Aeruginosin 525 (AER525) from Cyanobacterium Aphanizomenon Sp. (KUCC C2): A New Serine Proteases Inhibitor
Abstract
:1. Introduction
2. Results
2.1. Isolation and Structure Characterisation of AER525
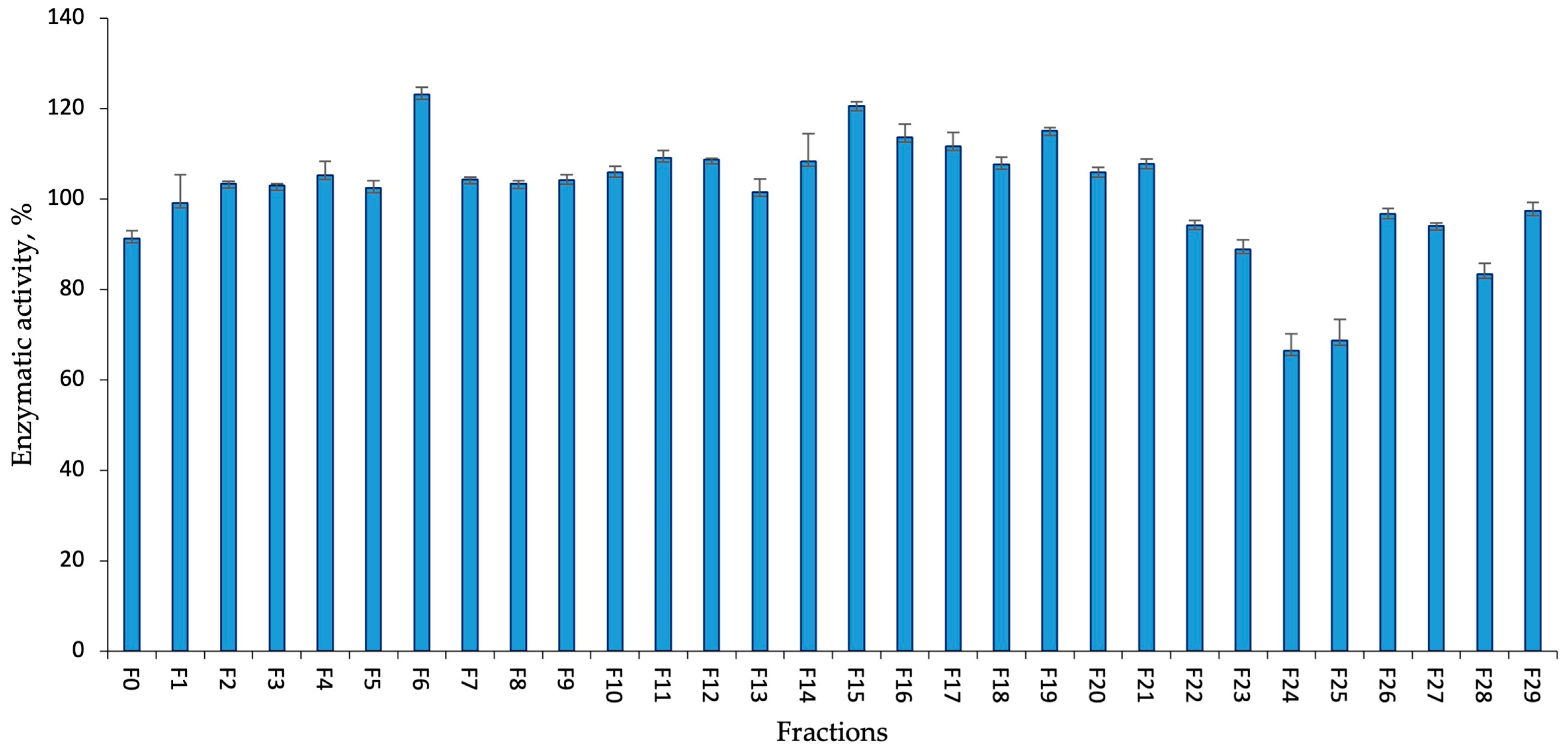
2.2. Enzymatic Assay of Aphanizomenon Sp. KUCC C2 Fractions
2.3. Enzymatic Assay of AER525
3. Discussion
4. Materials and Methods
4.1. Fractionation of Aphanizomenon Sp. KUCC C2 and Isolation of AER525
4.2. LC–MS/MS and HRMS Analysis
4.3. Enzymatic Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bishop, M.W.; Zubeck, M.H. Evaluation of Microalgae for Use as Nutraceuticals and Nutritional Supplements. J. Nutr. Food Sci. 2012, 2, 147. [Google Scholar] [CrossRef]
- Kownacka, J.; Busch, S.; Göbel, J.; Gromisz, S.; Hällfors, H.; Höglander, H.; Huseby, S.; Jaanus, A.; Jakobsen, H.H.; Johansen, M.; et al. Cyanobacteria Biomass 1990–2020. HELCOM Baltic Sea Environment Fact Sheets. 2021. Available online: https://helcom.fi/baltic-sea-trends/environment-fact-sheets/eutrophication/ (accessed on 17 September 2024).
- Scoglio, S.; Scoglio, G.D. Extracts and Benefits. In Insights into Algae-Fundamentals, Culture Techniques and Biotechnological Uses of Microalgae and Cyanobacteria; Severo, A.S., Martínez-Burgos, J.W., Ordonez, J., Eds.; IntechOpen Limited: London, UK, 2024. [Google Scholar]
- Grewe, C.B.; Pulz, O. The Biotechnology of Cyanobacteria. In Ecology of Cyanobacteria II: Their Diversity in Space and Time; Whitton, B., Ed.; Springer: Dordrecht, The Netherlands, 2012; pp. 707–739. [Google Scholar]
- Scoglio, G.D.; Jackson, H.O.; Purton, S. The Commercial Potential of Aphanizomenon Flos-Aquae, a Nitrogen-Fixing Edible Cyanobacterium. J. Appl. Phycol. 2024, 36, 1593–1617. [Google Scholar] [CrossRef]
- Gugger, M.; Lyra, C.; Henriksen, P.; Couté, A.; Humbert, J.F.; Sivonen, K. Phylogenetic Comparison of the Cyanobacterial Genera Anabaena and Aphanizomenon. Int. J. Syst. Evol. Microbiol. 2002, 52, 1867–1880. [Google Scholar] [CrossRef] [PubMed]
- Šulčius, S.; Pilkaityte, R.; Mazur-Marzec, H.; KasperovičienE, J.; Ezhova, E.; Błaszczyk, A.; Paškauskas, R. Increased Risk of Exposure to Microcystins in the Scum of the Filamentous Cyanobacterium Aphanizomenon flos-aquae Accumulated on the Western Shoreline of the Curonian Lagoon. Mar. Pollut. Bull. 2015, 99, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M.; Suzuki, S.; Itou, Y.; Kodani, S.; Ishida, K. New Anabaenopeptins, Carboxypeptidaze-A Inhibitors from the Cyanobacterium Aphanizomenon flos-aquae. J. Nat. Prod. 2000, 63, 1280–1282. [Google Scholar] [CrossRef]
- Ferreira, F.A.H. Peptides in Cyanobacteria under Different Environmental Conditions. Ph.D. Dissertation, Technical University of Berlin, Berlin, Germany, 2006. [Google Scholar]
- Genazzani, A.D.; Chierchia, E.; Lanzoni, C.; Santagni, S.; Veltri, F.; Ricchieri, F.; Rattighieri, E.; Nappi, R.E. Effects of Klamath Algae Extract on Psychological Disorders and Depression in Menopausal Women: A Pilot Study. Minerva Ginecol. 2010, 62, 381–388. [Google Scholar]
- Cremonte, M.; Sisti, D.; Maraucci, I.; Giribone, S.; Colombo, E.; Rocchi, M.B.L.; Scoglio, S. The Effect of Experimental Supplementation with the Klamath Algae Extract Klamin on Attention-Deficit/Hyperactivity Disorder. J. Med. Food 2017, 20, 1233–1239. [Google Scholar] [CrossRef]
- Scoglio, S.; Lo Curcio, V.; Catalani, S.; Palma, F.; Battistelli, S.; Benedetti, S. Inhibitory Effects of Aphanizomenon flos-aquae Constituents on Human UDP-Glucose Dehydrogenase Activity. J. Enzyme Inhib. Med. Chem. 2016, 31, 1492–1497. [Google Scholar] [CrossRef]
- Xue, X.; Lv, Y.; Liu, Q.; Zhang, X.; Zhao, Y.; Zhang, L.; Xu, S. Extracellular Polymeric Substance from Aphanizomenon Flos-Aquae Induces Apoptosis via the Mitochondrial Pathway in A431 Human Epidermoid Carcinoma Cells. Exp. Ther. Med. 2015, 10, 927–932. [Google Scholar] [CrossRef]
- Overlingė, D.; Toruńska-Sitarz, A.; Cegłowska, M.; Szubert, K.; Mazur-Marzec, H. Phylogenetic and Molecular Characteristics of Two Aphanizomenon Strains from the Curonian Lagoon, Southeastern Baltic Sea and Their Biological Activities. Sci. Rep. 2024, 14, 24686. [Google Scholar] [CrossRef]
- Ishida, K.; Christiansen, G.; Yoshida, W.Y.; Kurmayer, R.; Welker, M.; Valls, N.; Bonjoch, J.; Hertweck, C.; Börner, T.; Hemscheidt, T.; et al. Biosynthesis and Structure of Aeruginoside 126A and 126B, Cyanobacterial Peptide Glycosides Bearing a 2-Carboxy-6-Hydroxyoctahydroindole Moiety. Chem. Biol. 2007, 14, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Ersmark, K.; Del Valle, J.R.; Hanessian, S. Chemistry and Biology of the Aeruginosin Family of Serine Protease Inhibitors. Angew. Chem.-Int. Ed. 2008, 47, 1202–1223. [Google Scholar] [CrossRef] [PubMed]
- Welker, M.; Von Döhren, H. Cyanobacterial Peptides—Nature’s Own Combinatorial Biosynthesis. FEMS Microbiol. Rev. 2006, 30, 530–563. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M.; Okita, Y.; Matsuda, H.; Okino, T.; Yamaguchi, K. Aeruginosin 298-A, a Thrombin and Trypsin Inhibitor from the Blue-Green Alga Microcystis Aeruginosa (NIES-298). Tetrahedron Lett. 1994, 35, 3129–3132. [Google Scholar] [CrossRef]
- Jones, M.R.; Pinto, E.; Torres, M.A.; Dörr, F.; Mazur-Marzec, H.; Szubert, K.; Tartaglione, L.; Dell’Aversano, C.; Miles, C.O.; Beach, D.G.; et al. CyanoMetDB, a Comprehensive Public Database of Secondary Metabolites from Cyanobacteria. Water Res. 2021, 196, 117017. [Google Scholar] [CrossRef]
- Janssen, E.M.L.; Jones, M.R.; Pinto, E.; Dörr, F.; Torres, M.; Rios Jacinavicius, F.; Mazur-Marzec, H.; Szubert, K.; Konkel, R.; Tartaglione, L.; et al. Comprehensive database of secondary metabolites from cyanobacteria (NORMAN-SLE-S75.0.3.0) [Data set]. Zenodo 2024. [Google Scholar] [CrossRef]
- Entfellner, E.; Baumann, K.B.L.; Edwards, C.; Kurmayer, R. High Structural Diversity of Aeruginosins in Bloom-Forming Cyanobacteria of the Genus Planktothrix as a Consequence of Multiple Recombination Events. Mar. Drugs 2023, 21, 638. [Google Scholar] [CrossRef]
- Welker, M.; Maršálek, B.; Šejnohová, L.; von Döhren, H. Detection and Identification of Oligopeptides in Microcystis (Cyanobacteria) Colonies: Toward an Understanding of Metabolic Diversity. Peptides 2006, 27, 2090–2103. [Google Scholar] [CrossRef]
- Carroll, A.R.; Buchanan, M.S.; Edser, A.; Hyde, E.; Simpson, M.; Quinn, R.J. Dysinosins B-D, Inhibitors of Factor VIIa and Thrombin from the Australian Sponge Lamellodysidea chlorea. J. Nat. Prod. 2004, 67, 1291–1294. [Google Scholar] [CrossRef]
- Schorn, A.M.; Jordan, A.P.; Podell, S.; Jessica, B.M.; Agarwal, V.; Blggs, S.J.; Allen, E.E.; Moore, S.B. Comparative Genomics of Cyanobacterial Symbionts Reveals Distinct, Specialized Metabolism in Tropical Dysideidae Sponges. Appl. Environ. Sci. 2019, 10, 1–15. [Google Scholar] [CrossRef]
- Kapuścik, A.; Hrouzek, P.; Kuzma, M.; Bártová, S.; Novák, P.; Jokela, J.; Pflüger, M.; Eger, A.; Hundsberger, H.; Kopecký, J. Novel Aeruginosin-865 from Nostoc Sp. as a Potent Anti-Inflammatory Agent. ChemBioChem 2013, 14, 2329–2337. [Google Scholar] [CrossRef] [PubMed]
- Hanessian, S.; Del Valle, J.R.; Xue, Y.; Blomberg, N. Total Synthesis and Structural Confirmation of Chlorodysinosin A. J. Am. Chem. Soc. 2006, 128, 10491–10495. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Liu, H.; Yuan, L.; Wang, Y.; Ma, Y.; Wang, R.; Chen, X.; Losiewicz, M.D.; Guo, H.; Zhang, H. The Diversity of Cyanobacterial Toxins on Structural Characterization, Distribution and Identification: A Systematic Review. Toxins 2019, 11, 530. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Budnjo, A.; Jokela, J.; Haug, B.E.; Fewer, D.P.; Wahlsten, M.; Rouhiainen, L.; Permi, P.; Fossen, T.; Sivonen, K. Pseudoaeruginosins, Nonribosomal Peptides in Nodularia spumigena. ACS Chem. Biol. 2014, 10, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Banker, R.; Carmeli, S. Inhibitors of Serine Proteases from a Waterbloom of the Cyanobacterium Microcystis sp. Tetrahedron 1999, 55, 10835–10844. [Google Scholar] [CrossRef]
- Fewer, D.P.; Jokela, J.; Paukku, E.; Österholm, J.; Wahlsten, M.; Permi, P.; Aitio, O.; Rouhiainen, L.; Gomez-Saez, G.V.; Sivonen, K. New Structural Variants of Aeruginosin Produced by the Toxic Bloom Forming Cyanobacterium Nodularia spumigena. PLoS ONE 2013, 8, e73618. [Google Scholar] [CrossRef]
- Elkobi-Peer, S.; Carmeli, S. New Prenylated Aeruginosin, Microphycin, Anabaenopeptin and Micropeptin Analogues from a Microcystis Bloom Material Collected in Kibbutz Kfar Blum, Israel. Mar. Drugs 2015, 13, 2347–2375. [Google Scholar] [CrossRef]
- Adiv, S.; Carmeli, S. Protease Inhibitors from Microcystis Aeruginosa Bloom Material Collected from the Dalton Reservoir, Israel. J. Nat. Prod. 2013, 76, 2307–2315. [Google Scholar] [CrossRef]
- Vegman, M.; Carmeli, S. Three Aeruginosins and a Microviridin from a Bloom Assembly of Microcystis Spp. Collected from a Fishpond near Kibbutz Lehavot HaBashan, Israel. Tetrahedron 2014, 70, 6817–6824. [Google Scholar] [CrossRef]
- Kohler, E.; Grundler, V.; Häussinger, D.; Kurmayer, R.; Gademann, K.; Pernthaler, J.; Blom, J.F. The Toxicity and Enzyme Activity of a Chlorine and Sulfate Containing Aeruginosin Isolated from a Non-Microcystin-Producing Planktothrix Strain. Harmful Algae 2014, 39, 154–160. [Google Scholar] [CrossRef]
- Sanz, M.; Andreote, A.P.D.; Fiore, M.F.; Dörr, F.A.; Pinto, E. Structural Characterization of New Peptide Variants Produced by Cyanobacteria from the Brazilian Atlantic Coastal Forest Using Liquid Chromatography Coupled to Quadrupole Time-of-Flight Tandem Mass Spectrometry. Mar. Drugs 2015, 13, 3892–3919. [Google Scholar] [CrossRef] [PubMed]
- Carroll, A.R.; Pierens, G.K.; Fechner, G.; De Almeida Leone, P.; Ngo, A.; Simpson, M.; Hyde, E.; Hooper, J.N.A.; Boström, S.L.; Musil, D.; et al. Dysinosin A: A Novel Inhibitor of Factor Viia and Thrombin from a New Genus and Species of Australian Sponge of the Family Dysideidae. J. Am. Chem. Soc. 2002, 124, 13340–13341. [Google Scholar] [CrossRef] [PubMed]
- Vaičiūtė, D.; Bučas, M.; Bresciani, M.; Dabulevičienė, T.; Gintauskas, J.; Mėžinė, J.; Tiškus, E.; Umgiesser, G.; Morkūnas, J.; De Santi, F.; et al. Hot Moments and Hotspots of Cyanobacteria Hyperblooms in the Curonian Lagoon (SE Baltic Sea) Revealed via Remote Sensing-Based Retrospective Analysis. Sci. Total Environ. 2021, 769, 145053. [Google Scholar] [CrossRef] [PubMed]
- Zilius, M.; Vybernaite-Lubiene, I.; Vaiciute, D.; Petkuviene, J.; Zemlys, P.; Liskow, I.; Voss, M.; Bartoli, M.; Bukaveckas, P.A. The Influence of Cyanobacteria Blooms on the Attenuation of Nitrogen Throughputs in a Baltic Coastal Lagoon. Biogeochemistry 2018, 141, 143–165. [Google Scholar] [CrossRef]
- Overlingė, D.; Kataržytė, M.; Vaičiūtė, D.; Gyraite, G.; Gečaitė, I.; Jonikaitė, E.; Mazur-Marzec, H. Are There Concerns Regarding CHAB in Coastal Bathing Waters Affected by Freshwater-Brackish Continuum? Mar. Pollut. Bull. 2020, 159, 111500. [Google Scholar] [CrossRef]
- Pilkaitytė, R.; Overlingė, D.; Gasiūnaitė, Z.R.; Mazur-Marzec, H. Spatial and Temporal Diversity of Cyanometabolites in the Eutrophic Curonian Lagoon (SE Baltic Sea). Water 2021, 13, 1760. [Google Scholar] [CrossRef]
- Syrpas, M.; Bukauskaitė, J.; Paškauskas, R.; Bašinskienė, L.; Venskutonis, P.R. Recovery of Lipophilic Products from Wild Cyanobacteria (Aphanizomenon flos-aquae) Isolated from the Curonian Lagoon by Means of Supercritical Carbon Dioxide Extraction. Algal Res. 2018, 35, 10–21. [Google Scholar] [CrossRef]
- Syrpas, M.; Bukauskaite, J.; Ramanauskiene, K.; Karosiene, J.R.; Majiene, D.; Bašinskiene, L.; Venskutonis, P.R. Ultrasound-Assisted Extraction and Assessment of Biological Activity of Phycobiliprotein-Rich Aqueous Extracts from Wild Cyanobacteria (Aphanizomenon flos-aquae). J. Agric. Food Chem. 2019, 68, 1896–1909. [Google Scholar] [CrossRef]
- Available online: https://algaeservice.gamtostyrimai.lt/wp-content/uploads/2021/07/AlgaeService_for-LIFE_Mid-term-Report.pdf (accessed on 10 October 2024).
- Overlingė, D.; Toruńska-sitarz, A.; Cegłowska, M.; Błaszczyk, A.; Szubert, K.; Pilkaitytė, R.; Mazur-marzec, H. Phytoplankton of the Curonian Lagoon as a New Interesting Source for Bioactive Natural Products. Special Impact on Cyanobacterial Metabolites. Biomolecules 2021, 11, 1139. [Google Scholar] [CrossRef]
- Šulčius, S.; Montvydienė, D.; Mazur-Marzec, H.; Kasperovičienė, J.; Rulevičius, R.; Cibulskaitė, Ž. The Profound Effect of Harmful Cyanobacterial Blooms: From Food-Web and Management Perspectives. Sci. Total Environ. 2017, 609, 1443–1450. [Google Scholar] [CrossRef]
- Matsuda, H.; Okino, T.; Murakami, M.; Yamaguchi, K. Aeruginosins 102-A and B, New Thrombin Inhibitors from the Cyanobacterium Microcystis Viridis (NIES-102). Tetrahedron 1996, 52, 14501–14506. [Google Scholar] [CrossRef]
- Ishida, K.; Okita, Y.; Matsuda, H.; Okino, T.; Murakami, M. Aeruginosins, Protease Inhibitors from the Cyanobacterium Microcystis aeruginosa. Tetrahedron 1999, 55, 10971–10988. [Google Scholar] [CrossRef]
- Scherer, M.; Bezold, D.; Gademann, K. Investigating the Toxicity of the Aeruginosin Chlorosulfopeptides by Chemical Synthesis. Angew. Chem.-Int. Ed. 2016, 55, 9427–9431. [Google Scholar] [CrossRef] [PubMed]
- Elkobi-Peer, S.; Faigenbaum, R.; Carmeli, S. Bromine-and Chlorine-Containing Aeruginosins from Microcystis aeruginosa Bloom Material Collected in Kibbutz Geva, Israel. J. Nat. Prod. 2012, 75, 2144–2151. [Google Scholar] [CrossRef]
- Raveh, A.; Carmeli, S. Two Novel Biological Active Modified Peptides from the Cyanobacterium Microcystis sp. Phytochem. Lett. 2009, 2, 10–14. [Google Scholar] [CrossRef]
- Murakami, M.; Ishida, K.; Okino, T.; Okita, Y.; Matsuda, H.; Yamaguchi, K. Aeruginosins 98-A and B, Trypsin Inhibitors from the Blue-Green Alga Microcystis aeruginosa (NIES-98). Tetrahedron Lett. 1995, 36, 2785–2788. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, M.; Huang, Z.; Fang, J.; Wang, Z.; Zou, C.; Qui, X. Diversity, Biosynthesis and Bioactivity of Aeruginosins, a Family of Cyanobacteria-Derived Nonribosomal Linear Tetrapeptides. Mar. Drugs 2023, 21, 217. [Google Scholar] [CrossRef]
- Heinilä, L.M.P.; Jokela, J.; Ahmed, M.N.; Wahlsten, M.; Kumar, S.; Hrouzek, P.; Permi, P.; Koistinen, H.; Fewer, D.P.; Sivonen, K. Discovery of Varlaxins, New Aeruginosin-Type Inhibitors of Human Trypsins. Org. Biomol. Chem. 2022, 20, 2681–2692. [Google Scholar] [CrossRef]
- Faltermann, S.; Hutter, S.; Christen, V.; Hettich, T.; Fent, K. Anti-Inflammatory Activity of Cyanobacterial Serine Protease Inhibitors Aeruginosin 828A and Cyanopeptolin 1020 in Human Hepatoma Cell Line Huh7 and Effects in Zebrafish (Danio rerio). Toxins 2016, 8, 219. [Google Scholar] [CrossRef]
- Pluotno, A.; Carmeli, S. Banyasin A and Banyasides A and B, Three Novel Modified Peptides from a Water Bloom of the Cyanobacterium Nostoc sp. Tetrahedron 2005, 61, 575–583. [Google Scholar] [CrossRef]
- Ocampo Bennet, X. Peptide au Seiner Cyanobakterien Wasserblütte (1998) aus dem Wannsee/Berli: Strukturen and Biologische Wirksamkeit; University of Freiburg: Freiburg, Germany, 2007. [Google Scholar]
- Kwan, J.C.; Taori, K.; Paul, V.J.; Luesch, H. Lyngbyastatins 8–10, Elastase Inhibitors with Cyclic Depsipeptide Scaffolds Isolated from the Marine Cyanobacterium Lyngbya semiplena. Mar. Drugs 2009, 7, 528–538. [Google Scholar] [CrossRef] [PubMed]
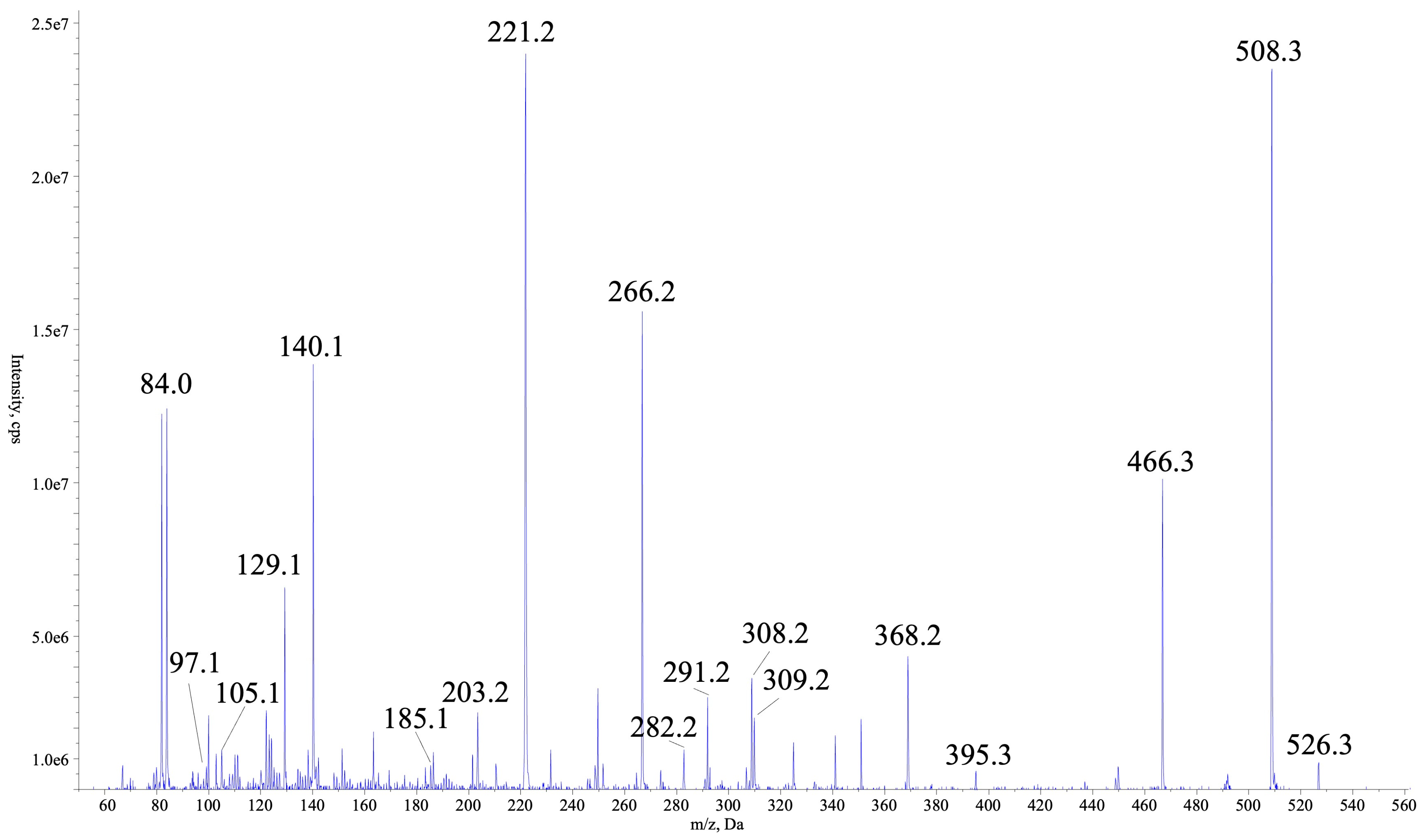


| Fragment | Exact Mass | Calculated m/z [M+H]+ | Observed m/z | Shift Mass |
|---|---|---|---|---|
| M | 525.3275 | 526.3353 | 526.3359 | Δ = 0.0006 |
| M-H2O | 507.3169 | 508.3247 | 508.3250 | Δ = 0.0003 |
| M+H-CH3N2-H2O | 465.2951 | 466.3030 | 466.3006 | Δ = 0.0024 |
| M+H-Argal | 368.21855 | 368.2185 | 368.2181 | Δ = 0.0004 |
| M+H-Argal-Choi | 202.1317 | 203.1396 | 203.1550 | Δ = 0.0154 |
| (Argal-CH3N2)-H2O | 96.0688 | 97.0766 | 97.0757 | Δ = 0.0009 |
| (Argal-CH3N2)+Choi | 281.1740 | 282.1818 | 282.1824 | Δ = 0.0006 |
| (Argal-CN2H5)+Choi-H2O | 265.1790 | 266.1869 | 266.1863 | Δ = 0.0006 |
| (Argal-CH3N2)+Choi+Leu | 394.2580 | 395.2659 | 395.2732 | Δ = 0.0073 |
| M+H-Leu-Ser-H2O | 307.2008 | 308.2087 | 308.2061 | Δ = 0.0025 |
| Leu+Ser-H2O | 184.1212 | 185.1290 | 185.1311 | Δ = 0.0021 |
| C3H8N2O2 | 104.0586 | 105.0664 | 105.0798 | Δ = 0.0134 |
| Choi+Argal-NH2 | 308.1848 | 309.1927 | 309.1945 | Δ = 0.0018 |
| Choi+Argal-NH2-H2O | 290.1743 | 291.1821 | 291.1848 | Δ = 0.0027 |
| Choi immonium | 140.1075 | 140.1083 | Δ = 0.0008 |
| Compound | Enzyme Inhibition (IC50 [µM]) | ||||
|---|---|---|---|---|---|
| Trypsin | Thrombin | Carboxypeptidase A | Elastase | Chymotrypsin | |
| AER525 | 71.7 | 0.59 | 89.7 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Overlingė, D.; Cegłowska, M.; Konkel, R.; Mazur-Marzec, H. Aeruginosin 525 (AER525) from Cyanobacterium Aphanizomenon Sp. (KUCC C2): A New Serine Proteases Inhibitor. Mar. Drugs 2024, 22, 506. https://doi.org/10.3390/md22110506
Overlingė D, Cegłowska M, Konkel R, Mazur-Marzec H. Aeruginosin 525 (AER525) from Cyanobacterium Aphanizomenon Sp. (KUCC C2): A New Serine Proteases Inhibitor. Marine Drugs. 2024; 22(11):506. https://doi.org/10.3390/md22110506
Chicago/Turabian StyleOverlingė, Donata, Marta Cegłowska, Robert Konkel, and Hanna Mazur-Marzec. 2024. "Aeruginosin 525 (AER525) from Cyanobacterium Aphanizomenon Sp. (KUCC C2): A New Serine Proteases Inhibitor" Marine Drugs 22, no. 11: 506. https://doi.org/10.3390/md22110506
APA StyleOverlingė, D., Cegłowska, M., Konkel, R., & Mazur-Marzec, H. (2024). Aeruginosin 525 (AER525) from Cyanobacterium Aphanizomenon Sp. (KUCC C2): A New Serine Proteases Inhibitor. Marine Drugs, 22(11), 506. https://doi.org/10.3390/md22110506








