Therapeutic Potential of Rosmarinic Acid: A Comprehensive Review
Abstract
:1. Introduction
2. Bioavailability of Rosmarinic Acid and Its Metabolic Changes in the Human Body
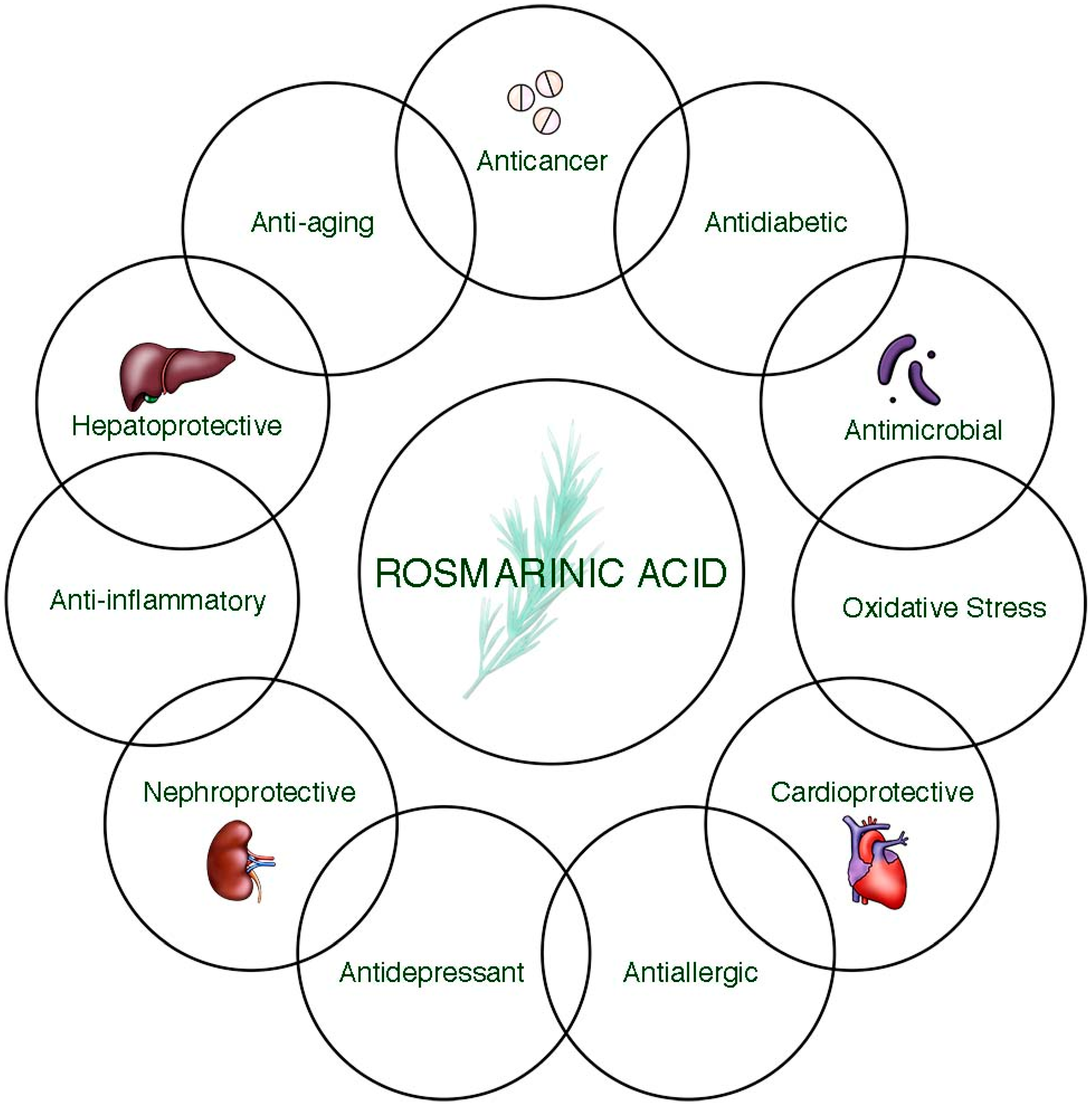
3. Health Benefits of Rosmarinic Acid
3.1. Anticancer Potential
3.2. Antidiabetic Activity
3.3. Antimicrobial Activity
3.4. Cardioprotective Activity
3.5. Antioxidant Activity
3.6. Hepatoprotective Activity
3.7. Antidepressant Potential
3.8. Nephroprotective Activity
3.9. Anti-Aging Activity
3.10. Anti-Allergic Activity
3.11. Anti-Inflammatory Activity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gordo, J.; Maximo, P.; Cabrita, E.; Lourenco, A.; Oliva, A.; Almeida, J.; Filipe, M.; Cruz, P.; Barcia, R.; Santos, M.; et al. Thymus mastichina: Chemical constituents and their anti-cancer activity. Nat. Prod. Commun. 2012, 7, 1491–1494. [Google Scholar] [CrossRef] [PubMed]
- Baliga, M.S.; Jimmy, R.; Thilakchand, K.R.; Sunitha, V.; Bhat, N.R.; Saldanha, E.; Rao, S.; Rao, P.; Arora, R.; Palatty, P.L. Ocimum sanctum L. (Holy Basil or Tulsi) and its phytochemicals in the prevention and treatment of cancer. Nutr. Cancer 2013, 65 (Suppl. 1), 26–35. [Google Scholar] [CrossRef] [PubMed]
- Andrade, J.M.; Faustino, C.; Garcia, C.; Ladeiras, D.; Reis, C.P.; Rijo, P. Rosmarinus officinalis L.: An update review of its phytochemistry and biological activity. Future Sci. OA 2018, 4, FSO283. [Google Scholar] [CrossRef] [PubMed]
- Ngo, S.N.; Williams, D.B.; Head, R.J. Rosemary and cancer prevention: Preclinical perspectives. Crit. Rev. Food Sci. Nutr. 2011, 51, 946–954. [Google Scholar] [CrossRef] [PubMed]
- Baba, S.; Osakabe, N.; Natsume, M.; Terao, J. Orally administered rosmarinic acid is present as the conjugated and/or methylated forms in plasma, and is degraded and metabolized to conjugated forms of caffeic acid, ferulic acid and m-coumaric acid. Life Sci. 2004, 75, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Cho, A.S.; Jeon, S.M.; Kim, M.J.; Yeo, J.; Seo, K.I.; Choi, M.S.; Lee, M.K. Chlorogenic acid exhibits anti-obesity property and improves lipid metabolism in high-fat diet-induced-obese mice. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2010, 48, 937–943. [Google Scholar] [CrossRef] [PubMed]
- Zych, M.; Kaczmarczyk-Sedlak, I.; Wojnar, W.; Folwarczna, J. The Effects of Sinapic Acid on the Development of Metabolic Disorders Induced by Estrogen Deficiency in Rats. Oxid. Med. Cell. Longev. 2018, 2018, 9274246. [Google Scholar] [CrossRef] [PubMed]
- Folwarczna, J.; Pytlik, M.; Zych, M.; Cegiela, U.; Nowinska, B.; Kaczmarczyk-Sedlak, I.; Sliwinski, L.; Trzeciak, H.; Trzeciak, H.I. Effects of caffeic and chlorogenic acids on the rat skeletal system. Eur. Rev. Med Pharmacol. Sci. 2015, 19, 682–693. [Google Scholar] [PubMed]
- Jayanthy, G.; Subramanian, S. RA abrogates hepatic gluconeogenesis and insulin resistance by enhancing IRS-1 and AMPK signalling in experimental type 2 diabetes. RSC Adv. 2015, 5, 44053–44067. [Google Scholar] [CrossRef]
- Han, J.; Wang, D.; Ye, L.; Li, P.; Hao, W.; Chen, X.; Ma, J.; Wang, B.; Shang, J.; Li, D.; et al. Rosmarinic Acid Protects against Inflammation and Cardiomyocyte Apoptosis during Myocardial Ischemia/Reperfusion Injury by Activating Peroxisome Proliferator-Activated Receptor Gamma. Front. Pharmacol. 2017, 8, 456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amoah, S.K.; Sandjo, L.P.; Kratz, J.M.; Biavatti, M.W. Rosmarinic Acid—Pharmaceutical and Clinical Aspects. Planta Med. 2016, 82, 388–406. [Google Scholar] [CrossRef] [PubMed]
- Ou, J.; Huang, J.; Zhao, D.; Du, B.; Wang, M. Protective effect of rosmarinic acid and carnosic acid against streptozotocin-induced oxidation, glycation, inflammation and microbiota imbalance in diabetic rats. Food Funct. 2018, 9, 851–860. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Assessment Report on Melissa officinalis L., Folium; EMA/HMPC/196746/2012; European Medicines Agency: London, UK, 2013; pp. 1–20. [Google Scholar]
- Fecka, I.; Turek, S. Determination of water-soluble polyphenolic compounds in commercial herbal teas from Lamiaceae: Peppermint, melissa, and sage. J. Agric. Food Chem. 2007, 55, 10908–10917. [Google Scholar] [CrossRef]
- Sotnikova, R.; Kaprinay, B.; Navarova, J. Rosmarinic acid mitigates signs of systemic oxidative stress in streptozotocin-induced diabetes in rats. Gen. Physiol. Biophys. 2015, 34, 449–452. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Zou, Y.; Liu, Y.; Niu, Y. Rosmarinic acid counteracts activation of hepatic stellate cells via inhibiting the ROS-dependent MMP-2 activity: Involvement of Nrf2 antioxidant system. Toxicol. Appl. Pharmacol. 2017, 318, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Enns, G.M.; Cowan, T.M. Glutathione as a Redox Biomarker in Mitochondrial Disease-Implications for Therapy. J. Clin. Med. 2017, 6, 50. [Google Scholar] [CrossRef]
- Venkatachalam, K.; Gunasekaran, S.; Namasivayam, N. Biochemical and molecular mechanisms underlying the chemopreventive efficacy of rosmarinic acid in a rat colon cancer. Eur. J. Pharmacol. 2016, 791, 37–50. [Google Scholar] [CrossRef]
- Wu, J.; Zhu, Y.; Li, F.; Zhang, G.; Shi, J.; Ou, R.; Tong, Y.; Liu, Y.; Liu, L.; Lu, L.; et al. Spica prunellae and its marker compound rosmarinic acid induced the expression of efflux transporters through activation of Nrf2-mediated signaling pathway in HepG2 cells. J. Ethnopharmacol. 2016, 193, 1–11. [Google Scholar] [CrossRef]
- Wu, C.F.; Hong, C.; Klauck, S.M.; Lin, Y.L.; Efferth, T. Molecular mechanisms of rosmarinic acid from Salvia miltiorrhiza in acute lymphoblastic leukemia cells. J. Ethnopharmacol. 2015, 176, 55–68. [Google Scholar] [CrossRef]
- Xu, Y.; Han, S.; Lei, K.; Chang, X.; Wang, K.; Li, Z.; Liu, J. Anti-Warburg effect of rosmarinic acid via miR-155 in colorectal carcinoma cells. Eur. J. Cancer Prev. Off. J. Eur. Cancer Prev. Organ. 2016, 25, 481–489. [Google Scholar] [CrossRef]
- Han, S.; Yang, S.; Cai, Z.; Pan, D.; Li, Z.; Huang, Z.; Zhang, P.; Zhu, H.; Lei, L.; Wang, W. Anti-Warburg effect of rosmarinic acid via miR-155 in gastric cancer cells. Drug Des. Dev. Ther. 2015, 9, 2695–2703. [Google Scholar] [CrossRef]
- Saiko, P.; Steinmann, M.T.; Schuster, H.; Graser, G.; Bressler, S.; Giessrigl, B.; Lackner, A.; Grusch, M.; Krupitza, G.; Bago-Horvath, Z.; et al. Epigallocatechin gallate, ellagic acid, and rosmarinic acid perturb dNTP pools and inhibit de novo DNA synthesis and proliferation of human HL-60 promyelocytic leukemia cells: Synergism with arabinofuranosylcytosine. Phytomed. Int. J. Phytother. Phytopharm. 2015, 22, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.K.; Noh, E.K.; Yoon, D.J.; Jo, J.C.; Koh, S.; Baek, J.H.; Park, J.H.; Min, Y.J.; Kim, H. Rosmarinic acid potentiates ATRA-induced macrophage differentiation in acute promyelocytic leukemia NB4 cells. Eur. J. Pharmacol. 2015, 747, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Karthikkumar, V.; Sivagami, G.; Viswanathan, P.; Nalini, N. Rosmarinic acid inhibits DMH-induced cell proliferation in experimental rats. J. Basic Clin. Physiol. Pharmacol. 2015, 26, 185–200. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.Y.; Hong, C.O.; Lee, G.P.; Kim, C.T.; Lee, K.W. The hepatoprotection of caffeic acid and rosmarinic acid, major compounds of Perilla frutescens, against t-BHP-induced oxidative liver damage. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2013, 55, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Karthikkumar, V.; Sivagami, G.; Vinothkumar, R.; Rajkumar, D.; Nalini, N. Modulatory efficacy of rosmarinic acid on premalignant lesions and antioxidant status in 1.2-dimethylhydrazine induced rat colon carcinogenesis. Environ. Toxicol. Pharmacol. 2012, 34, 949–958. [Google Scholar] [CrossRef] [PubMed]
- Karmokar, A.; Marczylo, T.H.; Cai, H.; Steward, W.P.; Gescher, A.J.; Brown, K. Dietary intake of rosmarinic acid by Apc(Min) mice, a model of colorectal carcinogenesis: Levels of parent agent in the target tissue and effect on adenoma development. Mol. Nutr. Food Res. 2012, 56, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Sharmila, R.; Manoharan, S. Anti-tumor activity of rosmarinic acid in 7.12-dimethylbenz(a)anthracene (DMBA) induced skin carcinogenesis in Swiss albino mice. Indian J. Exp. Biol. 2012, 50, 187–194. [Google Scholar]
- Tai, J.; Cheung, S.; Wu, M.; Hasman, D. Antiproliferation effect of Rosemary (Rosmarinus officinalis) on human ovarian cancer cells in vitro. Phytomed. Int. J. Phytother. Phytopharm. 2012, 19, 436–443. [Google Scholar] [CrossRef]
- Jang, Y.G.; Hwang, K.A.; Choi, K.C. Rosmarinic Acid, a Component of Rosemary Tea, Induced the Cell Cycle Arrest and Apoptosis through Modulation of HDAC2 Expression in Prostate Cancer Cell Lines. Nutrients 2018, 10, 1784. [Google Scholar] [CrossRef]
- Venkatachalam, K.; Gunasekaran, S.; Jesudoss, V.A.; Namasivayam, N. The effect of rosmarinic acid on 1.2-dimethylhydrazine induced colon carcinogenesis. Exp. Toxicol. Pathol. Off. J. Ges. Fur Toxikol. Pathol. 2013, 65, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Xu, G.; Liu, L.; Xu, D.; Liu, J. Anti-invasion effect of rosmarinic acid via the extracellular signal-regulated kinase and oxidation-reduction pathway in Ls174-T cells. J. Cell. Biochem. 2010, 111, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Jiang, Z.; Ji, G.; Liu, J. Inhibition of bone metastasis from breast carcinoma by rosmarinic acid. Planta Med. 2010, 76, 956–962. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Chen, D.Q.; Liu, H.X.; Li, W.B.; Lu, J.W.; Feng, J.F. Rosmarinic acid reduces the resistance of gastric carcinoma cells to 5-fluorouracil by downregulating FOXO4-targeting miR-6785-5p. Biomed. Pharmacother. 2019, 109, 2327–2334. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, Q.; Wei, L.; Pan, X.; Huang, D.; Gan, J.; Tang, S. Rosmarinic Acid Analogue-11 Induces Apoptosis of Human Gastric Cancer SGC-7901 Cells via the Epidermal Growth Factor Receptor (EGFR)/Akt/Nuclear Factor kappa B (NF-κB) Pathway. Med. Sci. Monit. Basic Res. 2019, 25, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Jayanthy, G.; Roshana Devi, V.; Ilango, K.; Subramanian, S.P. Rosmarinic Acid Mediates Mitochondrial Biogenesis in Insulin Resistant Skeletal Muscle Through Activation of AMPK. J. Cell. Biochem. 2017, 118, 1839–1848. [Google Scholar] [CrossRef]
- Runtuwene, J.; Cheng, K.C.; Asakawa, A.; Amitani, H.; Amitani, M.; Morinaga, A.; Takimoto, Y.; Kairupan, B.H.; Inui, A. Rosmarinic acid ameliorates hyperglycemia and insulin sensitivity in diabetic rats, potentially by modulating the expression of PEPCK and GLUT4. Drug Des. Dev. Ther. 2016, 10, 2193–2202. [Google Scholar] [CrossRef]
- Hasanein, P.; Felehgari, Z.; Emamjomeh, A. Preventive effects of Salvia officinalis L. against learning and memory deficit induced by diabetes in rats: Possible hypoglycaemic and antioxidant mechanisms. Neurosci. Lett. 2016, 622, 72–77. [Google Scholar] [CrossRef]
- Mushtaq, N.; Schmatz, R.; Ahmed, M.; Pereira, L.B.; da Costa, P.; Reichert, K.P.; Dalenogare, D.; Pelinson, L.P.; Vieira, J.M.; Stefanello, N.; et al. Protective effect of rosmarinic acid against oxidative stress biomarkers in liver and kidney of strepotozotocin-induced diabetic rats. J. Physiol. Biochem. 2015, 71, 743–751. [Google Scholar] [CrossRef]
- Govindaraj, J.; Sorimuthu Pillai, S. Rosmarinic acid modulates the antioxidant status and protects pancreatic tissues from glucolipotoxicity mediated oxidative stress in high-fat diet: Streptozotocin-induced diabetic rats. Mol. Cell. Biochem. 2015, 404, 143–159. [Google Scholar] [CrossRef]
- Vujicic, M.; Nikolic, I.; Kontogianni, V.G.; Saksida, T.; Charisiadis, P.; Orescanin-Dusic, Z.; Blagojevic, D.; Stosic-Grujicic, S.; Tzakos, A.G.; Stojanovic, I. Methanolic extract of Origanum vulgare ameliorates type 1 diabetes through antioxidant, anti-inflammatory and anti-apoptotic activity. Br. J. Nutr. 2015, 113, 770–782. [Google Scholar] [CrossRef]
- Mushtaq, N.; Schmatz, R.; Pereira, L.B.; Ahmad, M.; Stefanello, N.; Vieira, J.M.; Abdalla, F.; Rodrigues, M.V.; Baldissarelli, J.; Pelinson, L.P.; et al. Rosmarinic acid prevents lipid peroxidation and increase in acetylcholinesterase activity in brain of streptozotocin-induced diabetic rats. Cell Biochem. Funct. 2014, 32, 287–293. [Google Scholar] [CrossRef]
- Sotnikova, R.; Okruhlicova, L.; Vlkovicova, J.; Navarova, J.; Gajdacova, B.; Pivackova, L.; Fialova, S.; Krenek, P. Rosmarinic acid administration attenuates diabetes-induced vascular dysfunction of the rat aorta. J. Pharm. Pharmacol. 2013, 65, 713–723. [Google Scholar] [CrossRef]
- Azevedo, M.F.; Lima, C.F.; Fernandes-Ferreira, M.; Almeida, M.J.; Wilson, J.M.; Pereira-Wilson, C. Rosmarinic acid, major phenolic constituent of Greek sage herbal tea, modulates rat intestinal SGLT1 levels with effects on blood glucose. Mol. Nutr. Food Res. 2011, 55 (Suppl. 1), S15–S25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ekambaram, S.P.; Perumal, S.S.; Balakrishnan, A.; Marappan, N.; Gajendran, S.S.; Viswanathan, V. Antibacterial synergy between rosmarinic acid and antibiotics against methicillin-resistant Staphylococcus aureus. J. Intercult. Ethnopharmacol. 2016, 5, 358–363. [Google Scholar] [CrossRef]
- Slobodnikova, L.; Fialova, S.; Hupkova, H.; Grancai, D. Rosmarinic acid interaction with planktonic and biofilm Staphylococcus aureus. Nat. Prod. Commun. 2013, 8, 1747–1750. [Google Scholar] [CrossRef]
- Suriyarak, S.; Bayrasy, C.; Schmidt, H.; Villeneuve, P.; Weiss, J. Impact of fatty acid chain length of rosmarinate esters on their antimicrobial activity against Staphylococcus carnosus LTH1502 and Escherichia coli K-12 LTH4263. J. Food Prot. 2013, 76, 1539–1548. [Google Scholar] [CrossRef]
- Dubois, M.; Bailly, F.; Mbemba, G.; Mouscadet, J.F.; Debyser, Z.; Witvrouw, M.; Cotelle, P. Reaction of rosmarinic acid with nitrite ions in acidic conditions: Discovery of nitro- and dinitrorosmarinic acids as new anti-HIV-1 agents. J. Med. Chem. 2008, 51, 2575–2579. [Google Scholar] [CrossRef]
- Raeisi, M.; Tabaraei, A.; Hashemi, M.; Behnampour, N. Effect of sodium alginate coating incorporated with nisin, Cinnamomum zeylanicum, and rosemary essential oils on microbial quality of chicken meat and fate of Listeria monocytogenes during refrigeration. Int. J. Food Microbiol. 2016, 238, 139–145. [Google Scholar] [CrossRef]
- Honorio, V.G.; Bezerra, J.; Souza, G.T.; Carvalho, R.J.; Gomes-Neto, N.J.; Figueiredo, R.C.; Melo, J.V.; Souza, E.L.; Magnani, M. Inhibition of Staphylococcus aureus cocktail using the synergies of oregano and rosemary essential oils or carvacrol and 1.8-cineole. Front. Microbiol. 2015, 6, 1223. [Google Scholar] [CrossRef]
- Zhuang, Y.; Jiang, J.; Bi, H.; Yin, H.; Liu, S.; Liu, T. Synthesis of rosmarinic acid analogues in Escherichia coli. Biotechnol. Lett. 2016, 38, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Suriyarak, S.; Gibis, M.; Schmidt, H.; Villeneuve, P.; Weiss, J. Antimicrobial mechanism and activity of dodecyl rosmarinate against Staphylococcus carnosus LTH1502 as influenced by addition of salt and change in pH. J. Food Prot. 2014, 77, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Li, X.L.; Liu, J.X.; Li, P.; Zheng, Y.Q. Protective effect of rosmarinic acid on hypoxia/reoxygenation injury in cardiomyocytes. J. Chin. Mater. Med. 2014, 39, 1897–1901. [Google Scholar]
- Ku, S.K.; Yang, E.J.; Song, K.S.; Bae, J.S. Rosmarinic acid down-regulates endothelial protein C receptor shedding in vitro and in vivo. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2013, 59, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.G.; Celotto, A.C.; Capellini, V.K.; Albuquerque, A.A.; Nadai, T.R.; Carvalho, M.T.; Evora, P.R. Is rosmarinic acid underestimated as an experimental cardiovascular drug? Acta Cir. Bras. 2013, 28 (Suppl. 1), 83–87. [Google Scholar] [CrossRef]
- Karthik, D.; Viswanathan, P.; Anuradha, C.V. Administration of rosmarinic acid reduces cardiopathology and blood pressure through inhibition of p22phox NADPH oxidase in fructose-fed hypertensive rats. J. Cardiovasc. Pharmacol. 2011, 58, 514–521. [Google Scholar] [CrossRef]
- Sinkovic, A.; Suran, D.; Lokar, L.; Fliser, E.; Skerget, M.; Novak, Z.; Knez, Z. Rosemary extracts improve flow-mediated dilatation of the brachial artery and plasma PAI-1 activity in healthy young volunteers. Phytother. Res. 2011, 25, 402–407. [Google Scholar] [CrossRef]
- Huang, S.S.; Zheng, R.L. Rosmarinic acid inhibits angiogenesis and its mechanism of action in vitro. Cancer Lett. 2006, 239, 271–280. [Google Scholar] [CrossRef]
- Kim, D.S.; Kim, H.R.; Woo, E.R.; Hong, S.T.; Chae, H.J.; Chae, S.W. Inhibitory effects of rosmarinic acid on adriamycin-induced apoptosis in H9c2 cardiac muscle cells by inhibiting reactive oxygen species and the activations of c-Jun N-terminal kinase and extracellular signal-regulated kinase. Biochem. Pharmacol. 2005, 70, 1066–1078. [Google Scholar] [CrossRef]
- Chlopcikova, S.; Psotova, J.; Miketova, P.; Sousek, J.; Lichnovsky, V.; Simanek, V. Chemoprotective effect of plant phenolics against anthracycline-induced toxicity on rat cardiomyocytes. Part II. caffeic, chlorogenic and rosmarinic acids. Phytother. Res. 2004, 18, 408–413. [Google Scholar] [CrossRef]
- Adomako-Bonsu, A.G.; Chan, S.L.; Pratten, M.; Fry, J.R. Antioxidant activity of rosmarinic acid and its principal metabolites in chemical and cellular systems: Importance of physico-chemical characteristics. Toxicol. Vitr. Int. J. Publ. Assoc. 2017, 40, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Bayomy, N.A.; Elbakary, R.H.; Ibrahim, M.A.A.; Abdelaziz, E. Effect of Lycopene and Rosmarinic Acid on Gentamicin Induced Renal Cortical Oxidative Stress, Apoptosis, and Autophagy in Adult Male Albino Rat. Anat. Rec. 2017, 300, 1137–1149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Govindarajan, R.; Vijayakumar, M.; Pushpangadan, P. Antioxidant approach to disease management and the role of ‘RasayanA’ herbs of Ayurveda. J. Ethnopharmacol. 2005, 99, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.R.; Rizvi, W.; Khan, G.N.; Khan, R.A.; Shaheen, S. Carbon tetrachloride-induced nephrotoxicity in rats: Protective role of Digera muricata. J. Ethnopharmacol. 2009, 122, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Gandy, S. The role of cerebral amyloid beta accumulation in common forms of Alzheimer disease. J. Clin. Investig. 2005, 115, 1121–1129. [Google Scholar] [CrossRef]
- Lee, A.Y.; Wu, T.T.; Hwang, B.R.; Lee, J.; Lee, M.H.; Lee, S.; Cho, E.J. The Neuro-Protective Effect of the Methanolic Extract of Perilla frutescens var. japonica and Rosmarinic Acid against H(2)O(2)-Induced Oxidative Stress in C6 Glial Cells. Biomol. Ther. 2016, 24, 338–345. [Google Scholar] [CrossRef]
- Hase, T.; Shishido, S.; Yamamoto, S.; Yamashita, R.; Nukima, H.; Taira, S.; Toyoda, T.; Abe, K.; Hamaguchi, T.; Ono, K.; et al. Rosmarinic acid suppresses Alzheimer’s disease development by reducing amyloid beta aggregation by increasing monoamine secretion. Sci. Rep. 2019, 9, 8711. [Google Scholar] [CrossRef]
- Shang, A.J.; Yang, Y.; Wang, H.Y.; Tao, B.Z.; Wang, J.; Wang, Z.F.; Zhou, D.B. Spinal cord injury effectively ameliorated by neuroprotective effects of rosmarinic acid. Nutr. Neurosci. 2017, 20, 172–179. [Google Scholar] [CrossRef]
- Fernando, P.M.; Piao, M.J.; Kang, K.A.; Ryu, Y.S.; Hewage, S.R.; Chae, S.W.; Hyun, J.W. Rosmarinic Acid Attenuates Cell Damage against UVB Radiation-Induced Oxidative Stress via Enhancing Antioxidant Effects in Human HaCaT Cells. Biomol. Ther. 2016, 24, 75–84. [Google Scholar] [CrossRef] [Green Version]
- Seo, J.S.; Choi, J.; Leem, Y.H.; Han, P.L. Rosmarinic Acid Alleviates Neurological Symptoms in the G93A-SOD1 Transgenic Mouse Model of Amyotrophic Lateral Sclerosis. Exp. Neurobiol. 2015, 24, 341–350. [Google Scholar] [CrossRef] [Green Version]
- Bacanli, M.; Aydin, S.; Taner, G.; Goktas, H.G.; Sahin, T.; Basaran, A.A.; Basaran, N. Does rosmarinic acid treatment have protective role against sepsis-induced oxidative damage in Wistar Albino rats? Hum. Exp. Toxicol. 2016, 35, 877–886. [Google Scholar] [CrossRef] [PubMed]
- Fetoni, A.R.; Paciello, F.; Rolesi, R.; Eramo, S.L.; Mancuso, C.; Troiani, D.; Paludetti, G. Rosmarinic acid up-regulates the noise-activated Nrf2/HO-1 pathway and protects against noise-induced injury in rat cochlea. Free Radic. Biol. Med. 2015, 85, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Khamse, S.; Sadr, S.S.; Roghani, M.; Hasanzadeh, G.; Mohammadian, M. Rosmarinic acid exerts a neuroprotective effect in the kainate rat model of temporal lobe epilepsy: Underlying mechanisms. Pharm. Biol. 2015, 53, 1818–1825. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, X.; Yang, L.; Zu, Y.; Lu, Q. Effects of rosmarinic acid on liver and kidney antioxidant enzymes, lipid peroxidation and tissue ultrastructure in aging mice. Food Funct. 2015, 6, 927–931. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, S.F.; Tenore, G.C.; Daglia, M.; Tundis, R.; Loizzo, M.R.; Nabavi, S.M. The cellular protective effects of rosmarinic acid: From bench to bedside. Curr. Neurovascular Res. 2015, 12, 98–105. [Google Scholar] [CrossRef]
- Yazihan, N.; Ataoglu, H.; Kavas, G.O.; Akyurek, N.; Yener, B.; Aydm, C. The effect of K-ATP channel blockage during erythropoietin treatment in renal ischemia-reperfusion injury. J. Investig. Surg. Off. J. Acad. Surg. Res. 2008, 21, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.H.; He, L.; Cao, Z.Q.; Xiang, B.; Liu, L. Effect of ischemia preconditioning on renal ischemia/reperfusion injury in rats. Int. Braz. J. Urol. Off. J. Braz. Soc. Urol. 2012, 38, 842–854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sedaghat, Z.; Kadkhodaee, M.; Seifi, B.; Salehi, E.; Najafi, A.; Dargahi, L. Remote preconditioning reduces oxidative stress, downregulates cyclo-oxygenase-2 expression and attenuates ischaemia-reperfusion-induced acute kidney injury. Clin. Exp. Pharmacol. Physiol. 2013, 40, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Elufioye, T.O.; Habtemariam, S. Hepatoprotective effects of rosmarinic acid: Insight into its mechanisms of action. Biomed. Pharmacother. 2019, 112, 108600. [Google Scholar] [CrossRef] [PubMed]
- Ferlemi, A.V.; Katsikoudi, A.; Kontogianni, V.G.; Kellici, T.F.; Iatrou, G.; Lamari, F.N.; Tzakos, A.G.; Margarity, M. Rosemary tea consumption results to anxiolytic- and anti-depressant-like behavior of adult male mice and inhibits all cerebral area and liver cholinesterase activity; phytochemical investigation and in silico studies. Chem. Biol. Interact. 2015, 237, 47–57. [Google Scholar] [CrossRef]
- Ramalho, L.N.; Pasta, A.A.; Terra, V.A.; Augusto, M.; Sanches, S.C.; Souza-Neto, F.P.; Cecchini, R.; Gulin, F.; Ramalho, F.S. Rosmarinic acid attenuates hepatic ischemia and reperfusion injury in rats. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2014, 74, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Kamyab, A.A.; Eshraghian, A. Anti-Inflammatory, gastrointestinal and hepatoprotective effects of Ocimum sanctum Linn: An ancient remedy with new application. Inflamm. Allergy Drug Targets 2013, 12, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Domitrovic, R.; Skoda, M.; Vasiljev Marchesi, V.; Cvijanovic, O.; Pernjak Pugel, E.; Stefan, M.B. Rosmarinic acid ameliorates acute liver damage and fibrogenesis in carbon tetrachloride-intoxicated mice. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2013, 51, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.D.; Chiang, Y.M.; Higashiyama, R.; Asahina, K.; Mann, D.A.; Mann, J.; Wang, C.C.; Tsukamoto, H. Rosmarinic acid and baicalin epigenetically derepress peroxisomal proliferator-activated receptor gamma in hepatic stellate cells for their antifibrotic effect. Hepatology 2012, 55, 1271–1281. [Google Scholar] [CrossRef] [PubMed]
- Tandogan, B.; Kuruuzum-Uz, A.; Sengezer, C.; Guvenalp, Z.; Demirezer, L.O.; Ulusu, N.N. In vitro effects of rosmarinic acid on glutathione reductase and glucose 6-phosphate dehydrogenase. Pharm. Biol. 2011, 49, 587–594. [Google Scholar] [CrossRef]
- Zhang, J.J.; Wang, Y.L.; Feng, X.B.; Song, X.D.; Liu, W.B. Rosmarinic acid inhibits proliferation and induces apoptosis of hepatic stellate cells. Biol. Pharm. Bull. 2011, 34, 343–348. [Google Scholar] [CrossRef]
- Li, G.S.; Jiang, W.L.; Tian, J.W.; Qu, G.W.; Zhu, H.B.; Fu, F.H. In vitro and in vivo antifibrotic effects of rosmarinic acid on experimental liver fibrosis. Phytomed. Int. J. Phytother. Phytopharm. 2010, 17, 282–288. [Google Scholar] [CrossRef]
- Jiang, W.L.; Chen, X.G.; Qu, G.W.; Yue, X.D.; Zhu, H.B.; Tian, J.W.; Fu, F.H. Rosmarinic acid protects against experimental sepsis by inhibiting proinflammatory factor release and ameliorating hemodynamics. Shock 2009, 32, 608–613. [Google Scholar] [CrossRef]
- Renzulli, C.; Galvano, F.; Pierdomenico, L.; Speroni, E.; Guerra, M.C. Effects of rosmarinic acid against aflatoxin B1 and ochratoxin-A-induced cell damage in a human hepatoma cell line (Hep G2). J. Appl. Toxicol. 2004, 24, 289–296. [Google Scholar] [CrossRef]
- Lin, S.Y.; Wang, Y.Y.; Chen, W.Y.; Liao, S.L.; Chou, S.T.; Yang, C.P.; Chen, C.J. Hepatoprotective activities of rosmarinic acid against extrahepatic cholestasis in rats. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2017, 108, 214–223. [Google Scholar] [CrossRef]
- Grigoletto, J.; Oliveira, C.V.; Grauncke, A.C.; Souza, T.L.; Souto, N.S.; Freitas, M.L.; Furian, A.F.; Santos, A.R.; Oliveira, M.S. Rosmarinic acid is anticonvulsant against seizures induced by pentylenetetrazol and pilocarpine in mice. Epilepsy Behav. 2016, 62, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Berton, O.; Nestler, E.J. New approaches to antidepressant drug discovery: Beyond monoamines. Nat. Rev. Neurosci. 2006, 7, 137–151. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Liu, P.; Yang, F.; Zhang, Y.H.; Miao, D. Rosmarinic acid ameliorates depressive-like behaviors in a rat model of CUS and Up-regulates BDNF levels in the hippocampus and hippocampal-derived astrocytes. Neurochem. Res. 2013, 38, 1828–1837. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; El Omri, A.; Kondo, S.; Han, J.; Isoda, H. Rosmarinus officinalis polyphenols produce anti-depressant like effect through monoaminergic and cholinergic functions modulation. Behav. Brain Res. 2013, 238, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Dashti-Khavidaki, S.; Moghaddas, A.; Heydari, B.; Khalili, H.; Lessan-Pezeshki, M.; Lessan-Pezeshki, M. Statins against drug-induced nephrotoxicity. J. Pharm. Pharm. Sci. 2013, 16, 588–608. [Google Scholar] [CrossRef] [PubMed]
- Balakumar, P.; Rohilla, A.; Thangathirupathi, A. Gentamicin-induced nephrotoxicity: Do we have a promising therapeutic approach to blunt it? Pharmacol. Res. 2010, 62, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.; Lee, H.; Hah, D.Y.; Heo, J.H.; Kim, C.H.; Kim, E.; Kim, J.S. Protective Effects of Houttuynia cordata Thunb. on Gentamicin-induced Oxidative Stress and Nephrotoxicity in Rats. Toxicol. Res. 2013, 29, 61–67. [Google Scholar] [CrossRef]
- Ozturk, H.; Ozturk, H.; Terzi, E.H.; Ozgen, U.; Duran, A.; Uygun, I. Protective effects of rosmarinic acid against renal ischaemia/reperfusion injury in rats. J. Pak. Med Assoc. 2014, 64, 260–265. [Google Scholar]
- Domitrovic, R.; Potocnjak, I.; Crncevic-Orlic, Z.; Skoda, M. Nephroprotective activities of rosmarinic acid against cisplatin-induced kidney injury in mice. Food Chem. Toxicol. 2014, 66, 321–328. [Google Scholar] [CrossRef]
- Tavafi, M.; Ahmadvand, H. Effect of rosmarinic acid on inhibition of gentamicin induced nephrotoxicity in rats. Tissue Cell 2011, 43, 392–397. [Google Scholar] [CrossRef]
- Ren, P.; Jiang, H.; Li, R.; Wang, J.; Song, N.; Xu, H.M.; Xie, J.X. Rosmarinic acid inhibits 6-OHDA-induced neurotoxicity by anti-oxidation in MES23.5 cells. J. Mol. Neurosci. 2009, 39, 220–225. [Google Scholar] [CrossRef]
- Shan, Y.; Wang, D.D.; Xu, Y.X.; Wang, C.; Cao, L.; Liu, Y.S.; Zhu, C.Q. Aging as a Precipitating Factor in Chronic Restraint Stress-Induced Tau Aggregation Pathology, and the Protective Effects of Rosmarinic Acid. J. Alzheimer’s Dis. 2016, 49, 829–844. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, H.J.; Lee, K.W. Naturally occurring phytochemicals for the prevention of Alzheimer’s disease. J. Neurochem. 2010, 112, 1415–1430. [Google Scholar] [CrossRef]
- Holland, D.; Desikan, R.S.; Dale, A.M.; McEvoy, L.K. Rates of decline in Alzheimer disease decrease with age. PLoS ONE 2012, 7, e42325. [Google Scholar] [CrossRef]
- Farr, S.A.; Niehoff, M.L.; Ceddia, M.A.; Herrlinger, K.A.; Lewis, B.J.; Feng, S.; Welleford, A.; Butterfield, D.A.; Morley, J.E. Effect of botanical extracts containing carnosic acid or rosmarinic acid on learning and memory in SAMP8 mice. Physiol. Behav. 2016, 165, 328–338. [Google Scholar] [CrossRef] [Green Version]
- Shimojo, Y.; Kosaka, K.; Noda, Y.; Shimizu, T.; Shirasawa, T. Effect of rosmarinic acid in motor dysfunction and life span in a mouse model of familial amyotrophic lateral sclerosis. J. Neurosci. Res. 2010, 88, 896–904. [Google Scholar] [CrossRef] [PubMed]
- Luft, J.G.; Steffens, L.; Moras, A.M.; da Rosa, M.S.; Leipnitz, G.; Regner, G.G.; Pfluger, P.F.; Goncalves, D.; Moura, D.J.; Pereira, P. Rosmarinic acid improves oxidative stress parameters and mitochondrial respiratory chain activity following 4-aminopyridine and picrotoxin-induced seizure in mice. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2019. [Google Scholar] [CrossRef]
- Airoldi, C.; Sironi, E.; Dias, C.; Marcelo, F.; Martins, A.; Rauter, A.P.; Nicotra, F.; Jimenez-Barbero, J. Natural compounds against Alzheimer’s disease: Molecular recognition of Abeta1-42 peptide by Salvia sclareoides extract and its major component, rosmarinic acid, as investigated by NMR. Chem. Asian J. 2013, 8, 596–602. [Google Scholar] [CrossRef]
- Iuvone, T.; De Filippis, D.; Esposito, G.; D’Amico, A.; Izzo, A.A. The spice sage and its active ingredient rosmarinic acid protect PC12 cells from amyloid-beta peptide-induced neurotoxicity. J. Pharmacol. Exp. Ther. 2006, 317, 1143–1149. [Google Scholar] [CrossRef]
- Dashti, A.; Soodi, M.; Amani, N. Cr (VI) induced oxidative stress and toxicity in cultured cerebellar granule neurons at different stages of development and protective effect of Rosmarinic acid. Environ. Toxicol. 2016, 31, 269–277. [Google Scholar] [CrossRef]
- Lin, C.; Xiao, J.; Xi, Y.; Zhang, X.; Zhong, Q.; Zheng, H.; Cao, Y.; Chen, Y. Rosmarinic acid improved antioxidant properties and healthspan via the IIS and MAPK pathways in Caenorhabditis elegans. BioFactors 2019. [Google Scholar] [CrossRef] [PubMed]
- Ghaffari, H.; Venkataramana, M.; Jalali Ghassam, B.; Chandra Nayaka, S.; Nataraju, A.; Geetha, N.P.; Prakash, H.S. Rosmarinic acid mediated neuroprotective effects against H2O2-induced neuronal cell damage in N2A cells. Life Sci. 2014, 113, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Braidy, N.; Matin, A.; Rossi, F.; Chinain, M.; Laurent, D.; Guillemin, G.J. Neuroprotective effects of rosmarinic acid on ciguatoxin in primary human neurons. Neurotox. Res. 2014, 25, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Fallarini, S.; Miglio, G.; Paoletti, T.; Minassi, A.; Amoruso, A.; Bardelli, C.; Brunelleschi, S.; Lombardi, G. Clovamide and rosmarinic acid induce neuroprotective effects in in vitro models of neuronal death. Br. J. Pharmacol. 2009, 157, 1072–1084. [Google Scholar] [CrossRef] [PubMed]
- Yoou, M.S.; Park, C.L.; Kim, M.H.; Kim, H.M.; Jeong, H.J. Inhibition of MDM2 expression by rosmarinic acid in TSLP-stimulated mast cell. Eur. J. Pharmacol. 2016, 771, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.S.; Carneiro, T.C.; Cerqueira-Lima, A.T.; Queiroz, N.V.; Alcantara-Neves, N.M.; Pontes-de-Carvalho, L.C.; Velozo Eda, S.; Oliveira, E.J.; Figueiredo, C.A. Ocimum gratissimum Linn. and rosmarinic acid, attenuate eosinophilic airway inflammation in an experimental model of respiratory allergy to Blomia tropicalis. Int. Immunopharmacol. 2012, 13, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Asada, T.; Sato, A.; Koi, Y.; Nishiwaki, H.; Tamura, H. Rosmarinic acid extract for antioxidant, antiallergic, and alpha-glucosidase inhibitory activities, isolated by supramolecular technique and solvent extraction from Perilla leaves. J. Agric. Food Chem. 2014, 62, 885–892. [Google Scholar] [CrossRef]
- Jang, A.H.; Kim, T.H.; Kim, G.D.; Kim, J.E.; Kim, H.J.; Kim, S.S.; Jin, Y.H.; Park, Y.S.; Park, C.S. Rosmarinic acid attenuates 2.4-dinitrofluorobenzene-induced atopic dermatitis in NC/Nga mice. Int. Immunopharmacol. 2011, 11, 1271–1277. [Google Scholar] [CrossRef]
- Oh, H.A.; Park, C.S.; Ahn, H.J.; Park, Y.S.; Kim, H.M. Effect of Perilla frutescens var. acuta Kudo and rosmarinic acid on allergic inflammatory reactions. Exp. Biol. Med. 2011, 236, 99–106. [Google Scholar] [CrossRef]
- Lee, J.; Jung, E.; Koh, J.; Kim, Y.S.; Park, D. Effect of rosmarinic acid on atopic dermatitis. J. Dermatol. 2008, 35, 768–771. [Google Scholar] [CrossRef]
- Inoue, K.; Takano, H.; Shiga, A.; Fujita, Y.; Makino, H.; Yanagisawa, R.; Ichinose, T.; Kato, Y.; Yamada, T.; Yoshikawa, T. Effects of volatile constituents of a rosemary extract on allergic airway inflammation related to house dust mite allergen in mice. Int. J. Mol. Med. 2005, 16, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Osakabe, N.; Takano, H.; Sanbongi, C.; Yasuda, A.; Yanagisawa, R.; Inoue, K.; Yoshikawa, T. Anti-inflammatory and anti-allergic effect of rosmarinic acid (RA); inhibition of seasonal allergic rhinoconjunctivitis (SAR) and its mechanism. BioFactors 2004, 21, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Sanbongi, C.; Takano, H.; Osakabe, N.; Sasa, N.; Natsume, M.; Yanagisawa, R.; Inoue, K.I.; Sadakane, K.; Ichinose, T.; Yoshikawa, T. Rosmarinic acid in Perilla extract inhibits allergic inflammation induced by mite allergen, in a mouse model. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2004, 34, 971–977. [Google Scholar] [CrossRef] [PubMed]
- Takano, H.; Osakabe, N.; Sanbongi, C.; Yanagisawa, R.; Inoue, K.; Yasuda, A.; Natsume, M.; Baba, S.; Ichiishi, E.; Yoshikawa, T. Extract of Perilla frutescens enriched for rosmarinic acid, a polyphenolic phytochemical, inhibits seasonal allergic rhinoconjunctivitis in humans. Exp. Biol. Med. 2004, 229, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Ghasemzadeh Rahbardar, M.; Amin, B.; Mehri, S.; Mirnajafi-Zadeh, S.J.; Hosseinzadeh, H. Anti-inflammatory effects of ethanolic extract of Rosmarinus officinalis L. and rosmarinic acid in a rat model of neuropathic pain. Biomed. Pharmacother. 2017, 86, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Hu, C.; Wu, L.; Xu, L.; Jiang, W. Rosmarinic acid inhibits inflammation and angiogenesis of hepatocellular carcinoma by suppression of NF-kappaB signaling in H22 tumor-bearing mice. J. Pharmacol. Sci. 2016, 132, 131–137. [Google Scholar] [CrossRef] [PubMed]
- So, Y.; Lee, S.Y.; Han, A.R.; Kim, J.B.; Jeong, H.G.; Jin, C.H. Rosmarinic Acid Methyl Ester Inhibits LPS-Induced NO Production via Suppression of MyD88-Dependent and -Independent Pathways and Induction of HO-1 in RAW 264.7 Cells. Molecules 2016, 21, 1083. [Google Scholar] [CrossRef]
- Liang, Z.; Xu, Y.; Wen, X.; Nie, H.; Hu, T.; Yang, X.; Chu, X.; Yang, J.; Deng, X.; He, J. Rosmarinic Acid Attenuates Airway Inflammation and Hyperresponsiveness in a Murine Model of Asthma. Molecules 2016, 21, 769. [Google Scholar] [CrossRef]
- Rocha, J.; Eduardo-Figueira, M.; Barateiro, A.; Fernandes, A.; Brites, D.; Bronze, R.; Duarte, C.M.; Serra, A.T.; Pinto, R.; Freitas, M.; et al. Anti-inflammatory effect of rosmarinic acid and an extract of Rosmarinus officinalis in rat models of local and systemic inflammation. Basic Clin. Pharmacol. Toxicol. 2015, 116, 398–413. [Google Scholar] [CrossRef]
- Boonyarikpunchai, W.; Sukrong, S.; Towiwat, P. Antinociceptive and anti-inflammatory effects of rosmarinic acid isolated from Thunbergia laurifolia Lindl. Pharmacol. Biochem. Behav. 2014, 124, 67–73. [Google Scholar] [CrossRef]
- Usha, T.; Middha, S.K.; Bhattacharya, M.; Lokesh, P.; Goyal, A.K. Rosmarinic Acid, a New Polyphenol from Baccaurea ramiflora Lour. Leaf: A Probable Compound for Its Anti-Inflammatory Activity. Antioxidants 2014, 3, 830–842. [Google Scholar] [CrossRef] [PubMed]
- Lucarini, R.; Bernardes, W.A.; Ferreira, D.S.; Tozatti, M.G.; Furtado, R.; Bastos, J.K.; Pauletti, P.M.; Januario, A.H.; Silva, M.L.; Cunha, W.R. In vivo analgesic and anti-inflammatory activities of Rosmarinus officinalis aqueous extracts, rosmarinic acid and its acetyl ester derivative. Pharm. Biol. 2013, 51, 1087–1090. [Google Scholar] [CrossRef] [PubMed]
- Gautam, R.K.; Gupta, G.; Sharma, S.; Hatware, K.; Patil, K.; Sharma, K.; Goyal, S.; Chellappan, D.K.; Dua, K. Rosmarinic acid attenuates inflammation in experimentally induced arthritis in Wistar rats, using Freund’s complete adjuvant. Int. J. Rheum. Dis. 2019, 22, 1247–1254. [Google Scholar] [CrossRef] [PubMed]
- Luan, H.; Kan, Z.; Xu, Y.; Lv, C.; Jiang, W. Rosmarinic acid protects against experimental diabetes with cerebral ischemia: Relation to inflammation response. J. Neuroinflammation 2013, 10, 28. [Google Scholar] [CrossRef] [PubMed]
- Pearson, W.; Fletcher, R.S.; Kott, L.S. Oral rosmarinic acid-enhanced Mentha spicata modulates synovial fluid biomarkers of inflammation in horses challenged with intra-articular LPS. J. Vet. Pharmacol. Ther. 2012, 35, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.; Hauck, C.; Yum, M.Y.; Rizshsky, L.; Widrlechner, M.P.; McCoy, J.A.; Murphy, P.A.; Dixon, P.M.; Nikolau, B.J.; Birt, D.F. Rosmarinic acid in Prunella vulgaris ethanol extract inhibits lipopolysaccharide-induced prostaglandin E2 and nitric oxide in RAW 264.7 mouse macrophages. J. Agric. Food Chem. 2009, 57, 10579–10589. [Google Scholar] [CrossRef] [PubMed]
- Abdelhalim, A.; Karim, N.; Chebib, M.; Aburjai, T.; Khan, I.; Johnston, G.A.; Hanrahan, J. Antidepressant, Anxiolytic and Antinociceptive Activities of Constituents from Rosmarinus officinalis. J. Pharm. Pharm. Sci. 2015, 18, 448–459. [Google Scholar] [CrossRef] [PubMed]
- Du, T.; Li, L.; Song, N.; Xie, J.; Jiang, H. Rosmarinic acid antagonized 1-methyl-4-phenylpyridinium (MPP+)-induced neurotoxicity in MES23.5 dopaminergic cells. Int. J. Toxicol. 2010, 29, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Demirezer, L.O.; Gurbuz, P.; Kelicen Ugur, E.P.; Bodur, M.; Ozenver, N.; Uz, A.; Guvenalp, Z. Molecular docking and ex vivo and in vitro anticholinesterase activity studies of Salvia sp. and highlighted rosmarinic acid. Turk. J. Med Sci. 2015, 45, 1141–1148. [Google Scholar] [CrossRef] [PubMed]


| Bioactive Effects | Mechanisms | References |
|---|---|---|
| Anticancer | Prevent tumor formation development, reduce lipid peroxidation byproducts and proapoptotic proteins expression | [18] |
| Cause cell cycle arrest and stimulate MMP dysfunction-activated PARP-cleavage Block p65 translocation from cytosol to the nucleus | [20] | |
| Inhibit HL-60 promyelocytic leukemia cells’ growth and development Induce apoptosis | [23,24] | |
| Inhibit transcription factor HIF-1α expression | [21] | |
| Promote Nrf2 translocation from cytoplasm to the nucleus Increase MRP2 activity efflux | [19] | |
| Stop tumor formation and proliferation Reduce TNF-α, COX-2, IL-6 levels and modulates p65 expression | [25] | |
| Modulate histone deacetylases expression | [31] | |
| Antidiabetic | Increase key genes expression involved in mitochondrial biogenesis like PGC-1α, SIRT-1, and TFAM via AMPK activation Decrease serine IRS-1phosphorylation and enhance GLUT4 translocation | [37] |
| Increase GLUT4 expression and decrease PEPCK expression | [38] | |
| Enhance antioxidant defense system | [39] | |
| Reduce blood glucose, advanced glycation end-products, HbA1c, IL,1β, TNFα, IL6, p-JNK, P38 MAPK, and NF-kB Reduce FFA, triglycerides, serum cholesterol, AOPPs, lipid peroxides, and protein carbonyls levels | [41] | |
| Preserve normal insulin secretion Attenuate pro-inflammatory T helper 2 and T regulatory cells | [42] | |
| Increase the population of diabetes-resistant bacteria and decrease the number of diabetes-sensitive bacteria | [12] | |
| Antimicrobial | Exhibit antibacterial activity against S. aureus Suppress MSCRAMM’s protein expression in S. aureus | [46] |
| Exert antimicrobial activity against Enterobacteriaceae, lactic acid bacteria, Pseudomonas spp., psychotropic, yeast, and mold | [50] | |
| Inhibit S. carnosus LTH1502 and E. coli K-12 LTH4263 growth | [48] | |
| Cardioprotective | Inhibit H/R-induced cardiomyocyte apoptosis and down-regulate the expression of cleaved caspase of p-AKt | [54] |
| Improve insulin sensitivity, reduce lipid levels and p22phox subunit of nicotinamide adenine dinucleotide phosphate reduced oxidase expression | [57] | |
| Inhibit PMA, TNF-α, IL-induced EPCR shedding by TACE expression suppression Reduce ERK1/2, PMA-stimulated p38 and JNK phosphorylation | [55,56] | |
| Oxidative stress | Enhance cognitive function Reduce nitric oxide and MDA levels | [67] |
| Inhibit cellular lipid peroxidation and decrease H₂O₂-induced COX-2 expression | [67] | |
| Enhance defense system of endogenous antioxidant Decrease 4-HNE expression | [73,74] | |
| Down-regulate NF-kB | [69] | |
| Increase CAT, HO-1, SOD activity and expression Reduce factor Nrf2 transcription | [70] | |
| Inhibit liver fibrosis progression and activation | [85,86] | |
| Prevent α-SMA expression and TGF-β1 | [84] | |
| Antidepressant | Restore hippocampal BDNF and pERK1/2 protein expression | [94] |
| Inhibit monoamine oxidase and monoamine transporters | [138] | |
| Up-regulate PC and TH | [95] | |
| Nephroprotective | Decrease serum levels of blood urea nitrogen and creatinine Decrease myeloperoxidase and MDA levels | [99] |
| Repress TNF-α and NF-κB expression, demonstrating inhibition of inflammation Reduce p53, phosphorylated p53, and active caspase-3-expression | [100] | |
| Anti-aging | Reduce protein carbonyls in the hippocampus | [106] |
| Attenuate disruption of LDH, intercellular ROS, and mitochondrial membrane potential | [113] | |
| Improves oxidative stress parameters and mitochondrial respiratory chain activity | [108] | |
| Inhibit phosphorylated p38 MAPK | [110,111] | |
| Exert antioxidant effects against 6-hydroxydopamine facilitate neurotoxicity Restore activity of complex 1 of mitochondrial respiratory chain | [139,140] | |
| Prevent amyloid peptide aggregation | [68,109] | |
| Anti-allergy | Decrease eosinophils number | [118] |
| Inhibit IgE, protein levels and mRNA expressions of IL-6, IL-1β, and TNF-α and reduce histamine levels | [120,121] | |
| Suppress IL-4 and INF production Inhibit skin lesions development and ears thickness | [119] | |
| Anti-inflammatory | Inhibit Th2 cytokines, ameliorate AHR Reduce total IgE and Ova-specific IgE concentrations Reduce Ym2, CCR3, CCL11, AMCase, and E-selectin mRNA expression | [129] |
| Inhibit LPS-induced NO production Repress LPS-induced pro-inflammatory cytokines expression including INF-β, monocyte chemo attractant protein-1, iNOS, IL-1β, IL-6, IL-10, and activation of NF-κB | [92,128] | |
| Decrease serum transaminases (ALT and AST) and LDH levels Inhibit NF-κB | [130] | |
| Reduce oxidative stress levels and down-regulate NF-κB | [69] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nadeem, M.; Imran, M.; Aslam Gondal, T.; Imran, A.; Shahbaz, M.; Muhammad Amir, R.; Wasim Sajid, M.; Batool Qaisrani, T.; Atif, M.; Hussain, G.; et al. Therapeutic Potential of Rosmarinic Acid: A Comprehensive Review. Appl. Sci. 2019, 9, 3139. https://doi.org/10.3390/app9153139
Nadeem M, Imran M, Aslam Gondal T, Imran A, Shahbaz M, Muhammad Amir R, Wasim Sajid M, Batool Qaisrani T, Atif M, Hussain G, et al. Therapeutic Potential of Rosmarinic Acid: A Comprehensive Review. Applied Sciences. 2019; 9(15):3139. https://doi.org/10.3390/app9153139
Chicago/Turabian StyleNadeem, Muhammad, Muhammad Imran, Tanweer Aslam Gondal, Ali Imran, Muhammad Shahbaz, Rai Muhammad Amir, Muhammad Wasim Sajid, Tahira Batool Qaisrani, Muhammad Atif, Ghulam Hussain, and et al. 2019. "Therapeutic Potential of Rosmarinic Acid: A Comprehensive Review" Applied Sciences 9, no. 15: 3139. https://doi.org/10.3390/app9153139
APA StyleNadeem, M., Imran, M., Aslam Gondal, T., Imran, A., Shahbaz, M., Muhammad Amir, R., Wasim Sajid, M., Batool Qaisrani, T., Atif, M., Hussain, G., Salehi, B., Adrian Ostrander, E., Martorell, M., Sharifi-Rad, J., C. Cho, W., & Martins, N. (2019). Therapeutic Potential of Rosmarinic Acid: A Comprehensive Review. Applied Sciences, 9(15), 3139. https://doi.org/10.3390/app9153139