Abstract
Legume seeds are rich sources of protein, fiber, and minerals. In addition, their phenolic compounds as secondary metabolites render health benefits beyond basic nutrition. Lowering apolipoprotein B secretion from HepG2 cells and decreasing the level of low-density lipoprotein (LDL)-cholesterol oxidation are mechanisms related to the prevention of cardiovascular diseases (CVD). Likewise, low-level chronic inflammation and related disorders of the immune system are clinical predictors of cardiovascular pathology. Furthermore, DNA-damage signaling and repair are crucial pathways to the etiology of human cancers. Along CVD and cancer, the prevalence of obesity and diabetes is constantly increasing. Screening the ability of polyphenols in inactivating digestive enzymes is a good option in pre-clinical studies. In addition, in vivo studies support the role of polyphenols in the prevention and/or management of diabetes and obesity. Soybean, a well-recognized source of phenolic isoflavones, exerts health benefits by decreasing oxidative stress and inflammation related to the above-mentioned chronic ailments. Similar to soybeans, chickpeas are good sources of nutrients and phenolic compounds, especially isoflavones. This review summarizes the potential of chickpea as a substitute for soybean in terms of health beneficial outcomes. Therefore, this contribution may guide the industry in manufacturing functional foods and/or ingredients by using an undervalued feedstock.
Keywords:
legume seeds; genetics; phenolic antioxidants; inflammation; cardiovascular disease; cancer; diabetes; obesity 1. Introduction
Legumes are a staple food with considerable importance for human nutrition due to their high content of carbohydrates and proteins, especially when diets are plant-based or have restrictions to animal-based products [1]. Historical evidence suggests soybean as a domesticated species that originated in Northeast China between 6000 and 9000 years ago [2,3]. The cultivated soybean (Glycine max) is considered to have evolved from the wild species Glycine soja [4] and from the transitional evolutionary species Glycine gracilis, which is considered a landrace [5]. Xu et al. [6] reported that cultivated soybean with different cpDNA haploytypes originated independently in different zones from different wild gene pools and/or hybrid swarms between wild and cultivated genotypes, evidencing major gene flow from G. soja to G. gracilis and the latter to G. max, with moderate gene contribution from G. soja to G. max [5,7,8]. The oldest records of soybean cultivation appear in bronze inscriptions and in early writings that date not much earlier than 1100 BC. With the expansion of the Shang dynasty, trade of soybean migrated to South China, Korea, Japan, and South East Asia, where it progressively became a dietary staple [9]. Soybean was introduced to Europe in about 1691, although it became a known food plant only in the 18th century. The introduction of soybean from Europe to the USA occurred around 1804, when its utilization rapidly spread out to the rest of the western world, especially in the 20th century [10]. According to Food and Agriculture Organization (FAO), world production of soybeans is currently around 352.6 million tons, associated to 123.6 million hectares grown globally, with average yields of 3.1 t/ha in 2017, and is presently a major crop in the United States, Brazil, Argentina, India, and China, and the most important legume crop cultivated in the world [11]. In Chile, national soybean production is only for seed exports and not for domestic consumption, and being a secondary producer of this crop worldwide, the cultivated area was 1458 ha of herbicide-tolerant transgenic soybeans in 2017 [12].
Chickpea (Cicer arietinum) is the only cultivated species in the genus Cicer, and it has never been found in the wild but in the closely related taxa [13]. Recently, Cicer reticulatum has been postulated to be the wild progenitor of modern chickpea [13,14]. The area of present-day southeastern Turkey and adjoining Syria is the most probable center of origin of chickpea, which was domesticated with wheat, barley, peas, and lentil as a member of West Asian Neolithic crops during the origin of agriculture around 10,000 years ago in the Fertile Crescent [15], with the oldest available archaeological evidence of chickpea from 7500 BC [16,17]. It is considered to have spread throughout the Mediterranean area about 6000 years ago and to have reached India about 4000 years ago [10], and then to the rest of the world. In chickpea, a particular and drastic narrowing of genetic diversity during domestication has occurred due to a series of bottlenecks unique to this crop [17]. Consequently, chickpea displays a lack of adaptive diversity for an assortment of biotic and abiotic stresses [18]. Unlike cultivated chickpea, wild Cicer spp. possess useful variation for several of those traits [19,20,21]. Globally, it is currently cultivated in over 14.5 million ha with an annual production of 14.8 million tons and productivity of around 1 t/ha in 2017 [11], which is very much lower than the estimated potential of 6 t/ha under optimum growing conditions [14]. The top chickpea producing countries in the world are India, Australia, Pakistan, Turkey, and Mexico [11]. In Chile, chickpea is a marginal crop, yielding on average 0.86 t/ha and a surface grown of 409 ha, which is mainly cultivated in the rainfed area of Central Chile, according to the data of the 2015/2016 season [22].
In plants, phenolic compounds are responsible for a plethora of functions, including structural components, UV protection, and antioxidant, signaling, and defense molecules [23]. Lignins and lignans are the phenolic polymers that compose the plant secondary cell wall, giving the physical structure and being tightly related to the growth of different plant parts, mostly stem, roots, and seeds [24]. These polymers are responsible for providing a protective barrier against herbivores, fungi, bacteria, and virus, as well as forming an intricate network of biochemical compounds that play scavenger roles of reactive oxygen species (ROS), antimicrobials, and wound signaling [25,26,27]. Moreover, hydrolyzable tannins are known anti-herbivory molecules, which act by precipitating proteins of the digestive tract of insects, impairing enzyme functions while reducing the digestibility of the ingested proteins [28]. The phenolic acids are important signaling molecules in plant-microbe interactions, for example, salicylic acid, and can improve nutrient uptake and protect against infections [29]. Flavonoids are the main source of plants non-enzymatic antioxidant apparatus; they provide a UV screen effect and play a central role in scavenging ROS derived from photorespiration in high light conditions as well as in low/high-temperature stresses or other oxidative stresses [30]. In addition, anthocyanins display a visual effect of colors ranging from yellow/orange to purple/bluish, which function as an important mechanism of attraction of pollinators and seed dispersers [31,32,33].
Food macronutrients play a crucial role in human nutrition and health. The proximate composition of chickpea and soybean is summarized in Table 1. The same amount of lipids, on a weight basis, provide more than two-fold the energy generated by the intake of proteins and carbohydrates. In this sense, due to its lower lipid content (up to twenty-one-fold less) compared to that of soybean, chickpea stands out as a good option in weight management. Furthermore, the content of insoluble fiber in chickpea is comparable or even higher than that of soybean. Due to their high contents of insoluble fiber, consumption of chickpea and soybean may promote regular bowel movement, thus preventing constipation [34]. Individuals that consume higher contents of fiber may show a lower risk of developing diabetes, cardiovascular diseases, and colorectal cancer [35,36,37]. However, the concept of antioxidant dietary fiber [38] has been gaining attention as several studies support the significant contribution of insoluble-bound phenolics in fruits, legumes, and vegetables and their processing by-products [39,40,41,42,43,44]. Therefore, the transport of dietary antioxidants through the gastrointestinal system was highlighted as an essential function of insoluble fibers [45]. In addition, protein concentrates are potential sources of healthy isoflavones [46]. Although some individuals may be allergenic to chickpea proteins, this feedstock is not consumed as a raw material. Gupta et al. [47] have summarized some industrial and home processed chickpeas and their potential to prevent adverse health effects caused by the presence of allergic components.

Table 1.
Proximate composition (g/100 g) of chickpea and soybean.
According to Valdez and Bolling [55], inappropriate activation and expression of nuclear factor-κB (NF-κB) p65 protein, which leads to inflammation, has been found to be positively correlated with the severity of inflammation and neutrophil inflammation in biopsies from inflammatory bowel disease patients. In addition, a recent study [56] has demonstrated that phenolic extracts containing phenolic acids and flavonoids were able to decrease the activation of NF-κB. The literature provides sufficient evidence on the link between oxidative stress and NF-κB activation [57,58]. Accordingly, it has been proposed that screening the antioxidant potential of plant food phenolics could be considered a first step in the studies aiming to prospect novel food bioactive, functional ingredients, and/or nutraceuticals [56]. Besides scavenging ROS, an “ideal antioxidant” should not only be a good reducing agent but must exhibit chelating capacity, and some isoflavones fit these characteristics. Therefore, as for the antioxidant potential, this review focuses on isoflavonoids from chickpea and soybean, the latter being the reference feedstock. When appropriate, the remaining phenolic compounds are cited to support a discussion on the role of structure/activity. The health benefits are discussed in terms of the prevention of cardiovascular diseases (CVD), certain types of cancer, diabetes, and obesity, among others, as inflammation and oxidative stress are common to all of them. In short, this contribution may guide the industry in manufacturing functional foods and/or ingredients by using chickpea which, thus far, is an undervalued feedstock.
2. Genetics
Although the benefits of phenolic compounds (e.g., phenolic acids and flavonoids), in food, feed, cosmetics, and medicine are well known, the genetic basis regulating their content in edible seeds is still not fully understood [59,60,61,62,63]. Isoflavones are the main compounds contributing to the phenolic profile of soybeans (2n = 40 chromosomes) [64]. Unraveling the genetics underlying their accumulation in seeds has been difficult because of several factors. Soybean has many quantitative trait loci (QTL) with small individual effects involved in an additive fashion that affects the phenotype and, by consequence, the accumulation of secondary metabolites [65,66,67]. Cai et al. [60] found that qIF5-1 can explain 49–52% of the isoflavone accumulation in Huachun 2 × Wayao F7:8–10 recombinant inbred lines, still far from being an easy trait to be determined and in line with other authors [59] who list several small-effect impact of QTLs on isoflavone seed content. However, in this intricate network, Li et al. [68] found four multidrug and toxic compound extrusion (MATE) transporter-encoding genes in wild soybean that increased antioxidant content and are unrelated to the phenylpropanoid pathway, which indicates phenolic intercellular transportation processes with a potential to be determinant. Besides, soybean possesses a complex genome that has passed by several whole genome duplication events [69,70]. Another concern stems from the fact that isoflavone content is strongly modulated by environmental conditions during seed development, thus affecting their synthesis and accumulation [69,71]. The variation of a warmer to a normal year in a two-year trial planted in eastern Maryland reduced isoflavones by c.a. 50% due to the higher temperature, while a cooler central location on the same state was not affected [72]. Individual identification and quantification of 12 different isoflavones of soybean seeds (including the most cited glycitein, daidzein, and genistein) have been technically difficult, cumbersome, and expensive, with liquid chromatography coupled to spectrophotometric detection being the most commonly used technique [60,73]. In order to properly determine the underlying genetic nuances of isoflavone accumulation, it is pivotal to have the most common compounds consistently determined and preferably in a high throughput setup. In addition, epistatic interactions, that is, the particular combination of alleles that results in a change in the phenotype, meaning that the same allele can produce different phenotypes in a different genetic background, are responsible for a great proportion of the observed phenotypic variance [74,75]. Furthermore, adding complexity to QTL analysis for validating isoflavone accumulation involves multiple interactions between those QTLs with different environmental variables that affect this trait [76,77]. The literature shows contrasting values of the heritability, that is, the proportion of observed phenotypic variability among individuals that is due to genetic differences among them, for isoflavones content of soybean seeds. These heritability values range between lower than 40% [66,67,78] and higher than 80% [79,80]. Thus, in the latter case, indicating that most of the variation among genotypes is explained by genetic effects allows to fine map major QTLs associated with both individual and total isoflavone content in soybean seeds, that could potentially be introgressed into elite cultivars through marker-assisted selection (MAS) [81].
The first study of DNA markers related to isoflavone content of soybean seeds used the recombinant inbred line (RIL) population ‘Essex’ × ‘Forrest’, and the markers associated to this trait was reported by Njiti et al. [82]. Using the same RIL population, subsequent works identified new QTLs associated with genistein, glycitein, and daidzein on six different soybean chromosomes [65,78,83]. Another RIL population ‘AC756’ × ‘RCAT Angora’ was employed for the identification of QTLs associated with genistein, glycitein, and daidzein [66]. In parallel, the same major QTL (on chromosome 7) was detected in another population based on the parental lines ‘Zhongdou 27’ × ‘Jiunong 20’ [67]. Other two major QTLs associated to total isoflavone content, genistein, glycitein, and daidzein were reported on chromosomes 5 (QDZGT1) and 8 (QDZGT2), by utilizing the ‘Hwangkeum’ (Glycine max) × ‘ITI82932’ (Glycine soja) population [84]. Other groups developed additional RILs populations (‘Essex’ × ‘PI 437654’, ‘Magellan’ × ‘PI 437654’) with the objective to identify major isoflavone-associated QTLs, and coincided with the previous reported QTLs, although several isoflavones-associated QTLs were detected in these studies [59,75,76]. This research group identified five QTLs that contributed to the concentration of isoflavones, possessing single or multiple additive effects on isoflavone component traits, and a major locus on chromosome 5 (Gm05) was validated that alone accounted for up to 10% of the phenotypic variance for glycitein, and 35–37% for daidzein, genistein, and the sum of all three soybean isoflavones. This Gm05 QTL was consistently associated with isoflavones concentration across different crosses, locations, and years [76]. More recently, a high-density soybean genetic map was constructed using RILs (Luheidou2 × Nanhuizao, F5:8), and 41 QTLs were identified that contributed to the isoflavone content, standing out one novel major QTL on chromosome 20, qIF20-2, which contributed to a majority of isoflavone components across various environments and explained a high amount of phenotypic variance (up to 35.3%) [85]. Using specific-locus amplified fragment sequencing in the F5:7 of the same RIL cross, Pei et al. [86] found four new QTLs (qG8, qMD19, qMG18, and qTIF19) associated to genistin, malonyldaidzin, malonylgenistin, and total isoflavones, respectively. Meanwhile, another study identified 44 isoflavone-associated QLTs using soybean landraces and restriction site-associated DNA tag sequencing (RAD-seq), including 55 candidate genes on 16 chromosomes, explaining 72.2% of the total phenotypic variation, reflecting the complex genetic nature of the synthesis and accumulation of isoflavones on soybean seeds [87]. Subsequently, 15 stable QTLs associated to individual and total isoflavone content were identified using a high-density soybean genetic map across multiple environments of China, by utilizing a population of 196 F7:8–10 RILs (Huachun 2 × Wayao). In this study, one major QTL on chromosome 5, qIF5-1, contributed significantly to the total isoflavone content and explained between 43.3 and 52.5% of the total isoflavone content phenotypic variance [60]. Taking all together, the research results of the last 20 years have generated knowledge for a better understanding of the genetics of isoflavone accumulation in soybean seeds, especially with the fact that there are genetic regions on chromosome 5 that appear to play an important role in the regulation of this trait. In addition, there is scope available for improvement of isoflavone content through MAS [88], which could be a powerful tool to increase isoflavone content by the introgression of valuable QTLs/genes/alleles (i.e., identified on chromosome 5) into elite cultivars of soybean that would certainly augment their seed quality. Another promising way of finding candidate genes that could further be validated by QTL is the use of genome-wide association studies (GWAS). Recently, an MYB transcription factor was found to regulate isoflavone contents in soybean hairy roots, the GmMYB29 activated the promoters IFS2 and CHS8, while its overexpression or silencing by RNAi had positive and negative effects on isoflavone content [89]. Nonetheless, further studies to verify and confirm biosynthetic pathways using RNA-sequencing and co-expression network analysis can lead to the identification of specialized varieties, for example, producing coumestrol [90].
Contrary to soybean, there is very little information in the literature on the role of genetics with respect to the polyphenolic composition of chickpea (2n = 16 chromosomes) [91], and to the best of our knowledge, there is no study associated with the genetic regulation of isoflavones on chickpea seeds. One important field of research in the last decades has been the alteration of isoflavone contents and composition upon germination [92]. However, the available literature demonstrates that variations in the isoflavone contents and profile are dependent on the process [93,94]. Changes of the isoflavones content upon germination has very likely a genetic regulation explanation, although no QTLs/genes associated with this phenomenon have been reported so far. Most of the genetic studies of chickpea have focused on the identification of genetic regions using different methodologies associated to improving agronomic traits, such as increasing seed yield and the yield of components [95,96,97,98,99,100,101,102,103]. In addition, a genetic approach has been used to develop drought/heat tolerance [70,104,105,106,107,108], as well as disease resistance, for example, Ascochyta blight [109,110]. Solid and broad genetic studies on chickpeas are emerging and paving the way for breeding approaches targeting phenolic compounds in general [111,112,113]. From a nutritional and functional food perspective, genetic studies have been carried out on chickpeas to identify genetic regions associated to seed color [114,115,116], seed protein content [117], and seed-zinc/iron concentrations [118]. Based on the existing literature, it seems clear that increasing the levels of isoflavones on chickpea seeds by germination could have valuable implications, to gain insight into the genetic basis regulating this process, which would be of scientific interest to identify QTLs/genes/alleles of relevance that could be transferred into elite chickpea cultivars through MAS and/or traditional breeding. In addition, genetic comparative studies between soybeans and chickpeas are of importance to understand whether the existing knowledge of the genetics underlying the regulation of isoflavone synthesis in soybeans might be transferable to chickpea.
3. Main Phenolic Bioactives and Their Quantities
In addition to being rich sources of carbohydrates, protein, fibers, minerals, and vitamins, chickpea, soybean, and other legumes also contain bioactive substances [119,120,121,122,123]. Phenolic compounds are present in both soluble and insoluble-bound forms. Therefore, one should bear in mind that optimizing the extraction of phenolics in the soluble form is desirable (Table 2).

Table 2.
Total phenolic contents (mg GAE/g) of chickpea and soybeans.
Xu and Chang [121] carried out a comparative study on the yield of phenolics of legumes as affected by the extraction solvent. Their results support the use of 50% acetone from chickpea and yellow soybean while black soybean showed a higher total phenolic content (TPC) when acidic 70% acetone (0.5% acetic acid) was employed. The contrast found by these authors might be related to differences in specific phenolics present. Supporting this assumption, it is worth to highlight the study carried out by Yoshiara et al. [120]. These researchers used a centroid design and made a valuable contribution by demonstrating that glycosidic isoflavones were better extracted with the polar ternary mixture (water, acetone, and acetonitrile, 2:1:1, v/v/v) while malonyl-glycosidic isoflavones showed higher extraction yields with mixtures of water, acetone, and ethanol (2:1:1, v/v/v). Finally, water and acetone (1:1, v/v) rendered a higher extraction of isoflavones as aglycones. Examples for the total phenolic contents (TPC) of soybean and chickpea are summarized in Table 2, while the isoflavone profile of chickpea and soybean is summarized in Table 3.

Table 3.
Isoflavone profile (%) of chickpea and soybean *.
Chickpea and soybean have significant amounts of flavonoids, especially isoflavones. According to the literature, the total isoflavone content (TIC) varied from 153 to 340 mg/100 g of chickpea and from 165 to 336 mg/100 g of soybean [51,126]. In chickpea, the major isoflavones found were biochanin A and formononetin while smaller amounts of genistein and daidzein might also occur [94]. Considering the contribution of each isoflavone to TIC, the isoflavone profile of chickpea and soybean is summarized in Table 3. Formononetin and biochanin A, which are present in chickpeas, have not been reported in soybeans. In addition, the presence of phenolic acids and other flavonoids in chickpeas were reported by Thavarajah and Thavarajah [133]. However, isoflavonoids have been reported as the main bioactive phenolics of chickpea [93]. As for the absolute concentration, biochanin A (180 μg/g) and its derivatives, biochanin glucoside (80 μg/g), and biochanin A glucoside malonylated (60 μg/g) made the highest contribution to the isoflavone profile of chickpeas, while formononetin (100 μg/g) rendered a lower contribution [93]. The same study [93] also reported the concentration of daidzein (120 μg/g) and genistein (60 μg/g). It is important to highlight that these concentrations were in good agreement with those found in soybeans, which were in the range of 18.5–242.7 μg/g for daidzein and 13.0–158 μg/g for genistein, which suggests that chickpeas may be considered a good substitute for soybeans as a source of isoflavone aglycones [132]. Finally, it is noteworthy that current identification and quantification of isoflavones in these feedstocks were mainly carried out using hyphenated techniques, such as liquid chromatography coupled to tandem mass spectrometry (LC–MSn), thus increasing the reliability of the results.
4. Potential Health Benefits
4.1. Antioxidant Potential
Dietary phytochemicals, specifically phenolic compounds, are known by their potency in scavenging ROS. Likewise, the antioxidant activity of phenolic compounds may be explained by their reducing power and/or metal-chelating activity towards ferric and ferrous ions, respectively. The Fenton reaction, also known as the Haber-Weiss cycle, is an important biological model. Peroxyl and hydroxyl radicals may be pointed among the most important ROS as both participate in the Fenton reaction. The role of ferric and ferrous ions in the Fenton reaction has also been addressed [23]. Peroxyl radicals show a longer half-life compared to that of hydroxyl radicals. Consequently, while hydroxyl radicals may damage intracellular components, the deleterious effects of peroxyl radicals may be extended to biological fluids [137,138].
The antioxidant properties of soybean are mainly associated with the presence and/or profile of isoflavones. Furthermore, a highly positive correlation existed between the concentration of genistein in phenolic extracts of soybean and their ability in scavenging 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) diammonium salt (ABTS) radical cation [130]. The presence of genistein as aglycone has been found in only one sample (out of seven) in Egyptian cultivars of chickpeas as evaluated by RP-HPLC-DAD-ESI-QTOF-MS (RP: reversed-phase; DAD: diode array detection; ESI: electrospray ionization; QTOF: quadrupole time of flight; MS: mass spectrometry), while its conjugated counterpart was detected in all samples [139]. The experience of our group with various feedstocks has demonstrated that the chelating ability of polyphenols is not simple to be confirmed, which is the opposite of the reducing power as, in general, the latter has been correlated with TPC [137,138].
As for the structure/activity, phytochemicals with one or more methoxy substitutions are regarded as potent chelating agents [140]. In contrast, compounds lacking catechol or galloyl moiety do not show any complex formation [141]. The properties of genistein and biochanin A as metal chelators have been confirmed by elemental analysis, Fourier-transform infrared spectroscopy, thermogravimetric analysis, and electrospray ionization mass spectrometry [142]. However, daidzein does not chelate Cu(II) or Fe(III), thus supporting a structure/activity relationship. These authors [142] also suggested that isoflavones bind metals at the 4-keto and the 5-OH sites. Additionally, it has been hypothesized that the ratio of ferric to ferrous ion is important for rapid initiation of lipid peroxidation through the Fenton reaction and the ratios of 1:1 to 7:1 (Fe3+/Fe2+) are optimum [143]. Therefore, reducing the concentration of ferric ions in the system could be beneficial. However, due to their cyclic nature, ferrous ions are oxidized to the ferric form, and the latter is again reduced to the ferrous form. In this scenario, it has been suggested that an “ideal antioxidant” should not only be a good reducing agent but must exhibit chelating capacity [23], hence genistein and biochanin A could be classified as ideal antioxidants.
Trolox, a water-soluble analog of vitamin E, is used as a standard to express the antioxidant activity as evaluated by several methods (e.g., 2,2-diphenyl-1-(2,4,6-trinitrophenyl)hydrazyl (DPPH), ABTS, ferric reducing antioxidant power (FRAP), oxygen radical absorbance capacity (ORAC)). Therefore, by examining the antioxidant activity in the available literature (Table 4), especially from the same research team and feedstock, it is easy to understand that the antioxidant activity should not be faced as a single number but rather as an index and/or trend. Yoshiara et al. [130] evaluated the antiradical activity of soybean seeds. The antioxidant potential towards DPPH radical and ABTS radical cation was 289 ± 1.0 and 252 ± 6.0 µmol Trolox equivalent/g, respectively. In general, these differences have been explained by their operative mechanisms. In fact, the ability of phenolic compounds in scavenging free radicals stems from single electron transfer (SET) or hydrogen atom transfer (HAT). Furthermore, the same authors [130] also showed that, regardless of the method, germination increased the antioxidant properties of the test material.

Table 4.
Scavenging of peroxyl radical and reducing power of chickpea and soybean.
A deeper evaluation of the data reported by Yoshiara et al. [130] shows that the greatest increase (up to 138%) was found against ABTS radical cation compared to the improvement towards DPPH radical (up to 12%). Likewise, while a significant difference between the antioxidant activity (ABTS assay) of free and insoluble-bound phenolics of soybean was found by Ademiluyi and Oboh [129], these authors did not find any difference with respect to the reducing power. Therefore, these studies [129,130] demonstrate that specific phenolics (e.g., conjugated versus isoflavones as aglycones) or fractions (free versus insoluble-bound phenolics) respond differently to each method. Additionally, besides the degree of glycosylation, the number and position of hydroxyl groups in polyphenols may also influence their antioxidant potential [23,126].
Beyond the starting material, their fractions (e.g., cotyledons, epicotyls, radicles, and hypocotyls) may show different antioxidant properties [130]. According to Sreerama et al. [119], the antioxidant activity towards hydrogen peroxide and DPPH radical was in the decreasing order of seed coat > embryonic axe > cotyledons. Therefore, evaluating the whole material, that is, along with non-edible portions, may not provide the best picture of the potential antioxidant activity under physiological conditions. Like plant foods, the human body relies on antioxidant systems to eliminate free radicals and the excessive increase in their levels can damage proteins, lipids, and nucleic acids. In addition, oxidative stress and lipid peroxidation are major causes of chronic ailments, such as neurodegenerative, inflammatory, and cardiovascular diseases. Likewise, the role of ROS in allergies, immune system dysfunctions, type 2 diabetes, obesity, certain types of cancer, and aging have been reported [146,147].
In humans, endogenous antioxidant enzymes, such as catalase (CAT), superoxide dismutase (SOD), glutathione peroxidase (GPx), glutathione reductase (GR), and peroxiredoxins (PRXs), exert antioxidant function during oxidative stress [148,149]. Phenolic compounds may also modulate the activity of antioxidant enzymes. Genistein has been found to improve the activity of antioxidant enzymes, as well as decreasing the levels of ROS and lipoperoxide in the brain and liver of C57BL/6J streptozotocin (STZ) diabetic mice, thus reverting the overproduction of ROS and restoring glutathione content and the reduced glutathione (GSH) and oxidized glutathione (GSSG) ratio [150]. Although these authors [150] did not address the bioaccessibility and further bioavailability of genistein, the improved oxidative status of brain and liver found by them suggests that the test compound was bioavailable. It is well accepted that isoflavones as aglycones are usually more bioavailable than their conjugated counterparts. The bioaccessibility and bioavailability of several phenolic compounds have recently been revised and published elsewhere [151]. Furthermore, genistein also increased the activity of hepatic superoxide dismutase, catalase, and glutathione peroxidase of STZ-induced diabetic rats [152]. Genistein and daidzein decreased malondialdehyde (MDA) production against anoxia-glucopenia and reperfusion damage in rat urinary bladder (A-G/R) [153], thus supporting their antioxidant activity in vivo.
Biochanin A, which is found in chickpeas [154], has been gaining attention due to its therapeutic value. Biochanin A reduces oxidative stress by decreasing MDA levels and by increasing the levels of CAT, SOD, and total antioxidant status in diabetic rats [155]. Exposure to arsenic through the consumption of contaminated drinking water and foods may cause significant negative health effects, including cancer and non-cancerous diseases. Jalaludeen et al. [140] tested the therapeutic efficacy of biochanin A against arsenic-induced renal and cardiac damage in rats. According to these authors, Biochanin A alleviated oxidative stress in the kidney of arsenic-treated rats. In addition, biochanin A exhibited a protective effect against oxidative stress in a rat model of Parkinson’s disease, inhibiting the nicotinamide adenine dinucleotide phosphate oxidase (NADPH oxidase) activation and MDA production and increasing SOD and GPx activities in the rat brain [156].
4.2. Anti-Inflammatory Effects
During oxidative stress, interleukins and inflammatory mediators are produced. Isoflavones from natural products have shown anti-inflammatory properties in vitro and in vivo [157]. Verdrengh et al. [158] showed that subcutaneous treatment with genistein was able to suppress the delayed-type hypersensitivity reaction to oxazolone and the granulocyte-mediated response. Likewise, Zhang et al. [159] demonstrated that genistein treatment inhibited the proliferation of rheumatoid synoviocytes by reducing the matrix metallopeptidase 9 (MMP-9) expression [159]. Genistein may also prevent periodontal disease. Bhattarai et al. [160] tested this hypothesis in a mice model of periodontitis. According to these authors, genistein was able to protect against alveolar bone loss and periodontal tissue degradation. Neuroinflammation, a phenomenon that is primarily mediated by microglial cells, may culminate in Parkinson and Alzheimer disease, multiple sclerosis, amyotrophic lateral sclerosis, among other chronic ailments. The potential of genistein in treating and/or preventing neuroinflammation has already been discussed [161]. The modulatory effects induced by flavonoids are mediated by their impact, mainly, on mitogen-activated protein kinase (MAPKs) and nuclear factor-kappa B (NF-κB) [161]. Lending support to the statements of Spagnuolo et al. [161], Ji et al. [162] demonstrated that tumor necrosis factor alpha (TNF-α) and interleukin 6 (IL-6) release in lipopolysaccharide (LPS)-stimulated macrophages was inhibited upon genistein treatment via NF-κB, specifically by stimulating adenosine monophosphate-activated protein kinase (AMPK). It has been reported that in vitro LPS-stimulated microglia cells (BV2) liberate less nitric oxide, prostaglandin E2, IL-1β, and TNF-α after genistein treatment [163]. In this scenario, the authors also attributed the inhibitory effects of genistein on the NF-κB. Furthermore, genistein reduced the binding of the LPS to the toll-like receptor 4 (TLR-4) receptor on microglial cells [163].
Biochanin A, a derivative of genistein, is also able to reduce neuronal inflammatory damage of the articular cartilage [164,165]. Wu et al. [166] showed that biochanin A attenuated LPS-induced pro-inflammatory responses and inhibited the activation of the MAPK pathway in BV2 microglial cells and decreased the expression of TNF-α and interleukin 1 beta (IL-1β). Therefore, the mechanisms of action of biochanin A are attributed to its ability in reducing phosphorylation of MAPK, c-Jun N-terminal kinases (JNK), extracellular signal-regulated kinase (ERK), and p38 [166]. However, biochanin A acts simultaneously on other targets. Ming et al. [167] reported that biochanin A suppressed the rolling and adhesion molecules expression, specifically the vascular cell adhesion molecule 1 (VCAM-1), intercellular adhesion molecule 1 (ICAM-1), and E-selectin molecules on human umbilical vein endothelial cells. The effects of biochanin A are related to the activation of Peroxisome proliferator-activated receptor (PPAR)-γ. Furthermore, its ability to reduce NF-κB activation has also been reported [167]. E-Selectin reduction is a marker for endothelial function improvement, controlled metabolic condition, and arteriosclerosis and inflammation reduction in diabetic patients [168,169].
Daidzein, another isoflavone aglycone, also shows anti-inflammatory potential [170]. According to Ahmad et al. [171], daidzein reduced the evolution of rheumatoid arthritis in Wistar albino rats. These authors showed that the levels of TNF-α and rheumatoid arthritis scores were improved when compared to the control group. Furthermore, the same study suggested protective effects towards CVD as it reduced the levels of low-density lipoproteins and triacylglycerols. Beneficial effects of daidzein in the lung were also observed in mice. Feng et al. [172] used an LPS-induced acute lung injury model. The test material reduced important inflammatory parameters, that is, the number of infiltrated cells and levels of inflammatory cytokines in bronchoalveolar lavage fluids. The inhibition of TLR4-MyD88-NF-κB signaling pathway is possibly involved in the protective action of daidzein. NF-κB and signal transducer and activator of transcription 1 (STAT-1) routes are important targets related to nitric oxide production, an important inflammatory mediator [173]. Besides reducing the NF-κB route, other authors have also shown that daidzein and other flavonoids (e.g., kaempferol and quercetin) are able to simultaneously affect the STAT-1 route [173]. Penga et al. [174] attempted to improve the pharmacokinetic properties of daidzein. These authors synthesized new sulfonic acid ester derivatives with much better pharmacologic effects than the original molecule. Furthermore, the derivatives inhibited phosphorylation of JNK and showed significantly enhanced anti-inflammatory activities by two to four orders of magnitudes over that of the parent daidzein in TNF-α-stimulated Caco-2 cells.
Formononetin, a daidzein derivative, has in vivo antinociceptive effects in mice models and reduced the neutrophil migration to the peritoneal cavity and hind paw edema [175]. Formononetin was also evaluated using an LPS-stimulated lung injury model on mice and showed promising protective effects. Ma et al. [176] showed that formononetin treatment reduced the bronchoalveolar lavage fluid cell numbers, increased PPAR-γ gene expression, and improved the superoxidase dismutase activity while simultaneously inhibited the myeloperoxidase activity. Wu et al. [177] evaluated the effects of formononetin on dextran sulfate sodium- (DSS-) induced acute colitis model in mice. Dose-dependent attenuation of colitis was noted, and, according to these authors, the effects of formononetin may stem from the inhibition of NLRP3 inflammasome signaling pathway.
4.3. Polyphenols in the Prevention of CVD
A recent report stated that CVD is the most common non-communicable ailment, claiming one-third of total global death [178]. In general, low-density lipoprotein-cholesterol (LDL-c) levels are predictors of death from cardiovascular diseases (CVD) [179]. Development of atheromatous Aplaques stems from the uptake of oxidized LDL-c, via scavenger receptors, thus leading to cholesterol accumulation and foam cell formation [23]. Therefore, it is important to highlight that oxidized LDL-c is the actual entity involved in the early event of atherosclerosis. Tarantino et al. [180] carried out a study with obese patients and showed that altered copper bioavailability is a predictor of early atherosclerosis, the main CVD risk in obese patients with hepatic steatosis. Accordingly, cupric ion-induced human LDL-c peroxidation in vitro has been employed to anticipate the potential benefits of polyphenols in reducing the risk of CVD, although some authors have used 2,2′-azobis (2-methylpropionamidine) dihydrochloride (AAPH) to induce the oxidation process [181].
The generation of conjugated dienes and trienes, as well as malondialdehyde-thiobarbituric acid (MDA-TBA) or TBA reactive substances (TBARS), have been used to monitor the level of LDL-c oxidation [181,182]. Genistein (200 μmol/L) has shown inhibitory activity by protecting LDL-c from peroxyl radical (azo-initiated)-induced oxidation in vitro [183]. Kerry and Abbey [183] supported their results in the lower time required (3 h) for MDA formation compared to that of the control (7 h) as well as evidenced by a decrease in relative electrophoretic mobility (REM) of LDL-c.
Xu et al. [184] carried out a comparative evaluation on the ability of common food legumes, including soybeans and chickpea, towards copper-induced human LDL-c oxidation in vitro. Total phenolics and total flavonoids were correlated (r = 0.79 to 0.94, p < 0.001) with the antioxidant activity in this model system, and the authors suggested that phenolic compounds played a possible role in the overall antioxidant activity of legumes. In addition, high correlations existed between ORAC assay (scavenging of peroxyl radical) and the protection of LDL-c against oxidation (r = 0.81 to 0.83, p < 0.001).
The potential of dietary chickpeas in reversing dyslipidemia in rats induced by a chronic high-fat diet was studied by Yang et al. [185]. Chickpea supplementation (10% w/w) induced a favorable lipid profile by decreasing the levels of triacylglycerols, LDL-c, and LDL-c:HDL-c ratio. Pittaway et al. [186] suggested that dietary supplementation with chickpeas results in lowering total serum and LDL-c levels in hypercholesterolemic women and men as compared to a wheat-supplemented diet. Improvements in serum lipid profile (e.g., lower serum total cholesterol and LDL-c) were confirmed in another study from the same research team [187]. These authors [185,186,187] did not evaluate the phenolic profile of their starting material. However, the role of phenolics in cardiovascular diseases is well substantiated [188]. Supporting the role of legumes in the prevention of CVD, Sedaghat et al. [189] carried out a clinical trial and demonstrated that soybean consumption decreased the level of total serum cholesterol and LDL-c in patients with type 2 diabetes. Other clinical trials [190,191] showed similar results with respect to the role of soybean, their products and related phytochemicals in reducing total cholesterol and LDL-c levels, thereby improving the cardiovascular risk profile.
According to the literature [192], cellular cholesterol synthesis was inhibited by genistein (41%) and daidzein (18%), and the effect was associated with increases in 3-hydroxy-3-methylglutaryl-CoA reductase mRNA. After univariate analysis, Pittaway et al. [187] concluded that dietary fiber exerted the greatest single effect by reducing serum total cholesterol, but increasing pieces of evidence show that dietary fibers act as carriers of phenolic antioxidants [45]. Lipopolysaccharides (LPS) are present in the cell wall of Gram-negative bacteria. Intestinal barrier dysfunction due to high intake of saturated fatty acids facilitates LPS migration into the bloodstream, thus enabling systemic inflammation, which is common to cardiovascular disease. Furthermore, phenolic acids and flavonoids from legume crops show antimicrobial activity and protect barrier integrity [193,194]. Therefore, phenolic compounds may be helpful in the prevention of CVD by inhibiting the growth of Gram-negative bacteria, protecting barrier integrity [193,194], and possible LPS release into the bloodstream, in addition to their well-documented anti-inflammatory action [157].
4.4. Polyphenols as Adjuvants in Cancer Prevention and Treatment
An unhealthy diet is among the risk factors responsible for cancer, a leading cause of death worldwide. Colorectal, female breast, liver, lung, and stomach cancer are among the most common causes of cancer death [195]. Lung cancer is most common in men, while breast cancer is most prevalent in women. Different from other chronic ailments (e.g., obesity and diabetes), clinical trials which are reporting the effects of soybean, chickpea, and other legumes in cancer development and/or management are not available. However, a meta-analysis of epidemiologic studies supports the negative correlation between soybean intake and lung [196] as well as breast cancer [197].
Girón-Calle [198] investigated the cell growth-regulating properties of chickpea seed extracts as possible responsible anti-cancer factors. Their results demonstrated that chickpea extracts inhibited the growth of Caco-2 cells exhibiting a cancerous phenotype. The effects of biochanin A on cell growth in the mammary carcinoma cell line MCF-7 has also been reported [199]. The preventive action of phenolic compounds against lung, liver, and female breast cancer is dependent on the bioaccessibility of the compounds as these must reach the plasma and different tissues. In contrast, stomach and colorectal cancer, which are also major causes of cancer death, do not necessarily stem from the bioaccessibility of polyphenols. Insoluble-bound phenolics have been found in soybean and other legume seeds [200]. Insoluble-bound phenolics are not readily bioaccessible. However, it has been hypothesized that upon human colonic microbiota fermentation, insoluble-bound bioactive phenolics can be released in the colon [200], thus potentially preventing colorectal cancer.
DNA-damage signaling/repair are crucial pathways to the etiology of human cancers [201]. In fact, it has been well accepted that DNA strand breakage may culminate in mutagenesis and affect replication and transcription of DNA, which are pointed among the causes of cancer initiation [23]. The presence of phenolic extracts from different legume seeds inhibits peroxyl radical-induced DNA damage [202,203]. Soluble and insoluble-bound fractions of black soybean seed coat and cotyledon contain isoflavones as aglycones, as well as their conjugated forms [204]. Phenolics from back soybean seed coat showed antioxidant activity by preventing AAPH-induced oxidative DNA-damage in HepG2 cells as noted by the inhibition of formation of 8-hydroxy-2′-deoxyguanosine (8-OHdG) as a biomarker for oxidative stress [203].
Mycotoxins, as chemical carcinogens, induce DNA-damage in cell models as well as in vivo [205,206]. The occurrence of aflatoxins, deoxynivalenol, and zearalenone has been reported in soybeans [207,208], while chickpea and chickpea-based products tested positive for aflatoxin and ochratoxin A [209]. However, several studies [210,211,212,213,214,215] have demonstrated that phenolic compounds protected against mycotoxin-induced dysfunctions (e.g., aflatoxins, deoxynivalenol, and zearalenone) in animal and cell models. Therefore, as a source of phenolic compounds, soybeans, chickpea, and their processing by-products may counteract potential health concerns caused by mycotoxins.
The role of transcription factors, such as NF-κB, AP-1, and STAT3 and their gene (e.g., tumor necrosis factor, interleukin-1, interleukin-6, chemokines, cyclooxygenase-2, 5 lipoxygenase, matrix metalloproteases, and vascular endothelial growth factor, adhesion molecules, and others), in inflammation and cancer has been well discussed in the available literature [216]. Acrylamide is probably carcinogenic to humans. Accordingly, chickpea flour has been used as a strategy to decrease the formation of acrylamide in processed foods (e.g., baked goods and fried potato) [217]. Some authors [217,218] have suggested that the beneficial effects of protein hydrolyzates stem from the ability of chickpea and soybean peptides in reacting with acrylamide, thus forming new derivatives. However, another study [219] has supported the ability of flavonoids (e.g., daidzin, genistin, daidzein, and genistein) in reducing acrylamide formation, which was correlated to the number of phenolic hydroxyl groups of flavonoids, their antiradical activity, and reducing power.
Surgery, chemotherapy, radiation, or their combination are the most common treatments for cancer [195]. Furthermore, the oxidative stress-based hypothesis involving the production of ROS due to the use of anticancer drugs has gained acceptance [220]. Side effects of chemotherapy include cardiotoxicity, hepatotoxicity, nephrotoxicity, neurotoxicity, and gastrointestinal and pulmonary toxicity [221]. In addition, hepatotoxicity and cardiotoxicity may be linked to drug-induced oxidative stress [220,222]. Anthracycline (ANT), which has been used in the treatment of leukemias, breast, and lung cancer, go through redox cycling where ROS are generated [220] due to the presence of ANT-Fe2+ complexes. The lower oxidative stress in anthracycline-treated rats, compared to control, was attributed to treatment with phenolic compounds [222].
The antioxidant activity and radioprotection isoflavone against gamma-irradiation were investigated by Dixit et al. [223]. These authors concluded that soy isoflavone scavenged free radicals induced by gamma-irradiation exposure and inhibited radiation-induced cellular damages. They also found increased survival rate of the animals pretreated with soy isoflavone and improved hematological and histological parameters as compared to the irradiated control group. Singh et al. [224] evaluated the effect of X-ray irradiation exposure on human lymphocytes isolated from the peripheral blood in vitro and suggested an age-related decline in DNA repair competence. The potential of genistein in preventing DNA damage, chromosomal aberration, and apoptosis triggered by X-ray irradiation in HL-7702 (L-02) cells was studied by Song et al. [225]. According to them, the molecular mechanism underlying its radioprotective properties might be explained by the inhibition of the endoplasmic reticulum stress marker GRP78, the promotion of the expression of HERP, a multifunctional protein, and the up-regulated expression of HUS1 and hHR23A, which are DNA repair-related genes. Therefore, consumption of soybeans and chickpea as natural sources of antioxidants may play an adjuvant’s role in cancer treatment.
4.5. Polyphenols in the Management and/or Treatment of Type 2 Diabetes and Obesity
Overweight (BMI [body mass index] 25–29.9) and obesity (BMI > 30) are defined by the World Health Organization (WHO) as abnormal or excessive fat accumulation that may impair health. Their fundamental cause is an imbalance between energy intake and energy expenditure, and their prevalence is on the rise around the world. From the 7500 million world population, it is estimated that 774 million are obese, and more than 50% of them live in the USA, China, or India [226]. Type II diabetes mellitus, which represents at least 90% of all diagnosed cases of diabetes, is one of the major diseases commonly associated with obesity. Diabetes is the fourth or fifth cause of death in the developed world, and together with obesity, they are projected to rise if measures are not taken to diminish this escalation [227]. Several strategies have been used to control and reduce these two diseases, among them dietary interventions, increased physical activity, medication, and consumption of functional foods and/or nutraceuticals are important. However, employing a combination of several strategies is probably more effective. Soybeans and chickpeas contain several kinds of bioactive compounds that may have positive effects on the control of obesity and type II diabetes. Thus, it is important to describe and discuss the main findings in this research area.
4.5.1. Digestive Enzymes as Biochemical Targets of Bioactive Compounds
Food macronutrients (carbohydrates, lipids, and proteins) must be hydrolyzed, and their hydrolysis products are absorbed through the digestive tract before they can be used as a source of energy and biosynthetic precursors in the tissues of higher animals. Many secondary metabolites present in soybeans, chickpeas, and other pulses are potent inhibitors of the enzymes responsible for the hydrolysis of food macronutrients. Protease inhibitors are especially abundant and may be considered as antinutritional factors because they reduce protein digestibility and hence, diet quality. They can have a negative health impact, especially in monogastric animals and in protein-restricted diets, but their effects can usually be eliminated or, at least, reduced by cooking, since many protease inhibitors are themselves proteins or peptides [228,229]. Other antinutritional factors, such as phytic acid, reduce mineral bioavailability by forming insoluble complexes with metal ions.
Likewise, inhibitors of the enzymes responsible for dietary carbohydrate and lipid hydrolysis used to be considered as antinutritional factors as they could reduce the bioavailability of these high energy nutrients enough to cause growth inhibition [228]. However, in recent times, the capacity of secondary metabolites in inhibiting carbohydrate and lipid hydrolysis has been viewed as a beneficial property. In fact, reducing the absorption of these macronutrients has been pointed out as one of the mechanisms by which some bioactive compounds decrease the risk of developing obesity and type II diabetes. Phenolic compounds are among the secondary metabolites that, in addition to their many biological activities, are being recognized as inhibitors of digestive enzymes. They can inhibit protein, carbohydrate, and lipid hydrolysis to different degrees, depending on several factors, including the structure of the enzyme as well as the phenolic compounds [230,231,232].
Starch (composed of two distinct polysaccharides, amylose and amylopectin) is the most abundant dietary carbohydrate. It is broken-down to monosaccharides (glucose) through the action of two types of enzymes present in the human digestive tract: α-amylases and α-glucosidases. There are two isoforms of human α-amylase: salivary and pancreatic. They both hydrolyze internal α1→4 glycosidic linkages of starch, yielding short-chain oligosaccharides. Pancreatic α-amylase, which is secreted by the pancreas but acts in the small intestine, carries out most of the starch hydrolysis (70 %) and its final products are mainly maltose, maltotriose, and dextrins (fragments of amylopectin containing α1→6 branched units). Human pancreatic α-amylase, a 55 kDa enzyme, contains 496 amino acid residues organized in three structural domains (A, B, and C). Domain A contains the catalytic triad (Asp197, Glu223, and Asp300) inside a (β/α)8 barrel [231].
Intestinal α-glucosidases act upon the products of pancreatic α-amylase, producing free glucose that is absorbed through specific transporters in the membrane of enterocytes. They consist of four different individual enzymes organized in two complexes: maltase-glucoamylase (MGAM) and sucrase-isomaltase (SI). Each complex possesses a molecular weight of approximately 260 kDa and contains a C-terminal (Ct) luminal domain and an N-terminal (Nt) domain covalently linked with a transmembrane protein of the intestinal brush border. Each domain has a distinct catalytic activity: MGAM Ct domain is glucoamylase and Nt domain is maltase, SI Ct domain is sucrase and Nt domain is isomaltase [233]. All four enzymes exhibit exo hydrolytic activity towards the non-reducing ends of α1→4 glycosidic linkages, but each catalytic unit possesses unique enzymatic characteristics. For example, glucoamylase (MGAM Ct) has the highest α1→4 hydrolytic activity, while isomaltase (SI Nt) can also hydrolyze α1→6 bonds [234]. α-Glucosidases from different origins, including those from rat and mouse intestine as well as yeast and bacterial α-glucosidases, have been used in studies aiming to screen potential α-glucosidase inhibitors [235]. Therefore, comparison among them may be difficult.
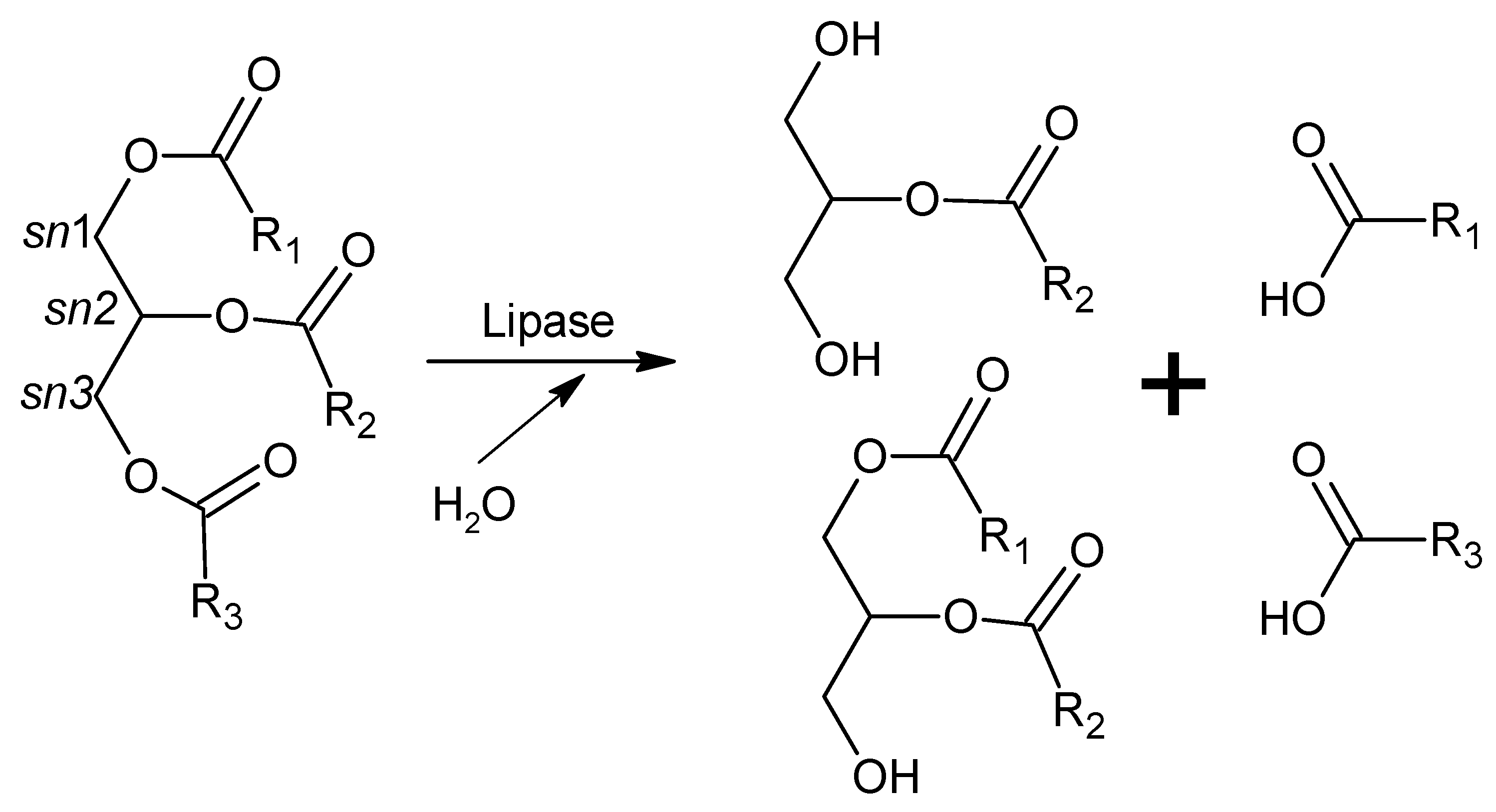
Fatty acids, which are the major lipid fuels of animal cells, are released from dietary triacylglycerols (found in fats and oils) by the action of intestinal lipases and then diffuse into the enterocytes crossing their apical membrane and are used as energy sources in many tissues. Although two pre-duodenal lipases are present in the human digestive system (lingual and gastric lipases), pancreatic lipase (secreted by the pancreas but acting in the small intestine) is the main enzyme responsible for lipid absorption (70%) [231]. Pancreatic lipase releases fatty acids from sn1 and sn3 positions of dietary triacylglycerols, yielding monoacylglycerols, diacylglycerols, and free fatty acids (Figure 1). It is a 50 kDa, 449-amino acid protein with two structural domains (Ct and Nt). The catalytic triad (Ser152, Asp176, and His263) is located in the Nt domain, while the Ct domain contains the interaction site with colipase [231]. Colipase is a small protein (10 kDa), also secreted by the pancreas, that acts as a cofactor required for the activity of pancreatic lipase in the presence of bile salts. Colipase anchors pancreatic lipase to the lipid/water interface of the micelles formed between bile salts and long-chain triacylglycerols, allowing it to display its catalytic activity [236].
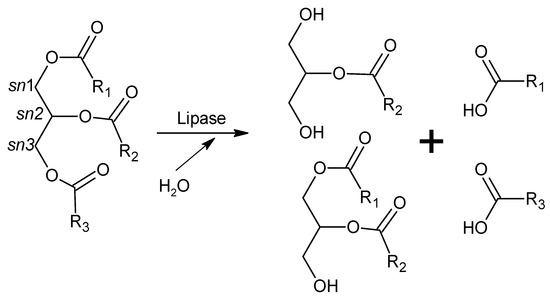
Figure 1.
Hydrolysis of triglyceride by lipase activity. R1, R2, and R3 are fatty acid residues.
4.5.2. Phenolics from Soybean and Chickpea as Inhibitors of α-amylase, α-glucosidase, and Lipase
Few studies have explored the inhibition of carbohydrate and lipid hydrolases by chickpea and soybean phenolic compounds. Most have only worked with phenolic-rich extracts, and just a few have thoroughly characterized the extracts and identified the bioactive compound(s). In Table 5 and Table 6, the main findings of these studies, most of which have focused on α-glucosidase (Table 5) and α-amylase (Table 6), are summarized.

Table 5.
Inhibition of α-glucosidase by soybean and chickpea phenolic extracts.

Table 6.
Inhibition of α-amylase by soybean and chickpea phenolic extracts.
The inhibition of both carbohydrate hydrolases (α-amylase and α-glucosidase) by phenolic extracts of chickpea [125,127] and soybean [129,237] has been studied in the last decades. All these studies found a similar inhibitory activity for both enzymes, except McCue et al. [237] who found that soybean extracts were better inhibitors of α-amylase than α-glucosidase. The chickpea extract studied by Sreerama et al. [119,127] contained phenolic acids and flavonoids, such as flavonols, isoflavones, and proanthocyanidins, although the authors did not identify the most bioactive component responsible for enzyme inhibition. The chickpea extract had lower α-glucosidase inhibitory activity and intermediate α-amylase inhibitory activity, compared with other legumes (cowpea [Vigna unguiculata] and horse gram [Macrotyloma uniflorum]), and it was a better inhibitor of both enzymes than the soybean extracts studied by Ademiluyi and Oboh [129]. The studies of Sánchez-Magaña et al. [125] and McCue et al. [237] showed that fermentation increased the enzyme inhibitory activity of chickpea and soybean phenolic extracts, respectively.
Several studies have analyzed the inhibition of α-glucosidases by phenolic-rich chickpea and soybean extracts. Yao et al. [131] compared the α-glucosidase inhibition (as a percentage) elicited by phenolic-rich ethanolic extracts of chickpea, soybean, and other legumes, such as peas and different kinds of beans. The chickpea extract was found to be a better α-glucosidase inhibitor than the soybean extract, but both showed considerably lower activity than extracts of various bean species, of which Adzuki bean (Vigna angularis) had the highest activity. The authors found no correlation between α-glucosidase inhibition and phenolic acid content or antioxidant activity [131]. This lack of correlation was also reported by Tiwari et al. [238], who studied the effect of sprouting on the α-glucosidase inhibitory activity, antioxidant, and other biological activities of chickpea extracts. Interestingly, the authors found mitigation of postprandial glycemic response in rats pretreated with extracts (sprouted and non-sprouted) of one chickpea variety, but this effect was not correlated with the α-glucosidase inhibitory activity [238]. In contrast, Lee et al. [132] did find a correlation between phenolic content of soybean extracts obtained with different solvent polarity (different methanol content, acetone, dichloromethane) and α-glucosidase inhibition. Authors observed the highest α-glucosidase inhibitory activity in the 70% methanol extract (higher than acarbose, positive control), which also presented the highest total phenol content and individual isoflavones (mainly glucosides, malonylglucosides, and acetylglucosides). In another study, Lee et al. [239] evaluated the effect of fermentation on ten varieties of soymilk over the inhibitory activity against α-glucosidase and lipase. Fermentation increased the inhibitory activity toward both enzymes, and the authors observed a correlation between total phenolic compounds and enzyme inhibition. As expected, upon fermentation, glycosylated isoflavones content decreased while aglycone content increased. Other studies have found that food products containing chickpea and soybean also possess α-glucosidase inhibitory activity. Furthermore, treatments like cooking, sprouting, or fermentation generally increases the capacity of the phenolics obtained from these feedstocks, inhibiting the activity of the aforementioned enzyme [132,238,239,240,241]. None of the studies analyzed the inhibitory mechanisms of the extracts or explored the molecular interactions between α-glucosidase and the chickpea or soybean bioactive.
Most studies published to date on the inhibition of α-amylase by chickpea extracts have used water as a solvent and considered the presence of inhibitors as an antinutritional factor. However, the extracts have generally shown low inhibition of α-amylase (as compared with other pulses, such as soybean and other beans), which is further decreased by cooking, suggesting the presence of peptide inhibitors and a low antinutritional potential of chickpea [243,244].
The work of Moussou et al. [245] found a moderate inhibitory activity of a chickpea water extract, which was slightly enhanced by thermal treatment; therefore, although they did not identify the inhibitory compounds in the extract, their heat-stability could indicate the presence of phenolic compounds. The authors also highlighted the health-promoting potential of α-amylase inhibitors, for their ability to avoid a rapid rise in postprandial glucose, which is a risk factor for the development of diabetic complications, in opposition to their antinutritional effects, which would be evident only in case of excessive α-amylase inhibition.
Shi et al. [243] compared the α-amylase inhibitory activity of aqueous extracts of chickpea, soybean, and other pulses (peas, lentils, and beans) and found that only beans (Phaseolus vulgaris) and soybean possessed this activity. The authors also found high protease inhibitory activity in the soybean extracts, but this was due to the presence of inhibitory peptides, which are significantly degraded upon cooking [243]. Another study showed that the α-amylase inhibitory activity of soybean aqueous extracts increased during fermentation, depending on the time of fermentation and the species of microorganism [246].
Inhibition of pancreatic lipase by chickpea and soybean has been less studied. In Table 7, the main findings of these studies are summarized. Lee et al. [247] studied the inhibition of aqueous methanolic extracts obtained from defatted chickpea and soybean flours. Although the authors did not identify the bioactive compounds in the extract, aqueous methanol was one of the preferred solvents for the extraction of phenolic compounds. The chickpea and soybean extracts showed similar EC50 values, slightly lower than that of mung bean (Vigna radiata), but in the range of red bean (Vigna angularis) and black-eyed pea (Vigna unguiculata). Interestingly, the activity of chickpea was not affected by simulated in vitro digestion. Two studies that analyzed the effect of fermentation on soybean anti-lipase potential found a decrease in glycosylated flavonoids (including isoflavones), an increase in isoflavone and other flavonoid aglycones, and an increase in lipase inhibitory activity in the extracts of fermented soy products [239,248]. This indicated that the anti-lipase activity of aglycones was superior to that of glycosides.

Table 7.
Inhibition of α-glucosidase by soybean and chickpea phenolic extracts.
Tan et al. [242] carried out a detailed study on the inhibition of the three digestive enzymes (α-glucosidase, α-amylase, and lipase) by crude, semi-purified, and fractionated phenolic extracts, as well as pure phenolic compounds of black soybean and other foods. They found that the soybean crude and semi-purified extracts were among the most potent inhibitors of the three enzymes with greater activity against α-glucosidase > lipase > α-amylase. In addition, some extract fractions rich in total phenolic compounds, flavonoids, and condensed tannins were better α-glucosidase and α-amylase inhibitors than commercially available inhibitors. Correlations were found between total phenol and total flavonoid content and IC50 values against α-glucosidase; and between condensed tannin content and IC50 against α-amylase. The authors observed that phytochemical content and antioxidant activity were not the only predictors for enzyme inhibition and suggested that some specific structures might have a stronger inhibitory effect on certain enzymes [242].
4.5.3. Phenolic Compounds and Digestive Enzymes and Their Structure-Activity Relationship (SAR)
As described in the previous section, the published studies regarding the inhibition of digestive enzymes by chickpea and soybean phenolic compounds are scarce, and their inhibition results are only reported as percentage of inhibition, half maximal inhibitory concentration (EC50), or enzymatic inhibition index, without giving information on the inhibition kinetics or the mechanism of action. However, considering the abundance of total and individual phenolic compounds found in chickpeas and soybeans, it is possible to propose the mechanism of action of these phenolic compounds as digestive enzyme inhibitors.
Some authors have summarized the possible mechanisms of action of phenolic compounds on the inhibition of digestive enzymes. They have also proposed the main structure-activity relationship (SAR) that regulates these inhibitory processes [231,249,250,251,252,253,254]. Even though, in some cases, semi-purified extracts have been used to evaluate the inhibition patterns of phenolic compounds, in order to propose the inhibitory mechanisms, it is preferable to evaluate the inhibitory activity of pure phenolic compounds since synergistic or antagonistic effects can be observed among them or with other compounds, such as sugars or organic acids, normally present in phenolic extracts, sometimes even at higher concentrations than the phenolic compounds [250,255].
In general terms, it is accepted that the inhibitory activity of phenolic compounds against digestive enzymes depends on two main factors: (i) structural characteristics of the enzyme, including hydrophobicity, isoelectric point, size, and amino acid composition [252]. A higher inhibition of α-amylase (55 kDa) than α-glucosidase (260 kDa) has been reported by some authors, suggesting that the size of the enzyme plays an important role in their inhibition by phenolic compounds [125,256]. Phenolic compounds are more active toward carbohydrate-hydrolyzing enzymes, compared to lipases, probably because lipases are active in a less polar environment [231]. Extraction solvent polarity also plays an important role in enzyme inhibition by phenolic compounds. In this context, Benatti Justino et al. [257] reported that phenolic extracts obtained with low polarity solvents (butanol, ethyl acetate) showed higher inhibitory activities toward the three digestive enzymes compared to extracts obtained with polar solvents (water, ethanol). In another study, Tan et al. [242] observed that while amylase and glucosidase enzymes were highly inhibited by condensed tannins extracted from soybeans, lipase was better inhibited by flavonoids rich extracts. (ii) structural characteristics of phenolic compounds, including molecular weight, number of hydroxyl groups, glycosylation, and hydrogenation [231,252]. It has been extensively reported that phenolic acids show lower inhibitory activity than flavonoids and that the activity of phenolic acids increases with the number of hydroxyl groups present in their structure [231]. In the case of polymeric compounds (condensed tannins), oligomeric structures (<10 monomeric units) show higher inhibitory activity compared to polymeric structures (>10 monomeric units) and monomeric phenolic compounds, probably because oligomeric structures are able to form specific interactions with the active site of the enzyme, while bigger structures form non-specific interactions within all the enzyme surface [258] and monomeric compounds form less or weaker interactions. Interestingly, a synergic effect of monomeric phenolic compounds and condensed tannins with different degree of polymerization from black bean extracts has been reported for the inhibition of α-amylase, α-glucosidase, and lipase [242].
In order to gain information on the inhibitory mechanism of action of phenolic compounds against digestive enzymes, kinetic studies, in combination with other techniques, such as fluorescence spectroscopy, calorimetric studies, and in silico analyses by molecular docking of pure compounds, are necessary. The inhibition of digestive enzymes by phenolic compounds is mainly due to spontaneous (ΔG −) reversible non-covalent specific interactions [231,250], even though in the case of high molecular weight proanthocyanidins (condensed tannins), non-specific covalent and non-covalent interactions may occur [258]. Hydrophobic interactions, hydrogen bonds, van der Waals, and electrostatic interactions are the main forces involved in the phenolic compounds-digestive enzymes interactions [231,250]. Hydrophobic interactions (ΔH +, ΔS +) occur mainly between two aromatic groups (π stacking interactions), one from the phenolic compound, and the second one from an aromatic residue in the enzyme. van der Waals forces (ΔH −, ΔS −) are present between the aromatic ring of the phenolic compound and methyl groups or aliphatic chains in the enzyme. Hydrogen bonds (ΔH −, ΔS −) occur between hydroxyl groups from the phenolic compound and oxo or hydroxyl groups in the enzyme. Finally, electrostatic interactions occur between hydroxyl groups from the phenolic compound and charged amino groups from Lys or Arg residues [231,250]. The number and intensity of these non-covalent interactions are regulated by physicochemical parameters, such as temperature, solvent polarity, pH, and saline concentration.
The main inhibition mechanisms reported for phenolic compounds as inhibitors of digestive enzymes is mixed-type inhibition, in which both competitive (ki) and non-competitive (ki’) components are present [231,250,259,260], indicating that the phenolic compound may bind both near the binding site and/or in another region of the enzyme and that they can bind both the free enzyme and the enzyme-substrate complex. Flavonoids have shown mixed-type inhibition of both α-amylase and lipase, in which the competitive component (inhibitor binds tighter with free enzyme) regulates the process [259,260]. Similar results were observed for caffeic and p-coumaric acids with lipase [259].
Structure-activity relationship (SAR) is an analytical (qualitative or quantitative) technique that allows us to determine the association between the structure of bioactive compounds to their biological/chemical activities. For phenolic compounds, it has been used to determine the association between their main structural characteristics (number and position of hydroxyl groups, number of conjugated double bonds, methylation, glycation, galloylation) and their antioxidant, antiproliferative, and enzyme inhibitory activities. SAR analysis combines the kinetic, spectroscopic, and in silico modeling (molecular docking) results in order to better understand the main structural characteristics of phenolic compounds that allow them to inhibit the digestive enzymes. With these studies, it is possible to propose the binding site as well as the type of interaction present between the enzyme and the different phenolic compounds.
SAR analysis has been used mainly to describe the structural characteristics of flavonoid-enzyme interactions, because, as previously described, phenolic acids showed lower inhibitory activity toward digestive enzymes. Table 8 summarizes the main structural characteristics of flavonoids that affect their inhibitory activity of α-amylase, α-glucosidase, and lipase [231,249,250,251,252,253,254,261].

Table 8.
Structure-activity relationship of flavonoids-digestive enzymes inhibition.
As shown in Table 8, the unsaturation of the C2–C3 bond in combination with the 4-oxo group (both in ring C), which provides rigidity and planarity to the flavonoid structure, increases the inhibitory activity toward the three digestive enzymes. In this context, flavan-3-ol (catechin), which lacks both the double bond at C2–C3 and 4-oxo group, showed neither lipase nor α-amylase inhibitory activity [231,260].
Increasing the number of hydroxyl groups in all rings is another relevant characteristic for enzyme inhibition. This inhibitory effect is more evident when ortho diphenols (catechol) are present in the structure. Substitution of hydroxyl groups in any of the rings for methoxy or glycoside moieties drastically reduces the enzymatic activity due to the loss of hydrogen bonds and steric hindrance. Interestingly, when this glycoside moiety is replaced by a phenolic acid (gallic acid), the α-glucosidase activity of the flavonoids is increased [253]. Finally, the substitution of C3 hydroxyl group in ring C of flavonoids by a galloyl moiety increases the enzymatic inhibition of the three digestive enzymes, due to the increase in the number of hydroxyl groups in the molecule.
Few SAR studies have been carried out to evaluate the effect of isoflavones against digestive enzymes [249,251,253,254,262]. Tadera et al. [262] reported lower inhibition of α-glucosidase than α-amylase in the presence of daidzein (2 hydroxyl groups at positions 7 and 4’) and genistein (3 hydroxyl groups at positions 5, 7, and 4’). They also observed that the inhibition of both enzymes was higher with genistein, indicating that hydroxylation of isoflavone ring A increased the inhibitory activity against α-amylase and α-glucosidase. In the case of ring B, no systematic effect of hydroxylation was observed. Methoxylation of hydroxyl groups in all rings decreased the inhibitory activity of isoflavones. The same effect was observed upon glycosylation of hydroxyl groups in ring C [253,254].
In order to confirm the structure-activity relationship and the mechanism of action of phenolic compounds in digestive enzyme inhibition, in silico studies by molecular docking and molecular potential analyses are performed. Docking analysis predicts the possible binding sites between phenolic compounds and digestive enzymes [258,260]. For this, the minimal free energy change (ΔG) and the structure of ligand (phenolic compound)-protein complex is determined, in order to propose the most favorable binding pockets and ligand conformation. Docking results predict the main interactions between amino acid residues with phenolic compounds, as well as the main non-specific covalent interactions present in the complex formation. Docking results confirm that the main interactions between digestive enzymes and monomeric phenolic compounds (phenolic acids and flavonoids) occurred near the active site, through hydrophobic interactions, hydrogen bonds, van der Waals, and electrostatic interactions, in agreement with kinetic and spectroscopic results. Interestingly, docking results obtained between proanthocyanidin oligomers (heptamers) and α-amylase showed multiple interaction sites with the surface of the enzyme through numerous hydrophobic interactions, instead of one single interaction site with the catalytic cavity [258]. Similar results were obtained for the interaction of proanthocyanidin oligomers with lipase. Trimer and tetramer proanthocyanidins interacted in the lipase-colipase binding region through hydrogen bonding, resulting in a higher inhibition compared to a heptamer proanthocyanidin, which interacted only with colipase in a region not critical for the lipase-colipase union, necessary to catalyze the triacylglycerol hydrolysis [258].
From the analysis of the information given in the present review, it is evident that both chickpea and soybean may inhibit digestive enzymes by the action of monomeric and polymeric phenolic compounds present in their matrix. However, further studies are necessary in order to be able to identify the individual inhibitory compounds and propose their mechanism of action against digestive enzymes, as well as to evaluate the possible synergistic or antagonistic effect of these phenolic compounds between them and with other molecules present in the extracts.
4.5.4. Prevention of Type 2 Diabetes and Obesity Beyond Inhibition of Digestive Enzymes
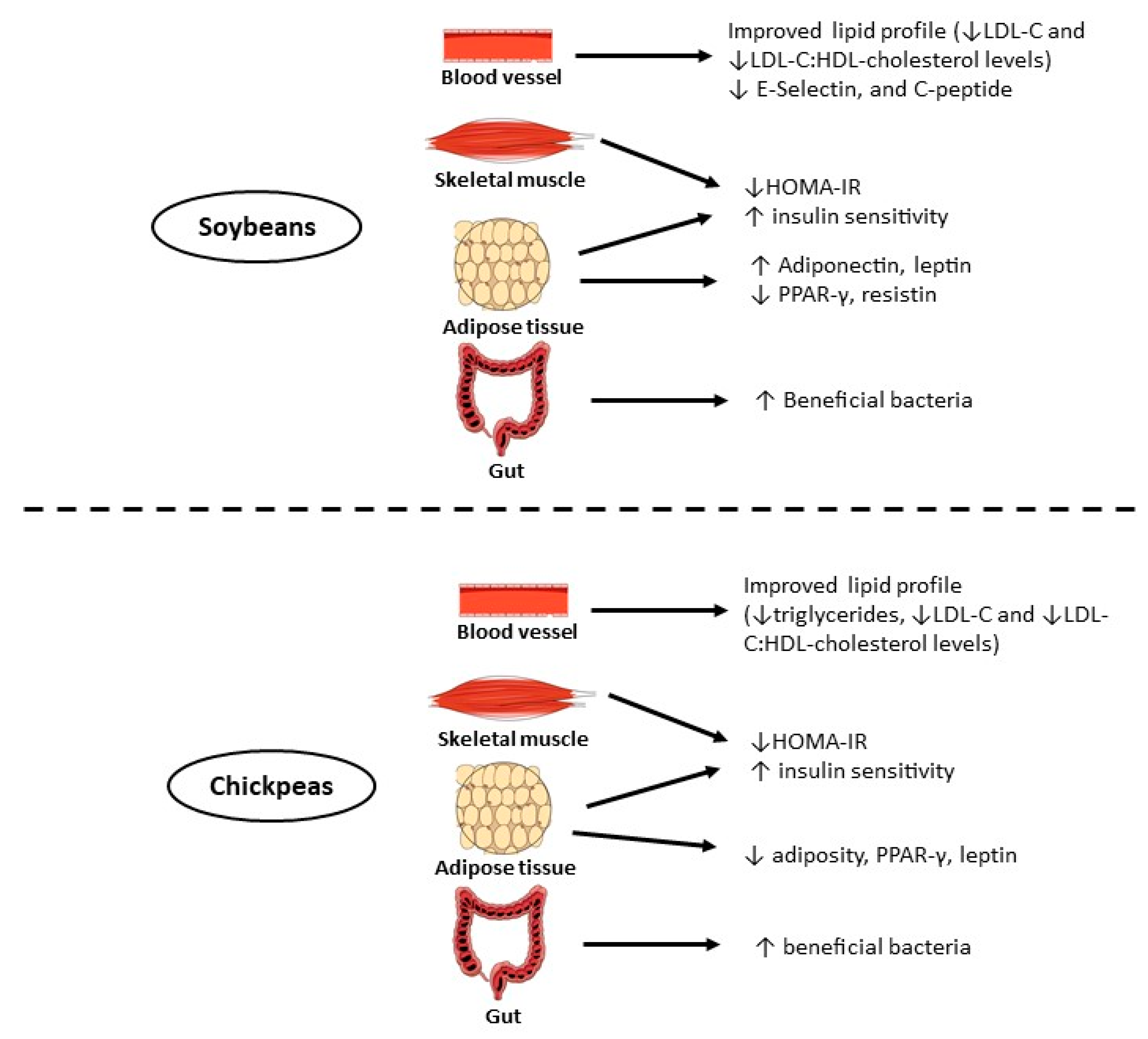
The evidence about chickpea in relation to obesity and diabetes prevention have mainly been focused on the impact of the bioactive phenolic compounds (phenolic acids and flavonoids), bioactive peptides, carotenoids, phytoesterols, and fermentable fibers [47]. In addition, soybean, chickpea, and other legumes have a low glycemic index, which is important for patients with type 2 diabetes [263]. The proposed mechanisms of action of soybean, chickpea, and their bioactive compounds on insulin resistance, obesity, and lipid metabolism are summarized in Figure 2 and Table 9.
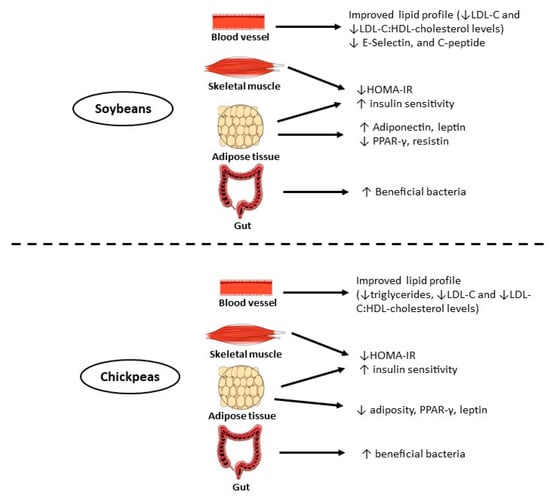
Figure 2.
Proposed mechanisms of action for the reduction of insulin resistance by soybeans and chickpeas. Abbreviations: ↓, decrease; ↑, increase; PPAR-γ, peroxisome proliferator-activated receptor-γ; HOMA-IR, homeostasis model assessment-insulin resistance; LDL-c: low-density lipoprotein cholesterol; HDL: high-density lipoprotein.

Table 9.
Chickpea, soybean, and isoflavones in the prevention of type 2 diabetes and obesity and associated metabolic biomarkers in vivo.
Leptin, a hormone produced by adipose cells, modulates energy balance and inhibits hunger. A decreased sensitivity to leptin in obesity jeopardizes detection of satiety even when high levels of this hormone and high energy stores occur. Among the mechanisms listed in Figure 2, human evidence on the role of soybean isoflavones in modulating sensitivity to leptin is still inconclusive [264,265,266] and needs further investigation. Furthermore, to the best of our knowledge, there is still a gap in the available literature with respect to the effects of isoflavones from chickpea.
Supplementation with soybean, chickpea, or their respective isoflavones can attenuate insulin resistance, possibly by reducing adiposity [94,185]. Furthermore, non-alcoholic fatty liver disease (NAFLD) is associated with an increase in the prevalence of obesity and diabetes [267]. The effects of soy isoflavones on the development of NAFLD in animal models, as well as the mechanisms involved (e.g., lipid metabolism, oxidative stress, and inflammation), were summarized by Van De Wier et al. [268]. According to these authors, histological studies related to inflammation support the anti-inflammatory effect of isoflavones. Finally, the same report suggests that clinical trials on the use of soy isoflavones in NAFLD patients are still necessary.
Yang et al. [185] showed that chickpea attenuated hyperglycemic, hyperinsulinemic, visceral adiposity, and lipid accumulation in rats induced by a chronic high-fat diet. Animal studies have shown that soy protein and soy isoflavones could improve insulin sensitivity and glucose control [269,270,271], and population studies suggested a lower risk of insulin resistance and type 2 diabetes [272,273]. A meta-analysis suggested an inverse association between soy food consumption (soy protein and isoflavones) and risk of type 2 diabetes mellitus [274]. Post-menopausal women who consumed a high soy diet had a lower body mass index and fasting insulin [275]. These protective effects can be found, especially, in women probably because phytoestrogen is structurally similar to female hormone estradiol with receptor-mediated estrogenic activity, and probably may affect insulin modulation [274].
Several in vivo studies have reported the beneficial effect of daidzein and genistein intake in the glycemic regulation among different animal models. A high-fat diet supplemented with soy isoflavones (0.4%, w/w) reduced weight gain, improved glucose metabolism and insulin sensitivity, reduced hepatic lipid accumulation, and increased gene expression of Cpt1α and Acox-1 associated with lipid oxidation in C57BL/6J mice [276]. Genistein and daidzein showed to exert anti-diabetic effects by modulating hepatic glucose (glucokinase, glucose-6-phosphate dehydrogenase) and lipid (fatty acid synthase, carnitine palmitoyltransferase, β-oxidation), regulating enzyme activities in db/db mice [277]. Genistein and daidzein treatment for 9 weeks caused a reduction in fasting blood glucose with concomitant decrease in plasma insulin and C peptide levels by down-regulating glucose-6-phosphate dehydrogenase (G6PD), phosphoenolpyruvate carboxykinase, fatty acid β-oxidation, and carnitine palmitoyltransferase activities, while up-regulating malic enzyme and G6PD activities in liver with preservation of pancreatic β-cells in non-obese diabetic mice [278].
Many studies have reported regulatory mechanisms of isoflavones on glucose tolerance, insulin secretion, and pancreatic β-cells in streptozotocin (STZ)-induced diabetic rats. El-Kordy and Alshahrani [285] demonstrated a protective and regenerative effect of genistein on pancreatic β-cell damage and improvement in serum levels of insulin and glucose in STZ-induced diabetic rats in a dose-dependent manner. Isolated soy protein and genistein were shown to regulate hyperglycemia in STZ-induced diabetic rats [152]. Another study also demonstrated the effect of genistein on glycemia, glucose tolerance, and insulin levels in STZ-induced diabetic mice [286]. Wright et al. [287] suggested that genistein has a direct inhibitory effect on GLUT4 in rat adipocytes and soleus muscles.
Dietary soy isoflavone supplementation (genistein, daidzein, and glycitein 0.1% w/w) reduced fat deposition in an animal model of obesity and diabetes [282]. Administration of soybean isoflavones (150 and 450 mg/kg) in rats lowered fasting insulin levels and HOMA-IR, reduced total white adipose tissue weight, lowered plasma resistin levels, and increased circulating protein and mRNA levels of adiponectin in perirenal white adipose tissue compared to the insulin resistant control group [283]. Furthermore, according to the same study [283], the higher supplementation dose of soy isoflavones (450 mg/kg/day) increased circulating protein and adipose mRNA levels of leptin and lowered adipose mRNA levels of resistin compared to the insulin resistant control group.
Higher risk for obesity and insulin resistance due to the decline of estrogen has been reported in postmenopausal women [288]. However, phytoestrogens (e.g., isoflavones) may reduce the risk of these metabolic diseases. Accordingly, Choi et al. [268] investigated the effect of genistein supplementation (0.1%, w/w) in a high-fat diet ovariectomized rats. Treated rats showed smaller-sized adipocytes and decreased HOMA-IR index, reaching levels comparable to those of the non-ovariectomized group [288]. In addition, genistein supplementation resulted in the reduction of hepatic fatty acid synthase activity, fatty acid synthesis genes, and showed an up-regulation in carnitine palmitoyltransferase, β-oxidation, succinate dehydrogenase activity, and fat utilization genes [288].
Peroxisome proliferator-activated receptor (PPAR) and their subtypes (alpha, beta, gamma) are related to insulin sensitivity [289]. PPAR-γ from adipose tissue indirectly modulates glucose and lipid homeostasis due to its regulation of adipocyte differentiation [290]. It has been suggested that chickpeas and soybeans isoflavones can down-regulate PPAR-γ, preventing the development of large and dysfunctional adipocytes that occurs in obesity and insulin resistance [291]. Luo et al. [276] evaluated the effect of soy isoflavones (genistein and daidzein) on C57BL/6J mice fed with a low-fat diet, western diet (WD), WD + 0.16% (w/w) of genistein or daidzein. Genistein and daidzein decreased food intake, body weight gain and induced liver X Receptor (LXR)-mediated pathways. Furthermore, a higher reduction of energy intake (26%) was found in genistein-treated mice compared to that of daidzein (8%). According to these authors, the reduction of food intake may stem from the activation of transcription factor PPARα due to genistein and daidzein intake. PPARα agonist transiently decreases the intake of food. In addition, reduced food intake associated with PPARα agonist treatment may be associated with cholecystokinin (CCK)-A receptor production. CCK is secreted from duodenal and jejunal mucosal cells in response to the intake of fat and protein. CCK has several physiological effects, including slowing gastric emptying and suppressing energy intake.
Phosphoenolpyruvate carboxykinase (PEPCK) activity regulate normal blood glucose levels and has been shown to be increased in diabetic animal models. Dkhar et al. [284] investigated the effect of genistein on the expression of PEPCK and glucose production in alloxan-induced diabetic mice. Genistein (50 mg/kg body weight) reduced fasting glucose levels, PEPCK-C expression and increased AMPK and ERK1/2 phosphorylation states in the liver of the genistein-treated alloxan-induced diabetic mice [284]. The same study also showed that glucose production in HepG2 cells was reduced by about 50% in cells treated with genistein (30 µM).
Epidemiological and prospective studies have shown the beneficial effects of soy-foods consumption in lowering the incidence of diabetes type 2 [292,293,294]. A randomized, cross-over, double-blinded trial of dietary supplementation with soy phytoestrogens in postmenopausal women resulted in lower values for fasting insulin, insulin resistance, and glycated hemoglobin A1c, when compared with placebo [190]. Soybean inclusion in the diet decreased insulin resistance and fasting plasma glucose in a randomized cross-over clinical trial with postmenopausal women with metabolic syndrome [191]. Li et al. [281] compared the effects of a soy-based meal replacement versus an individualized diet plan on weight loss and metabolic profile in diabetic patients randomized to treatment. The soy-based group underwent a greater weight loss, fasting plasma glucose (6 months), glycated hemoglobin A1c, and high sensitivity C-reactive protein. Furthermore, a greater number of subjects in a soy-based meal replacement group reduced their use of sulfonylureas and metformin as compared to the individualized diet plan. Medications, such as anti-hyperglycemic, are provided by the government in some places like Brazil [295]. Therefore, by improving health and reducing the use of anti-hyperglycemic medications, the inclusion of functional foods in the diet may also be helpful to overcome an eventual economic burden.
High serum concentrations of soluble E-selectin have been found to correlate with obesity in patients with type 2 diabetes mellitus [169]. Human intervention studies focusing on the health effects of chickpea and its components are also available in the literature. Incorporation of chickpeas in the diet reduced serum total fasting insulin concentration and HOMA-IR in healthy subjects [187]. Chickpea-based meal led to a lower glucose and insulin response in an acute consumption compared with a wheat-based meal [280]. A systematic review and meta-analysis of randomized controlled experimental trials in people with and without diabetes were carried out by Sievenpiper et al. [296]. According to these authors, pulses alone or in low-glycemic-index or high-fiber diets improve markers of longer-term glycemic control in humans.
Polyphenols can also play a role by modulating gut microbiota in such a prebiotic-like effect [23,297]. In addition, the literature has shown that the addition of excess fructooligosaccharide (FOS) may preserve genistein in human gut microflora in vitro [298]. Guadamuro [299] suggested that isoflavones may act as an alternative energy source, thus increasing the production of equol and short-chain fatty acids (SCFA), which are pivotal bacterial metabolites related to intestinal health benefits. The possible role of SCFA on specific receptors that influence gut epithelial, enteroendocrine, and pancreatic β-cells, as well as blood and renal vessels, adipose tissue, and peripheral nervous system, has been summarized by Li et al. [300]. Therefore, as sources of isoflavones, chickpea and soybean may promote the secretion of SCFA and, hence, their potential benefits do not rely only on the release of bioavailable isoflavones in the plasma and other organs but also in indirectly mediating receptors related to several chronic ailments.
5. Conclusions
Isoflavones as aglycones are usually more bioavailable than their conjugated counterparts. Furthermore, the literature has supported their higher antioxidant activity compared to that of their conjugates. The contents of daidzein and genistein in chickpeas are comparable to the concentrations reported in soybeans, which suggests that this legume seed may potentially substitute soybeans as a source of isoflavone aglycones. Contrary to soybean, there is very little information in the literature on the role of genetics with respect to the polyphenolic composition of chickpea, and, to the best of our knowledge, there is no study associated with the genetic regulation of isoflavones in chickpea seeds. Much has been discussed about the role of oxidative stress and inflammation in several chronic ailments, and we have demonstrated that both legumes show antioxidant and anti-inflammatory potential. Hence, their ability in preventing cardiovascular diseases and certain types of cancer appears to be irrefutable. In contrast, due to their lower lipid content, chickpea seems to offer a better option in weight management and prevention of type 2 diabetes. Several biomarkers related to these two diseases were discussed, and the effect of isoflavones in inhibiting digestive enzymes must be better clarified. Likewise, among the mechanisms discussed, human evidence on the role of soybean isoflavones in modulating sensitivity to leptin is still inconclusive, which warrants further investigation.
Author Contributions
Conceptualization, A.C.d.C., A.R.S., and F.S.; Writing—Original Draft Preparation, A.C.d.C. (Abstract, Conclusion, Section 1, Section 3, Section 4.1, Section 4.3, Section 4.4, Section 4.5.4 and Section 5); A.R.S. and B.T.F. (Section 1 and Section 2); M.C.M. (Section 3 and Section 4.1); E.A.-P. and L.A.d.l.R. (Section 4.5, Section 4.5.1, Section 4.5.2 and Section 4.5.3); M.R.M.J. and M.V.G. (Section 4.5.4); M.F. (Section 4.2); Final Review and Editing, A.C.d.C., A.R.S., and F.S.
Funding
The article processing charge was funded by Conicyt (Fondecyt Regular n° 1161298).
Acknowledgments
A.C.C. and A.R.S. acknowledge Conicyt (Fondecyt Postdoctorado n°3180432 and Fondecyt Regular n° 1161298). M.F. acknowledges FAPESP (Process 2016/15563-9). M.R.M.J. thanks Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) code 001; CNPq (403328/2016-0; 301108/2016-1) and FAPESP (2015/50333-1). F.S. thanks the Natural Science and Engineering Research Council (NSERC) of Canada for partial financial support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Messina, M.J. Legumes and soybeans: Overview of their nutritional profiles and health effects. Am. J. Clin. Nutr. 1999, 70, 439s–450s. [Google Scholar] [CrossRef]
- Carter, T.E.; Hymowitz, T.; Nelson, R.L. Biogeography, local adaptation, Vavilov, and genetic diversity in soybean. In Proceedings of Biological Resources and Migration; Springer: Berlin/Heidelberg, Germany, 2004; pp. 47–59. [Google Scholar]
- Kim, M.Y.; Van, K.; Kang, Y.J.; Kim, K.H.; Lee, S.H. Tracing soybean domestication history: From nucleotide to genome. Breed. Sci. 2012, 61, 445–452. [Google Scholar] [CrossRef]
- Leamy, L.J.; Zhang, H.; Li, C.; Chen, C.Y.; Song, B.-H. A genome-wide association study of seed composition traits in wild soybean (Glycine soja). BMC Genomics 2017, 18, 18. [Google Scholar]
- Wang, K.J.; Li, X.H. Interspecific gene flow and the origin of semi-wild soybean revealed by capturing the natural occurrence of introgression between wild and cultivated soybean populations. Plant Breed. 2011, 130, 117–127. [Google Scholar] [CrossRef]
- Xu, D.; Abe, J.; Gai, J.; Shimamoto, Y. Diversity of chloroplast DNA SSRs in wild and cultivated soybeans: Evidence for multiple origins of cultivated soybean. Theor. Appl. Genet. 2002, 105, 645–653. [Google Scholar] [CrossRef]
- Qiu, J.; Wang, Y.; Wu, S.; Wang, Y.-Y.; Ye, C.-Y.; Bai, X.; Li, Z.; Yan, C.; Wang, W.; Wang, Z.; et al. Genome re-sequencing of semi-wild soybean reveals a complex Soja population structure and deep introgression. PLoS ONE 2014, 9, e108479. [Google Scholar] [CrossRef] [PubMed]
- Sedivy, E.J.; Wu, F.; Hanzawa, Y. Soybean domestication: The origin, genetic architecture and molecular bases. New Phytol. 2017, 214, 539–553. [Google Scholar] [CrossRef]
- Pratap, A.; Gupta, S.K.; Kumar, J.; Solanki, R.K. Technological innovations in major world oil crops. In Soybean; Springer New York: New York, NY, USA, 2012; Volume 1, pp. 293–321. [Google Scholar]
- Black, M.; Bewley, J.D.; Halmer, P. The encyclopedia of seeds: Science, technology and uses. Ann. Bot. 2006, 100, 1379. [Google Scholar]
- FAOSTAT. Food and Agriculture Organization of the United Nations, Statistics Division. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 29 January 2019).
- ISAAA. Global Status of Commercialized Biotech/GM Crops in 2017: Biotech Crop Adoption Surges as Economic Benefits Accumulate in 22 Years. ISAAA Brief No. 53. Available online: http://www.isaaa.org/resources/publications/briefs/53/download/isaaa-brief-53-2017.pdf (accessed on 29 January 2019).
- Gupta, S.; Nawaz, K.; Parween, S.; Roy, R.; Sahu, K.; Kumar Pole, A.; Khandal, H.; Srivastava, R.; Kumar Parida, S.; Chattopadhyay, D. Draft genome sequence of Cicer reticulatum L., the wild progenitor of chickpea provides a resource for agronomic trait improvement. DNA Res. 2017, 24, 1–10. [Google Scholar] [PubMed]
- Thudi, M.; Khan, A.W.; Kumar, V.; Gaur, P.M.; Katta, K.; Garg, V.; Roorkiwal, M.; Samineni, S.; Varshney, R.K. Whole genome re-sequencing reveals genome-wide variations among parental lines of 16 mapping populations in chickpea (Cicer arietinum L.). BMC Plant Biol. 2016, 16, 10. [Google Scholar] [CrossRef]
- Preece, C.; Livarda, A.; Christin, P.-A.; Wallace, M.; Martin, G.; Charles, M.; Jones, G.; Rees, M.; Osborne, C.P. How did the domestication of Fertile Crescent grain crops increase their yields? Funct. Ecol. 2017, 31, 387–397. [Google Scholar] [CrossRef]
- Harlan, J.R. Agricultural origins: Centers and noncenters. Science 1971, 174, 468–474. [Google Scholar] [CrossRef]
- Abbo, S.; Berger, J.; Turner, N.C. Evolution of cultivated chickpea: Four bottlenecks limit diversity and constrain adaptation. Funct. Plant Biol. 2003, 30, 1081–1087. [Google Scholar] [CrossRef]
- Jha, U.C. Current advances in chickpea genomics: Applications and future perspectives. Plant Cell Rep. 2018, 37, 947–965. [Google Scholar] [CrossRef]
- Smýkal, P.; Coyne, C.J.; Ambrose, M.J.; Maxted, N.; Schaefer, H.; Blair, M.W.; Berger, J.; Greene, S.L.; Nelson, M.N.; Besharat, N.; et al. Legume crops phylogeny and genetic diversity for science and breeding. CRC Crit. Rev. Plant Sci. 2015, 34, 43–104. [Google Scholar]
- Amalraj, A.; Taylor, J.; Bithell, S.; Li, Y.; Moore, K.; Hobson, K.; Sutton, T. Mapping resistance to Phytophthora root rot identifies independent loci from cultivated (Cicer arietinum L.) and wild (Cicer echinospermum P.H. Davis) chickpea. Theor. Appl. Genet. 2018, 132, 1017–1033. [Google Scholar] [CrossRef]
- Çevik, S.; Akpinar, G.; Yildizli, A.; Kasap, M.; Karaosmanoğlu, K.; Ünyayar, S. Comparative physiological and leaf proteome analysis between drought-tolerant chickpea Cicer reticulatum and drought-sensitive chickpea C. arietinum. J. Biosci. 2019, 44, 20. [Google Scholar] [CrossRef]
- Baginsky, G.C.; Ramos, C.L. Situación de las legumbres en Chile: Una mirada agronómica. Rev. Chil. Nutr. 2018, 45, 21–31. [Google Scholar] [CrossRef]
- de Camargo, A.C.; Schwember, A.R.; Parada, R.; Garcia, S.; Maróstica, M.R.; Franchin, M.; Regitano-d’Arce, M.A.B.; Shahidi, F. Opinion on the hurdles and potential health benefits in value-added use of plant food processing by-products as sources of phenolic compounds. Int. J. Mol. Sci. 2018, 19, 3498. [Google Scholar] [CrossRef]
- Marriott, P.E.; Gomez, L.D.; McQueen-Mason, S.J. Unlocking the potential of lignocellulosic biomass through plant science. New Phytol. 2016, 209, 1366–1381. [Google Scholar] [CrossRef]
- Whitehill, J.G.; Henderson, H.; Schuetz, M.; Skyba, O.; Yuen, M.M.; King, J.; Samuels, A.L.; Mansfield, S.D.; Bohlmann, J. Histology and cell wall biochemistry of stone cells in the physical defence of conifers against insects. Plant Cell Environ. 2016, 39, 1646–1661. [Google Scholar] [CrossRef]
- Wojtasik, W.; Kulma, A.; Dyminska, L.; Hanuza, J.; Czemplik, M.; Szopa, J. Evaluation of the significance of cell wall polymers in flax infected with a pathogenic strain of Fusarium oxysporum. BMC Plant Biol. 2016, 16, 75. [Google Scholar] [CrossRef]
- Stavolone, L.; Lionetti, V. Extracellular matrix in plants and animals: Hooks and locks for viruses. Front. Microbiol. 2017, 8, 1760. [Google Scholar] [CrossRef]
- Castagneyrol, B.; Jactel, H.; Moreira, X. Anti-herbivore defences and insect herbivory: Interactive effects of drought and tree neighbours. J. Ecol. 2018, 106, 2043–2057. [Google Scholar] [CrossRef]
- Qi, G.; Chen, J.; Chang, M.; Chen, H.; Hall, K.; Korin, J.; Liu, F.; Wang, D.; Fu, Z.Q. Pandemonium breaks out: Disruption of salicylic acid-mediated defense by plant pathogens. Mol Plant. 2018, 11, 1427–1439. [Google Scholar] [CrossRef]
- Cooper-Driver, G.A.; Bhattacharya, M.; Harborne, J.B. Role of phenols in plant evolution. Phytochem 1998, 49, 1165–1174. [Google Scholar]
- Petroni, K.; Tonelli, C. Recent advances on the regulation of anthocyanin synthesis in reproductive organs. Plant Sci. 2011, 181, 219–229. [Google Scholar] [CrossRef]
- Davies, K.M.; Albert, N.W.; Schwinn, K.E. From landing lights to mimicry: The molecular regulation of flower colouration and mechanisms for pigmentation patterning. Funct. Plant Biol. 2012, 39, 619–638. [Google Scholar] [CrossRef]
- de Camargo, A.C.; Schewember, A.R. Phenolic-driven sensory changes in functional foods. J. Food Bioact. 2019, 5, 6–7. [Google Scholar] [CrossRef]
- Sanchez Almaraz, R.; Martin Fuentes, M.; Palma Milla, S.; Lopez Plaza, B.; Bermejo Lopez, L.M.; Gomez Candela, C. Fiber-type indication among different pathologies. Nutr. Hosp. 2015, 31, 2372–2383. [Google Scholar]
- Bingham, S.A.; Day, N.E.; Luben, R.; Ferrari, P.; Slimani, N.; Norat, T.; Clavel-Chapelon, F.; Kesse, E.; Nieters, A.; Boeing, H.; et al. Dietary fibre in food and protection against colorectal cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC): An observational study. Lancet 2003, 361, 1496–1501. [Google Scholar] [CrossRef]
- Eshak, E.S.; Iso, H.; Date, C.; Kikuchi, S.; Watanabe, Y.; Wada, Y.; Wakai, K.; Tamakoshi, A.; JACC Study Group. Dietary fiber intake is associated with reduced risk of mortality from cardiovascular disease among Japanese men and women. J. Nutr. 2010, 140, 1445–1453. [Google Scholar]
- Post, R.E.; Mainous, A.G.; King, D.E.; Simpson, K.N. Dietary fiber for the treatment of type 2 diabetes mellitus: A meta-analysis. J. Am. Board Fam. Med. 2012, 25, 16–23. [Google Scholar] [CrossRef]
- Saura-Calixto, F. Antioxidant dietary fiber product: A new concept and a potential food ingredient. J. Agric. Food Chem. 1998, 46, 4303–4306. [Google Scholar] [CrossRef]
- Albishi, T.; John, J.A.; Al-Khalifa, A.S.; Shahidi, F. Antioxidant, anti-inflammatory and DNA scission inhibitory activities of phenolic compounds in selected onion and potato varieties. J. Funct. Foods. 2013, 5, 930–939. [Google Scholar] [CrossRef]
- Albishi, T.; John, J.A.; Al-Khalifa, A.S.; Shahidi, F. Phenolic content and antioxidant activities of selected potato varieties and their processing by-products. J. Funct. Foods 2013, 5, 590–600. [Google Scholar] [CrossRef]
- Zhang, B.; Peng, H.; Deng, Z.; Tsao, R. Phytochemicals of lentil (Lens culinaris) and their antioxidant and anti-inflammatory effects. J. Food Bioact. 2018, 1, 93–103. [Google Scholar] [CrossRef]
- Ambigaipalan, P.; de Camargo, A.C.; Shahidi, F. Identification of phenolic antioxidants and bioactives of pomegranate seeds following juice extraction using HPLC-DAD-ESI-MSn. Food Chem. 2017, 221, 1883–1894. [Google Scholar] [CrossRef]
- Abeywickrama, G.; Debnath, S.C.; Ambigaipalan, P.; Shahidi, F. Phenolics of selected cranberry genotypes (Vaccinium macrocarpon Ait.) and their antioxidant efficacy. J. Agric. Food Chem. 2016, 64, 9342–9351. [Google Scholar] [CrossRef]
- de Camargo, A.C.; Regitano-d’Arce, M.A.B.; Shahidi, F. Phenolic profile of peanut by-products: Antioxidant potential and inhibition of alpha-glucosidase and lipase activities. J. Am. Oil Chem. Soc. 2017, 94, 959–971. [Google Scholar] [CrossRef]
- Saura-Calixto, F. Dietary fiber as a carrier of dietary antioxidants: An essential physiological function. J. Agric. Food Chem. 2011, 59, 43–49. [Google Scholar] [CrossRef]
- Megías, C.; Cortés-Giraldo, I.; Alaiz, M.; Vioque, J.; Girón-Calle, J. Isoflavones in chickpea (Cicer arietinum) protein concentrates. J. Funct. Foods 2016, 21, 186–192. [Google Scholar] [CrossRef]
- Gupta, R.K.; Gupta, K.; Sharma, A.; Das, M.; Ansari, I.A.; Dwivedi, P.D. Health risks and benefits of chickpea (Cicer arietinum) consumption. J. Agric. Food Chem. 2017, 65, 6–22. [Google Scholar] [CrossRef]
- Avilés-Gaxiola, S.; Chuck-Hernández, C.; Rocha-Pizaña, M.D.R.; García-Lara, S.; López-Castillo, L.M.; Serna-Saldívar, S.O. Effect of thermal processing and reducing agents on trypsin inhibitor activity and functional properties of soybean and chickpea protein concentrates. LWT—Food Sci. Technol. 2018, 98, 629–634. [Google Scholar] [CrossRef]
- Sharma, S.; Yadav, N.; Singh, A.; Kumar, R. Nutritional and antinutritional profile of newly developed chickpea (Cicer arietinum L) varieties. Int. Food Res. J. 2013, 20, 805–810. [Google Scholar]
- Wang, N.; Hatcher, D.W.; Tyler, R.T.; Toews, R.; Gawalko, E.J. Effect of cooking on the composition of beans (Phaseolus vulgaris L.) and chickpeas (Cicer arietinum L.). Food Res. Int. 2010, 43, 589–594. [Google Scholar] [CrossRef]
- Cantelli, K.C.; Schmitd, J.T.; Oliveira, M.A.D.; Steffens, J.; Steffens, C.; Leite, R.S.; Carrão-Panizzi, M.C. Brotos de linhagens genéticas de soja: Avaliação das propriedades físico-químicas. Braz. J. Food. Technol. 2017, 20. [Google Scholar] [CrossRef]
- Kan, L.; Nie, S.; Hu, J.; Wang, S.; Bai, Z.; Wang, J.; Zhou, Y.; Jiang, J.; Zeng, Q.; Song, K. Comparative study on the chemical composition, anthocyanins, tocopherols and carotenoids of selected legumes. Food Chem. 2018, 260, 317–326. [Google Scholar] [CrossRef]
- Ramírez-Ojeda, A.M.; Moreno-Rojas, R.; Cámara-Martos, F. Mineral and trace element content in legumes (lentils, chickpeas and beans): Bioaccesibility and probabilistic assessment of the dietary intake. J. Food Compos. Anal. 2018, 73, 17–28. [Google Scholar] [CrossRef]
- Houx Iii, J.H.; Wiebold, W.J.; Fritschi, F.B. Rotation and tillage affect soybean grain composition, yield, and nutrient removal. Field Crop Res. 2014, 164, 12–21. [Google Scholar] [CrossRef]
- Valdez, J.C.; Bolling, B.W. Anthocyanins and intestinal barrier function: A review. J. Food Bioact. 2019, 5, 18–30. [Google Scholar] [CrossRef]
- de Camargo, A.C.; Biasoto, A.C.T.; Schwember, A.R.; Granato, D.; Rasera, G.B.; Franchin, M.; Rosalen, P.L.; Alencar, S.M.; Shahidi, F. Should we ban total phenolics and antioxidant screening methods? The link between antioxidant potential and activation of NF-κB using phenolic compounds from grape by-products. Food Chem. 2019, 290, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Lingappan, K. NF-κB in oxidative stress. Curr. Opin. Toxicol. 2018, 7, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Thakur, V.; Pritchard, M.T.; McMullen, M.R.; Wang, Q.; Nagy, L.E. Chronic ethanol feeding increases activation of NADPH oxidase by lipopolysaccharide in rat Kupffer cells: Role of increased reactive oxygen in LPS-stimulated ERK1/2 activation and TNF-α production. J. Leukoc. Biol. 2006, 79, 1348–1356. [Google Scholar] [CrossRef]
- Gutierrez-Gonzalez, J.J.; Wu, X.; Gillman, J.D.; Lee, J.-D.; Zhong, R.; Yu, O.; Shannon, G.; Ellersieck, M.; Nguyen, H.T.; Sleper, D.A. Intricate environment-modulated genetic networks control isoflavone accumulation in soybean seeds. BMC Plant Biol. 2010, 10, 105. [Google Scholar] [CrossRef]
- Cai, Z.; Cheng, Y.; Ma, Z.; Liu, X.; Ma, Q.; Xia, Q.; Zhang, G.; Mu, Y.; Nian, H. Fine-mapping of QTLs for individual and total isoflavone content in soybean (Glycine max L.) using a high-density genetic map. Theor. Appl. Genet. 2018, 131, 555–568. [Google Scholar] [CrossRef] [PubMed]
- Nigro, D.; Laddomada, B.; Mita, G.; Blanco, E.; Colasuonno, P.; Simeone, R.; Gadaleta, A.; Pasqualone, A.; Blanco, A. Genome-wide association mapping of phenolic acids in tetraploid wheats. J. Cereal Sci. 2017, 75, 25–34. [Google Scholar] [CrossRef]
- Patil, N.Y.; Pugh, N.A.; Klein, R.R.; Martinez, H.S.; Martinez, R.S.; Rodriguez-Herrera, R.; Rooney, W.L.; Klein, P.E. Heritability and quantitative trait loci of composition and structural characteristics in sorghum grain. J. Crop Improv. 2019, 33, 1–24. [Google Scholar] [CrossRef]
- Hong, M.J.; Kim, D.Y.; Nam, B.M.; Ahn, J.W.; Kwon, S.J.; Seo, Y.W.; Kim, J.B. Characterization of novel mutants of hexaploid wheat (Triticum aestivum L.) with various depths of purple grain color and antioxidant capacity. J. Sci. Food Agric. 2019, 99, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Sanz, T.; Luyten, H. Release, partitioning and stability of isoflavones from enriched custards during mouth, stomach and intestine in vitro simulations. Food Hydrocoll. 2006, 20, 892–900. [Google Scholar] [CrossRef]
- Kassem, M.A.; Meksem, K.; Iqbal, M.J.; Njiti, V.N.; Banz, W.J.; Winters, T.A.; Wood, A.; Lightfoot, D.A. Definition of soybean genomic regions that control seed phytoestrogen amounts. J. Biomed. Biotechnol. 2004, 2004, 52–60. [Google Scholar] [CrossRef]
- Primomo, V.S.; Poysa, V.; Ablett, G.R.; Jackson, C.-J.; Gijzen, M.; Rajcan, I. Mapping QTL for individual and total isoflavone content in soybean seeds. Crop Sci. 2005, 45, 2454–2464. [Google Scholar] [CrossRef]
- Zeng, G.; Li, D.; Han, Y.; Teng, W.; Wang, J.; Qiu, L.; Li, W. Identification of QTL underlying isoflavone contents in soybean seeds among multiple environments. Theor. Appl. Genet. 2009, 118, 1455–1463. [Google Scholar] [CrossRef]
- Li, M.-W.; Muñoz, N.B.; Wong, C.-F.; Wong, F.-L.; Wong, K.-S.; Wong, J.W.-H.; Qi, X.; Li, K.-P.; Ng, M.-S.; Lam, H.-M. QTLs regulating the contents of antioxidants, phenolics, and flavonoids in soybean seeds share a common genomic region. Front. Plant Sci. 2016, 7, 854. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Murphy, P.A. Isoflavone composition of American and Japanese soybeans in Iowa: Effects of variety, crop year, and location. J. Agric. Food Chem. 1994, 42, 1674–1677. [Google Scholar] [CrossRef]
- Serraj, R.; Krishnamurthy, L.; Kashiwagi, J.; Kumar, J.; Chandra, S.; Crouch, J.H. Variation in root traits of chickpea (Cicer arietinum L.) grown under terminal drought. Field Crop Res. 2004, 88, 115–127. [Google Scholar] [CrossRef]
- Eldridge, A.C.; Kwolek, W.F. Soybean isoflavones: Effect of environment and variety on composition. J. Agric. Food Chem. 1983, 31, 394–396. [Google Scholar] [CrossRef] [PubMed]
- Britz, S.J.; Schomburg, C.J.; Kenworthy, W.J. Isoflavones in seeds of field-grown soybean: Variation among genetic lines and environmental effects. J. Am. Oil. Chem. Soc. 2011, 88, 827–832. [Google Scholar] [CrossRef]
- Bustamante-Rangel, M.; Delgado-Zamarreño, M.M.; Pérez-Martín, L.; Rodríguez-Gonzalo, E.; Domínguez-Álvarez, J. Analysis of isoflavones in foods. Compr. Rev. Food Sci. Food Saf. 2018, 17, 391–411. [Google Scholar] [CrossRef]
- Phillips, P.C. Epistasis—The essential role of gene interactions in the structure and evolution of genetic systems. Nat. Rev. Genet. 2008, 9, 855–867. [Google Scholar] [CrossRef]
- Gutierrez-Gonzalez, J.J.; Wu, X.; Zhang, J.; Lee, J.-D.; Ellersieck, M.; Shannon, J.G.; Yu, O.; Nguyen, H.T.; Sleper, D.A. Genetic control of soybean seed isoflavone content: Importance of statistical model and epistasis in complex traits. Theor. Appl. Genet. 2009, 119, 1069–1083. [Google Scholar] [CrossRef]
- Gutierrez-Gonzalez, J.J.; Vuong, T.D.; Zhong, R.; Yu, O.; Lee, J.-D.; Shannon, G.; Ellersieck, M.; Nguyen, H.T.; Sleper, D.A. Major locus and other novel additive and epistatic loci involved in modulation of isoflavone concentration in soybean seeds. Theor. Appl. Genet. 2011, 123, 1375–1385. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Han, Y.; Zhao, X.; Li, Y.; Teng, W.; Li, D.; Zhan, Y.; Li, W. Mapping isoflavone QTL with main, epistatic and QTL × environment effects in recombinant inbred lines of soybean. PLoS ONE 2015, 10, e0118447. [Google Scholar] [CrossRef]
- Kassem, M.A.; Shultz, J.; Meksem, K.; Cho, Y.; Wood, A.J.; Iqbal, M.J.; Lightfoot, D.A. An updated ‘Essex’ by ‘Forrest’ linkage map and first composite interval map of QTL underlying six soybean traits. Theor. Appl. Genet. 2006, 113, 1015–1026. [Google Scholar] [CrossRef]
- Chiari, L.; Piovesan, N.D.; Naoe, L.K.; José, I.C.; Viana, J.M.S.; Moreira, M.A.; de Barros, E.G. Genetic parameters relating isoflavone and protein content in soybean seeds. Euphytica 2004, 138, 55–60. [Google Scholar] [CrossRef]
- Yoshikawa, T.; Okumoto, Y.; Ogata, D.; Sayama, T.; Teraishi, M.; Terai, M.; Toda, T.; Yamada, K.; Yagasaki, K.; Yamada, N.; et al. Transgressive segregation of isoflavone contents under the control of four QTLs in a cross between distantly related soybean varieties. Breed. Sci. 2010, 60, 243–254. [Google Scholar] [CrossRef]
- Collard, B.C.Y.; Jahufer, M.Z.Z.; Brouwer, J.B.; Pang, E.C.K. An introduction to markers, quantitative trait loci (QTL) mapping and marker-assisted selection for crop improvement: The basic concepts. Euphytica 2005, 142, 169–196. [Google Scholar] [CrossRef]
- Njiti, V.N.; Meksem, K.; Yuan, J.; Lightfoot, D.A.; Banz, W.J.; Winters, T.A. DNA markers associated with loci underlying seed phytoestrogen content in soybeans. J. Med. Food. 1999, 2, 185–187. [Google Scholar] [CrossRef] [PubMed]
- Meksem, K.; Njiti, V.N.; Banz, W.J.; Iqbal, M.J.; Kassem, M.M.; Hyten, D.L.; Yuang, J.; Winters, T.A.; Lightfoot, D.A. Genomic regions that underlie soybean seed isoflavone content. J. Biomed. Biotechnol. 2001, 1, 38–44. [Google Scholar] [CrossRef]
- Yang, K.; Moon, J.-K.; Jeong, N.; Chun, H.-K.; Kang, S.-T.; Back, K.; Jeong, S.-C. Novel major quantitative trait loci regulating the content of isoflavone in soybean seeds. Genes Genom. 2011, 33, 685–692. [Google Scholar] [CrossRef]
- Li, B.; Tian, L.; Zhang, J.; Huang, L.; Han, F.; Yan, S.; Wang, L.; Zheng, H.; Sun, J. Construction of a high-density genetic map based on large-scale markers developed by specific length amplified fragment sequencing (SLAF-seq) and its application to QTL analysis for isoflavone content in Glycine max. BMC Genomics 2014, 15, 1086. [Google Scholar] [CrossRef]
- Pei, R.; Zhang, J.; Tian, L.; Zhang, S.; Han, F.; Yan, S.; Wang, L.; Li, B.; Sun, J. Identification of novel QTL associated with soybean isoflavone content. Crop J. 2018, 6, 244–252. [Google Scholar] [CrossRef]
- Meng, S.; He, J.; Zhao, T.; Xing, G.; Li, Y.; Yang, S.; Lu, J.; Wang, Y.; Gai, J. Detecting the QTL-allele system of seed isoflavone content in Chinese soybean landrace population for optimal cross design and gene system exploration. Theor. Appl. Genet. 2016, 129, 1557–1576. [Google Scholar] [CrossRef]
- Collard, B.C.Y.; Mackill, D.J. Marker-assisted selection: An approach for precision plant breeding in the twenty-first century. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 557–572. [Google Scholar] [CrossRef]
- Chu, S.; Wang, J.; Zhu, Y.; Liu, S.; Zhou, X.; Zhang, H.; Wang, C.-E.; Yang, W.; Tian, Z.; Cheng, H.; et al. An R2R3-type MYB transcription factor, GmMYB29, regulates isoflavone biosynthesis in soybean. PLoS Genet. 2017, 13, e1006770. [Google Scholar] [CrossRef] [PubMed]
- Ha, J.; Kang, Y.-G.; Lee, T.; Kim, M.; Yoon, M.Y.; Lee, E.; Yang, X.; Kim, D.; Kim, Y.-J.; Lee, T.R.; et al. Comprehensive RNA sequencing and co-expression network analysis to complete the biosynthetic pathway of coumestrol, a phytoestrogen. Sci. Rep. 2019, 9, 1934. [Google Scholar] [CrossRef]
- McNeil, D.; Ahmad, F.; Abbo, S.; Bahl, P.N. Chickpea breeding and management. In Genetics and Cytogenetics; Yadav, S.S., Redden, R.R., Chen, W., Sharma, B., Eds.; CABI International: Wallingford, UK, 2007; pp. 321–337. [Google Scholar]
- Kim, E.H.; Kim, S.H.; Chung, J.I.; Chi, H.Y.; Kim, J.A.; Chung, I.M. Analysis of phenolic compounds and isoflavones in soybean seeds (Glycine max (L.) Merill) and sprouts grown under different conditions. Eur. Food Resd Technol. 2005, 222, 201. [Google Scholar] [CrossRef]
- Wu, Z.; Song, L.; Feng, S.; Liu, Y.; He, G.; Yioe, Y.; Liu, S.Q.; Huang, D. Germination dramatically increases isoflavonoid content and diversity in chickpea (Cicer arietinum L.) seeds. J. Agric. Food Chem. 2012, 60, 8606–8615. [Google Scholar] [CrossRef]
- Gao, Y.; Yao, Y.; Zhu, Y.; Ren, G. Isoflavone content and composition in chickpea (Cicer arietinum L.) sprouts germinated under different conditions. J. Agric. Food Chem. 2015, 63, 2701–2707. [Google Scholar] [CrossRef]
- Cobos, M.J.; Rubio, J.; Fernández-Romero, M.D.; Garza, R.; Moreno, M.T.; Millán, T.; Gil, J. Genetic analysis of seed size, yield and days to flowering in a chickpea recombinant inbred line population derived from a Kabuli × Desi cross. Ann. Appl. Biol. 2007, 151, 33–42. [Google Scholar] [CrossRef]
- Vadez, V.; Rashmi, M.; Sindhu, K.; Muralidharan, M.; Pushpavalli, R.; Turner, N.C.; Krishnamurthy, L.; Gaur, P.M.; Colmer, T.D. Large number of flowers and tertiary branches, and higher reproductive success increase yields under salt stress in chickpea. Eur. J. Agron. 2012, 41, 42–51. [Google Scholar] [CrossRef]
- Kashiwagi, J.; Krishnamurthy, L.; Purushothaman, R.; Upadhyaya, H.D.; Gaur, P.M.; Gowda, C.L.L.; Ito, O.; Varshney, R.K. Scope for improvement of yield under drought through the root traits in chickpea (Cicer arietinum L.). Field Crop Res. 2015, 170, 47–54. [Google Scholar] [CrossRef]
- Gupta, S.; Kumar, T.; Verma, S.; Bharadwaj, C.; Bhatia, S. Development of gene-based markers for use in construction of the chickpea (Cicer arietinum L.) genetic linkage map and identification of QTLs associated with seed weight and plant height. Mol. Biol. Rep. 2015, 42, 1571–1580. [Google Scholar] [CrossRef]
- Das, S.; Singh, M.; Srivastava, R.; Bajaj, D.; Saxena, M.S.; Rana, J.C.; Bansal, K.C.; Tyagi, A.K.; Parida, S.K. mQTL-seq delineates functionally relevant candidate gene harbouring a major QTL regulating pod number in chickpea. DNA Res. 2016, 23, 53–65. [Google Scholar] [CrossRef]
- Srivastava, R.; Singh, M.; Bajaj, D.; Parida, S.K. A High-Resolution InDel (Insertion–Deletion) Markers-anchored consensus genetic map identifies major QTLs governing pod number and seed yield in chickpea. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef]
- Shimray, P.W.; Bajaj, D.; Srivastava, R.; Daware, A.; Upadhyaya, H.D.; Kumar, R.; Bharadwaj, C.; Tyagi, A.K.; Parida, S.K. Identifying transcription factor genes associated with yield traits in chickpea. Plant. Mol. Biol. Report. 2017, 35, 562–574. [Google Scholar] [CrossRef]
- Basu, U.; Bajaj, D.; Sharma, A.; Malik, N.; Daware, A.; Narnoliya, L.; Thakro, V.; Upadhyaya, H.D.; Kumar, R.; Tripathi, S.; et al. Genetic dissection of photosynthetic efficiency traits for enhancing seed yield in chickpea. Plant Cell Environ. 2019, 42, 158–173. [Google Scholar] [CrossRef]
- Basu, U.; Srivastava, R.; Bajaj, D.; Thakro, V.; Daware, A.; Malik, N.; Upadhyaya, H.D.; Parida, S.K. Genome-wide generation and genotyping of informative SNPs to scan molecular signatures for seed yield in chickpea. Sci. Rep. 2018, 8, 13240. [Google Scholar] [CrossRef]
- Gaur, P.M.; Krishnamurthy, L.; Kashiwagi, J. Improving drought-avoidance root traits in chickpea (Cicer arietinum L.)—Current status of research at ICRISAT. Plant Prod. Sci. 2008, 11, 3–11. [Google Scholar] [CrossRef]
- Hamwieh, A.; Imtiaz, M.; Malhotra, R.S. Multi-environment QTL analyses for drought-related traits in a recombinant inbred population of chickpea (Cicer arientinum L.). Theor. Appl. Genet. 2013, 126, 1025–1038. [Google Scholar] [CrossRef]
- Varshney, R.K.; Thudi, M.; Nayak, S.N.; Gaur, P.M.; Kashiwagi, J.; Krishnamurthy, L.; Jaganathan, D.; Koppolu, J.; Bohra, A.; Tripathi, S.; et al. Genetic dissection of drought tolerance in chickpea (Cicer arietinum L.). Theor. Appl. Genet. 2014, 127, 445–462. [Google Scholar] [CrossRef]
- Paul, J.P.; Samineni, S.; Thudi, M.; Sajja, B.S.; Rathore, A.; Das, R.R.; Khan, W.A.; Chaturvedi, K.S.; Lavanya, R.G.; Varshney, K.R.; et al. Molecular mapping of QTLs for heat tolerance in chickpea. Int. J. Mol. Sci. 2018, 19, 2166. [Google Scholar] [CrossRef]
- Paul, P.J.; Samineni, S.; Sajja, S.B.; Rathore, A.; Das, R.R.; Chaturvedi, S.K.; Lavanya, G.R.; Varshney, R.K.; Gaur, P.M. Capturing genetic variability and selection of traits for heat tolerance in a chickpea recombinant inbred line (RIL) population under field conditions. Euphytica 2018, 214, 27. [Google Scholar] [CrossRef]
- Kumar, K.; Purayannur, S.; Kaladhar, V.C.; Parida, S.K.; Verma, P.K. mQTL-seq and classical mapping implicates the role of an AT-HOOK MOTIF CONTAINING NUCLEAR LOCALIZED (AHL) family gene in Ascochyta blight resistance of chickpea. Plant. Cell Environ. 2018, 41, 2128–2140. [Google Scholar] [CrossRef]
- Garg, V.; Khan, A.W.; Kudapa, H.; Kale, S.M.; Chitikineni, A.; Qiwei, S.; Sharma, M.; Li, C.; Zhang, B.; Xin, L.; et al. Integrated transcriptome, small RNA and degradome sequencing approaches provide insights into Ascochyta blight resistance in chickpea. Plant Biotechnol. J. 2018, 17, 914–931. [Google Scholar] [CrossRef]
- Deokar, A.A.; Ramsay, L.; Sharpe, A.G.; Diapari, M.; Sindhu, A.; Bett, K.; Warkentin, T.D.; Tar’an, B. Genome wide SNP identification in chickpea for use in development of a high density genetic map and improvement of chickpea reference genome assembly. BMC Genomics 2014, 15, 708. [Google Scholar] [CrossRef]
- Das, S.; Upadhyaya, H.D.; Bajaj, D.; Kujur, A.; Badoni, S.; Laxmi; Kumar, V.; Tripathi, S.; Gowda, C.L.L.; Sharma, S.; et al. Deploying QTL-seq for rapid delineation of a potential candidate gene underlying major trait-associated QTL in chickpea. DNA Res. 2015, 22, 193–203. [Google Scholar] [CrossRef]
- Kudapa, H.; Garg, V.; Chitikineni, A.; Varshney, R.K. The RNA-Seq-based high resolution gene expression atlas of chickpea (Cicer arietinum L.) reveals dynamic spatio-temporal changes associated with growth and development. Plant Cell Environ. 2018, 41, 2209–2225. [Google Scholar] [CrossRef]
- Abbo, S.; Molina, C.; Jungmann, R.; Grusak, M.A.; Berkovitch, Z.; Reifen, R.; Kahl, G.; Winter, P.; Reifen, R. Quantitative trait loci governing carotenoid concentration and weight in seeds of chickpea (Cicer arietinum L.). Theor. Appl. Genet. 2005, 111, 185–195. [Google Scholar] [CrossRef]
- Hossain, S.; Panozzo, J.; Pittock, C.; Ford, R. Quantitative trait loci analysis of seed coat color components for selective breeding in chickpea (Cicer arietinum L.). Can. J. Plant Sci. 2011, 91, 49–55. [Google Scholar] [CrossRef]
- Bajaj, D.; Das, S.; Upadhyaya, H.D.; Ranjan, R.; Badoni, S.; Kumar, V.; Tripathi, S.; Gowda, C.L.L.; Sharma, S.; Singh, S.; et al. A genome-wide combinatorial strategy dissects complex genetic architecture of seed coat color in chickpea. Front. Plant Sci. 2015, 6, 979. [Google Scholar] [CrossRef]
- Upadhyaya, H.D.; Bajaj, D.; Narnoliya, L.; Das, S.; Kumar, V.; Gowda, C.L.L.; Sharma, S.; Tyagi, A.K.; Parida, S.K. Genome-wide scans for delineation of candidate genes regulating seed-protein content in chickpea. Front. Plant Sci. 2016, 7, 302. [Google Scholar] [CrossRef]
- Upadhyaya, H.D.; Bajaj, D.; Das, S.; Kumar, V.; Gowda, C.L.L.; Sharma, S.; Tyagi, A.K.; Parida, S.K. Genetic dissection of seed-iron and zinc concentrations in chickpea. Sci. Rep. 2016, 6, 24050. [Google Scholar] [CrossRef] [PubMed]
- Sreerama, Y.N.; Sashikala, V.B.; Pratape, V.M. Variability in the distribution of phenolic compounds in milled fractions of chickpea and horse gram: Evaluation of their antioxidant properties. J. Agric. Food Chem. 2010, 58, 8322–8330. [Google Scholar] [CrossRef]
- Yoshiara, L.Y.; Madeira, T.B.; Delaroza, F.; Da Silva, J.B.; Ida, E.I. Optimization of soy isoflavone extraction with different solvents using the simplex-centroid mixture design. Int. J. Food Sci. Nutr. 2012, 63, 978–986. [Google Scholar] [CrossRef]
- Xu, B.J.; Chang, S.K.C. A comparative study on phenolic profiles and antioxidant activities of legumes as affected by extraction solvents. J. Food Sci. 2007, 72, S159–S166. [Google Scholar] [CrossRef]
- Duranti, M. Grain legume proteins and nutraceutical properties. Fitoterapia 2006, 77, 67–82. [Google Scholar] [CrossRef]
- Ryszard, A.; Fereidoon, S. Antioxidant activity of faba bean extract and fractions thereof. J. Food Bioact. 2018, 2, 112–118. [Google Scholar]
- Jogihalli, P.; Singh, L.; Kumar, K.; Sharanagat, V.S. Physico-functional and antioxidant properties of sand-roasted chickpea (Cicer arietinum). Food Chem. 2017, 237, 1124–1132. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Magaña, L.M.; Cuevas-Rodríguez, E.O.; Gutiérrez-Dorado, R.; Ayala-Rodríguez, A.E.; Valdez-Ortiz, A.; Milán-Carrillo, J.; Reyes-Moreno, C. Solid-state bioconversion of chickpea (Cicer arietinum L.) by Rhizopus oligosporus to improve total phenolic content, antioxidant activity and hypoglycemic functionality. Int. J. Food. Sci. Nutr. 2014, 65, 558–564. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic composition and antioxidant potential of grain legume seeds: A review. Food Res. Int. 2017, 101, 1–16. [Google Scholar] [CrossRef]
- Sreerama, Y.N.; Sashikala, V.B.; Pratape, V.M. Phenolic compounds in cowpea and horse gram flours in comparison to chickpea flour: Evaluation of their antioxidant and enzyme inhibitory properties associated with hyperglycemia and hypertension. Food Chem. 2012, 133, 156–162. [Google Scholar] [CrossRef]
- Xu, B.; Chang, S.K.C. Effect of soaking, boiling, and steaming on total phenolic contentand antioxidant activities of cool season food legumes. Food Chem. 2008, 110, 1–13. [Google Scholar] [CrossRef]
- Ademiluyi, A.O.; Oboh, G. Soybean phenolic-rich extracts inhibit key-enzymes linked to type 2 diabetes (α-amylase and α-glucosidase) and hypertension (angiotensin I converting enzyme) in vitro. Exp. Toxicol. Pathol. 2013, 65, 305–309. [Google Scholar] [CrossRef]
- Yoshiara, L.Y.; Mandarino, J.M.G.; Carrão-Panizzi, M.C.; Madeira, T.B.; da Silva, J.B.; de Camargo, A.C.; Shahidi, F.; Ida, E.I. Germination changes the isoflavone profile and increases the antioxidant potential of soybean. J. Food Bioact. 2018, 3, 144–150. [Google Scholar] [CrossRef]
- Yao, Y.; Cheng, X.; Wang, L.; Wang, S.; Ren, G. Biological potential of sixteen legumes in China. Int. J. Mol. Sci. 2011, 12, 7048–7058. [Google Scholar] [CrossRef]
- Lee, Y.H.; Kim, B.; Hwang, S.-R.; Kim, K.; Lee, J.H. Rapid characterization of metabolites in soybean using ultra high performance liquid chromatography coupled with electrospray ionization quadrupole time-of-flight mass spectrometry (UPLC-ESI-Q-TOF-MS/MS) and screening for α-glucosidase inhibitory and antioxidant properties through different solvent systems. J. Food Drug Anal. 2018, 26, 277–291. [Google Scholar]
- Thavarajah, D.; Thavarajah, P. Evaluation of chickpea (Cicer arietinum L.) micronutrient composition: Biofortification opportunities to combat global micronutrient malnutrition. Food Res. Int. 2012, 49, 99–104. [Google Scholar] [CrossRef]
- Konar, N.; Poyrazoğlu, E.S.; Demir, K.; Artik, N. Determination of conjugated and free isoflavones in some legumes by LC–MS/MS. J. Food Compos. Anal. 2012, 25, 173–178. [Google Scholar] [CrossRef]
- Aguilera, Y.; Duenas, M.; Estrella, I.; Hernandez, T.; Benitez, V.; Esteban, R.M.; Martin-Cabrejas, M.A. Phenolic profile and antioxidant capacity of chickpeas (Cicer arietinum L.) as affected by a dehydration process. Plant Foods Hum. Nutr. 2011, 66, 187–195. [Google Scholar] [CrossRef]
- Ebert, A.W.; Chang, C.H.; Yan, M.R.; Yang, R.Y. Nutritional composition of mungbean and soybean sprouts compared to their adult growth stage. Food Chem. 2017, 237, 15–22. [Google Scholar] [CrossRef]
- de Camargo, A.C.; Regitano-d’Arce, M.A.B.; Biasoto, A.C.T.; Shahidi, F. Low molecular weight phenolics of grape juice and winemaking byproducts: Antioxidant activities and inhibition of oxidation of human low-density lipoprotein cholesterol and DNA strand breakage. J. Agric. Food Chem. 2014, 62, 12159–12171. [Google Scholar] [CrossRef]
- de Camargo, A.C.; Regitano-d’Arce, M.A.B.; Gallo, C.R.; Shahidi, F. Gamma-irradiation induced changes in microbiological status, phenolic profile and antioxidant activity of peanut skin. J. Funct. Foods 2015, 12, 129–143. [Google Scholar] [CrossRef]
- Mekky, R.H.; Contreras, M.D.; El-Gindi, M.R.; Abdel-Monem, A.R.; Abdel-Sattar, E.; Segura-Carretero, A. Profiling of phenolic and other compounds from Egyptian cultivars of chickpea (Cicer arietinum L.) and antioxidant activity: A comparative study. RSC Adv. 2015, 5, 17751–17767. [Google Scholar] [CrossRef]
- Jalaludeen, A.M.; Lee, W.Y.; Kim, J.H.; Jeong, H.Y.; Ki, K.S.; Kwon, E.G.; Song, H. Therapeutic efficacy of biochanin A against arsenic-induced renal and cardiac damage in rats. Environ. Toxicol. Pharmacol. 2015, 39, 1221–1231. [Google Scholar] [CrossRef]
- Andjelković, M.; Van Camp, J.; De Meulenaer, B.; Depaemelaere, G.; Socaciu, C.; Verloo, M.; Verhe, R. Iron-chelation properties of phenolic acids bearing catechol and galloyl groups. Food Chem. 2006, 98, 23–31. [Google Scholar] [CrossRef]
- Dowling, S.; Regan, F.; Hughes, H. The characterisation of structural and antioxidant properties of isoflavone metal chelates. J. Inorg. Biochem. 2010, 104, 1091–1098. [Google Scholar] [CrossRef]
- Braughler, J.M.; Duncan, L.A.; Chase, R.L. The involvement of iron in lipid peroxidation. Importance of ferric to ferrous ratios in initiation. J. Biol. Chem. 1986, 261, 10282–10289. [Google Scholar]
- Kumari, S.; Chang, S.K. Effect of cooking on isoflavones, phenolic acids, and antioxidant activity in sprouts of prosoy soybean (Glycine max). J. Food Sci. 2016, 81, C1679–C1691. [Google Scholar] [CrossRef]
- Zia-Ul-Haq, M.; Iqbal, S.; Ahmad, S.; Bhanger, M.I.; Wiczkowski, W.; Amarowicz, R. Antioxidant potential of Desi chickpea varieties commonly consumed in Pakistan. J. Food Lipids. 2008, 15, 326–342. [Google Scholar] [CrossRef]
- Sosa, V.; Moline, T.; Somoza, R.; Paciucci, R.; Kondoh, H.; ME, L.L. Oxidative stress and cancer: An overview. Ageing Res. Rev. 2013, 12, 376–390. [Google Scholar] [CrossRef]
- Marseglia, L.; Manti, S.; D’Angelo, G.; Nicotera, A.; Parisi, E.; Di Rosa, G.; Gitto, E.; Arrigo, T. Oxidative stress in obesity: A critical component in human diseases. Int. J. Mol. Sci. 2015, 16, 378–400. [Google Scholar] [CrossRef]
- Sarangarajan, R.; Meera, S.; Rukkumani, R.; Sankar, P.; Anuradha, G. Antioxidants: Friend or foe? Asian Pac. J. Trop. Med. 2017, 10, 1111–1116. [Google Scholar] [CrossRef]
- Zhang, H.; Tsao, R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr. Opin. Food Sci. 2016, 8, 33–42. [Google Scholar] [CrossRef]
- Valsecchi, A.E.; Franchi, S.; Panerai, A.E.; Rossi, A.; Sacerdote, P.; Colleoni, M. The soy isoflavone genistein reverses oxidative and inflammatory state, neuropathic pain, neurotrophic and vasculature deficits in diabetes mouse model. Eur. J. Pharmacol. 2011, 650, 694–702. [Google Scholar] [CrossRef]
- Shahidi, F.; Peng, H. Bioaccessibility and bioavailability of phenolic compounds. J. Food Bioact. 2018, 4, 11–68. [Google Scholar] [CrossRef]
- Lee, J.S. Effects of soy protein and genistein on blood glucose, antioxidant enzyme activities, and lipid profile in streptozotocin-induced diabetic rats. Life Sci. 2006, 79, 1578–1584. [Google Scholar] [CrossRef]
- Valeri, A.; Fiorenzani, P.; Rossi, R.; Aloisi, A.M.; Valoti, M.; Pessina, F. The soy phytoestrogens genistein and daidzein as neuroprotective agents against anoxia-glucopenia and reperfusion damage in rat urinary bladder. Pharmacol. Res. 2012, 66, 309–316. [Google Scholar] [CrossRef]
- Raheja, S.; Girdhar, A.; Lather, V.; Pandita, D. Biochanin A: A phytoestrogen with therapeutic potential. Trends Food Sci. Technol. 2018, 79, 55–66. [Google Scholar] [CrossRef]
- Sadri, H.; Goodarzi, M.T.; Salemi, Z.; Seifi, M. Antioxidant effects of biochanin A in streptozotocin induced diabetic rats. Braz. Arch. Biol. Technol. 2017, 60, e17160741. [Google Scholar] [CrossRef]
- Wang, J.; He, C.; Wu, W.Y.; Chen, F.; Wu, Y.Y.; Li, W.Z.; Chen, H.Q.; Yin, Y.Y. Biochanin A protects dopaminergic neurons against lipopolysaccharide-induced damage and oxidative stress in a rat model of Parkinson’s disease. Pharmacol. Biochem. Behav. 2015, 138, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Danciu, C.; Avram, S.; Pavel, I.Z.; Ghiulai, R.; Dehelean, C.A.; Ersilia, A.; Minda, D.; Petrescu, C.; Moaca, E.A.; Soica, C. Main isoflavones found in dietary sources as natural anti-inflammatory agents. Curr. Drug Targets. 2018, 19, 841–853. [Google Scholar] [CrossRef]
- Verdrengh, M.; Jonsson, I.M.; Holmdahl, R.; Tarkowski, A. Genistein as an anti-inflammatory agent. Inflamm. Res. 2003, 52, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Dong, J.; He, P.; Li, W.; Zhang, Q.; Li, N.; Sun, T. Genistein inhibit cytokines or growth factor-induced proliferation and transformation phenotype in fibroblast-like synoviocytes of rheumatoid arthritis. Inflammation 2012, 35, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, G.; Poudel, S.B.; Kook, S.H.; Lee, J.C. Anti-inflammatory, anti-osteoclastic, and antioxidant activities of genistein protect against alveolar bone loss and periodontal tissue degradation in a mouse model of periodontitis. J. Biomed. Mater. Res. A. 2017, 105, 2510–2521. [Google Scholar] [CrossRef] [PubMed]
- Spagnuolo, C.; Moccia, S.; Russo, G.L. Anti-inflammatory effects of flavonoids in neurodegenerative disorders. Eur. J. Med. Chem. 2018, 153, 105–115. [Google Scholar] [CrossRef]
- Ji, G.; Zhang, Y.; Yang, Q.; Cheng, S.; Hao, J.; Zhao, X.; Jiang, Z. Genistein suppresses LPS-induced inflammatory response through inhibiting NF-κB following AMP kinase activation in RAW 264.7 macrophages. PLoS ONE 2012, 7, e53101. [Google Scholar] [CrossRef]
- Jeong, J.W.; Lee, H.H.; Han, M.H.; Kim, G.Y.; Kim, W.J.; Choi, Y.H. Anti-inflammatory effects of genistein via suppression of the toll-like receptor 4-mediated signaling pathway in lipopolysaccharide-stimulated BV2 microglia. Chem. Biol. Interact. 2014, 212, 30–39. [Google Scholar] [CrossRef]
- Wu, D.Q.; Zhong, H.M.; Ding, Q.H.; Ba, L. Protective effects of biochanin A on articular cartilage: In vitro and in vivo studies. BMC Complement. Altern. Med. 2014, 14, 444. [Google Scholar] [CrossRef]
- Wu, L.Y.; Ye, Z.N.; Zhuang, Z.; Gao, Y.; Tang, C.; Zhou, C.H.; Wang, C.X.; Zhang, X.S.; Xie, G.B.; Liu, J.P.; et al. Biochanin A reduces inflammatory injury and neuronal apoptosis following subarachnoid hemorrhage via suppression of the TLRs/TIRAP/MyD88/NF-κB Pathway. Behav. Neurol. 2018, 2018, 1960106. [Google Scholar] [CrossRef]
- Wu, W.Y.; Wu, Y.Y.; Huang, H.; He, C.; Li, W.Z.; Wang, H.L.; Chen, H.Q.; Yin, Y.Y. Biochanin A attenuates LPS-induced pro-inflammatory responses and inhibits the activation of the MAPK pathway in BV2 microglial cells. Int. J. Mol. Med. 2015, 35, 391–398. [Google Scholar] [CrossRef]
- Ming, X.; Ding, M.; Zhai, B.; Xiao, L.; Piao, T.; Liu, M. Biochanin A inhibits lipopolysaccharide-induced inflammation in human umbilical vein endothelial cells. Life Sci. 2015, 136, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Cominacini, L.; Garbin, U.; Fratta Pasini, A.; Campagnola, M.; Davoli, A.; Foot, E.; Sighieri, G.; Sironi, A.M.; Lo Cascio, V.; Ferrannini, E. Troglitazone reduces LDL oxidation and lowers plasma E-selectin concentration in NIDDM patients. Diabetes 1998, 47, 130–133. [Google Scholar] [CrossRef]
- Matsumoto, K.; Sera, Y.; Abe, Y.; Tominaga, T.; Horikami, K.; Hirao, K.; Ueki, Y.; Miyake, S. High serum concentrations of soluble E-selectin correlate with obesity but not fat distribution in patients with type 2 diabetes mellitus. Metabolism 2002, 51, 932–934. [Google Scholar] [CrossRef]
- Franchin, M.; Freires, I.A.; Lazarini, J.G.; Nani, B.D.; da Cunha, M.G.; Colón, D.F.; de Alencar, S.M.; Rosalen, P.L. The use of Brazilian propolis for discovery and development of novel anti-inflammatory drugs. Eur. J. Med. Chem. 2018, 153, 49–55. [Google Scholar] [CrossRef]
- Ahmad, S.; Alam, K.; Hossain, M.M.; Fatima, M.; Firdaus, F.; Zafeer, M.F.; Arif, Z.; Ahmed, M.; Nafees, K.A. Anti-arthritogenic and cardioprotective action of hesperidin and daidzein in collagen-induced rheumatoid arthritis. Mol. Cell. Biochem. 2016, 423, 115–127. [Google Scholar] [CrossRef]
- Feng, G.; Sun, B.; Li, T.Z. Daidzein attenuates lipopolysaccharide-induced acute lung injury via toll-like receptor 4/NF-kappaB pathway. Int. Immunopharmacol. 2015, 26, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Hämäläinen, M.; Nieminen, R.; Vuorela, P.; Heinonen, M.; Moilanen, E. Anti-inflammatory effects of flavonoids: Genistein, kaempferol, quercetin, and daidzein inhibit STAT-1 and NF-κB activations, whereas flavone, isorhamnetin, naringenin, and pelargonidin inhibit only NF-κB activation along with their inhibitory effect on iNOS expression and NO production in activated macrophages. Mediators Inflamm. 2007, 2007, 45673. [Google Scholar]
- Penga, Y.; Shi, Y.; Zhang, H.; Mine, Y.; Tsao, R. Anti-inflammatory and anti-oxidative activities of daidzein and its sulfonic acid ester derivatives. J. Funct. Foods 2017, 35, 635–640. [Google Scholar] [CrossRef]
- Lima Cavendish, R.; de Souza Santos, J.; Belo Neto, R.; Oliveira Paixão, A.; Valéria Oliveira, J.; Divino de Araujo, E.; Berretta, E.; Silva, A.A.; Maria Thomazzi, S.; Cordeiro Cardoso, J.; et al. Antinociceptive and anti-inflammatory effects of Brazilian red propolis extract and formononetin in rodents. J. Ethnopharmacol. 2015, 173, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Ji, W.; Fu, Q.; Ma, S. Formononetin inhibited the inflammation of LPS-induced acute lung injury in mice associated with induction of PPAR gamma expression. Inflammation 2013, 36, 1560–1566. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Wu, K.; Zhu, Q.; Xiao, W.; Shan, Q.; Yan, Z.; Wu, J.; Deng, B.; Xue, Y.; Gong, W.; et al. Formononetin administration ameliorates dextran sulfate sodium-induced acute colitis by inhibiting NLRP3 inflammasome signaling pathway. Mediators Inflamm. 2018, 2018, 3048532. [Google Scholar] [PubMed]
- Hui-Fang, C.; You-Cheng, S.; Kamesh, V.; Chin-Kun, W. Popular functional foods and nutraceuticals with lipid lowering activity and in relation to cardiovascular disease, dyslipidemia, and related complications: An overview. J. Food Bioact. 2018, 2, 16–27. [Google Scholar]
- Pekkanen, J.; Linn, S.; Heiss, G.; Suchindran, C.M.; Leon, A.; Rifkind, B.M.; Tyroler, H.A. Ten-year mortality from cardiovascular disease in relation to cholesterol level among men with and without preexisting cardiovascular disease. N. Engl. J. Med. 1990, 322, 1700–1707. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, G.; Porcu, C.; Arciello, M.; Andreozzi, P.; Balsano, C. Prediction of carotid intima-media thickness in obese patients with low prevalence of comorbidities by serum copper bioavailability. J. Gastroenterol. Hepatol. 2018, 33, 1511–1517. [Google Scholar] [CrossRef]
- Amarowicz, R. Natural phenolic compounds protect LDL against oxidation. Eur. J. Lipid Sci. Technol. 2016, 118, 677–679. [Google Scholar] [CrossRef]
- Orrego, R.; Leiva, E.; Cheel, J. Inhibitory effect of three C-glycosylflavonoids from Cymbopogon citratus (Lemongrass) on human low density lipoprotein oxidation. Molecules 2009, 14, 3906–3913. [Google Scholar] [CrossRef]
- Kerry, N.; Abbey, M. The isoflavone genistein inhibits copper and peroxyl radical mediated low density lipoprotein oxidation in vitro. Atherosclerosis 1998, 140, 341–347. [Google Scholar] [CrossRef]
- Xu, B.J.; Yuan, S.H.; Chang, S.K. Comparative studies on the antioxidant activities of nine common food legumes against copper-induced human low-density lipoprotein oxidation in vitro. J. Food Sci. 2007, 72, S522–S527. [Google Scholar] [CrossRef]
- Yang, Y.; Zhou, L.; Gu, Y.; Zhang, Y.; Tang, J.; Li, F.; Shang, W.; Jiang, B.; Yue, X.; Chen, M. Dietary chickpeas reverse visceral adiposity, dyslipidaemia and insulin resistance in rats induced by a chronic high-fat diet. Br. J. Nutr. 2007, 98, 720–726. [Google Scholar] [CrossRef]
- Pittaway, J.K.; Ahuja, K.D.; Cehun, M.; Chronopoulos, A.; Robertson, I.K.; Nestel, P.J.; Ball, M.J. Dietary supplementation with chickpeas for at least 5 weeks results in small but significant reductions in serum total and low-density lipoprotein cholesterols in adult women and men. Ann. Nutr. Metab. 2006, 50, 512–518. [Google Scholar] [CrossRef]
- Pittaway, J.K.; Robertson, I.K.; Ball, M.J. Chickpeas may influence fatty acid and fiber intake in an ad libitum diet, leading to small improvements in serum lipid profile and glycemic control. J. Am. Diet. Assoc. 2008, 108, 1009–1013. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Sedaghat, A.; Shahbazian, H.; Rezazadeh, A.; Haidari, F.; Jahanshahi, A.; Mahmoud Latifi, S.; Shirbeigi, E. The effect of soy nut on serum total antioxidant, endothelial function and cardiovascular risk factors in patients with type 2 diabetes. Diabetes Metab. Res. Rev. 2019, 13, 1387–1391. [Google Scholar] [CrossRef]
- Jayagopal, V.; Albertazzi, P.; Kilpatrick, E.S.; Howarth, E.M.; Jennings, P.E.; Hepburn, D.A.; Atkin, S.L. Beneficial effects of soy phytoestrogen intake in postmenopausal women with type 2 diabetes. Diabetes Care. 2002, 25, 1709–1714. [Google Scholar] [CrossRef]
- Azadbakht, L.; Kimiagar, M.; Mehrabi, Y.; Esmaillzadeh, A.; Padyab, M.; Hu, F.B.; Willett, W.C. Soy inclusion in the diet improves features of the metabolic syndrome: A randomized crossover study in postmenopausal women. Am. J. Clin. Nutr. 2007, 85, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Borradaile, N.M.; de Dreu, L.E.; Wilcox, L.J.; Edwards, J.Y.; Huff, M.W. Soya phytoestrogens, genistein and daidzein, decrease apolipoprotein B secretion from HepG2 cells through multiple mechanisms. Biochem. J. 2002, 366, 531–539. [Google Scholar] [CrossRef] [PubMed]
- de Camargo, A.C.; Regitano-d’Arce, M.A.B.; Rasera, G.B.; Canniatti-Brazaca, S.G.; do Prado Silva, L.; Alvarenga, V.O.; Sant’Ana, A.S.; Shahidi, F. Phenolic acids and flavonoids of peanut by-products: Antioxidant capacity and antimicrobial effects. Food Chem. 2017, 237, 538–544. [Google Scholar] [CrossRef]
- De Santis, S.; Cavalcanti, E.; Mastronardi, M.; Jirillo, E.; Chieppa, M. Nutritional keys for intestinal barrier modulation. Front. immunol. 2015, 6, 612. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; de Camargo, A.C. Tocopherols and tocotrienols in common and emerging dietary sources: Occurrence, applications, and health benefits. Int. J. Mol. Sci. 2016, 17, 1745. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.S.; Va, P.; Wong, M.Y.; Zhang, H.L.; Xiang, Y.B. Soy intake is associated with lower lung cancer risk: Results from a meta-analysis of epidemiologic studies. Am. J. Clin. Nutr. 2011, 94, 1575–1583. [Google Scholar] [CrossRef]
- Fritz, H.; Seely, D.; Flower, G.; Skidmore, B.; Fernandes, R.; Vadeboncoeur, S.; Kennedy, D.; Cooley, K.; Wong, R.; Sagar, S.; et al. Soy, red clover, and isoflavones and breast cancer: A systematic review. PLoS ONE 2013, 8, e81968. [Google Scholar] [CrossRef]
- Girón-Calle, J.; Vioque, J.; del Mar Yust, M.; Pedroche, J.; Alaiz, M.; Millán, F. Effect of chickpea aqueous extracts, organic extracts, and protein concentrates on cell proliferation. J. Med. Food. 2004, 7, 122–129. [Google Scholar] [CrossRef]
- Hsu, J.T.; Hung, H.C.; Chen, C.J.; Hsu, W.L.; Ying, C. Effects of the dietary phytoestrogen biochanin A on cell growth in the mammary carcinoma cell line MCF-7. J. Nutr. Biochem. 1999, 10, 510–517. [Google Scholar] [CrossRef]
- Shahidi, F.; Yeo, J. Insoluble-bound phenolics in food. Molecules 2016, 21, 1216. [Google Scholar] [CrossRef]
- Khanna, K.K.; Jackson, S.P. DNA double-strand breaks: Signaling, repair and the cancer connection. Nat. Genet. 2001, 27, 247–254. [Google Scholar] [CrossRef]
- Alshikh, N.; de Camargo, A.C.; Shahidi, F. Phenolics of selected lentil cultivars: Antioxidant activities and inhibition of low-density lipoprotein and DNA damage. J. Funct. Foods. 2015, 18, 1022–1038. [Google Scholar] [CrossRef]
- Yoshioka, Y.; Li, X.; Zhang, T.; Mitani, T.; Yasuda, M.; Nanba, F.; Toda, T.; Yamashita, Y.; Ashida, H. Black soybean seed coat polyphenols prevent AAPH-induced oxidative DNA-damage in HepG2 cells. J. Clin. Biochem. Nutr. 2017, 60, 108–114. [Google Scholar] [CrossRef]
- Peng, H.; Li, W.; Li, H.; Deng, Z.; Zhang, B. Extractable and non-extractable bound phenolic compositions and their antioxidant properties in seed coat and cotyledon of black soybean (Glycinemax (L.) merr). J. Funct. Foods 2017, 32, 296–312. [Google Scholar] [CrossRef]
- Abid-Essefi, S.; Baudrimont, I.; Hassen, W.; Ouanes, Z.; Mobio, T.A.; Anane, R.; Creppy, E.E.; Bacha, H. DNA fragmentation, apoptosis and cell cycle arrest induced by zearalenone in cultured DOK, Vero and Caco-2 cells: Prevention by vitamin E. Toxicology 2003, 192, 237–248. [Google Scholar] [CrossRef]
- Miranda, D.D.C.; Arçari, D.P.; Ladeira, M.S.P.; Calori-Domingues, M.A.; Romero, A.C.; Salvadori, D.M.F.; Gloria, E.M.; Pedrazzoli, J., Jr.; Ribeiro, M.L. Analysis of DNA damage induced by aflatoxin B1 in Dunkin–Hartley guinea pigs. Mycopathologia 2007, 163, 275–280. [Google Scholar] [CrossRef]
- Pleadin, J.; Frece, J.; Lešić, T.; Zadravec, M.; Vahčić, N.; Malenica Staver, M.; Markov, K. Deoxynivalenol and zearalenone in unprocessed cereals and soybean from different cultivation regions in Croatia. Food Addit. Contam. Part B Surveill. 2017, 10, 268–274. [Google Scholar] [CrossRef]
- Calori-Domingues, M.A.; Iwahashi, P.M.R.; Ponce, G.H.; da Gloria, E.M.; Dias, C.T.S.; Button, D.C.; de Camargo, A.C. Aflatoxin B1 and zearalenone in soybeans: Occurrence and distribution in whole and defective kernels. Food Addit. Contam. Part B Surveill. 2018, 273–280. [Google Scholar] [CrossRef]
- Ramirez, M.L.; Cendoya, E.; Nichea, M.J.; Zachetti, V.G.L.; Chulze, S.N. Impact of toxigenic fungi and mycotoxins in chickpea: A review. Curr. Opin. Food Sci. 2018, 23, 32–37. [Google Scholar] [CrossRef]
- Wan, L.Y.; Ling, K.H.; El-Nezamy, H.S.; Wang, M. Oxyresveratrol protective effects against deoxynivalenol-induced intestinal barrier dysfunction and bacterial translocation on porcine intestinal epithelial IPEC-J2 cells. J. Food Bioact. 2018, 1, 116. [Google Scholar] [CrossRef]
- Taranu, I.; Marin, D.E.; Palade, M.; Pistol, G.C.; Chedea, V.S.; Gras, M.A.; Rotar, C. Assessment of the efficacy of a grape seed waste in counteracting the changes induced by aflatoxin B1 contaminated diet on performance, plasma, liver and intestinal tissues of pigs after weaning. Toxicon 2019, 162, 24–31. [Google Scholar] [CrossRef]
- Long, M.; Yang, S.-H.; Han, J.-X.; Li, P.; Zhang, Y.; Dong, S.; Chen, X.; Guo, J.; Wang, J.; He, J.-B. The protective effect of grape-seed proanthocyanidin extract on oxidative damage induced by zearalenone in Kunming mice liver. Int. J. Mol. Sci. 2016, 17, 808. [Google Scholar] [CrossRef]
- Long, M.; Yang, S.; Zhang, Y.; Li, P.; Han, J.; Dong, S.; Chen, X.; He, J. Proanthocyanidin protects against acute zearalenone-induced testicular oxidative damage in male mice. Environ. Sci. Pollut. Res. Int. 2017, 24, 938–946. [Google Scholar] [CrossRef]
- Ben Salem, I.; Prola, A.; Boussabbeh, M.; Guilbert, A.; Bacha, H.; Lemaire, C.; Abid-Essefi, S. Activation of ER stress and apoptosis by α- and β-zearalenol in HCT116 cells, protective role of quercetin. Neurotoxicology 2016, 53, 334–342. [Google Scholar] [CrossRef]
- Ben Salem, I.; Prola, A.; Boussabbeh, M.; Guilbert, A.; Bacha, H.; Abid-Essefi, S.; Lemaire, C. Crocin and quercetin protect HCT116 and HEK293 cells from zearalenone-induced apoptosis by reducing endoplasmic reticulum stress. Cell. Stress Chaperones. 2015, 20, 927–938. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Gehlot, P. Inflammation and cancer: How friendly is the relationship for cancer patients? Curr. Opin. Pharmacol. 2009, 9, 351–369. [Google Scholar] [CrossRef]
- Rachwa-Rosiak, D.; Nebesny, E.; Budryn, G. Chickpeas-composition, nutritional value, health benefits, application to bread and snacks: A review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1137–1145. [Google Scholar] [CrossRef]
- Friedman, M.; Levin, C.E. Review of methods for the reduction of dietary content and toxicity of acrylamide. J. Agric. Food Chem. 2008, 56, 6113–6140. [Google Scholar] [CrossRef]
- Cheng, J.; Chen, X.; Zhao, S.; Zhang, Y. Antioxidant-capacity-based models for the prediction of acrylamide reduction by flavonoids. Food Chem. 2015, 168, 90–99. [Google Scholar] [CrossRef]
- Simunek, T.; Sterba, M.; Popelova, O.; Adamcova, M.; Hrdina, R.; Gersl, V. Anthracycline-induced cardiotoxicity: Overview of studies examining the roles of oxidative stress and free cellular iron. Pharmacol. Rep. 2009, 61, 154–171. [Google Scholar] [CrossRef]
- Cheung-Ong, K.; Giaever, G.; Nislow, C. DNA-damaging agents in cancer chemotherapy: Serendipity and chemical biology. Chem. Biol. 2013, 20, 648–659. [Google Scholar] [CrossRef]
- Kalender, Y.; Yel, M.; Kalender, S. Doxorubicin hepatotoxicity and hepatic free radical metabolism in rats: The effects of vitamin E and catechin. Toxicology 2005, 209, 39–45. [Google Scholar] [CrossRef]
- Dixit, A.K.; Bhatnagar, D.; Kumar, V.; Chawla, D.; Fakhruddin, K.; Bhatnagar, D. Antioxidant potential and radioprotective effect of soy isoflavone against gamma irradiation induced oxidative stress. J. Funct. Foods 2012, 4, 197–206. [Google Scholar] [CrossRef]
- Singh, N.P.; Danner, D.B.; Tice, R.R.; Brant, L.; Schneider, E.L. DNA damage and repair with age in individual human lymphocytes. Mutat. Res. 1990, 237, 123–130. [Google Scholar] [CrossRef]
- Song, L.; Ma, L.; Cong, F.; Shen, X.; Jing, P.; Ying, X.; Zhou, H.; Jiang, J.; Fu, Y.; Yan, H. Radioprotective effects of genistein on HL-7702 cells via the inhibition of apoptosis and DNA damage. Cancer Lett. 2015, 366, 100–111. [Google Scholar] [CrossRef]
- Karri, S.; Sharma, S.; Hatware, K.; Patil, K. Natural anti-obesity agents and their therapeutic role in management of obesity: A future trend perspective. Biomed. Pharmacother. 2019, 110, 224–238. [Google Scholar] [CrossRef]
- Njume, C.; Donkor, O.; McAinch, A.J. Predisposing factors of type 2 diabetes mellitus and the potential protective role of native plants with functional properties. J. Funct. Foods 2019, 53, 115–124. [Google Scholar] [CrossRef]
- Campos-Vega, R.; Loarca-Piña, G.; Oomah, B.D. Minor components of pulses and their potential impact on human health. Food Res. Int. 2010, 43, 461–482. [Google Scholar] [CrossRef]
- Jukanti, A.K.; Gaur, P.M.; Gowda, C.L.L.; Chibbar, R.N. Nutritional quality and health benefits of chickpea (Cicer arietinum L.): A review. Br. J. Nutr. 2012, 108, S11–S26. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Lv, Y.; Yao, K. Effects of tea polyphenols on the activities of α-amylase, pepsin, trypsin and lipase. Food Chem. 2007, 101, 1178–1182. [Google Scholar] [CrossRef]
- Martinez-Gonzalez, A.I.; Díaz-Sánchez, Á.G.; Rosa, L.A.d.l.; Vargas-Requena, C.L.; Bustos-Jaimes, I.; Alvarez-Parrilla, A.E. Polyphenolic compounds and digestive enzymes: In vitro non-covalent interactions. Molecules 2017, 22, 669. [Google Scholar] [CrossRef]
- Vazquez-Flores, A.A.; Wong-Paz, J.E.; Lerma-Herrera, M.A.; Martinez-Gonzalez, A.I.; Olivas-Aguirre, F.J.; Aguilar, C.N.; Wall-Medrano, A.; Gonzalez-Aguilar, G.A.; Alvarez-Parrilla, E.; de la Rosa, L.A. Proanthocyanidins from the kernel and shell of pecan (Carya illinoinensis): Average degree of polymerization and effects on carbohydrate, lipid, and peptide hydrolysis in a simulated human digestive system. J. Funct Foods. 2017, 28, 227–234. [Google Scholar] [CrossRef]
- Lee, B.-H.; Rose, D.R.; Lin, A.H.-M.; Quezada-Calvillo, R.; Nichols, B.L.; Hamaker, B.R. Contribution of the individual small intestinal α-glucosidases to digestion of unusual α-linked glycemic disaccharides. J. Agric. Food Chem. 2016, 64, 6487–6494. [Google Scholar] [CrossRef]
- Shin, H.; Seo, D.-H.; Seo, J.; Lamothe, L.M.; Yoo, S.-H.; Lee, B.-H. Optimization of in vitro carbohydrate digestion by mammalian mucosal α-glucosidases and its applications to hydrolyze the various sources of starches. Food Hydrocoll. 2019, 87, 470–476. [Google Scholar] [CrossRef]
- Yin, Z.; Zhang, W.; Feng, F.; Zhang, Y.; Kang, W. α-Glucosidase inhibitors isolated from medicinal plants. Food Sci. Hum. Wellness 2014, 3, 136–174. [Google Scholar] [CrossRef]
- Egloff, M.P.; Sarda, L.; Verger, R.; Cambillau, C.; van Tilbeurgh, H. Crystallographic study of the structure of colipase and of the interaction with pancreatic lipase. Protein Sci. 1995, 4, 44–57. [Google Scholar] [CrossRef]
- McCue, P.; Kwon, Y.I.; Shetty, K. Anti-diabetic and anti-hypertensive potential of sprouted and solid-state bioprocessed soybean. Asia Pac. J. Clin. Nutr. 2005, 14, 145–152. [Google Scholar]
- Tiwari, A.K.; Sahana, C.; Zehra, A.; Madhusudana, K.; Kumar, D.A.; Agawane, S.B. Mitigation of starch-induced postprandial glycemic spikes in rats by antioxidants-rich extract of Cicer arietinum Linn. seeds and sprouts. J. Pharm. Bioallied. Sci. 2013, 5, 270–276. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, B.; Hwang, C.E.; Haque, M.A.; Kim, S.C.; Lee, C.S.; Kang, S.S.; Cho, K.M.; Lee, D.H. Changes in conjugated linoleic acid and isoflavone contents from fermented soymilks using Lactobacillus plantarum P1201 and screening for their digestive enzyme inhibition and antioxidant properties. J. Funct. Foods 2018, 43, 17–28. [Google Scholar] [CrossRef]
- Shukla, S.; Park, J.; Kim, D.-H.; Hong, S.-Y.; Lee, J.S.; Kim, M. Total phenolic content, antioxidant, tyrosinase and α-glucosidase inhibitory activities of water soluble extracts of noble starter culture Doenjang, a Korean fermented soybean sauce variety. Food Control 2016, 59, 854–861. [Google Scholar] [CrossRef]
- Medina, J.J.R.; Ramírez, K.; Rangel-Peraza, J.G.; Aguayo-Rojas, J. Incremento del valor nutrimental, actividad antioxidante y potencial inhibitorio de α-glucosida en brownies a base de leguminosas cocidas. Arch. Latinoam. Nutr. 2018, 68. [Google Scholar]
- Tan, Y.; Chang, S.K.C.; Zhang, Y. Comparison of α-amylase, α-glucosidase and lipase inhibitory activity of the phenolic substances in two black legumes of different genera. Food Chem. 2017, 214, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Mu, K.; Arntfield, S.D.; Nickerson, M.T. Changes in levels of enzyme inhibitors during soaking and cooking for pulses available in Canada. J. Food. Sci. Technol. 2017, 54, 1014–1022. [Google Scholar] [CrossRef]
- Singh, U. Antinutritional factors of chickpea and pigeonpea and their removal by processing. Plant Foods Hum. Nutr. 1988, 38, 251–261. [Google Scholar] [CrossRef]
- Moussou, N.; Corzo-Martínez, M.; Sanz, M.L.; Zaidi, F.; Montilla, A.; Villamiel, M. Assessment of Maillard reaction evolution, prebiotic carbohydrates, antioxidant activity and α-amylase inhibition in pulse flours. J. Food Sci. Technol. 2017, 54, 890–900. [Google Scholar] [CrossRef]
- Wittanalai, S.; Deming, R.L.; Rakariyatham, N. Characterization of soybean kapi during fermentation with Bacillus spp. Food Biotechnol. 2012, 26, 199–217. [Google Scholar] [CrossRef]
- Lee, S.S.; Mohd Esa, N.; Loh, S.P. In vitro inhibitory activity of selected legumes against pancreatic lipase. J. Food Biochem. 2015, 39, 485–490. [Google Scholar] [CrossRef]
- Suh, D.H.; Jung, E.S.; Park, H.M.; Kim, S.H.; Lee, S.; Jo, Y.H.; Lee, M.K.; Jung, G.; Do, S.-G.; Lee, C.H. Comparison of metabolites variation and antiobesity effects of fermented versus nonfermented mixtures of Cudrania tricuspidata, Lonicera caerulea, and soybean according to fermentation in vitro and in vivo. PLoS ONE 2016, 11, e0149022. [Google Scholar] [CrossRef]
- Hui Cao and Xiaoqing, C. Structures required of flavonoids for inhibiting digestive enzymes. Anticancer Agents Med. Chem. 2012, 12, 929–939. [Google Scholar]
- Di Stefano, E.; Oliviero, T.; Udenigwe, C.C. Functional significance and structure–activity relationship of food-derived α-glucosidase inhibitors. Curr. Opin. Food Sci. 2018, 20, 7–12. [Google Scholar] [CrossRef]
- Gonzales, G.B.; Smagghe, G.; Grootaert, C.; Zotti, M.; Raes, K.; Camp, J.V. Flavonoid interactions during digestion, absorption, distribution and metabolism: A sequential structure–activity/property relationship-based approach in the study of bioavailability and bioactivity. Drug Metab. Rev. 2015, 47, 175–190. [Google Scholar] [CrossRef]
- Ozdal, T.; Capanoglu, E.; Altay, F. A review on protein–phenolic interactions and associated changes. Food Res. Int. 2013, 51, 954–970. [Google Scholar] [CrossRef]
- Xiao, J.; Kai, G.; Yamamoto, K.; Chen, X. Advance in dietary polyphenols as α-glucosidases inhibitors: A review on structure-activity relationship aspect. Crit. Rev. Food Sci. Nutr. 2013, 53, 818–836. [Google Scholar] [CrossRef]
- Xiao, J.; Ni, X.; Kai, G.; Chen, X. Review on structure–activity relationship of dietary polyphenols inhibiting α-amylase. Crit. Rev. Food Sci. Nutr. 2013, 53, 497–506. [Google Scholar] [CrossRef]
- Akkarachiyasit, S.; Charoenlertkul, P.; Yibchok-Anun, S.; Adisakwattana, S. Inhibitory activities of cyanidin and its glycosides and synergistic effect with acarbose against intestinal α-glucosidase and pancreatic α-amylase. Int. J. Mol. Sci. 2010, 11, 3387–3396. [Google Scholar] [CrossRef]
- Ercan, P.; El, S.N. Inhibitory effects of chickpea and Tribulus terrestris on lipase, α-amylase and α-glucosidase. Food Chem. 2016, 205, 163–169. [Google Scholar] [CrossRef]
- Justino, A.B.; Miranda, N.C.; Franco, R.R.; Martins, M.M.; Silva, N.M.d.; Espindola, F.S. Annona muricata Linn. leaf as a source of antioxidant compounds with in vitro antidiabetic and inhibitory potential against α-amylase, α-glucosidase, lipase, non-enzymatic glycation and lipid peroxidation. Biomed. Pharmacother. 2018, 100, 83–92. [Google Scholar] [CrossRef]
- Vazquez-Flores, A.A.; Martinez-Gonzalez, A.I.; Alvarez-Parrilla, E.; Díaz-Sánchez, Á.G.; de la Rosa, L.A.; González-Aguilar, G.A.; Aguilar, C.N. Proanthocyanidins with a low degree of polymerization are good inhibitors of digestive enzymes because of their ability to form specific interactions: A hypothesis. J. Food Sci. 2018, 83, 2895–2902. [Google Scholar] [CrossRef]
- Martinez-Gonzalez, A.I.; Alvarez-Parrilla, E.; Díaz-Sánchez, Á.G.; de la Rosa, L.A.; Núñez-Gastélum, J.A.; Vazquez-Flores, A.A.; Gonzalez-Aguilar, G.A. In vitro inhibition of pancreatic lipase by polyphenols: A kinetic, fluorescence spectroscopy and molecular docking study. Food Technol. Biotechnol. 2017, 55, 519–530. [Google Scholar] [CrossRef]
- Martinez-Gonzalez, A.I.; Díaz-Sánchez, Á.G.; de la Rosa, L.A.; Bustos-Jaimes, I.; Alvarez-Parrilla, E. Inhibition of α-amylase by flavonoids: Structure activity relationship (SAR). Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 206, 437–447. [Google Scholar] [CrossRef]
- Lunagariya, N.A.; Patel, N.K.; Jagtap, S.C.; Bhutani, K.K. Inhibitors of pancreatic lipase: State of the art and clinical perspectives. EXCLI J. 2014, 13, 897–921. [Google Scholar]
- Tadera, K.; Minami, Y.; Takamatsu, K.; Matsuoka, T. Inhibition of alpha-glucosidase and alpha-amylase by flavonoids. J. Nutr. Sci. Vitaminol. (Tokyo) 2006, 52, 149–153. [Google Scholar] [CrossRef]
- Cassidy, Y.M.; McSorley, E.M.; Allsopp, P.J. Effect of soluble dietary fibre on postprandial blood glucose response and its potential as a functional food ingredient. J. Funct. Foods 2018, 46, 423–439. [Google Scholar] [CrossRef]
- Konig, D.; Deibert, P.; Frey, I.; Landmann, U.; Berg, A. Effect of meal replacement on metabolic risk factors in overweight and obese subjects. Ann. Nutr. Metab. 2008, 52, 74–78. [Google Scholar] [CrossRef]
- Rebholz, C.M.; Reynolds, K.; Wofford, M.R.; Chen, J.; Kelly, T.N.; Mei, H.; Whelton, P.K.; He, J. Effect of soybean protein on novel cardiovascular disease risk factors: A randomized controlled trial. Eur. J. Clin. Nutr. 2013, 67, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Kani, A.H.; Alavian, S.M.; Esmaillzadeh, A.; Adibi, P.; Haghighatdoost, F.; Azadbakht, L. Effects of a low-calorie, low-carbohydrate soy containing diet on systemic inflammation among patients with nonalcoholic fatty liver disease: A parallel randomized clinical trial. Horm. Metab. Res. 2017, 49, 687–692. [Google Scholar] [CrossRef] [PubMed]
- Adams, L.A.; Lindor, K.D. Nonalcoholic fatty liver disease. Ann. Epidemiol. 2007, 17, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Van De Wier, B.; Koek, G.H.; Bast, A.; Haenen, G.R. The potential of flavonoids in the treatment of non-alcoholic fatty liver disease. Crit. Rev. Food Sci. Nutr. 2017, 57, 834–855. [Google Scholar] [CrossRef] [PubMed]
- Iritani, N.; Sugimoto, T.; Fukuda, H.; Komiya, M.; Ikeda, H. Dietary soybean protein increases insulin receptor gene expression in Wistar fatty rats when dietary polyunsaturated fatty acid level is low. J. Nutr. 1997, 127, 1077–1083. [Google Scholar] [CrossRef] [PubMed]
- Lavigne, C.; Marette, A.; Jacques, H. Cod and soy proteins compared with casein improve glucose tolerance and insulin sensitivity in rats. Am. J. Physiol. Endocrinol. Metab. 2000, 278, E491–E500. [Google Scholar] [CrossRef]
- Lim, S.S.; Vos, T.; Flaxman, A.D.; Danaei, G.; Shibuya, K.; Adair-Rohani, H.; Amann, M.; Anderson, H.R.; Andrews, K.G.; Aryee, M.; et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: A systematic analysis for the global burden of disease study 2010. Lancet 2012, 380, 2224–2260. [Google Scholar] [CrossRef]
- Kwon, D.Y.; Daily, J.W., 3rd; Kim, H.J.; Park, S. Antidiabetic effects of fermented soybean products on type 2 diabetes. Nutr. Res. 2010, 30, 1–13. [Google Scholar] [CrossRef]
- Li, S.; Camp, S.; Finck, J.; Winter, M.; Chapman-Novakofski, K. Behavioral control is an important predictor of soy intake in adults in the USA concerned about diabetes. Asia Pac. J. Clin. Nutr. 2010, 19, 358–364. [Google Scholar] [PubMed]
- Li, W.; Ruan, W.; Peng, Y.; Wang, D. Soy and the risk of type 2 diabetes mellitus: A systematic review and meta-analysis of observational studies. Diabetes Res. Clin. Pract. 2018, 137, 190–199. [Google Scholar] [CrossRef]
- Goodman-Gruen, D.; Kritz-Silverstein, D. Usual dietary isoflavone intake is associated with cardiovascular disease risk factors in postmenopausal women. J. Nutr. 2001, 131, 1202–1206. [Google Scholar] [CrossRef]
- Luo, T.; Miranda-Garcia, O.; Sasaki, G.; Wang, J.; Shay, N.F. Genistein and daidzein decrease food intake and body weight gain in mice, and alter LXR signaling in vivo and in vitro. Food Funct. 2018, 9, 6257–6267. [Google Scholar] [CrossRef]
- Ae Park, S.; Choi, M.S.; Cho, S.Y.; Seo, J.S.; Jung, U.J.; Kim, M.J.; Sung, M.K.; Park, Y.B.; Lee, M.K. Genistein and daidzein modulate hepatic glucose and lipid regulating enzyme activities in C57BL/KsJ-db/db mice. Life Sci. 2006, 79, 1207–1213. [Google Scholar] [CrossRef]
- Choi, M.S.; Jung, U.J.; Yeo, J.; Kim, M.J.; Lee, M.K. Genistein and daidzein prevent diabetes onset by elevating insulin level and altering hepatic gluconeogenic and lipogenic enzyme activities in non-obese diabetic (NOD) mice. Diabetes Metab. Res. Rev. 2008, 24, 74–81. [Google Scholar] [CrossRef]
- Crujeiras, A.B.; Parra, D.; Abete, I.; Martinez, J.A. A hypocaloric diet enriched in legumes specifically mitigates lipid peroxidation in obese subjects. Free Radic. Res. 2007, 41, 498–506. [Google Scholar] [CrossRef]
- Nestel, P.; Cehun, M.; Chronopoulos, A. Effects of long-term consumption and single meals of chickpeas on plasma glucose, insulin, and triacylglycerol concentrations. Am. J. Clin. Nutr. 2004, 79, 390–395. [Google Scholar] [CrossRef]
- Li, Z.; Hong, K.; Saltsman, P.; DeShields, S.; Bellman, M.; Thames, G.; Liu, Y.; Wang, H.J.; Elashoff, R.; Heber, D. Long-term efficacy of soy-based meal replacements vs an individualized diet plan in obese type II DM patients: Relative effects on weight loss, metabolic parameters, and C-reactive protein. Eur. J. Clin. Nutr. 2005, 59, 411–418. [Google Scholar] [CrossRef]
- Ali, A.A.; Velasquez, M.T.; Hansen, C.T.; Mohamed, A.I.; Bhathena, S.J. Effects of soybean isoflavones, probiotics, and their interactions on lipid metabolism and endocrine system in an animal model of obesity and diabetes. J. Nutr. Biochem. 2004, 15, 583–590. [Google Scholar] [CrossRef]
- Zhang, H.M.; Chen, S.W.; Zhang, L.S.; Feng, X.F. The effects of soy isoflavone on insulin sensitivity and adipocytokines in insulin resistant rats administered with high-fat diet. Nat. Prod. Res. 2008, 22, 1637–1649. [Google Scholar] [CrossRef]
- Dkhar, B.; Khongsti, K.; Thabah, D.; Syiem, D.; Satyamoorthy, K.; Das, B. Genistein represses PEPCK-C expression in an insulin-independent manner in HepG2 cells and in alloxan-induced diabetic mice. J. Cell Biochem. 2018, 119, 1953–1970. [Google Scholar] [CrossRef]
- El-Kordy, E.A.; Alshahrani, A.M. Effect of genistein, a natural soy isoflavone, on pancreatic β-cells of streptozotocin-induced diabetic rats: Histological and immunohistochemical study. J. Microsc. Ultrastruct. 2015, 3, 108–119. [Google Scholar] [CrossRef]
- Fu, Z.; Zhang, W.; Zhen, W.; Lum, H.; Nadler, J.; Bassaganya-Riera, J.; Jia, Z.; Wang, Y.; Misra, H.; Liu, D. Genistein induces pancreatic beta-cell proliferation through activation of multiple signaling pathways and prevents insulin-deficient diabetes in mice. Endocrinology 2010, 151, 3026–3037. [Google Scholar] [CrossRef]
- Wright, D.C.; Geiger, P.C.; Han, D.H.; Holloszy, J.O. Are tyrosine kinases involved in mediating contraction-stimulated muscle glucose transport? Am. J. Physiol. Endocrinol. Metab. 2006, 290, E123–E128. [Google Scholar] [CrossRef][Green Version]
- Choi, J.S.; Koh, I.U.; Song, J. Genistein reduced insulin resistance index through modulating lipid metabolism in ovariectomized rats. Nutr. Res. 2012, 32, 844–855. [Google Scholar] [CrossRef]
- Haluzik, M.M.; Haluzik, M. PPAR-α and insulin sensitivity. Physiol. Res. 2006, 55, 115–122. [Google Scholar] [PubMed]
- Leonardini, A.; Laviola, L.; Perrini, S.; Natalicchio, A.; Giorgino, F. Cross-talk between PPARγ and insulin signaling and modulation of insulin sensitivity. PPAR Res. 2009, 2009, 818945. [Google Scholar] [CrossRef]
- Kubota, N.; Terauchi, Y.; Miki, H.; Tamemoto, H.; Yamauchi, T.; Komeda, K.; Satoh, S.; Nakano, R.; Ishii, C.; Sugiyama, T.; et al. PPARγ mediates high-fat diet-induced adipocyte hypertrophy and insulin resistance. Mol. Cell. 1999, 4, 597–609. [Google Scholar] [CrossRef]
- Mueller, N.T.; Odegaard, A.O.; Gross, M.D.; Koh, W.P.; Yu, M.C.; Yuan, J.M.; Pereira, M.A. Soy intake and risk of type 2 diabetes in Chinese Singaporeans [corrected]. Eur. J. Nutr. 2012, 51, 1033–1040. [Google Scholar] [CrossRef]
- Nanri, A.; Mizoue, T.; Takahashi, Y.; Kirii, K.; Inoue, M.; Noda, M.; Tsugane, S. Soy product and isoflavone intakes are associated with a lower risk of type 2 diabetes in overweight Japanese women. J. Nutr. 2010, 140, 580–586. [Google Scholar] [CrossRef]
- Villegas, R.; Gao, Y.T.; Yang, G.; Li, H.L.; Elasy, T.A.; Zheng, W.; Shu, X.O. Legume and soy food intake and the incidence of type 2 diabetes in the Shanghai Women’s Health Study. Am. J. Clin. Nutr. 2008, 87, 162–167. [Google Scholar] [CrossRef]
- de Camargo, A.C.; Regitano-d’Arce, M.A.B.; Biasoto, A.C.T.; Shahidi, F. Enzyme-assisted extraction of phenolics from winemaking by-products: Antioxidant potential and inhibition of alpha-glucosidase and lipase activities. Food Chem. 2016, 212, 395–402. [Google Scholar] [CrossRef]
- Sievenpiper, J.L.; Kendall, C.W.; Esfahani, A.; Wong, J.M.; Carleton, A.J.; Jiang, H.Y.; Bazinet, R.P.; Vidgen, E.; Jenkins, D.J. Effect of non-oil-seed pulses on glycaemic control: A systematic review and meta-analysis of randomised controlled experimental trials in people with and without diabetes. Diabetologia 2009, 52, 1479–1495. [Google Scholar] [CrossRef] [PubMed]
- Etxeberria, U.; Fernández-Quintela, A.; Milagro, F.I.; Aguirre, L.; Martínez, J.A.; Portillo, M.P. Impact of polyphenols and polyphenol-rich dietary sources on gut microbiota composition. J. Agric. Food Chem. 2013, 61, 9517–9533. [Google Scholar] [CrossRef]
- Steer, T.E.; Johnson, I.T.; Gee, J.M.; Gibson, G.R. Metabolism of the soybean isoflavone glycoside genistin in vitro by human gut bacteria and the effect of prebiotics. Br. J. Nutr. 2003, 90, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Guadamuro, L.; Dohrmann, A.B.; Tebbe, C.C.; Mayo, B.; Delgado, S. Bacterial communities and metabolic activity of faecal cultures from equol producer and non-producer menopausal women under treatment with soy isoflavones. BMC Microbiol. 2017, 17, 93. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Shimizu, Y.; Kimura, I. Gut microbial metabolite short-chain fatty acids and obesity. Biosci. Microbiota Food Health 2017, 36, 135–140. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).