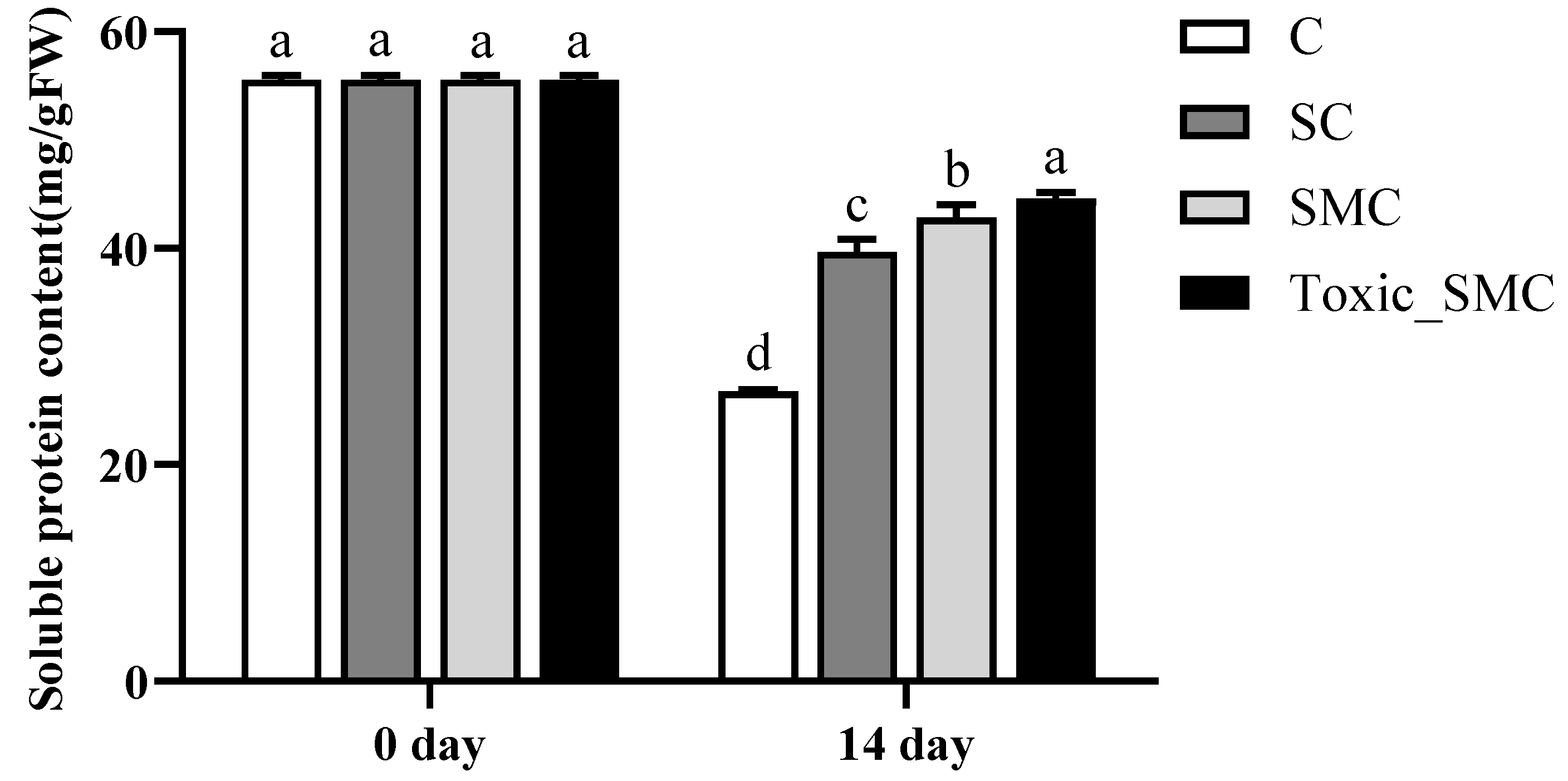
At the end of the experiment, on the 14th day, the soluble protein content of C. demersum (group C) was the lowest, and it was higher when co-cultivated with S. obliquus. Furthermore, the protein content of C. demersum significantly increased after the addition of M. aeruginosa, with toxic M. aeruginosa showing a more pronounced effect compared to non-toxic M. aeruginosa (p < 0.05) (Figure 4).
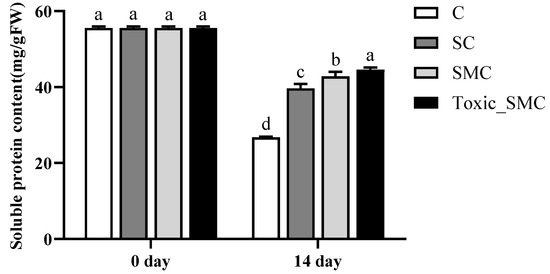
Figure 4.
The soluble protein contents in each group on the 0th day and 14th day of the experiment. Mean values and standard deviations were calculated for the different replicates (n = 3); different letters represent significant differences detected (p < 0.05).
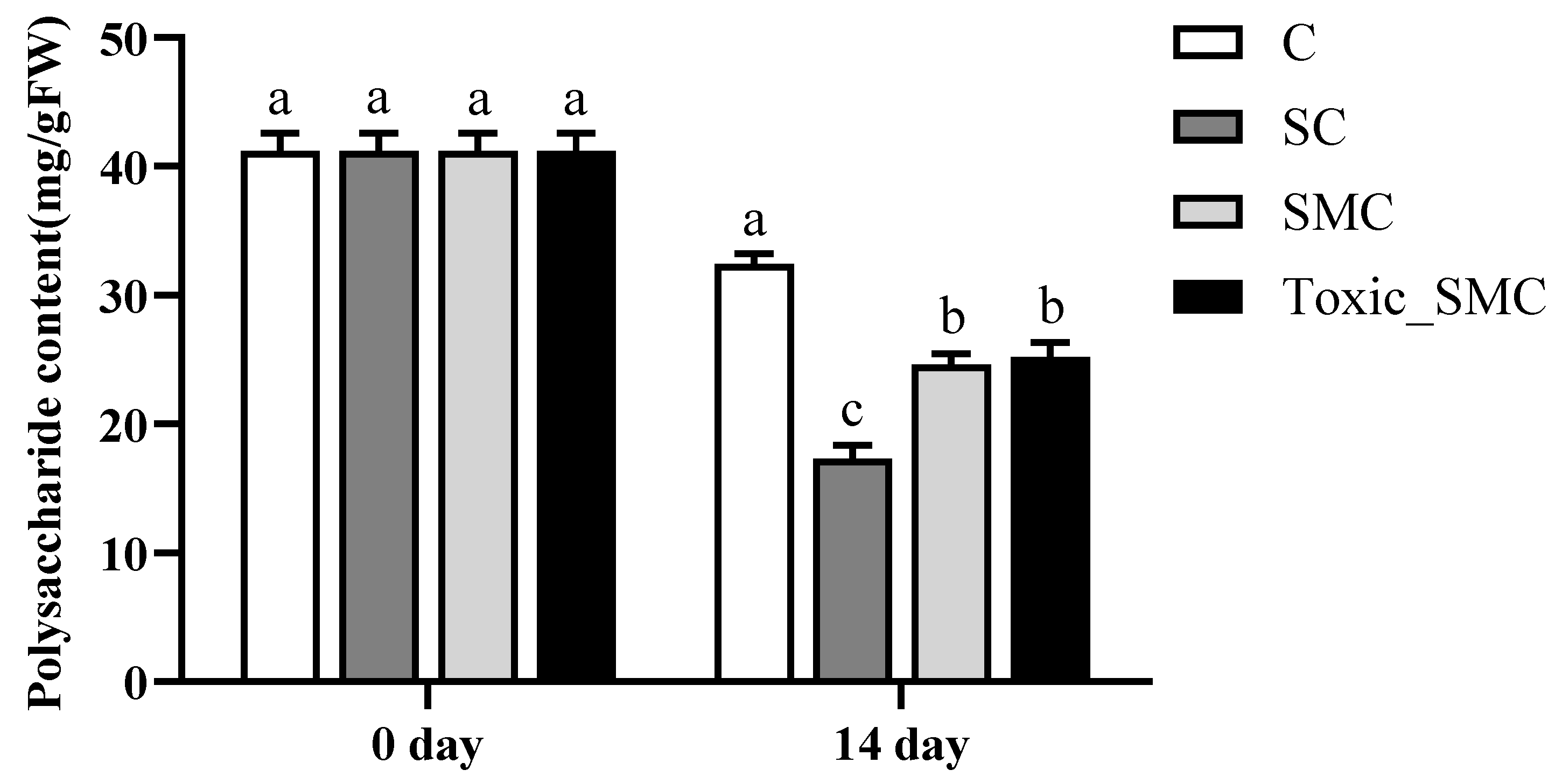
In addition, at the end of the experiment, the soluble polysaccharide content in C. demersum (group C) was the highest, but it significantly decreased when co-cultured with S. obliquus (p < 0.05). The presence of M. aeruginosa significantly improved the soluble polysaccharide content in the SC group, with no significant differences observed between toxic and non-toxic M. aeruginosa (Figure 5).
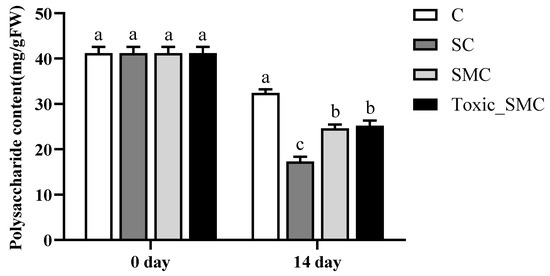
Figure 5.
The soluble polysaccharide contents in each group on the 0th day and 14th day of the experiment. Mean values and standard deviations were calculated for the different replicates (n = 3); different letters represent significant differences detected (p < 0.05).
3.3. Analysis of Epiphytic Microorganisms in C. demersum
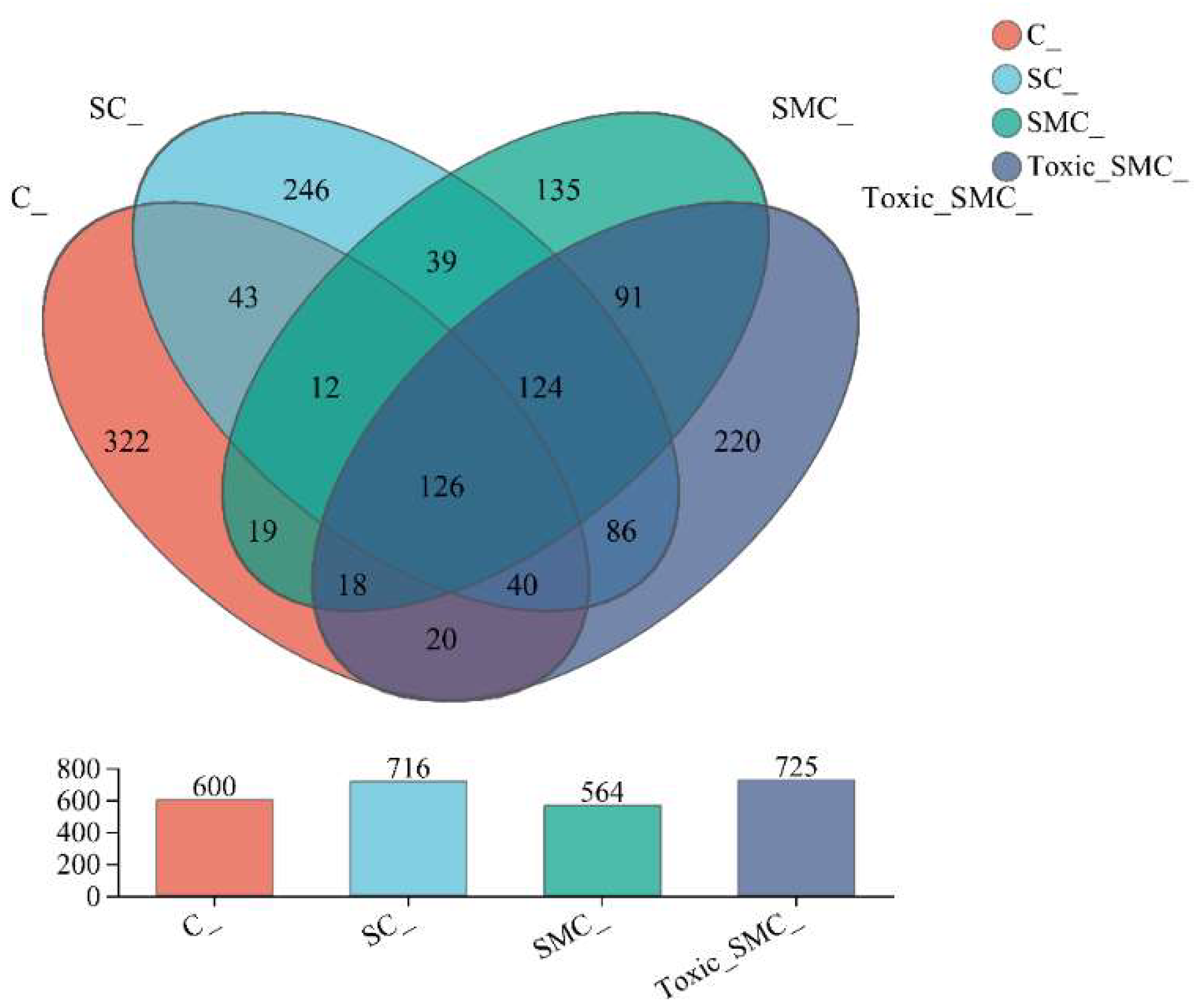
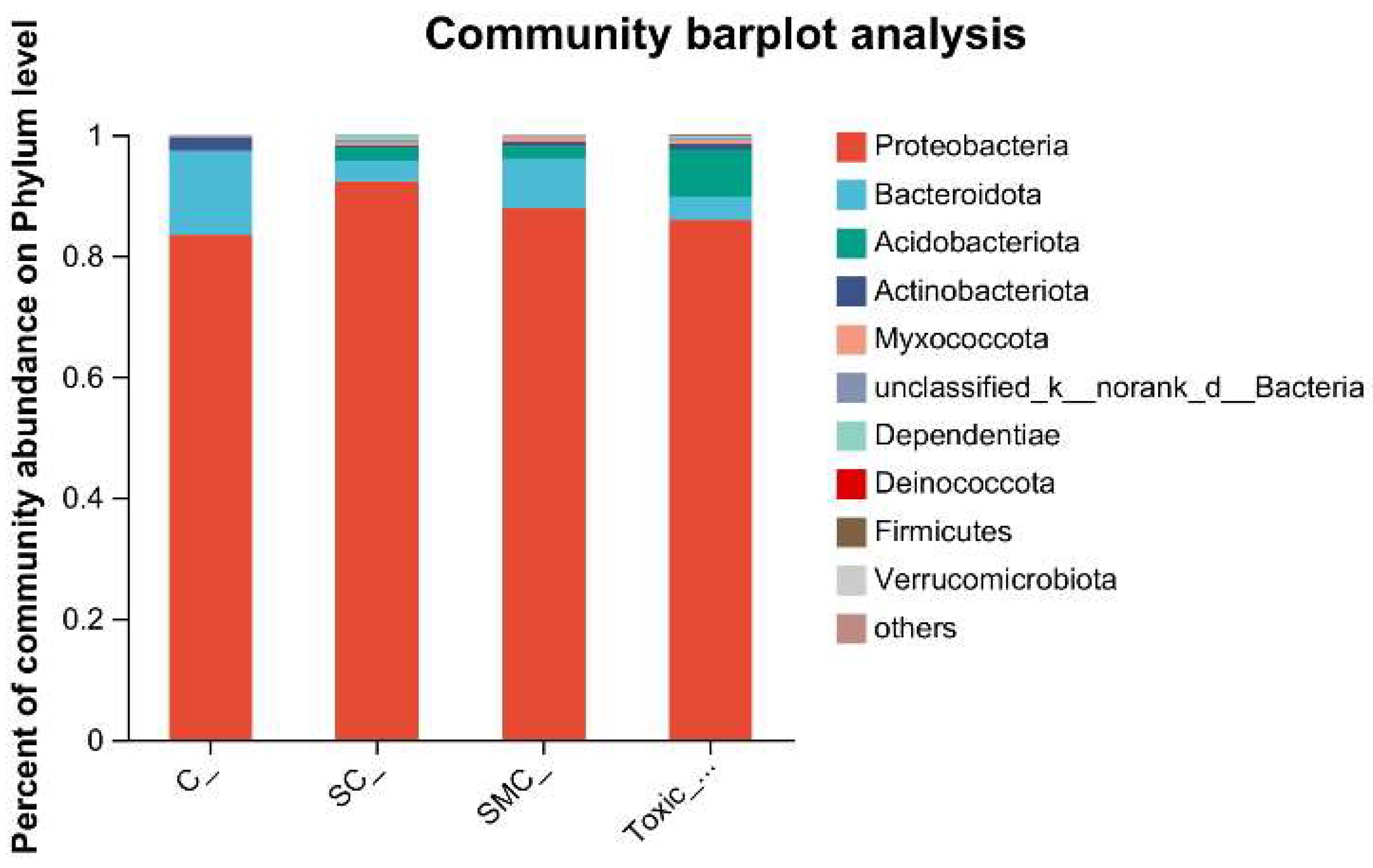
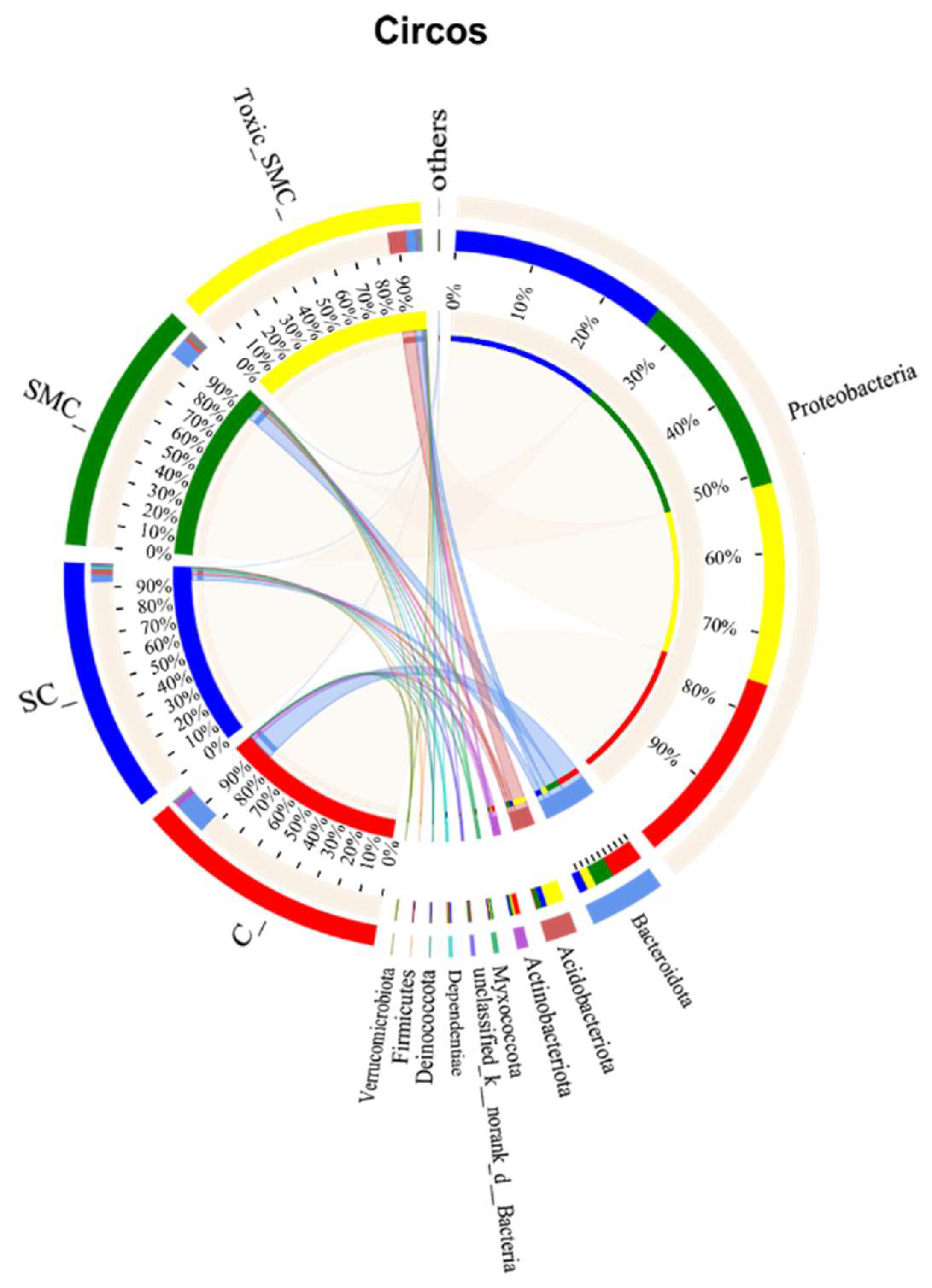
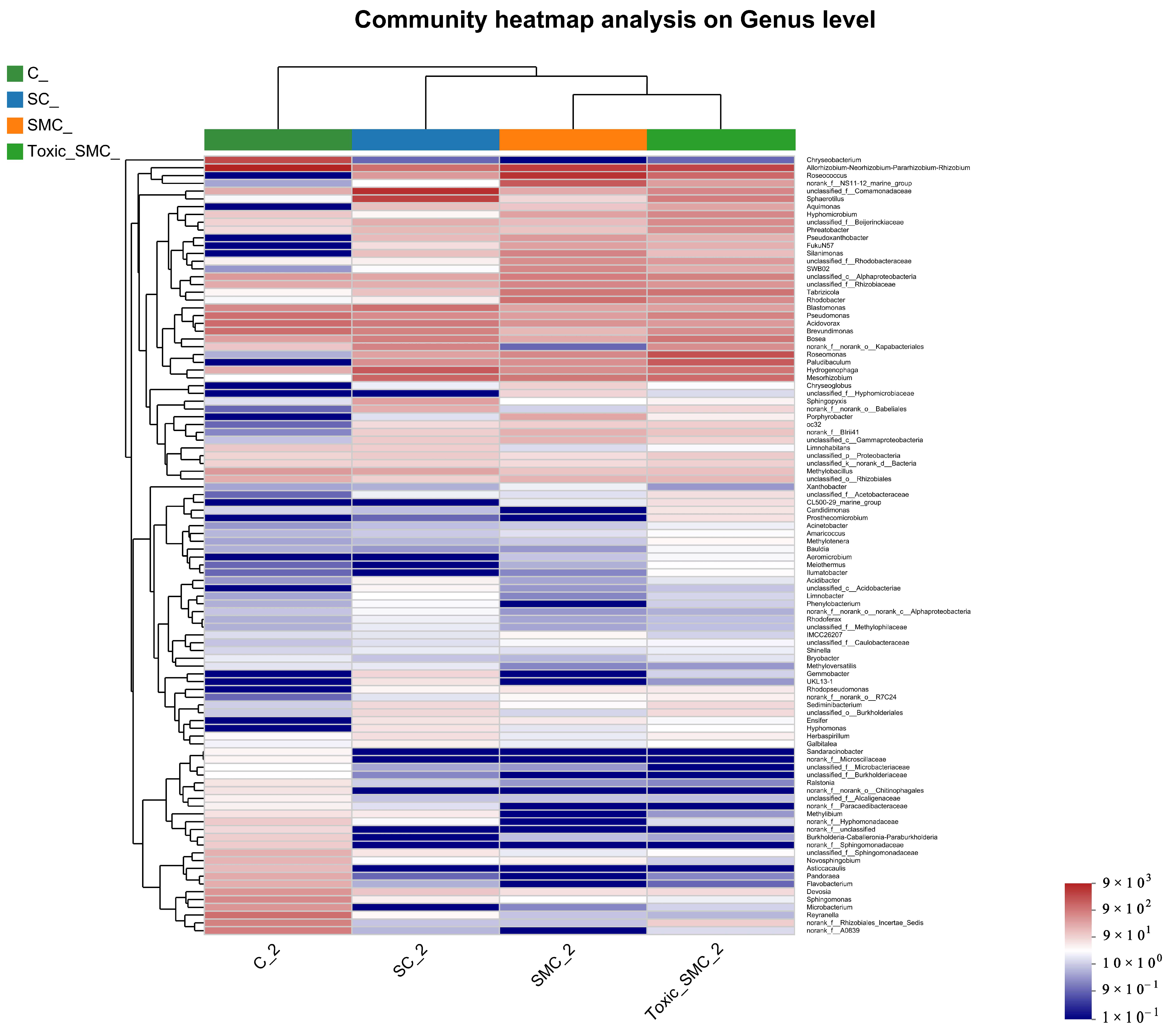
The Venn diagram in Figure 6 shows that the unique OTU values were highest in the C group and lowest in the SMC group, with 322 (C), 246 (SC), 135 (SMC), and 220 (Toxic_SMC) unique OTUs observed. The Circos diagram and column diagram depicting the percentage accumulation of bacterial community at the phylum level revealed that Proteobacteria was the dominant phylum, with an abundance of over 80% in each treatment. Additionally, Bacteroidetes and Actinobacteriota were the most abundant in the group of C. demersum cultivation alone. Acidobacteriota and Myxococcota were found in the co-cultivation of C. demersum and S. obliquus, with their abundance being highest in the Toxic_SMC group (Figure 7 and Figure 8).
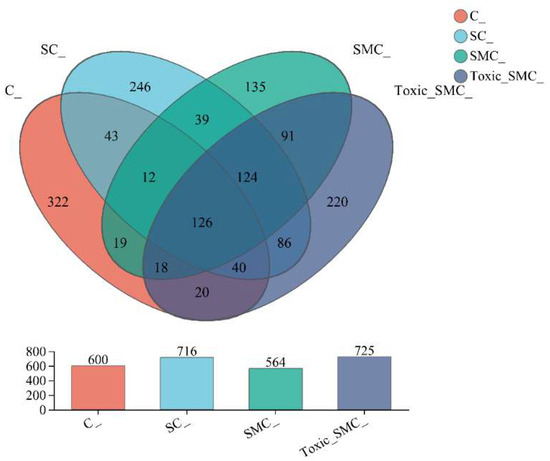
Figure 6.
Microbial community analysis: Venn diagram; C: C. demersum; EU: C. demersum and S. obliquus are co-cultured; DU: C. demersum, S. obliquus, and non-toxic M. aeruginosa are co-cultured; DU: C. demersum, S. obliquus, and toxic M. aeruginosa are co-cultured.
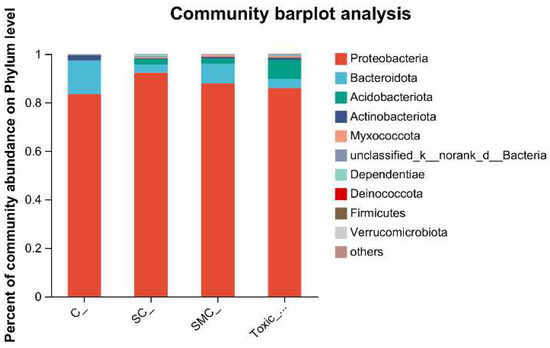
Figure 7.
Microbial community analysis: percentage community abundance of phylum.
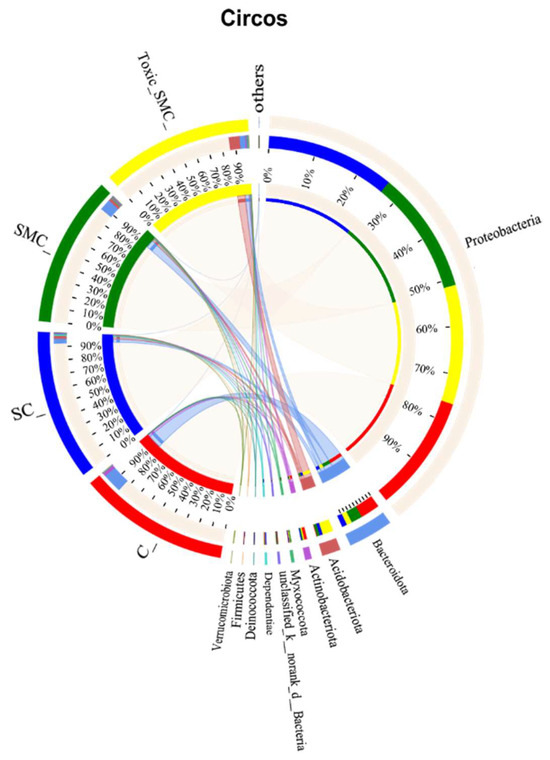
Figure 8.
Microbial community analysis: Circos plot.
The cluster heatmap in Figure 9 displays the top 100 genera at the genera level. Group C stands out, with two major bacterial genera, Chryseobacterium and Allorhizobium–Neorhizobium–Pararhizobium, dominated by C. demersum alone. Additionally, Sandaracinobacter, Asticcacaulis, and Microbacterium were more prevalent in group C compared to the other three groups. Conversely, the abundance of Hyphomonas in the SC, SMC, and Toxic_SMC groups significantly increased in comparison to the cultivation of C. demersum alone. Moreover, the SMC group exhibited significantly lower abundances of Candidimonas and Prosthecomicrobium when compared to the Toxic_SMC group (Figure 9).
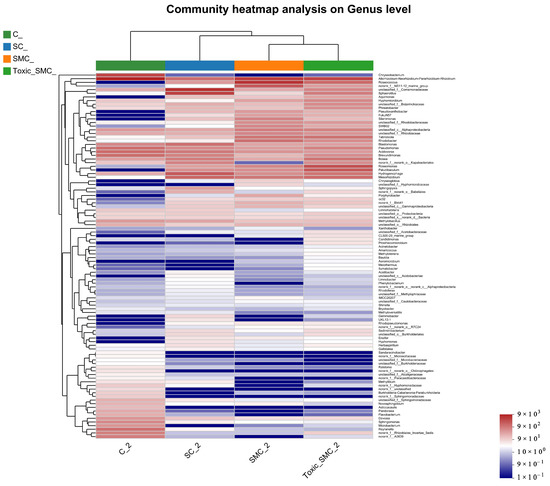
Figure 9.
Microbial structure at genus level: heatmap of genus levels in different samples.
4. Discussion
4.1. Growth and Morphology Changes of S. obliquus
The morphological changes induced by submerged macrophytes are a defense strategy employed by green algae when confronted with adverse conditions. These changes promote colony formation, leading to increased sedimentation rates, reduced interspecific competition among photosynthetic organisms, and the prevention of predation. Consequently, green algae can maintain their dominance in aquatic ecosystems [49,50]. In this study, it was further demonstrated that C. demersum can enhance the colony formation of S. obliquus. This finding aligns with previous research that has shown the promoting effects of C. demersum and Elodea densa on S. obliquus and C. vulgaris colony formation through co-cultivation, extract application, and simulation experiments [51,52]. Meanwhile, the presence of M. aeruginosa in the experimental system greatly disrupted the induced colony formation of S. obliquus by C. demersum. There are two possible explanations for this. Firstly, cyanobacteria are more sensitive to allelochemicals compared to green algae [53], which means that the chemical signals produced by C. demersum may have been transmitted more effectively to M. aeruginosa than to S. obliquus. In the process of transmission, the chemical information substances produced diversion, and the decrease in the allelochemicals accepted by S. obliquus resulted in a decrease in the population proportion. Secondly, the toxicity of MC-LR could have caused physiological damage to C. demersum [54], thereby interfering with the secretion of chemically active substances by aquatic macrophytes. Previous study has been reported that MC-LR can increase the soluble polysaccharide content in and out of S. obliquus cells [55], which may explain why the colony proportion of S. obliquus was higher in the Toxic_SMC group compared to the SMC group in our study. Additionally, the average cell number per colony of S. obliquus in the Toxic_SMC group was slightly lower than that in the SMC group on the 1st, 7th, and 14th days of the experiment, indicating that the active substances secreted by toxic M. aeruginosa could inhibit the formation of larger colonies by S. obliquus.
In the present study, the cell abundance of S. obliquus in the SC group was significantly higher than that in the S group. This finding is consistent with El-Darier et al.’s observations (2021) [56], suggesting that the allelopathic effects of C. demersum on S. obliquus exhibit a dose-dependent promotion and inhibition pattern. The possible reason for this is that the response of S. obliquus to allelopathy is in dose-dependent promotion and inhibition mode. Some green algae also have a similar phenomenon in response to allelopathy from other organisms [56], that is, a low concentration of allelopathy can promote growth, while a high concentration of allelopathy can even inhibit growth. At the same time, the interspecific competition, including nutrient salts and light, as well as the interaction of chemical active substances, will affect the growth of S. obliquus, and M. aeruginosa has a competitive advantage over S. obliquus [57]. When green algae and M. aeruginosa co-existed, MC-LR released by the latter caused damage to the photosynthetic system, and activated the protective mechanism of the photosynthetic system by reducing the concentration of chlorophyll a and carotenoid, triggering oxidative stress and inhibiting the growth of green algae [58]. Moreover, MC-LR can slow down the growth of S. obliquus [59].
4.2. Soluble Proteins and Polysaccharides of C. demersum
Photosynthetic organisms produce ATP and NADPH in the light reaction stage of photosynthesis, so that H2O and CO2 can be synthesized into compounds in the dark reaction stage and stable chemical energy can be obtained to generate photosynthetic products [60]. There will be interspecific competition between C. demersum and Scenedesmus in co-culture. A decrease in photosynthesis makes C. demersum produce fewer photosynthetic products than when it grows alone, and its monosaccharide production is bound to be affected. At the same time, S. obliquus forms a population due to allelopathy, and this phenotypic defense is related to the soluble polysaccharide level of S. obliquus [15,22], which requires S. obliquus to increase the productivity of soluble polysaccharide. The decrease in raw materials for polysaccharide production in the environment makes the distribution of soluble photosynthetic products of C. demersum tilt towards protein. Under stress, such as exposure to microcystins, large plants can increase their soluble protein and polysaccharide synthesis as one of their defensive measures [54], and at the same time, S. obliquus changes from the colony to the single cell phenotype, which reduces the demand and competition of polysaccharide synthesis raw materials, which can also affect the recovery of the soluble polysaccharide content of C. demersum.
4.3. Epiphytic Microorganism of C. demersum
The results from the Venn diagram indicate significant changes in the species composition of the microbial community in each experimental group compared to the control group. The highest abundance of OTUs was observed in the Toxic_SMC group, suggesting that toxic M. aeruginosa has an impact on the diversity of biofilm microbial communities. Furthermore, the bacterial community of C. demersum varied at the phylum and genus levels across different treatments. Proteobacteria, which are commonly found in freshwater environments and attached bacterial communities of aquatic macrophytes [61,62,63], were the dominant phyla in all the experimental groups. The presence of Proteobacteria is known to enhance the degradation of cyanobacterial toxins, potentially contributing to the control of cyanobacteria by C. demersum [64]. The Allorhizobium–Neorhizobium–Pararhizobium–Rhizobium bacteria primarily utilize ammonia as their nitrogen source [65]. The abundance of this genus remained consistently high across all the experimental groups, possibly due to the low oxygen content in the subaqueous environment. Actinobacteriota, which typically inhabit water with low nutrient concentrations, were most abundant in the C. demersum-alone cultivation. Bacteria belonging to the phylum Bacteroides, such as Flavobacterium, are known for their efficient hydrocarbon degradation capabilities and are commonly found in a range of aerobic and anaerobic environments, where they degrade carbohydrates [66]. The higher abundance of Bacteroides in the C. demersum-alone cultivation compared to the other treatment groups may be attributed to the higher polysaccharide content in this group. The abundance of Hyphomonas increased in the presence of toxic M. aeruginosa, suggesting its potential role as an organic pollutant and toxin-degrading bacterium. Sandaracinobacter, which contains chlorophyll a [67], was not dominant in the co-culture system of C. demersum and S. obliquus, where competition for photosynthesis is intensified. Asticcacaulis and Microbacterium are aerobic and chemoheterotrophic bacteria that are more likely to thrive in low-competition environments, and their occurrence was not suitable in the water body where the MCs were produced. Candidimonas, known for its ability to degrade organic pollutants [68], showed higher abundance in the Toxic_SMC group compared to the SMC group, which may be attributed to the higher concentration of MC-LR in the Toxic_SMC group.
5. Conclusions
In this study, the effects of M. aeruginosa on the formation of S. obliquus colonies induced by C. demersum, and the response of the C. demersu–S. obliquus system to M. aeruginosa were investigated. The growth of S. obliquus was promoted under the influence of C demersum, but it was inhibited under the influence of M. aeruginosa. M. aeruginosa threatened the C. demersum-induced colony formation of S. obliquus, reduced colony proportion, improved the grazing sensitivity of S. obliquus, and restored the interspecific competition between C. demersum and S. obliquus. The soluble polysaccharide synthesis and soluble protein production of C. demersum were increased under the stimulation of declustering and M. aeruginosa. The response of epiphytic microorganisms in C. demersum to MCs is evident in a change in community species diversity. The predominance of Proteobacteria remained unchanged, and the diversity of Bacteroidetes decreased significantly with the decrease in soluble polysaccharide content in C. demersum. The survival of aerobic chemoheterotrophic bacteria such as Asticcacaulis and Microbacterium was threatened by MCs, and their diversity decreased. Under MCs exposure, the species diversity of organic-degrading bacteria such as Hyphomonas and Candidimonas increased. These changes to the attached microorganisms of C. demersum tend to adapt to and alleviate the environmental conditions stressed by M. aeruginosa.
Author Contributions
Y.S.: Data analysis and draft writing; S.Z.: Experiment design and performance of the experiments; J.D. and X.L.: Revision and supervision; X.G. and Y.G.: Language improvement and reviewing of the original draft; H.Y. and J.Z.: Assistance in microbial community analysis. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the Key Scientific and Technological Project of Henan Province (No. 232102321056), the Young Backbone Teachers Project of Henan Province (No. 2020GGJS064), the Key Scientific and Technological Project of Henan Province (No. 232102320256), the Project of Huanghe River Fisheries Resources and Environment Investigation from the MARA, P.R. China, and the Henan Provincial Natural Science Foundation (No. 242300420496 and 242300421578).
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Amorim, C.A.; Moura, A.N. Effects of the manipulation of submerged macrophytes, large zooplankton, and nutrients on a cyanobacterial bloom: A mesocosm study in a tropical shallow reservoir. Environ. Pollut. 2020, 265, 114997. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhao, K.; Zhang, H.; He, L.; Niu, Y.; Zhang, M.; Xu, J. Linking macrophyte community structure with food chain length: A case study in the largest freshwater lake in China and ecological restoration implications. Ecol. Indic. 2021, 123, 107363. [Google Scholar] [CrossRef]
- Mowe, M.A.; Song, Y.; Sim, D.Z.; Lu, J.; Mitrovic, S.M.; Tan, H.T.; Yeo, D.C. Comparative study of six emergent macrophyte species for controlling cyanobacterial blooms in a tropical reservoir. Ecol. Eng. 2019, 129, 11–21. [Google Scholar] [CrossRef]
- Kurbatova, S.; Berezina, N.; Sharov, A.; Chernova, E.; Kurashov, E.; Krylova, Y.; Yershov, I.; Mavrin, A.; Otyukova, N.; Borisovskaya, E.; et al. Effects of Algicidal Macrophyte Metabolites on Cyanobacteria, Microcystins, Other Plankton, and Fish in Microcosms. Toxins 2023, 15, 529. [Google Scholar] [CrossRef]
- Wang, T.; Liu, H. Aquatic plant allelochemicals inhibit the growth of microalgae and cyanobacteria in aquatic environments. Environ. Sci. Pollut. Res. 2023, 30, 105084–105098. [Google Scholar] [CrossRef]
- Wang, H.; Liu, F.; Luo, P.; Li, Z.; Zheng, L.; Wang, H.; Zou, D.; Wu, J. Allelopathic Effects of Myriophyllum aquaticum on Two Cyanobacteria of Anabaena flos-aquae and Microcystis aeruginosa. Bull. Environ. Contam. Toxicol. 2017, 98, 556–561. [Google Scholar] [CrossRef]
- Yu, S.; Miao, C.; Song, H.; Huang, Y.; Chen, W.; He, X. Efficiency of nitrogen and phosphorus removal by six macrophytes from eutrophic water. Int. J. Phytoremediation 2019, 21, 643–651. [Google Scholar] [CrossRef]
- Rice, E.L. Allelopathy, 2nd ed.; Academic Press: Orlando, FL, USA, 1984; pp. 1–501. [Google Scholar]
- Santonja, M.; Le Rouzic, B.; Thiébaut, G. Seasonal dependence and functional implications of macrophyte–phytoplankton allelopathic interactions. Freshw. Biol. 2018, 63, 1161–1172. [Google Scholar] [CrossRef]
- Zhu, X.; Dao, G.; Tao, Y.; Zhan, X.; Hu, H. A review on control of harmful algal blooms by plant-derived allelochemicals. J. Hazard. Mater. 2021, 401, 123403. [Google Scholar] [CrossRef]
- Peterson, B.C.; Burr, G.S.; Barrows, F.T.; Block, S.; Bowzer, J.; Buentello, A. Growth Performance of Atlantic Salmon Smolts Fed Diets Containing Heterotrophic Algal Biomass as Replacement of Fish Oil. N. Am. J. Aquac. 2019, 81, 364–371. [Google Scholar] [CrossRef]
- Gross, E.M.; Erhard, D.; Iványi, E. Allelopathic activity of Ceratophyllum demersum L. and Najas marina ssp. intermedia (Wolfgang) Casper. Hydrobiologia 2003, 506–509, 583–589. [Google Scholar] [CrossRef]
- Dong, J.; Yang, K.; Li, S.; Li, G.; Song, L. Submerged vegetation removal promotes shift of dominant phytoplankton functional groups in a eutrophic lake. J. Environ. Sci. 2014, 26, 1699–1707. [Google Scholar] [CrossRef] [PubMed]
- Mulderij, G.; Mooij, W.M.; Donk, E.V. Allelopathic growth inhibition and colony formation of the green alga Scenedesmus obliquus by the aquatic macrophyte Stratiotes aloides. Aquat. Ecol. 2005, 39, 11–21. [Google Scholar] [CrossRef]
- Dong, J.; Gao, Y.; Chang, M.; Ma, H.; Han, K.; Tao, X.; Li, Y. Colony formation by the green alga Chlorella vulgaris in response to the competitor Ceratophyllum demersum. Hydrobiologia 2018, 805, 177–187. [Google Scholar] [CrossRef]
- Dong, J.; Chang, M.; Li, C.; Dai, D.; Gao, Y. Allelopathic effects and potential active substances of Ceratophyllum demersum L. on Chlorella vulgaris Beij. Aquat. Ecol. 2019, 53, 651–663. [Google Scholar] [CrossRef]
- Lu, Q. Effects of Five Aquatic Plant Extracts on the Growth of Scenedesmus Obliquus; Jiangxi Normal University: Nanchang, China, 2019; (In Chinese). [Google Scholar] [CrossRef]
- Zhu, X.; Wang, J.; Chen, Q.; Chen, G.; Huang, Y.; Yang, Z. Costs and trade-offs of grazer-induced defenses in Scenedesmus under deficient resource. Sci. Rep. 2016, 6, 22594. [Google Scholar] [CrossRef]
- Bišová, K.; Zachleder, V. Cell-cycle regulation in green algae dividing by multiple fission. J. Exp. Bot. 2014, 65, 2585–2602. [Google Scholar] [CrossRef]
- Li, M.; Gao, L.; Lin, L. Specific growth rate, colonial morphology and extracellular polysaccharides (EPS) content ofScenedesmus obliquusgrown under different levels of light limitation. Ann. De Limnol.—Int. J. Limnol. 2015, 51, 329–334. [Google Scholar] [CrossRef]
- Yang, Z.; Kong, F.; Shi, X.; Xing, P.; Zhang, M. Effects of Daphnia -Associated Infochemicals on the Morphology, Polysaccharides Content and PSII-Efficiency in Scenedesmus obliquus. Int. Rev. Hydrobiol. 2007, 92, 618–625. [Google Scholar] [CrossRef]
- Khona, D.K.; Shirolikar, S.M.; Gawde, K.K.; Hom, E.; Deodhar, M.A.; D’Souza, J.S. Characterization of salt stress-induced palmelloids in the green alga, Chlamydomonas reinhardtii. Algal Res. 2016, 16, 434–448. [Google Scholar] [CrossRef]
- Fisher, R.M.; Bell, T.; West, S.A. Multicellular group formation in response to predators in the alga Chlorella vulgaris. J. Evol. Biol. 2016, 29, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Liu, C.; Li, F.; Zhou, C.; Yan, S.; Dong, J.; Li, T.; Duan, C. Norfloxacin disrupts Daphnia magna -induced colony formation in Scenedesmus quadricauda and facilitates grazing. Ecol. Eng. 2017, 102, 255–261. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, Z.; Sun, Y.; Gu, L.; Zhang, L.; Wang, J.; Huang, Y.; Yang, Z. Surfactants at environmentally relevant concentrations interfere the inducible defense of Scenedesmus obliquus and the implications for ecological risk assessment. Environ. Pollut. 2020, 261, 114131. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Nan, H.; Zhu, X.; Li, B.; Zhang, Z.; Yang, Z. Waterborne copper impairs grazer-induced colony formation and photosynthetic efficiency inScenedesmus obliquus. Limnol. Oceanogr. 2016, 61, 625–634. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, Y.; Hou, X.; Kong, Q.; Sun, Y.; Wang, J.; Huang, Y.; Yang, Z. High temperature promotes the inhibition effect of Zn2+ on inducible defense of Scenedesmus obliquus. Chemosphere 2019, 216, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Metcalf, J.S.; Banack, S.A.; Wessel, R.A.; Lester, M.; Pim, J.G.; Cassani, J.R.; Cox, P.A. Toxin Analysis of Freshwater Cyanobacterial and Marine Harmful Algal Blooms on the West Coast of Florida and Implications for Estuarine Environments. Neurotox. Res. 2020, 39, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Long, S.; Hamilton, P.B.; Yang, Y.; Ma, J.; Chobet, O.C.; Chen, C.; Dang, A.; Liu, Z.; Dong, X.; Chen, J. Multi-year succession of cyanobacteria blooms in a highland reservoir with changing nutrient status, Guizhou Province, China. J. Limnol. 2018, 77, 232–246. [Google Scholar] [CrossRef]
- Du, C.; Zheng, S.; Yang, Y.; Feng, X.; Chen, J.; Tang, Y.; Wang, H.; Yang, F. Chronic exposure to low concentration of MC-LR caused hepatic lipid metabolism disorder. Ecotoxicol. Environ. Saf. 2022, 239, 113649. [Google Scholar] [CrossRef]
- Gu, S.; Yan, M.; Wang, C.; Meng, X.; Xiang, Z.; Qiu, Y.; Han, X. Microcystin-leucine-arginine induces liver fibrosis by activating the Hedgehog pathway in hepatic stellate cells. Biochem. Biophys. Res. Commun. 2020, 533, 770–778. [Google Scholar] [CrossRef]
- Li, F.; Liu, R.; Qin, S.; Deng, Z.; Li, W. Progress in culture technology and active substance research on Nostoc sphaeroides Kützing. J. Sci. Food Agric. 2024; Online ahead of print. [Google Scholar] [CrossRef]
- Nejatian, M.; Yazdi, A.P.G.; Saberian, H.; Bazsefidpar, N.; Karimi, A.; Soltani, A.; Assadpour, E.; Toker, O.S.; Jafari, S.M. Application of Spirulina as an innovative ingredient in pasta and bakery products. Food Biosci. 2024, 62, 105170. [Google Scholar] [CrossRef]
- Du, C.; Li, G.; Xia, R.; Li, C.; Zhu, Q.; Li, X.; Li, J.; Zhao, C.; Tian, Z.; Zhang, L. New insights into cyanobacterial blooms and the response of associated microbial communities in freshwater ecosystems. Environ. Pollut. 2022, 309, 119781. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-F.; Xing, P.; Liu, S.; Wu, Q.L. Enhanced Microbial Interactions and Deterministic Successions During Anoxic Decomposition of Microcystis Biomass in Lake Sediment. Front. Microbiol. 2019, 10, 2474. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wei, J.; Massey, I.Y.; Peng, T.; Yang, F. Immobilization of Microbes for Biodegradation of Microcystins: A Mini Review. Toxins 2022, 14, 573. [Google Scholar] [CrossRef] [PubMed]
- Do Carmo Bittencourt-Oliveira, M.; Chia, M.A.; de Oliveira, H.S.B.; Araújo, M.K.C.; Molica, R.J.R.; Dias, C.T.S. Allelopathic interactions between microcystin-producing and non-microcystin-producing cyanobacteria and green microalgae: Implications for microcystins production. J. Appl. Phycol. 2015, 27, 275–284. [Google Scholar] [CrossRef]
- Li, Q.; Gu, P.; Zhang, H.; Luo, X.; Zhang, J.; Zheng, Z. Response of submerged macrophytes and leaf biofilms to the decline phase of Microcystis aeruginosa: Antioxidant response, ultrastructure, microbial properties, and potential mechanism. Sci. Total. Environ. 2020, 699, 134325. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Q.; Feng, J.; Lv, J.-P.; Xie, S.-L. Effect of high-doses pyrogallol on oxidative damage, transcriptional responses and microcystins synthesis in Microcystis aeruginosa TY001 (Cyanobacteria). Ecotoxicol. Environ. Saf. 2016, 134, 273–279. [Google Scholar] [CrossRef]
- Gao, Y.; Lu, J.; Orr, P.T.; Chuang, A.; Franklin, H.M.; Burford, M.A. Enhanced resistance of co-existing toxigenic and non-toxigenic Microcystis aeruginosa to pyrogallol compared with monostrains. Toxicon 2020, 176, 47–54. [Google Scholar] [CrossRef]
- Zhu, X.X. Tradeoff Strategy and Cost Analysis of Inducible Defense in Scenedesmus Obliquus and Differences at Tran-Scriptomic Level; Nanjing Normal University: Nanjing, China, 2017. (In Chinese) [Google Scholar]
- Rippka, R.; Deruelles, J.; Waterbury, J.B.; Herdman, M.; Stanier, R.Y. Generic Assignments, Strain Histories and Properties of Pure Cultures of Cyanobacteria. Microbiology 1979, 111, 1–61. [Google Scholar] [CrossRef]
- Ha, M.-H.; Contardo-Jara, V.; Pflugmacher, S. Uptake of the cyanobacterial neurotoxin, anatoxin-a, and alterations in oxidative stress in the submerged aquatic plant Ceratophyllum demersum. Ecotoxicol. Environ. Saf. 2014, 101, 205–212. [Google Scholar] [CrossRef]
- Romero-Oliva, C.S.; Contardo-Jara, V.; Pflugmacher, S. Antioxidative response of the three macrophytes Ceratophyllum demersum, Egeria densa, and Hydrilla verticillata to a time dependent exposure of cell-free crude extracts containing three microcystins from cyanobacterial blooms of Lake Amatitlán, Guatemala. Aquat. Toxicol. 2015, 163, 130–139. [Google Scholar] [CrossRef]
- Cao, Q.; Liu, W.; Gu, Y.; Xie, L.; Jiang, W.; Gao, Y.; Yang, L. Synergetic enhancement toxicity of copper, cadmium and microcystin-LR to the Ceratophyllum demersum L. Toxicon 2020, 186, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Li, H.S.; Sun, Q.; Zhao, S.J. The Experiment Principle and Technique on Plant Physiology and Biochemistry; Higher Education Press: Beijing, China, 2000; pp. 194–197. [Google Scholar]
- Li, Q.; Gu, P.; Zhang, C.; Luo, X.; Zhang, H.; Zhang, J.; Zheng, Z. Combined toxic effects of anatoxin-a and microcystin-LR on submerged macrophytes and biofilms. J. Hazard. Mater. 2020, 389, 122053. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Sha, Y.; Tang, Y.; Li, L.; Wang, F.; Dong, J.; Li, X.; Gao, Y.; Gao, X.; Yuan, H.; et al. Laboratory-Simulated Inhibitory Effects of the Floating-Bed Plants on Microcystis aeruginosa and Their Microbial Communities’ Responses to Microcystins. Microorganisms 2024, 12, 2035. [Google Scholar] [CrossRef] [PubMed]
- Pančić, M.; Kiørboe, T. Phytoplankton defence mechanisms: Traits and trade-offs. Biol. Rev. 2018, 93, 1269–1303. [Google Scholar] [CrossRef]
- Herron, M.D.; Borin, J.M.; Boswell, J.C.; Walker, J.; Chen, I.-C.K.; Knox, C.A.; Boyd, M.; Rosenzweig, F.; Ratcliff, W.C. De novo origins of multicellularity in response to predation. Sci. Rep. 2019, 9, 2328. [Google Scholar] [CrossRef]
- Lürling, M.; Van Donk, E. Grazer-induced colony formation in Scenedesmus: Are there costs to being colonial? Oikos 2000, 88, 111–118. [Google Scholar] [CrossRef]
- Dai, D.J. Preliminary Study on the Mechanisms of the Growth and Morphology Response of Two Common Green Algae to Egeria Densa; Henan Normal University: Xinxiang, China, 2022. (In Chinese) [Google Scholar]
- Körner, S.; Nicklisch, A. Allelopathic Growth Inhibition of Selected Phytoplankton Species by Submerged Macrophytes. J. Phycol. 2002, 38, 862–871. [Google Scholar] [CrossRef]
- Dong, J.; Yang, Y.; Dai, D.; Wang, F.; Zhang, Y.; Chen, Y.; Yuan, J.; Guo, C.; Gao, Y.; Zhang, M.; et al. Response of submerged macrophyte Ceratophyllum demersum to the exponential phase (EP) and declining phase (DP) of toxic Microcystis aeruginosa. Hydrobiologia 2022, 849, 3581–3596. [Google Scholar] [CrossRef]
- Mohamed, Z.A. Polysaccharides as a protective response against microcystin-induced oxidative stress in Chlorella vulgaris and Scenedesmus quadricauda and their possible significance in the aquatic ecosystem. Ecotoxicology 2008, 17, 504–516. [Google Scholar] [CrossRef]
- El-Darier, S.M.; Metwally, A.-F.K.; Nasser, A.W.; Taha, H.M. Biointerference relationship between the macroalga Ulva lactuca and two green microalgae. Egypt. J. Aquat. Res. 2021, 47, 163–169. [Google Scholar] [CrossRef]
- Zhen, Z.; Cai, R.; Salam, M.; Hu, J.; Yang, B.; Liu, M.; Li, H.; Tang, B. The competitive advantage of Microcystis aeruginosa over Scenedesmus obliquus weakened by exposure to polylactic acid microplastics. Ecotoxicol. Environ. Saf. 2023, 267, 115620. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zheng, Y.; Ma, H.; Cui, F. Microcystin-LR (MC-LR) inhibits green algae growth by regulating antioxidant and photosynthetic systems. Harmful Algae 2024, 134, 102623. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xu, Y.; Yang, J.; Li, Y.; Sun, Y.; Zhang, L.; Yang, Z. Adverse role of colonial morphology and favorable function of microcystins for Microcystis to compete with Scenedesmus. Harmful Algae 2022, 117, 102293. [Google Scholar] [CrossRef]
- Harris, E.H. Chlamydomonas as Model Organism. Annu. Rev. Plant Biol. 2001, 52, 363–406. [Google Scholar] [CrossRef]
- Bianchelli, J.; Sagua, M.I.; Quiroga, M.P.; Nuozzi, G.; Fernández, J.; Schiaffino, M.R. Temporal dynamics of Legionella (Proteobacteria, Legionellaceae) in two Pampean shallow lakes from Argentina. Environ. Sci. Pollut. Res. 2024, 31, 59058–59070. [Google Scholar] [CrossRef] [PubMed]
- Burke, C.; Thomas, T.; Lewis, M.; Steinberg, P.; Kjelleberg, S. Composition, uniqueness and variability of the epiphytic bacterial community of the green alga Ulva australis. ISME J. 2011, 5, 590–600. [Google Scholar] [CrossRef]
- Liu, M.; Nie, H.; Luo, X.; Yang, S.; Chen, H.; Cai, P. A Polysaccharide Biosynthesis Locus in Vibrio parahaemolyticus Important for Biofilm Formation Has Homologs Widely Distributed in Aquatic Bacteria Mainly from Gammaproteobacteria. mSystems 2022, 7, e0122621. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, J.; Shi, G.; Mei, Z.; Wang, R.; Li, D. Discerning biodegradation and adsorption of microcystin-LR in a shallow semi-enclosed bay and bacterial community shifts in response to associated process. Ecotoxicol. Environ. Saf. 2016, 132, 123–131. [Google Scholar] [CrossRef]
- Zheng, S.M.; Wei, Q.; Ma, X.M.; Chen, R.G.; Yuan, S.H.; Huang, Y.F. Experimental study on the combined treatment of ammonia nitrogen wastewater by Chlorella and bacteria agent. Energy Environ. Prot. 2022, 36, 44–53. (In Chinese) Available online: https://engine.scichina.com/doi/pdf/E5D55590127A4AF0BCDCB392AA8743F5 (accessed on 28 October 2024).
- Larsbrink, J.; Zhu, Y.; Kharade, S.S.; Kwiatkowski, K.J.; Eijsink, V.G.H.; Koropatkin, N.M.; McBride, M.J.; Pope, P.B. A polysaccharide utilization locus from Flavobacterium johnsoniae enables conversion of recalcitrant chitin. Biotechnol. Biofuels 2016, 9, 260. [Google Scholar] [CrossRef]
- Tang, Y.; Huang, J.; Zhang, C.; Bi, S.; Guo, Z.; Liu, Q.; Lei, P. Complete Genome Sequence of Sandaracinobacter sp. Strain M6, Isolated from a Rocky Mountain in China. Genome Announc. 2021, 10, 110–128. [Google Scholar] [CrossRef] [PubMed]
- Vaz-Moreira, I.; Figueira, V.; Lopes, A.R.; De Brandt, E.; Vandamme, P.; Nunes, O.C.; Manaia, C.M. Candidimonas nitroreducens gen. nov., sp. nov. and Candidimonas humi sp. nov., isolated from sewage sludge compost. Int. J. Syst. Evol. Microbiol. 2011, 61, 2238–2246. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).