Difficulties in Translating Appetite Sensations Effect of Turmeric-Based Beverage When Given Prior to Isoenergetic Medium- or High-Fat Meals in Healthy Subjects
Abstract
:1. Introduction
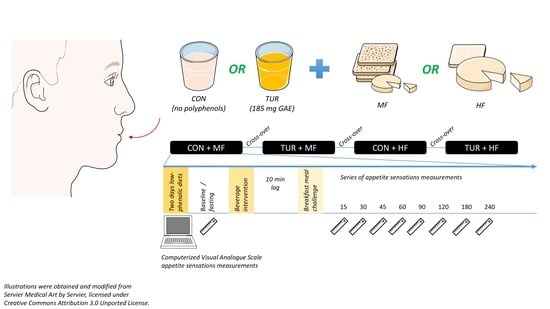
2. Ethical Aspect, Participants, and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Halford, J.C.G.; Harrold, J.A. Satiety-enhancing products for appetite control: Science and regulation of functional foods for weight management. Proc. Nutr. Soc. 2012, 71, 350–362. [Google Scholar] [CrossRef] [PubMed]
- Chambers, L.; McCrickerd, K.; Yeomans, M.R. Optimising foods for satiety. Trends Food Sci. Technol. 2015, 41, 149–160. [Google Scholar] [CrossRef] [Green Version]
- Zanzer, Y.C.; Plaza, M.; Dougkas, A.; Turner, C.; Björck, I.; Östman, E. Polyphenol-rich spice-based beverages modulated postprandial early glycaemia, appetite and pyy after breakfast challenge in healthy subjects: A randomized, single blind, crossover study. J. Funct. Foods 2017, 35, 574–583. [Google Scholar] [CrossRef]
- Zanzer, Y.C.; Plaza, M.; Dougkas, A.; Turner, C.; Ostman, E. Black pepper-based beverage induced appetite-suppressing effects without altering postprandial glycaemia, gut and thyroid hormones or gastrointestinal well-being: A randomized crossover study in healthy subjects. Food Funct. 2018, 9, 2774–2786. [Google Scholar] [CrossRef] [PubMed]
- Hlebowicz, J.; Darwiche, G.; Bjorgell, O.; Almer, L.O. Effect of cinnamon on postprandial blood glucose, gastric emptying, and satiety in healthy subjects. Am. J. Clin. Nutr. 2007, 85, 1552–1556. [Google Scholar] [CrossRef] [Green Version]
- Hlebowicz, J.; Hlebowicz, A.; Lindstedt, S.; Bjorgell, O.; Hoglund, P.; Holst, J.J.; Darwiche, G.; Almer, L.O. Effects of 1 and 3 g cinnamon on gastric emptying, satiety, and postprandial blood glucose, insulin, glucose-dependent insulinotropic polypeptide, glucagon-like peptide 1, and ghrelin concentrations in healthy subjects. Am. J. Clin. Nutr. 2009, 89, 815–821. [Google Scholar] [CrossRef] [Green Version]
- Markey, O.; McClean, C.M.; Medlow, P.; Davison, G.W.; Trinick, T.R.; Duly, E.; Shafat, A. Effect of cinnamon on gastric emptying, arterial stiffness, postprandial lipemia, glycemia, and appetite responses to high-fat breakfast. Cardiovasc. Diabetol. 2011, 10, 78. [Google Scholar] [CrossRef]
- Mansour, M.S.; Ni, Y.M.; Roberts, A.L.; Kelleman, M.; Roychoudhury, A.; St-Onge, M.P. Ginger consumption enhances the thermic effect of food and promotes feelings of satiety without affecting metabolic and hormonal parameters in overweight men: A pilot study. Metabolism 2012, 61, 1347–1352. [Google Scholar] [CrossRef] [Green Version]
- De Silva, A.; Bloom, S.R. Gut hormones and appetite control: A focus on pyy and glp-1 as therapeutic targets in obesity. Gut Liver 2012, 6, 10–20. [Google Scholar] [CrossRef]
- Flint, A.; Verdich, C.; Astrup, A.; Näslund, E.; Morgan, L.M.; Long, S.J.; Beglinger, C.; Gutzwiller, J.-P.; Hellström, P.M.; Holst, J.J. A meta-analysis of the effect of glucagon-like peptide-1 (7–36) amide on ad libitum energy intake in humans. J. Clin. Endocrinol. Metab. 2001, 86, 4382–4389. [Google Scholar]
- Takikawa, M.; Kurimoto, Y.; Tsuda, T. Curcumin stimulates glucagon-like peptide-1 secretion in glutag cells via ca2+/calmodulin-dependent kinase ii activation. Biochem. Biophys. Res. Commun. 2013, 435, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Nishikawa, S.; Ikehata, A.; Dochi, K.; Tani, T.; Takahashi, T.; Imaizumi, A.; Tsuda, T. Curcumin improves glucose tolerance via stimulation of glucagon-like peptide-1 secretion. Mol. Nutr. Food Res. 2017, 61, 1600471. [Google Scholar] [CrossRef]
- Marsh-Richard, D.M.; Hatzis, E.S.; Mathias, C.W.; Venditti, N.; Dougherty, D.M. Adaptive visual analog scales (avas): A modifiable software program for the creation, administration, and scoring of visual analog scales. Behav. Res. Methods 2009, 41, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Flint, A.; Raben, A.; Blundell, J.E.; Astrup, A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int. J. Obes. Relat. Metab. Disord. 2000, 24, 38–48. [Google Scholar] [CrossRef] [Green Version]
- Holt, S.H.A.; Delargy, H.J.; Lawton, C.L.; Blundell, J.E. The effects of high-carbohydrate vs. high-fat breakfasts on feelings of fullness and alertness, and subsequent food intake. Int. J. Food Sci. Nutr. 1999, 50, 13–28. [Google Scholar] [CrossRef]
- Yang, N.; Liu, X.; Ding, E.L.; Xu, M.; Wu, S.; Liu, L.; Sun, X.; Hu, F.B. Impaired ghrelin response after high-fat meals is associated with decreased satiety in obese and lean chinese young adults. J. Nutr. 2009, 139, 1286–1291. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, M.; Gibbons, C.; Caudwell, P.; Blundell, J.E.; Finlayson, G. Differing effects of high-fat or high-carbohydrate meals on food hedonics in overweight and obese individuals. Br. J. Nutr. 2016, 115, 1875–1884. [Google Scholar] [CrossRef]
- Shin, H.S.; Ingram, J.R.; McGill, A.T.; Poppitt, S.D. Lipids, chos, proteins: Can all macronutrients put a ‘brake’ on eating? Physiol. Behav. 2013, 120, 114–123. [Google Scholar] [CrossRef]
- Goetze, O.; Steingoetter, A.; Menne, D.; van der Voort, I.R.; Kwiatek, M.A.; Boesiger, P.; Weishaupt, D.; Thumshirn, M.; Fried, M.; Schwizer, W. The effect of macronutrients on gastric volume responses and gastric emptying in humans: A magnetic resonance imaging study. Am. J. Physiol.-Gastrointest. Liver Physiol. 2007, 292, G11–G17. [Google Scholar] [CrossRef] [PubMed]
- Cecil, J.E.; Francis, J.; Read, N.W. Comparison of the effects of a high-fat and high-carbohydrate soup delivered orally and intragastrically on gastric emptying, appetite, and eating behaviour. Physiol. Behav. 1999, 67, 299–306. [Google Scholar] [CrossRef]
- Marciani, L.; Cox, E.F.; Pritchard, S.E.; Major, G.; Hoad, C.L.; Mellows, M.; Hussein, M.O.; Costigan, C.; Fox, M.; Gowland, P.A.; et al. Additive effects of gastric volumes and macronutrient composition on the sensation of postprandial fullness in humans. Eur. J. Clin. Nutr. 2014, 69, 380–384. [Google Scholar] [CrossRef] [Green Version]
- Jain, N.K.; Boivin, M.; Zinsmeister, A.R.; Brown, M.L.; Malagelada, J.-R.; DiMagno, E.P. Effect of ileal perfusion of carbohydrates and amylase inhibitor on gastrointestinal hormones and emptying. Gastroenterology 1989, 96, 377–387. [Google Scholar] [CrossRef]
- Jain, N.K.; Boivin, M.; Zinsmeister, A.R.; DiMagno, E.P. The ileum and carbohydrate-mediated feedback regulation of postprandial pancreaticobiliary secretion in normal humans. Pancreas 1991, 6, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Chaikomin, R.; Wu, K.-L.; Doran, S.; Meyer, J.H.; Jones, K.L.; Feinle-Bisset, C.; Horowitz, M.; Rayner, C.K. Effects of mid-jejunal compared to duodenal glucose infusion on peptide hormone release and appetite in healthy men. Regul. Pept. 2008, 150, 38–42. [Google Scholar] [CrossRef]
- Gibbons, C.; Finlayson, G.; Caudwell, P.; Webb, D.-L.; Hellström, P.M.; Näslund, E.; Blundell, J.E. Postprandial profiles of cck after high fat and high carbohydrate meals and the relationship to satiety in humans. Peptides 2016, 77, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Camacho, S.; Michlig, S.; de Senarclens-Bezençon, C.; Meylan, J.; Meystre, J.; Pezzoli, M.; Markram, H.; le Coutre, J. Anti-obesity and anti-hyperglycemic effects of cinnamaldehyde via altered ghrelin secretion and functional impact on food intake and gastric emptying. Sci. Rep. 2015, 5, 7919. [Google Scholar] [CrossRef]
- Bédard, A.; Hudon, A.-M.; Drapeau, V.; Corneau, L.; Dodin, S.; Lemieux, S. Gender differences in the appetite response to a satiating diet. J. Obes. 2015, 2015, 140139. [Google Scholar] [CrossRef]
- Gregersen, N.T.; Møller, B.K.; Raben, A.; Kristensen, S.T.; Holm, L.; Flint, A.; Astrup, A. Determinants of appetite ratings: The role of age, gender, bmi, physical activity, smoking habits, and diet/weight concern. Food Nutr. Res. 2011, 55. [Google Scholar] [CrossRef] [PubMed]


| Mean ± SEM | |
|---|---|
| Age, y | 26.5 ± 1.1 |
| BMI, kg·m−2 | 23.8 ± 0.6 |
| BMR, kcal | 1615 ± 109 |
| Body fat, % | 24 ± 2.4 |
| Blood glucose, mmol·L−1 | 4.98 ± 0.09 |
| Insulin, pmol·L−1 | 67.14 ± 9.3 |
| Triacylglycerol, mmol·L−1 | 0.85 ± 0.11 |
| Total cholesterol, mmol·L−1 | 4.39 ± 0.26 |
| Hb, g·L−1 | 146.7 ± 4.7 |
| Creatinine, µmol·L−1 | 77.3 ± 3.4 |
| ASAT, µkat·L−1 | 0.38 ± 0.03 |
| ALAT, µkat·L−1 | 0.38 ± 0.07 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zanzer, Y.C.; Batista, Â.G.; Dougkas, A.; Tovar, J.; Granfeldt, Y.; Östman, E. Difficulties in Translating Appetite Sensations Effect of Turmeric-Based Beverage When Given Prior to Isoenergetic Medium- or High-Fat Meals in Healthy Subjects. Nutrients 2019, 11, 736. https://doi.org/10.3390/nu11040736
Zanzer YC, Batista ÂG, Dougkas A, Tovar J, Granfeldt Y, Östman E. Difficulties in Translating Appetite Sensations Effect of Turmeric-Based Beverage When Given Prior to Isoenergetic Medium- or High-Fat Meals in Healthy Subjects. Nutrients. 2019; 11(4):736. https://doi.org/10.3390/nu11040736
Chicago/Turabian StyleZanzer, Yoghatama Cindya, Ângela Giovana Batista, Anestis Dougkas, Juscelino Tovar, Yvonne Granfeldt, and Elin Östman. 2019. "Difficulties in Translating Appetite Sensations Effect of Turmeric-Based Beverage When Given Prior to Isoenergetic Medium- or High-Fat Meals in Healthy Subjects" Nutrients 11, no. 4: 736. https://doi.org/10.3390/nu11040736