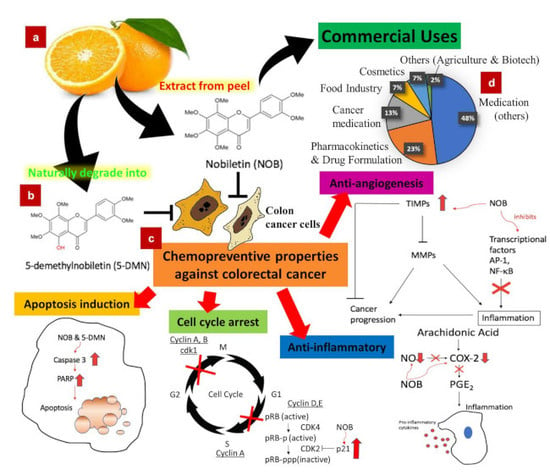
Nobiletin and Derivatives: Functional Compounds from Citrus Fruit Peel for Colon Cancer Chemoprevention
Abstract
:1. Introduction
2. Research Methodology
3. Nobiletin and Its Derivatives
4. Pathogenesis of Colorectal Cancer
5. Chemopreventive Effects of Nobiletin, 5-DMN and NOB-Metabolites
5.1. Cell Cycle Arrest
5.1.1. Action of NOB and Its Metabolites Inducing Cell Arrest
5.1.2. Action of 5-DMN Inducing Cell Cycle Arrest
5.2. Programmed Cell Death
Action of NOB and Metabolites Inducing Programmed Cell Death
5.3. Anti-Inflammation
Anti-Inflammation Effect of NOB and Its Metabolites
5.4. Anti-Angiogenesis
Anti-Angiogenesis Effect of NOB
6. Pharmacokinetics, Bioavailability and Delivery Systems of NOB
7. Toxicity
8. Commercial Uses
9. Future Directions
10. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2017. CA Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernstein, C.N.; Blanchard, J.F.; Kliewer, E.; Wajda, A. Cancer risk in patients with inflammatory bowel disease: A population-based study. Cancer 2001, 91, 854–862. [Google Scholar] [CrossRef]
- Rubin, D.C.; Shaker, A.; Levin, M.S. Chronic intestinal inflammation: Inflammatory bowel disease and colitis-associated colon cancer. Front. Immunol. 2012, 3, 107. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fedewa, S.A.; Ahnen, D.J.; Meester, R.G.; Barzi, A.; Jemal, A. Colorectal cancer statistics, 2017. CA Cancer J. Clin. 2017, 67, 177–193. [Google Scholar] [CrossRef] [PubMed]
- Simon, K. Colorectal cancer development and advances in screening. Clin. Interv. Aging 2016, 11, 967–976. [Google Scholar] [PubMed]
- Tan, L.T.H.; Lee, L.H.; Yin, W.F.; Chan, C.K.; Abdul Kadir, H.; Chan, K.G.; Goh, B.H. Traditional Uses, Phytochemistry, and Bioactivities of Cananga odorata (Ylang-Ylang). Evid. Based Complement. Altern. Med. 2015, 2015, 30. [Google Scholar] [CrossRef]
- Chan, W.-K.; Tan, L.T.-H.; Chan, K.-G.; Lee, L.-H.; Goh, B.-H. Nerolidol: A Sesquiterpene Alcohol with Multi-Faceted Pharmacological and Biological Activities. Molecules 2016, 21, 529. [Google Scholar] [CrossRef]
- Tan, H.-L.; Chan, K.-G.; Pusparajah, P.; Saokaew, S.; Duangjai, A.; Lee, L.-H.; Goh, B.-H. Anti-Cancer Properties of the Naturally Occurring Aphrodisiacs: Icariin and Its Derivatives. Front. Pharmacol. 2016, 7. [Google Scholar] [CrossRef] [Green Version]
- Steward, W.P.; Brown, K. Cancer chemoprevention: A rapidly evolving field. Br. J. Cancer 2013, 109, 1. [Google Scholar] [CrossRef]
- Tang, C.; Hoo, P.C.-X.; Tan, L.T.-H.; Pusparajah, P.; Khan, T.M.; Lee, L.-H.; Goh, B.-H.; Chan, K.-G. Golden Needle Mushroom: A Culinary Medicine with Evidenced-Based Biological Activities and Health Promoting Properties. Front. Pharmacol. 2016, 7. [Google Scholar] [CrossRef]
- Alam, M.N.; Almoyad, M.; Huq, F. Polyphenols in Colorectal Cancer: Current State of Knowledge including Clinical Trials and Molecular Mechanism of Action. BioMed Res. Int. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Cai, D.; Pearce, K.; Sun, P.Y.; Roberts, A.C.; Glanzman, D.L. Reinstatement of long-term memory following erasure of its behavioral and synaptic expression in Aplysia. eLife 2014, 3. [Google Scholar] [CrossRef] [PubMed]
- Surichan, S.; Arroo, R.R.; Ruparelia, K.; Tsatsakis, A.M.; Androutsopoulos, V.P. Nobiletin bioactivation in MDA-MB-468 breast cancer cells by cytochrome P450 CYP1 enzymes. Food Chem. Toxicol. 2018, 113, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Morley, K.L.; Ferguson, P.J.; Koropatnick, J. Tangeretin and nobiletin induce G1 cell cycle arrest but not apoptosis in human breast and colon cancer cells. Cancer Lett. 2007, 251, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.-P.; Guo, H.; Wang, X.-B. Nobiletin (NOB) suppresses autophagic degradation via over-expressing AKT pathway and enhances apoptosis in multidrug-resistant SKOV3/TAX ovarian cancer cells. Biomed. Pharmacother. 2018, 103, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.Y.; Cho, M.; Ahn, K.S.; Cho, S.K. Nobiletin induces apoptosis and potentiates the effects of the anticancer drug 5-fluorouracil in p53-mutated SNU-16 human gastric cancer cells. Nutr. Cancer 2013, 65, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.Y.; Cho, S.K. Nobiletin induces protective autophagy accompanied by ER-stress mediated apoptosis in human gastric cancer SNU-16 cells. Molecules 2016, 21, 914. [Google Scholar] [CrossRef] [PubMed]
- Uesato, S.; Yamashita, H.; Maeda, R.; Hirata, Y.; Yamamoto, M.; Matsue, S.; Nagaoka, Y.; Shibano, M.; Taniguchi, M.; Baba, K. Synergistic antitumor effect of a combination of paclitaxel and carboplatin with nobiletin from Citrus depressa on non-small-cell lung cancer cell lines. Planta Med. 2014, 80, 452–457. [Google Scholar] [CrossRef]
- Song, M.; Wu, X.; Charoensinphon, N.; Wang, M.; Zheng, J.; Gao, Z.; Xu, F.; Li, Z.; Li, F.; Zhou, J. Dietary 5-demethylnobiletin inhibits cigarette carcinogen NNK-induced lung tumorigenesis in mice. Food Funct. 2017, 8, 954–963. [Google Scholar] [CrossRef]
- Ma, X.; Jin, S.; Zhang, Y.; Wan, L.; Zhao, Y.; Zhou, L. Inhibitory effects of nobiletin on hepatocellular carcinoma in vitro and in vivo. Phytother. Res. 2014, 28, 560–567. [Google Scholar] [CrossRef]
- Cheng, H.-L.; Hsieh, M.-J.; Yang, J.-S.; Lin, C.-W.; Lue, K.-H.; Lu, K.-H.; Yang, S.-F. Nobiletin inhibits human osteosarcoma cells metastasis by blocking ERK and JNK-mediated MMPs expression. Oncotarget 2016, 7, 35208–35223. [Google Scholar] [CrossRef] [PubMed]
- Braidy, N.; Behzad, S.; Habtemariam, S.; Ahmed, T.; Daglia, M.; Mohammad Nabavi, S.; Sobarzo-Sanchez, E.; Fazel Nabavi, S. Neuroprotective effects of citrus fruit-derived flavonoids, nobiletin and tangeretin in Alzheimer’s and Parkinson’s disease. CNS Neurol. Disord. Drug Targets (Former. Curr. Drug Targets CNS Neurol. Disord.) 2017, 16, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Qi, G.; Guo, R.; Tian, H.; Li, L.; Liu, H.; Mi, Y.; Liu, X. Nobiletin protects against insulin resistance and disorders of lipid metabolism by reprogramming of circadian clock in hepatocytes. Biochim. Biophys. Acta Mol. Cell Biolo. Lipids 2018, 1863, 549–562. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Veeranjaneyulu, A. Complications of Diabetes and Role of a Citrus Flavonoid Nobiletin in Its Treatment. In Herbs for Diabetes and Neurological Disease Management; Apple Academic Press: Oakville, ON, Canada, 2018; pp. 197–224. [Google Scholar]
- Morrow, N.M.; Telford, D.E.; Sutherland, B.G.; Edwards, J.Y.; Huff, M.W. Nobiletin Corrects Intestinal Lipid Metabolism in Ldlr-/-Mice Fed a High-Fat Diet. Atheroscler. Suppl. 2018, 32, 28. [Google Scholar] [CrossRef]
- Yuk, T.; Kim, Y.; Yang, J.; Sung, J.; Jeong, H.S.; Lee, J. Nobiletin Inhibits Hepatic Lipogenesis via Activation of AMP-Activated Protein Kinase. Evid. Based Complement. Altern. Med. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Tung, Y.-C.; Li, S.; Huang, Q.; Hung, W.-L.; Ho, C.-T.; Wei, G.-J.; Pan, M.-H. 5-Demethylnobiletin and 5-Acetoxy-6, 7, 8, 3′, 4′-pentamethoxyflavone Suppress Lipid Accumulation by Activating the LKB1-AMPK Pathway in 3T3-L1 Preadipocytes and High Fat Diet-Fed C57BL/6 Mice. J. Agric. Food Chem. 2016, 64, 3196–3205. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Zhu, X.; Pan, S.; Fang, Y.; Jiang, F.; Phillips, G.O.; Xu, X. Antimicrobial activity of nobiletin and tangeretin against Pseudomonas. Food Chem. 2012, 132, 1883–1890. [Google Scholar] [CrossRef]
- Onishi, S.; Nishi, K.; Yasunaga, S.; Muranaka, A.; Maeyama, K.; Kadota, A.; Sugahara, T. Nobiletin, a polymethoxy flavonoid, exerts anti-allergic effect by suppressing activation of phosphoinositide 3-kinase. J. Funct. Foods 2014, 6, 606–614. [Google Scholar] [CrossRef]
- Lai, C.-S.; Li, S.; Chai, C.-Y.; Lo, C.-Y.; Dushenkov, S.; Ho, C.-T.; Pan, M.-H.; Wang, Y.-J. Anti-inflammatory and antitumor promotional effects of a novel urinary metabolite, 3′, 4′-didemethylnobiletin, derived from nobiletin. Carcinogenesis 2008, 29, 2415–2424. [Google Scholar] [CrossRef]
- Narayana, J.L.; Huang, H.-N.; Wu, C.-J.; Chen, J.-Y. Epinecidin-1 antimicrobial activity: In vitro membrane lysis and In vivo efficacy against Helicobacter pylori infection in a mouse model. Biomaterials 2015, 61, 41–51. [Google Scholar] [CrossRef]
- Eguchi, A.; Murakami, A.; Li, S.; Ho, C.T.; Ohigashi, H. Suppressive effects of demethylated metabolites of nobiletin on phorbol ester-induced expression of scavenger receptor genes in THP-1 human monocytic cells. Biofactors 2007, 31, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, A.; Murakami, A.; Ohigashi, H. Nobiletin, a citrus flavonoid, suppresses phorbol ester-induced expression of multiple scavenger receptor genes in THP-1 human monocytic cells. FEBS Lett. 2006, 580, 3321–3328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.-S.; Asai, M.; Choi, S.-S.; Yonezawa, T.; Teruya, T.; Nagai, K.; Woo, J.-T.; Cha, B.-Y. Nobiletin prevents body weight gain and bone loss in ovariectomized C57BL/6J mice. Pharmaco. Pharm. 2014, 5, 959–965. [Google Scholar] [CrossRef]
- Tominari, T.; Hirata, M.; Matsumoto, C.; Inada, M.; Miyaura, C. Polymethoxy flavonoids, nobiletin and tangeretin, prevent lipopolysaccharide-induced inflammatory bone loss in an experimental model for periodontitis. J. Pharmacol. Sci. 2012, 119, 390–394. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Gao, W.; Zeng, S.-L.; Li, P.; Liu, E.-H. Chemical structures, bioactivities and molecular mechanisms of citrus polymethoxyflavones. J. Funct. Foods 2018, 40, 498–509. [Google Scholar] [CrossRef]
- Tung, Y.-C.; Chou, Y.-C.; Hung, W.-L.; Cheng, A.-C.; Yu, R.-C.; Ho, C.-T.; Pan, M.-H. Polymethoxyflavones: Chemistry and Molecular Mechanisms for Cancer Prevention and Treatment. Curr. Pharmacol. Rep. 2019, 5, 98–113. [Google Scholar] [CrossRef]
- Uckoo, R.M.; Jayaprakasha, G.; Vikram, A.; Patil, B.S. Polymethoxyflavones isolated from the peel of Miaray Mandarin (Citrus miaray) have biofilm inhibitory activity in Vibrio harveyi. J. Agric. Food Chem. 2015, 63, 7180–7189. [Google Scholar] [CrossRef] [PubMed]
- Itoh, N.; Iwata, C.; Toda, H. Molecular cloning and characterization of a flavonoid-O-methyltransferase with broad substrate specificity and regioselectivity from Citrus depressa. BMC Plant Biol. 2016, 16, 180. [Google Scholar] [CrossRef]
- Lee, Y.-H.; Charles, A.L.; Kung, H.-F.; Ho, C.-T.; Huang, T.-C. Extraction of nobiletin and tangeretin from Citrus depressa Hayata by supercritical carbon dioxide with ethanol as modifier. Ind. Crops Prod. 2010, 31, 59–64. [Google Scholar] [CrossRef]
- Kohno, H.; Yoshitani, S.-I.; Tsukio, Y.; Murakami, A.; Koshimizu, K.; Yano, M.; Tokuda, H.; Nishino, H.; Ohigashi, H.; Tanaka, T. Dietary administration of citrus nobiletin inhibits azoxymethane-induced colonic aberrant crypt foci in rats. Life Sci. 2001, 69, 901–913. [Google Scholar] [CrossRef]
- Murakami, A.; Nakamura, Y.; Torikai, K.; Tanaka, T.; Koshiba, T.; Koshimizu, K.; Kuwahara, S.; Takahashi, Y.; Ogawa, K.; Yano, M. Inhibitory effect of citrus nobiletin on phorbol ester-induced skin inflammation, oxidative stress, and tumor promotion in mice. Cancer Res. 2000, 60, 5059–5066. [Google Scholar] [PubMed]
- Uckoo, R.M.; Jayaprakasha, G.K.; Patil, B.S. Rapid separation method of polymethoxyflavones from citrus using flash chromatography. Sep. Purif. Technol. 2011, 81, 151–158. [Google Scholar] [CrossRef]
- Teruya, T.; Teruya, Y.; Sueyoshi, K.; Yamano, A.; Jitai, Y. Manufacturing method of fermentation treated products containing high-content nobiletin and tangeretin. Patent JP 2015202065, 16 November 2015. [Google Scholar]
- Kawaii, S.; Tomono, Y.; Katase, E.; Ogawa, K.; Yano, M. HL-60 differentiating activity and flavonoid content of the readily extractable fraction prepared from Citrus juices. J. Agric. Food Chem. 1999, 47, 128–135. [Google Scholar] [CrossRef]
- Tsukayama, M.; Ichikawa, R.; Yamamoto, K.; Sasaki, T.; Kawamura, Y. Microwave-assisted rapid extraction of polymethoxyflavones from dried peels of Citrus yuko Hort. ex Tanaka. J. Jpn. Soc. Food Sci. Technol. 2009, 56, 359–362. [Google Scholar] [CrossRef]
- Silva, I.; Estrada, M.F.; V. Pereira, C.; da Silva, A.B.; Bronze, M.R.; Alves, P.M.; Duarte, C.M.; Brito, C.; Serra, A.T. Polymethoxylated Flavones from Orange Peels Inhibit Cell Proliferation in a 3D Cell Model of Human Colorectal Cancer. Nutr. Cancer 2018, 70, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.W.; Jang, D.R.; Kim, Y.U. Preparation Method of Citrus Peel Extract with Increased Polymethoxyflavone Content by Supercritical Fluid Extraction. Patent KR 1838266, 14 March 2018. [Google Scholar]
- Chiou, Y.-S.; Zheng, Y.-N.; Tsai, M.-L.; Lai, C.-S.; Ho, C.-T.; Pan, M.-H. 5-Demethylnobiletin more potently inhibits colon cancer cell growth than nobiletin in vitro and in vivo. J. Food Bioact. 2018, 2, 91–97. [Google Scholar] [CrossRef]
- Zheng, J.; Bi, J.; Johnson, D.; Sun, Y.; Song, M.; Qiu, P.; Dong, P.; Decker, E.; Xiao, H. Analysis of 10 metabolites of polymethoxyflavones with high sensitivity by electrochemical detection in high-performance liquid chromatography. J. Agric. Food Chem. 2015, 63, 509–516. [Google Scholar] [CrossRef]
- Zheng, J.; Song, M.; Dong, P.; Qiu, P.; Guo, S.; Zhong, Z.; Li, S.; Ho, C.T.; Xiao, H. Identification of novel bioactive metabolites of 5-demethylnobiletin in mice. Mol. Nutr. Food Res. 2013, 57, 1999–2007. [Google Scholar] [CrossRef]
- Li, S.; Wang, Z.; Sang, S.; Huang, M.T.; Ho, C.T. Identification of nobiletin metabolites in mouse urine. Mol. Nutr. Food Res. 2006, 50, 291–299. [Google Scholar] [CrossRef]
- Yasuda, T.; Yoshimura, Y.; Yabuki, H.; Nakazawa, T.; Ohsawa, K.; Mimaki, Y.; Sashida, Y. Urinary metabolites of nobiletin orally administered to rats. Chem. Pharm. Bull. 2003, 51, 1426–1428. [Google Scholar] [CrossRef]
- Wu, X.; Song, M.; Wang, M.; Zheng, J.; Gao, Z.; Xu, F.; Zhang, G.; Xiao, H. Chemopreventive effects of nobiletin and its colonic metabolites on colon carcinogenesis. Mol. Nutr. Food Res. 2015, 59, 2383–2394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Li, S.; Jonca, M.; Lambros, T.; Ferguson, S.; Goodnow, R.; Ho, C.T. Comparison of supercritical fluid chromatography and liquid chromatography for the separation of urinary metabolites of nobiletin with chiral and non-chiral stationary phases. Biomed. Chromatogr. 2006, 20, 1206–1215. [Google Scholar] [CrossRef] [PubMed]
- Koga, N.; Ohta, C.; Kato, Y.; Haraguchi, K.; Endo, T.; Ogawa, K.; Ohta, H.; Yano, M. In vitro metabolism of nobiletin, a polymethoxy-flavonoid, by human liver microsomes and cytochrome P450. Xenobiotica 2011, 41, 927–933. [Google Scholar] [CrossRef] [PubMed]
- Wang, M. Biotransformation of Polymethoxyflavones and Its Implication on Biological Activities. Ph.D. Thesis, University of Massachusetts, Amherst, MA, USA, 2017. [Google Scholar]
- Xu, L.; He, Y.; Guo, X.; Lu, Y.; Wang, C.; Wang, Z. Identification of metabolites of nobiletin in rats using ultra-performance liquid chromatography coupled with triple-quadrupole mass spectrometry. Yao Xue Xue Bao (Acta Pharm. Sin.) 2011, 46, 1483–1487. [Google Scholar]
- Manthey, J.A.; Bendele, P. Anti-inflammatory activity of an orange peel polymethoxylated flavone, 3′, 4′, 3, 5, 6, 7, 8-heptamethoxyflavone, in the rat carrageenan/paw edema and mouse lipopolysaccharide-challenge assays. J. Agric. Food Chem. 2008, 56, 9399–9403. [Google Scholar] [CrossRef] [PubMed]
- Kemperman, R.A.; Bolca, S.; Roger, L.C.; Vaughan, E.E. Novel approaches for analysing gut microbes and dietary polyphenols: Challenges and opportunities. Microbiology 2010, 156, 3224–3231. [Google Scholar] [CrossRef] [PubMed]
- Ma, C. Biotransformation of Polymethoxyflavones by Gut Microbiome and Molecular Characterization of Polymethoxyflavones by Surface Enhanced Raman Spectroscopy. Ph.D. Thesis, University of Massachusetts, Amherst, MA, USA, 2015. [Google Scholar]
- Li, S.; Sang, S.; Pan, M.-H.; Lai, C.-S.; Lo, C.-Y.; Yang, C.S.; Ho, C.-T. Anti-inflammatory property of the urinary metabolites of nobiletin in mouse. Bioorg. Med. Chem. Lett. 2007, 17, 5177–5181. [Google Scholar] [CrossRef] [PubMed]
- Qiu, P.; Dong, P.; Guan, H.; Li, S.; Ho, C.T.; Pan, M.H.; McClements, D.J.; Xiao, H. Inhibitory effects of 5-hydroxy polymethoxyflavones on colon cancer cells. Mol. Nutr. Food Res. 2010, 54, S244–S252. [Google Scholar] [CrossRef] [PubMed]
- Fearon, E.R.; Vogelstein, B. A genetic model for colorectal tumorigenesis. Cell 1990, 61, 759–767. [Google Scholar] [CrossRef]
- Ciardiello, F.; Tortora, G. EGFR antagonists in cancer treatment. N. Engl. J. Med. 2008, 358, 1160–1174. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Massagué, J. Mechanisms of TGF-β signaling from cell membrane to the nucleus. Cell 2003, 113, 685–700. [Google Scholar] [CrossRef]
- Neuzillet, C.; Tijeras-Raballand, A.; Cohen, R.; Cros, J.; Faivre, S.; Raymond, E.; de Gramont, A. Targeting the TGFβ pathway for cancer therapy. Pharmacol. Ther. 2015, 147, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Said, A.H.; Raufman, J.-P.; Xie, G. The role of matrix metalloproteinases in colorectal cancer. Cancers 2014, 6, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Kang, S.-M.; Sawada, T.; Nishiguchi, Y.; Yashiro, M.; Ogawa, Y.; Ohira, M.; Ishikawa, T.; Hirakawa-YS Chung, K. Expression of intercellular adhesion molecule-1 and prognosis in colorectal cancer. Oncol. Rep. 2002, 9, 511–514. [Google Scholar] [CrossRef] [PubMed]
- Manthey, J.A.; Guthrie, N. Antiproliferative activities of citrus flavonoids against six human cancer cell lines. J. Agric. Food Chem. 2002, 50, 5837–5843. [Google Scholar] [CrossRef] [PubMed]
- Parang, B.; Barrett, C.W.; Williams, C.S. AOM/DSS Model of Colitis-Associated Cancer. In Gastrointestinal Physiology and Diseases; Springer: Berlin/Heidelberg, Germany, 2016; pp. 297–307. [Google Scholar]
- Ito, N.; Hasegawa, R.; Sano, M.; Tamano, S.; Esumi, H.; Takayama, S.; Sugimura, T. A new colon and mammary carcinogen in cooked food, 2-amino-1-methyl-6-phenylimidazo [4, 5-b] pyridine (PhIP). Carcinogenesis 1991, 12, 1503–1506. [Google Scholar] [CrossRef] [PubMed]
- Nakagama, H.; Nakanishi, M.; Ochiai, M. Modeling human colon cancer in rodents using a food-borne carcinogen, PhIP. Cancer Sci. 2005, 96, 627–636. [Google Scholar] [CrossRef]
- Suzuki, R.; Kohno, H.; Murakami, A.; Koshimizu, K.; Ohigashi, H.; Yano, M.; Tokuda, H.; Nishino, H.; Tanaka, T. Citrus nobiletin inhibits azoxymethane-induced large bowel carcinogenesis in rats. Biofactors 2004, 21, 111–114. [Google Scholar] [CrossRef]
- Tang, M.X.; Ogawa, K.; Asamoto, M.; Chewonarin, T.; Suzuki, S.; Tanaka, T.; Shirai, T. Effects of nobiletin on PhIP-induced prostate and colon carcinogenesis in F344 rats. Nutr. Cancer 2011, 63, 227–233. [Google Scholar] [CrossRef]
- Wu, X.; Song, M.; Gao, Z.; Sun, Y.; Wang, M.; Li, F.; Zheng, J.; Xiao, H. Nobiletin and its colonic metabolites suppress colitis-associated colon carcinogenesis by down-regulating iNOS, inducing antioxidative enzymes and arresting cell cycle progression. J. Nutr. Biochem. 2017, 42, 17–25. [Google Scholar] [CrossRef]
- Kawabata, K.; Murakami, A.; Ohigashi, H. Nobiletin, a citrus flavonoid, down-regulates matrix metalloproteinase-7 (matrilysin) expression in HT-29 human colorectal cancer cells. Biosci Biotechnol. Biochem. 2005, 69, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Wu, X.; Zheng, J.; Xiao, H. 5-Demethylnobiletin inhibits colon carcinogenesis in azoxymethane/dextran sulfate sodium-treated mice (123.3). FASEB J. 2014, 28, 123.3. [Google Scholar]
- Zheng, Q.; Hirose, Y.; Yoshimi, N.; Murakami, A.; Koshimizu, K.; Ohigashi, H.; Sakata, K.; Matsumoto, Y.; Sayama, Y.; Mori, H. Further investigation of the modifying effect of various chemopreventive agents on apoptosis and cell proliferation in human colon cancer cells. J. Cancer Res. Clin. Oncol. 2002, 128, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Qiu, P.; Guan, H.; Dong, P.; Li, S.; Ho, C.T.; Pan, M.H.; McClements, D.J.; Xiao, H. The p53-, Bax-and p21-dependent inhibition of colon cancer cell growth by 5-hydroxy polymethoxyflavones. Mol. Nutr. Food Res. 2011, 55, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.; Sato, T.; Takayama, Y.; Mimaki, Y.; Sashida, Y.; Yano, M.; Ito, A. Novel anti-inflammatory actions of nobiletin, a citrus polymethoxy flavonoid, on human synovial fibroblasts and mouse macrophages. Biochem. Pharmacol. 2003, 65, 2065–2071. [Google Scholar] [CrossRef]
- Yasunaga, S.; Domen, M.; Nishi, K.; Kadota, A.; Sugahara, T. Nobiletin suppresses monocyte chemoattractant protein-1 (MCP-1) expression by regulating MAPK signaling in 3T3-L1 cells. J. Funct. Foods 2016, 27, 406–415. [Google Scholar] [CrossRef]
- Miyamoto, S.; Yasui, Y.; Ohigashi, H.; Tanaka, T.; Murakami, A. Dietary flavonoids suppress azoxymethane-induced colonic preneoplastic lesions in male C57BL/KsJ-db/db mice. Chem. Biol. Interact. 2010, 183, 276–283. [Google Scholar] [CrossRef] [Green Version]
- Miyamoto, S.; Yasui, Y.; Tanaka, T.; Ohigashi, H.; Murakami, A. Suppressive effects of nobiletin on hyperleptinemia and colitis-related colon carcinogenesis in male ICR mice. Carcinogenesis 2008, 29, 1057–1063. [Google Scholar] [CrossRef] [Green Version]
- Armaghany, T.; Wilson, J.D.; Chu, Q.; Mills, G. Genetic alterations in colorectal cancer. Gastrointest. Cancer Res. 2012, 5, 19. [Google Scholar]
- Owa, T.; Yoshino, H.; Yoshimatsu, K.; Nagasu, T. Cell cycle regulation in the G1 phase: A promising target for the development of new chemotherapeutic anticancer agents. Curr. Med. Chem. 2001, 8, 1487–1503. [Google Scholar] [CrossRef]
- Johnson, D.; Walker, C. Cyclins and cell cycle checkpoints. Ann. Rev. Pharmacol. Toxicol. 1999, 39, 295–312. [Google Scholar] [CrossRef] [PubMed]
- Sherr, C.J. Cancer cell cycles. Science 1996, 274, 1672–1677. [Google Scholar] [CrossRef] [PubMed]
- Malumbres, M.; Barbacid, M. Cell cycle, CDKs and cancer: A changing paradigm. Nat. Rev. Cancer 2009, 9, 153. [Google Scholar] [CrossRef] [PubMed]
- Kurki, P.; Vanderlaan, M.; Dolbeare, F.; Gray, J.; Tan, E. Expression of proliferating cell nuclear antigen (PCNA)/cyclin during the cell cycle. Exp. Cell Res. 1986, 166, 209–219. [Google Scholar] [CrossRef]
- McKay, J.A.; Douglas, J.J.; Ross, V.G.; Curran, S.; Loane, J.F.; Ahmed, F.Y.; Cassidy, J.; McLeod, H.L.; Murray, G.I. Analysis of key cell-cycle checkpoint proteins in colorectal tumours. J. Pathol. J. Pathol. Soc. Great Br. Irel. 2002, 196, 386–393. [Google Scholar] [CrossRef]
- Kroker, A.J.; Bruning, J.B. p21 exploits residue Tyr151 as a tether for high-affinity PCNA binding. Biochemistry 2015, 54, 3483–3493. [Google Scholar] [CrossRef] [PubMed]
- Soria, G.; Gottifredi, V. PCNA-coupled p21 degradation after DNA damage: The exception that confirms the rule? DNA Repair 2010, 9, 358–364. [Google Scholar] [CrossRef] [Green Version]
- Morgan, D.O. Cyclin-dependent kinases: Engines, clocks, and microprocessors. Annu. Rev. Cell Dev. Biol. 1997, 13, 261–291. [Google Scholar] [CrossRef]
- Karimian, A.; Ahmadi, Y.; Yousefi, B. Multiple functions of p21 in cell cycle, apoptosis and transcriptional regulation after DNA damage. DNA Repair 2016, 42, 63–71. [Google Scholar] [CrossRef]
- Bertoli, C.; Skotheim, J.M.; De Bruin, R.A. Control of cell cycle transcription during G1 and S phases. Nat. Rev. Mol. Cell Biol. 2013, 14, 518–528. [Google Scholar] [CrossRef] [Green Version]
- Taylor, W.R.; Stark, G.R. Regulation of the G2/M transition by p53. Oncogene 2001, 20, 1803–1815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borgne, A.; Meijer, L. Sequential dephosphorylation of p34cdc2 on Thr-14 and Tyr-15 at the prophase/metaphase transition. J. Biol. Chem. 1996, 271, 27847–27854. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, L.; Shi, Z.; Zhao, S.; Wang, F.T.; Zhou, T.T.; Liu, B.; Bao, J.K. Programmed cell death pathways in cancer: A review of apoptosis, autophagy and programmed necrosis. Cell Prolif. 2012, 45, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Zong, W.-X.; Ditsworth, D.; Bauer, D.E.; Wang, Z.-Q.; Thompson, C.B. Alkylating DNA damage stimulates a regulated form of necrotic cell death. Genes Dev. 2004, 18, 1272–1282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sayers, T.J. Targeting the extrinsic apoptosis signaling pathway for cancer therapy. Cancer Immunol. Immunother 2011, 60, 1173–1180. [Google Scholar] [CrossRef]
- Wang, X. The expanding role of mitochondria in apoptosis. Genes Dev. 2001, 15, 2922–2933. [Google Scholar] [PubMed]
- Llambi, F.; Green, D.R. Apoptosis and oncogenesis: Give and take in the BCL-2 family. Curr. Opin. Genet. Dev. 2011, 21, 12–20. [Google Scholar] [CrossRef]
- Engel, T.; Henshall, D.C. Apoptosis, Bcl-2 family proteins and caspases: The ABCs of seizure-damage and epileptogenesis? Int. J. Physiol. Pathophysiol. Pharmacol. 2009, 1, 97–115. [Google Scholar]
- Chan, C.K.; Supriady, H.; Goh, B.H.; Kadir, H.A. Elephantopus scaber induces apoptosis through ROS-dependent mitochondrial signaling pathway in HCT116 human colorectal carcinoma cells. J. Ethnopharmacol. 2015, 168, 291–304. [Google Scholar] [CrossRef]
- Nuñez, G.; Benedict, M.A.; Hu, Y.; Inohara, N. Caspases: The proteases of the apoptotic pathway. Oncogene 1998, 17, 3237–3245. [Google Scholar] [CrossRef]
- Fernandes-Alnemri, T.; Litwack, G.; Alnemri, E.S. CPP32, a novel human apoptotic protein with homology to Caenorhabditis elegans cell death protein Ced-3 and mammalian interleukin-1 beta-converting enzyme. J. Biol. Chem. 1994, 269, 30761–30764. [Google Scholar] [PubMed]
- Kerr, J.F.; Wyllie, A.H.; Currie, A.R. Apoptosis: A basic biological phenomenon with wideranging implications in tissue kinetics. Br. J. Cancer 1972, 26, 239. [Google Scholar] [CrossRef] [PubMed]
- Oliver, F.J.; de la Rubia, G.; Rolli, V.; Ruiz-Ruiz, M.C.; de Murcia, G.; Ménissier-de Murcia, J. Importance of poly (ADP-ribose) polymerase and its cleavage in apoptosis Lesson from an uncleavable mutant. J. Biol. Chem. 1998, 273, 33533–33539. [Google Scholar] [CrossRef] [PubMed]
- Saraste, A.; Pulkki, K. Morphologic and biochemical hallmarks of apoptosis. Cardiovasc. Res. 2000, 45, 528–537. [Google Scholar] [CrossRef]
- Martin, S.J.; Green, D.R. Protease activation during apoptosis: Death by a thousand cuts? Cell 1995, 82, 349–352. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.-R.; Shen, S.-C.; Lin, H.-Y.; Hou, W.-C.; Yang, L.-L.; Chen, Y.-C. Wogonin and fisetin induce apoptosis in human promyeloleukemic cells, accompanied by a decrease of reactive oxygen species, and activation of caspase 3 and Ca2+-dependent endonuclease. Biochem. Pharmacol. 2002, 63, 225–236. [Google Scholar] [CrossRef]
- Fletcher, J.I.; Huang, D.C. Controlling the cell death mediators Bax and Bak: Puzzles and conundrums. Cell Cycle 2008, 7, 39–44. [Google Scholar] [CrossRef]
- Wei, M.C.; Zong, W.-X.; Cheng, E.H.-Y.; Lindsten, T.; Panoutsakopoulou, V.; Ross, A.J.; Roth, K.A.; MacGregor, G.R.; Thompson, C.B.; Korsmeyer, S.J. Proapoptotic BAX and BAK: A requisite gateway to mitochondrial dysfunction and death. Science 2001, 292, 727–730. [Google Scholar] [CrossRef]
- Wang, S.-Y.; Yu, Q.-J.; Zhang, R.-D.; Liu, B. Core signaling pathways of survival/death in autophagy-related cancer networks. Int. J. Biochem. Cell Biol. 2011, 43, 1263–1266. [Google Scholar] [CrossRef]
- Kundu, M.; Thompson, C.B. Autophagy: Basic principles and relevance to disease. Annu. Rev. Pathmechdis Mech. Dis. 2008, 3, 427–455. [Google Scholar] [CrossRef]
- Eum, K.-H.; Lee, M. Crosstalk between autophagy and apoptosis in the regulation of paclitaxel-induced cell death in v-Ha-ras-transformed fibroblasts. Mol. Cell. Biochem. 2011, 348, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Amelio, I.; Melino, G.; Knight, R.A. Cell death pathology: Cross-talk with autophagy and its clinical implications. Biochem. Biophys. Res. Commun. 2011, 414, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Terzić, J.; Grivennikov, S.; Karin, E.; Karin, M. Inflammation and colon cancer. Gastroenterology 2010, 138, 2101–2114.e5. [Google Scholar] [CrossRef] [PubMed]
- Klampfer, L. Cytokines, inflammation and colon cancer. Curr. Cancer Drug Targets 2011, 11, 451–464. [Google Scholar] [CrossRef] [PubMed]
- Meira, L.B.; Bugni, J.M.; Green, S.L.; Lee, C.-W.; Pang, B.; Borenshtein, D.; Rickman, B.H.; Rogers, A.B.; Moroski-Erkul, C.A.; McFaline, J.L. DNA damage induced by chronic inflammation contributes to colon carcinogenesis in mice. J. Clin. Investig. 2008, 118, 2516–2525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Westbrook, A.M.; Wei, B.; Braun, J.; Schiestl, R.H. Intestinal mucosal inflammation leads to systemic genotoxicity in mice. Cancer Res. 2009, 69, 4827–4834. [Google Scholar] [CrossRef]
- Kraus, S.; Arber, N. Inflammation and colorectal cancer. Curr. Opin. Pharmacol. 2009, 9, 405–410. [Google Scholar] [CrossRef]
- Itzkowitz, S.H.; Yio, X. Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: The role of inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 287, G7–G17. [Google Scholar] [CrossRef]
- Takahashi, M.; Wakabayashi, K. Gene mutations and altered gene expression in azoxymethane-induced colon carcinogenesis in rodents. Cancer Sci. 2004, 95, 475–480. [Google Scholar] [CrossRef]
- Xiong, Y.; Chen, D.; Yu, C.; Lv, B.; Peng, J.; Wang, J.; Lin, Y. Citrus nobiletin ameliorates experimental colitis by reducing inflammation and restoring impaired intestinal barrier function. Mol. Nutr. Food Res. 2015, 59, 829–842. [Google Scholar] [CrossRef]
- Kaidi, A.; Qualtrough, D.; Williams, A.C.; Paraskeva, C. Direct transcriptional up-regulation of cyclooxygenase-2 by hypoxia-inducible factor (HIF)-1 promotes colorectal tumor cell survival and enhances HIF-1 transcriptional activity during hypoxia. Cancer Res. 2006, 66, 6683–6691. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, J. Are prostaglandins proinflammatory, antiinflammatory, both or neither? J. Rheumatol. Suppl. 1991, 28, 26–29. [Google Scholar] [PubMed]
- Surh, Y.-J.; Chun, K.-S.; Cha, H.-H.; Han, S.S.; Keum, Y.-S.; Park, K.-K.; Lee, S.S. Molecular mechanisms underlying chemopreventive activities of anti-inflammatory phytochemicals: Down-regulation of COX-2 and iNOS through suppression of NF-κB activation. Mutat. Res./Fundam. Mol. Mech. Mutagen. 2001, 480, 243–268. [Google Scholar] [CrossRef]
- Rao, C.V. Nitric oxide signaling in colon cancer chemoprevention. Mutat. Res./Fundam. Mol. Mech. Mutagen. 2004, 555, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Rushworth, S.A.; MacEwan, D.J.; O’Connell, M.A. Lipopolysaccharide-induced expression of NAD (P) H: Quinone oxidoreductase 1 and heme oxygenase-1 protects against excessive inflammatory responses in human monocytes. J. Immunol. 2008, 181, 6730–6737. [Google Scholar] [CrossRef]
- Khor, T.O.; Huang, M.-T.; Kwon, K.H.; Chan, J.Y.; Reddy, B.S.; Kong, A.-N. Nrf2-deficient mice have an increased susceptibility to dextran sulfate sodium–induced colitis. Cancer Res. 2006, 66, 11580–11584. [Google Scholar] [CrossRef]
- Klaunig, J.E.; Kamendulis, L.M.; Hocevar, B.A. Oxidative stress and oxidative damage in carcinogenesis. Toxicol. Pathol. 2010, 38, 96–109. [Google Scholar] [CrossRef]
- Kwak, M.-K.; Kensler, T.W. Targeting NRF2 signaling for cancer chemoprevention. Toxicol. Appl. Pharmacol. 2010, 244, 66–76. [Google Scholar] [CrossRef] [Green Version]
- Nishida, N.; Yano, H.; Nishida, T.; Kamura, T.; Kojiro, M. Angiogenesis in cancer. Vasc. Health Risk Manag. 2006, 2, 213–219. [Google Scholar] [CrossRef]
- Prager, G.; Poettler, M. Angiogenesis in cancer. Hämostaseologie 2012, 32, 105–114. [Google Scholar]
- Park, M.H.; Hong, J.T. Roles of NF-κB in cancer and inflammatory diseases and their therapeutic approaches. Cells 2016, 5, 15. [Google Scholar] [CrossRef]
- Ferrara, N.; Gerber, H.-P.; LeCouter, J. The biology of VEGF and its receptors. Nat. Med. 2003, 9, 669. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P. VEGF as a key mediator of angiogenesis in cancer. Oncology 2005, 69, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Berra, E.; Pagès, G.; Pouysségur, J. MAP kinases and hypoxia in the control of VEGF expression. Cancer Metastasis Rev. 2000, 19, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Saxena, N.K.; Taliaferro-Smith, L.; Knight, B.B.; Merlin, D.; Anania, F.A.; O’Regan, R.M.; Sharma, D. Bidirectional crosstalk between leptin and insulin-like growth factor-I signaling promotes invasion and migration of breast cancer cells via transactivation of epidermal growth factor receptor. Cancer Res. 2008, 68, 9712–9722. [Google Scholar] [CrossRef] [PubMed]
- Fenton, J.I.; Hord, N.G.; Lavigne, J.A.; Perkins, S.N.; Hursting, S.D. Leptin, insulin-like growth factor-1, and insulin-like growth factor-2 are mitogens in ApcMin/+ but not Apc+/+ colonic epithelial cell lines. Cancer Epidemiol. Prev. Biomark. 2005, 14, 1646–1652. [Google Scholar] [CrossRef] [PubMed]
- Rouet-Benzineb, P.; Aparicio, T.; Guilmeau, S.; Pouzet, C.; Descatoire, V.; Buyse, M.; Bado, A. Leptin counteracts sodium butyrate-induced apoptosis in human colon cancer HT-29 cells via NF-κB signaling. J. Biol. Chem. 2004, 279, 16495–16502. [Google Scholar] [CrossRef] [PubMed]
- Miyata, Y.; Sato, T.; Yano, M.; Ito, A. Activation of protein kinase C βII/ε-c-Jun NH2-terminal kinase pathway and inhibition of mitogen-activated protein/extracellular signal-regulated kinase 1/2 phosphorylation in antitumor invasive activity induced by the polymethoxy flavonoid, nobiletin. Mol. Cancer Ther. 2004, 3, 839–847. [Google Scholar] [PubMed]
- Miyata, Y.; Sato, T.; Imada, K.; Dobashi, A.; Yano, M.; Ito, A. A citrus polymethoxyflavonoid, nobiletin, is a novel MEK inhibitor that exhibits antitumor metastasis in human fibrosarcoma HT-1080 cells. Biochem. Biophys. Res. Commun. 2008, 366, 168–173. [Google Scholar] [CrossRef]
- Fong, Y. Surgical therapy of hepatic colorectal metastasis. CA Cancer J. Clin. 1999, 49, 231–255. [Google Scholar] [CrossRef]
- Kim, Y.-S.; Kim, S.-H.; Kang, J.-G.; Ko, J.-H. Expression level and glycan dynamics determine the net effects of TIMP-1 on cancer progression. BMB Rep. 2012, 45, 623–628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chambers, A.F.; Matrisian, L.M. Changing views of the role of matrix metalloproteinases in metastasis. J. Natl. Cancer Inst. 1997, 89, 1260–1270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waas, E.; Wobbes, T.; Lomme, R.; DeGroot, J.; Ruers, T.; Hendriks, T. Matrix metalloproteinase 2 and 9 activity in patients with colorectal cancer liver metastasis. Br. J. Surg. 2003, 90, 1556–1564. [Google Scholar] [CrossRef] [PubMed]
- Zucker, S.; Vacirca, J. Role of matrix metalloproteinases (MMPs) in colorectal cancer. Cancer Metastasis Rev. 2004, 23, 101–117. [Google Scholar] [CrossRef] [PubMed]
- Brabletz, T.; Jung, A.; Dag, S.; Hlubek, F.; Kirchner, T. β-Catenin regulates the expression of the matrix metalloproteinase-7 in human colorectal cancer. Am. J. Pathol. 1999, 155, 1033–1038. [Google Scholar] [CrossRef]
- Crawford, H.C.; Fingleton, B.M.; Rudolph-Owen, L.A.; Goss, K.J.H.; Rubinfeld, B.; Polakis, P.; Matrisian, L.M. The metalloproteinase matrilysin is a target of β-catenin transactivation in intestinal tumors. Oncogene 1999, 18, 2883–2891. [Google Scholar] [CrossRef]
- Egeblad, M.; Werb, Z. New functions for the matrix metalloproteinases in cancer progression. Nat. Rev. Cancer 2002, 2, 161–174. [Google Scholar] [CrossRef]
- Bingle, á.; Brown, N.; Lewis, C. The role of tumour-associated macrophages in tumour progression: Implications for new anticancer therapies. J. Pathol. J. Pathol. Soc. Great Br. Irel. 2002, 196, 254–265. [Google Scholar] [CrossRef]
- Kerkelä, E.; Ala-aho, R.; Klemi, P.; Grénman, S.; Shapiro, S.D.; Kähäri, V.M.; Saarialho-Kere, U. Metalloelastase (MMP-12) expression by tumour cells in squamous cell carcinoma of the vulva correlates with invasiveness, while that by macrophages predicts better outcome. J. Pathol. 2002, 198, 258–269. [Google Scholar] [CrossRef]
- Li, S.; Pan, M.-H.; Lo, C.-Y.; Tan, D.; Wang, Y.; Shahidi, F.; Ho, C.-T. Chemistry and health effects of polymethoxyflavones and hydroxylated polymethoxyflavones. J. Funct. Foods 2009, 1, 2–12. [Google Scholar] [CrossRef]
- Scholz; Williamson. Interactions affecting the bioavailability of dietary polyphenols in vivo. Int. J. Vitam. Nutr. Res. 2007, 77, 224–235. [Google Scholar] [CrossRef] [PubMed]
- Murakami, A.; Kuwahara, S.; Takahashi, Y.; Ito, C.; Furukawa, H.; Ju-ichi, M.; Koshimizu, K.; OHIGASHI, H. In vitro absorption and metabolism of nobiletin, a chemopreventive polymethoxyflavonoid in citrus fruits. Biosci. Biotechnol. Biochem. 2001, 65, 194–197. [Google Scholar] [CrossRef] [PubMed]
- Kansy, M.; Senner, F.; Gubernator, K. Physicochemical high throughput screening: Parallel artificial membrane permeation assay in the description of passive absorption processes. J. Med. Chem. 1998, 41, 1007–1010. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Walle, T. Methylated flavonoids have greatly improved intestinal absorption and metabolic stability. Drug Metab. Dispos. 2006, 34, 1786–1792. [Google Scholar] [CrossRef] [PubMed]
- Van de Waterbeemd, H. Physico-Chemical Approaches to Drug Absorption. In Drug Bioavailability: Estimation of Solubility, Permeability, Absorption and Bioavailability; Wiley: Hoboken, NJ, USA, 2003; pp. 3–20. [Google Scholar]
- Murakami, A.; Koshimizu, K.; Ohigashi, H.; Kuwahara, S.; Kuki, W.; Takahashi, Y.; Hosotani, K.; Kawahara, S.; Matsuoka, Y. Characteristic rat tissue accumulation of nobiletin, a chemopreventive polymethoxyflavonoid, in comparison with luteolin. Biofactors 2002, 16, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zheng, J.; Zhong, Z.; Song, M.; Wu, X. Tissue Distribution of Nobiletin and Its Metabolites in Mice after Oral Administration of Nobiletin; Federation of American Societies for Experimental Biology: Bethesda, MD, USA, 2013. [Google Scholar]
- Wu, X.; Song, M.; Qiu, P.; Rakariyatham, K.; Li, F.; Gao, Z.; Cai, X.; Wang, M.; Xu, F.; Zheng, J. Synergistic chemopreventive effects of nobiletin and atorvastatin on colon carcinogenesis. Carcinogenesis 2017, 38, 455–464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, A.; Devaraj, V.; Giri, K.C.; Giri, S.; Rajagopal, S.; Mullangi, R. Development and validation of a highly sensitive LC-MS/MS-ESI method for the determination of nobiletin in rat plasma: Application to a pharmacokinetic study. Biomed. Chromatogr. 2012, 26, 1464–1471. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.P.; Tewari, D.; Patel, K.; Jain, G.K. Permeability determination and pharmacokinetic study of nobiletin in rat plasma and brain by validated high-performance liquid chromatography method. Fitoterapia 2011, 82, 1206–1214. [Google Scholar] [CrossRef] [PubMed]
- Manthey, J.A.; Cesar, T.B.; Jackson, E.; Mertens-Talcott, S. Pharmacokinetic Study of Nobiletin and Tangeretin in Rat Serum by High-Performance Liquid Chromatography—Electrospray Ionization—Mass Spectrometry. J. Agric. Food Chem. 2011, 59, 145–151. [Google Scholar] [CrossRef]
- McClements, D.J. Emulsion design to improve the delivery of functional lipophilic components. Annu. Rev. Food Sci. Technol. 2010, 1, 241–269. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhao, C.; Chen, J.; Tian, G.; McClements, D.J.; Xiao, H.; Zheng, J. Encapsulation of polymethoxyflavones in citrus oil emulsion-based delivery systems. J. Agric. Food Chem. 2017, 65, 1732–1739. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Lu, Y.; Zhou, J.P. Preparation of nobiletin in self-microemulsifying systems and its intestinal permeability in rats. J. Pharm. Pharm. Sci. 2008, 11, 22–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, W.; Yao, J.; Zhou, J. Preparation of self-assemble nobiletin proliposomes and its pharmacokinetics in rats. Yao Xue Xue Bao (Acta Pharm. Sin.) 2009, 44, 192–196. [Google Scholar]
- Chen, H.; An, Y.; Yan, X.; McClements, D.J.; Li, B.; Li, Y. Designing self-nanoemulsifying delivery systems to enhance bioaccessibility of hydrophobic bioactives (nobiletin): Influence of hydroxypropyl methylcellulose and thermal processing. Food Hydrocoll. 2015, 51, 395–404. [Google Scholar] [CrossRef]
- Lei, L.; Zhang, Y.; He, L.; Wu, S.; Li, B.; Li, Y. Fabrication of nanoemulsion-filled alginate hydrogel to control the digestion behavior of hydrophobic nobiletin. LWT Food Sci. Technol. 2017, 82, 260–267. [Google Scholar] [CrossRef]
- Onoue, S.; Uchida, A.; Takahashi, H.; Seto, Y.; Kawabata, Y.; Ogawa, K.; Yuminoki, K.; Hashimoto, N.; Yamada, S. Development of high-energy amorphous solid dispersion of nanosized nobiletin, a citrus polymethoxylated flavone, with improved oral bioavailability. J. Pharm. Sci. 2011, 100, 3793–3801. [Google Scholar] [CrossRef] [PubMed]
- Jordan, S.; Murty, M.; Pilon, K. Products containing bitter orange or synephrine: Suspected cardiovascular adverse reactions. Can. Med. Assoc. J. 2004, 171, 993–994. [Google Scholar]
- Yang, G.; Li, S.; Long, T.; Yang, Y.; Li, Y. Application of Polymethoxylflavone in Preparation of Prevention Drug for Cardiovascular Inflammation. Patent CN 107281179, 24 October 2017. [Google Scholar]
- Wu, X.; Zheng, D.; Qin, Y.; Liu, Z.; Zhu, X. Application of Nobiletin in Medicine for Preventing or Treating Heart Failure. Patent CN 106924241, 7 July 2017. [Google Scholar]
- Morimoto, T.; Hasegawa, K.; Murakami, A.; Fukuda, H.; Takahashi, K. Cardiac Disease Treatment Agents Containing Nobiletin. Patent JP 2011037798, 24 February 2011. [Google Scholar]
- Caramelli, G. Product with Blood Lipid-Lowering Activity. Patent IT 2008RM0232, 2 August 2008. [Google Scholar]
- Ohizumi, Y.; Kajima, K.; Maruyama, K.; Ishibashi, M. Pharmaceutical Composition and Food Containing Citrus Butanol Extract for Preventing and/or Treating Central Nervous System Disease. Patent WO 2017208869, 7 December 2017. [Google Scholar]
- Ohizumi, Y.; Kajima, K.; Maruyama, K. Pharmaceutical and food composition containing Anredera cordifolia and nobiletin. Patent JP 6238089, 29 November 2017. [Google Scholar]
- Jeon, M.R.; L, S.A.; Yoon, G.J.; Park, J.H. Composition for Preventing or Treating Neurodegenerative Disease Comprising Nobiletin as Active Ingredient. Patent KR 2017090073, 25 September 2017. [Google Scholar]
- Wu, X.; Mei, Z.; Zheng, D.; Liu, Z.; Zhu, X.; Zhou, Y.; Zeng, L.; Liang, Z. Application of Nobiletin in Preparation or Screening of Diabetic Cardiomyopathy Drug. Patent CN 108403684, 17 August 2018. [Google Scholar]
- Guthrie, N. Compositions Comprising at Least one Polymethoxyflavone, Flavonoid, Liminoid, and/or Tocotrienol Useful in Combination Therapies for Treating Diabetes. Patent WO 2014203059, 24 December 2014. [Google Scholar]
- Kim, T.J.; Kim, H.G.; Kwon, Y.I.; Lee, J.U. Obesity inhibiting Composition Comprising Powder of Citrus Grandis Cultivated by Eco Friendly Method as Active Ingredient. Patent KR 2016111554, 27 September 2016. [Google Scholar]
- Miyaura, C.; Inada, M. Preventive or Therapeutic Compositions Containing Heptamethoxyflavone for Bone Diseases. Patent JP 2012232916, 22 October 2012. [Google Scholar]
- Liao, X. Manufacture Method of Chinese Medicine Composition for Treatment of Halitosis. Patent CN 105434729, 30 March 2016. [Google Scholar]
- Wang, L.; Tian, A.; Li, S.; Chen, J.; Li, B. Mouth Smell-Improving Agent and Its Preparation Method. Patent CN 103893334, 2 July 2014. [Google Scholar]
- Huang, R.L.; Hsu, S.W. Polymethoxylated Flavone for Manufacturing Drugs Against Hepatitis-B with Drug Resistance. Patent TW I535439, 1 June 2016. [Google Scholar]
- Kim, D.H.; Han, M.J.; Cho, E.H.; Kim, Y.R. Natural Products for Treating Cancer and HIV-Related diseases. Patent KR 2012011169, 7 February 2012. [Google Scholar]
- Zhang, T.; Liao, M.; Gong, S.; Xie, X.; Sun, W.; Wang, L.; Zheng, Y. Application of Total Flavonoid Extract from Citrus Aurantium in Manufacturing Medicines for Treating Asthma. Patent CN 102935131, 20 February 2013. [Google Scholar]
- Li, K. Application of Nobiletin in Medicine for Treating Allergic Asthma. Patent CN 102552242, 11 July 2012. [Google Scholar]
- Sugawara, T.; Kadota, A.; Kikuchi, T. Antiallergic Oral Composition Containing β-Lactoglobulin and Nobiletin. Patent JP 2015036369, 23 February 2015. [Google Scholar]
- Seo, J.W.; Choi, B.G.; Cheng, J.H.; Cho, M.J. Citrus Pericarp Extracts for Preventing Hair Loss and Promoting Hair Growth. Patent KR 1651833, 19 September 2016. [Google Scholar]
- Ito, Y.; Hikiyama, E.; Yamada, S.; Woo, J.-T.; Teruya, Y.; Sugaya, K.; Nishijima, S.; Wakuda, H.; Shinozuka, K. Medicinal Composition for Preventing or Improving Dysuria, Antagonist Against Dysuria-Related Receptor, and Method for Preventing or Improving Dysuria Using Medicinal Composition or Antagonist. Patent WO 2016075960, 19 May 2016. [Google Scholar]
- Sakata, Y.; Nakamura, H.; Oshio, K. Muscular Atrophy Preventing Agent Containing Citrus Depressa Extract. Patent WO 2013099982, 4 July 2013. [Google Scholar]
- Li, S.; Yang, G.; Long, T. Application of (demethyl) polymethoxyflavone and taxol medicine in producing the medicine for treating non-small cell lung cancer. Patent CN 106562954, 19 April 2017. [Google Scholar]
- Nakano, S.; Ono, M.; Hayashi, C. Agent and Method for Inhibiting Breast Cancer Cell Proliferation Comprising Nobiletin. Patent JP 2016017042, 4 February 2016. [Google Scholar]
- Chen, G.; Wang, H. Application of Nobiletin in the Preparation of Health Products or Medicines for Preventing and/or Treating Oral Cancer. Patent CN 105030559, 11 November 2015. [Google Scholar]
- Ma, W.-Z.; Feng, S.-L.; Yao, X.-J.; Yuan, Z.-W.; Liu, L.; Xie, Y. Use of Nobiletin in Cancer Treatment. Patent AU 2015101287, 22 October 2015. [Google Scholar]
- Zhang, Z. Chinese Medicinal Composition Containing Extracts from Citrus and Scutellaria for Treating Cancer Chemotherapy Related Diarrhea. Patent CN 103655835, 26 March 2014. [Google Scholar]
- Li, M.; Jin, H.; Yang, Z.; Xu, G.; Lin, Y.; Lin, Q.; Zhang, Z. Medical Application of Flavonoids of Citrus Reticulata Pericarp as Angiogenesis Inhibitor. Patent CN 101947215, 19 January 2011. [Google Scholar]
- Zhou, H.; Xie, B.; Zang, X.; Cheng, L.; Liang, G. A Multiple Index Component content Determination, Fingerprint Construction and Preparation Method for Liver-Tonifying Eyesight-Improving Oral Liquid [Machine Translation]. Patent CN 105510452, 20 April 2016. [Google Scholar]
- Guo, J.; Liang, L.; Song, J.; Li, H.; Yang, J.; Chen, B.; Wang, S. Method for Extracting Nobiletin and Hesperetin from Citrus. Patent CN 106632196, 10 May 2017. [Google Scholar]
- Cao, J.; Hu, S.; Liu, X.; Cao, W.; Pang, X.; Dai, H.; Da, J. A method of Extracting Flavonoids active Ingredients in Citrus Reticulata Pericarp. Patent CN 104297026, 21 January 2015. [Google Scholar]
- Yamaguchi, K.; Mogami, K.; Yamaguchi, Y.; Hitomi, N.; Murata, K.; Tani, Y. Manufacture of Nobiletin by Solvent Extraction and Nobiletin-Containing Extract. Patent JP 2012056938, 22 March 2012. [Google Scholar]
- Sun, C.; Wang, Y.; Chen, K.; Li, X.; Cao, J. Process Forextn. And Purifn. of Polymethoxylated Flavonoids Compound from Fruit of Citrus Reticulate. Patent CN 107011308, 4 August 2017. [Google Scholar]
- Li, X.; Zhang, J.; Sun, C.; Chen, K. Method for Isolating and Purifying Seven Flavonoids from Citrus Tangerina oil Cell Layer. Patent CN 103610800, 5 March 2014. [Google Scholar]
- Liang, H.; Wu, D.; Li, B.; Li, Y.; Li, J. Stable Nobiletin liquid Preparation and Preparation Method Thereof. Patent CN 107998073, 8 May 2018. [Google Scholar]
- Yang, W.; Song, Y.; Chen, H.; Luo, X.; Yuan, J. A Technique Based on Multi-Solvents for Preparing Nobiletin. Patent CN 105669626, 15 June 2016. [Google Scholar]
- Iwashita, M.; Umehara, M.; Onishi, S.; Yamamoto, M.; Yamagami, K.; Ishigami, T. Method for Manufacturing Nobiletin-Containing Solid Dispersion. Patent WO 2018025871, 8 February 2018. [Google Scholar]
- Woo, J.T.; Komaki, M. Polymethoxyflavonoid Dissolved Composition and its Manufacturing Method. Patent JP 2015221761, 10 December 2015. [Google Scholar]
- Chen, Y.; Yu, Y.; Yang, D.; Wei, W.; He, Z.; Lin, X.; Xie, H. Measurement Method for Seventeen Kinds of Phenol Substances in Grape and Citrus Fruit Using High Performance Liquid Chromatography (HPLC). Patent CN 102706980, 3 October 2012. [Google Scholar]
- Kusano, S.; Tamasu, S. Composition Containing 4’-Demethylnobiletin for skin Whitening Cosmetics, Medicines, Foods and Drinks. Patent JP 2017226612, 28 December 2017. [Google Scholar]
- Choi, B.G.; Lee, D.R. Skin Moisturizers Containing Citrus Peel Extracts. Patent KR 2017000068, 6 January 2017. [Google Scholar]
- Karabey, F. Nobiletin Molecules in Cosmetic Preparationsuse. Patent TR 2014000324, 2015. [Google Scholar]
- Zhang, X.; Chen, S.; Wang, X.; Xie, F.; Liu, X.; Wang, J.; Yan, A.; Gao, N.; Li, F. A Snap Bean Preservative [Machine Translation]. Patent CN 106172719, 7 December 2016. [Google Scholar]
- Krohn, M.; Seibert, S.; Kleber, A.; Wonschik, J. Sweetener and/or Sweetness Enhancer, Sweetener Composition, Methods of Making the Same and Consumables Containing the Same. Patent WO 2012107203, 16 August 2012. [Google Scholar]
- Zhang, L.; Zhu, W.; Yang, C.; Guo, H.; Yu, A.; Ji, J.; Gao, Y.; Sun, M.; Zhai, G. A novel folate-modified self-microemulsifying drug delivery system of curcumin for colon targeting. Int. J. Nanomed. 2012, 7, 151–162. [Google Scholar] [CrossRef] [Green Version]
- Bansode, S.T.; Kshirsagar, S.J.; Madgulkar, A.R.; Bhalekar, M.R.; Bandivadekar, M.M. Design and development of SMEDDS for colon-specific drug delivery. Drug Dev. Ind. Pharm. 2016, 42, 611–623. [Google Scholar] [CrossRef]
- Low, L.E.; Tan, L.T.-H.; Goh, B.-H.; Tey, B.T.; Ong, B.H.; Tang, S.Y. Magnetic cellulose nanocrystal stabilized Pickering emulsions for enhanced bioactive release and human colon cancer therapy. Int. J. Biol. Macromol. 2019, 127, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Low, L.E.; Tey, B.T.; Ong, B.H.; Chan, E.S.; Tang, S.Y. Palm olein-in-water Pickering emulsion stabilized by Fe3O4-cellulose nanocrystal nanocomposites and their responses to pH. Carbohydr. Polym. 2017, 155, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Vangijzegem, T.; Stanicki, D.; Laurent, S. Magnetic iron oxide nanoparticles for drug delivery: Applications and characteristics. Exp. Opin. Drug Deliv. 2019, 16, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Xing, T.T.; Zhao, X.J.; Zhang, Y.D.; Li, Y.F. Fast separation and sensitive quantitation of polymethoxylated flavonoids in the peels of citrus using UPLC-Q-TOF-MS. J. Agric. Food Chem. 2017, 65, 2615–2627. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Rycaj, K.; Liu, Z.-M.; Tang, D.G. Cancer Stem Cells: Constantly Evolving and Functionally Heterogeneous Therapeutic Targets; AACR: Philadelphia, PA, USA, 2014. [Google Scholar]
- Chen, K.; Huang, Y.-H.; Chen, J.-L. Understanding and targeting cancer stem cells: Therapeutic implications and challenges. Acta Pharmacol. Sin. 2013, 34, 732–740. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.T.H.; Low, L.E.; Tang, S.Y.; Yap, W.H.; Chuah, L.H.; Chan, C.K.; Lee, L.H.; Goh, B.H. A reliable and affordable 3D tumor spheroid model for natural product drug discovery: A case study of curcumin. Prog. Drug Discov. Biomed. Sci. 2019, 2, 1–5. [Google Scholar]
- DiMarco-Crook, C.; Xiao, H. Diet-based strategies for cancer chemoprevention: The role of combination regimens using dietary bioactive components. Annu. Rev. Food Sci. Technol. 2015, 6, 505–526. [Google Scholar] [CrossRef]
- Funaro, A.; Wu, X.; Song, M.; Zheng, J.; Guo, S.; Rakariyatham, K.; Rodriguez-Estrada, M.T.; Xiao, H. Enhanced Anti-Inflammatory Activities by the Combination of Luteolin and Tangeretin. J. Food Sci. 2016, 81, H1320–H1327. [Google Scholar] [CrossRef]
- Wu, X.; Song, M.; Qiu, P.; Li, F.; Wang, M.; Zheng, J.; Wang, Q.; Xu, F.; Xiao, H. A metabolite of nobiletin, 4′-demethylnobiletin and atorvastatin synergistically inhibits human colon cancer cell growth by inducing G0/G1 cell cycle arrest and apoptosis. Food Funct. 2018, 9, 87–95. [Google Scholar] [CrossRef]

| Compounds | Activities | Cell lines | Treatment/Assay (Treatment Duration) | Assays/Results/Mechanisms | References |
|---|---|---|---|---|---|
| NOB | Anti-proliferative | HT-29 | H-thymidine uptake assay | - IC50 of NOB = 4.7 μM | [70] |
| - IC90 of NOB = 13.9 μM | |||||
| 5-DMN | - IC50 of 5-DMN = 8.5 μM | ||||
| - IC90 of 5-DMN = 171 μM | |||||
| NOB | Cytotoxicity | COLO320, SW480 and Caco-2 | MTS viability assay (48 h) | - IC50 for COLO320 = 40.4 ± 9.1 μM | [79] |
| - IC50 for SW480 = 245 ± 9.1 μΜ | |||||
| - IC50 for Caco-2 = 305.6 ± 41.9 μΜ | |||||
| Apoptosis-inducing | Apoptosis assays—DNA fragmentation | - DNA ladder pattern | |||
| 200 μΜ—2-fold increase DNA fragmentation in COLO320 | |||||
| - gel electrophoresis (48 h) | |||||
| Anti-proliferative | BrdU labelling index | - 34.7 ± 4.7% BrdU-binding cells at 100 μΜ | |||
| - 44.4 ± 6.4% BrdU-binding cells at 40 μΜ | |||||
| NOB | Anti-metastasis | HT-29 | ELISA | [77] | |
| - proMMP-7 expression | - At 100 μM, no detection of proMMP-7 in media, ~280 pg/mL proMMP-7 in media | ||||
| qPCR and Western blot | - >25 μM, reduced RNA and protein (both intracellular and supernatant) expression of proMMP-7 | ||||
| AP-1 binding activity | - Inhibited binding activity of AP-1 (transcription factor for MMP-7 gene) | ||||
| NOB | Anti-proliferative | HT-29 | Cell counting assay | - IC50 of NOB ≈ 50 μM | [14] |
| - Inhibited cell proliferation in a time- and dose-dependent manner | |||||
| Cell cycle arrest | |||||
| Cell cycle analysis | - Induced G1 phase cell cycle arrest (60 and 200 μM) | ||||
| - Propidium iodide staining | |||||
| Apoptosis-inducing | Apoptosis assay | - No significant apoptosis detected at 60 and 100 μM | |||
| Resumption of proliferation | - Resumed proliferation within 24 h of removal of NOB and achieve the same stage of growth as compared to control after four days of removal of NOB | ||||
| NOB 5-DMN | Cytotoxicity | HCT116, HT-29 | MTT viability assay (48 h) | - IC50 of NOB on HCT116 = 37 μM | [63] |
| - IC50 of 5-DMN on HCT116 = 8.7 μM | |||||
| - IC50 of NOB on HT-29 = 46.2 μM | |||||
| - IC50 of 5-DMN on HT-29 = 22 μM | |||||
| Cell cycle arrest | Cell cycle analysis - Propidium iodide staining (24 h) Western blot | - At 8 μM, 5-DMN induced G2/M phase arrest in HCT116 | |||
| - At 36 μM, 5-DMN induced G2/M phase arrest in HT-29 | |||||
| - At 16 μM, NOB reduced CDK-2 expression | |||||
| - At 4 μM and 8 μM, 5-DMN increased p21 and Rb, while decreased CDK-2 and p-Rb. | |||||
| Apoptosis-inducing | Apoptosis assay | - At 8 μM, 5-DMN increased early apoptosis by 2.2-fold in HCT116 | |||
| Annexin-V/PI (48 h) | - At 36 μM, 5-DMN increased early apoptosis by ~2-fold in HT-29 | ||||
| Western blot | - At 16 μM, NOB did not increase apoptotic cell population in HCT116/HT-29 | ||||
| - At 4 μM and 8 μM, 5-DMN increased expressions of cleaved caspase 8, cleaved caspase 3 and cleaved PARP. | |||||
| 5-DMN | Apoptosis-inducing | HCT116 (p53 +/+) and HCT116 (p53 −/−); HCT116 (Bax +/−) and HCT116 (Bax −/−); HCT116 (p21 −/−) | Apoptosis assay Annexin-V/PI | - At 15 μM, 5-DMN increased late apoptotic/necrotic cell in HCT116 (p53 −/−) > HCT115 (p53 +/+), suggesting the apoptotic inducing action is independent of p53 | [80] |
| - At 15 μM, 5-DMN increased early apoptotic cell in HCT116 (Bax +/−), but not in HCT116 (Bax −/−) | |||||
| Cell cycle arrest | Cell cycle analysis - Propidium iodide staining | - At 15 μM, 5-DMN arrested cells at G2/M and G0/G1 phases in HCT116 (p53 +/+) cells, but only caused G2/M phase arrest in HCT116 (p53 −/−) cells | |||
| - G0/G1 is p53 dependent and G2/M is p53-independent | |||||
| NOB; 3′-DMN; 4′-DMN; 3′,4′-DMN | Cytotoxicity | HCT116, HT-29 | MTT viability assay | - At 2.03 μM and 3.28 μM, NOB and 3′-DMN, respectively showed no significant cytotoxicity against HCT116 and HT-29 | [54] |
| - At 24.13 μM, 4′-DMN inhibited growth of HCT-116 by 45% and HT-29 by 33% | |||||
| - At 12.03 μM, 3’,4’-DMN inhibited growth of HCT116 by 30% and HT-29 by 9% | |||||
| - combination of all three NOB-metabolites inhibited growth of HCT116 by 64% and HT-29 by 62% (no significant difference to three NOB-metabolites + NOB) | |||||
| Cell cycle arrest | Cell cycle analysis - Propidium iodide staining (24 h) | - NOB (40 μM) arrested cells at G0/G1 phase in both HCT-116 and HT-29 | |||
| - 3′-DMN (40 μM) arrested cells at both S phase and G2/M phase in HCT-116; while arrested cells at both G0/G1 and G2/M phase in HT-29 | |||||
| - 4′-DMN (40 μM) induced a stronger effect than NOB in arresting cells at G0/G1 phase in HCT-116 and HT-29 | |||||
| - 3′,4′-DMN (20 μM) arrested cells at both S phase and G2/M phase in HCT-116; while arrested cells at both G0/G1 and G2/M phase in HT-29 | |||||
| Apoptosis inducing | Western blot | - NOB and all three NOB-metabolites cause profound increase in expression of p21Cip1/Waf1 | |||
| Annexin-V/PI (48 h) | - NOB (40 μM) increased early apoptotic cell population by 3.3-fold, increased late apoptotic cell population by 4.2-fold in HCT116 | ||||
| - 3′-DMN (40 μM) increased early apoptotic cell population by 5.0-fold, increased late apoptotic cell population by 3.5-fold in HCT116 | |||||
| - 4′-DMN (40 μM) increased early apoptotic cell population by 4.9-fold, increased late apoptotic cell population by 7.1-fold in HCT116 | |||||
| - 3′,4′-DMN (20 μM) increased early apoptotic cell population by 7.6-fold, increase late apoptotic cell population by 4.5-fold in HCT116 | |||||
| -3′-DMN (40 μM) and 4’-DMN (40 μM) did not cause significant apoptosis in HT-29 | |||||
| - 3′,4′-DMN (20 μM) exhibits stronger apoptosis effect than NOB (40 μM) in HT-29 | |||||
| Western blot | - NOB (40 μM) only increased activation of caspase-9 and did not affect caspase-3 or PARP levels in HCT116 | ||||
| - NOB-metabolites increased activation of caspase-3, caspase-9 and other downstream proteins like PARP in HCT116 | |||||
| NOB-Met (2.03 μM NOB: 3.28 μM 3′-DMN: 24.13 μM 4′-DMN: 12.03 μM 3′,4′-DMN | Anti-inflammatory | RAW264.7 | Western Blot | - At 0.5× concentration of NOB-Met, supressed LPS-induced iNOS expression by 56.4% | [76] |
| - At 1× and 2× concentration of NOB-Met, completely abrogated LPS-induced iNOS expression | |||||
| - At ×0.5, increased expression of NQO1 by 21% as compared to LPS-treated cells | |||||
| - At ×1, increased expression of HO-1 by 10%, increased expression of NQO1 by 34% as compared to LPS-treated cells | |||||
| - At ×2, increased expression of HO-1 by 37%, increased expression of NQO1 by 50% as compared to LPS-treated cells | |||||
| - Induced translocation of Nrf2 | |||||
| Cell cycle arrest | HCT116 | Cell cycle analysis - Propidium iodide staining Western blot | - At 1×, induced G0/G1 phase arrest; while at 2×, induced G0/G1 and G2/M phases arrest | ||
| - Reduced expressions of CDK-2, CDK-4, CDK-6 and cyclin D, while increased expressions of p53 and p27 | |||||
| NOB, 5-DMN | Cytotoxicity | HCT116, HT-29, COLO205 | MTT viability assay | - At 40 μM, NOB significantly reduced viability of HCT116, HT-29 and COLO205 by ~20–30% | [49] |
| - At >5 μM, 5-DMN significantly reduced viability of HCT116, HT-29 and COLO205 | |||||
| Apoptosis inducing | Cell cycle analysis - SubG1 quantification Western | - At 20 μM, 5-DMN increased apoptosis ratio by ~26%, while no increased in subG1 population in NOB-treated COLO205 | |||
| - At 10 and 20 μM, significantly increased expression of cleaved PARP in COLO205 | |||||
| NOB | Anti-inflammatory | Human synovial fibroblast, mouse macrophage J774A.1 | ELISA | - At >4 μM, NOB inhibited PGE2 induced by IL-1α in human synovial fibroblast | [81] |
| Western blot and qPCR | - At >16 μM, NOB reduced mRNA of COX-2 induced by IL-1α in human synovial fibroblast | ||||
| - At 64 μM, NOB inhibited COX-2 protein expression induced by IL-1α in human synovial fibroblast | |||||
| qPCR | - At 32 μM, NOB reduced mRNA of IL-1α, IL-1β, IL-6, TNF-α induced by LPS in J774A.1 | ||||
| Western blot | - At >16 μM, NOB reduced proMMP-1 and proMMP-3 induced by IL-1α in human synovial fibroblast | ||||
| - At >16 μM, NOB enhanced TIMP-1 expression in response to IL-1α in human synovial fibroblast | |||||
| NOB | Anti-inflammatory | Mouse adipocyte 3T3-L1 | ELISA | - At 50 and 100 μM, NOB suppressed MCP-1 secretion induced by TNF-α IN 3T3-L1 adipocytes | [82] |
| Western blot | - At 50 and 100 μM, NOB reduced ERK phosphorylation in 3T3-L1 adipocytes treated with TNF-α | ||||
| Animal Models | Treatment/Dosage | Mechanisms | Detailed Results | References |
|---|---|---|---|---|
Colitis-associated colon carcinogenesis model
| AIN93G diet containing 0.05% wt NOB (20 weeks) | Cell cycle arrest | Protein expression in colonic mucosa by Western blot - Reduced levels of CDK-2, CDK-4, CDK-6, cyclin D and cyclin E - Increased levels of p21, p27 and p53 | [76] |
| Anti-inflammatory effects | Immunohistochemical analysis - Reduced expression of iNOS reduced by 35% when compared to the positive control Protein expression in colonic mucosa by Western blot - Increased level of HO-1 - Increased level of NQO1 - Induced translocation of level of Nrf2 transcription factor (Nuclear fraction < Cytoplasmic fraction) | |||
Colitis-associated colon carcinogenesis model
| AIN93G diet containing 0.05% wt NOB (20 weeks) | Inhibit AOM/DSS-induced colon carcinogenesis | - Prevented shortening of colon length, reduced the increased colon weight/length ratio - Reduced tumor incidence by 40% and tumor multiplicity by 71% - Maintained histological characteristic of normal mucosa | [54] |
| Anti-proliferative effect | - Reduced PCNA-positive colonocytes by 69% in mucosal crypts | |||
| Apoptosis-inducing effect | - Increased cleaved caspase-3 positive cells by 2.3-fold in colonic tumor | |||
| Anti-inflammatory effects | - Reduced levels of proinflammatory cytokines - ELISA showed reduction of TNF-α by 51%, IL-1ß by 92% and IL-6 by 69% compared - qRT-PCR analysis showed reduction of TNF-α by 65%, IL-1ß by 69% and IL-6 by 45% | |||
Colon carcinogenesis model
| Diet containing 100 ppm NOB (0.1% wt) (10 weeks) | Inhibit AOM induced colon carcinogenesis | - Reduced frequency of preneoplastic lesions (colonic aberrant crypt foci (ACF) and β-catenin-accumulated crypts (BCAC)) - Reduced incidence of ACF by 68-91% and BCAC by 64–71% - Reduced PCNA-labeling index in ACF by 21% and BCAC by 19% | [83] |
Colon carcinogenesis model
| Diet containing 100 ppm NOB (0.1% wt) (for 17 weeks) | Inhibit AOM/DSS-induced colon carcinogenesis | - Suppressed incidence of neoplasms (adenoma and adenocarcinoma), lowered multiplicity of tumor | [84] |
| Inhibit leptin-induced colon carcinogenesis | ||||
| - Suppressed serum levels of leptin by 75–84% | ||||
Colon carcinogenesis model
| Diet containing NOB (0.01% wt and 0.05% wt) (34 weeks) | Inhibit AOM induced colon carcinogenesis | - Reduced incidence and multiplicity of colonic adenocarcinoma | [74] |
| Anti-proliferative effect | ||||
| - Increased apoptosis index of adenocarcinoma | ||||
| Anti-inflammatory effect | ||||
| - Reduced level of PGE2 in colonic adenocarcinoma and surrounding mucosa | ||||
Colon carcinogenesis model
| Diet containing NOB (0.01% wt and 0.05% wt) (5 weeks) | Inhibit AOM-induced colon carcinogenesis | - Reduced the frequency of colonic aberrant crypt foci formation - Reduced number of ACF in proximal, middle and distal colon | [41] |
| Anti-proliferative effect | ||||
| - Reduced MIB-5 labeling index of ACF but not of normal colonic crypts | ||||
| Anti-inflammatory effect | ||||
| - Reduced level of PGE2 in colonic mucosa | ||||
Colon carcinogenesis model
| Diet containing NOB (0.05% wt.) (50 weeks) | Inhibit PhIP-induced ACF in transverse colon | - Reduced the total colonic ACF indices in transverse colon | [75] |
Colorectal cancer xenograft mouse model
| NOB 100 mg/kg i.p. daily for 3 weeks 5-DMN 50 mg/kg and 100 mg/kg i.p. daily for 3 weeks | Anti-tumor effect | - NOB reduced tumor size and weight but not significant as compared to control - 5-DMN reduced tumor size and weight significantly as compared to control | [49] |
| Autophagy induction | - 5-DMN increased LC3 expression | |||
| Anti-inflammatory effect | ||||
| - 5-DMN increased p53 expression - 5-DMN reduced COX-2 expression | ||||
| Anti-angiogenesis | ||||
| - 5-DMN reduced VEGF expression |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goh, J.X.H.; Tan, L.T.-H.; Goh, J.K.; Chan, K.G.; Pusparajah, P.; Lee, L.-H.; Goh, B.-H. Nobiletin and Derivatives: Functional Compounds from Citrus Fruit Peel for Colon Cancer Chemoprevention. Cancers 2019, 11, 867. https://doi.org/10.3390/cancers11060867
Goh JXH, Tan LT-H, Goh JK, Chan KG, Pusparajah P, Lee L-H, Goh B-H. Nobiletin and Derivatives: Functional Compounds from Citrus Fruit Peel for Colon Cancer Chemoprevention. Cancers. 2019; 11(6):867. https://doi.org/10.3390/cancers11060867
Chicago/Turabian StyleGoh, Joanna Xuan Hui, Loh Teng-Hern Tan, Joo Kheng Goh, Kok Gan Chan, Priyia Pusparajah, Learn-Han Lee, and Bey-Hing Goh. 2019. "Nobiletin and Derivatives: Functional Compounds from Citrus Fruit Peel for Colon Cancer Chemoprevention" Cancers 11, no. 6: 867. https://doi.org/10.3390/cancers11060867
APA StyleGoh, J. X. H., Tan, L. T.-H., Goh, J. K., Chan, K. G., Pusparajah, P., Lee, L.-H., & Goh, B.-H. (2019). Nobiletin and Derivatives: Functional Compounds from Citrus Fruit Peel for Colon Cancer Chemoprevention. Cancers, 11(6), 867. https://doi.org/10.3390/cancers11060867