Computer Model of Synapse Loss During an Alzheimer’s Disease-Like Pathology in Hippocampal Subregions DG, CA3 and CA1—The Way to Chaos and Information Transfer
Abstract
:1. Introduction
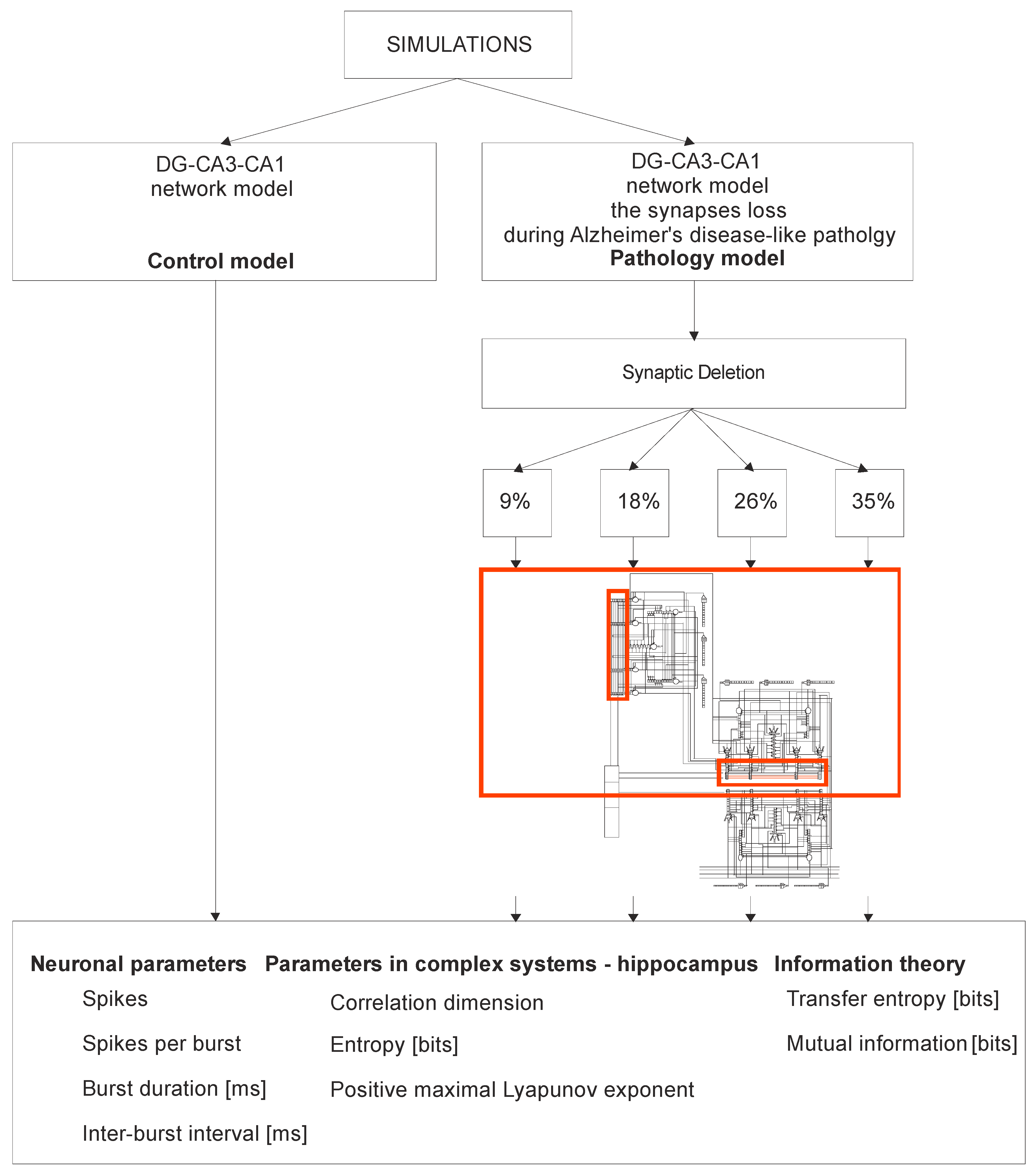
2. Materials and Methods
2.1. Study Design
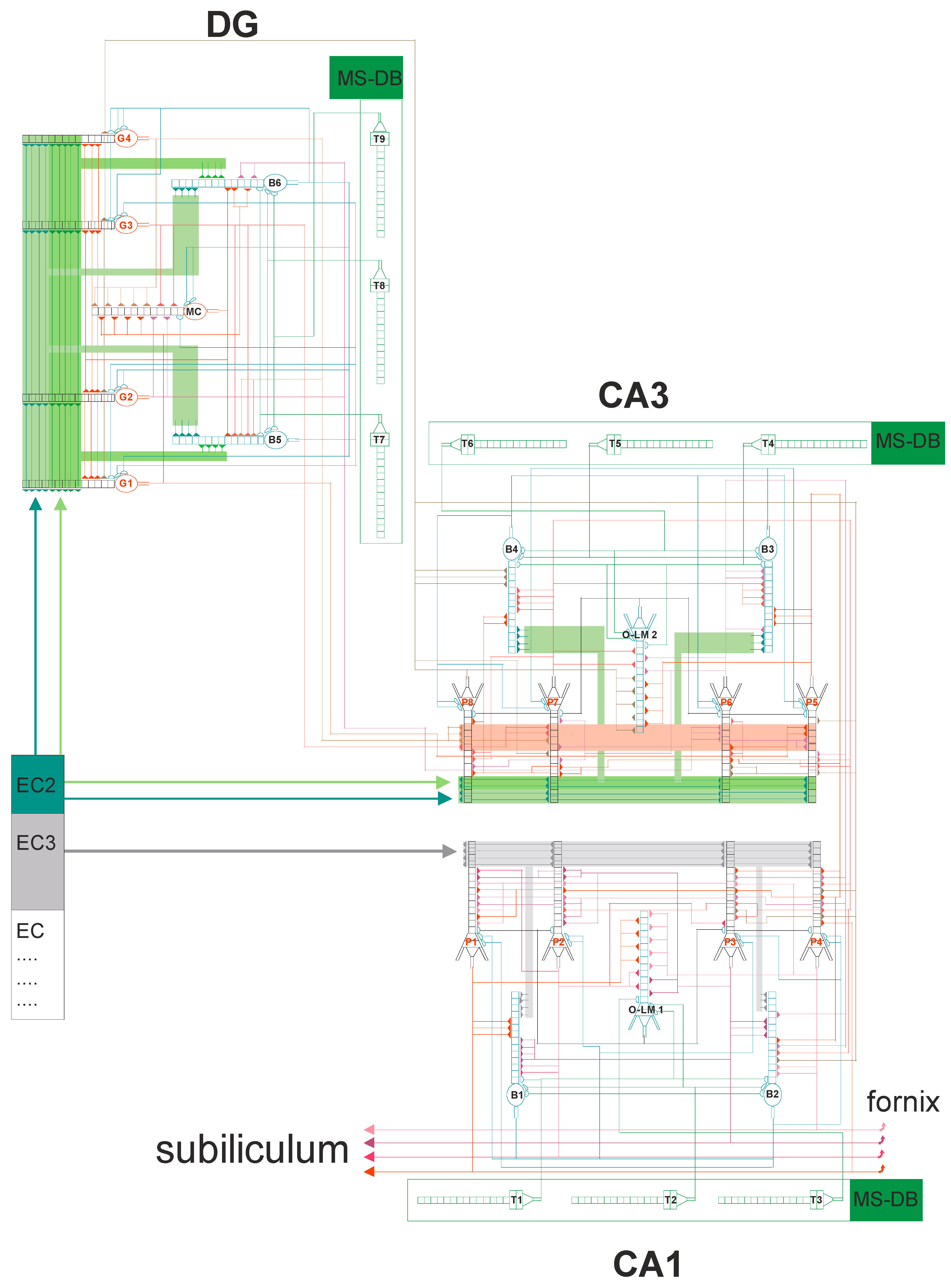
2.2. The Model Description
2.3. Synaptic Properties
2.4. Correlation Dimension, Shannon Entropy and the Positive Maximal Lyapunov Exponent
2.5. Mutual Information and Transfer Entropy
2.6. Statistical Methods and Software
3. Results
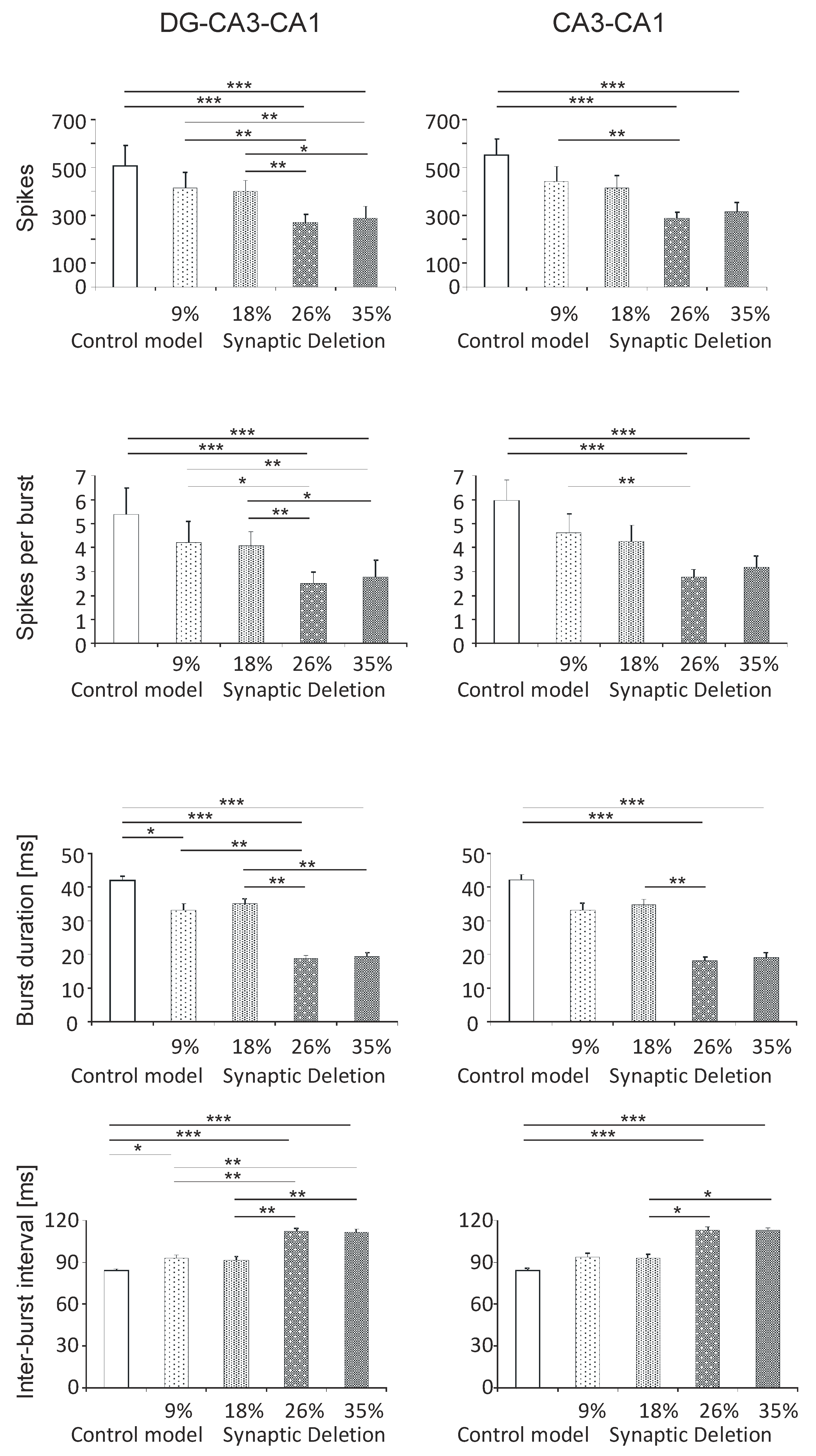
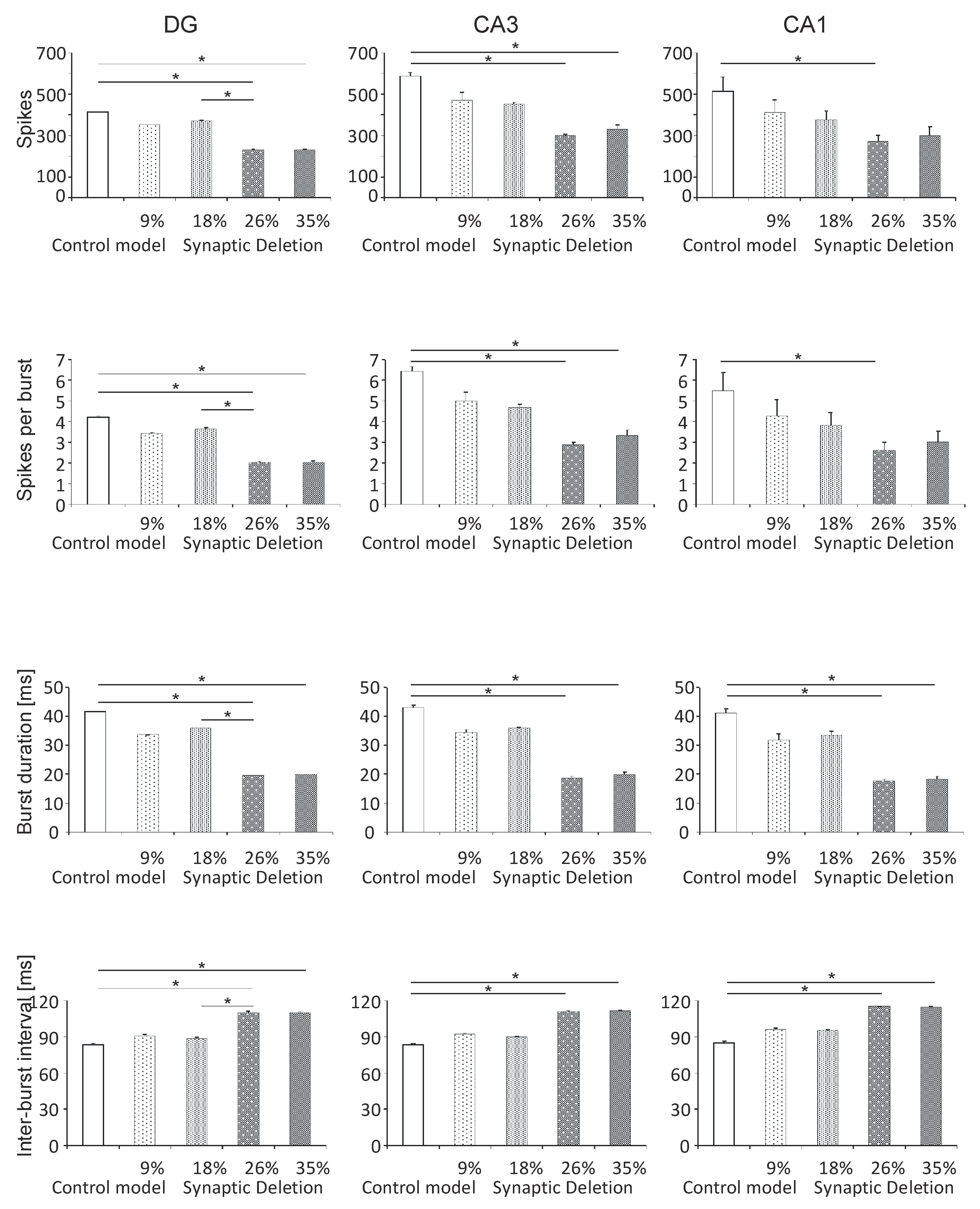
3.1. Neuronal Parameters
3.1.1. DG-CA3-CA1 and CA3-CA1 Areas
3.1.2. DG, CA3 and CA1 Regions
3.2. Parameters in a Complex System: Hippocampus
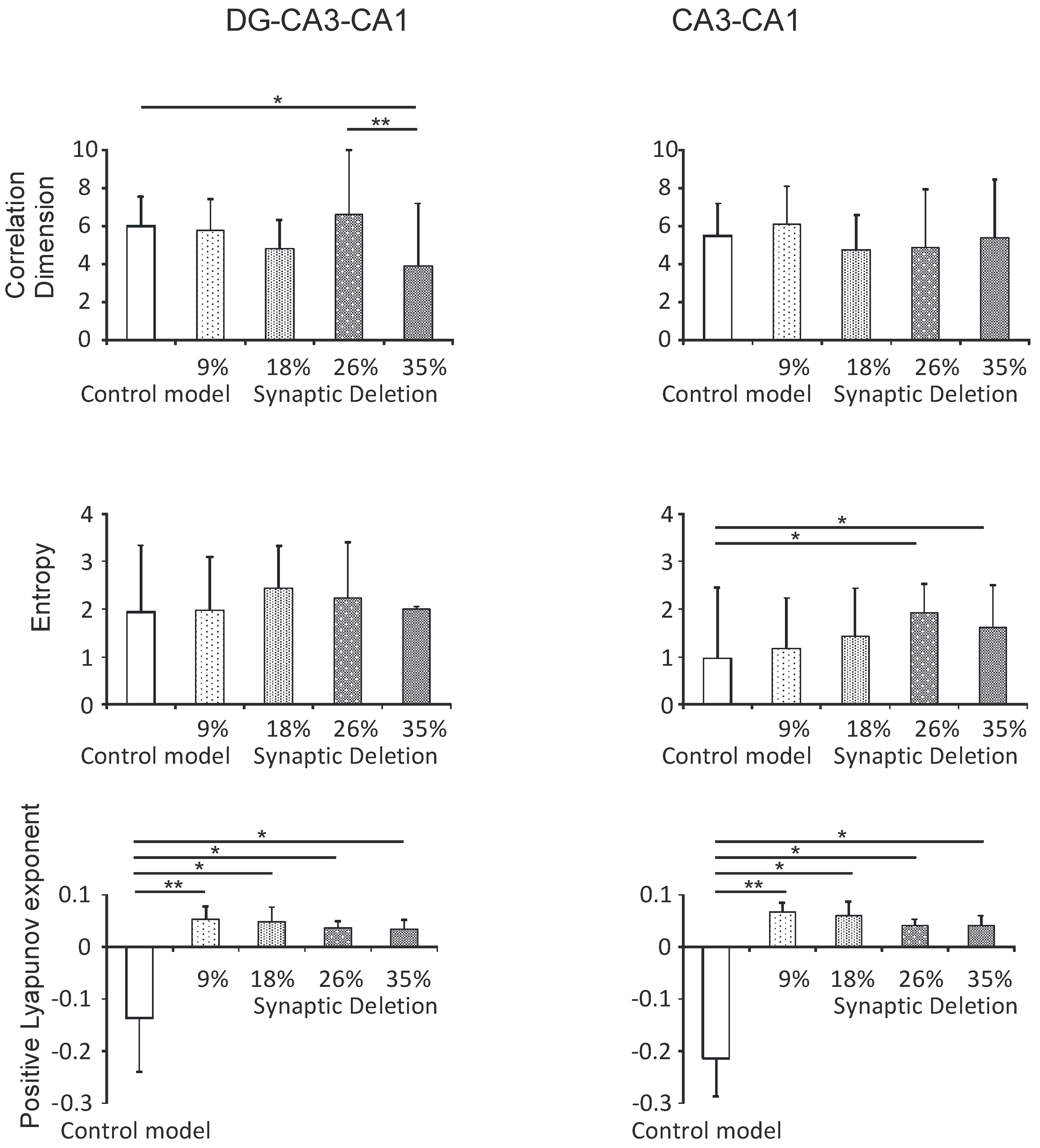
3.2.1. DG-CA3-CA1 and CA3-CA1 Regions
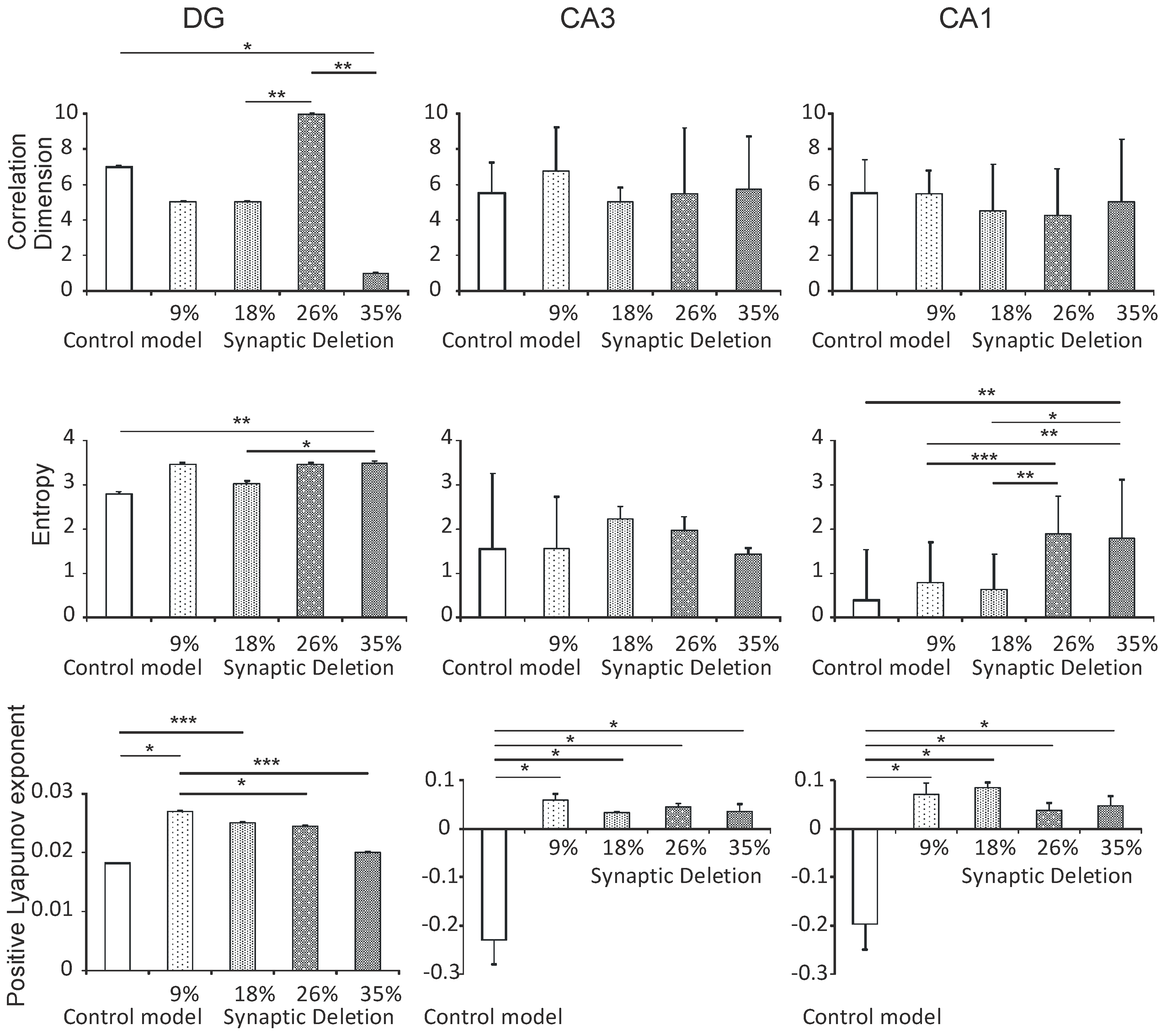
3.2.2. DG, CA3 and CA1 Regions
3.3. The Flow of Information in Hippocampus vs. Shannon Entropy, Transfer Entropy and Mutual Information
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Llorens-Martín, M.; Blazquez-Llorca, L.; Benavides-Piccione, R.; Rabano, A.; Hernandez, F.; Avila, J.; DeFelipe, J. Selective alterations of neurons and circuits related to early memory loss in Alzheimer’s disease. Front. Neuroanat. 2014, 27, 8–38. [Google Scholar] [CrossRef]
- Finkel, L. Neuroengineering models of brain disease. Annu. Rev. Biomed. Eng. 2000, 2, 577–606. [Google Scholar] [CrossRef]
- Horn, D.; Ruppin, E.; Usher, M.; Herrmann, M. Neural network modeling of memory deterioration in Alzheimer’s disease. Neural Comput. 1993, 5, 736–749. [Google Scholar] [CrossRef]
- Reggia, J.; Ruppin, E.; Berndt, R. Computer models: A new approach to the investigation of disease. MD Comput. Comput. Med. Pract. 1997, 14, 160–168. [Google Scholar]
- Ruppin, E.; Reggia, J. A neural model of memory impairment in diffuse cerebral atrophy. Br. J. Psychiatry 1995, 166, 19–28. [Google Scholar] [CrossRef]
- Hasselmo, M. Neuromodulation and cortical function: Modeling the physiological basis of behavior. Behav. Brain Res. 1995, 67, 1–27. [Google Scholar] [CrossRef]
- Hasselmo, M. Runaway synaptic modification in models of cortex: Implications for Alzheimer’s disease. Neural Netw. 1994, 7, 13–40. [Google Scholar] [CrossRef]
- Siegle, G.; Hasselmo, M. Using connectionist models to guide assessment of psychological disorder. Psychol. Assess. 2002, 14, 263–278. [Google Scholar] [CrossRef]
- Hasselmo, M.; McClelland, J.L. Neural models of memory. Curr. Opin. Neurobiol. 1999, 9, 184–188. [Google Scholar] [CrossRef]
- Duch, W. Computational models of dementia and neuro-logical problems. Methods Mol. Biol. 2007, 401, 305–336. [Google Scholar] [CrossRef]
- Duch, W. Therapeutic implications of computer models of brain activity for Alzheimer disease. J. Med. Inform. Technol. 2000, 5, 27–34. [Google Scholar]
- Menschik, E.; Finkel, L. Neuromodulatory control of hippocampal function: Towards a model of Alzheimer’s disease. Artif. Intell. Med. 1998, 13, 99–121. [Google Scholar] [CrossRef]
- Menschik, E.; Yen, S.-C.; Finkel, L. Model and scale-independent performance of a hippocampal CA3 network architecture. Neurocomputing 1999, 26–27, 443–453. [Google Scholar] [CrossRef]
- Menschik, E.; Finkel, L. Cholinergic neuromodulation of an anatomically reconstructed hippocampal CA3 pyramidal cell. Neurocomputing 2000, 32–33, 197–205. [Google Scholar] [CrossRef]
- Jeong, J.; Kim, S.Y.; Han, S.H. Non-linear dynamical analysis of the EEG in Alzheimer’s disease with optimal embeddingdimension. Electroencephalogr. Clin. Neurophysiol. 1998, 106, 220–228. [Google Scholar] [CrossRef]
- Jelles, B.; van Birgelen, J.H.; Slaets, J.P.J.; Hekster, R.E.M.; Jonkman, E.J.; Stam, C.J. Decrease of non-linear structure in the EEG of Alzheimer patients compared to healthy controls. Clin. Neurophysiol. 1999, 110, 1159–1167. [Google Scholar] [CrossRef]
- Abásolo, D.; Hornero, R.; Espino, P.; Poza, J.; Sánchez, C.I.; de la Rosa, R. Analysis of regularity in the EEG background activity of Alzheimer’s disease patients with approximate entropy. Clin. Neurophysiol. 2005, 116, 1826–1834. [Google Scholar] [CrossRef] [PubMed]
- Sleigh, J.W.; Steyn-Ross, D.A.; Grant, C.; Ludbrook, G. Cortical entropy changes with general anaesthesia: Theory and experiment. Physiol. Meas. 2004, 25, 921–934. [Google Scholar] [CrossRef]
- Gómez, C.; Hornero, R.; Abásolo, D.; Fernández, A.; Escudero, J. Analysis of MEG background activity in Alzheimer’s disease using non-linear methods and ANFIS. Ann. Biomed. Eng. 2009, 37, 586–594. [Google Scholar] [CrossRef]
- Escudero, J.; Abásolo, D.; Hornero, R.; Espino, P.; López, M. Analysis of electroencephalograms in Alzheimer’s disease patients with multiscale entropy. Physiol. Meas. 2006, 27, 1091–1106. [Google Scholar] [CrossRef]
- Hornero, R.; Escudero, J.; Fernández, A.; Poza, J.; Gómez, C. Spectral and non-linear analyses of MEG background activity in patients with Alzheimer’s disease. IEEE Trans. Biomed. Eng. 2008, 55, 1658–1665. [Google Scholar] [CrossRef] [PubMed]
- Świetlik, D.; Białowąs, J.; Kusiak, A.; Cichońska, D. Memory and forgetting processes with the firing neuron model. Folia Morphol. 2018, 77, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Świetlik, D.; Białowąs, J.; Kusiak, A.; Cichońska, D. A computational simulation of long-term synaptic potentiation inducing protocol processes with model of CA3 hippocampal microcircuit. Folia Morphol. 2018, 77, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Świetlik, D. Simulations of Learning, Memory, and Forgetting Processes with Model of CA1 Region of the Hippocampus. Complexity 2018, 2018, 1297150. [Google Scholar] [CrossRef]
- Aradi, I.; Holmes, W. Role of multiple calcium and calcium-dependent conductances in regulation of hippocampal dentate granule cell excitability. J. Comput. Neurosci. 1999, 6, 215–235. [Google Scholar] [CrossRef] [PubMed]
- Migliore, M.; Cook, E.P.; Jaffe, D.B.; Turner, D.A.; Johnston, D. Computer simulations of morphologically reconstructed CA3 hippocampal neurons. J. Neurophysiol. 1995, 73, 1157–1168. [Google Scholar] [CrossRef]
- Poirazi, P.; Brannon, T.; Mel, B.W. Arithmetic of subthreshold synaptic summation in a model CA1 pyramidal cell. Neuron 2003, 37, 977–987. [Google Scholar] [CrossRef]
- Santhakumar, V. Role of Mossy Fiber Sprouting and Mossy Cell Loss in Hyperexcitability: A Network Model of the Dentate Gyrus Incorporating Cell Types and Axonal Topography. J. Neurophysiol. 2004, 93, 437–453. [Google Scholar] [CrossRef]
- Saraga, F.; Wu, C.P.; Zhang, L.; Skinner, F.K. Active dendrites and spike propagation in multi-compartment models of oriens-lacunosum/moleculare hippocampal interneurons. J. Physiol. 2003, 552, 673–689. [Google Scholar] [CrossRef] [PubMed]
- Cutsuridis, V.; Cobb, S.; Graham, B. Encoding and retrieval in the hippocampal CA1 microcircuit model. Hippocampus 2010, 20, 423–446. [Google Scholar] [CrossRef] [PubMed]
- Borhegyi, Z.; Varga, V.; Szilagyi, N.; Fabo, D.; Freund, T. Phase segregation of medial septal GABAergic neurons during hippocampal theta activity. J. Neurosci. 2004, 24, 8470–8479. [Google Scholar] [CrossRef] [PubMed]
- Amaral, D.; Lavenex, P. Hippocampal Neuroanatomy. In The Hippocampus Book; Andersen, P., Morris, R., Amaral, D., Bliss, T., O’Keefe, J., Eds.; Oxford University Press: Oxford, UK, 2006; pp. 37–115. [Google Scholar]
- Andersen, P.; Morris, R.; Amaral, D.; Bliss, T.; O’Keefe, J. The Hippocampus Book; University Press: Oxford, UK, 2007. [Google Scholar]
- Witter, M. Connectivity of the hippocampus. In Hippocampal Microcircuits: A Computational Modeler’s Resource Book; Springer: New York, NY, USA, 2010. [Google Scholar]
- Buzsáki, G.; Leung, L.W.; Vanderwolf, C. Cellular bases of hippocampal EEG in the behaving rat. Brain Res. 1983, 287, 139–171. [Google Scholar] [CrossRef]
- Buzsáki, G. Two-stage model of memory trace formation: A role for “noisy” brain states. Neuroscience 1989, 31, 551–570. [Google Scholar] [CrossRef]
- Stewart, M.; Fox, S. Do septal neurons pace the hippocampal theta rhythm? Trends Neurosci. 1990, 13, 163–169. [Google Scholar] [CrossRef]
- Bragin, A.; Jandó, G.; Nádasdy, Z. Gamma (40–100 Hz) oscilla-tion in the hippocampus of the behaving rat. J. Neurosci. 1995, 15, 47–60. [Google Scholar] [CrossRef]
- Pietak, A.; Levin, M. Bioelectrical control of positional information in development and regeneration: A review of conceptual and computational advances. Prog. Biophys. Mol. Biol. 2018, 4, 52–68. [Google Scholar] [CrossRef]
- O’Keefe, J.; Recce, M.L. Phase relationship between hippocampal place units and the EEG theta rhythm. Hippocampus 1993, 3, 317–330. [Google Scholar] [CrossRef]
- Skaggs, W.; McNaughton, B.; Wilson, M. Theta phase precession in hippocampal neuronal populations and the compression of tem-poral sequences. Hippocampus 1996, 6, 149–172. [Google Scholar] [CrossRef]
- Omura, Y.; Carvalho, M.; Inokuchi, K.; Fukai, T. A Lognormal Recurrent Network Model for Burst Generation during Hippocampal Sharp Waves. J. Neurosci. 2015, 28, 14585–14601. [Google Scholar] [CrossRef]
- Eckman, J.P.; Ruelle, D. Ergodic theory of chaos and strange attractor. Rev. Mod. Phys. 1985, 57, 617–656. [Google Scholar] [CrossRef]
- Packard, N.H.; Crutchfield, J.P.; Farmer, J.D.; Shaw, R.S. Geometry from a Time Series. Phys. Rev. Lett. 1980, 45, 712–716. [Google Scholar] [CrossRef]
- Doyne, J.D.; Ott, E.; Yorke, J.A. The dimension of chaotic attractors. Phys. D Nonlinear Phenom. 1983, 7, 153–180. [Google Scholar]
- Fraser, A.M.; Swinney, H.L. Independent coordinates for strange attractors from mutual information. Phys. Rev. A 1986, 33, 1134–1140. [Google Scholar] [CrossRef]
- Kennel, M.; Brown, R.; Abarbanel, H. Determining embedding dimension for phase-space reconstruction using a geometrical construction. Phys. Rev. A 1992, 45, 3403–3411. [Google Scholar] [CrossRef] [PubMed]
- Trulla, L.L.; Giuliani, A.; Zbilut, J.P.; Webber, C.L. Recurrence quantification analysis of the logistic equation with transients. Phys. Lett. A 1996, 223, 255–260. [Google Scholar] [CrossRef]
- Shannon, C.E. A mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Hoyer, D. Mutual Information Function Assesses Autonomic Information Flow of Heart Rate Dynamisc at Different Time Scales. IEEE Trans. Biomed. Eng. 2005, 52, 584–592. [Google Scholar] [CrossRef]
- Pompe, B. Using Mutual Information to Measure Coupling in the Cardiorespiratory System. IEEE Eng. Med. Boil. 1998, 17, 32–39. [Google Scholar] [CrossRef]
- Schreiber, T. Measuring Information Transfer. Phys. Rev. Lett. 2000, 85, 461. [Google Scholar] [CrossRef]
- Nicholas, M.T.; Lapish, C. A Tutorial for Information Theory in Neuroscience. eNeuro 2018, 5. [Google Scholar] [CrossRef]
- Braak, H.; Braak, E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991, 82, 239–259. [Google Scholar] [CrossRef] [PubMed]
- Świetlik, D.; Białowąs, J. Application of Artificial Neural Networks to Identify Alzheimer’s Disease Using Cerebral Perfusion SPECT Data. Int. J. Environ. Res. Public Health 2019, 16, 1303. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Świetlik, D.; Białowąs, J.; Moryś, J.; Kusiak, A. Computer Model of Synapse Loss During an Alzheimer’s Disease-Like Pathology in Hippocampal Subregions DG, CA3 and CA1—The Way to Chaos and Information Transfer. Entropy 2019, 21, 408. https://doi.org/10.3390/e21040408
Świetlik D, Białowąs J, Moryś J, Kusiak A. Computer Model of Synapse Loss During an Alzheimer’s Disease-Like Pathology in Hippocampal Subregions DG, CA3 and CA1—The Way to Chaos and Information Transfer. Entropy. 2019; 21(4):408. https://doi.org/10.3390/e21040408
Chicago/Turabian StyleŚwietlik, Dariusz, Jacek Białowąs, Janusz Moryś, and Aida Kusiak. 2019. "Computer Model of Synapse Loss During an Alzheimer’s Disease-Like Pathology in Hippocampal Subregions DG, CA3 and CA1—The Way to Chaos and Information Transfer" Entropy 21, no. 4: 408. https://doi.org/10.3390/e21040408
APA StyleŚwietlik, D., Białowąs, J., Moryś, J., & Kusiak, A. (2019). Computer Model of Synapse Loss During an Alzheimer’s Disease-Like Pathology in Hippocampal Subregions DG, CA3 and CA1—The Way to Chaos and Information Transfer. Entropy, 21(4), 408. https://doi.org/10.3390/e21040408




