Sweet Pepper (Capsicum annuum L.) Fruits Contain an Atypical Peroxisomal Catalase That Is Modulated by Reactive Oxygen and Nitrogen Species
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Preparation of Crude Extracts for Enzyme Activity
2.3. Catalase Activity Assays: Spectrophotometry and Non-Denaturing Electrophoresis—Effect of Different Modulators
2.4. Electrophoretic Methods
2.5. Immunoblot Analysis
2.6. Determination of Native Molecular Weight of Catalase
2.7. Bovine Catalase as a Model to Analyze the S-Nitrosation Process
2.8. Statistical Analysis
3. Results
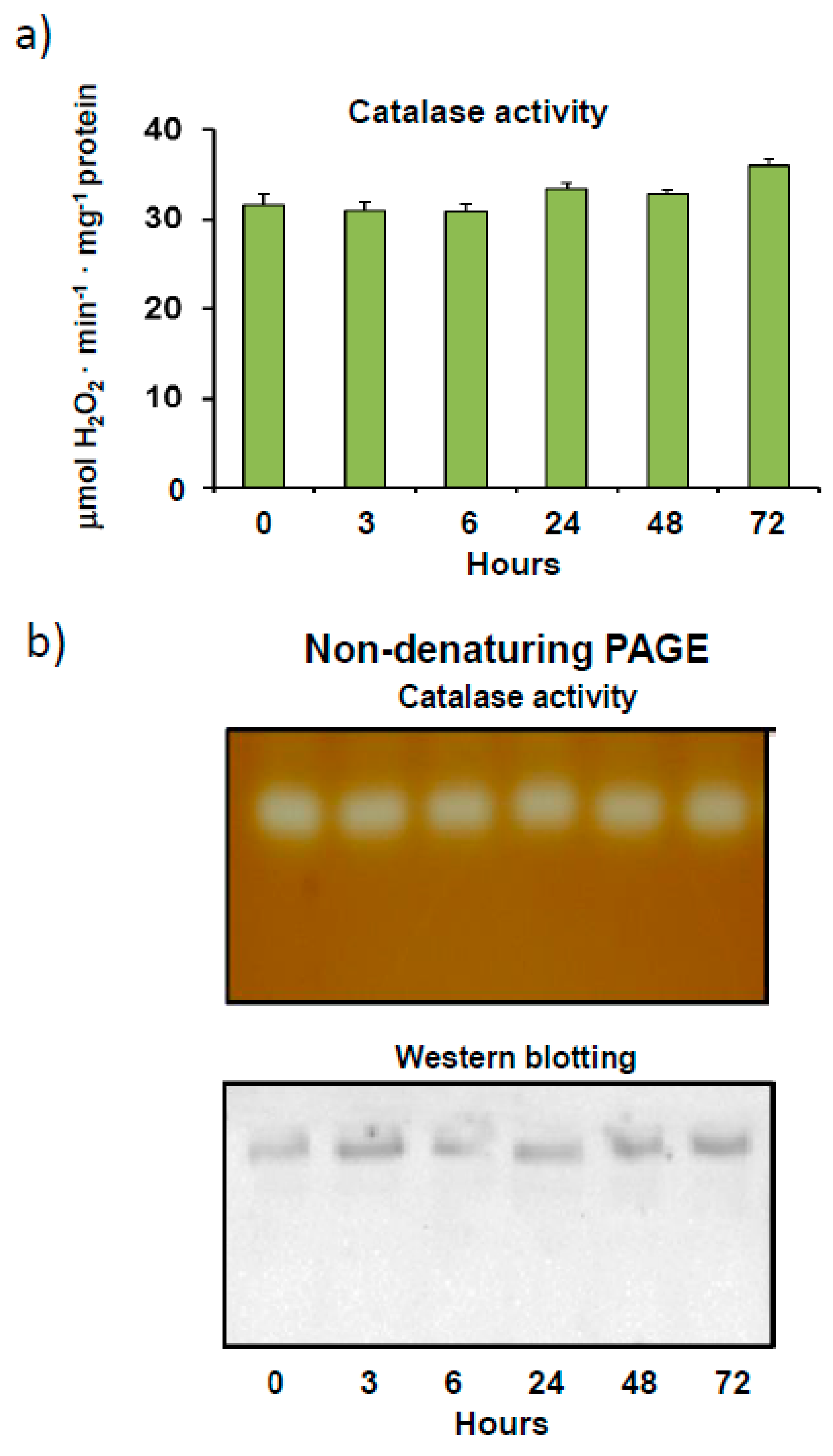
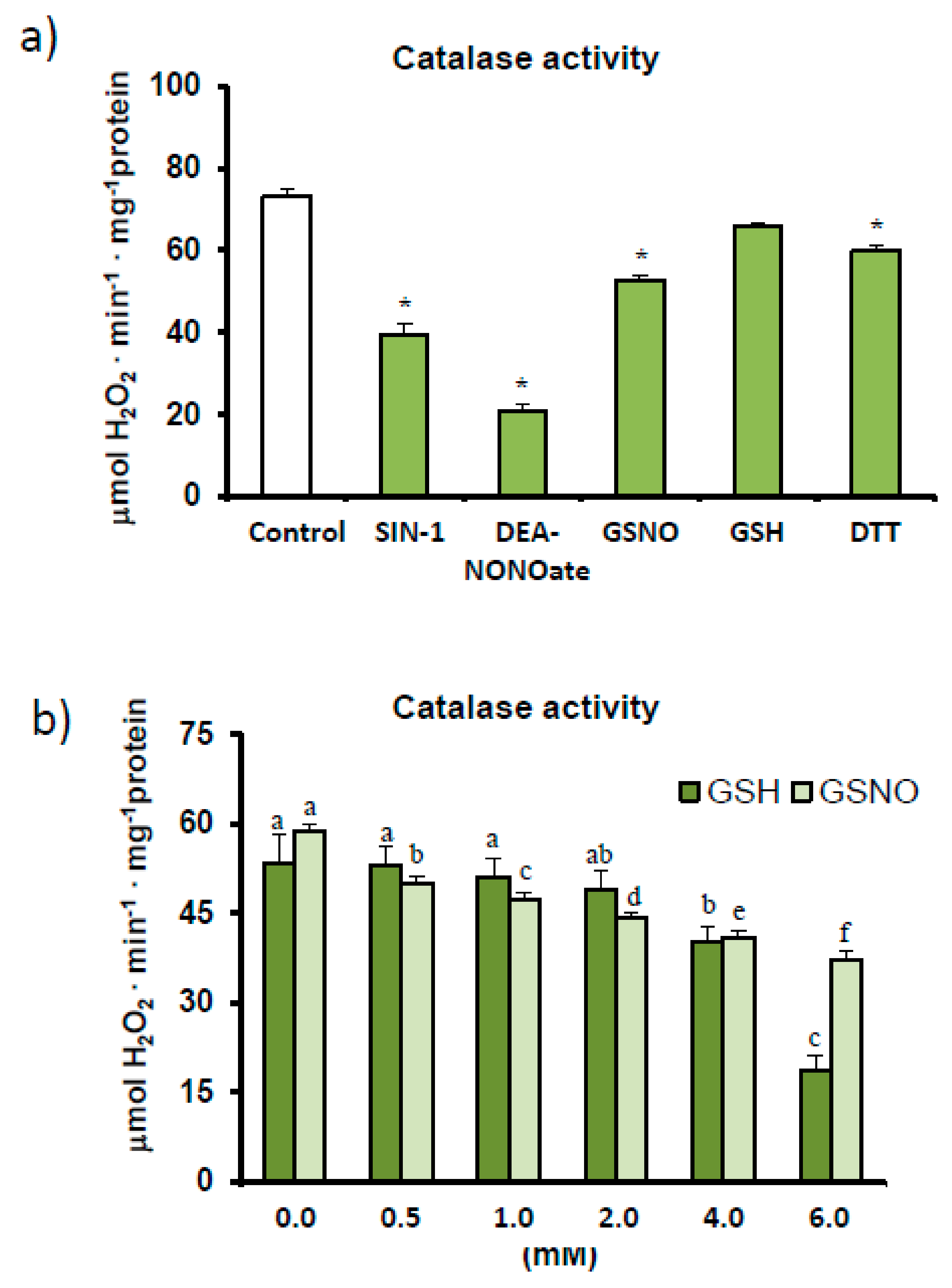
3.1. Pepper Fruit Catalase Activity under Different Assay Conditions
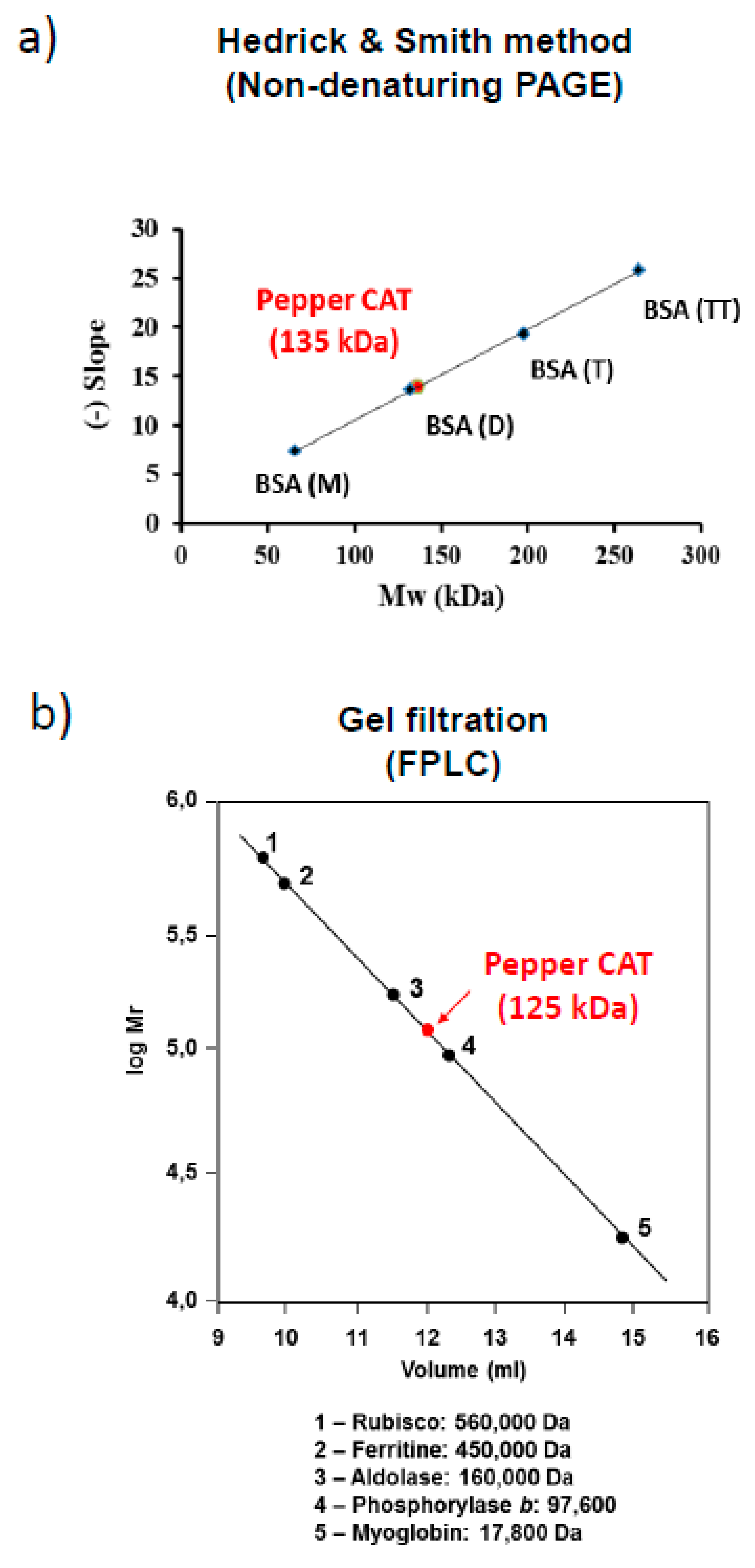
3.2. Quaternary Structure of Pepper Fruit Catalase
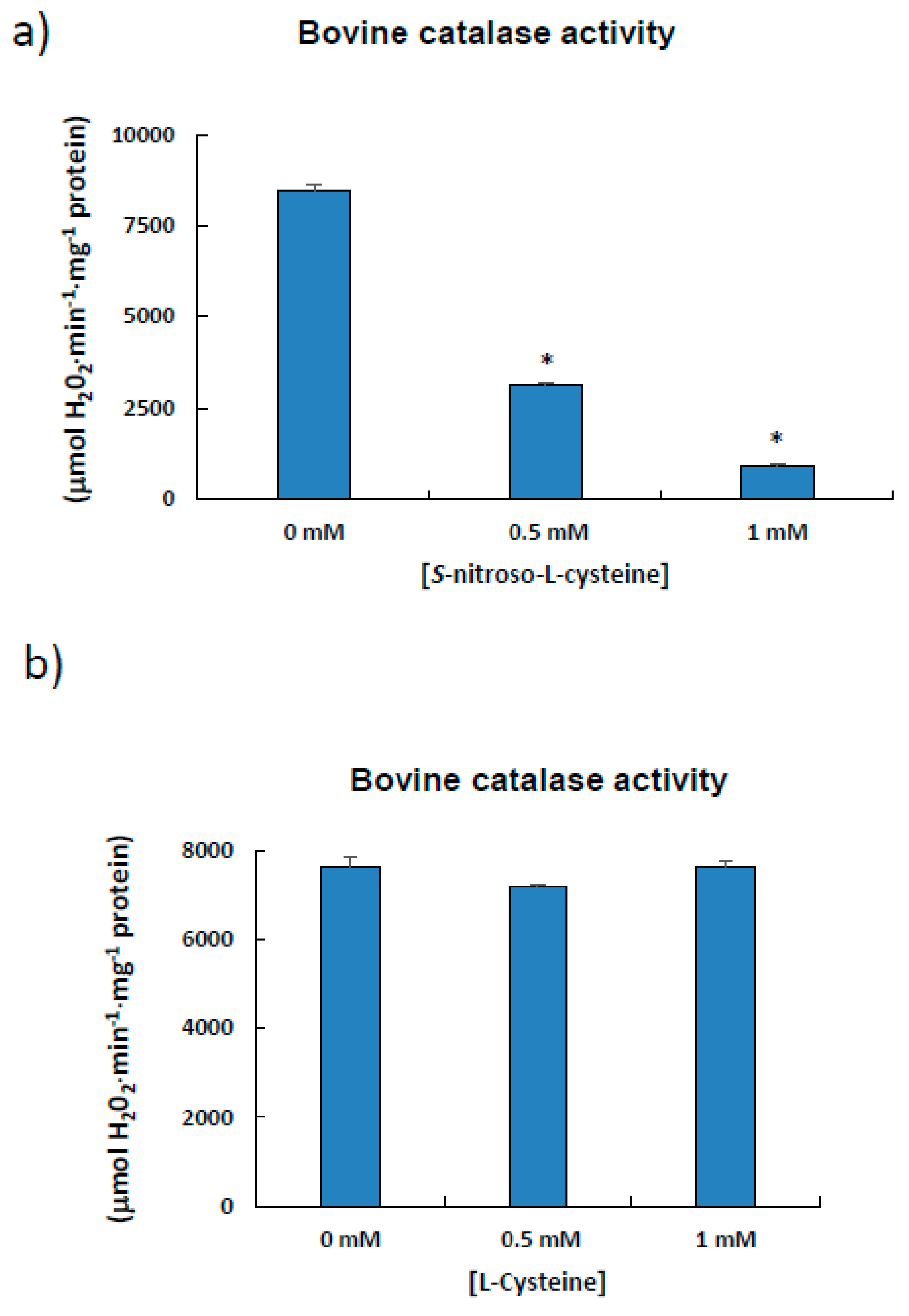
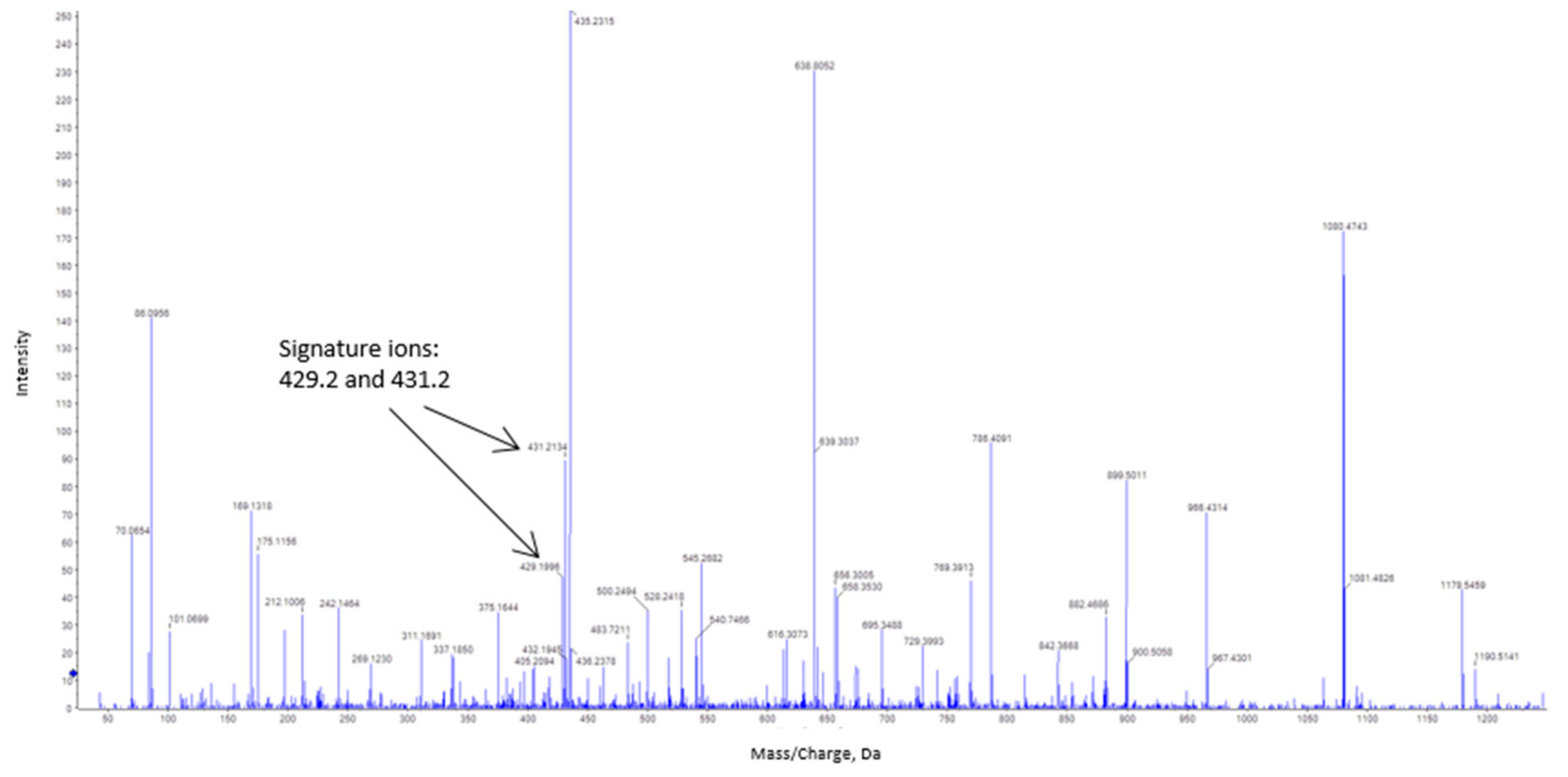
3.3. Estimation of the S-Nitrosated Cysteine from the Bovine Catalase Used a Model
4. Discussion
4.1. Finely Tuned Modulation of Catalase Activity during Sweet Pepper Fruit Ripening
4.2. Sweet Pepper Fruit Contains an Atypical Catalase Enzyme
4.3. Bovine Catalase (527 aa) is S-Nitrosated at Cys377
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Corpas, F.J.; Palma, J.M.; Sandalio, L.M.; López-Huertas, E.; Romero-Puertas, M.C.; Barroso, J.B.; Del Río, L.A. Purification of Catalase from Pea Leaf Peroxisomes: Identification of Five Different Isoforms. Free. Radic. Res. 1999, 31, 235–241. [Google Scholar] [CrossRef]
- Mhamdi, A.; Queval, G.; Chaouch, S.; Vanderauwera, S.; Van Breusegem, F.; Noctor, G. Catalase function in plants: A focus on Arabidopsis mutants as stress-mimic models. J. Exp. Bot. 2010, 61, 4197–4220. [Google Scholar] [CrossRef] [PubMed]
- Mhamdi, A.; Noctor, G.; Baker, A. Plant catalases: Peroxisomal redox guardians. Arch. Biochem. Biophys. 2012, 525, 181–194. [Google Scholar] [CrossRef] [PubMed]
- Palma, J.M.; Gupta, D.K.; Corpas, F.J. Metalloenzymes Involved in the Metabolism of Reactive Oxygen Species and Heavy Metal Stress. In Heavy Metal Stress in Plants; Springer Science and Business Media LLC: Berlin, Germany, 2013; pp. 1–17. [Google Scholar]
- Su, T.; Wang, P.; Li, H.; Zhao, Y.; Lü, Y.; Dai, P.; Ren, T.; Wang, X.; Li, X.; Shao, Q.; et al. The Arabidopsis catalase triple mutant reveals important roles of catalases and peroxisome-derived signaling in plant development. J. Integr. Plant Boil. 2018, 60, 591–607. [Google Scholar] [CrossRef] [PubMed]
- Anjum, N.A.; Sharma, P.; Gill, S.S.; Hasanuzzaman, M.; Khan, E.A.; Kachhap, K.; Mohamed, A.A.; Thangavel, P.; Devi, G.D.; Vasudhevan, P.; et al. Catalase and ascorbate peroxidase-representative H2O2-detoxifying heme enzymes in plants. Environ. Sci. Pollut. Res. 2016, 23, 19002–19029. [Google Scholar] [CrossRef] [PubMed]
- Goyal, M.M.; Basak, A. Human catalase: Looking for complete indentity. Prot. Cell 2010, 1, 888–897. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine; Oxford University Press (OUP): Oxford, UK,, 2015. [Google Scholar]
- Mullen, R.T.; Gifford, D.J. Purification and Characterization of Catalase from Loblolly Pine (Pinus taeda L.) Megagametophytes. Plant Physiol. 1993, 103, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Dinçler, A.; Aydemir, T. Purification and Characterization of Catalase from Chard (Beta vulgaris var. cicla). J. Enzym. Inhib. 2001, 16, 165–175. [Google Scholar] [CrossRef]
- Bagnoli, F.; Danti, S.; Magherini, V.; Cozza, R.; Innocenti, A.M.; Racchi, M.L. Molecular cloning, characterisation and expression of two catalase genes from peach. Funct. Plant Biol. 2002, 31, 349–357. [Google Scholar] [CrossRef]
- Teng, X.-L.; Chen, N.; Xiao, X.-G. Identification of a Catalase-Phenol Oxidase in Betalain Biosynthesis in Red Amaranth (Amaranthus cruentus). Front. Plant Sci. 2016, 6, 121. [Google Scholar] [CrossRef]
- Niewiadomska, E.; Miszalski, Z. Partial characterization and expression of leaf catalase in the CAM-inducible halophyte Mesembryanthemum crystallinum L. Plant Physiol. Biochem. 2008, 46, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Nie, Q.; Gao, G.-L.; Fan, Q.-J.; Qiao, G.; Wen, X.-P.; Liu, T.; Peng, Z.-J.; Cai, Y.-Q. Isolation and characterization of a catalase gene “HuCAT3” from pitaya (Hylocereus undatus) and its expression under abiotic stress. Gene 2015, 563, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Cheng, Y.; Chen, D.; Liu, D.; Hu, M.; Dong, J.; Zhang, X.; Song, L.; Shen, F. The Catalase Gene Family in Cotton: Genome-Wide Characterization and Bioinformatics Analysis. Cells 2019, 8, 86. [Google Scholar] [CrossRef] [PubMed]
- Lopez, A.P.; Gochicoa, M.T.N.; Franco, A.R. Activities of antioxidant enzymes during strawberry fruit development and ripening. Boil. Plant. 2010, 54, 349–352. [Google Scholar] [CrossRef]
- Nilo, R.; Saffie, C.; Lilley, K.; Baeza-Yates, R.; Cambiazo, V.; Campos-Vargas, R.; González, M.; Meisel, L.A.; Retamales, J.; Silva, H.; et al. Proteomic analysis of peach fruit mesocarp softening and chilling injury using difference gel electrophoresis (DIGE). BMC Genom. 2010, 11, 43. [Google Scholar] [CrossRef] [PubMed]
- Chaki, M.; Álvarez de Morales, P.; Ruiz, C.; Begara-Morales, J.C.; Barroso, J.B.; Corpas, F.J.; Palma, J.M. Ripening of pepper (Capsicum annuum) fruit is characterized by an enhancement of protein tyrosine nitration. Ann. Bot. 2015, 116, 637–647. [Google Scholar] [CrossRef] [PubMed]
- Huan, C.; Jiang, L.; An, X.; Yu, M.; Xu, Y.; Ma, R.; Yu, Z. Potential role of reactive oxygen species and antioxidant genes in the regulation of peach fruit development and ripening. Plant Physiol. Biochem. 2016, 104, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Palma, J.M.; Sevilla, F.; Jiménez, A.; Del Río, L.A.; Corpas, F.J.; Álvarez de Morales, P.; Camejo, D.M. Physiology of pepper fruit and the metabolism of antioxidants: Chloroplasts, mitochondria and peroxisomes. Ann. Bot. 2015, 116, 627–636. [Google Scholar] [CrossRef]
- Palma, J.M.; De Morales, P.Á.; Luis, A.; Corpas, F.J. The Proteome of Fruit Peroxisomes: Sweet Pepper (Capsicum annuum L.) as a Model. Subcell. Biochem. 2018, 89, 323–341. [Google Scholar]
- Chelikani, P.; Fita, I.; Loewen, P.C. Diversity of structures and properties among catalases. Cell. Mol. Life Sci. 2004, 61, 192–208. [Google Scholar] [CrossRef]
- U.S. National Library of Medicine. Available online: https://www.ncbi.nlm.nih.gov/search/all/?term=catalase (accessed on 2 September 2019).
- Scandalios, J.G.; Guan, L.; Polidoros, A.N. Catalases in plants, gene structure, properties, regulation and expression. In Oxidative Stress and the Molecular Biology of Antioxidant Defences; Scandalios, J.G., Ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 1997; pp. 343–406. [Google Scholar]
- Iwamoto, M.; Maekawa, M.; Saito, A.; Higo, H.; Higo, K. Evolutionary relationship of plant catalase genes inferred from exon-intron structures: Isozyme divergence after the separation of monocots and dicots. Theor. Appl. Genet. 1998, 97, 9–19. [Google Scholar] [CrossRef]
- Frugoli, J.; Zhong, H.H.; Nuccio, M.L.; McCourt, P.; McPeek, M.A.; Thomas, T.L.; McClung, C.R. Catalase is encoded by a multigene family in Arabidopsis thaliana (L.) Heynh. Plant Physiol. 1996, 112, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Jia, W.; Zhang, J. AtMEK1 mediates stress-induced gene expression of CAT1 catalase by triggering H2O2 production in Arabidopsis. J. Exp. Bot. 2007, 58, 2969–2981. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.-Y.; Wang, P.-C.; Chen, J.; Song, C.-P. Comprehensive Functional Analysis of the Catalase Gene Family inArabidopsis thaliana. J. Integr. Plant Boil. 2008, 50, 1318–1326. [Google Scholar] [CrossRef]
- Hu, Y.-Q.; Liu, S.; Yuan, H.-M.; Li, J.; Yan, D.-W.; Zhang, J.-F.; Lu, Y.-T. Functional comparison of catalase genes in the elimination of photorespiratory H2O2 using promoter- and 3′-untranslated region exchange experiments in the Arabidopsis cat2 photorespiratory mutant. Plant Cell Environ. 2010, 33, 1656–1670. [Google Scholar] [CrossRef] [PubMed]
- Skadsen, R.W.; Schulze-Lefert, P.; Herbst, J.M. Molecular cloning, characterization and expression analysis of two catalase isozyme genes in barley. Plant Mol. Boil. 1995, 29, 1005–1014. [Google Scholar] [CrossRef] [PubMed]
- Schiefer, S.; Teifel, W.; Kindl, H. Plant microbody proteins. 1. Purification and characterization of catalase from leaves of Lens culinaris. Hoppe-Seyler’ Z. Phys. Chem. 1976, 357, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Correa-Aragunde, N.; Foresi, N.; LaMattina, L. Nitric oxide is a ubiquitous signal for maintaining redox balance in plant cells: Regulation of ascorbate peroxidase as a case study. J. Exp. Bot. 2015, 66, 2913–2921. [Google Scholar] [CrossRef] [PubMed]
- Begara-Morales, J.C.; Sánchez-Calvo, B.; Chaki, M.; Valderrama, R.; Mata-Pérez, C.; Padilla, M.N.; Corpas, F.J.; Barroso, J.B. Antioxidant Systems are Regulated by Nitric Oxide-Mediated Post-translational Modifications (NO-PTMs). Front. Plant Sci. 2016, 7, 3222. [Google Scholar] [CrossRef] [Green Version]
- Corpas, F.J.; Barroso, J.B.; González-Gordo, S.; Muñoz-Vargas, M.A.; Palma, J.M. Hydrogen sulfide: A novel component in Arabidopsis peroxisomes which triggers catalase inhibition. J. Integr. Plant Boil. 2019, 61, 871–883. [Google Scholar] [CrossRef] [PubMed]
- Mateos, R.M.; León, A.M.; Sandalio, L.M.; Gómez, M.; Luis, A.; Palma, J.M. Peroxisomes from pepper fruits (Capsicum annuum L): Purification, characterization and antioxidant activity. J. Plant Physiol. 2003, 160, 1507–1516. [Google Scholar] [CrossRef] [PubMed]
- Campolo, N.; Bartesaghi, S.; Radi, R. Metal-catalyzed protein tyrosine nitration in biological systems. Redox Rep. 2014, 19, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Aicardo, A.; Martinez, D.M.; Campolo, N.; Bartesaghi, S.; Radi, R. Biochemistry of Nitric Oxide and Peroxynitrite: Sources, Targets and Biological Implications. In Biochemistry of Oxidative Stress; Springer Science and Business Media LLC: Berlin, Germany, 2016; Volume 16, pp. 49–77. [Google Scholar]
- Rodríguez-Ruiz, M.; Mioto, P.; Palma, J.M.; Corpas, F.J. S-nitrosoglutathione reductase (GSNOR) activity is down-regulated during pepper (Capsicum annuum L.) fruit ripening. Nitric Oxide 2017, 68, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Klie, S.; Osorio, S.; Tohge, T.; Drincovich, M.F.; Fait, A.; Giovannoni, J.J.; Fernie, A.R.; Nikoloski, Z. Conserved changes in the dynamics of metabolic processes during fruit development and ripening across species. Plant Physiol. 2014, 164, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Palma, J.M.; Corpas, F.J.; Del Río, L.A. Proteomics as an approach to the understanding of the molecular physiology of fruit development and ripening. J. Proteom. 2011, 74, 1230–1243. [Google Scholar] [CrossRef]
- Barsan, C.; Zouine, M.; Maza, E.; Bian, W.; Egea, I.; Rossignol, M.; Bouyssie, D.; Pichereaux, C.; Purgatto, E.; Bouzayen, M.; et al. Proteomic Analysis of Chloroplast-to-Chromoplast Transition in Tomato Reveals Metabolic Shifts Coupled with Disrupted Thylakoid Biogenesis Machinery and Elevated Energy-Production Components. Plant Physiol. 2012, 160, 708–725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corpas, F.J.; Palma, J.M. Nitric oxide on/off in fruit ripening. Plant Boil. 2018, 20, 805–807. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, M.; Ren, L.; Li, A.; Chen, G.; Hu, Z. The SlFSR gene controls fruit shelf-life in tomato. J. Exp. Bot. 2018, 69, 2897–2909. [Google Scholar] [CrossRef] [Green Version]
- Chu-Puga, A.; González-Gordo, S.; Rodríguez-Ruiz, M.; Palma, J.M.; Corpas, F.J. NADPH oxidase (Rboh) activity is up regulated during sweet pepper (Capsicum annuum L.) fruit ripening. Antioxidants 2019, 8, 9. [Google Scholar] [CrossRef]
- Mateos, R.M.; Jiménez, A.; Román, P.; Romojaro, F.; Bacarizo, S.; Leterrier, M.; Gomez, M.; Sevilla, F.; Del Río, L.A.; Corpas, F.J.; et al. Antioxidant Systems from Pepper (Capsicum annuum L.): Involvement in the Response to Temperature Changes in Ripe Fruits. Int. J. Mol. Sci. 2013, 14, 9556–9580. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Clare, D.A.; Duong, M.N.; Darr, D.; Archibald, F.; Fridovich, I. Effects of molecular oxygen on detection of superoxide radical with nitroblue tetrazolium and on activity stains for catalase. Anal. Biochem. 1984, 140, 532–537. [Google Scholar] [CrossRef]
- Muñoz-Vargas, M.A.; González-Gordo, S.; Cañas, A.; López-Jaramillo, J.; Palma, J.M.; Corpas, F.J. Endogenous hydrogen sulfide (H2S) is up-regulated during sweet pepper (Capsicum annuum L.) fruit ripening. In vitro analysis shows that NADP-dependent isocitrate dehydrogenase (ICDH) activity is inhibited by H2S and NO. Nitric Oxide 2018, 81, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Palma, J.M.; Pastori, G.M.; Bueno, P.; Distefano, S.; Del Río, L.A. Purification and Properties of Cytosolic Copper, Zinc Superoxide Dismutase from Watermelon (Citrullus vulgarisSchrad.) Cotyledons. Free. Radic. Res. 1997, 26, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Heukeshoven, J.; Dernick, R. Simplified method for silver staining of proteins in polyacrylamide gels and the mechanism of silver staining. Electrophoresis 1985, 6, 103–112. [Google Scholar] [CrossRef]
- Corpas, F.J.; Barroso, J.B.; Sandalio, L.M.; Distefano, S.; Palma, J.M.; Lupiáñez, J.A.; Del Río, L.A. A dehydrogenase-mediated recycling system of NADPH peroxisomes. Biochem. J. 1998, 330, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Hedrick, J.L.; Smith, A.J. Size and charge isomer separation and estimation of molecular weights of proteins by disc gel electrophoresis. Arch. Biochem. Biophys. 1968, 126, 155–164. [Google Scholar] [CrossRef]
- Jaffrey, S.R.; Erdjument-Bromage, H.; Ferris, C.D.; Tempst, P.; Snyder, S.H. Protein S-nitrosylation: A physiological signal for neuronal nitric oxide. Nat. Cell Biol. 2001, 3, 193–197. [Google Scholar] [CrossRef] [PubMed]
- González-Gordo, S.; Bautista, R.; Claros, M.G.; Cañas, A.; Palma, J.M.; Corpas, F.J. Nitric oxide-dependent regulation of sweet pepper fruit ripening. J. Exp. Bot. 2019, 70, 4557–4570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corpas, F.J.; Barroso, J.B. Lead-induced stress, which triggers the production of nitric oxide (NO) and superoxide anion (O2 -) in Arabidopsis peroxisomes, affects catalase activity. Nitric Oxide 2017, 68, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Ruíz-Torres, C.; Feriche-Linares, R.; Rodríguez-Ruíz, M.; Palma, J.M.; Corpas, F.J. Arsenic-induced stress activates sulfur metabolism in different organs of garlic (Allium sativum L.) plants accompanied by a general decline of the NADPH-generating systems in roots. J. Plant Physiol. 2017, 211, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Alam, N.B.; Ghosh, A. Comprehensive analysis and transcript profiling of Arabidopsis thaliana and Oryza sativa catalase gene family suggests their specific roles in development and stress responses. Plant Physiol. Biochem. 2018, 123, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Corpas, F.J.; Alché, J.D.D.; Barroso, J.B. Current overview of S-nitrosoglutathione (GSNO) in higher plants. Front. Plant Sci. 2013, 4, 126. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Ruiz, M.; Mateos, R.M.; Codesido, V.; Corpas, F.J.; Palma, J.M. Characterization of the galactono-1,4-lactone dehydrogenase from pepper fruits and its modulation in the ascorbate biosynthesis. Role of nitric oxide. Redox Boil. 2017, 12, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Oberley, L.W. The inhibition of catalase by glutathione. Free. Radic. Boil. Med. 1989, 7, 595–602. [Google Scholar] [CrossRef]
- Clark, D.; Durner, J.; Navarre, D.A.; Klessig, D.F. Nitric Oxide Inhibition of Tobacco Catalase and Ascorbate Peroxidase. Mol. Plant-Microbe Interact. 2000, 13, 1380–1384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortega-Galisteo, A.P.; Rodríguez-Serrano, M.; Pazmiño, D.M.; Gupta, D.K.; Sandalio, L.M.; Romero-Puertas, M.C. S-Nitrosylated proteins in pea (Pisum sativum L.) leaf peroxisomes: Changes under abiotic stress. J. Exp. Bot. 2012, 63, 2089–2103. [Google Scholar] [CrossRef]
- Begara-Morales, J.C.; Chaki, M.; Sánchez-Calvo, B.; Mata-Pérez, C.; Leterrier, M.; Palma, J.M.; Barroso, J.B.; Corpas, F.J. Protein tyrosine nitration in pea roots during development and senescence. J. Exp. Bot. 2013, 64, 1121–1134. [Google Scholar] [CrossRef] [Green Version]
- Arora, D.; Jain, P.; Singh, N.; Kaur, H.; Bhatla, S.C. Mechanisms of nitric oxide crosstalk with reactive oxygen species scavenging enzymes during abiotic stress tolerance in plants. Free. Radic. Res. 2016, 50, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Silveira, N.M.; Marcos, F.C.C.; Frungillo, L.; Moura, B.B.; Seabra, A.B.; Salgado, I.; Machado, E.C.; Hancock, J.T.; Ribeiro, R.V. S-nitrosoglutathione spraying improves stomatal conductance, Rubisco activity and antioxidant defense in both leaves and roots of sugarcane plants under water deficit. Physiol. Plant. 2017, 160, 383–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cassia, R.; Nocioni, M.; Correa-Aragunde, N.; LaMattina, L. Climate Change and the Impact of Greenhouse Gasses: CO2 and NO, Friends and Foes of Plant Oxidative Stress. Front. Plant Sci. 2018, 9, 273. [Google Scholar] [CrossRef] [PubMed]
- Corpas, F.J.; Barroso, J.B.; Palma, J.M.; Rodriguez-Ruiz, M. Plant peroxisomes: A nitro-oxidative cocktail. Redox Boil. 2017, 11, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Pedrajas, J.R.; Peinado, J.; LopezBarea, J. Oxidative stress in fish exposed to model xenobiotics. Oxidatively modified forms of Cu, Zn-superoxide dismutase as potential biomarkers. Chem.-Biol. Interact. 1995, 98, 267–282. [Google Scholar] [CrossRef]
- Barroso, J.B.; Peragón, J.; Contreras-Jurado, C.; García-Salguero, L.; Corpas, F.J.; Esteban, F.J.; Peinado, M.A.; De La Higuera, M.; Lupiáñez, J.A. Impact of starvation-refeeding on kinetics and protein expression of trout liver NADPH-production systems. Am. J. Physiol. Integr. Comp. Physiol. 1998, 274, R1578–R1587. [Google Scholar] [CrossRef]
- Poly, W.J. Nongenetic variation, genetic-environmental interactions and altered gene expression. III. Prosttranslational modifications. Comp. Biochem. Phys. Part A Phys. 1997, 118, 551–572. [Google Scholar] [CrossRef]
- Anand, P.; Kwak, Y.; Simha, R.; Donaldson, R.P. Hydrogen peroxide induced oxidation of peroxisomal malate synthase and catalase. Arch. Biochem. Biophys. 2009, 491, 25–31. [Google Scholar] [CrossRef]
- Palma, J.M.; Gómez, M.; Yáñez, J.; Del Río, L.A.; Beale, S.I.; Chen, N.C. Increased Levels of Peroxisomal Active Oxygen-Related Enzymes in Copper-Tolerant Pea Plants. Plant Physiol. 1987, 85, 570–574. [Google Scholar] [CrossRef] [Green Version]
- Contento, A.L.; Bassham, D.C. Increase in catalase-3 activity as a response to use of alternative catabolic substrates during sucrose starvation. Plant Physiol. Biochem. 2010, 48, 232–238. [Google Scholar] [CrossRef]
- Pena, L.B.; Azpilicueta, C.E.; Gallego, S.M. Sunflower cotyledons cope with copper stress by inducing catalase subunits less sensitive to oxidation. J. Trace Elem. Med. Boil. 2011, 25, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Guerrero, Y.; Martínez-González, L.; Dell’Amico, J.; Nunez, M.; Pieters, A.J. Reversion of deleterious effects of salt stress by activation of ROS detoxifying enzymes via foliar application of 24-epibrassinolide in rice seedlings. Theor. Exp. Plant Physiol. 2015, 27, 31–40. [Google Scholar] [CrossRef]
- Kerchev, P.; Muhlenbock, P.; Denecker, J.; Morreel, K.; Hoeberichts, F.A.; Van Der Kelen, K.; Vandorpe, M.; Nguyen, L.; Audenaert, D.; Van Breusegem, F. Activation of auxin signalling counteracts photorespiratory H2O2-dependent cell death. Plant. Cell Environ. 2015, 38, 253–265. [Google Scholar] [CrossRef] [PubMed]
- López-Huertas, E.; Del Río, L.A. Characterization of Antioxidant Enzymes and Peroxisomes of Olive (Olea europaea L.) Fruits. J. Plant Phys. 2014, 171, 1463–1471. [Google Scholar] [CrossRef] [PubMed]
- Kunce, C.M.; Trelease, R.N. Heterogeneity of Catalase in Maturing and Germinated Cotton Seeds. Plant Physiol. 1986, 81, 1134–1139. [Google Scholar] [CrossRef]
- Eising, R.; Trelease, R.N.; Ni, W.T. Biogenesis of catalase in glyoxysomes and leaf-type peroxisomes of sunflower cotyledons. Arch. Biochem. Biophys. 1990, 278, 258–264. [Google Scholar] [CrossRef]
- Ni, W.; Trelease, R.N.; Eising, R. Two temporally synthesized charge subunits interact to form the five isoforms of cottonseed (Gossypium hirsutum) catalase. Biochem. J. 1990, 269, 233–238. [Google Scholar] [CrossRef]
- Ni, W.; Trelease, R.N. Two genes encode the two subunits of cottonseed catalase. Arch. Biochem. Biophys. 1991, 289, 237–243. [Google Scholar] [CrossRef]
- Corpas, F.J. What is the role of hydrogen peroxide in plant peroxisomes? Plant Boil. 2015, 17, 1099–1103. [Google Scholar] [CrossRef]
- Kumar, S.; Yadav, P.; Jain, V.; Malhotra, S.P. Isozymes of antioxidative enzymes during ripening and storage of ber (Ziziphus mauritiana Lamk.). J. Food Sci. Technol. 2014, 51, 329–334. [Google Scholar] [CrossRef]
- Li, Q.; Jia, Y.; Burris, W.R.; Bridges, P.J.; Matthews, J.C. Forms of selenium in vitamin-mineral mixes differentially affect the expression of genes responsible for prolactin, ACTH, and alpha-MSH synthesis and mitochondrial dysfunction in pituitaries of steers grazing endophyte-infected tall fescue. J. Anim. Sci. 2019, 97, 631–643. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Imaishi, H.; Ohkawa, H. Molecular Cloning and Sequence Analysis of Catalase cDNA from Green Pepper Seedlings Elicited with Arachidonic Acid. Biosci. Biotechnol. Biochem. 1998, 62, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Noctor, G. Redox sensing and signalling associated with reactive oxygenin chloroplasts, peroxisomes and mitochondria. Physiol. Plant. 2003, 119, 355–364. [Google Scholar] [CrossRef]
- Del Río, L.A.; López-Huertas, E. ROS generation in peroxisomes and its role in cell signaling. Plant Cell Physiol. 2016, 57, 1364–1376. [Google Scholar] [CrossRef] [PubMed]
- Martí, M.C.; Camejo, D.; Vallejo, F.; Romojaro, F.; Bacarizo, S.; Palma, J.M.; Sevilla, F.; Jiménez, A. Influence of Fruit Ripening Stage and Harvest Period on the Antioxidant Content of Sweet Pepper Cultivars. Plant Foods Hum. Nutr. 2011, 66, 416–423. [Google Scholar] [CrossRef]
- Glorieux, C.; Calderon, P.B. Catalase, a remarkable enzyme: Targeting the oldest antioxidant enzyme to find a new cancer treatment approach. Boil. Chem. 2017, 398, 1095–1108. [Google Scholar] [CrossRef] [PubMed]
- Fransen, M.; Lismont, C. Redox Signaling from and to Peroxisomes: Progress, Challenges, and Prospects. Antioxid. Redox Signal. 2019, 30, 95–112. [Google Scholar] [CrossRef]
- Shin, S.-K.; Cho, H.-W.; Song, S.-E.; Song, D.-K. Catalase and nonalcoholic fatty liver disease. Pflüg. Arch.-Eur. J. Phys. 2018, 470, 1721–1737. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Ruiz, M.; González-Gordo, S.; Cañas, A.; Campos, M.J.; Paradela, A.; Corpas, F.J.; Palma, J.M. Sweet Pepper (Capsicum annuum L.) Fruits Contain an Atypical Peroxisomal Catalase That Is Modulated by Reactive Oxygen and Nitrogen Species. Antioxidants 2019, 8, 374. https://doi.org/10.3390/antiox8090374
Rodríguez-Ruiz M, González-Gordo S, Cañas A, Campos MJ, Paradela A, Corpas FJ, Palma JM. Sweet Pepper (Capsicum annuum L.) Fruits Contain an Atypical Peroxisomal Catalase That Is Modulated by Reactive Oxygen and Nitrogen Species. Antioxidants. 2019; 8(9):374. https://doi.org/10.3390/antiox8090374
Chicago/Turabian StyleRodríguez-Ruiz, Marta, Salvador González-Gordo, Amanda Cañas, María Jesús Campos, Alberto Paradela, Francisco J. Corpas, and José M. Palma. 2019. "Sweet Pepper (Capsicum annuum L.) Fruits Contain an Atypical Peroxisomal Catalase That Is Modulated by Reactive Oxygen and Nitrogen Species" Antioxidants 8, no. 9: 374. https://doi.org/10.3390/antiox8090374
APA StyleRodríguez-Ruiz, M., González-Gordo, S., Cañas, A., Campos, M. J., Paradela, A., Corpas, F. J., & Palma, J. M. (2019). Sweet Pepper (Capsicum annuum L.) Fruits Contain an Atypical Peroxisomal Catalase That Is Modulated by Reactive Oxygen and Nitrogen Species. Antioxidants, 8(9), 374. https://doi.org/10.3390/antiox8090374







