Long-Term Exposure to Oroxylin A Inhibits Metastasis by Suppressing CCL2 in Oral Squamous Cell Carcinoma Cells
Abstract
:1. Introduction
2. Results
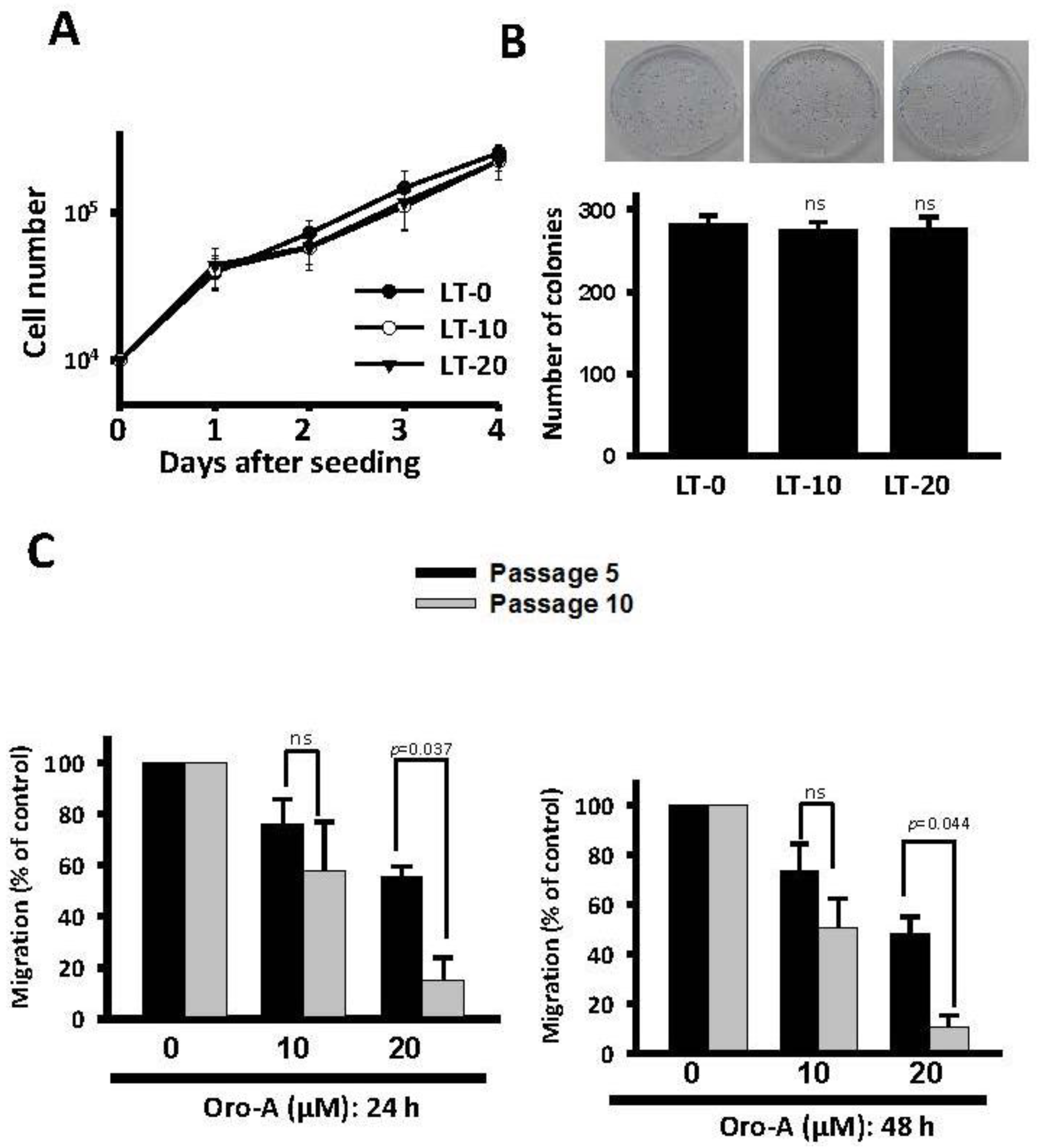
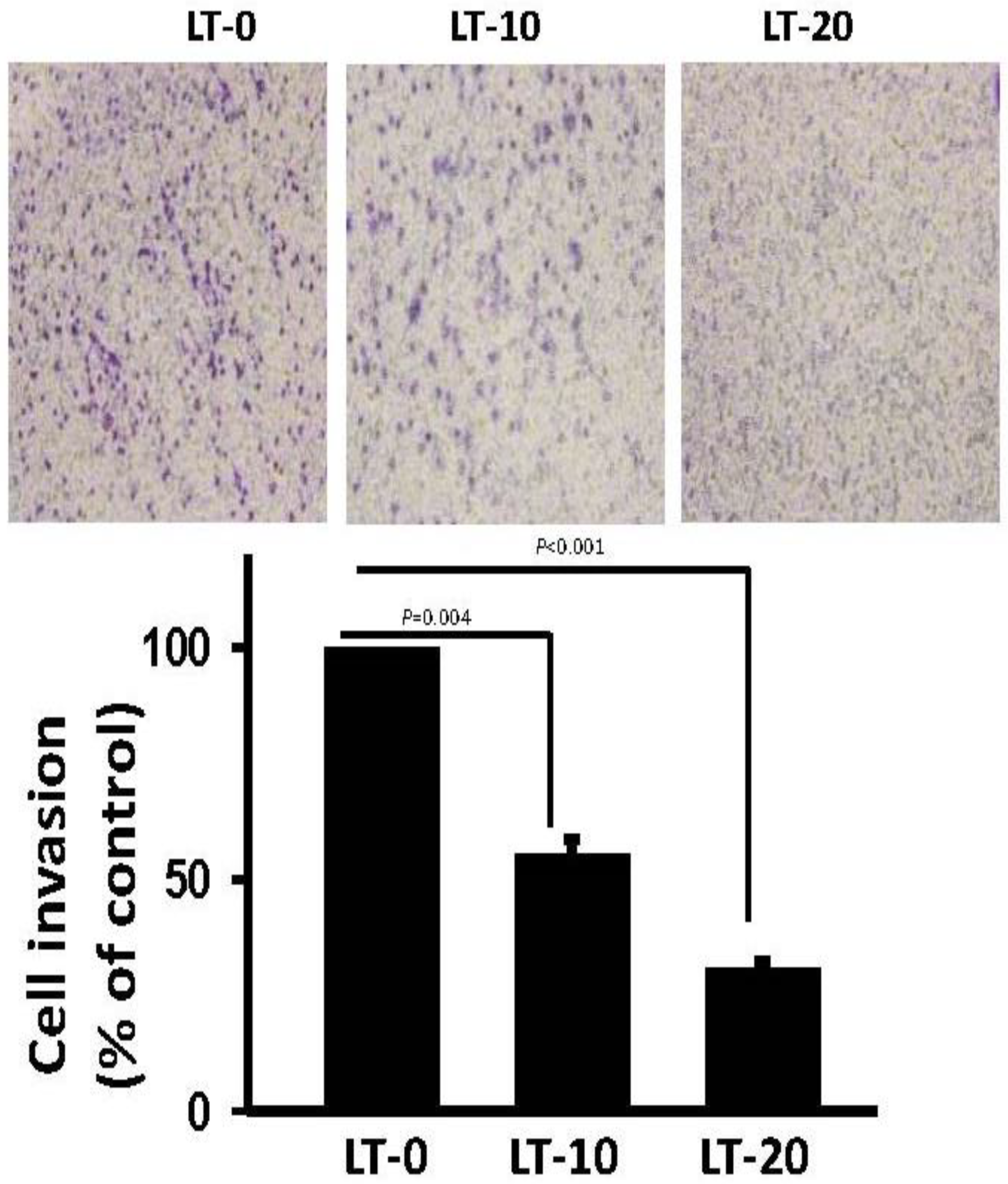
2.1. Long-Term Exposure to Oro-A Significantly Inhibited Migration of OSCC Cells with Non-Cytotoxic Effects
2.2. Migration-Related Genes Were Validated in Long-Term Oro-A-Exposed OSCC Cells
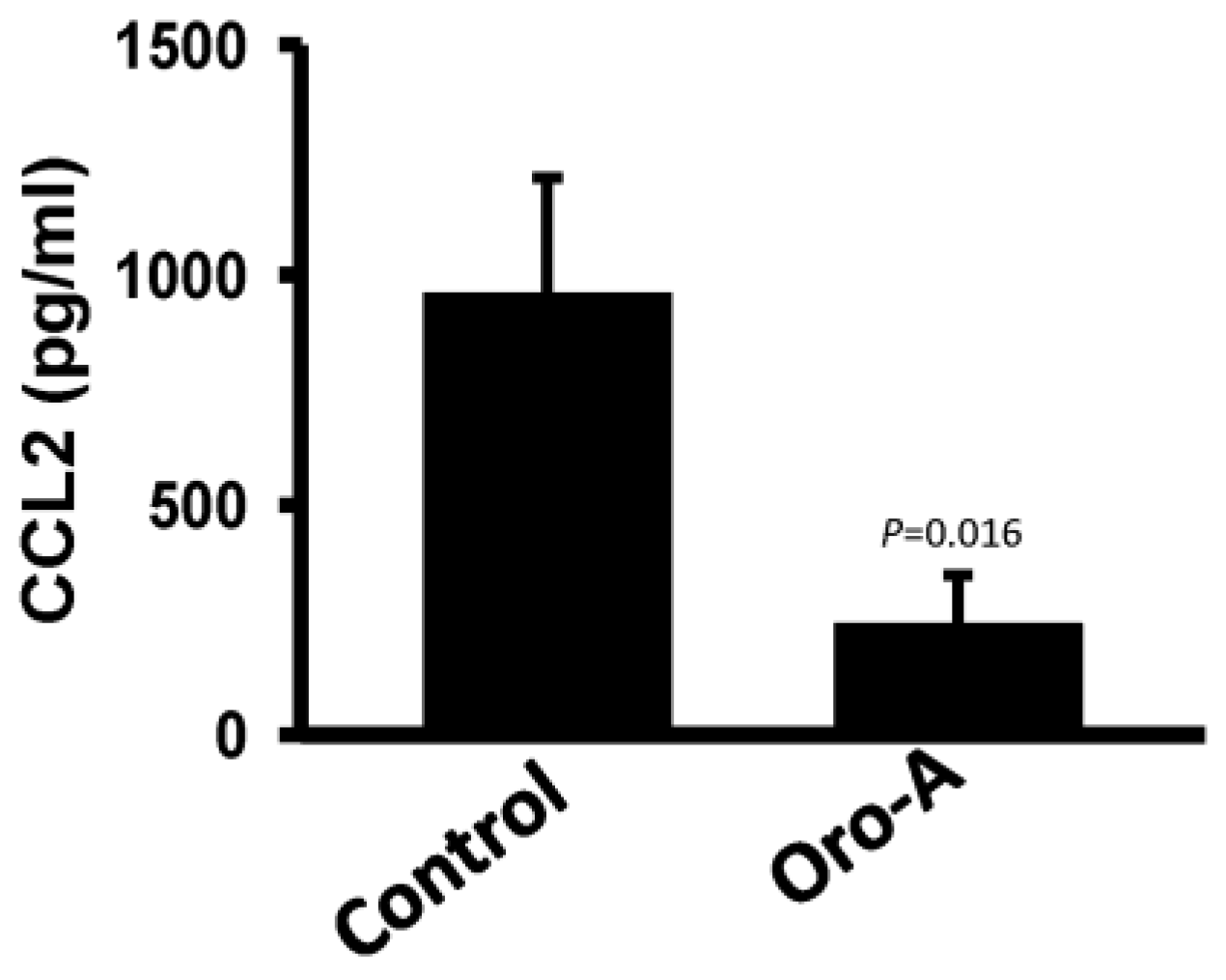
2.3. Long-Term Oro-A Exposure Downregulates CCL2 Signaling
2.4. Recombinant Human CCL2 Rescues Migration of Long-Term Oro-A Exposed OSCC Cells
2.5. Oro-A Inhibits OSCC Metastasis In Vivo
3. Discussion
4. Material and Methods
4.1. Medicine and Reagents
4.2. Cell Culture and Treatment
4.3. Measurement of Cell Viability
4.4. Wound-Healing Assay and Transwell Invasion Assay
4.5. Colony Formation Assay
4.6. cDNA Microarray Analysis
4.7. Quantitative Real-Time Polymerase Chain Reaction (Q-PCR) and Western Blotting Analysis
4.8. Enzyme-Linked Immunosorbent Assay (ELISA)
4.9. The Experimental Metastasis Model
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| OSCC | oral squamous cell carcinoma |
| COX-2 | cyclooxygenase-2 |
| Oro-A | oroxyiln A |
| EGFR | epidermal growth factor receptor |
| SRB | sulforhodamine B |
| ECM | extracellular matrix |
| MMPs | matrix metallopeptidases |
| TIMP-2 | tissue inhibitor of metalloproteinase-2 |
| IPA | Ingenuity Pathway Analysis |
References
- Noguti, J.; De Moura, C.F.; De Jesus, G.P.; Da Silva, V.H.; Hossaka, T.A.; Oshima, C.T.; Ribeiro, D.A. Metastasis from oral cancer: An overview. Cancer Genom. Proteom. 2012, 9, 329–335. [Google Scholar]
- da Silva, J.M.; Soave, D.F.; Moreira Dos Santos, T.P.; Batista, A.C.; Russo, R.C.; Teixeira, M.M.; da Silva, T.A. Significance of chemokine and chemokine receptors in head and neck squamous cell carcinoma: A critical review. Oral Oncol. 2016, 56, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.S.; Pajak, T.F.; Forastiere, A.A.; Jacobs, J.; Campbell, B.H.; Saxman, S.B.; Kish, J.A.; Kim, H.E.; Cmelak, A.J.; Rotman, M.; et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 2004, 350, 1937–1944. [Google Scholar] [CrossRef]
- Bhave, S.L.; Teknos, T.N.; Pan, Q.; James, A.G.; Solove, R.J. Molecular parameters of head and neck cancer metastasis. Crit. Rev. Eukaryot. Gene Expr. 2011, 21, 143–153. [Google Scholar] [CrossRef] [PubMed]
- de Heredia, F.P.; Gomez-Martinez, S.; Marcos, A. Obesity, inflammation and the immune system. Proc. Nutr. Soc. 2012, 71, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Bistrian, B. Systemic response to inflammation. Nutr. Rev. 2007, 65, S170–S172. [Google Scholar] [CrossRef] [PubMed]
- Colotta, F.; Allavena, P.; Sica, A.; Garlanda, C.; Mantovani, A. Cancer-related inflammation, the seventh hallmark of cancer: Links to genetic instability. Carcinogenesis 2009, 30, 1073–1081. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, B.P. Inflammation: A driving force speeds cancer metastasis. Cell Cycle 2009, 8, 3267–3273. [Google Scholar] [CrossRef]
- Sarode, G.S.; Sarode, S.C.; Patil, A.; Anand, R.; Patil, S.G.; Rao, R.S.; Augustine, D. Inflammation and oral cancer: An update review on targeted therapies. J. Contemp. Dent. Pract. 2015, 16, 595–602. [Google Scholar] [CrossRef]
- Maggioni, D.; Biffi, L.; Nicolini, G.; Garavello, W. Flavonoids in oral cancer prevention and therapy. Eur. J. Cancer Prev. 2015, 24, 517–528. [Google Scholar] [CrossRef]
- Serafini, M.; Peluso, I.; Raguzzini, A. Flavonoids as anti-inflammatory agents. Proc. Nutr. Soc. 2010, 69, 273–278. [Google Scholar] [CrossRef]
- Sonoda, M.; Nishiyama, T.; Matsukawa, Y.; Moriyasu, M. Cytotoxic activities of flavonoids from two scutellaria plants in chinese medicine. J. Ethnopharmacol. 2004, 91, 65–68. [Google Scholar] [CrossRef]
- Li, H.; Lu, N.; Yu, X.; Liu, X.; Hu, P.; Zhu, Y.; Shen, L.; Xu, J.; Li, Z.; Guo, Q.; et al. Oroxylin a, a natural compound, mitigates the negative effects of tnfalpha-treated acute myelogenous leukemia cells. Carcinogenesis 2018, 39, 1292–1303. [Google Scholar] [CrossRef]
- Liu, P.W.; Chen, M.F.; Tsai, A.P.; Lee, T.J. Stat1 mediates oroxylin a inhibition of inos and pro-inflammatory cytokines expression in microglial bv-2 cells. PLoS ONE 2012, 7, e50363. [Google Scholar] [CrossRef]
- Huen, M.S.; Leung, J.W.; Ng, W.; Lui, W.S.; Chan, M.N.; Wong, J.T.; Xue, H. 5,7-dihydroxy-6-methoxyflavone, a benzodiazepine site ligand isolated from scutellaria baicalensis georgi, with selective antagonistic properties. Biochem. Pharm. 2003, 66, 125–132. [Google Scholar] [CrossRef]
- Kim, D.H.; Jeon, S.J.; Son, K.H.; Jung, J.W.; Lee, S.; Yoon, B.H.; Choi, J.W.; Cheong, J.H.; Ko, K.H.; Ryu, J.H. Effect of the flavonoid, oroxylin a, on transient cerebral hypoperfusion-induced memory impairment in mice. Pharm. Biochem. Behav. 2006, 85, 658–668. [Google Scholar] [CrossRef]
- Li, C.; Lin, G.; Zuo, Z. Pharmacological effects and pharmacokinetics properties of radix scutellariae and its bioactive flavones. Biopharm. Drug Dispos. 2011, 32, 427–445. [Google Scholar] [CrossRef]
- Jin, J.; Chen, S.; Wang, D.; Chen, Y.; Wang, Y.; Guo, M.; Zhou, C.; Dou, J. Oroxylin a suppresses influenza a virus replication correlating with neuraminidase inhibition and induction of ifns. Biomed. Pharm. 2018, 97, 385–394. [Google Scholar] [CrossRef]
- Wei, L.; Dai, Q.; Zhou, Y.; Zou, M.; Li, Z.; Lu, N.; Guo, Q. Oroxylin a sensitizes non-small cell lung cancer cells to anoikis via glucose-deprivation-like mechanisms: C-src and hexokinase ii. Biochim. Biophys. Acta 2013, 1830, 3835–3845. [Google Scholar] [CrossRef]
- Wei, L.; Zhou, Y.; Qiao, C.; Ni, T.; Li, Z.; You, Q.; Guo, Q.; Lu, N. Oroxylin a inhibits glycolysis-dependent proliferation of human breast cancer via promoting sirt3-mediated sod2 transcription and hif1alpha destabilization. Cell Death Dis. 2015, 6, e1714. [Google Scholar] [CrossRef]
- Yang, Y.; Hu, Y.; Gu, H.Y.; Lu, N.; Liu, W.; Qi, Q.; Zhao, L.; Wang, X.T.; You, Q.D.; Guo, Q.L. Oroxylin a induces g2/m phase cell-cycle arrest via inhibiting cdk7-mediated expression of cdc2/p34 in human gastric carcinoma bgc-823 cells. J. Pharm. Pharm. 2008, 60, 1459–1463. [Google Scholar] [CrossRef]
- Wei, L.; Dai, Y.; Zhou, Y.; He, Z.; Yao, J.; Zhao, L.; Guo, Q.; Yang, L. Oroxylin a activates pkm1/hnf4 alpha to induce hepatoma differentiation and block cancer progression. Cell Death Dis. 2017, 8, e2944. [Google Scholar] [CrossRef] [PubMed]
- Qiao, C.; Wei, L.; Dai, Q.; Zhou, Y.; Yin, Q.; Li, Z.; Xiao, Y.; Guo, Q.; Lu, N. Ucp2-related mitochondrial pathway participates in oroxylin a-induced apoptosis in human colon cancer cells. J. Cell. Physiol. 2015, 230, 1054–1063. [Google Scholar] [CrossRef] [PubMed]
- Li, H.N.; Nie, F.F.; Liu, W.; Dai, Q.S.; Lu, N.; Qi, Q.; Li, Z.Y.; You, Q.D.; Guo, Q.L. Apoptosis induction of oroxylin a in human cervical cancer hela cell line in vitro and in vivo. Toxicology 2009, 257, 80–85. [Google Scholar] [CrossRef]
- Hui, H.; Chen, Y.; Yang, H.; Zhao, K.; Wang, Q.; Zhao, L.; Wang, X.; Li, Z.; Lu, N.; Guo, Q. Oroxylin a has therapeutic potential in acute myelogenous leukemia by dual effects targeting ppargamma and rxralpha. Int. J. Cancer 2014, 134, 1195–1206. [Google Scholar] [CrossRef] [PubMed]
- Mu, R.; Qi, Q.; Gu, H.; Wang, J.; Yang, Y.; Rong, J.; Liu, W.; Lu, N.; You, Q.; Guo, Q. Involvement of p53 in oroxylin a-induced apoptosis in cancer cells. Mol. Carcinog. 2009, 48, 1159–1169. [Google Scholar] [CrossRef]
- Sun, Y.; Lu, N.; Ling, Y.; Gao, Y.; Chen, Y.; Wang, L.; Hu, R.; Qi, Q.; Liu, W.; Yang, Y.; et al. Oroxylin a suppresses invasion through down-regulating the expression of matrix metalloproteinase-2/9 in mda-mb-435 human breast cancer cells. Eur. J. Pharm. 2009, 603, 22–28. [Google Scholar] [CrossRef]
- Lu, Z.; Lu, N.; Li, C.; Li, F.; Zhao, K.; Lin, B.; Guo, Q. Oroxylin a inhibits matrix metalloproteinase-2/9 expression and activation by up-regulating tissue inhibitor of metalloproteinase-2 and suppressing the erk1/2 signaling pathway. Toxicol. Lett. 2012, 209, 211–220. [Google Scholar] [CrossRef]
- Fang, W.B.; Jokar, I.; Zou, A.; Lambert, D.; Dendukuri, P.; Cheng, N. Ccl2/ccr2 chemokine signaling coordinates survival and motility of breast cancer cells through smad3 protein- and p42/44 mitogen-activated protein kinase (mapk)-dependent mechanisms. J. Biol. Chem. 2012, 287, 36593–36608. [Google Scholar] [CrossRef]
- Tang, C.H.; Tsai, C.C. Ccl2 increases mmp-9 expression and cell motility in human chondrosarcoma cells via the ras/raf/mek/erk/nf-kappab signaling pathway. Biochem. Pharm. 2012, 83, 335–344. [Google Scholar] [CrossRef]
- Bose, S.; Kim, S.; Oh, Y.; Moniruzzaman, M.; Lee, G.; Cho, J. Effect of ccl2 on bv2 microglial cell migration: Involvement of probable signaling pathways. Cytokine 2016, 81, 39–49. [Google Scholar] [CrossRef]
- Gao, L.; Wang, F.Q.; Li, H.M.; Yang, J.G.; Ren, J.G.; He, K.F.; Liu, B.; Zhang, W.; Zhao, Y.F. Ccl2/egf positive feedback loop between cancer cells and macrophages promotes cell migration and invasion in head and neck squamous cell carcinoma. Oncotarget 2016, 7, 87037–87051. [Google Scholar] [CrossRef]
- Yang, J.; Lv, X.; Chen, J.; Xie, C.; Xia, W.; Jiang, C.; Zeng, T.; Ye, Y.; Ke, L.; Yu, Y.; et al. Ccl2-ccr2 axis promotes metastasis of nasopharyngeal carcinoma by activating erk1/2-mmp2/9 pathway. Oncotarget 2016, 7, 15632–15647. [Google Scholar] [CrossRef]
- O’Connor, T.; Borsig, L.; Heikenwalder, M. Ccl2-ccr2 signaling in disease pathogenesis. Endocr. Metab. Immune Disord. Drug Targets 2015, 15, 105–118. [Google Scholar] [CrossRef]
- Chaffer, C.L.; Weinberg, R.A. A perspective on cancer cell metastasis. Science 2011, 331, 1559–1564. [Google Scholar] [CrossRef]
- Chow, M.T.; Luster, A.D. Chemokines in cancer. Cancer Immunol. Res. 2014, 2, 1125–1131. [Google Scholar] [CrossRef]
- Zhang, J.; Patel, L.; Pienta, K.J. Cc chemokine ligand 2 (ccl2) promotes prostate cancer tumorigenesis and metastasis. Cytokine Growth Factor Rev. 2010, 21, 41–48. [Google Scholar] [CrossRef]
- Borsig, L.; Wolf, M.J.; Roblek, M.; Lorentzen, A.; Heikenwalder, M. Inflammatory chemokines and metastasis--tracing the accessory. Oncogene 2014, 33, 3217–3224. [Google Scholar] [CrossRef]
- Lim, S.Y.; Yuzhalin, A.E.; Gordon-Weeks, A.N.; Muschel, R.J. Targeting the ccl2-ccr2 signaling axis in cancer metastasis. Oncotarget 2016, 7, 28697–28710. [Google Scholar] [CrossRef]
- Wolf, M.J.; Hoos, A.; Bauer, J.; Boettcher, S.; Knust, M.; Weber, A.; Simonavicius, N.; Schneider, C.; Lang, M.; Sturzl, M.; et al. Endothelial ccr2 signaling induced by colon carcinoma cells enables extravasation via the jak2-stat5 and p38mapk pathway. Cancer Cell 2012, 22, 91–105. [Google Scholar] [CrossRef]
- Qian, B.Z.; Li, J.; Zhang, H.; Kitamura, T.; Zhang, J.; Campion, L.R.; Kaiser, E.A.; Snyder, L.A.; Pollard, J.W. Ccl2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature 2011, 475, 222–225. [Google Scholar] [CrossRef]
- Zhao, L.; Lim, S.Y.; Gordon-Weeks, A.N.; Tapmeier, T.T.; Im, J.H.; Cao, Y.; Beech, J.; Allen, D.; Smart, S.; Muschel, R.J. Recruitment of a myeloid cell subset (cd11b/gr1 mid) via ccl2/ccr2 promotes the development of colorectal cancer liver metastasis. Hepatology 2013, 57, 829–839. [Google Scholar] [CrossRef]
- Pienta, K.J.; Machiels, J.P.; Schrijvers, D.; Alekseev, B.; Shkolnik, M.; Crabb, S.J.; Li, S.; Seetharam, S.; Puchalski, T.A.; Takimoto, C.; et al. Phase 2 study of carlumab (cnto 888), a human monoclonal antibody against cc-chemokine ligand 2 (ccl2), in metastatic castration-resistant prostate cancer. Investig. New Drugs 2013, 31, 760–768. [Google Scholar] [CrossRef]
- Newby, A.C. Matrix metalloproteinases regulate migration, proliferation, and death of vascular smooth muscle cells by degrading matrix and non-matrix substrates. Cardiovasc. Res. 2006, 69, 614–624. [Google Scholar] [CrossRef]
- Li, Y.; Ma, J.; Guo, Q.; Duan, F.; Tang, F.; Zheng, P.; Zhao, Z.; Lu, G. Overexpression of mmp-2 and mmp-9 in esophageal squamous cell carcinoma. Dis. Esophagus 2009, 22, 664–667. [Google Scholar] [CrossRef]
- Said, A.H.; Raufman, J.P.; Xie, G. The role of matrix metalloproteinases in colorectal cancer. Cancers 2014, 6, 366–375. [Google Scholar] [CrossRef]
- Patel, B.P.; Shah, P.M.; Rawal, U.M.; Desai, A.A.; Shah, S.V.; Rawal, R.M.; Patel, P.S. Activation of mmp-2 and mmp-9 in patients with oral squamous cell carcinoma. J. Surg. Oncol. 2005, 90, 81–88. [Google Scholar] [CrossRef]
- Egeblad, M.; Werb, Z. New functions for the matrix metalloproteinases in cancer progression. Nat. Rev. Cancer 2002, 2, 161–174. [Google Scholar] [CrossRef]
- Hoekstra, R.; Eskens, F.A.; Verweij, J. Matrix metalloproteinase inhibitors: Current developments and future perspectives. Oncologist 2001, 6, 415–427. [Google Scholar] [CrossRef]
- Lin, C.W.; Yang, W.E.; Lee, W.J.; Hua, K.T.; Hsieh, F.K.; Hsiao, M.; Chen, C.C.; Chow, J.M.; Chen, M.K.; Yang, S.F.; et al. Lipocalin 2 prevents oral cancer metastasis through carbonic anhydrase ix inhibition and is associated with favourable prognosis. Carcinogenesis 2016, 37, 712–722. [Google Scholar] [CrossRef]
- Hu, C.; Yang, K.; Li, M.; Huang, W.; Zhang, F.; Wang, H. Lipocalin 2: A potential therapeutic target for breast cancer metastasis. Onco Targets Ther. 2018, 11, 8099–8106. [Google Scholar] [CrossRef]
- Nishimine, M.; Nakamura, M.; Mishima, K.; Kishi, M.; Kirita, T.; Sugimura, M.; Konishi, N. Id proteins are overexpressed in human oral squamous cell carcinomas. J. Oral Pathol. Med. 2003, 32, 350–357. [Google Scholar] [CrossRef]
- Dong, Z.; Liu, S.; Zhou, C.; Sumida, T.; Hamakawa, H.; Chen, Z.; Liu, P.; Wei, F. Overexpression of id-1 is associated with tumor angiogenesis and poor clinical outcome in oral squamous cell carcinoma. Oral Oncol. 2010, 46, 154–157. [Google Scholar] [CrossRef]
- Kim, H.M.; Kang, Y.H.; Byun, J.H.; Jang, S.J.; Rho, G.J.; Lee, J.S.; Park, B.W. Midkine and nanog have similar immunohistochemical expression patterns and contribute equally to an adverse prognosis of oral squamous cell carcinoma. Int. J. Mol. Sci. 2017, 18, 2339. [Google Scholar] [CrossRef]
- Ota, K.; Fujimori, H.; Ueda, M.; Shiniriki, S.; Kudo, M.; Jono, H.; Fukuyoshi, Y.; Yamamoto, Y.; Sugiuchi, H.; Iwase, H.; et al. Midkine as a prognostic biomarker in oral squamous cell carcinoma. Br. J. Cancer 2008, 99, 655–662. [Google Scholar] [CrossRef]
- Fang, W.Y.; Chen, Y.W.; Hsiao, J.R.; Liu, C.S.; Kuo, Y.Z.; Wang, Y.C.; Chang, K.C.; Tsai, S.T.; Chang, M.Z.; Lin, S.H.; et al. Elevated s100a9 expression in tumor stroma functions as an early recurrence marker for early-stage oral cancer patients through increased tumor cell invasion, angiogenesis, macrophage recruitment and interleukin-6 production. Oncotarget 2015, 6, 28401–28424. [Google Scholar] [CrossRef]
- Liu, C.H.; Chen, M.F.; Tseng, T.L.; Chen, L.G.; Kuo, J.S.; Lee, T.J. Oroxylin a, but not vasopressin, ameliorates cardiac dysfunction of endotoxemic rats. Evid. Based Complement. Altern. Med. 2012, 2012, 408187. [Google Scholar] [CrossRef]
- Lai, K.C.; Liu, C.J.; Lin, T.J.; Mar, A.C.; Wang, H.H.; Chen, C.W.; Hong, Z.X.; Lee, T.C. Blocking tnf-alpha inhibits angiogenesis and growth of ifit2-depleted metastatic oral squamous cell carcinoma cells. Cancer Lett. 2016, 370, 207–215. [Google Scholar] [CrossRef]
- Lai, K.C.; Liu, C.J.; Chang, K.W.; Lee, T.C. Depleting ifit2 mediates atypical pkc signaling to enhance the migration and metastatic activity of oral squamous cell carcinoma cells. Oncogene 2013, 32, 3686–3697. [Google Scholar] [CrossRef]
- Lai, K.C.; Lee, T.C. Genetic damage in cultured human keratinocytes stressed by long-term exposure to areca nut extracts. Mutat. Res. 2006, 599, 66–75. [Google Scholar] [CrossRef]






| ID | Gene | Prediction # | Expression (Log 2) | Finding * |
|---|---|---|---|---|
| NM_005564.3 | LCN2 | Decreased | −1.613 | Increased (1) |
| NM_181353.1 | ID1 | Decreased | −1.41 | Increased (10) |
| NM_001012334.1 | MDK | Decreased | −1.237 | Increased (2) |
| NM_002965.2 | S100A9 | Decreased | −1.206 | Increased (5) |
| NM_002982.3 | CCL2 | Decreased | −1.081 | Increased (40) |
| NM_001038.4 | SCNN1A | Decreased | −0.981 | Increased (1) |
| NM_006113.4 | VAV3 | Decreased | −0.978 | Increased (2) |
| NM_004390.2 | CTSH | Decreased | −0.87 | Increased (3) |
| NM_003722.3 | TP63 | Increased | −0.735 | Decreased (5) |
| NM_004864.1 | GDF15 | Decreased | −0.729 | Increased (1) |
| ID | Gene | Prediction # | Expression (Log 2) | Finding * |
|---|---|---|---|---|
| NM_000758.2 | CSF2 | Increased | 2.158 | Increased (1) |
| NM_002658.2 | PLAU | Increased | 1.523 | Increased (25) |
| NM_001945.1 | HBEGF | Increased | 1.503 | Increased (1) |
| NM_000228.2 | LAMB3 | Increased | 1.482 | Increased (7) |
| NM_001964.2 | EGR1 | Increased | 1.385 | Increased (1) |
| NM_000584.2 | CXCL8 | Increased | 1.374 | Increased (10) |
| NM_000600.1 | IL6 | Increased | 1.280 | Increased (9) |
| NM_001005376.1 | PLAUR | Increased | 1.197 | Increased (11) |
| NM_002203.3 | ITGA2 | Increased | 1.173 | Increased (1) |
| NM_001001392.1 | CD44 | Increased | 1.139 | Increased (35) |
| Location of Tumor | Vehicle Control (n = 6) | Oro-A (n = 6) |
|---|---|---|
| Head and Neck | 4 | 1 |
| Thoracic cavity | 4 | 2 |
| Subcutaneous | 8 | 2 |
| Skeletal muscle | 8 | 5 |
| Total | 24 | 10 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ku, W.-T.; Tung, J.-J.; Lee, T.J.-F.; Lai, K.-C. Long-Term Exposure to Oroxylin A Inhibits Metastasis by Suppressing CCL2 in Oral Squamous Cell Carcinoma Cells. Cancers 2019, 11, 353. https://doi.org/10.3390/cancers11030353
Ku W-T, Tung J-J, Lee TJ-F, Lai K-C. Long-Term Exposure to Oroxylin A Inhibits Metastasis by Suppressing CCL2 in Oral Squamous Cell Carcinoma Cells. Cancers. 2019; 11(3):353. https://doi.org/10.3390/cancers11030353
Chicago/Turabian StyleKu, Wei-Ting, Jiun-Jia Tung, Tony Jer-Fu Lee, and Kuo-Chu Lai. 2019. "Long-Term Exposure to Oroxylin A Inhibits Metastasis by Suppressing CCL2 in Oral Squamous Cell Carcinoma Cells" Cancers 11, no. 3: 353. https://doi.org/10.3390/cancers11030353
APA StyleKu, W.-T., Tung, J.-J., Lee, T. J.-F., & Lai, K.-C. (2019). Long-Term Exposure to Oroxylin A Inhibits Metastasis by Suppressing CCL2 in Oral Squamous Cell Carcinoma Cells. Cancers, 11(3), 353. https://doi.org/10.3390/cancers11030353





