Gypenoside LXXV Promotes Cutaneous Wound Healing In Vivo by Enhancing Connective Tissue Growth Factor Levels Via the Glucocorticoid Receptor Pathway
Abstract
:1. Introduction
2. Results
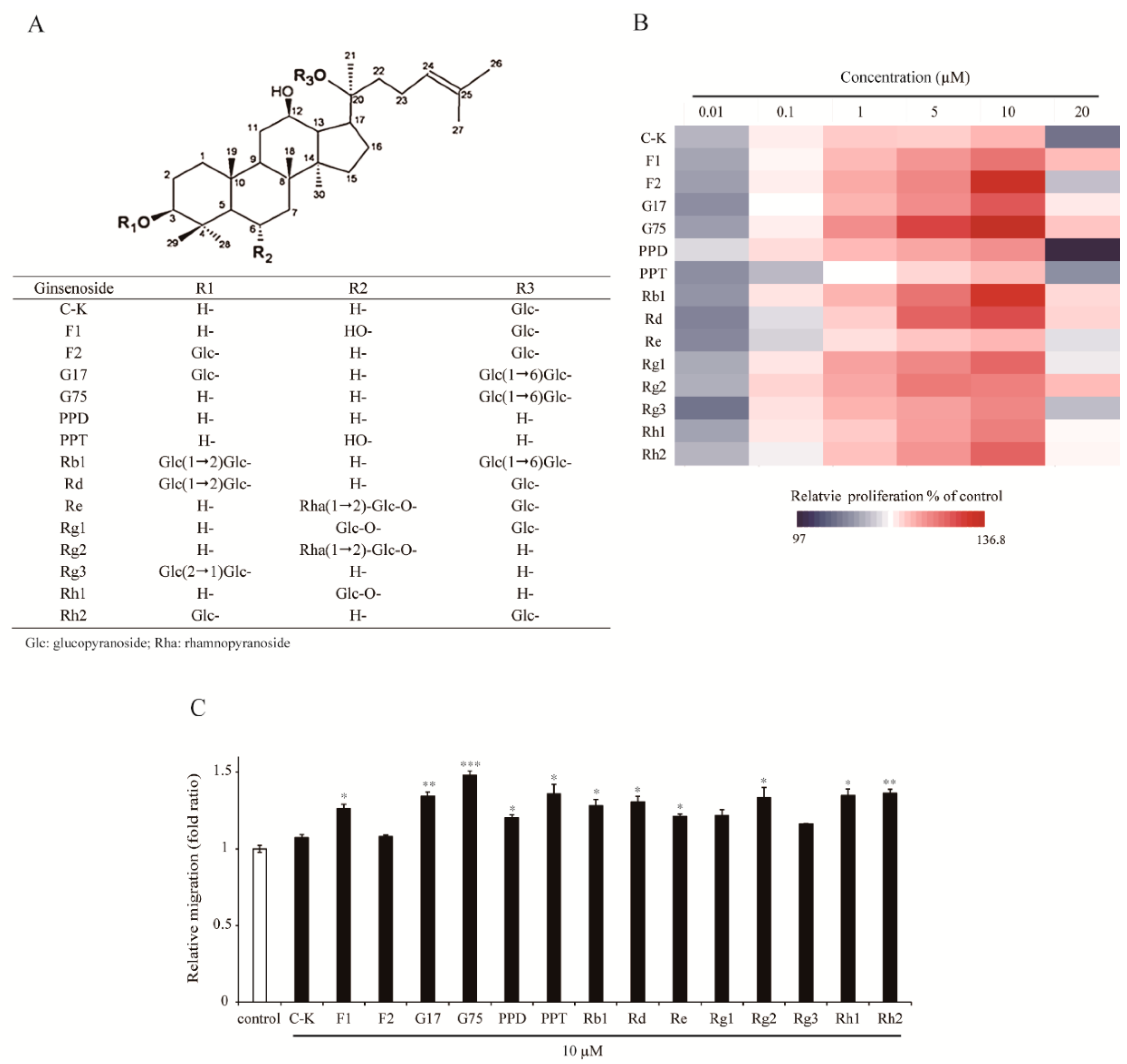
2.1. Effect of Different Types of Ginsenosides on the Proliferation and Migration of HaCaT Keratinocytes
2.2. Effect of G75 on Wound Healing In Vitro
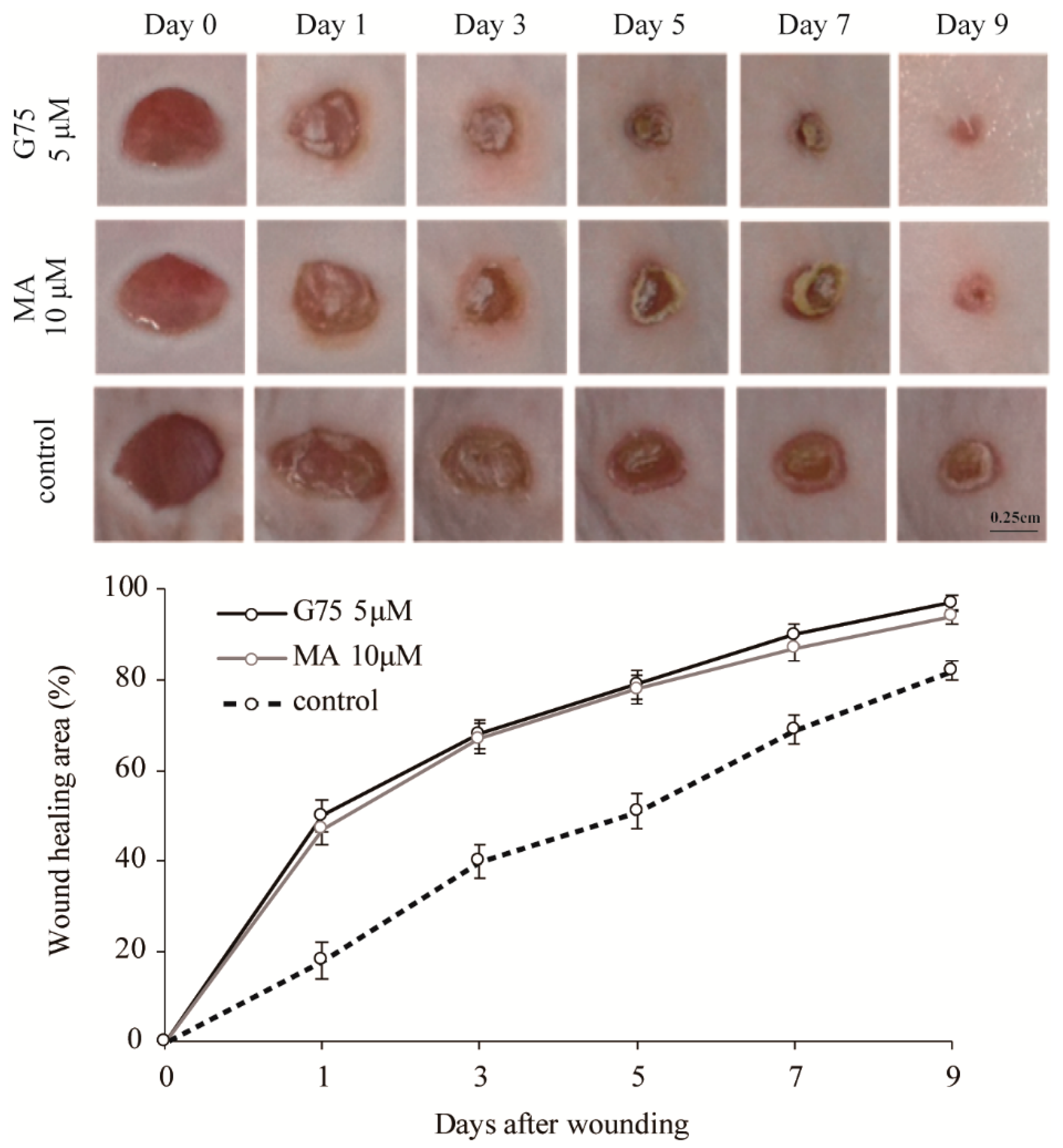
2.3. Effect of G75 on Wound Healing In Vivo
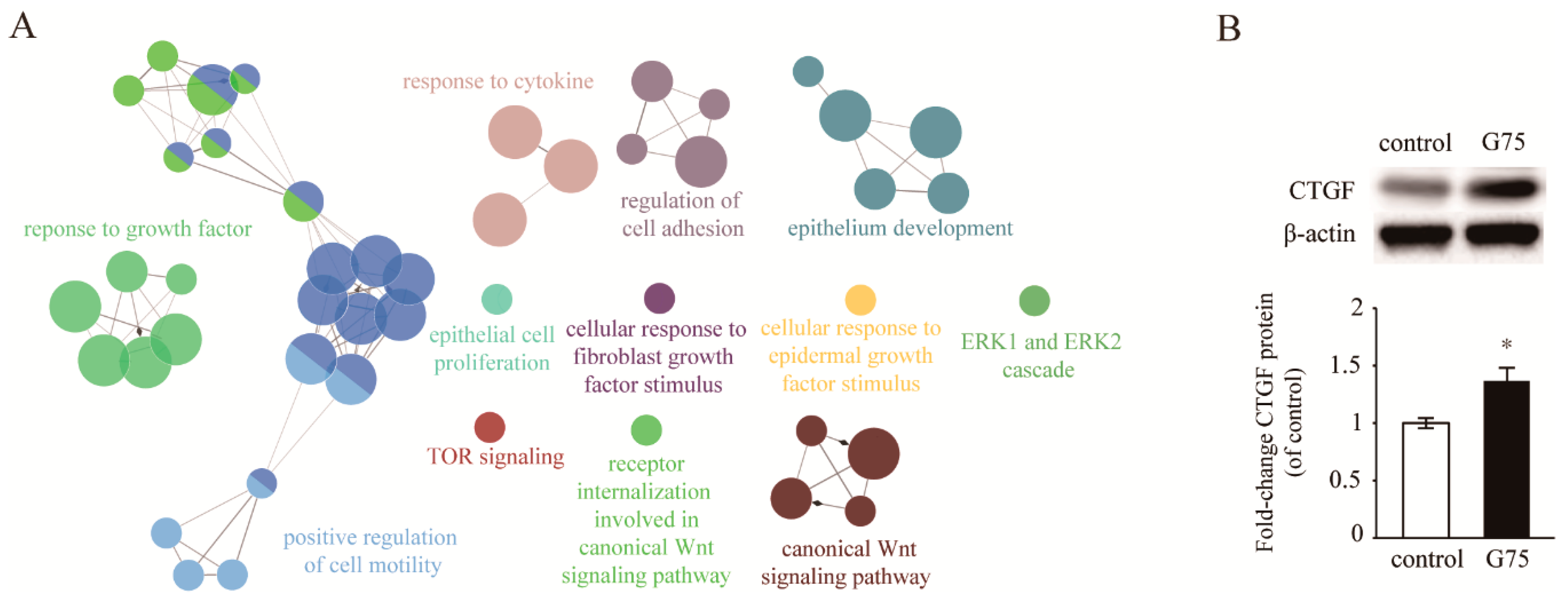
2.4. Effect of G75 on Biological Processes in Wound Healing
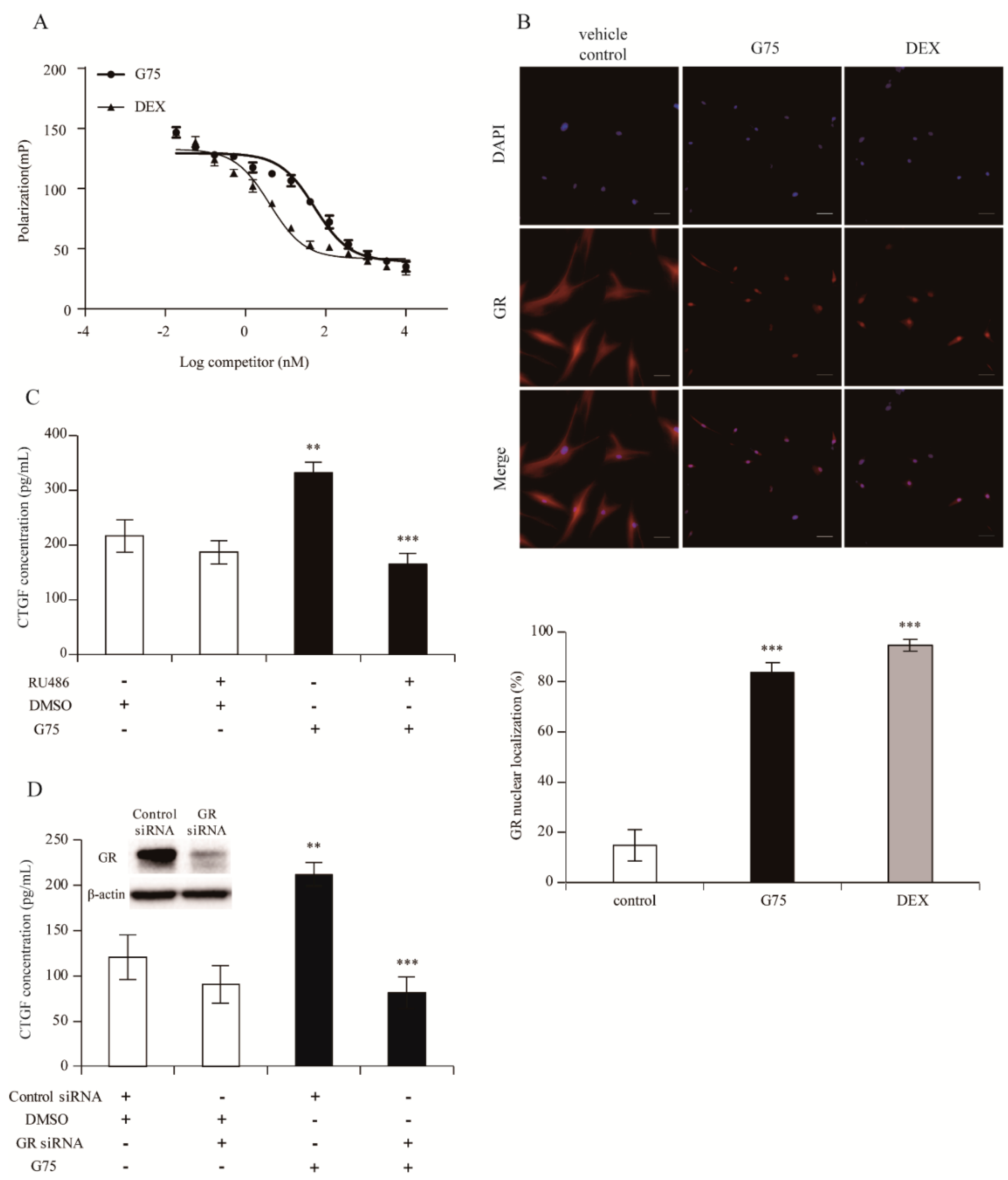
2.5. G75 Induces CTGF Production Via GR Pathway in Fibroblasts
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Reagents
4.2. Cell Proliferation Assay
4.3. Scratch Wound Closure Assay
4.4. Animals and Excisional Wound Mouse Model
4.5. NGS Analysis
4.6. Western Blot Analysis
4.7. ELISA
4.8. GR Binding Affinity Assay
4.9. GR Translocation Assay
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sabol, F.; Dancakova, L.; Gal, P.; Vasilenko, T.; Novotny, M.; Smetana, K.; Lenhardt, L. Immunohistological changes in skin wounds during the early periods of healing in a rat model. Vet. Med. 2012, 57, 77–82. [Google Scholar] [CrossRef] [Green Version]
- Sorg, H.; Tilkorn, D.J.; Hager, S.; Hauser, J.; Mirastschijski, U. Skin Wound Healing: An Update on the Current Knowledge and Concepts. Eur. Surg. Res. 2017, 58, 81–94. [Google Scholar] [CrossRef]
- Grazul-Bilska, A.T.; Johnson, M.L.; Bilski, J.J.; Redmer, D.A.; Reynolds, L.P.; Abdullah, A.; Abdullah, K.M. Wound healing: The role of growth factors. Drugs Today 2003, 39, 787–800. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.C.; Costa, T.F.; Andrade, Z.A.; Medrado, A.R. Wound healing—A literature review. An. Bras. Dermatol. 2016, 91, 614–620. [Google Scholar] [CrossRef]
- Qing, C. The molecular biology in wound healing & non-healing wound. Chin. J. Traumatol. 2017, 20, 189–193. [Google Scholar]
- Pastar, I.; Stojadinovic, O.; Yin, N.C.; Ramirez, H.; Nusbaum, A.G.; Sawaya, A.; Patel, S.B.; Khalid, L.; Isseroff, R.R.; Tomic-Canic, M. Epithelialization in Wound Healing: A Comprehensive Review. Adv. Wound Care 2014, 3, 445–464. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.K.; Mendis, E. Bioactive compounds from marine processing byproducts-A review. Food Res. Int. 2006, 39, 383–393. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, H.L.; Lee, M.H.; You, K.E.; Kwon, B.J.; Seo, H.J.; Park, J.C. Asiaticoside enhances normal human skin cell migration, attachment and growth in vitro wound healing model. Phytomedicine 2012, 19, 1223–1227. [Google Scholar] [CrossRef]
- Hou, J.; Kim, S. Possible role of ginsenoside Rb1 in skin wound healing via regulating senescent skin dermal fibroblast. Biochem. Biophys. Res. Commun. 2018, 499, 381–388. [Google Scholar] [CrossRef]
- Baeg, I.H.; So, S.H. The world ginseng market and the ginseng. J. Ginseng Res. 2013, 37, 1–7. [Google Scholar] [CrossRef]
- Lee, D.Y.; Lee, Y.H.; Yang, D.C. Physicochemical Characterization and NMR Assignments of Ginsenosides Rb1, Rb2, Rc, and Rd Isolated from Panax ginseng. J. Ginseng Res. 2010, 34, 113–121. [Google Scholar] [Green Version]
- Sun, M.; Ye, Y.; Xiao, L.; Duan, X.; Zhang, Y.; Zhang, H. Anticancer effects of ginsenoside Rg3. Int. J. Mol. Med. 2017, 39, 507–518. [Google Scholar] [CrossRef]
- Nie, L.; Xia, J.; Li, H.; Zhang, Z.; Yang, Y.; Huang, X.; He, Z.; Liu, J.; Yang, X. Ginsenoside Rg1 Ameliorates Behavioral Abnormalities and Modulates the Hippocampal Proteomic Change in Triple Transgenic Mice of Alzheimer’s Disease. Oxid. Med. Cell. Longev. 2017, 2017, 6473506. [Google Scholar] [CrossRef]
- Lee, I.S.; Uh, I.; Kim, K.S.; Kim, K.H.; Park, J.; Kim, Y.; Jung, J.H.; Jung, H.J.; Jang, H.J. Anti-Inflammatory Effects of Ginsenoside Rg3 via NF-κB Pathway in A549 Cells and Human Asthmatic Lung Tissue. J. Immunol. Res. 2016, 2016, 7521601. [Google Scholar] [CrossRef]
- Kimura, Y.; Sumiyoshi, M.; Kawahira, K.; Sakanaka, M. Effects of ginseng saponins isolated from Red Ginseng roots on burn wound healing in mice. Br. J. Pharmacol. 2006, 148, 860–870. [Google Scholar] [CrossRef] [Green Version]
- Kim, W.K.; Song, S.Y.; Oh, W.K.; Kaewsuwan, S.; Tran, T.L.; Kim, W.S.; Sung, J.H. Wound-healing effect of ginsenoside Rd from leaves of Panax ginseng via cyclic AMP-dependent protein kinase pathway. Eur. J. Pharmacol. 2013, 702, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.H.; Kim, D.J.; Jung, S.C.; Kim, S.C.; Im, W.T. Enhanced Production of Gypenoside LXXV Using a Novel Ginsenoside-Transforming β-Glucosidase from Ginseng-Cultivating Soil Bacteria and Its Anti-Cancer Property. Molecules 2017, 22, 844. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Bian, D.; Xia, Y.; Gong, Z.; Tan, Q.; Chen, J.; Dai, Y. Identification of Major Active Ingredients Responsible for Burn Wound Healing of Centella asiatica Herbs. Evid.-Based Complement. Altern. Med. 2012, 2012, 848093. [Google Scholar] [CrossRef]
- Liu, M.; Dai, Y.; Li, Y.; Luo, Y.; Huang, F.; Gong, Z.; Meng, Q. Madecassoside Isolated from Centella asiatica Herbs Facilitates Burn Wound Healing in Mice. Planta Med. 2008, 74, 809–815. [Google Scholar] [CrossRef]
- Bindea, G.; Mlecnik, B.; Hackl, H.; Charoentong, P.; Tosolini, M.; Kirilovsky, A.; Fridman, W.H.; Pagès, F.; Trajanoski, Z.; Galon, J. ClueGO: A Cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics 2009, 25, 1091–1093. [Google Scholar] [CrossRef]
- Dammeier, J.; Beer, H.D.; Brauchle, M.; Werner, S. Dexamethasone is a novel potent inducer of connective tissue growth factor expression. Implications for glucocorticoid therapy. J. Biol. Chem. 1998, 273, 18185–18190. [Google Scholar] [CrossRef]
- Attele, A.S.; Wu, J.A.; Yuan, C.S. Ginseng pharmacology: Multiple constituents and multiple actions. Biochem. Pharmacol. 1999, 58, 1685–1693. [Google Scholar] [CrossRef]
- Chen, X. Cardiovascular protection by ginsenosides and their nitric oxide releasing action. Clin. Exp. Pharmacol. Physiol. 1996, 23, 728–732. [Google Scholar] [CrossRef]
- Gillis, C.N. Panax ginseng pharmacology: A nitric oxide link? Biochem. Pharmacol. 1997, 54, 1–8. [Google Scholar] [CrossRef]
- Nag, S.A.; Qin, J.; Wang, W.; Wang, M.H.; Wang, H.; Zhang, R. Ginsenosides as anticancer agents: In vitro and in vivo activities, structure–activity relationships, and molecular mechanisms of action. Front. Pharmacol. 2012, 3, 25. [Google Scholar] [CrossRef]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic Canic, M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008, 16, 585–601. [Google Scholar] [CrossRef]
- Werner, S.; Grose, R. Regulation of Wound Healing by Growth Factors and Cytokines. Physiol Rev. 2003, 83, 835–870. [Google Scholar] [CrossRef] [Green Version]
- Pastore, S.; Mascia, F.; Mariani, V.; Girolomoni, G. The Epidermal Growth Factor Receptor System in Skin Repair and Inflammation. J. Invest. Dermatol. 2008, 128, 1365–1374. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Cui, W.; Qiu, W.; Zhu, M.; Zhao, R.; Zeng, D.; Dong, C.; Wang, X.; Guo, W.; Xing, W.; et al. Impaired wound healing results from the dysfunction of the Akt/mTOR pathway in diabetic rats. J. Dermatol. Sci. 2015, 79, 241–251. [Google Scholar] [CrossRef]
- Carre, A.L.; Hu, M.S.; James, A.W.; Kawai, K.; Galvez, M.G.; Longaker, M.T.; Lorenz, H.P. β-Catenin-Dependent Wnt Signaling: A Pathway in Acute Cutaneous Wounding. Plast. Reconstr. Surg. 2018, 141, 669–678. [Google Scholar] [CrossRef]
- Huang, C. MAP kinases and cell migration. J. Cell Sci. 2004, 117, 4619–4628. [Google Scholar] [CrossRef] [Green Version]
- Houschyar, K.S.; Momeni, A.; Pyles, M.N.; Maan, Z.N.; Whittam, A.J.; Siemers, F. Wnt signaling induces epithelial differentiation during cutaneous wound healing. Organogenesis 2015, 11, 95–104. [Google Scholar] [CrossRef] [Green Version]
- Squarize, C.H.; Castilho, R.M.; Bugge, T.H.; Gutkind, J.S. Accelerated wound healing by mTOR activation in genetically defined mouse models. PLoS ONE 2010, 5, e10643. [Google Scholar] [CrossRef] [PubMed]
- Greenhalgh, D.G. The Role of Growth Factors in Wound Healing. J. Trauma Acute Care Surg. 1996, 41, 159–167. [Google Scholar] [CrossRef]
- Borena, B.M.; Martens, A.; Broeckx, S.Y.; Meyer, E.; Chiers, K.; Duchateau, L.; Spaas, J.H. Regenerative Skin Wound Healing in Mammals: State-of-the-Art on Growth Factor and Stem Cell Based Treatments. Cell. Physiol. Biochem. 2015, 36, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Sherbet, G.V. Growth Factors and Their Receptors in Cell Differentiation, Cancer and Cancer Therapy; Elsevier: Amsterdam, The Netherlands, 2011; pp. 105–110. [Google Scholar]
- Henshaw, F.R.; Boughton, P.; Lo, L.; Mclennan, S.V.; Twigg, S.M. Topically Applied Connective Tissue Growth Factor/CCN2 Improves Diabetic Preclinical Cutaneous Wound Healing: Potential Role for CTGF in Human Diabetic Foot Ulcer Healing. J. Diabetes Res. 2015, 2015, 236238. [Google Scholar] [CrossRef]
- Leung, K.W.; Cheng, Y.K.; Mak, N.K.; Chan, K.K.C.; Fan, T.P.D.; Wong, R.N.S. Signaling pathway of ginsenoside-Rg1 leading to nitric oxide production in endothelial cells. FEBS Lett. 2006, 580, 3211–3216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leung, K.W.; Leung, F.P.; Huang, Y.; Mak, N.K.; Wong, R.N.S. Non-genomic effects of ginsenoside-Re in endothelial cells via glucocorticoid receptor. FEBS Lett. 2007, 581, 2423–2428. [Google Scholar] [CrossRef] [Green Version]
- Mohanan, P.; Subramaniyam, S.; Mathiyalagan, R.; Yang, D.C. Molecular signaling of ginsenosides Rb1, Rg1, and Rg3 and their mode of actions. J. Ginseng Res. 2018, 42, 123–132. [Google Scholar] [CrossRef]
- An, D.S.; Cui, C.H.; Siddiqi, M.Z.; Yu, H.S.; Jin, F.X.; Kim, S.G.; Im, W.T. Gram-Scale Production of Ginsenoside F1 Using a Recombinant Bacterial β-Glucosidase. J. Microbiol. Biotechnol. 2017, 27, 1559–1565. [Google Scholar]
- Du, J.; Cui, C.H.; Park, S.C.; Kim, J.K.; Yu, H.S.; Jin, F.-X.; Sun, C.; Kim, S.C.; Im, W.T. Identification and Characterization of a Ginsenoside-Transforming β-Glucosidase from Pseudonocardia sp. Gsoil 1536 and Its Application for Enhanced Production of Minor Ginsenoside Rg2(S). PLoS ONE 2014, 9, e96914. [Google Scholar] [CrossRef]
- Siddiqi, M.Z.; Cui, C.H.; Park, S.K.; Han, N.S.; Kim, S.C.; Im, W.T. Comparative analysis of the expression level of recombinant ginsenoside-transforming β-glucosidase in GRAS hosts and mass production of the ginsenoside Rh2-Mix. PLoS ONE 2017, 12, e0176098. [Google Scholar] [CrossRef]
- Cui, C.H.; Kim, S.C.; Im, W.T. Characterization of the ginsenoside-transforming recombinant β-glucosidase from Actinosynnema mirum and bioconversion of major ginsenosides into minor ginsenosides. Appl. Microbiol. Biotechnol. 2013, 97, 649–659. [Google Scholar] [CrossRef]
- Cui, C.H.; Liu, Q.M.; Kim, J.K.; Sung, B.H.; Kim, S.G.; Kim, S.C.; Im, W.T. Identification and Characterization of a Mucilaginibacter sp. Strain QM49-Glucosidase and Its Use in the Production of the Pharmaceutically Active Minor Ginsenosides (S)-Rh 1 and (S)-Rg 2. Appl. Environ. Microbiol. 2013, 79, 5788–5798. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.H.; Kim, J.K.; Kim, S.C.; Im, W.T. Characterization of a ginsenoside-transforming β-glucosidase from Paenibacillus mucilaginosus and its application for enhanced production of minor ginsenoside F2. PLoS ONE 2014, 9, 85727. [Google Scholar] [CrossRef]
- Kim, J.K.; Cui, C.H.; Liu, Q.; Yoon, M.H.; Kim, S.C.; Im, W.T. Mass production of the ginsenoside Rg3(S) through the combinative use of two glycoside hydrolases. Food Chem. 2013, 141, 1369–1377. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, T.; Yang, P.C. Western blot: Technique, theory, and trouble shooting. N. Am. J. Med. Sci. 2012, 4, 429–434. [Google Scholar] [PubMed]
- Mccloy, R.A.; Rogers, S.; Caldon, E.; Lorca, T.; Castro, A.; Burgess, A. Cell Cycle Partial inhibition of Cdk1 in G 2 phase overrides the SAC and decouples mitotic events. Cell Cycle 2014, 13, 1400–1412. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compound Gypenoside LXXV is available from the authors. |

| Growth Factors | Fold Change | p-Value |
|---|---|---|
| Ctgf (Connective tissue growth factor) | 1.879 | 0.022 * |
| Angpt1 (Angiopoietin 1) | 1.796 | 0.250 |
| Fgf7 (Fibroblast growth factor 7) | 1.789 | 0.070 |
| Fgf10 (Fibroblast growth factor 10) | 1.750 | 0.192 |
| Tgf alpha (Transforming growth factor alpha) | 1.504 | 0.278 |
| Tgf beta1 (Transforming growth factor beta 1) | 1.392 | 0.199 |
| Igf1 (Insulin-like growth factor 1) | 1.367 | 0.974 |
| Vegf alpha (Vascular endothelial growth factor alpha) | 1.281 | 0.343 |
| Hbegf (Heparin binding EFG like growth factor) | 1.267 | 0.685 |
| Hgf (Hepatocyte growth factor) | 1.191 | 0.826 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.; Ko, E.; Lee, J.H.; Song, Y.; Cui, C.-H.; Hou, J.; Jeon, B.M.; Kim, H.S.; Kim, S.C. Gypenoside LXXV Promotes Cutaneous Wound Healing In Vivo by Enhancing Connective Tissue Growth Factor Levels Via the Glucocorticoid Receptor Pathway. Molecules 2019, 24, 1595. https://doi.org/10.3390/molecules24081595
Park S, Ko E, Lee JH, Song Y, Cui C-H, Hou J, Jeon BM, Kim HS, Kim SC. Gypenoside LXXV Promotes Cutaneous Wound Healing In Vivo by Enhancing Connective Tissue Growth Factor Levels Via the Glucocorticoid Receptor Pathway. Molecules. 2019; 24(8):1595. https://doi.org/10.3390/molecules24081595
Chicago/Turabian StylePark, Sungjoo, Eunsu Ko, Jun Hyoung Lee, Yoseb Song, Chang-Hao Cui, Jingang Hou, Byeong Min Jeon, Hun Sik Kim, and Sun Chang Kim. 2019. "Gypenoside LXXV Promotes Cutaneous Wound Healing In Vivo by Enhancing Connective Tissue Growth Factor Levels Via the Glucocorticoid Receptor Pathway" Molecules 24, no. 8: 1595. https://doi.org/10.3390/molecules24081595
APA StylePark, S., Ko, E., Lee, J. H., Song, Y., Cui, C.-H., Hou, J., Jeon, B. M., Kim, H. S., & Kim, S. C. (2019). Gypenoside LXXV Promotes Cutaneous Wound Healing In Vivo by Enhancing Connective Tissue Growth Factor Levels Via the Glucocorticoid Receptor Pathway. Molecules, 24(8), 1595. https://doi.org/10.3390/molecules24081595






