Mesenchymal Stem Cell Expressing TRAIL as Targeted Therapy against Sensitised Tumour
Abstract
1. Introduction
2. Mesenchymal Stem Cells
3. MSCs and Its Effects in Tumour Tropism
4. Tumour Homing Capacity of Mesenchymal Stem Cells (MSCs)
5. Engineered MSCs for Anti-Tumour Therapy
5.1. Delivery of Anti-Tumour Cytokines
5.2. Delivery of Pro-Drug Converting Enzymes
5.3. MSCs as Vectors for Oncolytic Viruses
5.4. Safety Profile of Engineered MSCs
6. Tumour Necrosis Factor-Related Apoptosis Inducing Ligand (TRAIL) and Cancer Treatment
6.1. Tumour Necrosis Factor (TNF)-Related Apoptosis Inducing Ligand (TRAIL)
6.2. TRAIL Treatment in Solid Tumours
6.3. Synergistic Effects of TRAIL-Based Combination Therapy
7. The Existence of Cancer Stem Cells
8. Resistance of CSCs to TRAIL and Apoptosis
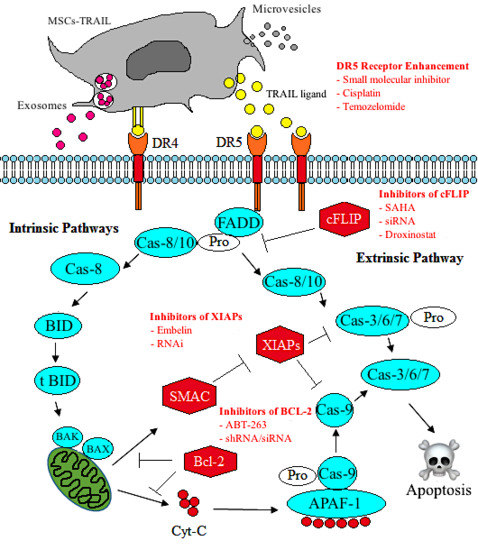
9. Sensitisation of CSCs to TRAIL and Apoptosis
10. Enhancing the Effect of MSC-TRAIL by Tumour Sensitisation
Challenges in MSC-TRAIL Applications: Discrepancies from In Vitro to In Vivo Models
11. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| MSCs | mesenchymal stem cells |
| TRAIL | tumour necrosis factor related apoptosis inducing ligand |
| CSCs | cancer stem cells |
| IL | interleukin |
| IFN | interferon |
| NSCLC | non-small cell lung cancer |
| mTOR | mammalian target of rapamycin |
| HDAC | histone deacetylases |
| BCL-2 | B-cell lymphoma 2 |
| ABCG2 | ATP-binding cassette sub-family G member 2 |
| ALDH1 | aldehyde dehydrogenase 1 |
| PARP | poly (ADP-ribose) polymerase |
| cFLIP | cellular FLICE (FADD-like IL-1β-converting enzyme)-inhibitory protein |
| XIAP | X-linked inhibitor of apoptosis protein |
| SAHA | suberoylanilide hydroxamic acid |
| shRNA | short hairpin RNA |
| RNAi | RNA interference |
| VEGF | vascular endothelial growth factor |
| PDGF | platelet-derived growth factor |
| FGF | fibroblast growth factors |
| IGF-1 | Insulin-like growth factor 1 |
| TGF-β | transforming growth factor beta |
| IDO | indoleamine 2,3-dioxygenase |
| HGF | hepatocyte growth factor |
| EGF | epidermal growth factor |
| WNT | proto-oncogene protein |
| Cas | caspase |
| FADD | fas-associated protein with death domain |
| BID | BH3 interacting-domain death agonist |
| P53 | tumor protein |
| Apaf-1 | apoptotic protease activating factor 1 |
References
- Bray, F.; Ferlay, J.; Laversanne, M.; Brewster, D.H.; Gombe Mbalawa, C.; Kohler, B.; Pineros, M.; Steliarova-Foucher, E.; Swaminathan, R.; Antoni, S.; et al. Cancer incidence in five continents: Inclusion criteria, highlights from Volume X and the global status of cancer registration. Int. J. Cancer 2015, 137, 2060–2071. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Huang, Y.H.; Chen, J.L. Understanding and targeting cancer stem cells: Therapeutic implications and challenges. Acta Pharmacol. Sin. 2013, 34, 732–740. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Kemp, K.C.; Hows, J.; Donaldson, C. Bone marrow-derived mesenchymal stem cells. Leuk. Lymphoma 2005, 46, 1531–1544. [Google Scholar] [CrossRef] [PubMed]
- Rubinstein, P.; Rosenfield, R.; Adamson, J.; Stevens, C. Stored placental blood for unrelated bone marrow reconstitution. Blood 1993, 81, 1679–1690. [Google Scholar] [PubMed]
- Rodriguez, A.M.; Elabd, C.; Amri, E.-Z.; Ailhaud, G.; Dani, C. The human adipose tissue is a source of multipotent stem cells. Biochimie 2005, 87, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Mamidi, M.K.; Nathan, K.G.; Singh, G.; Thrichelvam, S.T.; Mohd Yusof, N.A.; Fakharuzi, N.A.; Zakaria, Z.; Bhonde, R.; Das, A.K.; Majumdar, A.S. Comparative cellular and molecular analyses of pooled bone marrow multipotent mesenchymal stromal cells during continuous passaging and after successive cryopreservation. J. Cell. Biochem. 2012, 113, 3153–3164. [Google Scholar] [CrossRef] [PubMed]
- Le Blanc, K.; Tammik, C.; Rosendahl, K.; Zetterberg, E.; Ringdén, O. HLA expression and immunologic propertiesof differentiated and undifferentiated mesenchymal stem cells. Exp. Hematol. 2003, 31, 890–896. [Google Scholar] [CrossRef]
- Barry, F.P.; Murphy, J.M. Mesenchymal stem cells: Clinical applications and biological characterization. Int. J. Biochem. Cell Biol. 2004, 36, 568–584. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Amorin, B.; Alegretti, A.P.; Valim, V.; Pezzi, A.; Laureano, A.M.; da Silva, M.A.; Wieck, A.; Silla, L. Mesenchymal stem cell therapy and acute graft-versus-host disease: A review. Hum. Cell 2014, 27, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Introna, M.; Lucchini, G.; Dander, E.; Galimberti, S.; Rovelli, A.; Balduzzi, A.; Longoni, D.; Pavan, F.; Masciocchi, F.; Algarotti, A.; et al. Treatment of graft versus host disease with mesenchymal stromal cells: A phase I study on 40 adult and pediatric patients. Biol. Blood Marrow Transplant. 2014, 20, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Lou, R.; Huang, F.; Peng, Y.; Jiang, Z.; Huang, K.; Wu, X.; Zhang, Y.; Fan, Z.; Zhou, H.; et al. Immunomodulation effects of mesenchymal stromal cells on acute graft-versus-host disease after hematopoietic stem cell transplantation. Biol. Blood Marrow Transplant. 2015, 21, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Stuckey, D.W.; Shah, K. Stem cell-based therapies for cancer treatment: Separating hope from hype. Nature reviews. Cancer 2014, 14, 683–691. [Google Scholar] [PubMed]
- Chu, Y.; Liu, H.; Lou, G.; Zhang, Q.; Wu, C. Human placenta mesenchymal stem cells expressing exogenous kringle1-5 protein by fiber-modified adenovirus suppress angiogenesis. Cancer Gene Ther. 2014, 21, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.P. Tumor progression: Potential role of unstable genomic changes. Cancer Metastasis Rev. 1990, 9, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Tlsty, T.D.; Coussens, L.M. Tumor stroma and regulation of cancer development. Annu. Rev. Pathol. Mech. Dis. 2006, 1, 119–150. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Sun, R.; Origuchi, M.; Kanehira, M.; Takahata, T.; Itoh, J.; Umezawa, A.; Kijima, H.; Fukuda, S.; Saijo, Y. Mesenchymal stromal cells promote tumor growth through the enhancement of neovascularization. Mol. Med. 2011, 17, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Sung, S.Y.; Hsieh, C.L.; Wu, D.; Chung, L.W.; Johnstone, P.A. Tumor microenvironment promotes cancer progression, metastasis, and therapeutic resistance. Curr. Probl. Cancer 2007, 31, 36–100. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, S.; Venugopal, P.; Sundarraj, S.; Zakaria, Z.; Majumdar, A.; Ta, M. Comparison of chemokine and receptor gene expression between Wharton’s jelly and bone marrow-derived mesenchymal stromal cells. Cytotherapy 2012, 14, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Stagg, J. Mesenchymal stem cells in cancer. Stem Cell Rev. 2008, 4, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Klopp, A.H.; Gupta, A.; Spaeth, E.; Andreeff, M.; Marini, F., 3rd. Concise review: Dissecting a discrepancy in the literature: Do mesenchymal stem cells support or suppress tumor growth? Stem Cells 2011, 29, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Xu, Z.; Zhao, T.; Zhao, Z.; Shi, M.; Zhao, R.C.; Ye, L.; Zhang, X. Suppression of tumorigenesis by human mesenchymal stem cells in a hepatoma model. Cell Res. 2008, 18, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, R.; Lam, E.W.F.; Soeiro, I.; Tisato, V.; Bonnet, D.; Dazzi, F. Mesenchymal stem cells inhibit proliferation and apoptosis of tumor cells: Impact on in vivo tumor growth. Leukemia 2006, 21, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Khakoo, A.Y.; Pati, S.; Anderson, S.A.; Reid, W.; Elshal, M.F.; Rovira, I.I.; Nguyen, A.T.; Malide, D.; Combs, C.A.; Hall, G.; et al. Human mesenchymal stem cells exert potent antitumorigenic effects in a model of Kaposi’s sarcoma. J. Exp. Med. 2006, 203, 1235–1247. [Google Scholar] [CrossRef] [PubMed]
- Martin, F.T.; Dwyer, R.M.; Kelly, J.; Khan, S.; Murphy, J.M.; Curran, C.; Miller, N.; Hennessy, E.; Dockery, P.; Barry, F.P.; et al. Potential role of mesenchymal stem cells (MSCS) in the breast tumour microenvironment: Stimulation of epithelial to mesenchymal transition (EMT). Breast Cancer Res. Treat. 2010, 124, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Wang, L.; Li, H.; Han, Q.; Li, J.; Qu, X.; Huang, S.; Zhao, R.C. Mesenchymal stem cells play a potential role in regulating the establishment and maintenance of epithelial-mesenchymal transition in MCF7 human breast cancer cells by paracrine and induced autocrine TGF-β. Int. J. Oncol. 2012, 41, 959–968. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhai, W.; Xie, Y.; Chen, Q.; Zhu, W.; Sun, X. Mesenchymal stem cells derived from breast cancer tissue promote the proliferation and migration of the MCF-7 cell line. Oncol. Lett. 2013, 6, 1577–1582. [Google Scholar] [CrossRef] [PubMed]
- Timaner, M.; Letko-Khait, N.; Kotsofruk, R.; Benguigui, M.; Beyar-Katz, O.; Rachman-Tzemach, C.; Raviv, Z.; Bronshtein, T.; Machluf, M.; Shaked, Y. Therapy-educated mesenchymal stem cells enrich for tumor initiating cells. Cancer Res. 2018. [CrossRef] [PubMed]
- Kern, S.; Eichler, H.; Stoeve, J.; Klüter, H.; Bieback, K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells 2006, 24, 1294–1301. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Jia, Z.; Yin, X.; Zhang, X.; Liu, Y.; Chen, P.; Ma, K.; Zhou, C. Comparative analysis of mesenchymal stem cells from bone marrow, cartilage, and adipose tissue. Stem Cells Dev. 2008, 17, 761–773. [Google Scholar] [CrossRef] [PubMed]
- Kidd, S.; Spaeth, E.; Klopp, A.; Andreeff, M.; Hall, B.; Marini, F.C. The (in) auspicious role of mesenchymal stromal cells in cancer: Be it friend or foe. Cytotherapy 2008, 10, 657–667. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, N.; Burns, J.S.; Grisendi, G.; Candini, O.; Veronesi, E.; Piccinno, S.; Horwitz, E.M.; Paolucci, P.; Conte, P.; Dominici, M. MSC and tumors: Homing, differentiation, and secretion influence therapeutic potential. In Advances in Biochemical Engineering/Biotechnology; Springer: New York, NY, USA, 2012. [Google Scholar]
- Wang, H.; Cao, F.; De, A.; Cao, Y.; Contag, C.; Gambhir, S.S.; Wu, J.C.; Chen, X. Trafficking mesenchymal stem cell engraftment and differentiation in tumor-bearing mice by bioluminescence imaging. Stem Cells 2009, 27, 1548–1558. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Yong, X.; Li, C.; Lu, M.; Liu, D.; Chen, L.; Hu, J.; Teng, M.; Zhang, D.; Fan, Y.; et al. CXCL12/CXCR4 axis promotes mesenchymal stem cell mobilization to burn wounds and contributes to wound repair. J. Surg. Res. 2013, 183, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Yellowley, C. CXCL12/CXCR4 signalling and other recruitment and homing pathways in fracture repair. BoneKEy Rep. 2013, 2, 300. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, R.M.; Potter-Beirne, S.M.; Harrington, K.A.; Lowery, A.J.; Hennessy, E.; Murphy, J.M.; Barry, F.P.; O’Brien, T.; Kerin, M.J. Monocyte chemotactic protein-1 secreted by primary breast tumors stimulates migration of mesenchymal stem cells. Clin. Cancer Res. 2007, 13, 5020–5027. [Google Scholar] [CrossRef] [PubMed]
- Orimo, A.; Gupta, P.B.; Sgroi, D.C.; Arenzana-Seisdedos, F.; Delaunay, T.; Naeem, R.; Carey, V.J.; Richardson, A.L.; Weinberg, R.A. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell 2005, 121, 335–348. [Google Scholar] [CrossRef] [PubMed]
- Ip, J.E.; Wu, Y.; Huang, J.; Zhang, L.; Pratt, R.E.; Dzau, V.J. Mesenchymal stem cells use integrin β1 not CXC chemokine receptor 4 for myocardial migration and engraftment. Mol. Boil. Cell 2007, 18, 2873–2882. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.X.; Zhang, N.; Wang, H.W.; Gao, P.; Yang, Q.P.; Wen, Q.P. CXCR4 receptor overexpression in mesenchymal stem cells facilitates treatment of acute lung injury in rats. J. Boil. Chem. 2015, 290, 1994–2006. [Google Scholar] [CrossRef] [PubMed]
- Rengasamy, M.; Singh, G.; Fakharuzi, N.A.; Siddikuzzaman; Balasubramanian, S.; Swamynathan, P.; Thej, C.; Sasidharan, G.; Gupta, P.K.; Das, A.K.; et al. Transplantation of human bone marrow mesenchymal stromal cells reduces liver fibrosis more effectively than Wharton’s jelly mesenchymal stromal cells. Stem Cell Res. Ther. 2017, 8, 143. [Google Scholar] [CrossRef] [PubMed]
- Studeny, M.; Marini, F.C.; Dembinski, J.L.; Zompetta, C.; Cabreira-Hansen, M.; Bekele, B.N.; Champlin, R.E.; Andreeff, M. Mesenchymal stem cells: Potential precursors for tumor stroma and targeted-delivery vehicles for anticancer agents. J. Natl. Cancer Inst. 2004, 96, 1593–1603. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.A.; Hwang, S.-K.; Wang, K.-C.; Cho, B.-K.; Phi, J.H.; Lee, J.Y.; Jung, H.W.; Lee, D.-H.; Kim, S.-K. Therapeutic efficacy and safety of TRAIL-producing human adipose tissue–derived mesenchymal stem cells against experimental brainstem glioma. Neuro-Oncology 2011, 13, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.-Y.; Nong, J.; Qin, K.; Lu, H.; Moniri, M.R.; Dai, L.-J.; Warnock, G.L. MSC TRAIL-mediated HepG2 cell death in direct and indirect co-cultures. Anticancer Res. 2011, 30, 3705–3712. [Google Scholar]
- Mohr, A.; Lyons, M.; Deedigan, L.; Harte, T.; Shaw, G.; Howard, L.; Barry, F.; O’Brien, T.; Zwacka, R. Mesenchymal stem cells expressing TRAIL lead to tumour growth inhibition in an experimental lung cancer model. J. Cell. Mol. Med. 2008, 12, 2628–2643. [Google Scholar] [CrossRef] [PubMed]
- Ciavarella, S.; Grisendi, G.; Dominici, M.; Tucci, M.; Brunetti, O.; Dammacco, F.; Silvestris, F. In vitro anti-myeloma activity of TRAIL-expressing adipose-derived mesenchymal stem cells. Br. J. Haematol. 2012, 157, 586–598. [Google Scholar] [CrossRef] [PubMed]
- Grisendi, G.; Bussolari, R.; Cafarelli, L.; Petak, I.; Rasini, V.; Veronesi, E.; De Santis, G.; Spano, C.; Tagliazzucchi, M.; Barti-Juhasz, H.; et al. Adipose-derived mesenchymal stem cells as stable source of tumor necrosis factor–related apoptosis-inducing ligand delivery for cancer therapy. Cancer Res. 2010, 70, 3718–3729. [Google Scholar] [CrossRef] [PubMed]
- Campeau, P.M.; Rafei, M.; Francois, M.; Birman, E.; Forner, K.A.; Galipeau, J. Mesenchymal stromal cells engineered to express erythropoietin induce anti-erythropoietin antibodies and anemia in allorecipients. Mol. Ther. 2009, 17, 369–372. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Li, H.; Han, M.; Ruan, M.; Liu, Z.; Zhang, F.; Zhang, C.; Choi, Y.; Liu, B. Mesenchymal stem cells with enhanced Bcl-2 expression promote liver recovery in a rat model of hepatic cirrhosis. Cell. Physiol. Biochem. 2016, 40, 1117–1128. [Google Scholar] [CrossRef] [PubMed]
- Kuttappan, S.; Anitha, A.; Minsha, M.G.; Menon, P.M.; Sivanarayanan, T.B.; Vijayachandran, L.S.; Nair, M.B. BMP2 expressing genetically engineered mesenchymal stem cells on composite fibrous scaffolds for enhanced bone regeneration in segmental defects. Mater. Sci. Eng. C Mater. Boil. Appl. 2018, 85, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Ito, Y.; Kawano, Y.; Kurozumi, K.; Kobune, M.; Tsuda, H.; Bizen, A.; Honmou, O.; Niitsu, Y.; Hamada, H. Antitumor effect of genetically engineered mesenchymal stem cells in a rat glioma model. Gene Ther. 2004, 11, 1155–1164. [Google Scholar] [CrossRef] [PubMed]
- You, Q.; Yao, Y.; Zhang, Y.; Fu, S.; Du, M.; Zhang, G. Effect of targeted ovarian cancer therapy using amniotic fluid mesenchymal stem cells transfected with enhanced green fluorescent protein-human interleukin-2 in vivo. Mol. Med. Rep. 2015, 12, 4859–4866. [Google Scholar] [CrossRef] [PubMed]
- Studeny, M.; Marini, F.C.; Champlin, R.E.; Zompetta, C.; Fidler, I.J.; Andreeff, M. Bone marrow-derived mesenchymal stem cells as vehicles for interferon-β delivery into tumors. Cancer Res. 2002, 62, 3603–3608. [Google Scholar] [PubMed]
- Jing, W.; Chen, Y.; Lu, L.; Hu, X.; Shao, C.; Zhang, Y.; Zhou, X.; Zhou, Y.; Wu, L.; Liu, R.; et al. Human umbilical cord blood-derived mesenchymal stem cells producing IL-15 eradicate established pancreatic tumor in syngeneic mice. Mol. Cancer Ther. 2014. [Google Scholar] [CrossRef]
- Abdul Halim, N.S.; Fakiruddin, K.S.; Ali, S.A.; Yahaya, B.H. A comparative study of non-viral gene delivery techniques to human adipose-derived mesenchymal stem cell. Int. J. Mol. Sci. 2014, 15, 15044–15060. [Google Scholar] [CrossRef] [PubMed]
- Fakiruddin, K.S.; Baharuddin, P.; Lim, M.N.; Fakharuzi, N.A.; Yusof, N.A.N.M.; Zakaria, Z. Nucleofection optimization and in vitro anti-tumourigenic effect of TRAIL-expressing human adipose-derived mesenchymal stromal cells. Cancer Cell Int. 2014, 14, 122. [Google Scholar] [CrossRef] [PubMed]
- Cavarretta, I.T.; Altanerova, V.; Matuskova, M.; Kucerova, L.; Culig, Z.; Altaner, C. Adipose tissue–derived mesenchymal stem cells expressing prodrug-converting enzyme inhibit human prostate tumor growth. Mol. Ther. 2010, 18, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Chung, T.; Na, J.; Kim, Y.I.; Chang, D.Y.; Kim, Y.I.; Kim, H.; Moon, H.E.; Kang, K.W.; Lee, D.S.; Chung, J.K.; et al. Dihydropyrimidine dehydrogenase is a prognostic marker for mesenchymal stem cell-mediated cytosine deaminase gene and 5-fluorocytosine prodrug therapy for the treatment of recurrent gliomas. Theranostics 2016, 6, 1477–1490. [Google Scholar] [CrossRef] [PubMed]
- NguyenThai, Q.A.; Sharma, N.; Luong do, H.; Sodhi, S.S.; Kim, J.H.; Kim, N.; Oh, S.J.; Jeong, D.K. Targeted inhibition of osteosarcoma tumor growth by bone marrow-derived mesenchymal stem cells expressing cytosine deaminase/5-fluorocytosine in tumor-bearing mice. J. Gene Med. 2015, 17, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Jo, E.B.; Kim, S.J.; Yang, H.M.; Kim, Y.M.; Sung, Y.C.; Park, J.B.; Hong, D.; Park, H.; Choi, Y.L.; et al. Therapeutic strategies for locally recurrent and metastatic de-differentiated liposarcoma with herpes simplex virus-thymidine kinase-expressing mesenchymal stromal cells. Cytotherapy 2017, 19, 1035–1047. [Google Scholar] [CrossRef] [PubMed]
- Nouri, F.S.; Wang, X.; Hatefi, A. Genetically engineered theranostic mesenchymal stem cells for the evaluation of the anticancer efficacy of enzyme/prodrug systems. J. Control. Release 2015, 200, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Chiocca, E.A.; Rabkin, S.D. Oncolytic viruses and their application to cancer immunotherapy. Cancer Immunol. Res. 2014, 2, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Nagasato, M.; Rin, Y.; Henmi, M.; Ino, Y.; Yachida, S.; Ohki, R.; Hiraoka, N.; Tagawa, M.; Aoki, K. Strong antitumor efficacy of a pancreatic tumor-targeting oncolytic adenovirus for neuroendocrine tumors. Cancer Med. 2017, 6, 2385–2397. [Google Scholar] [CrossRef] [PubMed]
- Berkey, S.E.; Thorne, S.H.; Bartlett, D.L. Oncolytic virotherapy and the tumor microenvironment. Adv. Exp. Med. Biol. 2017, 1036, 157–172. [Google Scholar] [PubMed]
- Ahmed, A.U.; Tyler, M.A.; Thaci, B.; Alexiades, N.G.; Han, Y.; Ulasov, I.V.; Lesniak, M.S. A comparative study of neural and mesenchymal stem cell-based carriers for oncolytic adenovirus in a model of malignant glioma. Mol. Pharm. 2011, 8, 1559–1572. [Google Scholar] [CrossRef] [PubMed]
- Parker Kerrigan, B.C.; Shimizu, Y.; Andreeff, M.; Lang, F.F. Mesenchymal stem cells for the delivery of oncolytic viruses in gliomas. Cytotherapy 2017, 19, 445–457. [Google Scholar] [CrossRef] [PubMed]
- Kaczorowski, A.; Hammer, K.; Liu, L.; Villhauer, S.; Nwaeburu, C.; Fan, P.; Zhao, Z.; Gladkich, J.; Gross, W.; Nettelbeck, D.M.; et al. Delivery of improved oncolytic adenoviruses by mesenchymal stromal cells for elimination of tumorigenic pancreatic cancer cells. Oncotarget 2016, 7, 9046–9059. [Google Scholar] [CrossRef] [PubMed]
- Mader, E.K.; Butler, G.; Dowdy, S.C.; Mariani, A.; Knutson, K.L.; Federspiel, M.J.; Russell, S.J.; Galanis, E.; Dietz, A.B.; Peng, K.-W. Optimizing patient derived mesenchymal stem cells as virus carriers for a phase I clinical trial in ovarian cancer. J. Transl. Med. 2013, 11, 20. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Seah, I.; Bougazzoul, O.; Choi, G.; Meeth, K.; Bosenberg, M.W.; Wakimoto, H.; Fisher, D.; Shah, K. Stem cell-released oncolytic herpes simplex virus has therapeutic efficacy in brain metastatic melanomas. Proc. Natl. Acad. Sci. USA 2017, 114, E6157–E6165. [Google Scholar] [CrossRef] [PubMed]
- Leoni, V.; Gatta, V.; Palladini, A.; Nicoletti, G.; Ranieri, D.; Dall’Ora, M.; Grosso, V.; Rossi, M.; Alviano, F.; Bonsi, L.; et al. Systemic delivery of HER2-retargeted oncolytic-hsv by mesenchymal stromal cells protects from lung and brain metastases. Oncotarget 2015, 6, 34774–34787. [Google Scholar] [CrossRef] [PubMed]
- Yong, R.L.; Shinojima, N.; Fueyo, J.; Gumin, J.; Vecil, G.G.; Marini, F.C.; Bogler, O.; Andreeff, M.; Lang, F.F. Human bone marrow-derived mesenchymal stem cells for intravascular delivery of oncolytic adenovirus δ24-RGD to human gliomas. Cancer Res. 2009, 69, 8932–8940. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Ji, T.; Chen, P.; Li, X.; Fang, Y.; Gao, Q.; Liao, S.; You, L.; Xu, H.; Ma, Q.; et al. Mesenchymal stem cells as carriers and amplifiers in CRAd delivery to tumors. Mol. Cancer 2011, 10, 134. [Google Scholar] [CrossRef] [PubMed]
- Gura, T. How TRAIL kills cancer cells, but not normal cells. Science 1997, 277, 768. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Kale, V.; Chen, M. Gene-directed enzyme prodrug therapy. AAPS J. 2015, 17, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Chiocca, E.A.; Abbed, K.M.; Tatter, S.; Louis, D.N.; Hochberg, F.H.; Barker, F.; Kracher, J.; Grossman, S.A.; Fisher, J.D.; Carson, K.; et al. A phase I open-label, dose-escalation, multi-institutional trial of injection with an e1b-attenuated adenovirus, ONYX-015, into the peritumoral region of recurrent malignant gliomas, in the adjuvant setting. Mol. Ther. 2004, 10, 958–966. [Google Scholar] [CrossRef] [PubMed]
- Prestwich, R.J.; Errington, F.; Harrington, K.J.; Pandha, H.S.; Selby, P.; Melcher, A. Oncolytic viruses: Do they have a role in anti-cancer therapy? Clin. Med. Oncol. 2008, 2, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, R.N.; Stover, L.; Dutcher, J.P. Managing toxicities of high-dose interleukin-2. Oncology 2002, 16, 11–20. [Google Scholar] [PubMed]
- Gao, P.; Ding, Q.; Wu, Z.; Jiang, H.; Fang, Z. Therapeutic potential of human mesenchymal stem cells producing IL-12 in a mouse xenograft model of renal cell carcinoma. Cancer Lett. 2010, 290, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Elzaouk, L.; Moelling, K.; Pavlovic, J. Anti-tumor activity of mesenchymal stem cells producing IL-12 in a mouse melanoma model. Exp. Dermatol. 2006, 15, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Leonard, J.P.; Sherman, M.L.; Fisher, G.L.; Buchanan, L.J.; Larsen, G.; Atkins, M.B.; Sosman, J.A.; Dutcher, J.P.; Vogelzang, N.J.; Ryan, J.L. Effects of single-dose interleukin-12 exposure on interleukin-12-associated toxicity and interferon-γ production. Blood 1997, 90, 2541–2548. [Google Scholar] [PubMed]
- Berger, C.; Berger, M.; Hackman, R.C.; Gough, M.; Elliott, C.; Jensen, M.C.; Riddell, S.R. Safety and immunologic effects of IL-15 administration in nonhuman primates. Blood 2009, 114, 2417–2426. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Hu, J.; Sun, S.; Li, F.; Cao, W.; Wang, Y.U.; Ma, Z.; Yu, Z. Mesenchymal stem cells expressing interleukin-18 suppress breast cancer cells in vitro. Exp. Ther. Med. 2015, 9, 1192–1200. [Google Scholar] [CrossRef] [PubMed]
- Robertson, M.J.; Mier, J.W.; Logan, T.; Atkins, M.; Koon, H.; Koch, K.M.; Kathman, S.; Pandite, L.N.; Oei, C.; Kirby, L.C.; et al. Clinical and biological effects of recombinant human interleukin-18 administered by intravenous infusion to patients with advanced cancer. Clin. Cancer Res. 2006, 12, 4265–4273. [Google Scholar] [CrossRef] [PubMed]
- Jonasch, E.; Haluska, F.G. Interferon in oncological practice: Review of interferon biology, clinical applications, and toxicities. Oncologist 2001, 6, 34–55. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R.S.; Eckhardt, S.G.; Kurzrock, R.; Ebbinghaus, S.; O’Dwyer, P.J.; Gordon, M.S.; Novotny, W.; Goldwasser, M.A.; Tohnya, T.M.; Lum, B.L.; et al. Phase I dose-escalation study of recombinant human Apo2L/TRAIL, a dual proapoptotic receptor agonist, in patients with advanced cancer. J. Clin. Oncol. 2010, 28, 2839–2846. [Google Scholar] [CrossRef] [PubMed]
- Castleton, A.; Dey, A.; Beaton, B.; Patel, B.; Aucher, A.; Davis, D.M.; Fielding, A.K. Human mesenchymal stromal cells deliver systemic oncolytic measles virus to treat acute lymphoblastic leukemia in the presence of humoral immunity. Blood 2014, 27, 1327–1335. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Quintanilla, J.; He, D.; Wakimoto, H.; Alemany, R.; Shah, K. Encapsulated stem cells loaded with hyaluronidase-expressing oncolytic virus for brain tumor therapy. Mol. Ther. 2015, 23, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Almasan, A.; Ashkenazi, A. Apo2L/TRAIL: Apoptosis signalling, biology, and potential for cancer therapy. Cytokine Growth Factor Rev. 2003, 14, 337–348. [Google Scholar] [CrossRef]
- Robertson, N.M.; Zangrilli, J.G.; Steplewski, A.; Hastie, A.; Lindemeyer, R.G.; Planeta, M.A.; Smith, M.K.; Innocent, N.; Musani, A.; Pascual, R.; et al. Differential expression of TRAIL and TRAIL receptors in allergic asthmatics following segmental antigen challenge: Evidence for a role of TRAIL in eosinophil survival. J. Immunol. 2002, 169, 5986–5996. [Google Scholar] [CrossRef] [PubMed]
- Han, L.H.; Sun, W.S.; Ma, C.H.; Zhang, L.N.; Liu, S.X.; Zhang, Q.; Gao, L.F.; Chen, Y.H. Detection of soluble TRAIL in HBV infected patients and its clinical implications. World J. Gastroenterol. 2002, 8, 1077–1080. [Google Scholar] [CrossRef] [PubMed]
- Snell, V.; Clodi, K.; Zhao, S.; Goodwin, R.; Thomas, E.K.; Morris, S.W.; Kadin, M.E.; Cabanillas, F.; Andreeff, M.; Younes, A. Activity of TNF-related apoptosis-inducing ligand (TRAIL) in haematological malignancies. Br. J. Haematol. 1997, 99, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Belyanskaya, L.L.; Ziogas, A.; Hopkins-Donaldson, S.; Kurtz, S.; Simon, H.U.; Stahel, R.; Zangemeister-Wittke, U. TRAIL-induced survival and proliferation of SCLC cells is mediated by ERK and dependent on TRAIL-R2/DR5 expression in the absence of caspase-8. Lung Cancer 2008, 60, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, D.; Shahrokh, Z.; Marsters, S.; Achilles, K.; Shih, D.; Mounho, B.; Hillan, K.; Totpal, K.; DeForge, L.; Schow, P.; et al. Differential hepatocyte toxicity of recombinant Apo2L/TRAIL versions. Nat. Med. 2001, 7, 383–385. [Google Scholar] [CrossRef] [PubMed]
- Younes, A.; Kadin, M.E. Emerging applications of the tumor necrosis factor family of ligands and receptors in cancer therapy. J. Clin. Oncol. 2003, 21, 3526–3534. [Google Scholar] [CrossRef] [PubMed]
- Keane, M.M.; Ettenberg, S.A.; Nau, M.M.; Russell, E.K.; Lipkowitz, S. Chemotherapy augments TRAIL-induced apoptosis in breast cell lines. Cancer Res. 1999, 59, 734–741. [Google Scholar] [PubMed]
- Naka, T.; Sugamura, K.; Hylander, B.L.; Widmer, M.B.; Rustum, Y.M.; Repasky, E.A. Effects of tumor necrosis factor-related apoptosis-inducing ligand alone and in combination with chemotherapeutic agents on patients’ colon tumors grown in scid mice. Cancer Res. 2002, 62, 5800–5806. [Google Scholar] [PubMed]
- Singh, T.R.; Shankar, S.; Chen, X.; Asim, M.; Srivastava, R.K. Synergistic interactions of chemotherapeutic drugs and tumor necrosis factor-related apoptosis-inducing ligand/Apo-2 ligand on apoptosis and on regression of breast carcinoma in vivo. Cancer Res. 2003, 63, 5390–5400. [Google Scholar] [PubMed]
- Marini, P.; Denzinger, S.; Schiller, D.; Kauder, S.; Welz, S.; Humphreys, R.; Daniel, P.T.; Jendrossek, V.; Budach, W.; Belka, C. Combined treatment of colorectal tumours with agonistic TRAIL receptor antibodies HGS-ETR1 and HGS-ETR2 and radiotherapy: Enhanced effects in vitro and dose-dependent growth delay in vivo. Oncogene 2006, 25, 5145–5154. [Google Scholar] [CrossRef] [PubMed]
- Nagane, M.; Pan, G.; Weddle, J.J.; Dixit, V.M.; Cavenee, W.K.; Huang, H.J. Increased death receptor 5 expression by chemotherapeutic agents in human gliomas causes synergistic cytotoxicity with tumor necrosis factor-related apoptosis-inducing ligand in vitro and in vivo. Cancer Res. 2000, 60, 847–853. [Google Scholar] [PubMed]
- Voortman, J.; Resende, T.P.; Abou El Hassan, M.A.; Giaccone, G.; Kruyt, F.A. TRAIL therapy in non-small cell lung cancer cells: Sensitization to death receptor-mediated apoptosis by proteasome inhibitor bortezomib. Mol. Cancer Ther. 2007, 6, 2103–2112. [Google Scholar] [CrossRef] [PubMed]
- Baader, E.; Toloczko, A.; Fuchs, U.; Schmid, I.; Beltinger, C.; Ehrhardt, H.; Debatin, K.M.; Jeremias, I. Tumor necrosis factor-related apoptosis-inducing ligand-mediated proliferation of tumor cells with receptor-proximal apoptosis defects. Cancer Res. 2005, 65, 7888–7895. [Google Scholar] [CrossRef] [PubMed]
- Ehrhardt, H.; Fulda, S.; Schmid, I.; Hiscott, J.; Debatin, K.M.; Jeremias, I. TRAIL induced survival and proliferation in cancer cells resistant towards TRAIL-induced apoptosis mediated by NF-kappaB. Oncogene 2003, 22, 3842–3852. [Google Scholar] [CrossRef] [PubMed]
- Kelley, S.K.; Harris, L.A.; Xie, D.; Deforge, L.; Totpal, K.; Bussiere, J.; Fox, J.A. Preclinical studies to predict the disposition of Apo2L/tumor necrosis factor-related apoptosis-inducing ligand in humans: Characterization of in vivo efficacy, pharmacokinetics, and safety. J. Pharmacol. Exp. Ther. 2001, 299, 31–38. [Google Scholar] [PubMed]
- Walczak, H.; Miller, R.E.; Ariail, K.; Gliniak, B.; Griffith, T.S.; Kubin, M.; Chin, W.; Jones, J.; Woodward, A.; Le, T.; et al. Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nat. Med. 1999, 5, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Davis, J.S.; Wu, X. Immunoglobulin fc domain fusion to TRAIL significantly prolongs its plasma half-life and enhances its antitumor activity. Mol. Cancer Ther. 2014, 13, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Butler, L.M.; Liapis, V.; Bouralexis, S.; Welldon, K.; Hay, S.; Thai le, M.; Labrinidis, A.; Tilley, W.D.; Findlay, D.M.; Evdokiou, A. The histone deacetylase inhibitor, suberoylanilide hydroxamic acid, overcomes resistance of human breast cancer cells to Apo2L/TRAIL. Int. J. Cancer 2006, 119, 944–954. [Google Scholar] [CrossRef] [PubMed]
- Panner, A.; Parsa, A.T.; Pieper, R.O. Use of Apo2L/TRAIL with mTOR inhibitors in the treatment of glioblastoma multiforme. Expert Rev. Anticancer Ther. 2006, 6, 1313–1322. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Shiraki, K.; Fuke, H.; Yamanaka, Y.; Miyashita, K.; Yamaguchi, Y.; Yamamoto, N.; Ito, K.; Sugimoto, K.; Nakano, T. Proteasome inhibition sensitizes hepatocellular carcinoma cells to TRAIL by suppressing caspase inhibitors and AKT pathway. Anti-Cancer Drugs 2006, 17, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Kasman, L.; Lu, P.; Voelkel-Johnson, C. The histone deacetylase inhibitors depsipeptide and MS-275, enhance TRAIL gene therapy of LNCAP prostate cancer cells without adverse effects in normal prostate epithelial cells. Cancer Gene Ther. 2007, 14, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhan, Y.; Wang, H.; Li, W. ABT-263 sensitizes TRAIL-resistant hepatocarcinoma cells by downregulating the Bcl-2 family of anti-apoptotic protein. Cancer Chemother. Pharmacol. 2012, 69, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Frese, S.; Frese-Schaper, M.; Andres, A.C.; Miescher, D.; Zumkehr, B.; Schmid, R.A. Cardiac glycosides initiate Apo2L/TRAIL-induced apoptosis in non-small cell lung cancer cells by up-regulation of death receptors 4 and 5. Cancer Res. 2006, 66, 5867–5874. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.O.; Kang, S.E.; Im, C.R.; Lee, J.H.; Ahn, K.S.; Yang, W.M.; Um, J.Y.; Lee, S.G.; Yun, M. Tanshinone IIA induces TRAIL sensitization of human lung cancer cells through selective ER stress induction. Int. J. Oncol. 2016, 48, 2205–2212. [Google Scholar] [CrossRef] [PubMed]
- Lacour, S.; Micheau, O.; Hammann, A.; Drouineaud, V.; Tschopp, J.; Solary, E.; Dimanche-Boitrel, M.T. Chemotherapy enhances TNF-related apoptosis-inducing ligand disc assembly in HT29 human colon cancer cells. Oncogene 2003, 22, 1807–1816. [Google Scholar] [CrossRef] [PubMed]
- O’Flaherty, J.D.; Barr, M.; Fennell, D.; Richard, D.; Reynolds, J.; O’Leary, J.; O’Byrne, K. The cancer stem-cell hypothesis: Its emerging role in lung cancer biology and its relevance for future therapy. J. Thorac. Oncol. 2012, 7, 1880–1890. [Google Scholar] [CrossRef] [PubMed]
- Wong, N.K.; Fuller, M.; Sung, S.; Wong, F.; Karsan, A. Heterogeneity of breast cancer stem cells as evidenced with notch-dependent and notch-independent populations. Cancer Med. 2012, 1, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.G. Understanding cancer stem cell heterogeneity and plasticity. Cell Res. 2012, 22, 457–472. [Google Scholar] [CrossRef] [PubMed]
- Al-Hajj, M.; Wicha, M.S.; Benito-Hernandez, A.; Morrison, S.J.; Clarke, M.F. Prospective identification of tumorigenic breast cancer cells. Proc. Natl. Acad. Sci. USA 2003, 100, 3983–3988. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Chen, B.O.; Xu, W.E.I.; Zhao, W.; Wu, J. Clinicopathological significance of CD133 in lung cancer: A meta-analysis. Mol. Clin. Oncol. 2014, 2, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Pellacani, D.; Oldridge, E.E.; Collins, A.T.; Maitland, N.J. Prominin-1 (CD133) expression in the prostate and prostate cancer: A marker for quiescent stem cells. Adv. Exp. Med. Boil. 2013, 777, 167–184. [Google Scholar]
- Reyes, E.E.; Kunovac, S.K.; Duggan, R.; Kregel, S.; Vander Griend, D.J. Growth kinetics of CD133-positive prostate cancer cells. Prostate 2013, 73, 724–733. [Google Scholar] [CrossRef] [PubMed]
- Vander Griend, D.J.; Karthaus, W.L.; Dalrymple, S.; Meeker, A.; DeMarzo, A.M.; Isaacs, J.T. The role of CD133 in normal human prostate stem cells and malignant cancer-initiating cells. Cancer Res. 2008, 68, 9703–9711. [Google Scholar] [CrossRef] [PubMed]
- Bi, C.L.; Fang, J.S.; Chen, F.H.; Wang, Y.J.; Wu, J. Chemoresistant of CD133+ tumor stem cells from human brain glioma. Zhong Nan Da Xue Xue Bao. Yi Xue Ban 2007, 32, 568–573. [Google Scholar] [PubMed]
- Choi, S.A.; Wang, K.C.; Phi, J.H.; Lee, J.Y.; Park, C.K.; Park, S.H.; Kim, S.K. A distinct subpopulation within CD133 positive brain tumor cells shares characteristics with endothelial progenitor cells. Cancer Lett. 2012, 324, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Li, M.C.; Deng, Y.W.; Wu, J.; Chen, F.H.; Liu, J.F.; Fang, J.S. Isolation and characterization of brain tumor stem cells in human medulloblastoma. Ai Zheng 2006, 25, 241–246. [Google Scholar] [PubMed]
- Singh, S.; Dirks, P.B. Brain tumor stem cells: Identification and concepts. Neurosurg. Clin. North Am. 2007, 18, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Kozovska, Z.; Gabrisova, V.; Kucerova, L. Colon cancer: Cancer stem cells markers, drug resistance and treatment. Biomed. Pharmacother. 2014, 68, 911–916. [Google Scholar] [CrossRef] [PubMed]
- Margaritescu, C.; Pirici, D.; Cherciu, I.; Barbalan, A.; Cartana, T.; Saftoiu, A. CD133/CD166/KI-67 triple immunofluorescence assessment for putative cancer stem cells in colon carcinoma. J. Gastrointest. Liver Dis. 2014, 23, 161–170. [Google Scholar] [CrossRef]
- Vincent, Z.; Urakami, K.; Maruyama, K.; Yamaguchi, K.; Kusuhara, M. CD133-positive cancer stem cells from Colo205 human colon adenocarcinoma cell line show resistance to chemotherapy and display a specific metabolomic profile. Genes Cancer 2014, 5, 250–260. [Google Scholar] [PubMed]
- Cogliati, B.; Aloia, T.P.; Bosch, R.V.; Alves, V.A.; Hernandez-Blazquez, F.J.; Dagli, M.L. Identification of hepatic stem/progenitor cells in canine hepatocellular and cholangiocellular carcinoma. Vet. Comparat. Oncol. 2010, 8, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Tomuleasa, C.; Soritau, O.; Rus-Ciuca, D.; Pop, T.; Todea, D.; Mosteanu, O.; Pintea, B.; Foris, V.; Susman, S.; Kacso, G.; et al. Isolation and characterization of hepatic cancer cells with stem-like properties from hepatocellular carcinoma. J. Gastrointest. Liver Dis. 2010, 19, 61–67. [Google Scholar]
- Yang, X.R.; Xu, Y.; Yu, B.; Zhou, J.; Qiu, S.J.; Shi, G.M.; Zhang, B.H.; Wu, W.Z.; Shi, Y.H.; Wu, B.; et al. High expression levels of putative hepatic stem/progenitor cell biomarkers related to tumour angiogenesis and poor prognosis of hepatocellular carcinoma. Gut 2010, 59, 953–962. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Sun, H.; Zhao, F.; Lu, P.; Ge, C.; Li, H.; Hou, H.; Yan, M.; Chen, T.; Jiang, G.; et al. BMP4 administration induces differentiation of CD133+ hepatic cancer stem cells, blocking their contributions to hepatocellular carcinoma. Cancer Res. 2012, 72, 4276–4285. [Google Scholar] [CrossRef] [PubMed]
- Christgen, M.; Ballmaier, M.; Lehmann, U.; Kreipe, H. Detection of putative cancer stem cells of the side population phenotype in human tumor cell cultures. Methods Mol. Biol. 2012, 878, 201–215. [Google Scholar] [PubMed]
- Zhang, W.C.; Shyh-Chang, N.; Yang, H.; Rai, A.; Umashankar, S.; Ma, S.; Soh, B.S.; Sun, L.L.; Tai, B.C.; Nga, M.E.; et al. Glycine decarboxylase activity drives non-small cell lung cancer tumor-initiating cells and tumorigenesis. Cell 2012, 148, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Eramo, A.; Lotti, F.; Sette, G.; Pilozzi, E.; Biffoni, M.; Di Virgilio, A.; Conticello, C.; Ruco, L.; Peschle, C.; De Maria, R. Identification and expansion of the tumorigenic lung cancer stem cell population. Cell Death Differ. 2008, 15, 504–514. [Google Scholar] [CrossRef] [PubMed]
- Karimi-Busheri, F.; Zadorozhny, V.; Li, T.; Lin, H.; Shawler, D.L.; Fakhrai, H. Pivotal role of CD38 biomarker in combination with CD24, epcam, and aldh for identification of H460 derived lung cancer stem cells. J. Stem Cells 2011, 6, 9–20. [Google Scholar] [PubMed]
- Shao, C.; Sullivan, J.P.; Girard, L.; Augustyn, A.; Yenerall, P.; Rodriguez-Canales, J.; Liu, H.; Behrens, C.; Shay, J.W.; Wistuba, I.I.; et al. Essential role of aldehyde dehydrogenase 1A3 for the maintenance of non-small cell lung cancer stem cells is associated with the STAT3 pathway. Clin. Cancer Res. 2014, 20, 4154–4166. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, N.; Yusoff, N.M.; Zakaria, Z.; Lim, M.N.; Baharuddin, P.J.N.; Fakiruddin, K.S.; Yahaya, B. Human non-small cell lung cancer expresses putative cancer stem cell markers and exhibits the transcriptomic profile of multipotent cells. BMC Cancer 2015, 15, 84. [Google Scholar] [CrossRef] [PubMed]
- De Beca, F.F.; Caetano, P.; Gerhard, R.; Alvarenga, C.A.; Gomes, M.; Paredes, J.; Schmitt, F. Cancer stem cells markers CD44, CD24 and ALDH1 in breast cancer special histological types. J. Clin. Pathol. 2013, 66, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Kai, K.; Arima, Y.; Kamiya, T.; Saya, H. Breast cancer stem cells. Breast Cancer 2010, 17, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Puglisi, M.A.; Sgambato, A.; Saulnier, N.; Rafanelli, F.; Barba, M.; Boninsegna, A.; Piscaglia, A.C.; Lauritano, C.; Novi, M.L.; Barbaro, F.; et al. Isolation and characterization of CD133+ cell population within human primary and metastatic colon cancer. Eur. Rev. Med. Pharmacol. Sci. 2009, 13 (Suppl. S1), 55–62. [Google Scholar] [PubMed]
- Choi, D.; Lee, H.W.; Hur, K.Y.; Kim, J.J.; Park, G.S.; Jang, S.H.; Song, Y.S.; Jang, K.S.; Paik, S.S. Cancer stem cell markers CD133 and CD24 correlate with invasiveness and differentiation in colorectal adenocarcinoma. World J. Gastroenterol. 2009, 15, 2258–2264. [Google Scholar] [CrossRef] [PubMed]
- Chikamatsu, K.; Takahashi, G.; Sakakura, K.; Ferrone, S.; Masuyama, K. Immunoregulatory properties of CD44+ cancer stem-like cells in squamous cell carcinoma of the head and neck. Head Neck 2011, 33, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Fujii, M.; Sonoda, A.; Tokumaru, Y.; Matsunaga, T.; Habu, N. Identification of stem-like cells in head and neck cancer cell lines. Anticancer Res. 2010, 30, 2005–2010. [Google Scholar] [PubMed]
- Erdogan, S.; Doganlar, Z.B.; Doganlar, O.; Turkekul, K.; Serttas, R. Inhibition of midkine suppresses prostate cancer CD133+ stem cell growth and migration. Am. J. Med. Sci. 2017, 354, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Miki, J.; Furusato, B.; Li, H.; Gu, Y.; Takahashi, H.; Egawa, S.; Sesterhenn, I.A.; McLeod, D.G.; Srivastava, S.; Rhim, J.S. Identification of putative stem cell markers, CD133 and CXCR4, in hTERT-immortalized primary nonmalignant and malignant tumor-derived human prostate epithelial cell lines and in prostate cancer specimens. Cancer Res. 2007, 67, 3153–3161. [Google Scholar] [CrossRef] [PubMed]
- Kahlert, U.D.; Bender, N.O.; Maciaczyk, D.; Bogiel, T.; Bar, E.E.; Eberhart, C.G.; Nikkhah, G.; Maciaczyk, J. CD133/CD15 defines distinct cell subpopulations with differential in vitro clonogenic activity and stem cell-related gene expression profile in in vitro propagated glioblastoma multiforme-derived cell line with a PNET-like component. Folia Neuropathol. 2012, 50, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Hawkins, C.; Clarke, I.D.; Squire, J.A.; Bayani, J.; Hide, T.; Henkelman, R.M.; Cusimano, M.D.; Dirks, P.B. Identification of human brain tumour initiating cells. Nature 2004, 432, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Suresh, R.; Ali, S.; Ahmad, A.; Philip, P.A.; Sarkar, F.H. The role of cancer stem cells in recurrent and drug-resistant lung cancer. Adv. Exp. Med. Boil. 2016, 890, 57–74. [Google Scholar]
- Sussman, R.T.; Ricci, M.S.; Hart, L.S.; Sun, S.Y.; El-Deiry, W.S. Chemotherapy-resistant side-population of colon cancer cells has a higher sensitivity to TRAIL than the non-SP, a higher expression of c-Myc and TRAIL-receptor DR4. Cancer Boil. Ther. 2007, 6, 1490–1495. [Google Scholar] [CrossRef]
- Ding, L.; Yuan, C.; Wei, F.; Wang, G.; Zhang, J.; Bellail, A.C.; Zhang, Z.; Olson, J.J.; Hao, C. Cisplatin restores TRAIL apoptotic pathway in glioblastoma-derived stem cells through up-regulation of DR5 and down-regulation of c-FLIP. Cancer Investig. 2011, 29, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Li, L.; Tang, H.; Xie, Y.; Puliyappadamba, V.T.; Raisanen, J.; Burma, S.; Boothman, D.A.; Cochran, B.; Wu, J.; et al. Cytoplasmic TRADD confers a worse prognosis in glioblastoma. Neoplasia 2013, 15, 888–897. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.S.; Wang, G.F.; Zhao, Z.Q.; Liang, Y.; Wang, H.B.; Wu, M.Y.; Min, P.; Chen, L.Z.; Feng, Q.S.; Bei, J.X.; et al. Smac mimetics in combination with TRAIL selectively target cancer stem cells in nasopharyngeal carcinoma. Mol. Cancer Ther. 2013, 12, 1728–1737. [Google Scholar] [CrossRef] [PubMed]
- Iida, H.; Suzuki, M.; Goitsuka, R.; Ueno, H. Hypoxia induces CD133 expression in human lung cancer cells by up-regulation of OCT3/4 and SOX2. Int. J. Oncol. 2012, 40, 71–79. [Google Scholar] [PubMed]
- Singh, S.; Trevino, J.; Bora-Singhal, N.; Coppola, D.; Haura, E.; Altiok, S.; Chellappan, S.P. EGFR/SRC/AKT signalling modulates Sox2 expression and self-renewal of stem-like side-population cells in non-small cell lung cancer. Mol. Cancer 2012, 11, 73. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, G.J.; Saya, H. Therapeutic strategies targeting cancer stem cells. Cancer Sci. 2016, 107, 5–11. [Google Scholar] [CrossRef] [PubMed]
- French, R.; Hayward, O.; Jones, S.; Yang, W.; Clarkson, R. Cytoplasmic levels of cFLIP determine a broad susceptibility of breast cancer stem/progenitor-like cells to TRAIL. Mol. Cancer 2015, 14, 209. [Google Scholar] [CrossRef] [PubMed]
- Piggott, L.; Omidvar, N.; Marti Perez, S.; French, R.; Eberl, M.; Clarkson, R.W. Suppression of apoptosis inhibitor c-FLIP selectively eliminates breast cancer stem cell activity in response to the anti-cancer agent, TRAIL. Breast Cancer Res. 2011, 13, R88. [Google Scholar] [CrossRef] [PubMed]
- Zobalova, R.; McDermott, L.; Stantic, M.; Prokopova, K.; Dong, L.F.; Neuzil, J. CD133-positive cells are resistant to TRAIL due to up-regulation of FLIP. Biochem. Biophys. Res. Commun. 2008, 373, 567–571. [Google Scholar] [CrossRef] [PubMed]
- Zobalova, R.; Stantic, M.; Prokopova, K.; Dong, L.F.; Neuzil, J. Cancer cells with high expression of CD133 exert FLIP upregulation and resistance to TRAIL-induced apoptosis. BioFactors 2008, 34, 231–235. [Google Scholar] [PubMed]
- Day, T.W.; Najafi, F.; Wu, C.H.; Safa, A.R. Cellular flice-like inhibitory protein (c-FLIP): A novel target for taxol-induced apoptosis. Biochem. Pharmacol. 2006, 71, 1551–1561. [Google Scholar] [CrossRef] [PubMed]
- Haag, C.; Stadel, D.; Zhou, S.; Bachem, M.G.; Moller, P.; Debatin, K.M.; Fulda, S. Identification of c-FLIP(L) and c-FLIP(S) as critical regulators of death receptor-induced apoptosis in pancreatic cancer cells. Gut 2011, 60, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Wood, T.E.; Dalili, S.; Simpson, C.D.; Sukhai, M.A.; Hurren, R.; Anyiwe, K.; Mao, X.; Suarez Saiz, F.; Gronda, M.; Eberhard, Y.; et al. Selective inhibition of histone deacetylases sensitizes malignant cells to death receptor ligands. Mol. Cancer Ther. 2010, 9, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Ren, K.; Fang, F.; Zhao, D.H.; Yang, N.J.; Li, Y. Over expression of BCL2 and low expression of caspase 8 related to TRAIL resistance in brain cancer stem cells. Asian Pac. J. Cancer Prev. 2015, 16, 4849–4852. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kim, M.J.; Kim, D.W.; Kang, C.D.; Kim, S.H. Amurensin g enhances the susceptibility to tumor necrosis factor-related apoptosis-inducing ligand-mediated cytotoxicity of cancer stem-like cells of HCT-15 cells. Cancer Sci. 2013, 104, 1632–1639. [Google Scholar] [CrossRef] [PubMed]
- Loebinger, M.R.; Sage, E.K.; Davies, D.; Janes, S.M. TRAIL-expressing mesenchymal stem cells kill the putative cancer stem cell population. Br. J. Cancer 2010, 103, 1692–1697. [Google Scholar] [CrossRef] [PubMed]
- Sasportas, L.S.; Kasmieh, R.; Wakimoto, H.; Hingtgen, S.; van de Water, J.A.; Mohapatra, G.; Figueiredo, J.L.; Martuza, R.L.; Weissleder, R.; Shah, K. Assessment of therapeutic efficacy and fate of engineered human mesenchymal stem cells for cancer therapy. Proc. Natl. Acad. Sci. USA 2009, 106, 4822–4827. [Google Scholar] [CrossRef] [PubMed]
- Kazimirsky, G.; Jiang, W.; Slavin, S.; Ziv-Av, A.; Brodie, C. Mesenchymal stem cells enhance the oncolytic effect of newcastle disease virus in glioma cells and glioma stem cells via the secretion of TRAIL. Stem Cell Res. Ther. 2016, 7, 149. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Woo, J.S.; Jeong, C.H.; Ryu, C.H.; Lim, J.Y.; Jeun, S.S. Effective combination therapy for malignant glioma with TRAIL-secreting mesenchymal stem cells and lipoxygenase inhibitor MK886. Cancer Res. 2012, 72, 4807–4817. [Google Scholar] [CrossRef] [PubMed]
- Xia, P.; Wang, W.; Bai, Y. Claudin-7 suppresses the cytotoxicity of TRAIL-expressing mesenchymal stem cells in H460 human non-small cell lung cancer cells. Apoptosis 2014, 19, 491–505. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Tang, S.-N.; Upadhyay, G.; Marsh, J.L.; Jackman, C.P.; Shankar, S.; Srivastava, R.K. Embelin suppresses growth of human pancreatic cancer xenografts, and pancreatic cancer cells isolated from Kras(G12D) mice by inhibiting Akt and sonic hedgehog pathways. PLoS ONE 2014, 9, e92161. [Google Scholar]
- Mohr, A.; Albarenque, S.M.; Deedigan, L.; Yu, R.; Reidy, M.; Fulda, S.; Zwacka, R.M. Targeting of XIAP combined with systemic mesenchymal stem cell-mediated delivery of sTRAIL ligand inhibits metastatic growth of pancreatic carcinoma cells. Stem Cells 2010, 28, 2109–2120. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Kim, S.J.; Park, S.H.; Yang, H.G.; Kang, M.C.; Choi, Y.W.; Kim, S.M.; Jeun, S.S.; Sung, Y.C. Complete regression of metastatic renal cell carcinoma by multiple injections of engineered mesenchymal stem cells expressing dodecameric TRAIL and HSV-TK. Clin. Cancer Res. 2013, 19, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Nowakowski, A.; Drela, K.; Rozycka, J.; Janowski, M.; Lukomska, B. Engineered mesenchymal stem cells as an anti-cancer trojan horse. Stem Cells Dev. 2016, 25, 1513–1531. [Google Scholar] [CrossRef] [PubMed]
- Redjal, N.; Zhu, Y.; Shah, K. Combination of systemic chemotherapy with local stem cell delivered s-TRAIL in resected brain tumors. Stem Cells 2015, 33, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Shan, H.; Li, D.; Li, Z.R.; Zhu, K.S.; Jiang, Z.B. The inhibitory effect of MSCs expressing TRAIL as a cellular delivery vehicle in combination with cisplatin on hepatocellular carcinoma. Cancer Boil. Ther. 2012, 13, 1175–1184. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Woo, J.S.; Jeong, C.H.; Ryu, C.H.; Jang, J.D.; Jeun, S.S. Potential application of temozolomide in mesenchymal stem cell-based TRAIL gene therapy against malignant glioma. Stem Cells Transl. Med. 2014, 3, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Yoon, N.; Park, M.S.; Peltier, G.C.; Lee, R.H. Pre-activated human mesenchymal stromal cells in combination with doxorubicin synergistically enhance tumor-suppressive activity in mice. Cytotherapy 2015, 17, 1332–1341. [Google Scholar] [CrossRef] [PubMed]
- Mohr, A.; Yu, R.; Zwacka, R.M. TRAIL-receptor preferences in pancreatic cancer cells revisited: Both TRAIL-R1 and TRAIL-R2 have a licence to kill. BMC Cancer 2015, 15, 494. [Google Scholar] [CrossRef] [PubMed]
- Song, N.M.; Jun, S.; Zang, D.Y.; Kim, S.G.; Park, H.R.; Kang, D. Differential susceptibility of gastric cancer cells to TRAIL-induced apoptosis. Oncol. Rep. 2013, 29, 1224–1230. [Google Scholar] [CrossRef] [PubMed]
- Mueller, L.P.; Luetzkendorf, J.; Widder, M.; Nerger, K.; Caysa, H.; Mueller, T. TRAIL-transduced multipotent mesenchymal stromal cells (TRAIL-MSC) overcome TRAIL resistance in selected CRC cell lines in vitro and in vivo. Cancer Gene Ther. 2011, 18, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Luetzkendorf, J.; Mueller, L.P.; Mueller, T.; Caysa, H.; Nerger, K.; Schmoll, H.J. Growth inhibition of colorectal carcinoma by lentiviral TRAIL-transgenic human mesenchymal stem cells requires their substantial intratumoral presence. J. Cell. Mol. Med. 2010, 14, 2292–2304. [Google Scholar] [CrossRef] [PubMed]
- Nesterenko, I.; Wanningen, S.; Bagci-Onder, T.; Anderegg, M.; Shah, K. Evaluating the effect of therapeutic stem cells on TRAIL resistant and sensitive medulloblastomas. PLoS ONE 2012, 7, e49219. [Google Scholar] [CrossRef] [PubMed]
- Stolfi, C.; Pallone, F.; Monteleone, G. Molecular targets of TRAIL-sensitizing agents in colorectal cancer. Int. J. Mol. Sci. 2012, 13, 7886–7901. [Google Scholar] [CrossRef] [PubMed]
- Loebinger, M.R.; Eddaoudi, A.; Davies, D.; Janes, S.M. Mesenchymal stem cell delivery of TRAIL can eliminate metastatic cancer. Cancer Res. 2009, 69, 4134–4142. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Yang, Z.; Suo, Y.; Chen, Q.; Wei, D.; Weng, X.; Gu, Z.; Wei, X. Systemically infused mesenchymal stem cells show different homing profiles in healthy and tumor mouse models. Stem Cells Transl. Med. 2017, 6, 1120–1131. [Google Scholar] [CrossRef] [PubMed]


| Biological Agents | Mechanism | Tumour Model | Toxicity and Safety Concern | References |
|---|---|---|---|---|
| IL-2 | Reduce and inhibit tumour growth dependent of natural killer (NK) cells | Renal cell carcinoma, glioma | May cause capillary leak syndrome and fluid accumulation | [53,54,79] |
| IL-12 | Inhibit tumour growth dependent of NK cells | Melanoma model, renal cell carcinoma | Haematological toxicity, such as neutropenia and thrombocytopenia | [80,81,82] |
| IL-15 | Abolished tumour growth dependent of NK and CD8+ T cells | Pancreatic tumour | Probability for autoimmune toxicity | [56,83] |
| IL-18 | Suppress proliferation, migration, and invasion | Breast tumour | Haematological toxicity, hypotension, and bradycardia | [84,85] |
| IFN-β | Inhibit tumour growth and metastasis in vivo | Melanoma, breast tumour | Haematological-, autoimmune-, and hepato-toxicity | [44,55,86] |
| TRAIL | Induce apoptosis, inhibit clonogenicity and tumour bulk | Lung metastasis, lung CSCs, glioma, pancreatic cancer, mesothelioma, | Mild constitutional toxicity (e.g., nausea, fever, and constipation) and anaemia | [45,47,48,58,87] |
| Pro-drug converting enzymes | Inhibition of tumour growth in vitro and in vivo | Glioma, prostate cancer, osteosarcoma | “Off site” activated drug accumulation | [59,60,61,63] |
| Oncolytic virus | Oncolytic viruses mediated tumour regression in vivo | Glioblastoma, brain metastasis, leukemia and pancreatic cancer | Potential for virus mutation, normal cell toxicity, and human viral transmission | [71,78,88,89] |
| Cancer Type | CSCs Markers | References |
|---|---|---|
| Non-small cell lung cancer (NSCLC) | ABCG2+, CD133+, CD44+, EpCAM+, CD166+, ALDH+ | [137,138,140] |
| Breast | CD44+/CD24−, ALDH+ | [141,142] |
| Colon | CD133+, EpCAM high/CD44+ | [128,129,143,144] |
| Head and neck | CD44+, SP, ALDH | [145,146] |
| Prostate | CD133+, CD44+, α2β1high | [147,148] |
| Brain tumour/glioma | CD133+, CD15+, CD90+, CD49f+ | [126,149,150] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaik Fakiruddin, K.; Ghazalli, N.; Lim, M.N.; Zakaria, Z.; Abdullah, S. Mesenchymal Stem Cell Expressing TRAIL as Targeted Therapy against Sensitised Tumour. Int. J. Mol. Sci. 2018, 19, 2188. https://doi.org/10.3390/ijms19082188
Shaik Fakiruddin K, Ghazalli N, Lim MN, Zakaria Z, Abdullah S. Mesenchymal Stem Cell Expressing TRAIL as Targeted Therapy against Sensitised Tumour. International Journal of Molecular Sciences. 2018; 19(8):2188. https://doi.org/10.3390/ijms19082188
Chicago/Turabian StyleShaik Fakiruddin, Kamal, Nadiah Ghazalli, Moon Nian Lim, Zubaidah Zakaria, and Syahril Abdullah. 2018. "Mesenchymal Stem Cell Expressing TRAIL as Targeted Therapy against Sensitised Tumour" International Journal of Molecular Sciences 19, no. 8: 2188. https://doi.org/10.3390/ijms19082188
APA StyleShaik Fakiruddin, K., Ghazalli, N., Lim, M. N., Zakaria, Z., & Abdullah, S. (2018). Mesenchymal Stem Cell Expressing TRAIL as Targeted Therapy against Sensitised Tumour. International Journal of Molecular Sciences, 19(8), 2188. https://doi.org/10.3390/ijms19082188