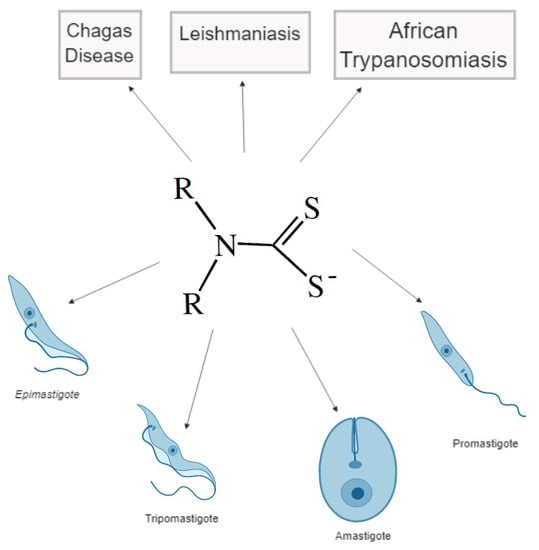
Application of Dithiocarbamates as Potential New Antitrypanosomatids-Drugs: Approach Chemistry, Functional and Biological
Abstract
:1. Introduction
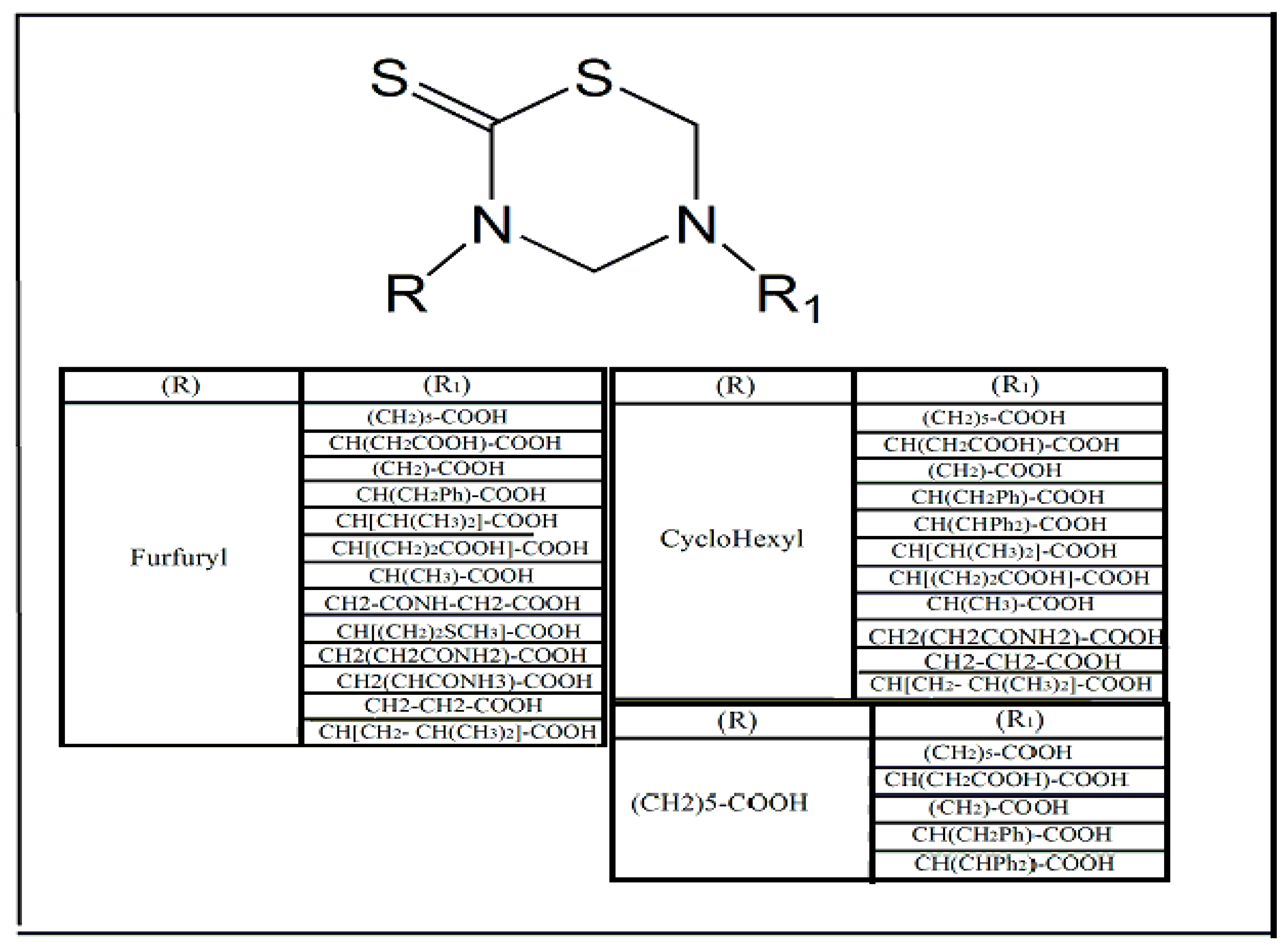
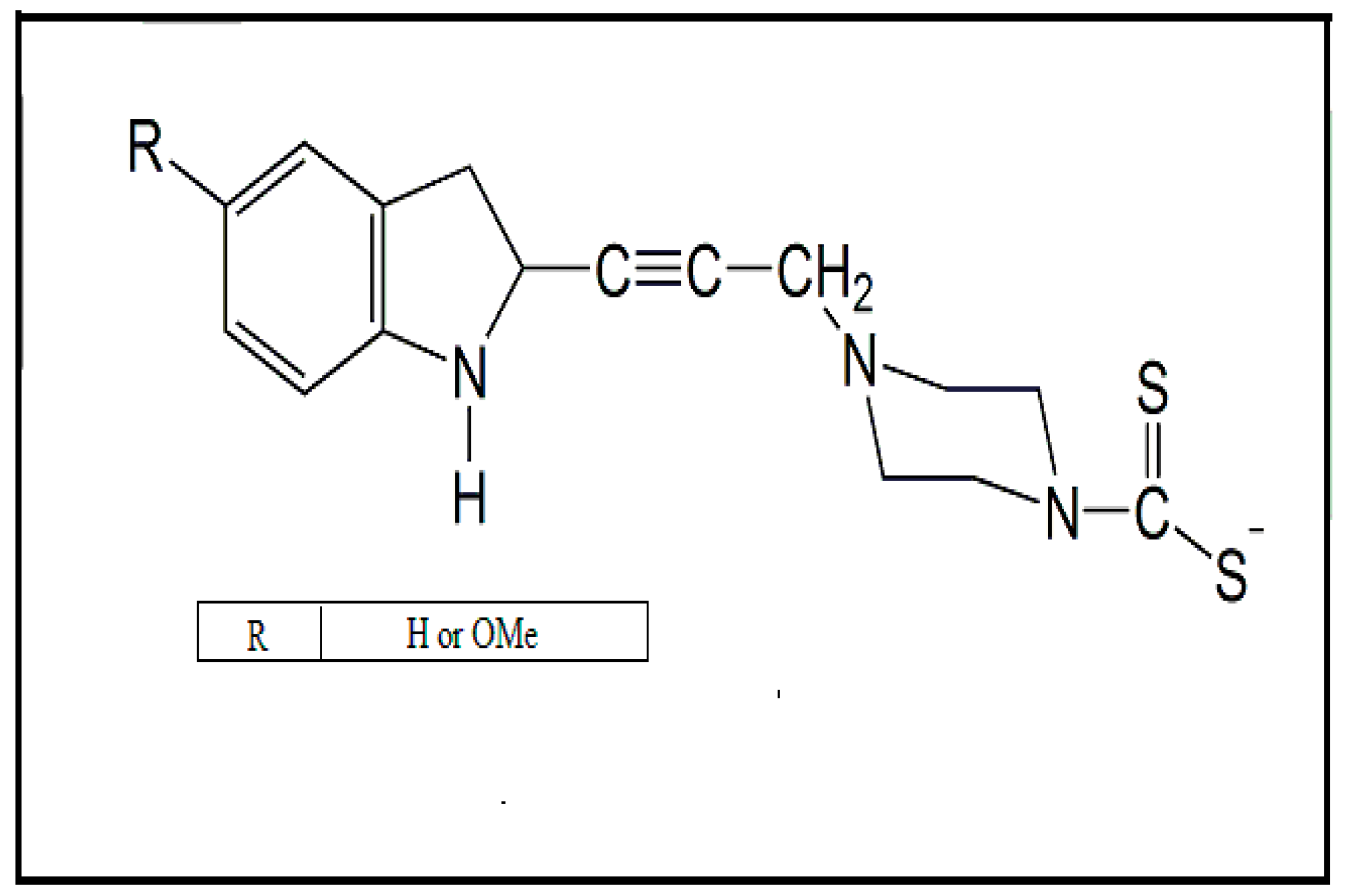
2. Main Chemical Groups Used in the Production of Dithiocarbamate Derivatives
3. Pharmacological Evaluation of Dithiocarbamate as Potential Antitrypanosomatids-Drugs
3.1. Evaluation of Dithiocarbamate as Potential Anti-Trypanosoma Cruzi Drugs
3.2. Evaluation of Dithiocarbamate as Potential Anti-Leishmania Drugs
3.3. Evaluation of Dithiocarbamate as Potential Anti-Trypanosoma Brucei Drugs
4. Final Considerations and Expectations
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Debus, H. Ueber die verbindungen der sulfocarbaminsäure. Justus Liebigs Ann. Chem. 1850, 73, 26–34. [Google Scholar] [CrossRef]
- Topping, R.J.; Jones, M.M. Optimal dithiocarbamate structure for immunomodulator action. Med. Hypotheses 1988, 27, 55–57. [Google Scholar] [CrossRef]
- Gucchait, A.; Joardar, N.; Parida, P.K.; Roy, P.; Mukherjee, N.; Dutta, A.; Yesuvadian, R.; SinhaBabu, S.P.; Jana, K.; Misra, A.K. Development of novel anti-filarial agents using carbamo (dithioperoxo) thioate derivatives. Eur. J. Med. Chem. 2018, 143, 598–610. [Google Scholar] [CrossRef] [PubMed]
- Buac, D.; Schmitt, S.; Ventro, G.; Rani Kona, F.; Ping Dou, Q. Dithiocarbamate-based coordination compounds as potent proteasome inhibitors in human cancer cells. Mini Rev. Med. Chem. 2012, 12, 1193–1201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pang, H.; Chen, D.; Cui, Q.C.; Dou, Q.P. Sodium diethyldithiocarbamate, an AIDS progression inhibitor and a copper-binding compound, has proteasome-inhibitory and apoptosis-inducing activities in cancer cells. Int. J. Mol. Med. 2007, 19, 809–816. [Google Scholar] [CrossRef]
- Sanchez-Cortes, S.; Domingo, C.; García-Ramos, J.V.; Aznárez, J.A. Surface-enhanced vibrational study (SEIR and SERS) of dithiocarbamate pesticides on gold films. Langmuir 2001, 17, 1157–1162. [Google Scholar] [CrossRef]
- Meco, G.; Bonifati, V.; Vanacore, N.; Fabrizio, E. Parkinsonism after chronic exposure to the fungicide maneb (manganese ethylene-bis-dithiocarbamate). Scand. J. Work Environ. Health 1994, 20, 301–305. [Google Scholar] [CrossRef]
- Bonamico, M.; Dessy, G.; Mugnoli, A.; Vaciago, A.; Zambonelli, L. Structural studies of metal dithiocarbamates. I. The crystal and molecular structure of the α-form of nickel diethyldithiocarbamate. Acta Crystallogr. 1965, 19, 619–626. [Google Scholar] [CrossRef]
- Bonamico, M.; Dessy, G.; Mugnoli, A.; Vaciago, A.; Zambonelli, L. Structural studies of metal dithiocarbamates. II. The crystal and molecular structure of copper diethyldithiocarbamate. Acta Crystallogr. 1965, 19, 886–897. [Google Scholar] [CrossRef]
- Bonamico, M.; Mazzone, G.; Vaciago, A.; Zambonelli, L. Structural studies of metal dithiocarbamates. III. The crystal and molecular structure of zinc diethyldithiocarbamate. Acta Crystallogr. 1965, 19, 898–909. [Google Scholar] [CrossRef]
- Hogarth, G. Transition metal dithiocarbamates: 1978–2003. In Progress in Inorganic Chemistry; Kenneth, D.K., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2005; Volume 53, pp. 71–561. [Google Scholar]
- Bond, A.M.; Martin, R.L. Electrochemistry and redox behaviour of transition metal dithiocarbamates. Coord. Chem. Rev. 1984, 54, 23–98. [Google Scholar] [CrossRef]
- Coucouvanis, D. The chemistry of the dithioacid and 1, 1-dithiolate complexes. Prog. Inorg. Chem. 1970, 11, 233–371. [Google Scholar]
- De Koning, P.D.; McAndrew, D.; Moore, R.; Moses, I.B.; Boyles, D.C.; Kissick, K.; Stanchina, C.L.; Cuthbertson, T.; Kamatani, A.; Rahman, L.; et al. Fit-for-purpose development of the enabling route to crizotinib (PF-02341066). Org. Proc. Res. Dev. 2011, 15, 1018–1026. [Google Scholar] [CrossRef]
- Corredor, C.; Tomasella, F.P.; Young, J. Drug interactions with potential rubber closure extractables: Identification of thiol–disulfide exchange reaction products of captopril and thiurams. J. Chromatogr. A 2009, 1216, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Heard, P.J. Main group dithiocarbamate complexes. In Progress in Inorganic Chemistry; Kenneth, D.K., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2005; Volume 53, pp. 1–69. [Google Scholar]
- Chaturvedi, D.; ZaidI, S. The role of Organic Dithiocarbamates in Drug Discovery Research. Res. Rev. J. Chem. 2016, 5, 10–12. [Google Scholar]
- Kanchi, S.; Singh, P.; Bisetty, K. Dithiocarbamates as hazardous remediation agent: A critical review on progress in environmental chemistry for inorganic species studies of 20th century. Arab. J. Chem. 2014, 7, 11–25. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Molina, J.A.; Molina, I. Chagas disease. Lancet 2018, 391, 82–94. [Google Scholar] [CrossRef]
- Piacenza, L.; Alvarez, M.N.; Peluffo, G.; Radi, R. Fighting the oxidative assault: The Trypanosoma cruzi journey to infection. Cur. Opin. Microbiol. 2009, 12, 415–421. [Google Scholar] [CrossRef]
- Milei, J.; Guerri-Guttenberg, R.A.; Grana, D.R.; Storino, R. Prognostic impact of Chagas disease in the United States. Am. Heart J. 2009, 157, 22–29. [Google Scholar] [CrossRef]
- Villela, M.M.; Rodrigues, V.L.C.C.; Casanova, C.; Dias, J.C.P. Analysis on the food source of Panstrongylus megistus (Hemiptera, Reduviidae, Triatominae) and its present importance as a vector for Trypanosoma cruzi, in the State of Minas Gerais. Rev. Soc. Bras. Med. Trop. 2010, 43, 125–128. [Google Scholar] [CrossRef]
- Castro, J.A.; Demecca, M.M.; Bartel, L.C. Toxic side effects of drugs used to treat Chagas’ disease (American trypanosomiasis). Hum. Exp. Toxicol. 2006, 25, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Giulivi, C.; Turrens, J.F.; Boveris, A. Chemiluminescence enhancement by trypanocidal drugs and by inhibitors of antioxidant enzymes in Trypanosoma cruzi. Mol. Biochem. Parasitol. 1988, 30, 243–251. [Google Scholar] [CrossRef]
- Rodrigues, R.R.; Elane, J.; Ecarter, C.; Jbogitsh, B.; Ksingh, P.; Jzimmerman, L.; Jmolenda, J.; Mjones, M. Chelating agent inhibition of Trypanosoma cruzi epimastigotes in vitro. J. Inorg. Biochem. 1995, 60, 277–288. [Google Scholar] [CrossRef]
- Winum, J.; Supuran, C.T. Recent advances in the discovery of zinc-binding motifs for the development of carbonic anhydrase inhibitors. J. Enzym. Inhib. Med. Chem. 2015, 30, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Pan, P.; Vermelho, A.B.; Scozzafava, A.; Parkkila, S.; Capasso, C.; Supuran, C.T. Anion inhibition studies of the α-carbonic anhydrase from the protozoan pathogen Trypanosoma cruzi, the causative agent of Chagas disease. Bioorganic. Med. Chem. 2013, 21, 4472–4476. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Dixit, R.; Pandey, K.C. Cysteine proteases: Modes of activation and future prospects as pharmacological targets. Front. Pharmacol. 2016, 7, 107. [Google Scholar] [CrossRef] [PubMed]
- North, M.J.; Mottram, J.C.; Coombs, G.H. Cysteine proteinases of parasitic protozoa. Parasitol. Today 1990, 6, 270–275. [Google Scholar] [CrossRef]
- Atienza, J.; Martínez Díaz, R.A.; Gómez Barrio, A.; Escario, J.A.; Herrero, A.; Ochoa, C.; Rodríguez, J. Activity assays of thiadiazine derivatives on Trichomonas vaginalis and amastigote forms of Trypanosoma cruzi. Chemotherapy 1992, 38, 441–446. [Google Scholar] [CrossRef]
- Ochoa, C.; Pérez, E.; Roland, P.; Suárez, M.; Ochoab, E.; Rodríguez, H.; Barrio, A.G.; Susana, M.; Nogal, J.J.; Martínez, R.A. Synthesis and antiprotozoan properties of new 3, 5—Disubstituted -tetrahydro-2H-1, 3, 5-thiadiazine-2-thione derivatives. Arzneimittelforschung 1999, 49, 764–769. [Google Scholar]
- Herrero, A.; Ochoa, C.; Atienza, J.; Antonio Escario, J.; Gómez-Barrio, A.; Martínez Fernández, A.R. Synthesis and Antiprotozoal Properties of 1, 2, 6-Thiadiazine 1, 1-Dioxide Derivatives. Arch. Pharm. 1992, 325, 509–514. [Google Scholar] [CrossRef]
- Houweling, T.A.; Karim-Kos, H.E.; Kulik, M.C.; Stolk, W.A.; Haagsma, J.A.; Lenk, E.J.; Richardus, J.H.; de Vlas, S.J. Socioeconomic inequalities in neglected tropical diseases: A systematic review. PLoS Negl. Trop. Dis. 2016, 10, e0004546. [Google Scholar] [CrossRef] [PubMed]
- Uliana, S.R.; Trinconi, C.T.; Coelho, A.C. Chemotherapy of leishmaniasis: Present challenges. Parasitology 2018, 145, 464–480. [Google Scholar] [CrossRef] [PubMed]
- Alvar, J.; Aparicio, P.; Aseffa, A.; Den Boer, M.; Cañavate, C.; Dedet, J.P.; Gradoni, L.; Ter Horst, R.; López-Vélez, R.; Moreno, J. The relationship between leishmaniasis and AIDS: The second 10 years. Clin. Microbiol. Rev. 2008, 21, 334–359. [Google Scholar] [CrossRef] [PubMed]
- Lestinova, T.; Rohousova, I.; Sima, M.; de Oliveira, C.I.; Volf, P. Insights into the sand fly saliva: Blood-feeding and immune interactions between sand flies, hosts, and Leishmania. PLoS Negl. Trop. Dis. 2017, 11, e0005600. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Leishmaniases: Epidemiological Report of the Americas. N° 7; World Health Organization: Genève, Switzerland, 2019. [Google Scholar]
- No, J.H. Visceral leishmaniasis: Revisiting current treatments and approaches for future discoveries. Acta Trop. 2016, 155, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Sundar, S.; Singh, A. Chemotherapeutics of visceral leishmaniasis: Present and future developments. Parasitology 2018, 145, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Laniado-Laborín, R.; Cabrales-Vargas, M.N. Amphotericin B: Side effects and toxicity. Rev. Iberoam. Micol. 2009, 26, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, S.R.; Bot, C.; Kelly, J.M.; Hall, B.S. Trypanocidal activity of nitroaromatic prodrugs: Current treatments and future perspectives. Curr. Top. Med. Chem. 2011, 11, 2072–2084. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.C.; Morais-Teixeira, E.; Reis, P.G.; Silva-Barcellos, N.M.; Salaün, P.; Campos, P.P.; Dias Corrêa-Junior, J.; Rabello, A.; Demicheli, C.; Frézard, F. Hepatotoxicity of pentavalent antimonial drug: Possible role of residual Sb (III) and protective effect of ascorbic acid. Antimicrob. Agents Chemother. 2014, 58, 481–488. [Google Scholar] [CrossRef]
- Brajtburg, J.; Elberg, S.; Schwartz, D.R.; Vertut-Croquin, A.; Schlessinger, D.; Kobayashi, G.S.; Medoff, G. Involvement of oxidative damage in erythrocyte lysis induced by amphotericin B. Antimicrob. Agents Chemother. 1985, 27, 172–176. [Google Scholar] [CrossRef] [Green Version]
- Lukášová, E.; Vojtísková, M.; Jelen, F.; Sticzay, T.; Palecek, E. Osmiuminduced alteration in DNA-structure. Gen. Physiol. Biophys. 1984, 3, 75–191. [Google Scholar]
- Rodriguez, M.; Bard, A.J. Electrochemical studies of the interaction of metal chelates with DNA. 4. Voltammetric and electrogenerated chemiluminescent studies of the interaction of tris (2, 2’-bipyridine) osmium (II) with DNA. Anal. Chem. 1990, 62, 2658–2662. [Google Scholar] [CrossRef] [PubMed]
- Castilla, J.J.; Mesa-Valle, C.M.; Sanchez-Moreno, M.; Arnedo, T.; Rosales, M.J.; Mascaro, C.; Craciunescu, D.; Osuna, A. In vitro activity and biochemical effectiveness of new organometallic complexes of osmium (III) against Leishmania donovani and Trypanosoma cruzi. Arzneimittel-Forschung 1996, 46, 990–996. [Google Scholar] [PubMed]
- Markham, A.; Hooper, M.; Fairlamb, A.H.; Berger, B.J.; Mung’ong’o, S.G. Activity of Novel Tryptophan Analogs against Mammalian and Trypanosomal Monoamine Oxidases. East Cent. Afr. J. Pharm. Sci. 2003, 6, 43–49. [Google Scholar]
- Mugoyela, V.K.; Hooper, M.; Fairlamb, A.H.; Mung’ong’o, S.G.; Croft, S. Activity of Novel Tryptophan Analogs against Trypanosoma cruzi and Leishmania donovani. East Cent. Afr. J. Pharm. Sci. 2008, 11. [Google Scholar] [CrossRef]
- Khouri, R.; Novais, F.; Santana, G.; de Oliveira, C.I.; Vannier dos Santos, M.A.; Barral, A.; Barral-Netto, M.; Van Weyenbergh, J. DETC induces Leishmania parasite killing in human in vitro and murine in vivo models: A promising therapeutic alternative in Leishmaniasis. PLoS ONE 2010, 5, e14394. [Google Scholar] [CrossRef] [PubMed]
- Celes, F.S.; Trovatti, E.; Khouri, R.; van Weyenbergh, J.; Ribeiro, S.J.L.; Borges, V.M.; Barud, H.S.; de Oliveira, C.I. DETC-based bacterial cellulose bio-curatives for topical treatment of cutaneous leishmaniasis. Sci. Rep. 2016, 6, 38330. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, S.; Goswami, S.; Adhya, S. Role of superoxide dismutase in survival of Leishmania within the macrophage. Biochem. J. 2003, 369, 447–452. [Google Scholar] [CrossRef]
- Pal, D.S.; Mondal, D.K.; Datta, R. Identification of metal dithiocarbamates as a novel class of antileishmanial agents. Antimicrob. Agents Chemother. 2015, 59, 2144–2152. [Google Scholar] [CrossRef]
- Vermelho, A.B.; Capaci, G.R.; Rodrigues, I.A.; Cardoso, V.S.; Mazotto, A.M.; Supuran, C.T. Carbonic anhydrases from Trypanosoma and Leishmania as anti-protozoan drug targets. Bioorganic Med. Chem. 2017, 25, 1543–1555. [Google Scholar] [CrossRef]
- Nocentini, A.; Cadoni, R.; Dumy, P.; Supuran, C.T.; Winum, J.Y. Carbonic anhydrases from Trypanosoma cruzi and Leishmania donovani chagasi are inhibited by benzoxaboroles. J. Enzym. Inhib. Med. Chem. 2018, 33, 286–289. [Google Scholar] [CrossRef]
- Da Silva Cardoso, V.; Vermelho, A.B.; Ricci Junior, E.; Almeida Rodrigues, I.; Mazotto, A.M.; Supuran, C.T. Antileishmanial activity of sulphonamide nanoemulsions targeting the β-carbonic anhydrase from Leishmania species. J. Enzym. Inhib. Med. Chem. 2018, 33, 850–857. [Google Scholar] [CrossRef]
- Barrett, M.P. The elimination of human African trypanosomiasis is in sight: Report from the third WHO stakeholders meeting on elimination of gambiense human African trypanosomiasis. PLoS Negl. Trop. Dis. 2018, 12, e0006925. [Google Scholar] [CrossRef]
- Wéry, M. Drug used in the treatment of sleeping sickness (human African trypanosomiasis: HAT). Int. J. Antimicrob. Agents 1994, 4, 227–238. [Google Scholar] [CrossRef]
- Rieche, A.; Hilgetag, G.; Martini, A.; Nejedly, O.; Schlegel, J. New compounds with bactericidal and fungicidal activity and which inhibit the growth of viruses. I. 2-thiotetrahydro-1, 3, 5-thiadiazine (“carbothialdine”) and dithiocarbamic acid salts. Arch. Pharm. Ber. Dtsch. Pharm. Ges. 1960, 293, 957–967. [Google Scholar] [CrossRef]
- Goksoyr, J. Chemical and fungicidal reactions of 3, 5-dimethyltetrahydro-1, 3, 5-thiadiazine-2-thione (3, 5-D). A comparison with sodium N-methyl dithiocarbamate and methyl isothiocyanate. Acta Chem. Scand. 1964, 18, 1341–1352. [Google Scholar] [CrossRef]
- El-Shorbagi, A. Model for delivery of amines through incorporation into a tetrahydro-2H-1, 3, 5-thiadiazine-2-thione structure. Eur. J. Med. Chem. 1994, 29, 11–15. [Google Scholar] [CrossRef]
- Aboul-Fadl, T.; El-Shorbagi, A. New prodrug approach for amino acids and amino-acid-like drugs. Eur. J. Med. Chem. 1996, 31, 165–169. [Google Scholar] [CrossRef]
- Bermello, J.C.; Piñeiro, R.P.; Fidalgo, L.M.; Cabrera, H.R.; Navarro, M.S. Thiadiazine derivatives as antiprotozoal new drugs. Open Med. Chem. J. 2011, 5, 51. [Google Scholar] [CrossRef]
- Micale, N.; Cinellu, M.A.; Maiore, L.; Sannella, A.R.; Severini, C.; Schirmeister, T.; Gabbiani, C.; Messori, L. Selected gold compounds cause pronounced inhibition of Falcipain 2 and effectively block P. falciparum growth in vitro. J. Inorg. Biochem. 2011, 105, 1576–1579. [Google Scholar] [CrossRef]
- Madeira, J.M.; Gibson, D.L.; Kean, W.F.; Klegeris, A. The biological activity of auranofin: Implications for novel treatment of diseases. Inflammopharmacology 2012, 20, 297–306. [Google Scholar] [CrossRef]
- Massai, L.; Messori, L.; Micale, N.; Schirmeister, T.; Maes, L.; Fregona, D.; Cinellu, M.A.; Gabbiani, C. Gold compounds as cysteine protease inhibitors: Perspectives for pharmaceutical application as antiparasitic agents. BioMetals 2017, 30, 313–320. [Google Scholar] [CrossRef]
- Cilibrasi, V.; Tsang, K.; Morellia, M.; Solfa, F.; Wiggins, H.L.; Jones, A.T.; Westwell, A.D. Synthesis of substituted carbamo (dithioperoxo) thioates as potential BCA2-inhibitory anticancer agents. Tetrahedron Lett. 2015, 56, 2583–2585. [Google Scholar] [CrossRef]
- Song, S.; Abdelmohsen, K.; Zhang, Y.; Becker, K.G.; Gorospe, M.; Bernier, M. Impact of pyrrolidine dithiocarbamate and interleukin-6 on mammalian target of rapamycin complex 1 regulation and global protein translation. J. Pharm. Exp. Ther. 2011, 339, 905–913. [Google Scholar] [CrossRef]
- Amir, M.K.; Hayat, F.; Khan, S.Z.; Hogarth, G.; Kondratyuk, T.; Pezzutode, J.M.; Tahir, M.N. Monofunctional platinum (II) dithiocarbamate complexes: Synthesis, characterization and anticancer activity. RSC Adv. 2016, 6, 110517–110524. [Google Scholar] [CrossRef]
- Altaf, M.; Monim-ul-Mehboob, M.; Kawde, A.-N.; Corona, G.; Larcher, R.; Ogasawara, M.; Casagrande, N.; Celegato, M.; Borghese, C.; Siddik, Z.H.; et al. New bipyridine gold (III) dithiocarbamate-containing complexes exerted a potent anticancer activity against cisplatin-resistant cancer cells independent of p53 status. Oncotarget 2017, 8, 490. [Google Scholar] [CrossRef]
- El-Aarag, B.Y.; Kasai, T.; Zahran, M.A.; Zakhary, N.I.; Shigehiro, T.; Sekhar, S.C.; Agwa, H.S.; Mizutani, A.; Murakami, H.; Kakuta, H.; et al. In vitro anti-proliferative and anti-angiogenic activities of thalidomide dithiocarbamate analogs. Int. Immunopharmacol. 2014, 21, 283–292. [Google Scholar] [CrossRef] [Green Version]
- Ding, P.-P.; Gao, M.; Mao, B.-B.; Cao, S.-L.; Liu, C.-H.; Yang, C.-R.; Li, Z.-F.; Liao, J.; Zhao, H.; Li, Z.; et al. Synthesis and biological evaluation of quinazolin-4(3H)-one derivatives bearing dithiocarbamate side chain at C2-position as potential antitumor agents. Eur. J. Med. Chem. 2016, 108, 364–373. [Google Scholar] [CrossRef]
- Ali, I.; Wani, W.A.; Saleem, K.; Hseih, M.-F. Design and synthesis of thalidomide based dithiocarbamate Cu (II), Ni (II) and Ru (III) complexes as anticancer agents. Polyhedron 2013, 56, 134–143. [Google Scholar] [CrossRef]
- Anthwal ASingh, K.; Rawat, M.S.M.; Kumar, A.; Aggarwal, B.B.; Rawat, D.S. C5-curcuminoid-dithiocarbamate based molecular hybrids: Synthesis and anti-inflammatory and anti-cancer activity evaluation. RSC Adv. 2014, 4, 28756–28764. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, J.; Jin, Z.; Zhang, W.Z.Y. Synthesis and biodistribution of novel 99m Tc labeled 4-nitroimidazole dithiocarbamate complexes as potential agents to target tumor hypoxia. Med. Chem. Commun. 2015, 6, 1143–1148. [Google Scholar] [CrossRef]
- Mansouri-Torshizi, H.; Saeidifar, M.; Divsalar, A.; Saboury, A.A. Interaction studies between a 1, 10-phenanthroline adduct of palladium (II) dithiocarbamate anti-tumor complex and calf thymus DNA. A synthesis spectral and in-vitro study. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2010, 77, 312–318. [Google Scholar] [CrossRef]
- Zheng, Y.C.; Duan, Y.C.; Ma, J.L.; Xu, R.M.; Zi, X.; Lv, W.L.; Wang, M.M.; Ye, X.W.; Zhu, S.; Mobley, D.; et al. Triazole–dithiocarbamate based selective lysine specific demethylase 1 (LSD1) inactivators inhibit gastric cancer cell growth, invasion, and migration. J. Med. Chem. 2013, 56, 8543–8560. [Google Scholar] [CrossRef]
- Keter, F.K.; Guzei, I.A.; Nell, M.; Zyl, W.E.; Darkwa, J. Phosphinogold (I) dithiocarbamate complexes: Effect of the nature of phosphine ligand on anticancer properties. Inorg. Chem. 2014, 53, 2058–2067. [Google Scholar] [CrossRef]
- Takii, T.; Horita, Y.; Kuroishi, R.; Chiba, T.; Mori, M.; Hasegawa, T.; Ito, T.; Tagami, T.; Ozeki, T.; Ito, S.; et al. The potential therapeutic usage of dithiocarbamate sugar derivatives for multi-drug resistant tuberculosis. In Understanding Tuberculosis-New Approaches to Fighting Against Drug Resistance; Cardona, P.J., Ed.; IntechOpen: Rijeka, Croatia, 2012; pp. 263–272. [Google Scholar]
- Horita, Y.; Takii, T.; Kuroishi, R.; Chiba, T.; Ogawa, K.; Kremer, L.; Sato, Y.; Lee, Y.; Hasegawa, T.; Onozaki, K. Synthesis and evaluation of anti-tubercular activity of new dithiocarbamate sugar derivatives. Bioorganic Med. Chem. Lett. 2011, 21, 899–903. [Google Scholar] [CrossRef]
- Wiesener, N.; Zimmer, C.; Jarasch-Althof, N.; Wutzler, P.; Henke, A. Therapy of experimental influenza virus infection with pyrrolidine dithiocarbamate. Med. Microbiol. Immunol. 2011, 200, 115–126. [Google Scholar] [CrossRef]
- Qiu, M.; Chen, Y.; Cheng, L.; Chu, Y.; Song, H.Y.; Wu, Z.W. Pyrrolidine dithiocarbamate inhibits herpes simplex virus 1 and 2 replication, and its activity may be mediated through dysregulation of the ubiquitin-proteasome system. J. Virol. 2013, 87, 8675–8686. [Google Scholar] [CrossRef]
- Li, Y.; Li, S. Facile synthesis and antifungal activity of dithiocarbamate derivatives bearing an amide moiety. J. Serb. Chem. Soc. 2015, 80, 1367–1374. [Google Scholar] [CrossRef]
- Ferreira, I.P.; de Lima, G.M.; Paniago, E.B.; Takahashi, J.A.; Pinheiro, C.B. Synthesis, characterization and antifungal activity of new dithiocarbamate-based complexes of Ni (II), Pd (II) and Pt (II). Inorg. Chem. Acta 2014, 423, 443–449. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, S.; Guo, H.; Wang, X. Synthesis and biological evaluation of a novel 99mTc (CO) 3 complex of ciprofloxacin dithiocarbamate as a potential agent to target infection. Bioorganic Med. Chem. Lett. 2010, 20, 3781–3784. [Google Scholar] [CrossRef]
- Yang, C.R.; Peng, B.; Cao, S.L.; Ren, T.T.; Jiang, W.; Wang, F.C.; Li, Y.S.; Wang, G.; Li, Z.; Xu, S.; et al. Synthesis, cytotoxic evaluation and target identification of thieno [2, 3-d] pyrimidine derivatives with a dithiocarbamate side chain at C2 position. Eur. J. Med. Chem. 2018, 154, 324–340. [Google Scholar] [CrossRef]
- Liao, J.; Zhao, H.; Zhou, L. Theoretical study on the bifunctional substitution reactions between gold (III) dithiocarbamate derivative Au (DMDT) Cl2 (DMDT = N,N-dimethyldithiocarbamate) and target molecules. Comput. Theor. Chem. 2014, 1048, 84–94. [Google Scholar] [CrossRef]
- Fu, D.; Zhang, S.Y.; Liu, Y.C.; Zhang, L.; Liu, J.J.; Song, J.; Zhao, R.H.; Li, F.; Sun, H.H.; Liu, H.M.; et al. Design, synthesis and antiproliferative activity studies of novel dithiocarbamate–chalcone derivates. Bioorganic Med. Chem. Lett. 2016, 26, 3918–3922. [Google Scholar] [CrossRef]
- Yurttaş, L.; Özkay, Y.; Demirci, F.; Göğer, G.; Yıldırım, Ş.U.; Mohsen, U.A.; Öztürk, Ö.Y.; Kaplancıklı, Z.A. Synthesis, anticandidal activity, and cytotoxicity of some thiazole derivatives with dithiocarbamate side chains. Turk. J. Chem. 2014, 38, 815–824. [Google Scholar] [CrossRef]
- Yurttaş, L.; Yusuf, Ö.; Murat, D.; Gülhan, T.; Ahmet, Ö.; Zerrin, C.; Kaan, K.; Asım, K.Z. Synthesis and antimicrobial activity evaluation of new dithiocarbamate derivatives bearing thiazole/benzothiazole rings. Phosphorus Sulfur Silicon Relat. Elem. 2016, 191, 1166–1173. [Google Scholar] [CrossRef]
- Duan, Y.-C.; Zheng, Y.-C.; Li, X.-C.; Wang, M.-M.; Ye, X.-W.; Guan, Y.-Y.; Liu, G.-Z.; Zheng, J.-X.; Liu, H.-M. Design, synthesis and antiproliferative activity studies of novel 1, 2, 3-triazole–dithiocarbamate–urea hybrids. Eur. J. Med. Chem. 2013, 64, 99–110. [Google Scholar] [CrossRef]
- Behar, M.; Barken, D.; Werner, S.L.; Hoffmann, A. The dynamics of signaling as a pharmacological target. Cell 2013, 155, 448–461. [Google Scholar] [CrossRef]
- Innocenti, A.; Scozzafava, A.; Supuran, C.T. Carbonic anhydrase inhibitors. Inhibition of transmembrane isoforms IX, XII, and XIV with less investigated anions including trithiocarbonate and dithiocarbamate. Bioorganic. Med. Chem. Lett. 2010, 20, 1548–1550. [Google Scholar] [CrossRef]
- Al-Jaroudi, S.S.; Altaf, M.; Seliman, A.A.; Yadav, S.; Arjmand, F.; Alhoshani, A.; Korashy, H.M.; Ahmad, S.; Isab, A.A. Synthesis, characterization, in vitro cytotoxicity and DNA interaction study of phosphanegold (I) complexes with dithiocarbamate ligands. Inorg. Chem. Acta 2017, 464, 37–48. [Google Scholar] [CrossRef]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, J.W.d.F.; Rocha, H.A.O.; de Medeiros, W.M.T.Q.; Silva, M.S. Application of Dithiocarbamates as Potential New Antitrypanosomatids-Drugs: Approach Chemistry, Functional and Biological. Molecules 2019, 24, 2806. https://doi.org/10.3390/molecules24152806
Oliveira JWdF, Rocha HAO, de Medeiros WMTQ, Silva MS. Application of Dithiocarbamates as Potential New Antitrypanosomatids-Drugs: Approach Chemistry, Functional and Biological. Molecules. 2019; 24(15):2806. https://doi.org/10.3390/molecules24152806
Chicago/Turabian StyleOliveira, Johny Wysllas de Freitas, Hugo Alexandre Oliveira Rocha, Wendy Marina Toscano Queiroz de Medeiros, and Marcelo Sousa Silva. 2019. "Application of Dithiocarbamates as Potential New Antitrypanosomatids-Drugs: Approach Chemistry, Functional and Biological" Molecules 24, no. 15: 2806. https://doi.org/10.3390/molecules24152806
APA StyleOliveira, J. W. d. F., Rocha, H. A. O., de Medeiros, W. M. T. Q., & Silva, M. S. (2019). Application of Dithiocarbamates as Potential New Antitrypanosomatids-Drugs: Approach Chemistry, Functional and Biological. Molecules, 24(15), 2806. https://doi.org/10.3390/molecules24152806