Structural Controlling of Highly-Oriented Polycrystal 3C-SiC Bulks via Halide CVD
Abstract
:1. Introduction
2. Materials and Methods
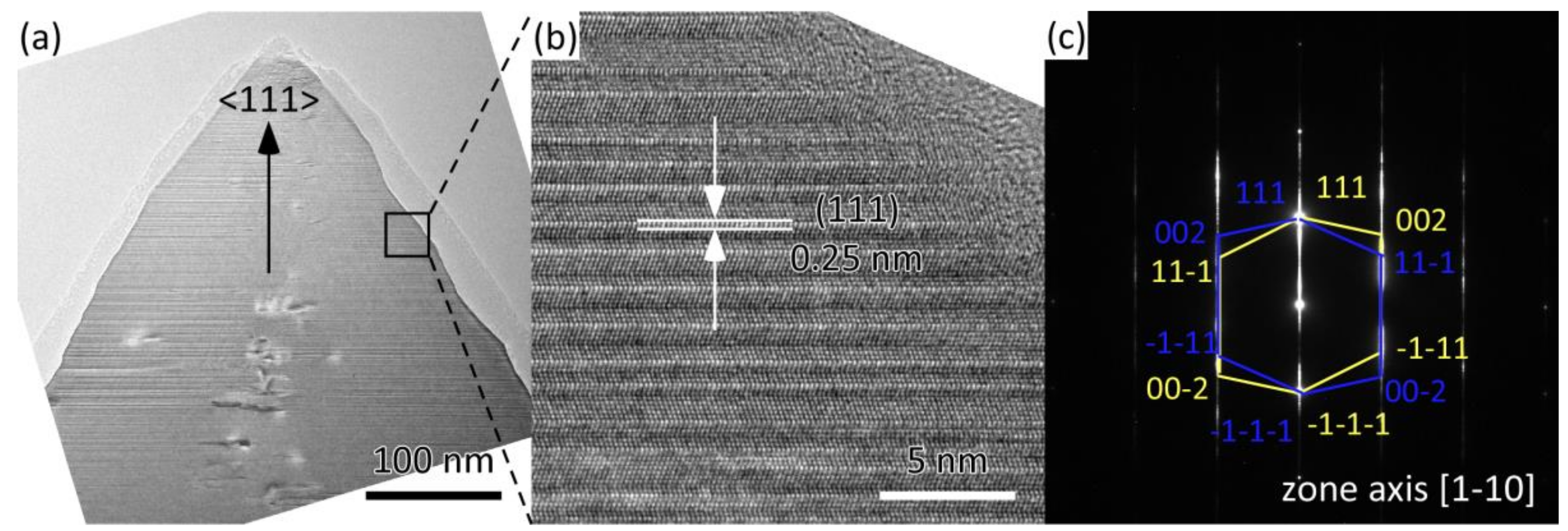
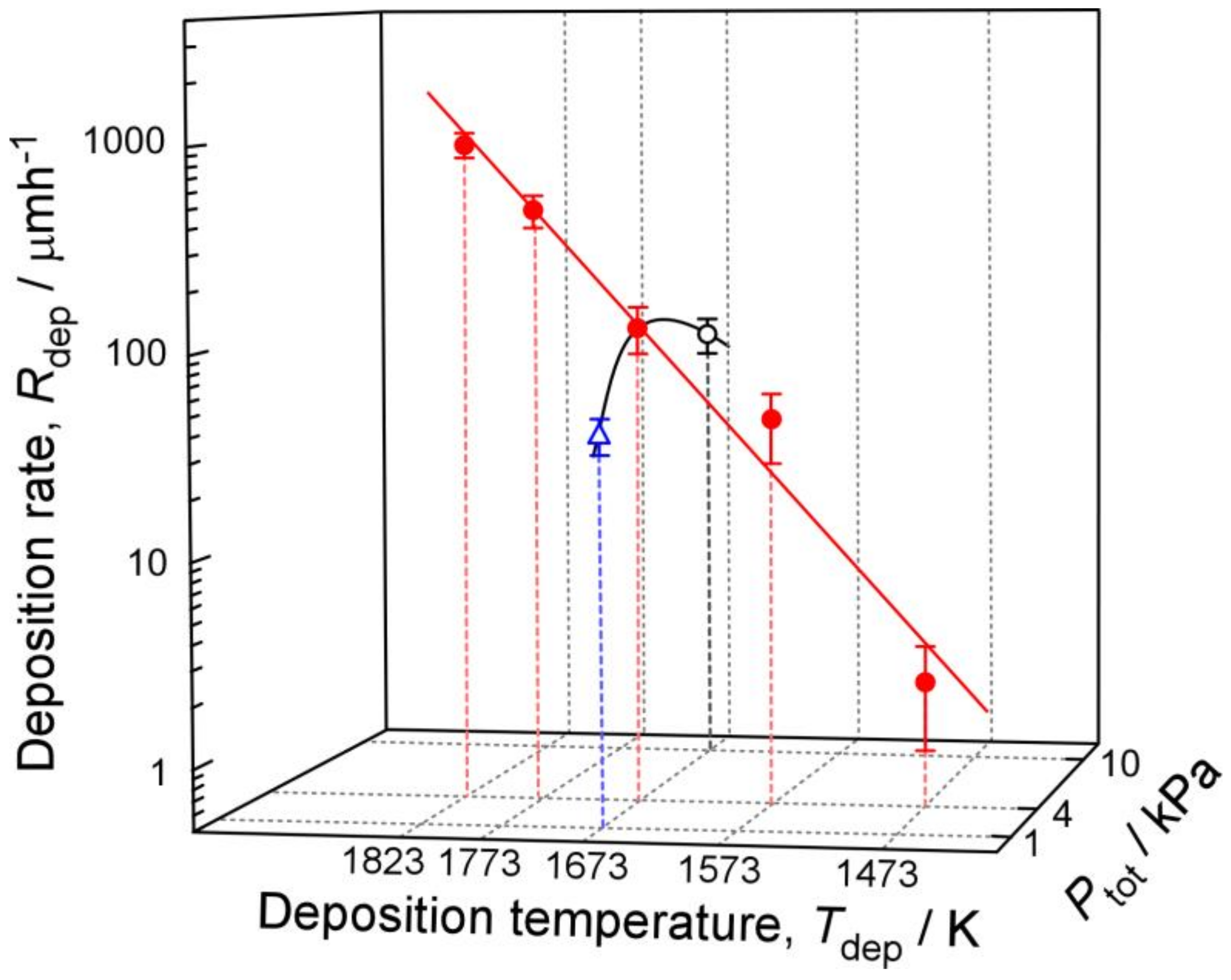
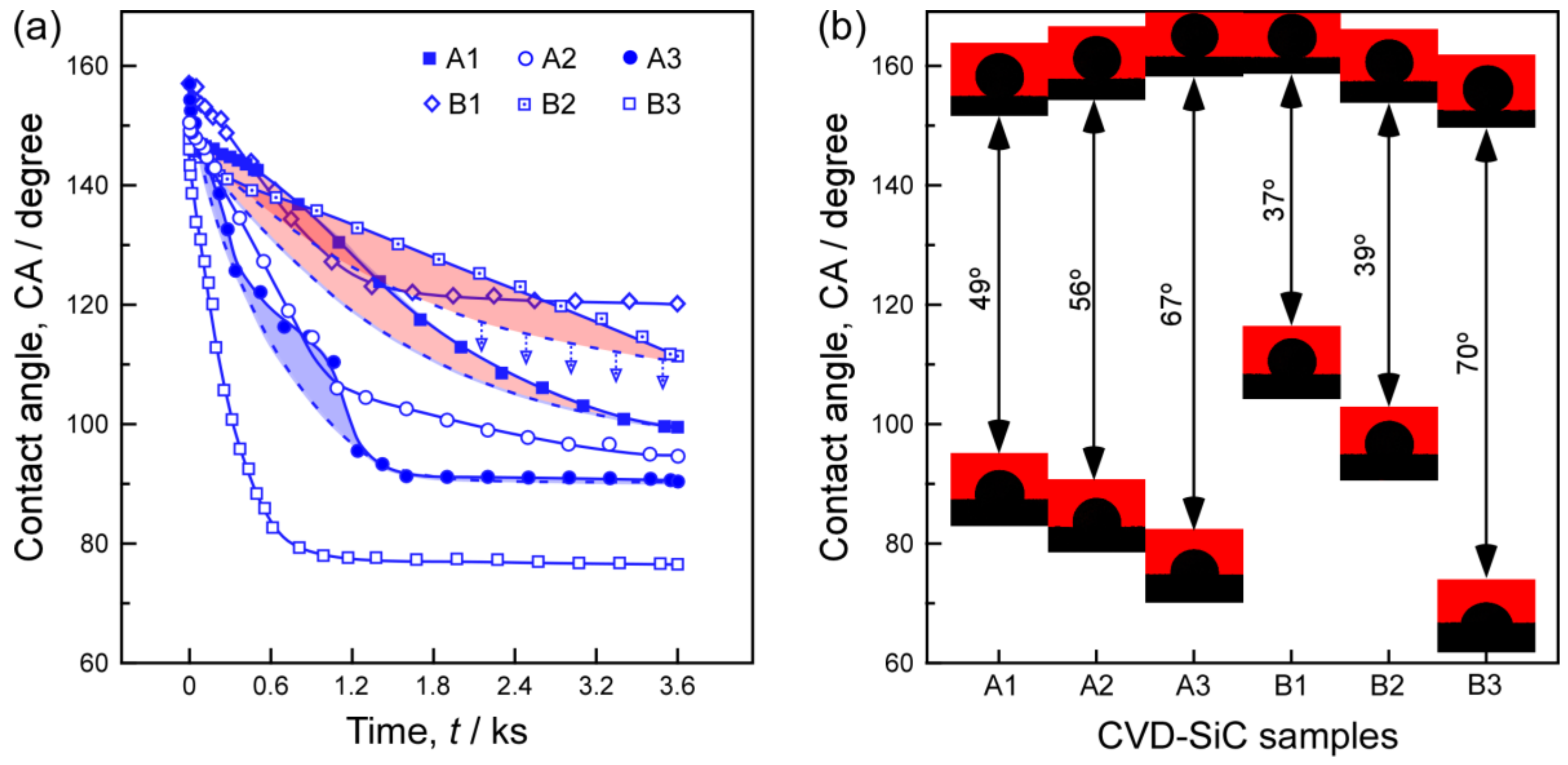
3. Results
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Seina, E.; Toulemonta, Y.; Safaa, F.; Durana, M.; Denyb, P.; Chamburec, D.D.E.; Passvogelc, T.; Pilbrattc, G. A Φ 3.5 M SiC Telescope for HERSCHEL Mission. Astron. Telesc. Instrum. 2007, 4850, 606–618. [Google Scholar]
- Balat, M.J.H. Determination of the Active-to-Passive Transition in the Oxidation of Silicon Carbide in Standard and Microwave-Excited Air. J. Eur. Ceram. Soc. 1996, 16, 55–62. [Google Scholar] [CrossRef]
- Bryant, P.A.; Lohstroh, A.; Sellin, P.J. Electrical Characteristics and Fast Neutron Response of Semi-Insulating Bulk Silicon Carbide. Trans. Nucl. Sci. 2013, 60, 1432–1435. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Yao, X.; Li, Y.; Liang, H.; Chen, J.; Zhang, J.; Yang, J.; Li, X.; Qiu, T.; Chen, Z.; et al. Effect of AlN addition on the thermal conductivity of pressureless sintered SiC ceramics. Ceram. Int. 2015, 41, 9107–9114. [Google Scholar] [CrossRef]
- Lanfant, B.; Leconte, Y.; Bonnefont, G.; Garnier, V.; Jorand, Y.; Le Gallet, S.; Pinault, M.; Herlin-Boime, N.; Bernard, F.; Fantozzi, G. Effects of carbon and oxygen on the spark plasma sintering additive-free densification and on the mechanical properties of nanostructured SiC ceramics. J. Eur. Ceram. Soc. 2015, 35, 3369–3379. [Google Scholar] [CrossRef]
- Li, Y.; Yin, J.; Wu, H.; Lu, P.; Yan, Y.; Liu, X.; Huang, Z.; Jiang, D. High thermal conductivity in pressureless densified SiC ceramics with ultra-low contents of additives derived from novel boron-carbon sources. J. Eur. Ceram. Soc. 2014, 34, 2591–2595. [Google Scholar] [CrossRef]
- Kim, Y.; Mimoto, M.; Nishimura, T. High-Temperature Strength of Liquid-Phase-Sintered SiC with AlN and Re2O3 (RE= Y, Yb). J. Am. Ceram. Soc. 2002, 85, 1007–1009. [Google Scholar] [CrossRef]
- Kovalčíková, A.; Dusza, J.; Šajgalík, P. Thermal shock resistance and fracture toughness of liquid-phase-sintered SiC-based ceramics. J. Eur. Ceram. Soc. 2009, 29, 2387–2394. [Google Scholar] [CrossRef]
- Kim, W.J.; Hwang, H.S.; Park, J.Y.; Ryu, W.S. Corrosion behaviors of sintered and chemically vapor deposited silicon carbide ceramics in water at 360 °C. J. Mater. Sci. Lett. 2003, 22, 581–584. [Google Scholar] [CrossRef]
- Choy, K.L. Chemical vapour deposition of coatings. Prog. Mater. Sci. 2003, 48, 57–170. [Google Scholar] [CrossRef]
- Lu, P.; Edgar, J.H.; Glembocki, O.J.; Klein, P.B.; Glaser, E.R.; Perrin, J.; Chaudhuri, J. High-speed homoepitaxy of SiC from methyltrichlorosilane by chemical vapor deposition. J. Cryst. Growth 2005, 285, 506–513. [Google Scholar] [CrossRef]
- Hoshino, N.; Kamata, I.; Tokuda, Y.; Makino, E.; Sugiyama, N.; Kojima, J.; Tsuchida, H. High-speed, high-quality crystal growth of 4H-SiC by high-temperature gas source method. Appl. Phys. Express 2014, 7, 065502. [Google Scholar] [CrossRef]
- Tu, R.; Zheng, D.; Cheng, H.; Hu, M.; Zhang, S.; Han, M.; Goto, T.; Zhang, L. Effect of CH4/SiCl4 ratio on the composition and microstructure of <110>-oriented β-SiC bulks by halide CVD. J. Eur. Ceram. Soc. 2017, 37, 1217–1223. [Google Scholar] [CrossRef]
- Han, M.; Zhou, W.; Zheng, D.; Tu, R.; Zhang, S.; Goto, T. Effects of C/Si Ratio on the Structure of β-SiC Film by Halide CVD. Key Eng. Mater. 2014, 616, 227–231. [Google Scholar] [CrossRef]
- Sandhyarani, M.; Rameshbabu, N.; Venkateswarlu, K.; Sreekanth, D.; Subrahmanyam, C. Surface morphology, corrosion resistance and in vitro bioactivity of P containing ZrO2 films formed on Zr by plasma electrolytic oxidation. J. Alloys Compd. 2013, 553, 324–332. [Google Scholar] [CrossRef]
- Lee, J.A.; McCarthy, T.J. Polymer surface modification: Topography effects leading to extreme wettability behavior. Macromolecules 2007, 40, 3965–3969. [Google Scholar] [CrossRef]
- Sommers, A.D.; Jacobi, A.M. Creating micro-scale surface topology to achieve anisotropic wettability on an aluminum surface. J. Micromech. Microeng. 2006, 16, 1571–1578. [Google Scholar] [CrossRef]
- Papenburg, B.J.; Rodrigues, E.D.; Wessling, M.; Stamatialis, D. Insights into the role of material surface topography and wettability on cell-material interactions. Soft Matter 2010, 6, 4377. [Google Scholar] [CrossRef]
- Zhang, J.M.; Ma, F.; Xu, K.W.; Xin, X.T. Anisotropy analysis of the surface energy of diamond cubic crystals. Surf. Interface Anal. 2003, 35, 805–809. [Google Scholar] [CrossRef]
- Van der Drift, A. Evolutionary selection, a principle governing growth orientation in vapour-deposited layers. Philips Res. Rep. 1967, 22, 267–288. [Google Scholar]
- Zhang, S.; Xu, Q.; Tu, R.; Goto, T.; Zhang, L. Growth Mechanism and Defects of <111>-Oriented β-SiC Films Deposited by Laser Chemical Vapor Deposition. J. Am. Ceram. Soc. 2015, 98, 236–241. [Google Scholar] [CrossRef]
- Lee, H.N.; Senz, S.; Pignolet, A.; Hesse, D. Epitaxial growth of (103)-oriented ferroelectric SrBi2Ta2O9 thin films on Si(100). Appl. Phys. Lett. 2001, 78, 2922–2924. [Google Scholar] [CrossRef]
- Tu, R.; Zheng, D.; Sun, Q.; Han, M.; Zhang, S.; Hu, Z.; Goto, T.; Zhang, L. Ultra-Fast Fabrication of <110>-Oriented β-SiC Wafers by Halide CVD. J. Am. Ceram. Soc. 2016, 99, 84–88. [Google Scholar] [CrossRef]
- Kajikawa, B.Y.; Noda, S.; Komiyama, H. Preferred Orientation of Chemical Vapor Deposited Polycrystalline Silicon Carbide Films. Chem. Vap. Depos. 2002, 8, 99–104. [Google Scholar] [CrossRef]
- Nishioka, K.; Mizutani, N.; Komiyama, H. A Model for Predicting Preferential Orientation of Chemical-Vapor-Deposited Films. J. Electrochem. Soc. 2000, 147, 1440. [Google Scholar] [CrossRef]
- Sun, Q.; Zhu, P.; Xu, Q.; Tu, R.; Zhang, S.; Shi, J.; Li, H.; Zhang, L.; Goto, T.; Yan, J.; et al. High-Speed Heteroepitaxial Growth of 3C-SiC (111) Thick Films on Si (110) by Laser Chemical Vapor Deposition. J. Am. Ceram. Soc. 2018, 12, 3218–3221. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, Q.; Tu, R.; Goto, T.; Zhang, L. Effect of Pressure on Microstructure of <111>-Oriented b-SiC Films: Research via Electron Backscatter Diffraction. J. Am. Ceram. Soc. 2014, 97, 952–958. [Google Scholar] [CrossRef]
- Chiew, Y.L.; Cheong, K.Y. A review on the synthesis of SiC from plant-based biomasses. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2011, 176, 951–964. [Google Scholar] [CrossRef]
- Shen, P.; Fujii, H.; Matsumoto, T.; Nogi, K. Critical Factors Affecting the Wettability of alpha-Alumina by Molten Aluminum. J. Am. Ceram. Soc. 2004, 87, 1265–1273. [Google Scholar] [CrossRef]
- Sarina, B.; Kai, T.; Kvithyld, A.; Engh, T.; Tangstad, M. Wetting of pure aluminium on graphite, SiC and Al2O3 in aluminium filtration. Trans. Nonferrous Met. Soc. China 2012, 22, 1930–1938. [Google Scholar]
- Laurent, V.; Chatain, D.; Eustathopoulos, N. Wettablity of SiC by aluminium and Al–Si alloys. J. Mater. Sci. 1987, 22, 244–250. [Google Scholar] [CrossRef]
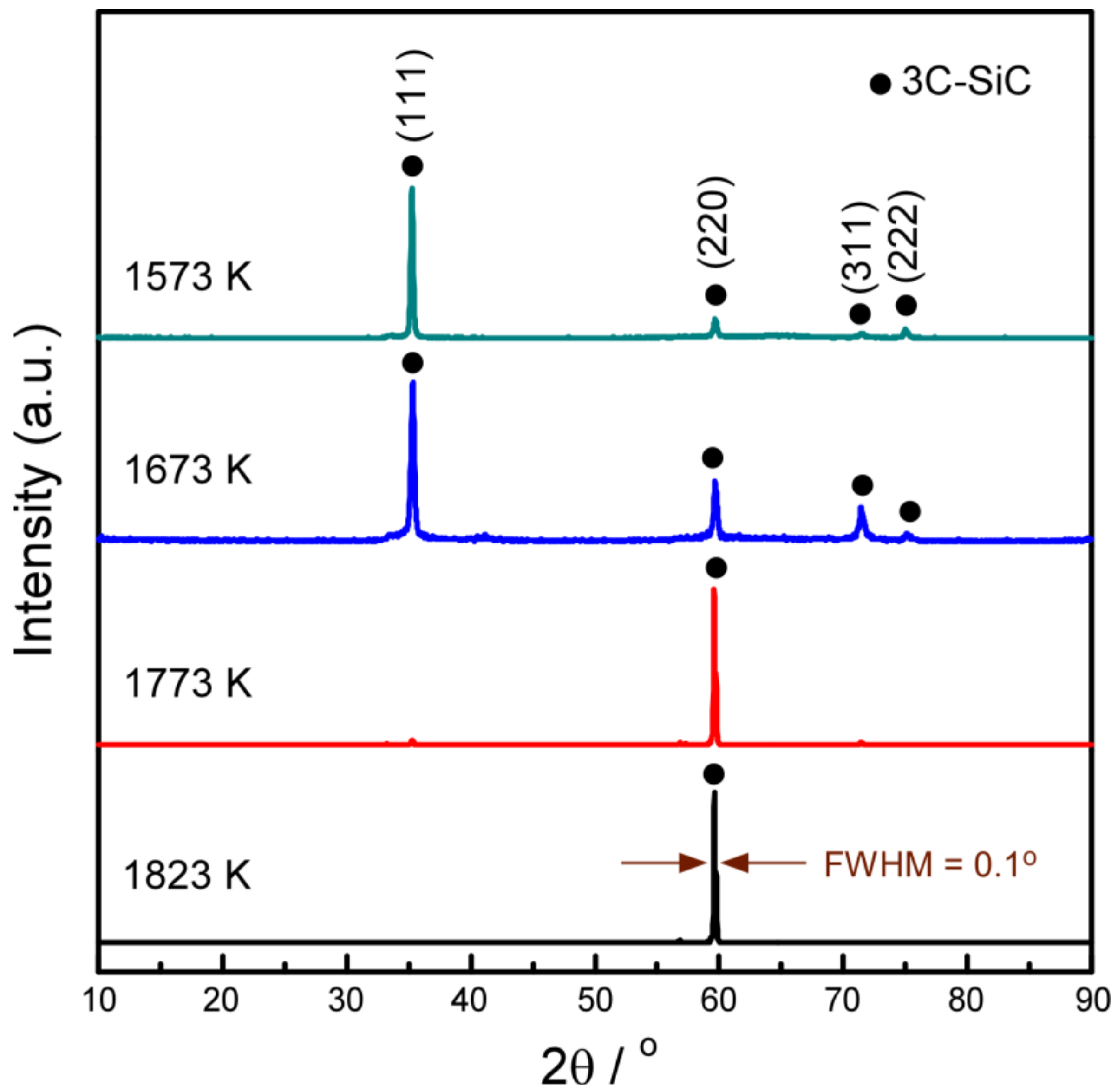
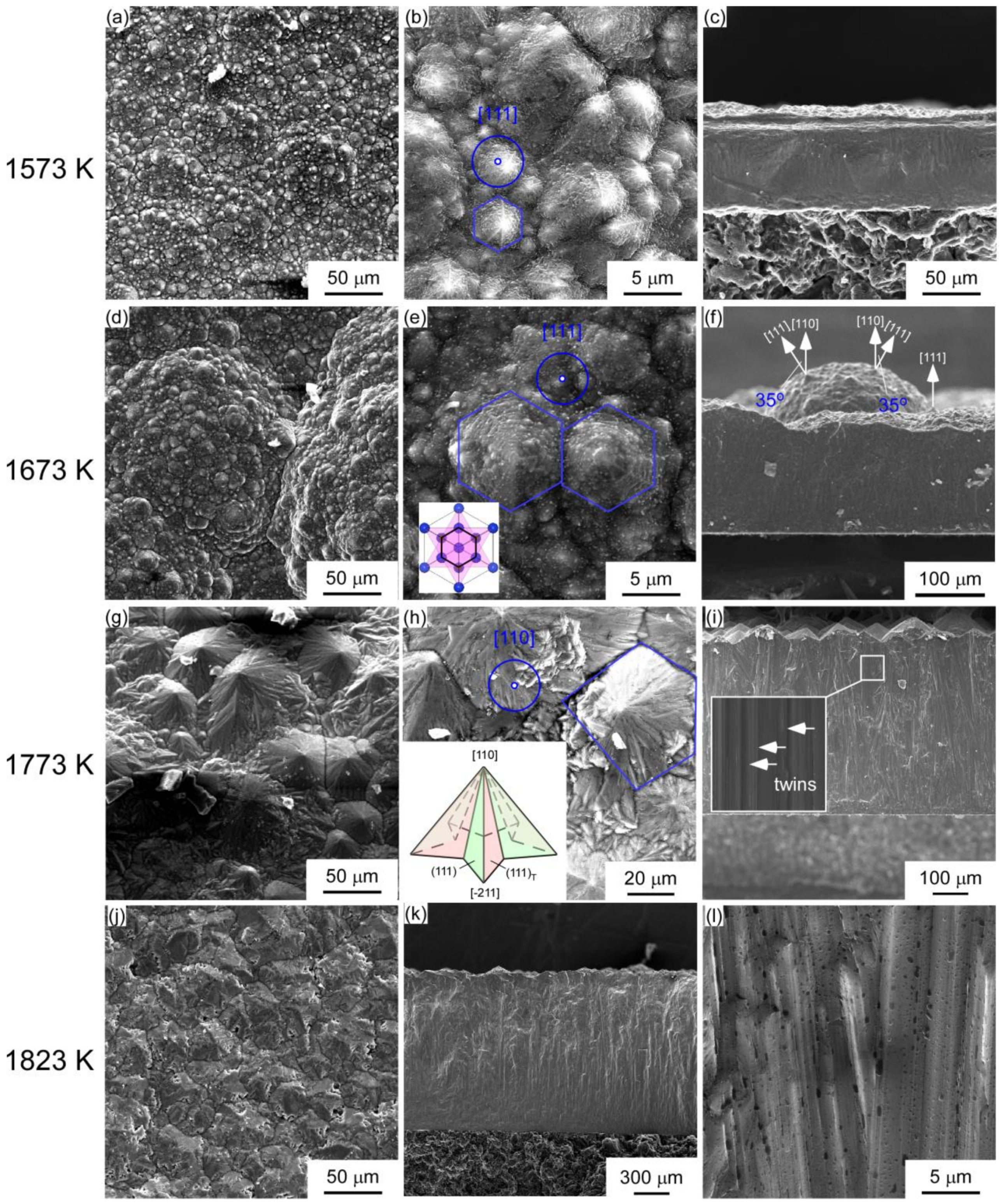
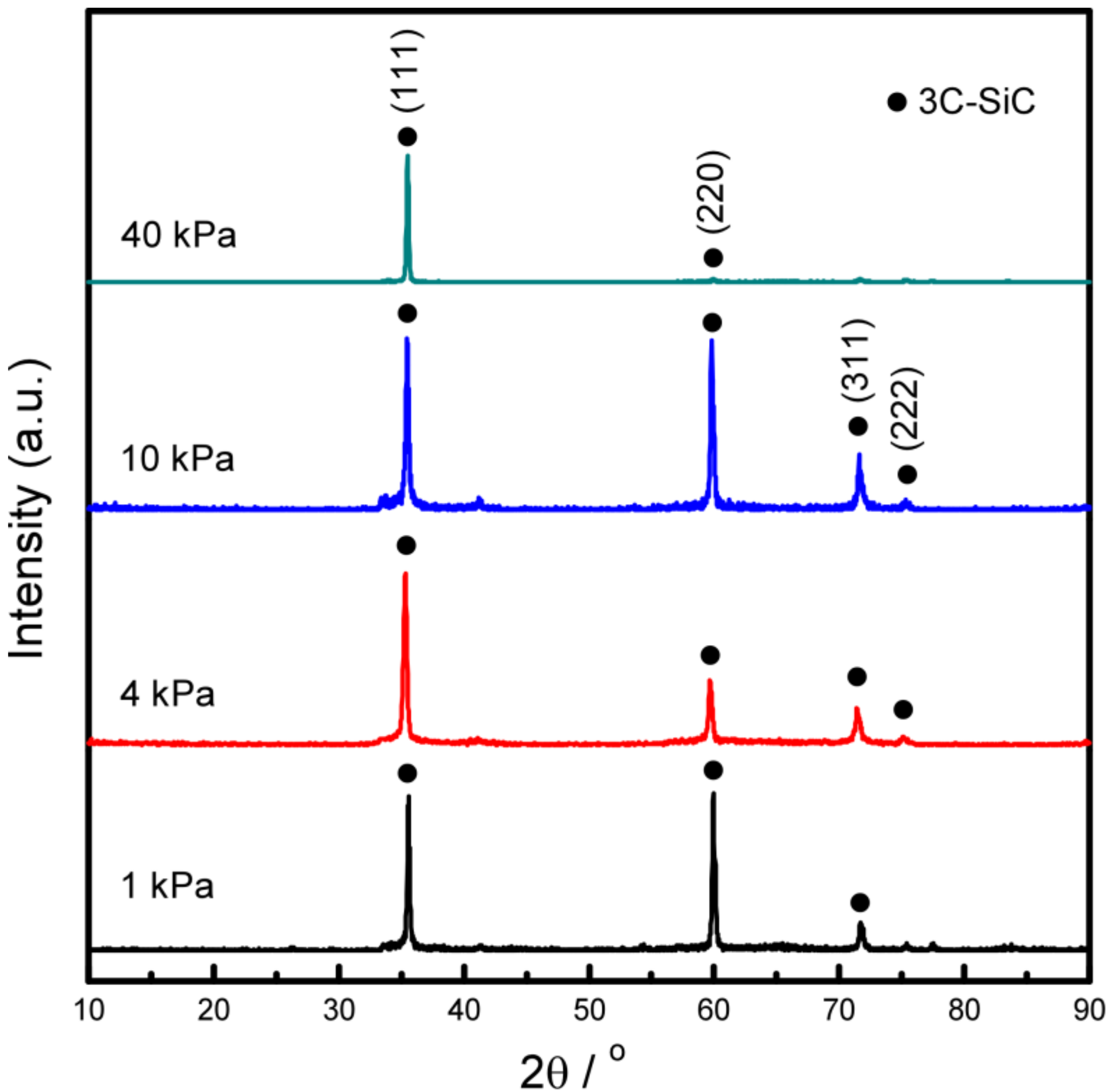
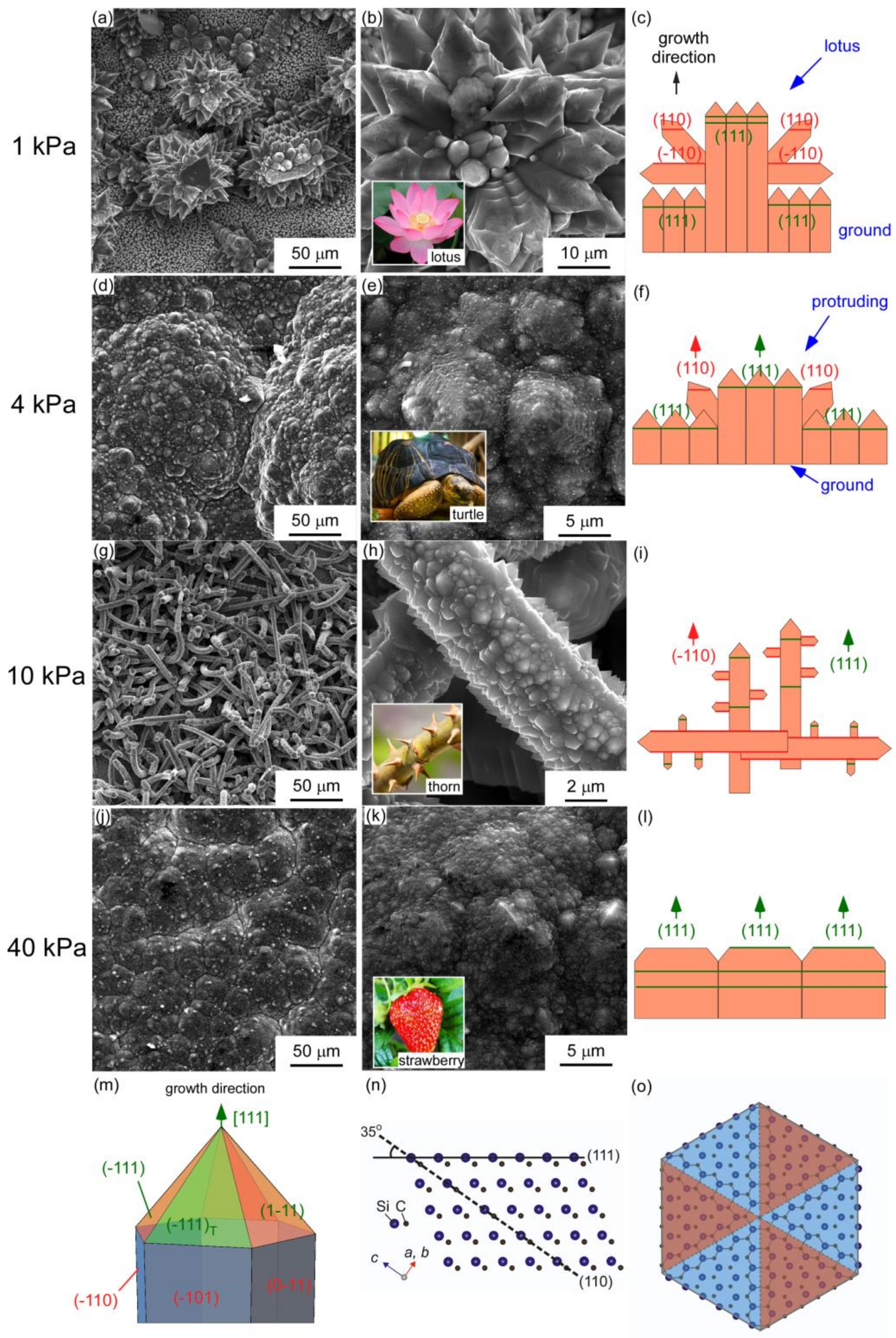
| Sample | A1 | A2 | A3 | A4 | B1 | B2 | B3 |
|---|---|---|---|---|---|---|---|
| Tdep (K) | 1573 | 1673 | 1773 | 1823 | 1673 | 1673 | 1673 |
| Ptot (kPa) | 4 | 4 | 4 | 4 | 1 | 10 | 40 |
| Flow rate of H2 | 700 sccm | ||||||
| Deposition time | 2 h | ||||||
| Si/C | 1.02 | 0.97 | 1.01 | 1.00 | 0.98 | 1.02 | 1.01 |
| Thickness (μm) | 85 | 201 | 492 | 1462 | 80 | 195 | 184 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Z.; Zheng, D.; Tu, R.; Yang, M.; Li, Q.; Han, M.; Zhang, S.; Zhang, L.; Goto, T. Structural Controlling of Highly-Oriented Polycrystal 3C-SiC Bulks via Halide CVD. Materials 2019, 12, 390. https://doi.org/10.3390/ma12030390
Hu Z, Zheng D, Tu R, Yang M, Li Q, Han M, Zhang S, Zhang L, Goto T. Structural Controlling of Highly-Oriented Polycrystal 3C-SiC Bulks via Halide CVD. Materials. 2019; 12(3):390. https://doi.org/10.3390/ma12030390
Chicago/Turabian StyleHu, Zhiying, Dingheng Zheng, Rong Tu, Meijun Yang, Qizhong Li, Mingxu Han, Song Zhang, Lianmeng Zhang, and Takashi Goto. 2019. "Structural Controlling of Highly-Oriented Polycrystal 3C-SiC Bulks via Halide CVD" Materials 12, no. 3: 390. https://doi.org/10.3390/ma12030390
APA StyleHu, Z., Zheng, D., Tu, R., Yang, M., Li, Q., Han, M., Zhang, S., Zhang, L., & Goto, T. (2019). Structural Controlling of Highly-Oriented Polycrystal 3C-SiC Bulks via Halide CVD. Materials, 12(3), 390. https://doi.org/10.3390/ma12030390






