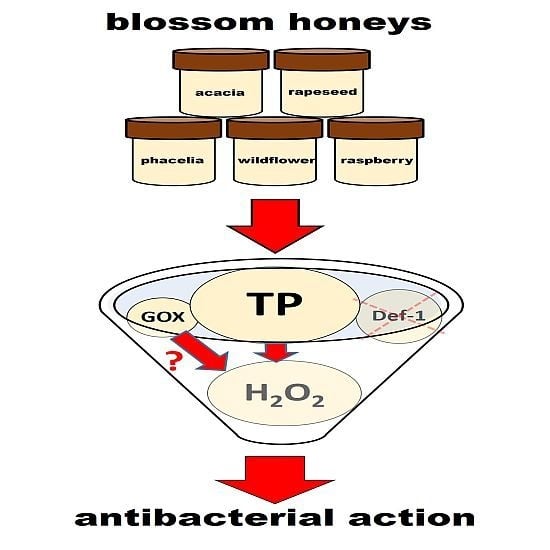
Antibacterial Activity of Different Blossom Honeys: New Findings
Abstract
1. Introduction
2. Results
2.1. Characterization of Pollen Profile in Different Honey Types
2.1.1. Acacia Honeys
2.1.2. Wildflower Honeys
2.1.3. Rapeseed Honeys
2.1.4. Raspberry and Phacelia Honeys
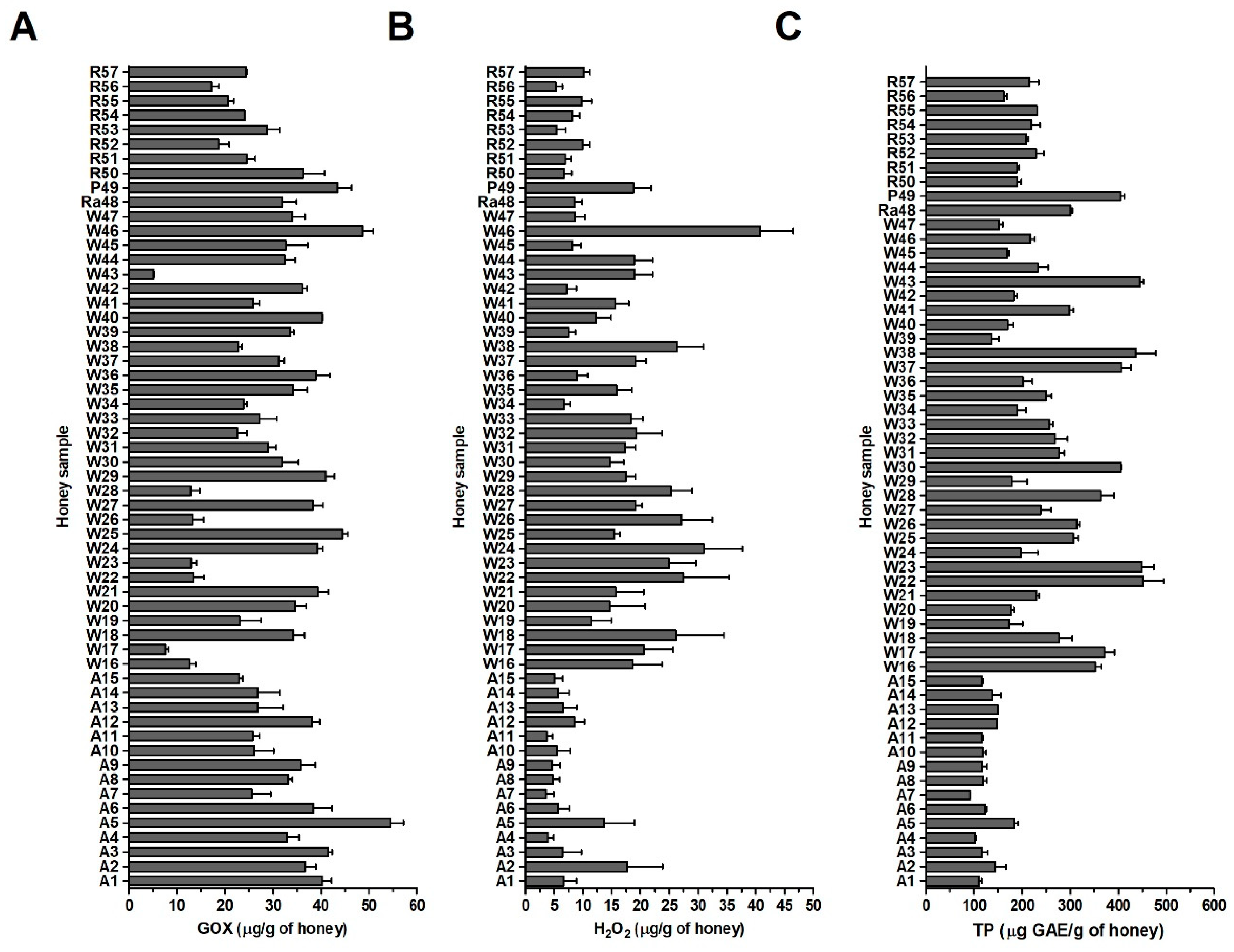
2.2. Antibacterial Activity of Blossom Honey Samples
2.3. GOX Content and Antibacterial Activity of Honey Samples
2.4. H2O2 and Total Polyphenol Contents in Honey Samples
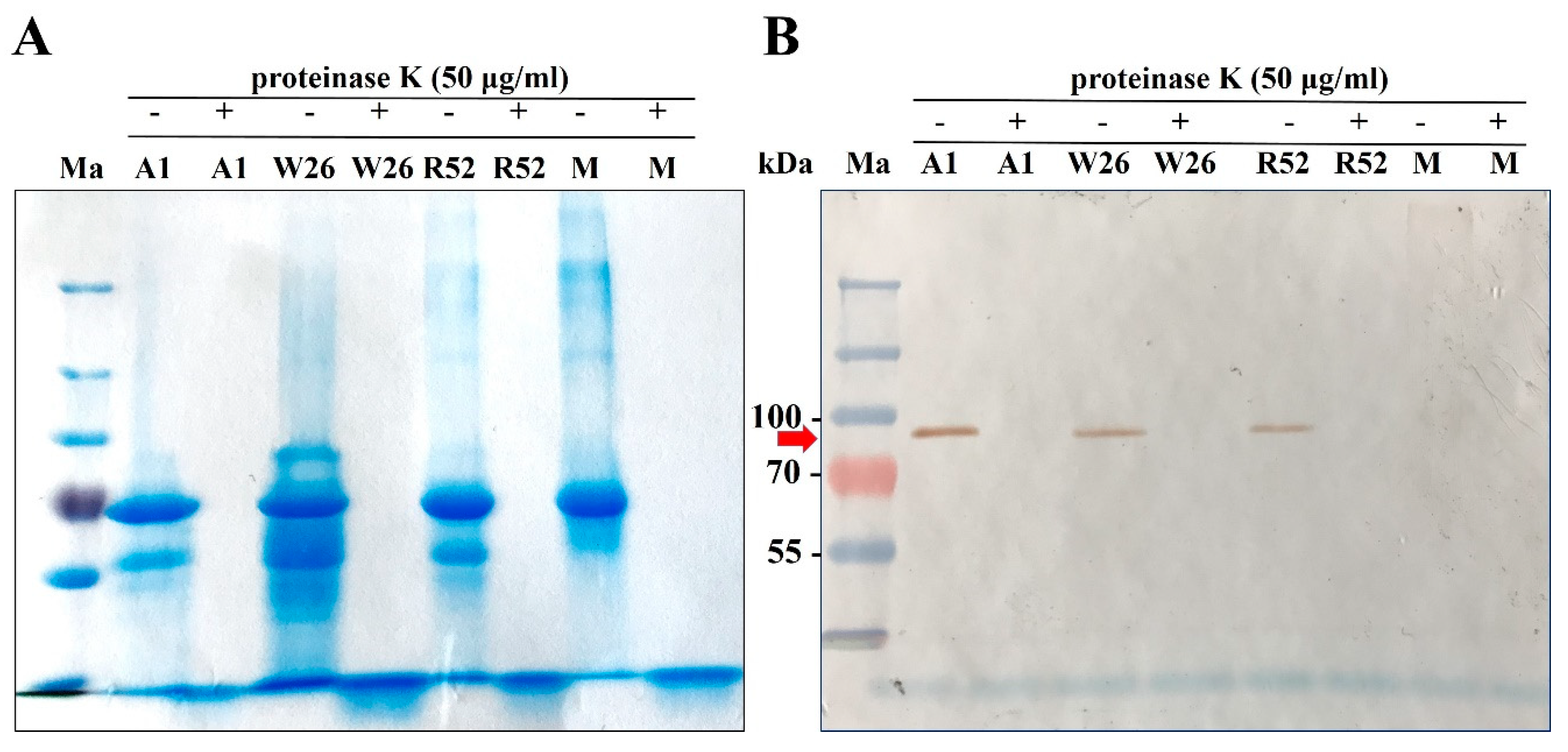
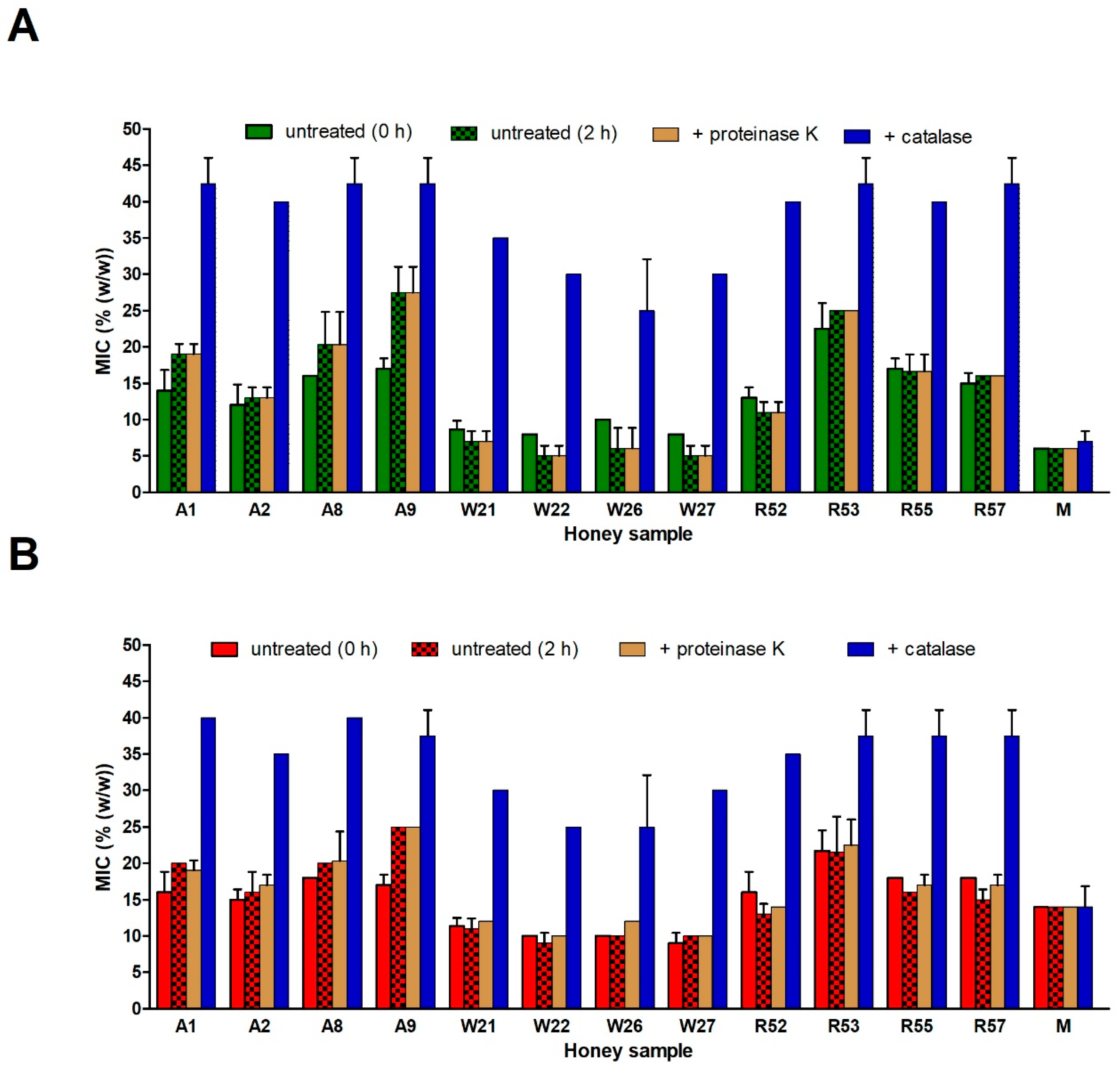
2.5. Role of Honey Proteins and H2O2 in the Antibacterial Effect of Blossom Honeys
2.6. Role of Polyphenols in the Antibacterial Effect of Blossom Honeys
3. Discussion
4. Materials and Methods
4.1. Honey Samples
4.2. Microorganisms
4.3. Strains, Vectors, Enzymes, and Reagents Used for GOX Gene Cloning
4.4. Construction of the GOX Expression Plasmid and Expression of Recombinant GOX (rGOX)
4.5. Purification of rGOX
4.6. Melissopalynological Analysis
4.7. Determination of Honey Antibacterial Activity
4.8. Determination of GOX Content
4.9. Determination of H2O2 Content
4.10. Determination of Total Polyphenolic Content
4.11. Enzymatic Treatment of Honey Samples with Catalase and Proteinase K
4.12. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Molan, P.C. The evidence supporting the use of honey as a wound dressing. Int. J. Low. Extrem. Wounds 2006, 5, 40–54. [Google Scholar] [CrossRef]
- Adams, C.J.; Boult, C.H.; Deadman, B.J.; Farr, J.M.; Grainger, M.N.C.; Manley-Harris, M.; Snow, M.J. Isolation by HPLC and characterisation of the bioactive fraction of New Zealand manuka (Leptospermum scoparium) honey. Carbohydr. Res. 2009, 343, 651–659. [Google Scholar] [CrossRef]
- Mavric, E.; Wittmann, S.; Barth, G.; Henle, T. Identification and quantification of methylglyoxal as the dominant antibacterial constituent of Manuka (Leptospermum scoparium) honeys from New Zealand. Mol. Nutr. Food Res. 2008, 52, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Kwakman, P.H.; te Velde, A.A.; De Boer, L.; Speijer, D.; Vandenbroucke-Grauls, C.M.; Zaat, S.A. How honey kills bacteria. FASEB J. 2010, 24, 2576–2582. [Google Scholar] [CrossRef] [PubMed]
- Kwakman, P.H.; Van der Akker, J.P.; Guclu, A.; Aslami, H.; Binnekade, J.M.; de Boer, L.; Boszhard, L.; Paulus, F.; Middelhoek, P.; te Velde, A.A.; et al. Medical-grade honey kills antibiotic-resistant bacteria in vitro and eradicates skin colonization. Clin. Infect. Dis. 2008, 46, 1677–1682. [Google Scholar] [CrossRef] [PubMed]
- Jull, A.; Walker, N.; Parag, V.; Molan, P.; Rodgers, A.; Honey as Adjuvant Leg Ulcer Therapy trial collaborators. Randomized clinical trial of honey-impregnated dressings for venous leg ulcers. Br. J. Surg. 2008, 95, 175–182. [Google Scholar] [CrossRef]
- Semprini, A.; Braithwaite, I.; Corin, A.; Sheahan, D.; Tofield, C.; Helm, C.; Montgomery, B.; Fingleton, J.; Weatherall, M.; Beasley, R. Randomised controlled trial of topical kanuka honey for the treatment of acne. BMJ Open 2016, 6, e009448. [Google Scholar] [CrossRef]
- Kwakman, P.H.; Te Velde, A.A.; De Boer, L.; Vandenbroucke-Grauls, C.M.; Zaat, S.A. Two major medicinal honeys have different mechanisms of bactericidal activity. PLoS ONE 2011, 6, e17709. [Google Scholar] [CrossRef] [PubMed]
- Matzen, R.D.; Zinck Leth-Espensen, J.; Jansson, T.; Nielsen, D.S.; Lund, M.N.; Matzen, S. The antibacterial effect In vitro of honey derived from various Danish flora. Dermatol. Res. Pract. 2018, 2018, 7021713. [Google Scholar] [CrossRef]
- Bucekova, M.; Buriova, M.; Pekarik, L.; Majtan, V.; Majtan, J. Phytochemicals-mediated production of hydrogen peroxide is crucial for high antibacterial activity of honeydew honey. Sci. Rep. 2018, 8, 9061. [Google Scholar] [CrossRef]
- Poli, J.P.; Guinoiseau, E.; Luciani, A.; Yang, Y.; Battesti, M.J.; Paolini, J.; Costa, J.; Quilichini, Y.; Berti, L.; Lorenzi, V. Key role of hydrogen peroxide in antimicrobial activity of spring, Honeydew maquis and chestnut grove Corsican honeys on Pseudomonas aeruginosa DNA. Lett. Appl. Microbiol. 2018, 66, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Grecka, K.; Kuś, P.M.; Worobo, R.W.; Szweda, P. Study of the anti-staphylococcal potential of honeys produced in Northern Poland. Molecules 2018, 23, 260. [Google Scholar] [CrossRef] [PubMed]
- Stagos, D.; Soulitsiotis, N.; Tsadila, C.; Papaeconomou, S.; Arvanitis, C.; Ntontos, A.; Karkanta, F.; Adamou-Androulaki, S.; Petrotos, K.; Spandidos, D.A.; et al. Antibacterial and antioxidant activity of different types of honey derived from Mount Olympus in Greece. Int. J. Mol. Med. 2018, 42, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Brudzynski, K.; Abubaker, A.; St-Martin, L.; Castle, A. Re-examining the role of hydrogen peroxide in bacteriostatic and bactericidal activities of honey. Front. Microbiol. 2011, 2, 1–9. [Google Scholar] [CrossRef] [PubMed]
- White, J.W.; Subers, M.H.; Schepartz, A.I. The identification of inhibine, the antibacterial factor in honey, as hydrogenperoxide and its origin in a honey glucose-oxidase system. Biochim. Biophys. Acta 1963, 73, 57–70. [Google Scholar] [CrossRef]
- Roshan, N.; Rippers, T.; Locher, C.; Hammer, K.A. Antibacterial activity and chemical characteristics of several Western Australian honeys compared to manuka honey and pasture honey. Arch. Microbiol. 2017, 199, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Juven, B.J.; Pierson, M.D. Antibacterial effects of hydrogen peroxide and methods for its detection and quantitation. J. Food Prot. 1996, 59, 1233–1241. [Google Scholar] [CrossRef]
- Brudzynski, K.; Lannigan, R. Mechanism of honey bacteriostatic action against MRSA and VRE involves hydroxyl radicals generated from honey’s hydrogen peroxide. Front. Microbiol. 2012, 3, 1–8. [Google Scholar] [CrossRef]
- Persano Oddo, L.; Piana, M.L.; Ricciardelli D'Albore, G. I Miele Regionali Italiani. Caratterizzazione Melissopalinologica; Ministero delle Politiche Agricole Alimentari e Forestali; C.R.A. ístituto Sperimentale per la Zoologia Agraria, Sezione di Apicoltura: Roma, Italy, 2007. [Google Scholar]
- Bucekova, M.; Valachova, I.; Kohutova, L.; Prochazka, E.; Klaudiny, J.; Majtan, J. Honeybee glucose oxidase —its expression in honeybee workers and comparative analyses of its content and H2O2-mediated antibacterial activity in natural honeys. Naturwissenschaften 2014, 101, 661–670. [Google Scholar] [CrossRef]
- Sakihama, Y.; Cohen, M.F.; Grace, S.C.; Yamasaki, H. Plant phenolic antioxidant and prooxidant activities: Phenolics-induced oxidative damage mediated by metals in plants. Toxicology 2002, 177, 67–80. [Google Scholar] [CrossRef]
- Akagawa, M.; Shigemitsu, T.; Suyama, K. Production of hydrogen peroxide by polyphenols and polyphenol-rich beverages under quasi-physiological conditions. Biosci. Biotechnol. Biochem. 2003, 67, 2632–2640. [Google Scholar] [CrossRef]
- Brudzynski, K.; Abubaker, K.; Miotto, D. Unraveling a mechanism of honey antibacterial action: Polyphenol/H₂O₂-induced oxidative effect on bacterial cell growth and on DNA degradation. Food Chem. 2012, 133, 329–336. [Google Scholar] [CrossRef]
- Brudzynski, K. Effect of hydrogen peroxide on antibacterial activities of Canadian honeys. Can. J. Microbiol. 2006, 52, 1228–1237. [Google Scholar] [CrossRef]
- Dogan-Guner, E.M.; Mohamed, H.; Orbey, N.; Goodyear, N. Stabilization and controlled release of micro-encapsulated hydrogen peroxide for wound treatment applications. J. Appl. Microbiol. 2018, 126, 965–972. [Google Scholar]
- Sowa, P.; Grabek-Lejko, D.; Wesołowska, M.; Swacha, S.; Dżugan, M. Hydrogen peroxide-dependent antibacterial action of Melilotus albus honey. Lett. Appl. Microbiol. 2017, 65, 82–89. [Google Scholar] [CrossRef]
- Di Girolamo, F.; D'Amato, A.; Righetti, P.G. Assessment of the floral origin of honey via proteomic tools. J. Proteomics 2012, 75, 3688–3693. [Google Scholar] [CrossRef]
- Chua, L.S.; Lee, J.Y.; Chan, G.F. Characterization of the proteins in honey. Anal. Lett. 2015, 48, 697–709. [Google Scholar] [CrossRef]
- Borutinskaite, V.; Treigyte, G.; Matuzevicius, D.; Zaikova, I.; Ceksteryte, V.; Navakauskas, D.; Kurtinaitiene, B.; Navakauskiene, R. Proteomic analysis of pollen and blossom honey from rape seed Brassica napus L. J. Apic. Sci. 2017, 61, 73–92. [Google Scholar] [CrossRef]
- Azevedo, M.S.; Valentim-Neto, P.A.; Seraglio, S.K.T.; da Luz, C.F.P.; Arisi, A.C.M.; Costa, A.C.O. Proteome comparison for discrimination between honeydew and floral honeys from botanical species Mimosa scabrella Bentham by principal component analysis. J. Sci. Food Agric. 2017, 97, 4515–4519. [Google Scholar] [CrossRef]
- Gismondi, A.; De Rossi, S.; Canuti, L.; Novelli, S.; Di Marco, G.; Fattorini, L.; Canini, A. From Robinia pseudoacacia L. nectar to acacia monofloral honey: Biochemical changes and variation of biological properties. J. Sci. Food Agric. 2018, 98, 4312–4322. [Google Scholar] [CrossRef] [PubMed]
- Borutinskaite, V.; Treigyte, G.; Ceksteryte, V.; Kurtinaitiene, B.; Navakauskiene, R. Proteomic identification and enzymatic activity of buckwheat (Fagopyrum esculentum) honey based on different assays. J. Food Nutr. Res. 2018, 57, 57–69. [Google Scholar]
- Gašić, U.M.; Milojković-Opsenica, D.M.; Tešić, Ž.L. Polyphenols as possible markers of botanical origin of honey. J. AOAC Int. 2017, 100, 852–861. [Google Scholar] [CrossRef] [PubMed]
- Al-Mamary, M.; Al-Meeri, A.; Al-Habori, M. Antioxidant activities and total phenolics of different types of honey. Nutr. Res. 2002, 22, 1041–1047. [Google Scholar] [CrossRef]
- Gheldof, N.; Engeseth, N.J. Antioxidant capacity of honeys from various floral sources based on the determination of oxygen radical absorbance capacity and inhibition of in vitro lipoprotein oxidation in human serum samples. J. Agric. Food Chem. 2002, 50, 3050–3055. [Google Scholar] [CrossRef] [PubMed]
- Kenjeric, D.; Mandic, M.L.; Primorac, L.; Bubalo, D.; Perl, A. Flavonoid profile of Robinia honeys produced in Croatia. Food Chem. 2007, 102, 683–690. [Google Scholar] [CrossRef]
- Dżugan, M.; Tomczyk, M.; Sowa, P.; Grabek-Lejko, D. Antioxidant activity as biomarker of honey variety. Molecules 2018, 23, 2069. [Google Scholar] [CrossRef]
- Alzahrani, H.A.; Alsabehi, R.; Boukraâ, L.; Abdellah, F.; Bellik, Y.; Bakhotmah, B.A. Antibacterial and antioxidant potency of floral honeys from different botanical and geographical origins. Molecules 2012, 17, 10540–10549. [Google Scholar] [CrossRef]
- Grzesik, M.; Bartosz, G.; Stefaniuk, I.; Pichla, M.; Namieśnik, J.; Sadowska-Bartosz, I. Dietary antioxidants as a source of hydrogen peroxide. Food Chem. 2019, 278, 692–699. [Google Scholar] [CrossRef]
- Valachova, I.; Bucekova, M.; Majtan, J. Quantification of bee-derived defensin-1 in honey by competitive enzyme-linked immunosorbent assay, a new approach in honey quality control. Czech J. Food Sci. 2016, 34, 233–243. [Google Scholar]
- Sojka, M.; Valachova, I.; Bucekova, M.; Majtan, J. Antibiofilm efficacy of honey and bee-derived defensin-1 on multispecies wound biofilm. J. Med. Microbiol. 2016, 65, 337–344. [Google Scholar] [CrossRef]
- Bucekova, M.; Sojka, M.; Valachova, I.; Martinotti, S.; Ranzato, E.; Szep, Z.; Majtan, V.; Klaudiny, J.; Majtan, J. Bee-derived antibacterial peptide, defensin-1, promotes wound re-epithelialisation in vitro and in vivo. Sci. Rep. 2017, 7, 7340. [Google Scholar] [CrossRef]
- McCarty, S.M.; Percival, S.L. Proteases and delayed wound healing. Adv. Wound Care 2013, 2, 438–447. [Google Scholar] [CrossRef] [PubMed]
- McCrudden, M.T.C.; McLean, D.T.F.; Zhou, M.; Shaw, J.; Linden, G.J.; Irwin, C.R.; Lundy, F.T. The host defence peptide LL-37 is susceptible to proteolytic degradation by wound fluid isolated from foot ulcers of diabetic patients. Int. J. Pept. Res. Ther. 2014, 20, 457–464. [Google Scholar] [CrossRef]
- Jull, A.B.; Cullum, N.; Dumville, J.C.; Westby, M.J.; Deshpande, S.; Walker, N. Honey as a topical treatment for wounds. Cochrane Databse. Syst. Rev. 2015, 3, CD005083. [Google Scholar] [CrossRef]
- Cooper, R. Impact of honey as a topical treatment for wounds remains unclear. Evid. Based Med. 2014, 19, 11. [Google Scholar] [CrossRef] [PubMed]
- Majtan, J.; Majtan, V. Is manuka honey the best type of honey for wound care? J. Hosp. Infect. 2010, 73, 305–306. [Google Scholar] [CrossRef]
- Von der Ohe, W.; Persano Oddo, L.; Piana, L.M.; Morlot, M.; Martin, P. Harmonized methods of melissopalynology. Apidologie 2004, 35, S18–S25. [Google Scholar] [CrossRef]
- Di Marco, G.; Manfredini, A.; Leonardi, D.; Canuti, L.; Impei, S.; Gismondi, A.; Canini, A. Geographical, botanical and chemical profile of monofloral Italian honeys as food quality guarantee and territory brand. Plant Biosyst. 2016, 151, 450–463. [Google Scholar] [CrossRef]
- Blair, S.E.; Cokcetin, N.N.; Harry, E.J.; Carter, D.A. The unusual antibacterial activity of medical-grade Leptospermum honey: Antibacterial spectrum, resistance and transcriptome analysis. Eur. J. Clin. Microbiol. Infect. Dis. 2009, 28, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the honeys are available from the authors. |






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bucekova, M.; Jardekova, L.; Juricova, V.; Bugarova, V.; Di Marco, G.; Gismondi, A.; Leonardi, D.; Farkasovska, J.; Godocikova, J.; Laho, M.; et al. Antibacterial Activity of Different Blossom Honeys: New Findings. Molecules 2019, 24, 1573. https://doi.org/10.3390/molecules24081573
Bucekova M, Jardekova L, Juricova V, Bugarova V, Di Marco G, Gismondi A, Leonardi D, Farkasovska J, Godocikova J, Laho M, et al. Antibacterial Activity of Different Blossom Honeys: New Findings. Molecules. 2019; 24(8):1573. https://doi.org/10.3390/molecules24081573
Chicago/Turabian StyleBucekova, Marcela, Lucia Jardekova, Valeria Juricova, Veronika Bugarova, Gabriele Di Marco, Angelo Gismondi, Donatella Leonardi, Jarmila Farkasovska, Jana Godocikova, Maros Laho, and et al. 2019. "Antibacterial Activity of Different Blossom Honeys: New Findings" Molecules 24, no. 8: 1573. https://doi.org/10.3390/molecules24081573
APA StyleBucekova, M., Jardekova, L., Juricova, V., Bugarova, V., Di Marco, G., Gismondi, A., Leonardi, D., Farkasovska, J., Godocikova, J., Laho, M., Klaudiny, J., Majtan, V., Canini, A., & Majtan, J. (2019). Antibacterial Activity of Different Blossom Honeys: New Findings. Molecules, 24(8), 1573. https://doi.org/10.3390/molecules24081573