Antidiabetic Potential of Medicinal Plants and Their Active Components
Abstract
1. Introduction
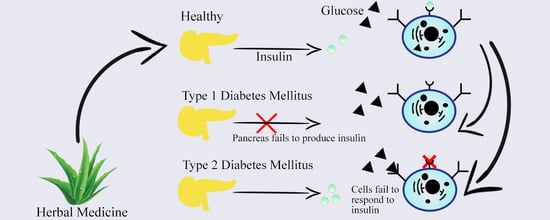
2. Types of Diabetes, Prevalence, and Management
3. Antidiabetic Drugs and Their Side Effects
4. Medicinal Plants as an Alternative Source of Antidiabetic Agents
- Pterocarpus marsupium (0.9) [136]
- Catharanthus roseus, Carthamus tinctorius, Momordica charantia, Gynostemma pentaphyllum, Glycyrrhiza glabra, Smilax glabra, Psidium guajava, and Rehmannia glutinosa (ranging from 2.5 to 48.8) [85]
- Santalum spicatum (5.43) [136]
- Ocimum tenuiflorum (8.9) [128]
- Rhizoma fagopyri, Rosa rugosa, Caulis polygoni, Fructus amomi, Rhizoma alpiniae officinarum, Folium ginkgo, and Cortex cinnamomi (16 to 2342.2) [109]
- Methanol extract of Marrubium radiatum (61.1) [137]
- Aloe vera (80) [138]
- Methanol extract of Salvia acetabulosa (91.2) [137]
- Paronychia argentea (200) [138]
- Methanol extracts of Terminalia arjuna (302) [127]
- Methanol extracts of Aegle marmelos (503) [127]
- Linum usitatisumum (540) [128]
- Methanol extracts of Eugenia cumini (632) [127]
- Morus alba (1440) [128]
- Moringa stenopetala (1470) [139]
- Nelumbo nucifera (2200) [140]
- Aqueous extract of Costus pictus (9900) [141]
- Beyeria leshnaultii (0.39) [136]
- Mucuna pruriens (0.8) [136]
- Acacia ligulata (1.01) [136]
- Pterocarpus marsupium (1.01) [136]
- Boerhaavia diffusa (1.72) [136]
- Hydroalcoholic extract of Juniperus oxycedrus (4.4) [142]
- Fagonia cretica (4.62) [143]
- Santalum spicatum (5.16) [136]
- Rhizoma fagopyri, Rosa rugosa, Caulis polygoni, Fructus amomi, Rhizoma alpiniae officinarum, Folium ginkgo, and Cortex cinnamomi (49 to 3385.5) [109]
- Methanol extract of Marrubium radiatum (68.8) [137]
- Methanol–water extract of Eugenia polyantha (71) [144]
- Methanol extract of Salvia acetabulosa (76.9) [137]
- Hydroalcoholic extracts of Ludwigia octovalvis (202) [145]
- Hydroalcoholic extracts of Camellia sinensis (299) [145]
- Aralia elata (450) [146]
- Hydroalcoholic extracts of Iostephane heterophylla (509) [145]
- Cinnamomum zeylanicum (670) [147]
- Nelumbo nucifera (1860) [140]
- Aqueous extract of Costus pictus (2510) [141]
5. Medicinal Plants with Antidiabetic Potential
5.1. Preclinical In Vitro/In Vivo (Animal) Studies
5.1.1. Acacia arabica (Fabaceae)
5.1.2. Achyranthes rubrofusca (Amaranthaceae)
5.1.3. Albizzia lebbeck (Fabaceae)
5.1.4. Aloe vera (Asphodelaceae)
5.1.5. Amaranthus tricolor (Amaranthaceae)
5.1.6. Anacardium occidentale (Anacardiaceae)
5.1.7. Azadirachta indica (Meliaceae)
5.1.8. Barleria prionitis (Acanthaceae)
5.1.9. Bauhinia thoningii (Fabaceae)
5.1.10. Caesalpinia ferrea (Fabaceae)
5.1.11. Camellia sinensis (Theaceae)
5.1.12. Casearia esculenta (Flacourtiaceae)
5.1.13. Cassia fistula (Fabaceae)
5.1.14. Cassia grandis (Fabaceae)
5.1.15. Catharanthus roseus (Apocynaceae)
5.1.16. Cecropia pachystachya (Urticaceae)
5.1.17. Ceriops decandra (Rhizophoraceae)
5.1.18. Chiliadenus iphionoides (Asteraceae)
5.1.19. Cinnamomum cassia and Cinnamomum japonica (Lauraceae)
5.1.20. Citrullus colocynthis (Cucurbitaceae)
5.1.21. Coscinium fenestratum (Menispermaceae)
5.1.22. Eucalyptus citriodora (Myrtaceae)
5.1.23. Gymnema sylvestre (Apocynaceae)
5.1.24. Heinsia crinata (Rubiaceae)
5.1.25. Helicteres isora (Sterculiaceae)
5.1.26. Momordica charantia (Cucurbitaceae)
5.1.27. Moringa oleifera (Moringaceae)
5.1.28. Murraya koenigii (Rutaceae)
5.1.29. Opuntia ficus-indica (Cactaceae)
5.1.30. Origanum vulgare (Lamiaceae)
5.1.31. Passiflora nitida (Passifloraceae)
5.1.32. Paspalum scrobiculatum (Poaceae)
5.1.33. Persea americana (Lauraceae)
5.1.34. Phoenix dactylifera (Arecaceae)
5.1.35. Phyllanthus niruri (Euphorbiaceae)
5.1.36. Phyllanthus simplex (Euphorbiaceae)
5.1.37. Picralima nitida (Magnoliopsida)
5.1.38. Piper longum (Piperaceae)
5.1.39. Sonchus oleraceus (Asteraceae)
5.1.40. Syzygium jambolana (Myrtaceae)
5.1.41. Tamarindus indica (Fabaceae)
5.1.42. Terminalia chebula (Combretaceae)
5.1.43. Terminalia catappa (Combretaceae)
5.1.44. Trigonella foenum-graecum (Fabaceae)
5.1.45. Vaccinium arctostaphylos (Ericaceae)
5.1.46. Vernonia amygdalina (Asteraceae)
5.1.47. Witheringia solanacea (Solanaceae)
5.1.48. Zaleya decandra (Aizoaceae)
5.1.49. Zizyphus mauritiana (Rhamnaceae)
6. Phytochemicals with Antidiabetic Potential
6.1. Alkaloids
6.2. Flavonoids
6.3. Terpenoids
6.3.1. Triterpenoids
6.3.2. Diterpenoids
6.3.3. Polysaccharides
6.3.4. Miscellaneous
7. In Human Evidence: Clinical Studies
7.1. Aloe vera (Asphodelaceae)
7.2. Cinnamon: Cinnamomum cassia, Cinnamomum verum, Cinnamomum burmanni, Cinnamomum zeylanicum (Lauraceae)
7.3. Ginkgo biloba (Ginkgoaceae)
7.4. Juglans regia (Juglandaceae)
7.5. Malvastrum coromandelianum (Malvaceae)
7.6. Sauropus androgynus (Phyllanthaceae)
7.7. Tinospora cordifolia (Menispermaceae)
7.8. Trigonella foenum-graecum (Fabaceae)
7.9. Vitis vinifera (Vitaceae)
7.10. Zingiber officinale (Zingiberaceae)
7.11. DBCare® (Ace Continental Exports Inc., London, UK)
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Soumya, D.; Srilatha, B. Late stage complications of diabetes and insulin resistance. J. Diabetes Metab. 2011, 2, 1000167. [Google Scholar]
- Arumugam, G.; Manjula, P.; Paari, N. A review: Anti diabetic medicinal plants used for diabetes mellitus. J. Acute Dis. 2013, 2, 196–200. [Google Scholar] [CrossRef]
- Murea, M.; Ma, L.; Freedman, B.I. Genetic and environmental factors associated with type 2 diabetes and diabetic vascular complications. Rev. Diabet. Stud. 2012, 9, 6–22. [Google Scholar] [CrossRef] [PubMed]
- Buowari, O. Chapter 8: Diabetes mellitus in developing countries and case series. In Diabetes Mellitus—Insights and Perspectives; InTechOpen: Rijeka, Croatia, 2013. [Google Scholar]
- Folorunso, O.; Oguntibeju, O. Chapter 5: The role of nutrition in the management of diabetes mellitus. In Diabetes Mellitus—Insights and Perspectives; InTechOpen: Rijeka, Croatia, 2013. [Google Scholar]
- Salsali, A.; Nathan, M. A review of types 1 and 2 diabetes mellitus and their treatment with insulin. Am. J. 2006, 13, 349–361. [Google Scholar] [CrossRef]
- Sperling, M.; Tamborlane, M.; Batteling, T.; Weinzimer, S.; Phillip, M. Pediatric endocrinology. In Chapter 19: Diabetes mellitus, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Spellman, C.W. Pathophysiology of type 2 diabetes: Targeting islet cell dysfunction. J. Am. Osteopath. Assoc. 2010, 110, S2–S7. [Google Scholar]
- Tripathy, D.; Chavez, A.O. Defects in insulin secretion and action in the pathogenesis of type 2 diabetes mellitus. Curr. Diabetes Rep. 2010, 10, 184–191. [Google Scholar] [CrossRef]
- Bahijri, S.M.; Jambi, H.A.; Al Raddadi, R.M.; Ferns, G.; Tuomilehto, J. The prevalence of diabetes and prediabetes in the adult population of Jeddah, Saudi Arabia—A community-based survey. PLoS ONE 2016, 11, e0152559. [Google Scholar] [CrossRef]
- Kakkar, R. Rising burden of diabetes-public health challenges and way out. Nepal J. Epidemiol. 2016, 6, 557–559. [Google Scholar] [CrossRef]
- Chijioke, A.; Adamu, A.; Makusidi, A. Mortality pattern among type 2 diabetes patients in Ilorin, Nigeria. JEMDSA 2010, 15, 1–4. [Google Scholar] [CrossRef]
- Owoaje, E.E.; Rotimi, C.N.; Kaufman, J.S.; Tracy, J.; Cooper, R.S. Prevalence of adult diabetes in Ibadan, Nigeria. E. Afr. Med. J. 1997, 74, 299–302. [Google Scholar]
- Narayan, K.M.V.; Zhang, P.; Williams, D.; Engelgau, M.; Imperatore, G.; Kanaya, A.; Ramachandran, A. How should developing countries manage diabetes? Can. Med Assoc. J. 2006, 175, 733–736. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Levitt, N. Diabetes in africa: Epidemiology, management, and health care challenges. Heart 2008, 94, 1376–1382. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A. Pharmacologic therapy for type 2 diabetes mellitus. Ann. Intern. Med. 1999, 131, 281–303. [Google Scholar] [CrossRef] [PubMed]
- Inzucchi, S.E. Oral antihyperglycemic therapy for type 2 diabetes—Scientific review. JAMA 2002, 287, 360–372. [Google Scholar] [CrossRef] [PubMed]
- Lebovitz, H.E. Alpha-glucosidase inhibitors. Endocrinol. Metab. Clin. N. Am. 1997, 26, 539–551. [Google Scholar] [CrossRef]
- Koski, R. Oral antidiabetic agents: A comparative review. J. Pharma. Pr. 2004, 17, 39–48. [Google Scholar] [CrossRef]
- Mayerson, A.B.; Inzucchi, S.E. Type 2 diabetes therapy. A pathophysiologically based approach. Postgrad. Med. 2002, 111, 83–95. [Google Scholar] [CrossRef]
- Rao, M.; Sreenivasulu, M.; Chengaiah, B.; Reddy, K.; Chetty, M. Herbal medicines for diabetes mellitus: A review. Int. J. Pharm. Tech. Res. 2010, 2, 1883–1892. [Google Scholar]
- Dey, L.; Attele, A.S.; Yuan, C.S. Alternative therapies for type 2 diabetes. Altern. Med. Rev. 2002, 7, 45–58. [Google Scholar]
- Wadkar, K.; Magdum, C.; Patil, S.; Naikwade, N. Antidiabetic potential and Indian medicinal plants. J. Herb. Med. Toxicol 2008, 2, 45–50. [Google Scholar]
- Sharifi-Rad, M.; Nazaruk, J.; Polito, L.; Morais-Braga, M.F.B.; Rocha, J.E.; Coutinho, H.D.M.; Salehi, B.; Tabanelli, G.; Montanari, C.; del Mar Contreras, M.; et al. Matricaria genus as a source of antimicrobial agents: From farm to pharmacy and food applications. Microbiol. Res. 2018, 215, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Kumar, N.V.A.; Şener, B.; Sharifi-Rad, M.; Kılıç, M.; Mahady, G.B.; Vlaisavljevic, S.; Iriti, M.; Kobarfard, F.; Setzer, W.N. Medicinal plants used in the treatment of human immunodeficiency virus. Int. J. Mol. Sci. 2018, 19, 1459. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, M.; Salehi, B.; Sharifi-Rad, J.; Setzer, W.N.; Iriti, M. Pulicaria vulgaris Gaertn. essential oil: An alternative or complementary treatment for leishmaniasis. Cell. Mol. Biol. 2018, 64, 18–21. [Google Scholar] [CrossRef] [PubMed]
- Arya, V.; Gupta, V.; Ranjeet, K. A review on fruits having anti-diabetic potential. J. Chem. Pharm. Res. 2011, 3, 204–212. [Google Scholar]
- Singab, A.; Youssef, F.; Ashour, M. Medicinal plants with potential antidiabetic activity and their assessment. Med. Aromat Plants 2014, 3. [Google Scholar] [CrossRef]
- Mishra, A.P.; Sharifi-Rad, M.; Shariati, M.A.; Mabkhot, Y.N.; Al-Showiman, S.S.; Rauf, A.; Salehi, B.; Župunski, M.; Sharifi-Rad, M.; Gusain, P. Bioactive compounds and health benefits of edible Rumex species—A review. Cell. Mol. Biol. 2018, 64, 27–34. [Google Scholar] [CrossRef]
- Mishra, A.P.; Saklani, S.; Salehi, B.; Parcha, V.; Sharifi-Rad, M.; Milella, L.; Iriti, M.; Sharifi-Rad, J.; Srivastava, M. Satyrium nepalense, a high altitude medicinal orchid of Indian Himalayan region: Chemical profile and biological activities of tuber extracts. Cell. Mol. Biol. 2018, 64, 35–43. [Google Scholar] [CrossRef]
- Abdolshahi, A.; Naybandi-Atashi, S.; Heydari-Majd, M.; Salehi, B.; Kobarfard, F.; Ayatollahi, S.A.; Ata, A.; Tabanelli, G.; Sharifi-Rad, M.; Montanari, C. Antibacterial activity of some lamiaceae species against Staphylococcus aureus in yoghurt-based drink (Doogh). Cell. Mol. Biol. 2018, 64, 71–77. [Google Scholar] [CrossRef]
- Mishra, A.P.; Saklani, S.; Sharifi-Rad, M.; Iriti, M.; Salehi, B.; Maurya, V.K.; Rauf, A.; Milella, L.; Rajabi, S.; Baghalpour, N. Antibacterial potential of Saussurea obvallata petroleum ether extract: A spiritually revered medicinal plant. Cell. Mol. Biol. 2018, 64, 65–70. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Tayeboon, G.S.; Niknam, F.; Sharifi-Rad, M.; Mohajeri, M.; Salehi, B.; Iriti, M.; Sharifi-Rad, M. Veronica persica Poir. Extract—antibacterial, antifungal and scolicidal activities, and inhibitory potential on acetylcholinesterase, tyrosinase, lipoxygenase and xanthine oxidase. Cell. Mol. Biol. 2018, 64, 50–56. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Roberts, T.H.; Matthews, K.R.; Bezerra, C.F.; Morais-Braga, M.F.B.; Coutinho, H.D.M.; Sharopov, F.; Salehi, B.; Yousaf, Z.; Sharifi-Rad, M.; et al. Ethnobotany of the genus Taraxacum—Phytochemicals and antimicrobial activity. Phytother. Res. 2018, 32, 2131–2145. [Google Scholar] [CrossRef] [PubMed]
- Durazzo, A.; D’Addezio, L.; Camilli, E.; Piccinelli, R.; Turrini, A.; Marletta, L.; Marconi, S.; Lucarini, M.; Lisciani, S.; Gabrielli, P. From plant compounds to botanicals and back: A current snapshot. Molecules 2018, 23, 1844. [Google Scholar] [CrossRef] [PubMed]
- Kooti, W.; Moradi, M.; Akbari, S.; Sharafi-Ahvazi, N.; AsadiSamani, M.; Ashtary-Larky, D. Therapeutic and pharmacological potential of Foeniculum vulgare Mill: A review. J. HerbMed Pharm. 2015, 4, 1–9. [Google Scholar]
- Afrisham, R.; Aberomand, M.; Ghaffari, M.; Siahpoosh, A.; Jamalan, M. Inhibitory effect of Heracleum persicum and Ziziphus jujuba on activity of alpha-amylase. J. Bot. 2015, 2015, 824683. [Google Scholar]
- Durazzo, A.; Lucarini, M. A current shot and re-thinking of antioxidant research strategy. Braz. J. Anal. Chem. 2017, 5, 9–11. [Google Scholar] [CrossRef]
- Durazzo, A. Study approach of antioxidant properties in foods: Update and considerations. Foods 2017, 6, 17. [Google Scholar] [CrossRef]
- Heo, H.J.; Kim, Y.J.; Chung, D.; Kim, D.-O. Antioxidant capacities of individual and combined phenolics in a model system. Food Chem. 2007, 104, 87–92. [Google Scholar] [CrossRef]
- Durazzo, A.; Turfani, V.; Azzini, E.; Maiani, G.; Carcea, M. Phenols, lignans and antioxidant properties of legume and sweet chestnut flours. Food Chem. 2013, 140, 666–671. [Google Scholar] [CrossRef]
- Tabart, J.; Kevers, C.; Pincemail, J.; Defraigne, J.-O.; Dommes, J. Comparative antioxidant capacities of phenolic compounds measured by various tests. Food Chem. 2009, 113, 1226–1233. [Google Scholar] [CrossRef]
- Saura-Calixto, F. Concept and health-related properties of nonextractable polyphenols: The missing dietary polyphenols. J. Agric. Food Chem. 2012, 60, 11195–11200. [Google Scholar] [CrossRef]
- Durazzo, A. Extractable and non-extractable polyphenols: An overview. In Non-Extractable Polyphenols and Carotenoids; Royal Society of Chemistry: London, UK, 2018; pp. 37–45. [Google Scholar]
- Durazzo, A.; Turfani, V.; Narducci, V.; Azzini, E.; Maiani, G.; Carcea, M. Nutritional characterisation and bioactive components of commercial carobs flours. Food Chem. 2014, 153, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Diaconeasa, Z.; Leopold, L.; Rugină, D.; Ayvaz, H.; Socaciu, C. Antiproliferative and antioxidant properties of anthocyanin rich extracts from blueberry and blackcurrant juice. Int. J. Mol. Sci. 2015, 16, 2352–2365. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; De, A. Diabetes mellitus and its herbal treatment. Int. J. Res. Pharm. Biomed. Sci. 2012, 3, 706–721. [Google Scholar]
- Ríos, J.L.; Francini, F.; Schinella, G.R. Natural products for the treatment of type 2 diabetes mellitus. Planta Med. 2015, 81, 975–994. [Google Scholar] [CrossRef] [PubMed]
- Jacob, B.; Narendhirakannan, R. Role of medicinal plants in the management of diabetes mellitus: A review. 3 Biotech 2019, 9, 4. [Google Scholar] [CrossRef]
- Asadi-Samani, M.; Moradi, M.T.; Mahmoodnia, L.; Alaei, S.; Asadi-Samani, F.; Luther, T. Traditional uses of medicinal plants to prevent and treat diabetes; an updated review of ethnobotanical studies in Iran. J. Nephropathol. 2017, 6, 118–125. [Google Scholar] [CrossRef]
- Bahmani, M.; Zargaran, A.; Rafieian-Kopaei, M.; Saki, K. Ethnobotanical study of medicinal plants used in the management of diabetes mellitus in the Urmia, Northwest Iran. Asian Pac. J. Trop. Med. 2014, 7, S348–S354. [Google Scholar] [CrossRef]
- Rashidi, A.A.; Mirhashemi, S.M.; Taghizadeh, M.; Sarkhail, P. Iranian medicinal plants for diabetes mellitus: A systematic review. Pak. J. Biol. Sci. 2013, 16, 401–411. [Google Scholar]
- Hasani-Ranjbar, S.; Larijani, B.; Abdollah, M. A systematic review of Iranian medicinal plants useful in diabetes mellitus. Arch. Med. Sci. 2008, 4, 285–292. [Google Scholar]
- Jarald, E.; Joshi, S.B.; Jain, D.C. Diabetes and herbal medicines. Iran. J. Pharmacol. Ther. 2008, 7, 97–106. [Google Scholar]
- Afifi-Yazar, F.U.; Kasabri, V.; Abu-Dahab, R. Medicinal plants from jordan in the treatment of diabetes: Traditional uses vs in vitro and in vivo evaluations part 2. Planta Med. 2011, 77, 1210–1220. [Google Scholar] [CrossRef] [PubMed]
- Al-Aboudi, A.; Afifi, F.U. Plants used for the treatment of diabetes in jordan: A review of scientific evidence. Pharm. Biol. 2011, 49, 221–239. [Google Scholar] [CrossRef] [PubMed]
- Al-Mustafa, A.H.; Al-Thunibat, O.Y. Antioxidant activity of some jordanian medicinal plants used traditionally for treatment of diabetes. Pak. J. Biol. Sci. 2008, 11, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Chin, Y.X.; Lim, P.E.; Maggs, C.A.; Phang, S.M.; Sharifuddin, Y.; Green, B.D. Anti-diabetic potential of selected malaysian seaweeds. J. Appl. Phycol. 2015, 27, 2137–2148. [Google Scholar] [CrossRef]
- Sekar, M.; Bin Abdullah, M.Z.; Bin Nor Azlan, A.Y.H.; Binti Nasir, S.N.; Binti Zakaria, Z.; Bin Abdullah, M.S. Ten commonly available medicinal plants in malaysia used for the treatment of diabetes—A review. Asian J. Pharm. Clin. Res. 2014, 7, 1–5. [Google Scholar]
- Khookhor, O.; Sato, Y. Mongolian plant extracts with potential glucose absorption inhibiting effects in rats. J. Tradit. Med. 2009, 26, 74–79. [Google Scholar]
- Mina, E.C.; Mina, J.F. Ethnobotanical survey of plants commonly used for diabetes in tarlac of central luzon Philippines. Int. Med. J. Malays. 2017, 16, 21–28. [Google Scholar]
- Chichioco-Hernandez, C.; Wudarski, J.; Gevaert, L.; Verschaeve, L. Evaluation of cytotoxicity and genotoxicity of some Philippine medicinal plants. Pharmacogn. Mag. 2011, 7, 171–175. [Google Scholar] [CrossRef]
- Kamel, F.O.; Magadmi, R.M.; Hagras, M.M.; Magadmi, B.; AlAhmad, R.A. Knowledge, attitude, and beliefs toward traditional herbal medicine use among diabetics in Jeddah Saudi Arabia. Complement. Ther. Clin. Pract. 2017, 29, 207–212. [Google Scholar] [CrossRef]
- Al-Awamy, B.H. Evaluation of commonly used tribal and traditional remedies in Saudi Arabia. Saudi Med. J. 2001, 22, 1065–1068. [Google Scholar]
- Mossa, J.S. A study on the crude antidiabetic drugs used in arabian folk medicine. Pharm. Biol. 1985, 23, 137–145. [Google Scholar] [CrossRef]
- Kim, H.; Song, M.J. Analysis of traditional knowledge about medicinal plants utilized in communities of Jirisan National Park (Korea). J. Ethnopharmacol. 2014, 153, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Kim, H.S. Korean traditional natural herbs and plants as immune enhancing, antidiabetic, chemopreventive, and antioxidative agents: A narrative review and perspective. J. Med. Food 2014, 17, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Lee, J.S. Mini review: Natural ingredients for diabetes which are approved by Korean FDA. Biomed. Res. 2013, 24, 164–169. [Google Scholar]
- Attanayake, A.P.; Jayatilaka, K.A.P.W.; Pathirana, C.; Mudduwa, L.K.B. Phytochemical screening and in vitro antioxidant potentials of extracts of ten medicinal plants used for the treatment of diabetes mellitus in Sri Lanka. Afr. J. Trad. Complement. Altern. Med. 2015, 12, 28–33. [Google Scholar] [CrossRef]
- Alachkar, A.; Jaddouh, A.; Elsheikh, M.S.; Bilia, A.R.; Vincieri, F.F. Traditional medicine in Syria: Folk medicine in Aleppo governorate. Nat. Pro. Comm. 2011, 6, 79–84. [Google Scholar] [CrossRef]
- Dej-Adisai, S.; Pitakbut, T. Determination of α-glucosidase inhibitory activity from selected Fabaceae plants. Pak. J. Pharma. Sci. 2015, 28, 1679–1683. [Google Scholar]
- Kasempitakpong, B.; Kusirisin, W.; Jaikang, C.; Sermpanich, N. Antioxidant and acetylcholinesterase inhibitory potential of thai medicinal plants. Curr. Nutr. Food Sci. 2015, 11, 99–104. [Google Scholar] [CrossRef]
- Neamsuvan, O.; Madeebing, N.; Mah, L.; Lateh, W. A survey of medicinal plants for diabetes treating from Chana and Nathawee district, Songkhla province, Thailand. J. Ethnopharmacol. 2015, 174, 82–90. [Google Scholar] [CrossRef]
- Tangjitman, K.; Wongsawad, C.; Winijchaiyanan, P.; Sukkho, T.; Kamwong, K.; Pongamornkul, W.; Trisonthi, C. Traditional knowledge on medicinal plant of the Karen in Northern Thailand: A comparative study. J. Ethnopharmacol. 2013, 150, 232–243. [Google Scholar] [CrossRef]
- Kusirisin, W.; Srichairatanakool, S.; Lerttrakarnnon, P.; Lailerd, N.; Suttajit, M.; Jaikang, C.; Chaiyasut, C. Antioxidative activity, polyphenolic content and anti-glycation effect of some Thai medicinal plants traditionally used in diabetic patients. Med. Chem. 2009, 5, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Durmuskahya, C.; Öztürk, M. Ethnobotanical survey of medicinal plants used for the treatment of diabetes in Manisa, Turkey. Sains Malays. 2013, 42, 1431–1438. [Google Scholar] [CrossRef]
- Bulut, G.; Biçer, M.; Tuzlaci, E. The folk medicinal plants of Yüksekova (Hakkari-Turkey). J. Pharm. Istanb. Univ. 2016, 46, 115–124. [Google Scholar]
- Kartal, Ç.; Güneş, F. Medicinal plants used in meriç town from Turkey. Indian J. Pharm. Educ. Res. 2017, 51, S249–S253. [Google Scholar] [CrossRef]
- Demirci, S.; Özhatay, N. An ethnobotanical study in Kahramanmaras (Turkey); wild plants used for medicinal purpose in Andirin, Kahramanmaraş. Turk. J. Pharm. Sci. 2012, 9, 75–92. [Google Scholar]
- Bulut, G. Folk medicinal plants of Silivri (Istanbul, Turkey). Marmara Pharm. J. 2011, 15, 25–29. [Google Scholar] [CrossRef]
- Sarikaya, S.; Öner, H.; Harput, U.S. Medicinal plants used for the treatment of diabetes in Turkey. Ank. Univ. Eczacilik Fak. Derg. 2010, 39, 317–342. [Google Scholar]
- Tuzlaci, E.; Şenkardeş, I. Turkish folk medicinal plants, X: Ürgüp (Nevşehir). Marmara Pharm. J. 2011, 15, 58–68. [Google Scholar] [CrossRef]
- Trinh, B.T.D.; Staerk, D.; Jäger, A.K. Screening for potential α-glucosidase and α-amylase inhibitory constituents from selected Vietnamese plants used to treat type 2 diabetes. J. Ethnopharmacol. 2016, 186, 189–195. [Google Scholar] [CrossRef]
- Hoa, N.K.; Phan, D.V.; Thuan, N.D.; Östenson, C.G. Screening of the hypoglycemic effect of eight Vietnamese herbal drugs. Methods Find. Exp. Clin. Pharmacol. 2009, 31, 165–169. [Google Scholar] [CrossRef]
- Tran, M.H.; Hoang, D.M.; Minh, P.T.H.; Ui, J.Y.; Na, M.; Won, K.O.; Byung, S.M.; Bae, K. α-amylase and protein tyrosine phosphatase 1B inhibitory of some Vietnamese medicinal plants used to treat diabetes. Nat. Prod. Sci. 2007, 13, 311–316. [Google Scholar]
- Bajpai, O.; Pandey, J.; Chaudhary, L.B. Ethnomedicinal uses of tree species by Tharu tribes in the Himalayan Terai region of India. Res. J. Med. Plant 2016, 10, 19–41. [Google Scholar] [CrossRef][Green Version]
- Bansal, R.; Jat, R.S.; Kumbhani, S.; Rathod, J.H. Ethnomedicinal survey of medicinal plants use from Narmada, Gujarat, India. Med. Plants 2016, 8, 233–237. [Google Scholar] [CrossRef]
- Kumar, R.B.; Suryanarayana, B. Ethnomedicinal recipes for diabetes from tribals of Sriharikota island, Andhra Pradesh. Pharm. Lett. 2016, 8, 111–118. [Google Scholar]
- Kumari, S.J.; Sangeetha, M.; Pavithra, R. A retrospective review on Indian traditional herbs and its biocompounds in diabetes. Int. J. Pharm. Res. 2016, 9, 444–460. [Google Scholar]
- Purohit, K.; Rathore, H.S.; Köhler-Rollefson, I. Increased risk of type 2 diabetes mellitus in the Maru Raika community of Rajasthan: A cross-sectional study. Int. J. Diabetes Dev. Ctries. 2017, 37, 494–501. [Google Scholar] [CrossRef]
- Smruthi, G.; Mahadevan, V.; Sahayam, S.; Rajalakshmi, P.; Vadivel, V.; Brindha, P. Anti-diabetic potential of selected Indian traditional medicinal plants—An updated review. J. Pharm. Sci. Res. 2016, 8, 1144–1158. [Google Scholar]
- Arora, A.; Paliwal, V.; Jain, H. An inventory of traditional herbal medicines used in management of diabetes mellitus II by ethnic people of south-east Rajasthan (India). Int. J. Pharm. Sci. Rev. Res. 2015, 30, 200–204. [Google Scholar]
- Bhatia, H.; Sharma, Y.P.; Manhas, R.K.; Kumar, K. Ethnomedicinal plants used by the villagers of district Udhampur, J&K, India. J. Ethnopharmacol. 2014, 151, 1005–1018. [Google Scholar]
- Chellappandian, M.; Pandikumar, P.; Mutheeswaran, S.; Paulraj, M.G.; Prabakaran, S.; Duraipandiyan, V.; Ignacimuthu, S.; Al-Dhabi, N.A. Documentation and quantitative analysis of local ethnozoological knowledge among traditional healers of Theni district, Tamil Nadu, India. J. Ethnopharmacol. 2014, 154, 116–130. [Google Scholar] [CrossRef]
- Tarafdar, R.G.; Nath, S.; Talukdar, A.D.; Choudhury, M.D. Antidiabetic plants used among the ethnic communities of Unakoti district of Tripura, India. J. Ethnopharmacol. 2015, 160, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Jerang, G.; Swamy, B.M.V.; Kotagiri, S.; Dey, T.; Fariyaz, S.M. Indian medicinal plants with antidiabetic and related beneficial effects: A review. Res. J. Pharm. Biol. Chem. Sci. 2015, 6, 31–38. [Google Scholar]
- Nongdam, P. Ethno-medicinal uses of some orchids of Nagaland, North-east India. Res. J. Med. Plant 2014, 8, 126–139. [Google Scholar] [CrossRef]
- Thirumalai, T.; Beverly, C.D.; Sathiyaraj, K.; Senthilkumar, B.; David, E. Ethnobotanical study of anti-diabetic medicinal plants used by the local people in Javadhu hills Tamilnadu, India. Asian Pac. J. Trop. Biomed. 2012, 2, S910–S913. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, J.; Chan, P. Treating type 2 diabetes mellitus with traditional Chinese and Indian medicinal herbs. Evid. Based Complement. Altern. Med. 2013, 2013, 343594. [Google Scholar] [CrossRef]
- Devi, W.I.; Devi, G.S.; Singh, C.B. Traditional herbal medicine used for the treatment of diabetes in Manipur, India. Res. J. Pharm. Biol. Chem. Sci. 2011, 2, 709–715. [Google Scholar]
- Joseph, B.; Jini, D. Insight into the hypoglycaemic effect of traditional Indian herbs used in the treatment of diabetes. Res. J. Med. Plant 2011, 5, 352–376. [Google Scholar] [CrossRef][Green Version]
- Basha, S.K.; Sudarsanam, G.; Mohammad, M.S.; Parveen, D.N. Investigations on anti-diabetic medicinal plants used by Sugali tribal inhabitants of Yerramalais of Kurnool district, Andhra Pradesh, India. Stamford J. Pharm. Sci. 2011, 4, 19–24. [Google Scholar] [CrossRef]
- Khan, M.H.; Yadava, P.S. Antidiabetic plants used in Thoubal district of Manipur, Northeast India. Indian J. Trad. Knowl. 2010, 9, 510–514. [Google Scholar]
- Tarak, D.; Namsa, N.D.; Tangjang, S.; Arya, S.C.; Rajbonshi, B.; Samal, P.K.; Mandal, M. An inventory of the ethnobotanicals used as anti-diabetic by a rural community of Dhemaji district of Assam, Northeast India. J. Ethnopharmacol. 2011, 138, 345–350. [Google Scholar] [CrossRef]
- Thakur, G.; Pal, K.; Mitra, A.; Mukherjee, S.; Basak, A.; Rousseau, D. Some common antidiabetic plants of the Indian subcontinent. Food Rev. Int. 2010, 26, 364–385. [Google Scholar] [CrossRef]
- Xie, W.; Zhao, Y.; Zhang, Y. Traditional Chinese medicines in treatment of patients with type 2 diabetes mellitus. Evid.Based Complement. Altern. Med. 2011, 2011, 726723. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.P.; Song, C.Q.; Yuan, P.; Mao, R.G. α-glucosidase and α-amylase inhibitory activity of common constituents from traditional Chinese medicine used for diabetes mellitus. Chin. J. Nat. Med. 2010, 8, 349–352. [Google Scholar] [CrossRef]
- Geng, S.Y.; Ouyang, X.Y.; Zhou, Q.; He, M.Z.; Qi, Y.R. Analysis of patents of antidiabetic traditional Chinese medicine. Chin. J. New Drugs 2016, 25, 1921–1927. [Google Scholar]
- Feng, S.; Song, L.; Liu, Y.; Lai, F.; Zuo, G.; He, G.; Chen, M.; Huang, D. Hypoglycemic activities of commonly-used traditional Chinese herbs. Am. J. Chin. Med. 2013, 41, 849–864. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.J.; Chiang, B.H. Anti-diabetic effect of a traditional Chinese medicine formula. Food. Funct. 2012, 3, 1161–1169. [Google Scholar] [CrossRef]
- Zhang, J.Q. Progress of diabetes research in traditional Chinese medicine in recent years. J. Chin. Integr. Med. 2007, 5, 373–377. [Google Scholar] [CrossRef]
- Li, Z.; Qian, Y.C.; Gao, F.; Qian, H.; Wang, X.J. Research progress of daibetes treatment by traditional Chinese medicine. Chin. J. Pharm. Biotechnol. 2015, 22, 373–376. [Google Scholar]
- Kar, A.; Choudhary, B.K.; Bandyopadhyay, N.G. Comparative evaluation of hypoglycaemic activity of some Indian medicinal plants in alloxan diabetic rats. J. Ethnopharmacol. 2003, 84, 105–108. [Google Scholar] [CrossRef]
- Gopukumar, S.T.; Praseetha, P.K. Ficus benghalensis linn—The sacred Indian medicinal tree with potent pharmacological remedies. Int. J. Pharm. Sci. Rev. Res. 2015, 32, 223–227. [Google Scholar]
- Deepa, P.; Sowndhararajan, K.; Kim, S.; Park, S.J. A role of ficus species in the management of diabetes mellitus: A review. J. Ethnopharmacol. 2018, 215, 210–232. [Google Scholar] [CrossRef] [PubMed]
- Shahreen, S.; Banik, J.; Hafiz, A.; Rahman, S.; Zaman, A.T.; Shoyeb, M.A.; Chowdhury, M.H.; Rahmatullah, M. Antihyperglycemic activities of leaves of three edible fruit plants (Averrhoa carambola, Ficus hispida and Syzygium samarangense) of Bangladesh. Afr. J. Tradit. Complement. Altern. Med. 2012, 9, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Rangika, B.S.; Dayananda, P.D.; Peiris, D.C. Hypoglycemic and hypolipidemic activities of aqueous extract of flowers from Nycantus arbor-tristis L. in male mice. BMC Complement. Altern. Med. 2015, 15, 289. [Google Scholar] [CrossRef] [PubMed]
- Doss, A.; Palaniswamy, M.; Angayarkanni, J.; Dhanabalan, R. Antidiabetic activity of water extract of Solanum trilobatum (Linn.) in alloxan-induced diabetes in rats. Afr. J. Biotechnol. 2009, 8, 5551–5553. [Google Scholar]
- Olaokun, O.O.; McGaw, L.J.; Awouafack, M.D.; Eloff, J.N.; Naidoo, V. The potential role of GLUT4 transporters and insulin receptors in the hypoglycaemic activity of Ficus lutea acetone leaf extract. BMC Complement. Altern. Med. 2014, 14, 269. [Google Scholar] [CrossRef]
- Zengin, G.; Mollica, A.; Aktumsek, A.; Picot, C.M.N.; Mahomoodally, M.F. In vitro and in silico insights of Cupressus sempervirens, Artemisia absinthium and Lippia triphylla: Bridging traditional knowledge and scientific validation. Eur. J. Integr. Med. 2017, 12, 135–141. [Google Scholar] [CrossRef]
- Liu, N.Q.; van der Kooy, F.; Verpoorte, R. Artemisia afra: A potential flagship for African medicinal plants? S. Afr. J. Bot. 2009, 75, 185–195. [Google Scholar] [CrossRef]
- Nedjimi, B.; Beladel, B. Assessment of some chemical elements in wild Shih (Artemisia herba-alba Asso) using INAA technique. J. Appl. Res. Med. Aromat. Plants 2015, 2, 203–205. [Google Scholar] [CrossRef]
- Al-Khazraji, S.M.; Al-Shamaony, L.A.; Twaij, H.A.A. Hypoglycaemic effect of Artemisia herba alba. I. Effect of different parts and influence of the solvent on hypoglycaemic activity. J. Ethnopharmacol. 1993, 40, 163–166. [Google Scholar] [CrossRef]
- Cruz, E.C.; Andrade-Cetto, A. Ethnopharmacological field study of the plants used to treat type 2 diabetes among the Cakchiquels in Guatemala. J. Ethnopharmacol. 2015, 159, 238–244. [Google Scholar] [CrossRef]
- Tag, H.; Kalita, P.; Dwivedi, P.; Das, A.K.; Namsa, N.D. Herbal medicines used in the treatment of diabetes mellitus in Arunachal Himalaya, Northeast, India. J. Ethnopharmacol. 2012, 141, 786–795. [Google Scholar] [CrossRef] [PubMed]
- Rafe, M.R. A review of five traditionally used anti-diabetic plants of Bangladesh and their pharmacological activities. Asian Pac. J. Trop. Med. 2017, 10, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Verma, R. Inhibitory potential of traditional herbs on α-amylase activity. Pharm. Biol. 2012, 50, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Sudha, P.; Zinjarde, S.S.; Bhargava, S.Y.; Kumar, A.R. Potent α-amylase inhibitory activity of Indian Ayurvedic medicinal plants. BMC Complement. Altern. Med. 2011, 11, 5. [Google Scholar]
- Ocvirk, S.; Kistler, M.; Khan, S.; Talukder, S.H.; Hauner, H. Traditional medicinal plants used for the treatment of diabetes in rural and urban areas of Dhaka, Bangladesh—An ethnobotanical survey. J. Ethnobiol. Ethnomedicine 2013, 9, 43. [Google Scholar] [CrossRef]
- Jokar, A.; Masoomi, F.; Sadeghpour, O.; Nassiri-Toosi, M.; Hamedi, S. Potential therapeutic applications for Terminalia chebula in Iranian traditional medicine. J. Tradit Chin Med. 2016, 36, 250–254. [Google Scholar] [CrossRef]
- Sharma, V. Microscopic studies and preliminary pharmacognostical evaluation of Euphorbia neriifolia L. Leaves. Ind. J. Nat. Prod. Resour. 2013, 4, 348–357. [Google Scholar]
- Goyal, M.; Sasmal, D.; Nagori, B.P. Review on medicinal plants used by local community of Jodhpur district of Thar desert. Int. J. Pharmacol. 2011, 7, 333–339. [Google Scholar] [CrossRef]
- Hossan, M.S.; Hanif, A.; Khan, M.; Bari, S.; Jahan, R.; Rahmatullah, M. Ethnobotanical survey of the Tripura tribe of Bangladesh. Am. Eurasian J. Sustain. Agric. 2009, 3, 253–261. [Google Scholar]
- Kim, S.J.; Jang, Y.W.; Hyung, K.E.; Lee, D.K.; Hyun, K.H.; Park, S.Y.; Park, E.S.; Hwang, K.W. Therapeutic effects of methanol extract from Euphorbia kansui radix on imiquimod-induced psoriasis. J. Immunol. Res. 2017, 2017, 7052560. [Google Scholar] [CrossRef]
- Dineshkumar, B.; Analava, M.; Manjunatha, M. Antidiabetic and hypolipidaemic effects of few common plants extract in type 2 diabetic patients at Bengal. Int. J. Diabetes Metabol. 2010, 18, 59–65. [Google Scholar] [CrossRef]
- Gulati, V.; Harding, I.H.; Palombo, E.A. Enzyme inhibitory and antioxidant activities of traditional medicinal plants: Potential application in the management of hyperglycemia. BMC Complement. Altern. Med. 2012, 12, 77. [Google Scholar] [CrossRef] [PubMed]
- Loizzo, M.R.; Saab, A.M.; Tundis, R.; Menichini, F.; Bonesi, M.; Piccolo, V.; Statti, G.A.; de Cindio, B.; Houghton, P.J.; Menichini, F. In vitro inhibitory activities of plants used in Lebanon traditional medicine against angiotensin converting enzyme (ACE) and digestive enzymes related to diabetes. J. Ethnopharmacol. 2008, 119, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Soud, R.S.A.; Hamdan, I.I.; Afifi, F.U. Alpha amylase inhibitory activity of some plant extracts with hypoglycemic activity. Sci. Pharm. 2004, 72, 25–33. [Google Scholar] [CrossRef]
- Toma, A.; Makonnen, E.; Mekonnen, Y.; Debella, A.; Addisakwattana, S. Intestinal α-glucosidase and some pancreatic enzymes inhibitory effect of hydroalcholic extract of Moringa stenopetala leaves. BMC Complement. Altern. Med. 2014, 14, 180. [Google Scholar] [CrossRef]
- Liu, S.; Li, D.; Huang, B.; Chen, Y.; Lu, X.; Wang, Y. Inhibition of pancreatic lipase, α-glucosidase, α-amylase, and hypolipidemic effects of the total flavonoids from Nelumbo nucifera leaves. J. Ethnopharmacol. 2013, 149, 263–269. [Google Scholar] [CrossRef]
- Rege, A.; Ambaye, R.; Chowdhary, A. Effect of Costus pictus D. Don on carbohydrate hydrolyzing enzymes. Int. J. Pharmcy Pharm. Sci. 2014, 6, 278–280. [Google Scholar]
- Orhan, N.; Hoşbaş, S.; Orhan, D.D.; Aslan, M.; Ergun, F. Enzyme inhibitory and radical scavenging effects of some antidiabetic plants of Turkey. Iran. J. Basic Med. Sci. 2014, 17, 426–432. [Google Scholar]
- Nazir, I.; Rahman, N.U.; Alvi, Z.; Rahman, M.H.; Sendker, J.; Zhang, T.; Frankish, N.; Sheridan, H. Antidiabetic activities of an LC/MS fingerprinted aqueous extract of Fagonia cretica L. in preclinical models. Planta Med. 2017, 83, 1141–1148. [Google Scholar]
- Lelono, R.A.A.; Tachibana, S. Preliminary studies of indonesian eugenia polyantha leaf extracts as inhibitors of key enzymes for type 2 diabetes. J. Med. Sci. 2013, 13, 103–110. [Google Scholar] [CrossRef]
- Ramírez, G.; Zavala, M.; Pérez, J.; Zamilpa, A. In vitro screening of medicinal plants used in Mexico as antidiabetics with glucosidase and lipase inhibitory activities. Evid.Based Complement. Altern. Med. 2012, 2012, 701261. [Google Scholar] [CrossRef] [PubMed]
- Ohno, H.; Nagai, J.; Kurokawa, T.; Sonoda, M.; Yumoto, R.; Takano, M. Effect of aqueous extract from the root cortex of Aralia elata on intestinal α-glucosidases and postprandial glycemic response in mice. Int. J. Phytomed. 2012, 4, 567–572. [Google Scholar]
- Shihabudeen, H.M.S.; Priscilla, D.H.; Thirumurugan, K. Cinnamon extract inhibits α-glucosidase activity and dampens postprandial glucose excursion in diabetic rats. Nutr. Metab. 2011, 8, 46. [Google Scholar] [CrossRef] [PubMed]
- Saha, M.R.; Dey, P.; Sarkar, I.; de Sarker, D.; Haldar, B.; Chaudhuri, T.K.; Sen, A. Acacia nilotica leaf improves insulin resistance and hyperglycemia associated acute hepatic injury and nephrotoxicity by improving systemic antioxidant status in diabetic mice. J. Ethnopharmacol. 2018, 210, 275–286. [Google Scholar] [CrossRef]
- Rahmatullah, M.; Hossain, M.; Mahmud, A.; Sultana, N.; Rahman, S.M.; Islam, M.R.; Khatoon, M.S.; Jahan, S.; Islam, F. Antihyperglycemic and antinociceptive activity evaluation of ‘khoyer’ prepared from boiling the wood of Acacia catechu in water. Afr. J. Tradit. Complement. Altern. Med. 2013, 10, 1–5. [Google Scholar] [CrossRef]
- Kunwar, R.M.; Shrestha, K.P.; Bussmann, R.W. Traditional herbal medicine in Far-west Nepal: A pharmacological appraisal. J. Ethnobiol. Ethnomedicine 2010, 6, 35. [Google Scholar] [CrossRef]
- Rao, P.K.; Hasan, S.S.; Bhellum, B.L.; Manhas, R.K. Ethnomedicinal plants of Kathua district, J&K, India. J. Ethnopharmacol. 2015, 171, 12–27. [Google Scholar]
- Kingsley, B.; Jesuraj, S.A.V.; Brindha, P.; Subramoniam, A.; Atif, M. Anti-diabetes activity of Acacia farnesiana (L.) willd in alloxan diabetic rats. Int. J. Pharm. Res. 2013, 5, 112–118. [Google Scholar]
- Mukhtar, M.H.; Almalki, W.H.; Azmat, A.; Abdalla, M.R.; Ahmed, M. Evaluation of anti-diabetic activity of Acacia tortilis (Forssk.) hayne leaf extract in streptozotocin-induced diabetic rats. Int. J. Pharmacol. 2017, 13, 438–447. [Google Scholar]
- Hilmi, Y.; Abushama, M.F.; Abdalgadir, H.; Khalid, A.; Khalid, H. A study of antioxidant activity, enzymatic inhibition and in vitro toxicity of selected traditional sudanese plants with anti-diabetic potential. BMC Complement. Altern. Med. 2014, 14, 149. [Google Scholar] [CrossRef]
- Deb, J.; Dash, G.K. Review on Acacia ferruginea DC. (Mimosaceae): An endangered medicinal plant. Int. J. Pharm. Res. 2013, 5, 1–3. [Google Scholar]
- Vadivel, V.; Biesalski, H.K. Total phenolic content, in vitro antioxidant activity and type II diabetes relevant enzyme inhibition properties of methanolic extract of traditionally processed underutilized food legume, Acacia nilotica (L.) Willd ex. Delile. Int. Food Res. J. 2012, 19, 593–601. [Google Scholar]
- Jawla, S.; Kumar, Y.; Khan, M.S.Y. Antimicrobial and antihyperglycemic activities of Acacia modesta leaves. Pharmacologyonline 2011, 2, 331–347. [Google Scholar]
- Yasir, M.; Jain, P.; Debajyoti, D.; Kharya, M.D. Hypoglycemic and antihyperglycemic effect of different extracts of Acacia arabica lamk bark in normal and alloxan induced diabetic rats. Int. J. Phytomed. 2010, 2, 133–138. [Google Scholar] [CrossRef][Green Version]
- Zahidin, N.S.; Saidin, S.; Zulkifli, R.M.; Muhamad, I.I.; Ya’akob, H.; Nur, H. A review of Acalypha indica L. (Euphorbiaceae) as traditional medicinal plant and its therapeutic potential. J. Ethnopharmacol. 2017, 207, 146–173. [Google Scholar] [CrossRef]
- Latiff, A.A.; Teoh, S.L.; Das, S. Wound healing in diabetes mellitus: Traditional treatment modalities. Clin. Ter. 2010, 161, 359–364. [Google Scholar]
- Ikewuchi, J.C.; Onyeike, E.N.; Uwakwe, A.A.; Ikewuchi, C.C. Effect of aqueous extract of the leaves of Acalypha wilkesiana ‘Godseffiana’ Muell Arg (Euphorbiaceae) on the hematology, plasma biochemistry and ocular indices of oxidative stress in alloxan induced diabetic rats. J. Ethnopharmacol. 2011, 137, 1415–1424. [Google Scholar] [CrossRef]
- Chang, I.A.; Shin, H.Y.; Youn, C.K.; Yun, Y.G.; Park, H. Immunostimulatory effect of Korean traditional medicine Acanthopanacis Cortex. Nat. Prod. Sci. 2007, 13, 283–288. [Google Scholar]
- Hong, C.E.; Lyu, S.Y. Evaluation of the mutagenic properties of two lignans from Acanthopanax koreanum Nakai. Toxicol. Res. 2013, 29, 279–283. [Google Scholar] [CrossRef]
- Saito, T.; Nishida, M.; Saito, M.; Tanabe, A.; Eitsuka, T.; Yuan, S.H.; Ikekawa, N.; Nishida, H. The fruit of Acanthopanax senticosus (Rupr. et Maxim.) Harms improves insulin resistance and hepatic lipid accumulation by modulation of liver adenosine monophosphate–activated protein kinase activity and lipogenic gene expression in high-fat diet–fed obese mice. Nutr. Res. 2016, 36, 1090–1097. [Google Scholar]
- Kim, J.H.; Shin, E.H.; Lee, H.Y.; Lee, B.G.; Park, S.H.; Moon, D.I.; Goo, G.C.; Kwon, D.Y.; Yang, H.J.; Kim, O.J.; et al. Immunostimulating effects of extract of Acanthopanax sessiliflorus. Exp. Anim. 2013, 62, 247–253. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Saeidnia, S.; Gohari, A.R.; Mokhber-Dezfuli, N.; Kiuchi, F. A review on phytochemistry and medicinal properties of the genus Achillea. DARU J. Pharm. Sci. 2011, 19, 173–186. [Google Scholar]
- Yazdanparast, R.; Ardestani, A.; Jamshidi, S. Experimental diabetes treated with Achillea santolina: Effect on pancreatic oxidative parameters. J. Ethnopharmacol. 2007, 112, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Kasabri, V.; Afifi, F.U.; Hamdan, I. In vitro and in vivo acute antihyperglycemic effects of five selected indigenous plants from jordan used in traditional medicine. J. Ethnopharmacol. 2011, 133, 888–896. [Google Scholar] [CrossRef]
- Tian, T.; Chen, H.; Zhao, Y.Y. Traditional uses, phytochemistry, pharmacology, toxicology and quality control of Alisma orientale (Sam.) Juzep: A review. J. Ethnopharmacol. 2014, 158, 373–387. [Google Scholar] [CrossRef]
- Li, Q.; Qu, H. Study on the hypoglycemic activities and metabolism of alcohol extract of Alismatis Rhizoma. Fitoterapia 2012, 83, 1046–1053. [Google Scholar] [CrossRef]
- Rahimi-Madiseh, M.; Heidarian, E.; Kheiri, S.; Rafieian-Kopaei, M. Effect of hydroalcoholic Allium ampeloprasum extract on oxidative stress, diabetes mellitus and dyslipidemia in alloxan-induced diabetic rats. Biomed. Pharmacother. 2017, 86, 363–367. [Google Scholar] [CrossRef]
- Grover, J.K.; Yadav, S.; Vats, V. Medicinal plants of India with anti-diabetic potential. J. Ethnopharmacol. 2002, 81, 81–100. [Google Scholar] [CrossRef]
- Mootoosamy, A.; Mahomoodally, M.F. Ethnomedicinal application of native remedies used against diabetes and related complications in Mauritius. J. Ethnopharmacol. 2014, 151, 413–444. [Google Scholar] [CrossRef]
- Amel, B. Traditional treatment of high blood pressure and diabetes in Souk Ahras District. J. Pharmacogn. Phytother. 2013, 5, 12–20. [Google Scholar]
- Roman-Ramos, R.; Flores-Saenz, J.L.; Alarcon-Aguilar, F.J. Anti-hyperglycemic effect of some edible plants. J. Ethnopharmacol. 1995, 48, 25–32. [Google Scholar] [CrossRef]
- Aslan, M.; Orhan, N.; Orhan, D.D.; Ergun, F. Hypoglycemic activity and antioxidant potential of some medicinal plants traditionally used in Turkey for diabetes. J. Ethnopharmacol. 2010, 128, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Sukandar, E.Y.; Adnyana, I.K.; Nurfitria, R.S. Antioxidant potential of garlic and turmeric mixture—A traditional Indonesian formulation. Indian J. Trad. Knowl. 2015, 14, 632–636. [Google Scholar]
- Moradabadi, L.; Kouhsari, S.M.; Sani, M.F. Hypoglycemic effects of three medicinal plants in experimental diabetes: Inhibition of rat intestinal α-glucosidase and enhanced pancreatic insulin and cardiac GLUT-4 mRNAs expression. Iran. J. Pharm. Res. 2013, 12, 385–397. [Google Scholar]
- Mesa, M.G. Hypolipidemic potential of plants used in Cuba. Pharmacologyonline 2014, 1, 73–80. [Google Scholar]
- Karou, S.D.; Tchacondo, T.; Tchibozo, M.A.D.; Abdoul-Rahaman, S.; Anani, K.; Koudouvo, K.; Batawila, K.; Agbonon, A.; Simpore, J.; de Souza, C. Ethnobotanical study of medicinal plants used in the management of diabetes mellitus and hypertension in the Central Region of Togo. Pharm. Biol. 2011, 49, 1286–1297. [Google Scholar] [CrossRef]
- Xie, W.; Du, L. Diabetes is an inflammatory disease: Evidence from traditional Chinese medicines. Diabetes Obes. Metab. 2011, 13, 289–301. [Google Scholar] [CrossRef]
- Bhaludra, C.S.S.; Bethapudi, R.R.; Murugulla, A.C.; Pullagummi, C.; Latha, T.; Venkatesh, K.; Bheemagani, A.J.; Pudutha, A.; Rani, A.R. Cultivation, phytochemical studies, biological activities and medicinal uses of Aloe ferox, grandfather of aloes an important amazing medicinal plant. Int. J. Pharmacol. 2013, 9, 405–415. [Google Scholar]
- Semenya, S.; Potgieter, M.; Erasmus, L. Ethnobotanical survey of medicinal plants used by Bapedi healers to treat diabetes mellitus in the Limpopo Province, South Africa. J. Ethnopharmacol. 2012, 141, 440–445. [Google Scholar] [CrossRef]
- Sharma, P.; Kharkwal, A.C.; Kharkwal, H.; Abdin, M.Z.; Varma, A. A review on pharmacological properties of Aloe vera. Int. J. Pharm. Sci. Rev. Res. 2014, 29, 31–37. [Google Scholar]
- Asase, A.; Yohonu, D.T. Ethnobotanical study of herbal medicines for management of diabetes mellitus in Dangme West District of southern Ghana. J. Herb. Med. 2016, 6, 204–209. [Google Scholar] [CrossRef]
- Mahomoodally, M.F.; Ramalingum, N. An investigation into the consumption patterns, attitude, and perception of Mauritians towards common medicinal food plants. J. Herb. Med. 2015, 5, 99–112. [Google Scholar] [CrossRef]
- Ssenyange, C.W.; Namulindwa, A.; Oyik, B.; Ssebuliba, J. Plants used to manage type II diabetes mellitus in selected districts of central Uganda. Afr. Health Sci. 2015, 15, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Stanifer, J.W.; Lunyera, J.; Boyd, D.; Karia, F.; Maro, V.; Omolo, J.; Patel, U.D. Traditionalmedicine practices among communitymembers with chronic kidney disease in northern Tanzania: An ethnomedical survey. BMC Nephrol. 2015, 16, 170. [Google Scholar] [CrossRef] [PubMed]
- Lans, C.A. Ethnomedicines used in Trinidad and Tobago for urinary problems and diabetes mellitus. J. Ethnobiol. Ethnomedicine 2006, 2, 45. [Google Scholar] [CrossRef]
- Waqar, M.A.; Shaukat, S.; Sohail, T. Study of glibenclamide with some traditional herbs used for the treatment of diabetes in Pakistan. J. Chem. Soc. Pak. 2008, 30, 147–154. [Google Scholar]
- Tripathi, P.; Swain, S.N. In-vitro antioxidant and free radical scavenging activity of Alpinia calcarata in Andaman Islands. Plant Arch. 2016, 16, 685–694. [Google Scholar]
- Arawwawala, L.D.A.M.; Arambewela, L.S.R.; Ratnasooriya, W.D. Alpinia calcarata Roscoe: A rich source of phytopharmaceuticals in Sri Lanka. Nat. Prod. J. 2012, 2, 263–269. [Google Scholar]
- Ayyanar, M.; Ignacimuthu, S. Ethnobotanical survey of medicinal plants commonly used by Kani tribals in Tirunelveli hills of Western Ghats, India. J. Ethnopharmacol. 2011, 134, 851–864. [Google Scholar] [CrossRef]
- Kunyanga, C.N.; Imungi, J.K.; Okoth, M.W.; Biesalski, H.K.; Vadivel, V. Total phenolic content, antioxidant and antidiabetic properties of methanolic extract of raw and traditionally processed Kenyan indigenous food ingredients. LWT Food Sci. Technol. 2012, 45, 269–276. [Google Scholar] [CrossRef]
- Lin, J.Y.; Li, C.Y.; Lin, B.F. Amaranthus spinosus L. inhibits spontaneous and dexamethasone-induced apoptosis in murine primary splenocytes. J. Food Drug Anal. 2008, 16, 52–61. [Google Scholar]
- Mondal, A.; Guria, T.; Maity, T.K. A new ester of fatty acid from a methanol extract of the whole plant of Amaranthus spinosus and its α-glucosidase inhibitory activity. Pharm. Biol. 2015, 53, 600–604. [Google Scholar] [CrossRef] [PubMed]
- Leu, Y.L.; Chen, Y.W.; Yang, C.Y.; Huang, C.F.; Lin, G.H.; Tsai, K.S.; Yang, R.S.; Liu, S.H. Extract isolated from Angelica hirsutiflora with insulin secretagogue activity. J. Ethnopharmacol. 2009, 123, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Ohnogi, H.; Kudo, Y.; Tahara, K.; Sugiyama, K.; Enoki, T.; Hayami, S.; Sagawa, H.; Tanimura, Y.; Aoi, W.; Naito, Y.; et al. Six new chalcones from Angelica keiskei inducing adiponectin production in 3T3-L1 adipocytes. Biosci. Biotechnol. Biochem. 2012, 76, 961–966. [Google Scholar] [CrossRef]
- Zhi, X.Y. Traditional Chinese medicine diagnosis and treatment of type 2 diabetes in Tianjin urban population. J. Chin. Integr. Med. 2009, 7, 823–826. [Google Scholar] [CrossRef]
- Bhat, Z.A.; Ali, M.; Ansari, S.H.; Naquvi, K.J. New phytoconstituents from the roots of Aralia cachemirica Decne. J. Saudi Chem. Soc. 2015, 19, 287–291. [Google Scholar] [CrossRef]
- Lee, Y.M.; Kim, H.; Choi, H.S.; Kang, B.H.; Han, Y.B.; Kim, S.J. Effects of water extract of 1:1 mixture of phellodendron cortex and aralia cortex on polyol pathway and oxidative damage in lenses of diabetic rats. Phytother. Res. 1999, 13, 555–560. [Google Scholar] [CrossRef]
- Shikov, A.N.; Pozharitskaya, O.N.; Makarov, V.G. Aralia elata var. Mandshurica (rupr. & maxim.) j.Wen: An overview of pharmacological studies. Phytomedicine 2016, 23, 1409–1421. [Google Scholar]
- Li, Y.; Park, J.; Wu, Y.; Cui, J.; Jia, N.; Xi, M.; Wen, A. Identification of ampk activator from twelve pure compounds isolated from aralia taibaiensis: Implication in antihyperglycemic and hypolipidemic activities. Korean J. Physiol. Pharmacol. 2017, 21, 279–286. [Google Scholar] [CrossRef]
- Dou, F.; Xi, M.; Wang, J.; Tian, X.; Hong, L.; Tang, H.; Wen, A. A glucosidase and α amylase inhibitory activities of saponins from traditional chinese medicines in the treatment of diabetes mellitus. Pharmazie 2013, 68, 300–304. [Google Scholar]
- Vouillamoz, J.F.; Carlen, C.; Taglialatela-Scafati, O.; Pollastro, F.; Appendino, G. The génépi artemisia species. Ethnopharmacology, cultivation, phytochemistry, and bioactivity. Fitoterapia 2015, 106, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Dib, I.; Tits, M.; Angenot, L.; Wauters, J.N.; Assaidi, A.; Mekhfi, H.; Aziz, M.; Bnouham, M.; Legssyer, A.; Frederich, M.; et al. Antihypertensive and vasorelaxant effects of aqueous extract of Artemisia campestris L. From eastern morocco. J. Ethnopharmacol. 2017, 206, 224–235. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.N.; Ishita, I.J.; Jung, H.A.; Choi, J.S. Vicenin 2 isolated from Artemisia capillaris exhibited potent anti-glycation properties. Food Chem. Toxicol. 2014, 69, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Ota, A.; Ulrih, N.P. An overview of herbal products and secondary metabolites used for management of type two diabetes. Front. Pharmacol. 2017, 8, 436. [Google Scholar] [CrossRef] [PubMed]
- Abu-Darwish, M.S.; Cabral, C.; Gonçalves, M.J.; Cavaleiro, C.; Cruz, M.T.; Zulfiqar, A.; Khan, I.A.; Efferth, T.; Salgueiro, L. Chemical composition and biological activities of Artemisia judaica essential oil from southern desert of jordan. J. Ethnopharmacol. 2016, 191, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Abu-Darwish, M.S.; Cabral, C.; Gonçalves, M.J.; Cavaleiro, C.; Cruz, M.T.; Efferth, T.; Salgueiro, L. Artemisia herba-alba essential oil from buseirah (south jordan): Chemical characterization and assessment of safe antifungal and anti-inflammatory doses. J. Ethnopharmacol. 2015, 174, 153–160. [Google Scholar] [CrossRef]
- Anaya-Eugenio, G.D.; Rivero-Cruz, I.; Rivera-Chávez, J.; Mata, R. Hypoglycemic properties of some preparations and compounds from Artemisia ludoviciana nutt. J. Ethnopharmacol. 2014, 155, 416–425. [Google Scholar] [CrossRef]
- Niranjan, A.; Barthwal, J.; Lehri, A.; Singh, D.P.; Govindrajan, R.; Rawat, A.K.S.; Amla, D.V. Development and validation of an hplc-uv-ms-ms method for identification and quantification of polyphenols in Artemisia pallens L. Acta Chromatogr. 2009, 21, 105–116. [Google Scholar] [CrossRef]
- Ahuja, J.; Suresh, J.; Paramakrishnan, N.; Mruthunjaya, K.; Naganandhini, M.N. An ethnomedical, phytochemical and pharmacological profile of Artemisia parviflora roxb. J. Essent. Oil Bear. Plant. 2011, 14, 647–657. [Google Scholar] [CrossRef]
- Yamamoto, N.; Kanemoto, Y.; Ueda, M.; Kawasaki, K.; Fukuda, I.; Ashida, H. Anti-obesity and anti-diabetic effects of ethanol extract of Artemisia princeps in c57bl/6 mice fed a high-fat diet. Food Funct. 2011, 2, 45–52. [Google Scholar] [CrossRef]
- Shah, M.R.; Ishtiaq, H.S.M.; Habtemariam, S.; Zarrelli, A.; Muhammad, A.; Collina, S.; Khan, I. Protein tyrosine phosphatase 1b inhibitors isolated from Artemisia roxburghiana. J. Enzym. Inhib. Med. Chem. 2016, 31, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.D.; Yuan, H.Y.; Chung, S.H.; Jin, G.Z.; Piao, G.C. An active part of Artemisia sacrorum ledeb. Attenuates hepatic lipid accumulation through activating amp-activated protein kinase in human hepg2 cells. Biosci. Biotechnol. Biochem. 2010, 74, 322–328. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wahyudin; Massi, M.N.; Natzir, R.; Alam, G.; Bukhari, A.S. Effect of sukun leaf extract [Artocarpus altilis (park.) fosberg] on insulin resistance in obese rats (rattus norvegicus): A study of free fatty acid (ffa) levels. Pak. J. Nutr. 2017, 16, 521–524. [Google Scholar]
- Adewole, S.O.; Ojewole, J.A.O. Artocarpus communis forst. Root-bark aqueous extract-and streptozotocin-induced ultrastructural and metabolic changes hepatic tissues of wistar rats. Afr. J. Trad. Complement. Altern. Med. 2007, 4, 397–410. [Google Scholar]
- Chandrika, U.G.; Wedage, W.S.; Wickramasinghe, S.M.D.N.; Fernando, W.S. Hypoglycaemic action of the flavonoid fraction of Artocarpus heterophyllus leaf. Afr. J. Trad. Complement. Altern. Med. 2006, 3, 42–50. [Google Scholar] [CrossRef]
- Kotowaroo, M.I.; Mahomoodally, M.F.; Gurib-Fakim, A.; Subratty, A.H. Screening of traditional antidiabetic medicinal plants of mauritius for possible α-amylase inhibitory effects in vitro. Phytother. Res. 2006, 20, 228–231. [Google Scholar] [CrossRef]
- Englberger, L.; Lorennij, R.; Taylor, M.; Tuia, V.S.; Aalbersberg, W.; Dolodolotawake, U.; Tibon, L.; Tibon, J.; Alfred, J. Carotenoid content and traditional knowledge of breadfruit cultivars of the republic of the marshall islands. J. Food Compos. Anal. 2014, 34, 192–199. [Google Scholar] [CrossRef]
- Fu, J.; Wang, Z.; Huang, L.; Zheng, S.; Wang, D.; Chen, S.; Zhang, H.; Yang, S. Review of the botanical characteristics, phytochemistry, and pharmacology of Astragalus membranaceus (huangqi). Phytother. Res. 2014, 28, 1275–1283. [Google Scholar] [CrossRef]
- Liu, Y.; Nyberg, N.T.; Jäger, A.K.; Staerk, D. Facilitated visual interpretation of scores in principal component analysis by bioactivity-labeling of 1h-nmr spectra-metabolomics investigation and identification of a new α-glucosidase inhibitor in radix astragali. Molecules 2017, 22, 411. [Google Scholar] [CrossRef]
- Alhassan, A.; Ahmed, Q. Averrhoa bilimbi linn: A review of its ethnomedicinal uses, phytochemistry, and pharmacology. J. Pharm. Bioallied Sci. 2016, 8, 265–271. [Google Scholar]
- Khan, I.; Najeebullah, S.; Ali, M.; Shinwari, Z. Phytopharmacological and ethnomedicinal uses of the genus Berberis (berberidaceae): A review. Trop. J. Pharm. Res. 2016, 15, 2047–2057. [Google Scholar] [CrossRef]
- Mishra, R.; Shuaib, M.; Shravan; Mishra, P.S. A review on herbal antidiabetic drugs. J. Appl. Pharm. Sci. 2011, 1, 235–237. [Google Scholar]
- Maithani, A.; Parcha, V.; Kumar, D. Quantitative estimation of berberine content of berberis asiatica from different altitude of garhwal himalaya. Asian J. Pharm. Clin. Res. 2014, 7, 165–167. [Google Scholar]
- Rahimi-Madiseh, M.; Lorigoini, Z.; Zamani-Gharaghoshi, H.; Rafieian-Kopaei, M. Berberis vulgaris: Specifications and traditional uses. Iran. J. Basic Med. Sci. 2017, 20, 569–587. [Google Scholar] [PubMed]
- Cui, G.; Qin, X.; Zhang, Y.; Gong, Z.; Ge, B.; Zang, Y.Q. Berberine differentially modulates the activities of erk, p38 mapk, and jnk to suppress th17 and th1 t cell differentiation in type 1 diabetic mice. J. Biol. Chem. 2009, 284, 28420–28429. [Google Scholar] [CrossRef] [PubMed]
- Namsa, N.D.; Mandal, M.; Tangjang, S.; Mandal, S.C. Ethnobotany of the monpa ethnic group at arunachal pradesh, india. J. Ethnobiol. Ethnomed. 2011, 7, 31. [Google Scholar] [CrossRef]
- Maiti, R.; Rodriguez, H.G.; Kumari, C.A.; Sarkar, N.C. Macro and micro-nutrient contents of 18 medicinal plants used traditionally to alleviate diabetes in nuevo leon, northeast of mexico. Pak. J. Bot. 2016, 48, 271–276. [Google Scholar]
- Yun, J.L.; Dae, G.K.; Jin, S.K.; Ho, S.L. Buddleja officinalis inhibits high glucose-induced matrix metalloproteinase activity in human umbilical vein endothelial cells. Phytother. Res. 2008, 22, 1655–1659. [Google Scholar]
- Kumar, M.; Malik, J. Pharmacognostical studies and evaluation of quality parameters of butea frondosa leaves. Int. J. Pharmcy Pharm. Sci. 2012, 4, 610–614. [Google Scholar]
- Bhutkar, M.A.; Bhise, S.B. In vitro assay of alpha amylase inhibitory activity of some indigenous plants. Int. J. Chem. Sci. 2012, 10, 457–462. [Google Scholar]
- Wyrepkowski, C.C.; Da Costa, D.L.M.G.; Sinhorin, A.P.; Vilegas, W.; De Grandis, R.A.; Resende, F.A.; Varanda, E.A.; Dos Santos, L.C. Characterization and quantification of the compounds of the ethanolic extract from caesalpinia ferrea stem bark and evaluation of their mutagenic activity. Molecules 2014, 19, 16039–16057. [Google Scholar] [CrossRef]
- Ghosal, M.; Mandal, P. In-vitro antidiabetic and antioxidant activity of Calamus erectus roxb. Fruit: A wild plant of darjeeling himalaya. Int. J. Pharma Bio Sci. 2013, 4, P671–P684. [Google Scholar]
- Haque, M.M.; Choudhury, M.S.; Hossain, M.S.; Haque, M.A.; Debnath, K.; Hossain, S.; Mou, S.M.; Malek, I.; Rahmatullah, M. Evaluation of antihyperglycemic and antinociceptive properties of leaves of Calotropis gigantea R. Br. (asclepiadaceae)—A medicinal plant of bangladesh. Adv. Nat. Appl. Sci. 2012, 6, 1508–1514. [Google Scholar]
- Parihar, G.; Balekar, N. Calotropis procera: A phytochemical and pharmacological review. Thai J. Pharm. Sci. 2016, 40, 115–131. [Google Scholar]
- Dangi, K.S.; Mishra, S.N. Antihyperglycemic, antioxidant and hypolipidemic effect of Capparis aphylla stem extract in streptozotocin induced diabetic rats. Biol. Med. 2010, 2, 35–44. [Google Scholar]
- Goyal, M. Traditional plants used for the treatment of diabetes mellitus in sursagar constituency, jodhpur, rajasthan—An ethnomedicinal survey. J. Ethnopharmacol. 2015, 174, 364–368. [Google Scholar] [CrossRef]
- Zia-Ul-Haq, M.; Ćavar, S.; Qayum, M.; Imran, I.; de Feo, V. Compositional studies: Antioxidant and antidiabetic activities of Capparis decidua (forsk.) edgew. Int. J. Mol. Sci. 2011, 12, 8846–8861. [Google Scholar] [CrossRef]
- Selvamani, P.; Latha, S.; Elayaraja, K.; Babu, P.; Gupta, J.; Pal, T.; Ghosh, L.; Sen, D. Antidiabetic activity of the ethanol extract of Capparis sepiaria L. leaves. Indian J. Pharm. Sci. 2008, 70, 378–380. [Google Scholar] [CrossRef]
- Sher, H.; Alyemeni, M.N. Ethnobotanical and pharmaceutical evaluation of Capparis spinosa L., validity of local folk and unani system of medicine. J. Med. Plant Res. 2010, 4, 1751–1756. [Google Scholar]
- Adnan, M.; Jan, S.; Mussarat, S.; Tariq, A.; Begum, S.; Afroz, A.; Shinwari, Z.K. A review on ethnobotany, phytochemistry and pharmacology of plant genus Caralluma R. Br. J. Pharm. Pharmacol. 2014, 66, 1351–1368. [Google Scholar] [CrossRef]
- Maheshu, V.; Priyadarsini, D.T.; Sasikumar, J.M. Antioxidant capacity and amino acid analysis of Caralluma adscendens (roxb.) haw var. Fimbriata (wall.) grav. & mayur. Aerial parts. J. Food Sci. Technol. 2012, 51, 2415–2424. [Google Scholar]
- Bellamakondi, P.K.; Godavarthi, A.; Ibrahim, M. Anti-hyperglycemic activity of Caralluma umbellata haw. BioImpacts 2014, 4, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Uppal, G.K. A review on carissa carandas-phytochemistry, ethno-pharmacology, and micropropagation as conservation strategy. Asian J. Pharm. Clin. Res. 2015, 8, 26–30. [Google Scholar]
- Maobe, M.A.G.; Gitu, L.; Gatebe, E.; Rotich, H.; Karanja, P.N.; Votha, D.M.; Nderitu, I.W.; Kungu, W. Antifungal activity of eight selected medicinal herbs used for the treatment of diabetes, malaria and pneumonia in kisii region, southwest kenya. World J. Med. Sci. 2013, 8, 74–78. [Google Scholar]
- Ayyanar, M.; Ignacimuthu, S. Pharmacological actions of Cassia auriculata L. And Cissus quadrangularis wall: A short review. J. Pharmacol. Toxicol. 2008, 3, 213–221. [Google Scholar]
- Moshi, M.J.; Mbwambo, Z.H. Experience of tanzanian traditional healers in the management of non-insulin dependent diabetes mellitus. Pharm. Biol. 2002, 40, 552–560. [Google Scholar] [CrossRef]
- Thakur, M.; Asrani, R.K.; Thakur, S.; Sharma, P.K.; Patil, R.D.; Lal, B.; Parkash, O. Observations on traditional usage of ethnomedicinal plants in humans and animals of kangra and chamba districts of himachal pradesh in north-western himalaya, india. J. Ethnopharmacol. 2016, 191, 280–300. [Google Scholar] [CrossRef]
- He, Z.W.; Wei, W.; Li, S.P.; Ling, Q.; Liao, K.J.; Wang, X. Anti-allodynic effects of obtusifolin and gluco-obtusifolin against inflammatory and neuropathic pain: Possible mechanism for neuroinflammation. Biol. Pharm. Bull. 2014, 37, 1606–1616. [Google Scholar] [CrossRef]
- Salihu Shinkafi, T.; Bello, L.; Wara Hassan, S.; Ali, S. An ethnobotanical survey of antidiabetic plants used by hausa-fulani tribes in sokoto, northwest nigeria. J. Ethnopharmacol. 2015, 172, 91–99. [Google Scholar] [CrossRef]
- Garg, R.; Mohana, D.C.; Manjunath, K. In vitro antibacterial activity and phytochemical analysis of some traditional herbs. Int. J. Pharma Bio Sci. 2013, 4, 994–1003. [Google Scholar]
- Dalar, A.; Uzun, Y.; Mukemre, M.; Turker, M.; Konczak, I. Centaurea karduchorum boiss. From eastern anatolia: Phenolic composition, antioxidant and enzyme inhibitory activities. J. Herb. Med. 2015, 5, 211–216. [Google Scholar] [CrossRef]
- Moradi, M.; Mojab, F.; Bidgoli, S.A. Toxicity assessment of asteraceae centaurea repens l extract in mice. Iran. J. Pharm. Res. 2017, 16, 1073–1081. [Google Scholar]
- Tüzün, B.S.; Hajdú, Z.; Orbán-Gyapai, O.; Zomborszki, Z.P.; Jedlinszki, N.; Forgo, P.; Vçak, B.; Hohmann, J. Isolation of chemical constituents of centaurea virgata lam. And xanthine oxidase inhibitory activity of the plant extract and compounds. Med. Chem. 2017, 13, 498–502. [Google Scholar] [CrossRef] [PubMed]
- Alkofahi, A.S.; Abdul-Razzak, K.K.; Alzoubi, K.H.; Khabour, O.F. Screening of the anti-hyperglycemic activity of some medicinal plants of jordan. Pak. J. Pharma. Sci. 2017, 30, 907–912. [Google Scholar]
- Dalar, A.; Konczak, I. Cichorium intybus from eastern anatolia: Phenolic composition, antioxidant and enzyme inhibitory activities. Ind. Crop. Prod. 2014, 60, 79–85. [Google Scholar] [CrossRef]
- Al-Dhubiab, B.E. Pharmaceutical applications and phytochemical profile of cinnamomum burmannii. Pharmacogn. Rev. 2012, 6, 125–131. [Google Scholar] [CrossRef]
- Zaidi, S.F.; Aziz, M.; Muhammad, J.S.; Kadowaki, M. Diverse pharmacological properties of Cinnamomum cassia: A review. Pak. J. Pharma. Sci. 2015, 28, 1433–1438. [Google Scholar]
- Boaduo, N.K.K.; Katerere, D.; Eloff, J.N.; Naidoo, V. Evaluation of six plant species used traditionally in the treatment and control of diabetes mellitus in south africa using in vitro methods. Pharm. Biol. 2014, 52, 756–761. [Google Scholar] [CrossRef]
- Mustaffa, F.; Hassan, Z.; Yusof, N.A.; Razak, K.N.A.; Asmawi, M.Z. Antidiabetic and antihyperlipidemic potential of standardized extract, fraction and subfraction of cinnamomum iners leaves. Int. J. Pharmcy Pharm. Sci. 2014, 6, 220–225. [Google Scholar]
- Seo, E.J.; Kuete, V.; Kadioglu, O.; Krusche, B.; Schröder, S.; Greten, H.J.; Arend, J.; Lee, I.S.; Efferth, T. Antiangiogenic activity and pharmacogenomics of medicinal plants from traditional Korean medicine. Evid. Based Complement. Altern. Med. 2013, 2013. [Google Scholar] [CrossRef]
- Gallo, M.; Ferracane, R.; Graziani, G.; Ritieni, A.; Fogliano, V. Microwave assisted extraction of phenolic compounds from four different spices. Molecules 2010, 15, 6365–6374. [Google Scholar] [CrossRef]
- Orhan, N.; Aslan, M.; Şüküroǧlu, M.; Deliorman Orhan, D. In vivo and in vitro antidiabetic effect of Cistus laurifolius L. And detection of major phenolic compounds by uplc-tof-ms analysis. J. Ethnopharmacol. 2013, 146, 859–865. [Google Scholar] [CrossRef] [PubMed]
- El Kabbaoui, M.; Chda, A.; El-Akhal, J.; Azdad, O.; Mejrhit, N.; Aarab, L.; Bencheikh, R.; Tazi, A. Acute and sub-chronic toxicity studies of the aqueous extract from leaves of Cistus ladaniferus L. In mice and rats. J. Ethnopharmacol. 2017, 209, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Sayah, K.; Marmouzi, I.; Naceiri Mrabti, H.; Cherrah, Y.; Faouzi, M.E.A. Antioxidant activity and inhibitory potential of Cistus salviifolius (L.) and Cistus monspeliensis (L.) aerial parts extracts against key enzymes linked to hyperglycemia. BioMed Res. Int. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.K.; Kim, K.S.; Yang, H.J.; Shin, M.H.; Suh, H.W.; Lee, K.B.; Ahn, K.S.; Um, J.Y.; Lee, S.G.; Lee, B.C.; et al. Hexane fraction of Citrus aurantium L. Stimulates glucagon-like peptide-1 (glp-1) secretion via membrane depolarization in nci-h716 cells. Bioch. J. 2012, 6, 41–47. [Google Scholar] [CrossRef]
- Tzeng, Y.M.; Rao, Y.K.; Lee, M.J.; Chen, K.; Lee, Y.C.; Wu, W.S. Insulin-mimetic action of rhoifolin and cosmosiin isolated from Citrus grandis (L.) osbeck leaves: Enhanced adiponectin secretion and insulin receptor phosphorylation in 3t3-l1 cells. Evid. Based Complement. Altern. Med. 2011, 2011. [Google Scholar] [CrossRef]
- Adeneye, A.A. Methanol seed extract of Citrus paradisi macfad lowers blood glucose, lipids and cardiovascular disease risk indices in normal wistar rats. Niger. Q. J. Hosp. Med. 2008, 18, 16–20. [Google Scholar] [CrossRef]
- Shakthi Deve, A.; Sathish kumar, T.; Kumaresan, K.; Rapheal, V.S. Extraction process optimization of polyphenols from indian Citrus sinensis—As novel antiglycative agents in the management of diabetes mellitus. J. Diabetes Metab. Disord. 2014, 13, 11. [Google Scholar] [CrossRef]
- Jadeja, R.N.; Thounaojam, M.C.; Ramani, U.V.; Devkar, R.V.; Ramachandran, A.V. Anti-obesity potential of clerodendron glandulosum.Coleb leaf aqueous extract. J. Ethnopharmacol. 2011, 135, 338–343. [Google Scholar] [CrossRef]
- Idoh, K.; Agbonon, A.; Potchoo, Y.; Gbeassor, M. Toxicological assessment of the hydroethanolic leaf extract of Clerodendrum capitatum in wistar rats. Pan Afr. Med. J. 2016, 24. [Google Scholar] [CrossRef]
- Gurudeeban, S.; Satyavani, K.; Shanmugapriya, R.; Ramanathan, T.; Umamaheswari, G.; Muthazagan, K. Antioxidant and radical scavenging effect of Clerodendrum inerme (L.). Glob. J. Pharmacol. 2010, 4, 91–94. [Google Scholar]
- Barman, T.K.; Kalita, P.; Pal, T.K. Comparative evaluation of total flavonoid content and antioxidant activity of methanolic root extract of Clerodendrum infortunatum and methanolic whole plant extract of biophytum sensitivum. Int. J. Pharm. Sci. Rev. Res. 2013, 22, 62–66. [Google Scholar]
- Mohan Maruga Raja, M.K.; Mishra, S.H. Comprehensive review of Clerodendrum phlomidis: A traditionally used bitter. J. Chin. Integr. Med. 2010, 8, 510–524. [Google Scholar] [CrossRef] [PubMed]
- Kuriyan, R.; Rajendran, R.; Bantwal, G.; Kurpad, A.V. Effect of supplementation of Coccinia cordifolia extract on newly detected diabetic patients. Diabetes Care 2008, 31, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Waisundara, V.Y.; Watawana, M.I. Evaluation of the antioxidant activity and additive effects of traditional medicinal herbs from sri lanka. Aust. J. Herb. Med. 2014, 26, 22–28. [Google Scholar]
- Attanayake, A.P.; Jayatilaka, K.A.P.W.; Pathirana, C.; Mudduwa, L.K.B. Antihyperglycemic activity of Coccinia grandis (L.) voigt in streptozotocin induced diabetic rats. Indian J. Trad. Knowl. 2015, 14, 376–381. [Google Scholar]
- Pulbutr, P.; Saweeram, N.; Ittisan, T.; Intrama, H.; Jaruchotikamol, A.; Cushnie, B. In vitro α-amylase and α-glucosidase inhibitory activities of Coccinia grandis aqueous leaf and stem extracts. J. Biol. Sci. 2017, 17, 61–68. [Google Scholar] [CrossRef]
- Yang, W.; She, L.; Yu, K.; Yan, S.; Zhang, X.; Tian, X.; Ma, S.; Zhang, X. Jatrorrhizine hydrochloride attenuates hyperlipidemia in a high-fat diet-induced obesity mouse model. Mol. Med. Rep. 2016, 14, 3277–3284. [Google Scholar] [CrossRef]
- Wang, M.F.; Zhu, Q.H.; He, Y.G. Treatment with cordyceps sinensis enriches treg population in peripheral lymph nodes and delays type i diabetes development in nod mice. Pharmazie 2013, 68, 768–771. [Google Scholar]
- Tian, J.Y.; Chen, L.; Zhang, X.L.; Li, J.; Han, J.; Fu, J.Y.; Yang, X.M.; Zhang, P.C.; Ye, F. Investigation of a compound, compatibility of rhodiola crenulata, cordyceps militaris, and rheum palmatum, on metabolic syndrome treatment ii-improving obesity. Zhongguo Zhongyao Zazhi 2013, 38, 1411–1415. [Google Scholar]
- Wang, W.; Xu, J.; Li, L.; Wang, P.; Ji, X.; Ai, H.; Zhang, L.; Li, L. Neuroprotective effect of morroniside on focal cerebral ischemia in rats. Brain Res. Bull. 2010, 83, 196–201. [Google Scholar] [CrossRef]
- Park, C.H.; Noh, J.S.; Tanaka, T.; Uebaba, K.; Cho, E.J.; Yokozawa, T. The effects of corni fructus extract and its fractions against α-glucosidase inhibitory activities in vitro and sucrose tolerance in normal rats. Am. J. Chin. Med. 2011, 39, 367–380. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Park, K.K.; Lee, S.K.; Lee, S.E.; Hwang, J.K. Cornus kousa F. Buerger ex miquel increases glucose uptake through activation of peroxisome proliferator-activated receptor γ and insulin sensitization. J. Ethnopharmacol. 2011, 133, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Soltani, R.; Gorji, A.; Asgary, S.; Sarrafzadegan, N.; Siavash, M. Evaluation of the effects of Cornus mas L. Fruit extract on glycemic control and insulin level in type 2 diabetic adult patients: A randomized double-blind placebo-controlled clinical trial. Evid. Based Complement. Altern. Med. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.J.; Hebda, R.J. Contemporary use of bark for medicine by two salishan native elders of southeast vancouver island, canada. J. Ethnopharmacol. 1990, 29, 59–72. [Google Scholar] [CrossRef]
- McCune, L.M.; Johns, T. Antioxidant activity in medicinal plants associated with the symptoms of diabetes mellitus used by the indigenous peoples of the north american boreal forest. J. Ethnopharmacol. 2002, 82, 197–205. [Google Scholar] [CrossRef]
- Krishnan, K.; Mathew, L.E.; Vijayalakshmi, N.R.; Helen, A. Anti-inflammatory potential of β-amyrin, a triterpenoid isolated from costus igneus. Inflammopharmacology 2014, 22, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Maciel, M.A.M.; Pinto, A.C.; Arruda, A.C.; Pamplona, S.G.S.R.; Vanderlinde, F.A.; Lapa, A.J.; Echevarria, A.; Grynberg, N.F.; Côlus, I.M.S.; Farias, R.A.F.; et al. Ethnopharmacology, phytochemistry and pharmacology: A successful combination in the study of croton cajucara. J. Ethnopharmacol. 2000, 70, 41–55. [Google Scholar] [CrossRef]
- Biscaro, F.; Parisotto, E.B.; Zanette, V.C.; Günther, T.M.F.; Ferreira, E.A.; Gris, E.F.; Correia, J.F.G.; Pich, C.T.; Mattivi, F.; Filho, D.W.; et al. Anticancer activity of flavonol and flavan-3-ol rich extracts from croton celtidifolius latex. Pharm. Biol. 2013, 51, 737–743. [Google Scholar] [CrossRef]
- Govindarajan, R.; Vijayakumar, M.; Rao, C.V.; Pushpangadan, P.; Asare-Anane, H.; Persaud, S.; Jones, P.; Houghton, P.J. Antidiabetic activity of croton klozchianus in rats and direct stimulation of insulin secretion in-vitro. J. Pharm. Pharmacol. 2008, 60, 371–376. [Google Scholar] [CrossRef]
- Okokon, J.E.; Bassey, A.L.; Obot, J. Antidiabetic activity of ethanolic leaf extract of croton zambesicus muell. (thunder plant) in alloxan diabetic rats. Afr. J. Trad. Complement. Altern. Med. 2006, 3, 21–26. [Google Scholar] [CrossRef]
- Panwar, N.S.; Pradheep, K.; Bhatt, K.C.; Deswal, R.P.S. Ethnobotany of a threatened medicinal plant “indravan” (Cucumis callosus) from central india. Med. Plants 2014, 6, 307–309. [Google Scholar] [CrossRef]
- Jamal, P.; Barkat, A.A.; Amid, A. Response surface optimization of the process conditions for anti-diabetic compounds from cucumis sativus. Afr. J. Biotechnol. 2011, 10, 18788–18794. [Google Scholar]
- Bayat, A.; Azizi-Soleiman, F.; Heidari-Beni, M.; Feizi, A.; Iraj, B.; Ghiasvand, R.; Askari, G. Effect of cucurbita ficifolia and probiotic yogurt consumption on blood glucose, lipid profile, and inflammatory marker in type 2 diabetes. Int. J. Prev. Med. 2016, 2016. [Google Scholar]
- Miranda-Perez, M.E.; Ortega-Camarillo, C.; Del Carmen Escobar-Villanueva, M.; Blancas-Flores, G.; Alarcon-Aguilar, F.J. Cucurbita ficifolia bouché increases insulin secretion in rinm5f cells through an influx of ca2+ from the endoplasmic reticulum. J. Ethnopharmacol. 2016, 188, 159–166. [Google Scholar] [CrossRef]
- Andrade-Cetto, A.; Heinrich, M. Mexican plants with hypoglycaemic effect used in the treatment of diabetes. J. Ethnopharmacol. 2005, 99, 325–348. [Google Scholar] [CrossRef]
- Sheh-Hong, L.; Darah, I. Assessment of anticandidal activity and cytotoxicity of root extract from curculigo latifolia on pathogenic candida albicans. J. Med. Sci. 2013, 13, 193–200. [Google Scholar] [CrossRef][Green Version]
- Thakur, M.; Chauhan, N.S.; Sharma, V.; Dixit, V.K.; Bhargava, S. Effect of curculigo orchioides on hyperglycemia-induced oligospermia and sexual dysfunction in male rats. Int. J. Impot. Res. 2012, 24, 31–37. [Google Scholar] [CrossRef][Green Version]
- Sushma, S.M.; Sharath, R.; Sujan Ganapathy, P.S.; Sivakamisundari, P.; Preetham, J. Pharmacognostic and phytochemical evaluation of Curcuma angustifolia roxb. (rhizome) indigenous ethno-medicinal plant used by tribal soliga community of biligirirangana hills. Int. J. Pharmacogn. Phytochem. Res. 2015, 7, 820–824. [Google Scholar]
- Yadav, K.D.; Chaudhury, A.K. Anti-obesity mechanism of Curcuma longa L.—An over view. Ind. J. Nat. Prod. Resour. 2016, 7, 99–106. [Google Scholar]
- Mahabub, A.H.; Hossain, M.; Karim, M.; Khan, M.; Jahan, R.; Rahmatullah, M. An ethnobotanical survey of jessore district in khulna division, bangladesh. Am. Eurasian J. Sustain. Agric. 2009, 3, 238–243. [Google Scholar]
- Peltzer, K.; Sydara, K.; Pengpid, S. Traditional, complementary and alternative medicine use in a community population in lao pdr. Afr. J. Trad. Complement. Altern. Med. 2016, 13, 95–100. [Google Scholar] [CrossRef][Green Version]
- Salleh, N.; Ismail, S.; Ab Halim, M.R. Effects of Curcuma xanthorrhiza extracts and their constituents on phase ii drug-metabolizing enzymes activity. Pharmacogn. Res. 2016, 8, 309–315. [Google Scholar]
- Yasni, S.; Imaizumi, K.; Sugano, M. Effects of an indonesian medicinal plant, Curcuma xanthorrhiza roxb., on the levels of serum glucose and triglyceride, fatty acid desaturation, and bile acid excretion in streptozotocin-induced diabetic rats. Agric. Biol. Chem. 1991, 55, 3005–3010. [Google Scholar] [CrossRef]
- Gao, J.M.; Li, R.; Zhang, L.; Jia, L.L.; Ying, X.X.; Dou, D.Q.; Li, J.C.; Li, H.B. Cuscuta chinensis seeds water extraction protecting murine osteoblastic mc3t3-e1 cells against tertiary butyl hydroperoxide induced injury. J. Ethnopharmacol. 2013, 148, 587–595. [Google Scholar] [CrossRef]
- Cui, Z.; Guo, Z.; Miao, J.; Wang, Z.; Li, Q.; Chai, X.; Li, M. The genus cynomorium in china: An ethnopharmacological and phytochemical review. J. Ethnopharmacol. 2013, 147, 1–15. [Google Scholar] [CrossRef]
- Sudipta, B.; Kumar, D.S.; Goutam, P.; Monalisha, D. Evaluation of antidiabetic activity and histological study of cyperus kyllinga endl. Roots. Ind. J. Nat. Prod. Resour. 2012, 3, 343–346. [Google Scholar]
- Elshamy, A.I.; El-Shazly, M.; Yassine, Y.M.; El-Bana, M.A.; Farrag, A.R.; Nassar, M.I.; Singab, A.N.; Noji, M.; Umeyama, A. Phenolic constituents, anti-inflammatory and antidiabetic activities of Cyperus laevigatus L. Pharm. J. 2014, 9, 828–833. [Google Scholar] [CrossRef]
- Pirzada, A.M.; Ali, H.H.; Naeem, M.; Latif, M.; Bukhari, A.H.; Tanveer, A. Cyperus rotundus L.: Traditional uses, phytochemistry, and pharmacological activities. J. Ethnopharmacol. 2015, 174, 540–560. [Google Scholar] [CrossRef]
- Rahman, M.M.; Hasan, M.N.; Das, A.K.; Hossain, M.T.; Jahan, R.; Khatun, M.A.; Rahmatullah, M. Effect of delonix regia leaf extract on glucose tolerance in glucose-induced hyperglycemic mice. Afr. J. Trad. Complement. Altern. Med. 2011, 8, 34–36. [Google Scholar]
- Nithya Devi, M.; Brindha, P. Herbal nutraceuticals in the management of cancer and chronic diseases—A select study. Int. J. Pharmcy Pharm. Sci. 2014, 6, 104–106. [Google Scholar]
- Yoo, S.R.; Jeong, S.J.; Lee, N.R.; Shin, H.K.; Seo, C.S. Simultaneous determination and anti-inflammatory effects of four phenolic compounds in dendrobii herba. Nat. Prod. Res. 2017, 31, 2923–2926. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Kuang, M.; Hu, G.P.; Wu, R.B.; Wang, J.; Liu, L.; Lin, Y.C. Loddigesiinols g-j: A-glucosidase inhibitors from Dendrobium loddigesii. Molecules 2014, 19, 8544–8555. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, S.; Pandey, M.M.; Rawat, A.K.S. An ethnomedicinal, phytochemical and pharmacological profile of Desmodium gangeticum (L.) DC. And Desmodium adscendens (Sw.) DC. J. Ethnopharmacol. 2011, 136, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zheng, C.; Hu, C.; Rahman, K.; Qin, L. The genus Desmodium (fabaceae)-traditional uses in chinese medicine, phytochemistry and pharmacology. J. Ethnopharmacol. 2011, 138, 314–332. [Google Scholar] [CrossRef]
- Wang, T.S.; Lii, C.K.; Huang, Y.C.; Chang, J.Y.; Yang, F.Y. Anticlastogenic effect of aqueous extract from water yam (Dioscorea alata L.). J. Med. Plant Res. 2011, 5, 6192–6202. [Google Scholar]
- Chopade, B.A.; Ghosh, S.; Ahire, M.; Patil, S.; Jabgunde, A.; Bhat Dusane, M.; Joshi, B.N.; Pardesi, K.; Jachak, S.; Dhavale, D.D. Antidiabetic activity of gnidia glauca and dioscorea bulbifera: Potent amylase and glucosidase inhibitors. Evid.-Based Complement. Altern. Med. 2012, 2012. [Google Scholar] [CrossRef]
- Kim, N.; Kim, S.H.; Kim, Y.J.; Kim, J.K.; Nam, M.K.; Rhim, H.; Yoon, S.K.; Choi, S.Z.; Son, M.; Kim, S.Y.; et al. Neurotrophic activity of da-9801, a mixture extract of Dioscorea japonica thunb. And Dioscorea nipponica makino, in vitro. J. Ethnopharmacol. 2011, 137, 312–319. [Google Scholar] [CrossRef]
- Wan Woo, K.; Wook Kwon, O.; Yeou Kim, S.; Zin Choi, S.; Won Son, M.; Hyun Kim, K.; Ro Lee, K. Phenolic derivatives from the rhizomes of Dioscorea nipponica and their anti-neuroinflammatory and neuroprotective activities. J. Ethnopharmacol. 2014, 155, 1164–1170. [Google Scholar] [CrossRef]
- Pi, W.X.; Feng, X.P.; Ye, L.H.; Cai, B.C. Combination of morroniside and diosgenin prevents high glucose-induced cardiomyocytes apoptosis. Molecules 2017, 22, 163. [Google Scholar] [CrossRef]
- Kuete, V.; Efferth, T. Pharmacogenomics of cameroonian traditional herbal medicine for cancer therapy. J. Ethnopharmacol. 2011, 137, 752–766. [Google Scholar] [CrossRef]
- Cho, B.O.; Yin, H.H.; Park, S.H.; Byun, E.B.; Ha, H.Y.; Jang, S.I. Anti-inflammatory activity of myricetin from Diospyros lotus through suppression of nf-κb and stat1 activation and nrf2-mediated ho-1 induction in lipopolysaccharide-stimulated raw264.7 macrophages. Biosci. Biotechnol. Biochem. 2016, 80, 1520–1530. [Google Scholar] [CrossRef] [PubMed]
- Kiran Kumar, A.N.D.E.; Gowrishankar, N.L.; Manju Bhargavi, V.; Nagarjuna, M.; Rajani, G.; Swetha, Y.; Vinay Reddy, P. Evaluation of anti ulcer activity of ethanol extract of Diospyros melanoxylon (roxb). Bark. Int. J. Pharmcy Pharm. Sci. 2012, 4, 537–539. [Google Scholar]
- Dewanjee, S.; Maiti, A.; Sahu, R.; Dua, T.K.; Mandal, V. Effective control of type 2 diabetes through antioxidant defense by edible fruits of diospyros peregrina. Evid.-Based Complement. Altern. Med. 2011, 2011. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; Bandyopadhyay, A. Promising phytomedicines from Elephantopus scaber L: A review. Res. J. Pharm. Biol. Chem. Sci. 2015, 6, 1508–1518. [Google Scholar]
- Ooi, K.L.; Muhammad, T.S.T.; Tan, M.L.; Sulaiman, S.F. Cytotoxic, apoptotic and anti-α-glucosidase activities of 3,4-di-o-caffeoyl quinic acid, an antioxidant isolated from the polyphenolic-rich extract of elephantopus mollis kunth. J. Ethnopharmacol. 2011, 135, 685–695. [Google Scholar] [CrossRef]
- Miura, T.; Kato, A. Hypoglycémie action ofembelia madagascariensis in normal and diabetic mice. Am. J. Chin. Med. 1997, 25, 169–173. [Google Scholar] [CrossRef]
- Bhandari, U.; Jain, N.; Ansari, M.N.; Pillai, K.K. Beneficial effect of embelia ribes ethanolic extract on blood pressure and glycosylated hemoglobin in streptozotocin-induced diabetes in rats. Fitoterapia 2008, 79, 351–355. [Google Scholar] [CrossRef]
- Ratnasooriya, W.D.; Somarathna, K.I.W.K.; Premakumara, G.A.S.; Ediriweera, E.R.H.S.S. Lack of antiglycation activity of fresh juice of whole plant of Enicostema axillare (lam.) raynal. J. Pharm. Negat. Results 2011, 2, 55–57. [Google Scholar] [CrossRef]
- Tripathi, A.K.; Bhoyar, P.K.; Baheti, J.R.; Biyani, D.M.; Khalique, M.; Kothmire, M.S.; Amgaonkar, Y.M.; Bhanarkar, A.B. Herbal antidiabetics: A review. Int. J. Res. Pharm. Sci. 2011, 2, 30–37. [Google Scholar]
- Sen, B.; Kessler, S.; Gurdal, B.; Kiemer, A.; Mat, A. The difference between the extracts of erica manipuliflora in flowering and fruiting periods in terms of the cytotoxic effects. J. Pharm. Istanb. Univ. 2016, 46, 71–78. [Google Scholar]
- Vadivel, V.; Biesalski, H.K. Phenolic content in traditionally processed Erythrina indica L. Seeds: Antioxidant potential and type ii diabetes related functionality. Curr. Nutr. Food Sci. 2011, 7, 200–208. [Google Scholar] [CrossRef]
- Bokaeian, M.; Nakhaee, A.; Moodi, B.; Khazaei, H.A. Eucalyptus globulus (eucalyptus) treatment of candidiasis in normal and diabetic rats. Iran. Biomed. J. 2010, 14, 121–126. [Google Scholar] [PubMed]
- Asgharpour, F.; Pouramir, M.; Moghadamnia, A.A. Evaluation of viscosity of traditional medicinal antihyperglycemic plant extracts and relationship with glucose diffusion in vitro. J. Med. Plants 2012, 11, 166–176. [Google Scholar]
- Ogunwande, I.A.; Matsui, T.; Fujise, T.; Matsumoto, K. A-glucosidase inhibitory profile of nigerian medicinal plants in immobilized assay system. Food Sci. Technol. Res. 2007, 13, 169–172. [Google Scholar] [CrossRef]
- Guillén, A.; Granados, S.; Rivas, K.E.; Estrada, O.; Echeverri, L.F.; Balcázar, N. Antihyperglycemic activity of eucalyptus tereticornis in insulin-resistant cells and a nutritional model of diabetic mice. Adv. Pharmacol. Sci. 2015, 2015. [Google Scholar] [CrossRef]
- Kumar, P.; Mehta, M.; Satija, S.; Garg, M. Enzymatic in vitro anti-diabetic activity of few traditional indian medicinal plants. J. Biol. Sci. 2013, 13, 540–544. [Google Scholar]
- Matsumura, T.; Kasai, M.; Hayashi, T.; Arisawa, M.; Momose, Y.; Arai, I.; Amagaya, S.; Komatsu, Y. A-glucosidase inhibitors from paraguayan natural medicine, nangapiry, the leaves of eugenia uniflora. Pharm. Biol. 2000, 38, 302–307. [Google Scholar] [CrossRef]
- Nguyen, Q.V.; Nguyen, N.H.; Wang, S.L.; Nguyen, V.B.; Nguyen, A.D. Free radical scavenging and antidiabetic activities of euonymus laxiflorus champ. Extract. Res. Chem. Intermed. 2017, 1–10, 5615–5624. [Google Scholar] [CrossRef]
- Hao, G.M.; Liu, Y.G.; Wu, Y.; Xing, W.; Guo, S.Z.; Wang, Y.; Wang, Z.L.; Li, C.; Lv, T.T.; Wang, H.L.; et al. The protective effect of the active components of erpc on diabetic peripheral neuropathy in rats. J. Ethnopharmacol. 2017, 202, 162–171. [Google Scholar] [CrossRef]
- Cristians, S.; Osuna-Fernández, H.R.; Ramírez-Ávila, G.; Muñóz-Ocotero, V.; Laguna-Hernández, G.; Brechú-Franco, A.E. Euphorbia dioeca kunth as a novel source for α-glucosidase inhibitors. Bol. Lat. Y Del Caribe De Plant. Med. Y Aromat. 2015, 14, 483–490. [Google Scholar]
- Gulati, V.; Gulati, P.; Harding, I.H.; Palombo, E.A. Exploring the anti-diabetic potential of australian aboriginal and indian ayurvedic plant extracts using cell-based assays. BMC Complement. Altern. Med. 2015, 15, 8. [Google Scholar] [CrossRef] [PubMed]
- Sheliya, M.A.; Rayhana, B.; Ali, A.; Pillai, K.K.; Aeri, V.; Sharma, M.; Mir, S.R. Inhibition of α-glucosidase by new prenylated flavonoids from Euphorbia hirta L. Herb. J. Ethnopharmacol. 2015, 176, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Alarcon-Aguilara, F.J.; Roman-Ramos, R.; Perez-Gutierrez, S.; Aguilar-Contreras, A.; Contreras-Weber, C.C.; Flores-Saenz, J.L. Study of the anti-hyperglycemic effect of plants used as antidiabetics. J. Ethnopharmacol. 1998, 61, 101–110. [Google Scholar] [CrossRef]
- Kareparamban, J.A.; Nikam, P.H.; Jadhav, A.P.; Kadam, V.J. Ferula foetida “hing”: A review. Res. J. Pharm. Biol. Chem. Sci. 2012, 3, 775–786. [Google Scholar]
- Zare, A.R.; Omidi, M.; Fallah Hoseini, H.; Yazdani, D.; Sh, R.; Irvani, N.; Oladzad, A. A review on pharmacological effects of Ferula assa-foetida L.: A systematic review. J. Med. Plants 2011, 10, 17–25. [Google Scholar]
- Sattar, Z.; Iranshahi, M. Phytochemistry and pharmacology of ferula hermonis boiss—A review. Drug Res. 2017, 67, 437–446. [Google Scholar] [CrossRef]
- Hamdan, I.I.; Afifi, F.U. Studies on the in vitro and in vivo hypoglycemic activities of some medicinal plants used in treatment of diabetes in jordanian traditional medicine. J. Ethnopharmacol. 2004, 93, 117–121. [Google Scholar] [CrossRef]
- Arunachalam, K.; Parimelazhagan, T. Antidiabetic activity of ficus amplissima smith. Bark extract in streptozotocin induced diabetic rats. J. Ethnopharmacol. 2013, 147, 302–310. [Google Scholar] [CrossRef]
- Joseph, B.; Justin Raj, S. Phytopharmacological and phytochemical properties of three ficus species—An overview. Int. J. Pharma Bio Sci. 2010, 1, 246–253. [Google Scholar]
- Joseph, B.; Justin Raj, S. An overview—Ficus bengalensis linn. Int. J. Pharm. Sci. Rev. Res. 2011, 6, 21–24. [Google Scholar]
- Marwat, S.K.; Fazal Ur, R.; Khan, E.A.; Khakwani, A.A.; Ullah, I.; Khan, K.U.; Khan, I.U. Useful ethnophytomedicinal recipes of angiosperms used against diabetes in south east asian countries (india, pakistan & sri lanka). Pak. J. Pharma. Sci. 2014, 27, 1333–1358. [Google Scholar]
- Joseph, B.; Justin Raj, S. Pharmacognostic and phytochemical properties of Ficus carica linn—An overview. Int. J. Pharm. Res. 2011, 3, 8–12. [Google Scholar]
- Badgujar, S.B.; Patel, V.V.; Bandivdekar, A.H.; Mahajan, R.T. Traditional uses, phytochemistry and pharmacology of ficus carica: A review. Pharm. Biol. 2014, 52, 1487–1503. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, Y.; Maibam, B.C.; Biswas, D.; Laisharm, S.; Deb, L.; Talukdar, N.C.; Borah, J.C. Anti-diabetic potential of selected ethno-medicinal plants of north east india. J. Ethnopharmacol. 2015, 171, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Misbah, H.; Aziz, A.A.; Aminudin, N. Antidiabetic and antioxidant properties of ficus deltoidea fruit extracts and fractions. BMC Complement. Altern. Med. 2013, 13, 118. [Google Scholar] [CrossRef]
- Farsi, E.; Shafaei, A.; Hor, S.Y.; Ahamed, M.B.K.; Yam, M.F.; Asmawi, M.Z.; Ismail, Z. Genotoxicity and acute and subchronic toxicity studies of a standardized methanolic extract of ficus deltoidea leaves. Clinics 2013, 68, 865–875. [Google Scholar] [CrossRef]
- Choo, C.Y.; Sulong, N.Y.; Man, F.; Wong, T.W. Vitexin and isovitexin from the leaves of ficus deltoidea with in-vivo α-glucosidase inhibition. J. Ethnopharmacol. 2012, 142, 776–781. [Google Scholar] [CrossRef]
- Ahmed, F.; Mueen Ahmed, K.; Abedin, M.; Karim, A. Traditional uses and pharmacological potential of ficus exasperata vahl. Syst. Rev. Pharm. 2012, 3, 15–23. [Google Scholar] [CrossRef]
- Vaishnav, R.; Agrawal, R.D.; Sandeep, S. Medicinal value and future perspective of some therapeutically important plants from indian western region. Int. J. Pharm. Sci. Rev. Res. 2015, 34, 88–93. [Google Scholar]
- Madubunyi, I.I.; Onoja, S.O.; Asuzu, I.U. In vitro antioxidant and in vivo antidiabetic potential of the methanol extract of ficus glumosa del (moraceae) stem bark in alloxan-induced diabetic mice. Comp. Clin. Pathol. 2012, 21, 389–394. [Google Scholar] [CrossRef]
- Fidele, N.; Abakar, D.; Emmanuel, T.; Sélestin, S.D.; Paulin, N.; Hamadjida, A.; Marcel, N.R.; Christian, B.; Samuel, G.; Nicolas, N.Y.; et al. Hypolipidemic and anti-atherogenic effect of aqueous extract leaves of Ficus glumosa (moraceae) in rats. Exp. Gerontol. 2015, 62, 53–62. [Google Scholar]
- Zayyanu Usman, U.; Mohammed, A.; Binti Mohamed, M. Role of ethanol leaf extracts of ficus glumosa on fasting blood glucose and liver function test results of diabetes treated rats. J. Med. Biomed. Res. 2015, 14, 64–71. [Google Scholar]
- Ali, M.; Chaudhary, N. Ficus hispida linn.: A review of its pharmacognostic and ethnomedicinal properties. Pharmacogn. Rev. 2011, 5, 96–102. [Google Scholar] [PubMed]
- Akhtar, N.; Syed, D.N.; Khan, M.I.; Adhami, V.M.; Mirza, B.; Mukhtar, H. The pentacyclic triterpenoid, plectranthoic acid, a novel activator of ampk induces apoptotic death in prostate cancer cells. Oncotarget 2016, 7, 3819–3831. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.W.C.; Tangah, J.; Inoue, T.; Kainuma, M.; Baba, K.; Oshiro, N.; Kezuka, M.; Kimura, N. Botany, uses, chemistry and pharmacology of ficus microcarpa: A short review. Syst. Rev. Pharm. 2017, 8, 103–111. [Google Scholar] [CrossRef]
- Singh, D.; Mukhija, M.; Singh, S.; Aggarwal, A.; Sundriyal, A. Anti-diabetic effect of hydroalcoholic extract of Ficus palmata forsk leaves in streptozotocin-induced diabetic rats. Int. J. Green Pharm. 2014, 8, 276–282. [Google Scholar]
- Shah, S.K.; Garg, G.; Jhade, D.; Pandey, H. Ficus racemosa linn: Its potentials food security and rural medicinal management. J. Pharm. Sci. Res. 2016, 8, 317–322. [Google Scholar]
- Solanki, N.D.; Bhavsar, S.K. Evaluation of phytochemical profile and antidiabetic activity of Ficus racemosa (linn.) stem bark in rats. Indian Drugs 2017, 54, 49–54. [Google Scholar]
- Patil, V.V.; Sutar, N.G.; Pimprikar, R.B.; Patil, A.P.; Chaudhari, R.Y.; Patil, V.R. Antihyperglycemic and hypoglycemic effect of ficus racemosa leaves. J. Nat. Rem. 2010, 10, 11–16. [Google Scholar]
- Sophia, D.; Manoharan, S. Hypolipidemic activities of Ficus racemosa linn. Bark in alloxan induced diabetic rats. Afr. J. Trad. Complement. Altern. Med. 2007, 4, 279–288. [Google Scholar] [CrossRef]
- Basar, M.H.; Hossain, S.J.; Sadhu, S.K.; Rahman, M.H. A comparative study of antioxidant potential of commonly used antidiabetic plants in bangladesh. Orient. Pharm. Exp. Med. 2013, 13, 21–28. [Google Scholar] [CrossRef]
- Singh, D.; Singh, B.; Goel, R.K. Traditional uses, phytochemistry and pharmacology of ficus religiosa: A review. J. Ethnopharmacol. 2011, 134, 565–583. [Google Scholar] [CrossRef] [PubMed]
- Awolola, G.V.; Koorbanally, N.A.; Chenia, H.; Shode, F.O.; Baijnath, H. Antibacterial and anti-biofilm activity of flavonoids and triterpenes isolated from the extracts of Ficus sansibarica warb. Subsp. Sansibarica (moraceae) extracts. Afr. J. Tradit. Complement. Altern. Med. 2014, 11, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Babu, K.; Gokul Shankar, S.; Rai, S. Comparative pharmacognostic studies on the barks of four ficus species. Turk. J. Bot. 2010, 34, 215–224. [Google Scholar]
- Hoshovs’ka, I.V.; Korkach, I.P.; Shymans’ka, T.V.; Kotsiuruba, A.V.; Sahach, V.F. Effects of uncoupling proteins on nitric oxide synthesis and oxidative stress development in ishemia-reperfusion of old rat hearts. Fiziolohichnyi zhurnal 2009, 55, 3–11. [Google Scholar] [PubMed]
- Suh, H.W.; Lee, K.B.; Kim, K.S.; Yang, H.J.; Choi, E.K.; Shin, M.H.; Park, Y.S.; Na, Y.C.; Ahn, K.S.; Jang, Y.P.; et al. A bitter herbal medicine gentiana scabra root extract stimulates glucagon-like peptide-1 secretion and regulates blood glucose in db/db mouse. J. Ethnopharmacol. 2015, 172, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, T.; Tanaka, Y.; Yamamoto, K.; Morii, H.; Kamisako, T.; Ogawa, H. Geranium dielsianum extract powder (miskamiskatm) improves the intestinal environment through alteration of microbiota and microbial metabolites in rats. J. Funct. Foods 2014, 11, 12–19. [Google Scholar] [CrossRef]
- Kasabri, V.; Abu-Dahab, R.; Afifi, F.U.; Naffa, R.; Majdalawi, L.; Shawash, H. In vitro effects of geranium graveolens, sarcopoterium spinosum and varthemia iphionoides extracts on pancreatic min6 proliferation and insulin secretion and on extrapancreatic glucose diffusion. Int. J. Diabetes Dev. Ctries. 2013, 33, 170–177. [Google Scholar] [CrossRef]
- Pandit, S.; Ponnusankar, S.; Bandyopadhyay, A.; Ota, S.; Mukherjee, P.K. Exploring the possible metabolism mediated interaction of glycyrrhiza glabra extract with cyp3a4 and cyp2d6. Phytother. Res. 2011, 25, 1429–1434. [Google Scholar] [CrossRef]
- Lee, M.; Son, M.; Ryu, E.; Shin, Y.S.; Kim, J.G.; Kang, B.W.; Cho, H.; Kang, H. Quercetin-induced apoptosis prevents ebv infection. Oncotarget 2015, 6, 12603–12624. [Google Scholar] [CrossRef]
- Shukla, R.; Sharma, D.C.; Baig, M.H.; Bano, S.; Roy, S.; Provazník, I.; Kamal, M.A. Antioxidant, antimicrobial activity and medicinal properties of Grewia asiatica L. Med. Chem. 2016, 12, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, A.; Sugumar, S.; Bitragunta, S.; Balasubramanyan, N. Molecular docking studies of (4z, 12z)-cyclopentadeca-4, 12-dienone from grewia hirsuta with some targets related to type 2 diabetes. BMC Complement. Altern. Med. 2015, 15, 73. [Google Scholar] [CrossRef] [PubMed]
- Meena, S.N.; Ghadi, S.C.; Janarthanam, M.K. Evaluation of medicinal properties of Grewia nervosa (lour.) panigrahi. Int. J. Pharma Bio Sci. 2013, 4, P821–P828. [Google Scholar]
- Xu, B.Q.; Zhang, Y.Q. Bioactive components of gynura divaricata and its potential use in health, food and medicine: A mini-review. Afr. J. Tradit. Complement. Altern. Med. AJTCAM 2017, 14, 113–127. [Google Scholar] [CrossRef]
- Ma, J.; Guo, C.; Pan, Y.; Lin, D.; Qiu, L.; Wen, L. Antioxidant and anti-inflammatory activities of ethyl acetate extract of Gynura formosana (kitam) leaves. Exp. Ther. Med. 2017, 14, 2303–2309. [Google Scholar] [CrossRef]
- Kusuma, D.Y.; Kristanti, A.N.; Wulan Manuhara, Y.S. Effect of sucrose and immersion frequency on production of adventitious roots and secondary metabolites of Gynura procumbens (lour.) merr in temporary immersion bioreactors. Asian J. Plant Sci. 2017, 16, 24–36. [Google Scholar]
- Vejanan, V.; Latip, J.; Chin, L.P.; Embi, N.; Sidek, H.M. In vitro and in vivo anti-plasmodial activities of gynura procumbens. Sains Malays. 2012, 41, 1535–1542. [Google Scholar]
- Puangpronpitag, D.; Kaewseejan, N.; Nakornriab, M. Evaluation of phytochemical composition and antibacterial property of gynura procumbens extract. Asian J. Plant Sci. 2012, 11, 77–82. [Google Scholar] [CrossRef][Green Version]
- Kwak, H.R.; Go, W.R.; Kim, M.; Kim, C.S.; Choi, H.S.; Seo, J.K.; Kim, J.G.; Kim, J.S. First report of broad bean wilt virus 2 in gynura procumbens in Korea. Plant Dis. 2017, 101, 514. [Google Scholar] [CrossRef]
- Yuandani; Jantan, I.; Husain, K. 4,5,4′-trihydroxychalcone, 8,8′-(ethene-1,2-diyl)-dinaphtalene-1,4,5-triol and rutin from gynura segetum inhibit phagocytosis, lymphocyte proliferation, cytokine release and nitric oxide production from phagocytic cells. BMC Complement. Altern. Med. 2017, 17, 211. [Google Scholar]
- Dong, Y.; Tang, D.; Zhang, N.; Li, Y.; Zhang, C.; Li, L.; Li, M. Phytochemicals and biological studies of plants in genus Hedysarum. Chem. Cent. J. 2013, 7, 124. [Google Scholar] [CrossRef]
- Pereira, C.G.; Barreira, L.; Bijttebier, S.; Pieters, L.; Neves, V.; Rodrigues, M.J.; Rivas, R.; Varela, J.; Custódio, L. Chemical profiling of infusions and decoctions of helichrysum italicum subsp. Picardii by uhplc-pda-ms and in vitro biological activities comparatively with green tea (Camellia sinensis) and rooibos tisane (Aspalathus linearis). J. Pharm. Biomed. Anal. 2017, 145, 593–603. [Google Scholar] [CrossRef] [PubMed]
- Pham, H.N.T.; Vuong, Q.V.; Bowyer, M.C.; Scarlett, C.J. Optimum conventional extraction conditions for phenolics, flavonoids, and antioxidant capacity of Helicteres hirsuta lour. Asia-Pac. J. Chem. Eng. 2017, 12, 332–347. [Google Scholar] [CrossRef]
- Varghese, E.; Pappachen, K.L.; Narayanan, S.S. Isolation and evaluation of antimicrobial properties of isolated phytoconstituents of fruits of Helicteres isora linn. Res. J. Pharm., Biol. Chem. Sci. 2012, 3, 959–964. [Google Scholar]
- Sinha, S.; Sharma, A.; Hemalatha Reddy, P.; Rathi, B.; Prasad, N.V.S.R.K.; Vashishtha, A. Evaluation of phytochemical and pharmacological aspects of Holarrhena antidysenterica (wall.): A comprehensive review. J. Pharm. Res. 2013, 6, 488–492. [Google Scholar] [CrossRef]
- Ogbole, O.O.; Aliu, L.O.; Abiodun, O.O.; Ajaiyeoba, E.O. Alpha-amylase inhibition and brine shrimp lethality activities of nine medicinal plant extracts from south-west nigerian ethnomedicine. J. Herbs Spices Med. Plants 2016, 22, 319–326. [Google Scholar] [CrossRef]
- Balamurugan, R.; Vendan, S.E.; Aravinthan, A.; Kim, J.H. Isolation and structural characterization of 2r, 3r taxifolin 3-o-rhamnoside from ethyl acetate extract of hydnocarpus alpina and its hypoglycemic effect by attenuating hepatic key enzymes of glucose metabolism in streptozotocin-induced diabetic rats. Biochimie 2015, 111, 70–81. [Google Scholar] [CrossRef]
- Reddy, S.V.; Tiwari, A.K.; Kumar, U.S.; Rao, R.J.; Rao, J.M. Free radical scavenging, enzyme inhibitory constituents from antidiabetic ayurvedic medicinal plant hydnocarpus wightiana blume. Phytother. Res. 2005, 19, 277–281. [Google Scholar] [CrossRef]
- Orhan, N.; Aslan, M.; Pekcan, M.; Orhan, D.D.; Bedir, E.; Ergun, F. Identification of hypoglycaemic compounds from berries of juniperus oxycedrus subsp. Oxycedrus through bioactivity guided isolation technique. J. Ethnopharmacol. 2012, 139, 110–118. [Google Scholar] [CrossRef]
- Gulfraz, M.; Ahmad, A.; Asad, M.J.; Sadiq, A.; Afzal, U.; Imran, M.; Anwar, P.; Zeenat, A.; Abbasi, K.S.; Maqsood, S.; et al. Antidiabetic activities of leaves and root extracts of Justicia adhatoda linn against alloxan induced diabetes in rats. Afr. J. Biotechnol. 2011, 10, 6101–6106. [Google Scholar]
- Periyanayagam, K.; Umamaheswari, B.; Suseela, L.; Padmini, M.; Ismail, M. Evaluation of antiangiogenic effect of the leaves of Justicia gendarussa (burm. F) (acanthaceae) by chrio allontoic membrane method. Am. J. Infect. Dis. 2009, 5, 187–189. [Google Scholar] [CrossRef]
- Carrington, S.; Cohall, D.H.; Gossell-Williams, M.; Lindo, J.F. The antimicrobial screening of a barbadian medicinal plant with indications for use in the treatment of diabetic wound infections. West Indian Med. J. 2012, 61, 861–864. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Andrade, R.; Cabañas-Wuan, A.; Arana-Argáez, V.E.; Alonso-Castro, A.J.; Zapata-Bustos, R.; Salazar-Olivo, L.A.; Domínguez, F.; Chávez, M.; Carranza-Álvarez, C.; García-Carrancá, A. Antidiabetic effects of Justicia spicigera schltdl (acanthaceae). J. Ethnopharmacol. 2012, 143, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Hanif, A.; Hossan, M.S.; Mia, M.M.K.; Islam, M.J.; Jahan, R.; Rahmatullah, M. Ethnobotanical survey of the rakhain tribe inhabiting the chittagong hill tracts region of bangladesh. Am. Eurasian J. Sustain. Agric. 2009, 3, 172–180. [Google Scholar]
- Bavarva, J.H.; Narasimhacharya, A.V.R.L. Leucas cephalotes regulates carbohydrate and lipid metabolism and improves antioxidant status in iddm and niddm rats. J. Ethnopharmacol. 2010, 127, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.Q.; Wang, Y.L.; Gan, S.R.; Chen, J.C. Polysaccharides from liriopes radix ameliorates hyperglycemia via various potential mechanisms in diabetic rats. J. Sci. Food Agric. 2014, 94, 975–982. [Google Scholar] [CrossRef]
- Liu, Y.; Wan, L.; Xiao, Z.; Wang, J.; Wang, Y.; Chen, J. Antidiabetic activity of polysaccharides from tuberous root of Liriope spicata var. Prolifera in kkay mice. Evid. Based Complement. Altern. Med. 2013, 2013. [Google Scholar] [CrossRef]
- Svarcova, I.; Heinrich, J.; Valentova, K. Berry fruits as a source of biologically active compounds: The case of lonicera caerulea. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 2007, 151, 163–174. [Google Scholar] [CrossRef]
- Chang, Y.X.; Ge, A.H.; Donnapee, S.; Li, J.; Bai, Y.; Liu, J.; He, J.; Yang, X.; Song, L.J.; Zhang, B.L.; et al. The multi-targets integrated fingerprinting for screening anti-diabetic compounds from a chinese medicine jinqi jiangtang tablet. J. Ethnopharmacol. 2015, 164, 210–222. [Google Scholar] [CrossRef]
- Dashora, N.; Chauhan, L.S.; Kumar, N. Luffa acutangula (linn.) roxb. Var. Amara (roxb.) a consensus review. Int. J. Pharma Bio Sci. 2013, 4, P835–P846. [Google Scholar]
- Balakrishnan, N.; Sharma, A. Preliminary phytochemical and pharmacological activities of Luffa cylindrica L. Fruit. Asian J. Pharm. Clin. Res. 2013, 6, 113–116. [Google Scholar]
- Modi, A.; Kumar, V. Luffa echinata roxb.-a review on its ethanomedicinal, phytochemical and pharmacological perspective. Asian Pac. J. Trop. Dis. 2014, 4, S7–S12. [Google Scholar] [CrossRef]
- Potterat, O. Goji (Lycium barbarum and L. Chinense): Phytochemistry, pharmacology and safety in the perspective of traditional uses and recent popularity. Planta Med. 2010, 76, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Ye, Z. Cortex lycii radicis extracts protect pancreatic beta cells under high glucose conditions. Curr. Mol. Med. 2016, 16, 591–595. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.Y.W.; Lam, F.C.; Leung, P.C.; Che, C.T.; Fung, K.P. Antihyperglycemic and antioxidative effects of a herbal formulation of radix astragali, radix codonopsis and cortex lycii in a mouse model of type 2 diabetes mellitus. Phytother. Res. 2009, 23, 658–665. [Google Scholar] [CrossRef] [PubMed]
- Dhar, P.; Tayade, A.; Ballabh, B.; Chaurasia, O.P.; Bhatt, R.P.; Srivastava, R.B. Lycium ruthenicum murray: A less-explored but high-value medicinal plant from trans-himalayan cold deserts of ladakh, india. Plant Arch. 2011, 11, 583–586. [Google Scholar]
- Aderibigbe, A.O.; Emudianughe, T.S.; Lawal, B.A.S. Antihyperglycaemic effect of mangifera indica in rat. Phytother. Res. 1999, 13, 504–507. [Google Scholar] [CrossRef]
- Nguyen, H.X.; Le, T.C.; Do, T.N.V.; Le, T.H.; Nguyen, N.T.; Nguyen, M.T.T. A-glucosidase inhibitors from the bark of mangifera mekongensis. Chem. Cent. J. 2016, 10, 45. [Google Scholar] [CrossRef][Green Version]
- Abd El-Mohsen, M.M.; Rabeh, M.A.; Abou-Setta, L.; El-Rashedy, A.A.; Hussein, A.A. Marrubiin: A potent α-glucosidase inhibitor from marrubium alysson. Int. J. Appl. Res. Nat. Prod. 2014, 7, 21–27. [Google Scholar]
- Edziri, H.; Mastouri, M.; Aouni, M.; Verschaeve, L. Polyphenols content, antioxidant and antiviral activities of leaf extracts of marrubium deserti growing in tunisia. South Afr. J. Bot. 2012, 80, 104–109. [Google Scholar] [CrossRef]
- Sweidan, N.I.; Zarga, M.H.A. Acylated flavonoid glucoside from marrubium vulgare. Lett. Org. Chem. 2016, 13, 277–282. [Google Scholar] [CrossRef][Green Version]
- Boudjelal, A.; Henchiri, C.; Siracusa, L.; Sari, M.; Ruberto, G. Compositional analysis and in vivo anti-diabetic activity of wild algerian Marrubium vulgare L. Infusion. Fitoterapia 2012, 83, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Ranganathan, R.; Vijayalakshmi, R.; Parameswari, P. Ethnomedicinal survey of jawadhu hills in tamil nadu. Asian J. Pharm. Clin. Res. 2012, 5, 45–49. [Google Scholar]
- Marimuthu, S.; Padmaja, B.; Nair, S. Phytochemical screening studies on melia orientalis by gc-ms analysis. Pharmacogn. Res. 2013, 5, 216–218. [Google Scholar]
- Baliga, M.; Rao, S. Radioprotective potential of mint: A brief review. J. Cancer Res. Ther. 2010, 6, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Manosroi, J.; Zaruwa, M.Z.; Manosroi, A. Potent hypoglycemic effect of nigerian anti-diabetic medicinal plants. J. Complement. Integr. Med. 2011, 8. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.; Imam, K.M.S.U.; Rahman, S.; Mou, S.M.; Choudhury, M.S.; Mahal, M.J.; Jahan, S.; Hossain, M.S.; Rahmatullah, M. Antihyperglycemic and antinociceptive activity of fabaceae family plants—An evaluation of Mimosa pigra L. Stems. Adv. Nat. Appl. Sci. 2012, 6, 1490–1495. [Google Scholar]
- Manosroi, J.; Moses, Z.Z.; Manosroi, W.; Manosroi, A. Hypoglycemic activity of thai medicinal plants selected from the thai/lanna medicinal recipe database manosroi ii. J. Ethnopharmacol. 2011, 138, 92–98. [Google Scholar] [CrossRef]
- Ganu, G.P.; Jadhav, S.S.; Deshpande, A.D. Antioxidant and antihyperglycemic potential of methanolic extract of bark of Mimusops elengi L. In mice. Int. J. Phytomed. 2010, 2, 116–123. [Google Scholar]
- Kumar, K.P.S.; Bhowmik, D. Traditional medicinal uses and therapeutic benefits of Momordica charantia linn. Int. J. Pharm. Sci. Rev. Res. 2010, 4, 23–28. [Google Scholar]
- Akharaiyi, F.C.; Akinyemi, A.J.; Isitua, C.C.; Ogunmefun, O.T.; Opakunle, S.O.; Fasae, J.K. Some antidiabetic medicinal plants used by traditional healers in Ado Ekiti, Nigeria. Bratisl. Med. J. 2017, 118, 504–505. [Google Scholar] [CrossRef]
- Wang, H.Y.; Kan, W.C.; Cheng, T.J.; Yu, S.H.; Chang, L.H.; Chuu, J.J. Differential anti-diabetic effects and mechanism of action of charantin-rich extract of taiwanese momordica charantia between type 1 and type 2 diabetic mice. Food Chem. Toxicol. 2014, 69, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Giovannini, P.; Howes, M.J.R.; Edwards, S.E. Medicinal plants used in the traditional management of diabetes and its sequelae in central america: A review. J. Ethnopharmacol. 2016, 184, 58–71. [Google Scholar] [CrossRef] [PubMed]
- Rammal, H.; Bouayed, J.; Desor, F.; Younos, C.; Soulimani, R. A study of the anti-hyperglycaemic effects of the medicinal plant, Momordica charantia L.: Validation and contribution. Phytotherapie 2009, 7, 191–196. [Google Scholar] [CrossRef]
- Balkhande, S.V.; Surwase, B.S. Antimicrobial activity of tuberous root extracts of momordica cymbalaria hook. Asian J. Pharm. Clin. Res. 2013, 6, 201–203. [Google Scholar]
- Van de Venter, M.; Roux, S.; Bungu, L.C.; Louw, J.; Crouch, N.R.; Grace, O.M.; Maharaj, V.; Pillay, P.; Sewnarian, P.; Bhagwandin, N.; et al. Antidiabetic screening and scoring of 11 plants traditionally used in south africa. J. Ethnopharmacol. 2008, 119, 81–86. [Google Scholar] [CrossRef]
- Di, R.; Huang, M.T.; Ho, C.T. Anti-inflammatory activities of mogrosides from momordica grosvenori in murine macrophages and a murine ear edema model. J. Agric. Food Chem. 2011, 59, 7474–7481. [Google Scholar] [CrossRef]
- Umar, A.N.; Mann, A.; Ajiboso, O.S.O. Ethnodietetics of moringa oleifera leaves amongst the ethnic groups in bida, niger state, nigeria and its hypoglycaemic effects in rats. Am. Eurasian J. Sustain. Agric. 2011, 5, 107–114. [Google Scholar]
- Geleta, B.; Makonnen, E.; Debella, A.; Abebe, A.; Fekadu, N. In vitro vasodilatory activity and possible mechanisms of the crude extracts and fractions of Moringa stenopetala (baker f.) cufod. Leaves in isolated thoracic aorta of guinea pigs. J. Exp. Pharm. 2016, 8, 35–42. [Google Scholar] [CrossRef]
- Dièye, A.M.; Sarr, A.; Diop, S.N.; Ndiaye, M.; Sy, G.Y.; Diarra, M.; Rajraji-Gaffary, I.; Ndiaye-Sy, A.; Faye, B. Medicinal plants and the treatment of diabetes in senegal: Survey with patients. Fundam. Clin. Pharmacol. 2008, 22, 211–216. [Google Scholar] [CrossRef]
- Ullah, M.F.; Bhat, S.H.; Abuduhier, F.M. Antidiabetic potential of hydro-alcoholic extract of moringa peregrina leaves: Implication as functional food for prophylactic intervention in prediabetic stage. J. Food Biochem. 2015, 39, 360–367. [Google Scholar] [CrossRef]
- Devi, B.; Sharma, N.; Kumar, D.; Jeet, K. Morus alba linn: A phytopharmacological review. Int. J. Pharmcy Pharm. Sci. 2013, 5, 14–18. [Google Scholar]
- Cai, S.; Sun, W.; Fan, Y.; Guo, X.; Xu, G.; Xu, T.; Hou, Y.; Zhao, B.; Feng, X.; Liu, T. Effect of mulberry leaf (folium mori) on insulin resistance via irs-1/pi3k/glut-4 signalling pathway in type 2 diabetes mellitus rats. Pharm. Biol. 2016, 54, 2685–2691. [Google Scholar] [CrossRef] [PubMed]
- Mussarat, S.; Abdel-Salam, N.M.; Tariq, A.; Wazir, S.M.; Ullah, R.; Adnan, M. Use of ethnomedicinal plants by the people living around indus river. Evid.-Based Complement. Altern. Med. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Oh, K.S.; Ryu, S.Y.; Lee, S.; Seo, H.W.; Oh, B.K.; Kim, Y.S.; Lee, B.H. Melanin-concentrating hormone-1 receptor antagonism and anti-obesity effects of ethanolic extract from morus alba leaves in diet-induced obese mice. J. Ethnopharmacol. 2009, 122, 216–220. [Google Scholar] [CrossRef]
- Lemus, I.; García, R.; Delvillar, E.; Knop, G. Hypoglycaemic activity of four plants used in chilean popular medicine. Phytother. Res. 1999, 13, 91–94. [Google Scholar] [CrossRef]
- Adisakwattana, S.; Ruengsamran, T.; Kampa, P.; Sompong, W. In vitro inhibitory effects of plant-based foods and their combinations on intestinal α-glucosidase and pancreatic α-amylase. BMC Complement. Altern. Med. 2012, 12, 110. [Google Scholar] [CrossRef]
- Yin, N.; Hong, X.; Han, Y.; Duan, Y.; Zhang, Y.; Chen, Z. Cortex mori radicis extract induces neurite outgrowth in pc12 cells activating erk signaling pathway via inhibiting ca2+ influx. Int. J. Clin. Exp. Med. 2015, 8, 5022–5032. [Google Scholar]
- Vadivel, V.; Biesalski, H.K. Total phenolic content, antioxidant activity, and type ii diabetes related functionality of traditionally processed ox-eye bean [Mucuna gigantea (Willd) DC.] seeds: An indian underutilized food legume. Food Sci. Biotechnol. 2011, 20, 783–791. [Google Scholar] [CrossRef]
- Kamat, N.; Pearline, D.; Thiagarajan, P. Murraya koenigii (L.) (curry leaf): A traditional indian plant. Res. J. Pharm. Biol. Chem. Sci. 2015, 6, 691–697. [Google Scholar]
- Dineshkumar, B.; Mitra, A.; Mahadevappa, M. Antidiabetic and hypolipidemic effects of mahanimbine (carbazole alkaloid) from murraya koenigii (rutaceae) leaves. Int. J. Phytomed. 2010, 2, 22–30. [Google Scholar]
- Narkhede, M.B. Evaluation of alpha amylase inhibitory potential of four traditional culinary leaves. Asian J. Pharm. Clin. Res. 2012, 5, 75–76. [Google Scholar]
- Kesari, A.N.; Gupta, R.K.; Watal, G. Hypoglycemic effects of murraya koenigii on normal and alloxan-diabetic rabbits. J. Ethnopharmacol. 2005, 97, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Vats, V.; Dhunnoo, Y.; Grover, J.K. Hypoglycemic and antihyperglycemic activity of murraya koenigii leaves in diabetic rats. J. Ethnopharmacol. 2002, 82, 111–116. [Google Scholar] [CrossRef]
- Venkatesh, K.V.; Girish Kumar, K.; Pradeepa, K.; Santosh Kumar, S.R. Antibacterial activity of ethanol extract of musa paradisiaca cv. Puttabale and musa acuminate cv. Grand naine. Asian J. Pharm. Clin. Res. 2013, 6, 167–170. [Google Scholar]
- Jayamurthy, P.; Aparna, B.; Gayathri, G.; Nisha, P. Evaluation of antioxidant potential of inflorescence and stalk of plantain (Musa sapientum). J. Food Biochem. 2013, 37, 2–7. [Google Scholar] [CrossRef]
- Parimala, M. In vitro antimicrobial activity and hptlc analysis of hydroalcoholic seed extract of nymphaea nouchali burm. F. BMC Complement. Altern. Med. 2014, 14, 361. [Google Scholar] [CrossRef] [PubMed]
- Mohan Maruga Raja, M.K.; Sethiya, N.K.; Mishra, S.H. A comprehensive review on nymphaea stellata: A traditionally used bitter. J. Adv. Pharm. Technol. Res. 2010, 1, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.N.; Zhao, Y.L.; Gao, X.L.; Zhao, Z.F.; Jing, Z.; Zeng, W.C.; Yang, R.; Peng, R.; Tong, T.; Wang, L.F.; et al. Intestinal α-glucosidase inhibitory activity and toxicological evaluation of nymphaea stellata flowers extract. J. Ethnopharmacol. 2010, 131, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Rajagopal, K.; Sasikala, K.; Ragavan, B. Hypoglycemic and antihyperglycemic activity of nymphaea stellata flowers in normal and alloxan diabetic rats. Pharm. Biol. 2008, 46, 654–659. [Google Scholar] [CrossRef]
- Berhow, M.A.; Affum, A.O.; Gyan, B.A. Rosmarinic acid content in antidiabetic aqueous extract of ocimum canum sims grown in ghana. J. Med. Food 2012, 15, 611–620. [Google Scholar] [CrossRef]
- Nyarko, A.K.; Asare-Anane, H.; Ofosuhene, M.; Addy, M.E. Extract of ocimum canum lowers blood glucose and facilitates insulin release by isolated pancreatic β-islet cells. Phytomedicine 2002, 9, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Egesie, U.G.; Adelaiye, A.B.; Ibu, J.O.; Egesie, O.J. Safety and hypoglycaemic properties of aqueous leaf extract of ocimum gratissimum in streptozotocin induced diabetic rats. Niger. J. Physiol. Sci. 2006, 21, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, R.K. Tulsi: A holy plant with high medicinal and therapeutic value. Int. J. Green Pharm. 2017, 11, S1–S12. [Google Scholar]
- Mahajan, N.; Rawal, S.; Verma, M.; Poddar, M.; Alok, S. A phytopharmacological overview on ocimum species with special emphasis on ocimum sanctum. Biomed. Prev. Nutr. 2013, 3, 185–192. [Google Scholar] [CrossRef]
- Mahabub, A.H.; Hossain, M.; Karim, M.; Khan, M.; Jahan, R.; Rahmatullah, M. An ethnobotanical survey of rajshahi district in rajshahi division, bangladesh. Am. Eurasian J. Sustain. Agric. 2009, 3, 143–150. [Google Scholar]
- Sethi, J.; Sood, S.; Seth, S.; Talwar, A. Evaluation of hypoglycemic and antioxidant effect of ocimum sanctum. Indian J. Clin. Biochem. 2004, 19, 152–155. [Google Scholar] [CrossRef]
- Mousavi, L.; Salleh, R.M.; Murugaiyah, V.; Asmawi, M.Z. Hypoglycemic and anti-hyperglycemic study of Ocimum tenuiflorum L. Leaves extract in normal and streptozotocin-induced diabetic rats. Asian Pac. J. Trop. Biomed. 2016, 6, 1029–1036. [Google Scholar] [CrossRef]
- Shikov, A.N.; Pozharitskaya, O.N.; Makarov, V.G.; Yang, W.Z.; Guo, D.A. Oplopanax elatus (nakai) nakai: Chemistry, traditional use and pharmacology. Chin. J. Nat. Med. 2014, 12, 721–729. [Google Scholar] [CrossRef]
- Tai, J.; Cheung, S.; Cheah, S.; Chan, E.; Hasman, D. In vitro anti-proliferative and antioxidant studies on devil’s club oplopanax horridus. J. Ethnopharmacol. 2006, 108, 228–235. [Google Scholar] [CrossRef]
- Tepe, B.; Cakir, A.; Sihoglu Tepe, A. Medicinal uses, phytochemistry, and pharmacology of Origanum onites (L.): A review. Chem. Biodivers. 2016, 13, 504–520. [Google Scholar] [CrossRef]
- McCue, P.; Vattem, D.; Shetty, K. Inhibitory effect of clonal oregano extracts against porcine pancreatic amylase in vitro. Asia Pac. J. Clin. Nutr. 2004, 13, 401–408. [Google Scholar] [PubMed]
- Singh, M.K.; Gidwani, B.; Gupta, A.; Dhongade, H.; Kaur, C.D.; Kashyap, P.P.; Tripathi, D.K. A review of the medicinal plants of genus Orthosiphon (lamiaceae). Int. J. Biol. Chem. 2015, 9, 318–331. [Google Scholar] [CrossRef]
- Man, S.; Kiong, L.S.; Ab’lah, N.A.; Abdullah, Z. Differentiation of the white and purple flower forms of Orthosiphon aristatus (blume) miq. By 1d and 2d correlation ir spectroscopy. J. Teknol. 2015, 77, 81–86. [Google Scholar] [CrossRef][Green Version]
- Muhammad, H.; Gomes-Carneiro, M.R.; Poa, K.S.; De-Oliveira, A.C.A.X.; Afzan, A.; Sulaiman, S.A.; Ismail, Z.; Paumgartten, F.J.R. Evaluation of the genotoxicity of orthosiphon stamineus aqueous extract. J. Ethnopharmacol. 2011, 133, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimpoor-Mashhadi, M.R.; Khaksar, Z.; Noorafshan, A.; Mogheisi, B. Stereological study of the effects of orally administrated otostegia persica extract on pancreatic beta cells in male diabetic rats. Comp. Clin. Pathol. 2014, 23, 761–767. [Google Scholar] [CrossRef]
- Shewamene, Z.; Abdelwuhab, M.; Birhanu, Z. Methanolic leaf exctract of otostegia integrifolia benth reduces blood glucose levels in diabetic, glucose loaded and normal rodents. BMC Complement. Altern. Med. 2015, 15, 19. [Google Scholar] [CrossRef]
- Wang, L.; Jiang, Y.; Han, T.; Zheng, C.; Qin, L. A phytochemical, pharmacological and clinical profile of paederia foetida and p. Scandens. Nat. Pro. Comm. 2014, 9, 879–886. [Google Scholar] [CrossRef]
- Yoon, I.S.; Jung, Y.; Kim, H.J.; Lim, H.J.; Cho, S.S.; Shim, J.H.; Kang, B.Y.; Cheon, S.H.; Kim, S.N.; Yoon, G. Hypoglycemic effect of paeonia lactiflora in high fat diet-induced type 2 diabetic mouse model. Korean J. Pharmacogn. 2014, 45, 194–199. [Google Scholar]
- Chen, J.; Hou, X.F.; Wang, G.; Zhong, Q.X.; Liu, Y.; Qiu, H.H.; Yang, N.; Gu, J.F.; Wang, C.F.; Zhang, L.; et al. Terpene glycoside component from moutan cortex ameliorates diabetic nephropathy by regulating endoplasmic reticulum stress-related inflammatory responses. J. Ethnopharmacol. 2016, 193, 433–444. [Google Scholar] [CrossRef]
- Chiabchalard, A.; Nooron, N. Antihyperglycemic effects of Pandanus amaryllifolius roxb. Leaf extract. Pharmacogn. Mag. 2015, 11, 117–122. [Google Scholar] [CrossRef]
- Madhavan, V.; Nagar, J.C.; Murali, A.; Mythreyi, R.; Yoganarasimhan, S.N. Antihyperglycemic activity of alcohol and aqueous extracts of pandanus fascicularis lam. Roots in alloxan induced diabetic rats. Pharmacologyonline 2008, 3, 529–536. [Google Scholar]
- Englberger, L.; Schierle, J.; Hofmann, P.; Lorens, A.; Albert, K.; Levendusky, A.; Paul, Y.; Lickaneth, E.; Elymore, A.; Maddison, M.; et al. Carotenoid and vitamin content of micronesian atoll foods: Pandanus (Pandanus tectorius) and garlic pear (crataeva speciosa) fruit. J. Food Compos. Anal. 2009, 22, 1–8. [Google Scholar] [CrossRef]
- Lee, H.; Choi, J.; Shik Shin, S.; Yoon, M. Effects of korean red ginseng (panax ginseng) on obesity and adipose inflammation in ovariectomized mice. J. Ethnopharmacol. 2016, 178, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Xia, P.; Guo, H.; Liang, Z.; Cui, X.; Liu, Y.; Liu, F. Nutritional composition of sanchi (panax notoginseng) seed and its potential for industrial use. Genet. Resour. Crop. Evol. 2014, 61, 663–667. [Google Scholar] [CrossRef]
- Yang, C.Y.; Wang, J.; Zhao, Y.; Shen, L.; Jiang, X.; Xie, Z.G.; Liang, N.; Zhang, L.; Chen, Z.H. Anti-diabetic effects of panax notoginseng saponins and its major anti-hyperglycemic components. J. Ethnopharmacol. 2010, 130, 231–236. [Google Scholar] [CrossRef]
- Mucalo, I.; Rahelić, D.; Jovanovski, E.; Božikov, V.; Romić, Z.; Vuksan, V. Effect of american ginseng (Panax quinquefolius L.) on glycemic control in type 2 diabetes. Coll. Antropol. 2012, 36, 1435–1440. [Google Scholar]
- Tokunaga, M.; Matsuda, H.; Iwahashi, H.; Naruto, S.; Tsuruoka, T.; Yagi, H.; Masuko, T.; Kubo, M. Studies on palauan medicinal herbs. Iv. Immunopotentiatory activities of ongael, leaves of phaleria cumingii (meisn.) f. Vill. In diabetic mice. J. Tradit. Med. 2006, 23, 24–26. [Google Scholar]
- Kavitha, N.; Ein Oon, C.; Chen, Y.; Kanwar, J.R.; Sasidharan, S. Phaleria macrocarpa (boerl.) fruit induce g0/g1 and g2/m cell cycle arrest and apoptosis through mitochondria-mediated pathway in mda-mb-231 human breast cancer cell. J. Ethnopharmacol. 2017, 201, 42–55. [Google Scholar] [CrossRef]
- Altaf, R.; Asmawi, M.Z.B.; Dewa, A.; Sadikun, A.; Umar, M.I. Phytochemistry and medicinal properties of Phaleria macrocarpa (scheff.) boerl. Extracts. Pharmacogn. Rev. 2013, 7, 73–80. [Google Scholar] [CrossRef]
- Nor Fariza, I.; Fadzureena, J.; Zunoliza, A.; Luqman Chuah, A.; Pin, K.Y.; Adawiah, I. Anti-inflammatory activity of the major compound from methanol extract of phaleria macrocarpa leaves. J. Appl. Sci. 2012, 12, 1195–1198. [Google Scholar] [CrossRef]
- Sabina, E.; Zaidul, I.S.M.; Ghafoor, K.; Jaffri, J.M.; Sahena, F.; Babiker, E.E.; Perumal, V.; Hamed, M.; Amid, M.; Khatib, A. Screening of various parts of phaleria macrocarpa plant for α-glucosidase inhibitory activity. J. Food Biochem. 2016, 40, 201–210. [Google Scholar] [CrossRef]
- Graz, B.; Kitalong, C.; Yano, V. Traditional local medicines in the republic of palau and non-communicable diseases (ncd), signs of effectiveness. J. Ethnopharmacol. 2015, 161, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Sarin, B.; Verma, N.; Martín, J.P.; Mohanty, A. An overview of important ethnomedicinal herbs of phyllanthus species: Present status and future prospects. Sci. World J. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Joseph, B.; Raj, S.J. An overview: Phannacognostic properties of Phyllanthus atnarus linn. Int. J. Pharmacol. 2011, 7, 40–45. [Google Scholar]
- Adedapo, A.A.; Ofuegbe, S.O. The evaluation of the hypoglycemic effect of soft drink leaf extract of Phyllanthus amarus (euphorbiaceae) in rats. J. Basic Clin. Physiol. Pharmacol. 2014, 25, 47–57. [Google Scholar] [CrossRef]
- Ali, H.; Houghton, P.J.; Soumyanath, A. A-amylase inhibitory activity of some malaysian plants used to treat diabetes; with particular reference to Phyllanthus amarus. J. Ethnopharmacol. 2006, 107, 449–455. [Google Scholar] [CrossRef]
- Baliga, M.S.; Meera, S.; Mathai, B.; Rai, M.P.; Pawar, V.; Palatty, P.L. Scientific validation of the ethnomedicinal properties of the ayurvedic drug triphala: A review. Chin. J. Integr. Med. 2012, 18, 946–954. [Google Scholar] [CrossRef]
- Moshi, M.J.; Mbwambo, Z.H.; Nondo, R.S.O.; Masimba, P.J.; Kamuhabwa, A.; Kapingu, M.C.; Thomas, P.; Richard, M. Evaluation of ethnomedical claims and brine shrimp toxicity of some plants used in tanzania as traditional medicines. Afr. J. Tradit. Complement. Altern. Med. 2006, 3, 48–58. [Google Scholar]
- Ali, A.; Jameel, M.; Ali, M. New fatty acid and acyl glycoside from the aerial parts of Phyllanthus fraternus webster. J. Pharm. Bioallied Sci. 2016, 8, 43–46. [Google Scholar] [CrossRef]
- Muthulakshmi, S.; Bhavani, K.; Manju, R.; Mohamed Shahila, N.A. Hepatoprotective activity of Phyllanthus gardnerianus (wight) baill. Against d-galactosamine induced hepatotoxicity. Biomedicine 2014, 34, 36–44. [Google Scholar]
- Bharati, D.; Rawat, S.; Sharma, P.; Shrivastava, B. Comparative evaluation of antidiabetic antihypertensive activity of Cynodon dactylon L. and Phyllanthus niruri L in ratswith simultaneous type 2 diabetic and hypertension. Der Pharm. Lett. 2016, 8, 255–263. [Google Scholar]
- Fernández, G.A.I.; Rodríguez, I.E.R.; Camarillo, E.E.S.; Urdaneta, M.A.M. Hypoglycemic effect of Azadirachta indica A. Juss. And Phyllanthus niruri L. and their combined use in normal rats. Rev. Cuba. Plantas Med. 2011, 16, 183–189. [Google Scholar]
- Hashim, A.; Khan, M.S.; Khan, M.S.; Baig, M.H.; Ahmad, S. Antioxidant and α; ylase inhibitory property of Phyllanthus virgatus L.: An in vitro and molecular interaction study. BioMed Res. Int. 2013, 2013, 729393. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, S.; Abdul Wahab, N.; Zainal Abidin, N.; Manickam, S. Effect of extracts from Phyllanthus watsonii airy shaw on cell apoptosis in cultured human breast cancer mcf-7 cells. Exp. Toxicol. Pathol. 2013, 65, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Bano, A.; Dhaliwal, H.S.; Sharma, V. A pharmacological comprehensive review on ‘rassbhary’ Physalis angulata (L.). Int. J. Pharmcy Pharm. Sci. 2015, 7, 34–38. [Google Scholar]
- Ranilla, L.G.; Kwon, Y.I.; Apostolidis, E.; Shetty, K. Phenolic compounds, antioxidant activity and in vitro inhibitory potential against key enzymes relevant for hyperglycemia and hypertension of commonly used medicinal plants, herbs and spices in Latin America. Bioresour. Technol. 2010, 101, 4676–4689. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Misra, P.; Dube, A.; Bhattacharya, S.; Dikshit, M.; Ranade, S. Piper betle linn. A maligned pan-asiatic plant with an array of pharmacological activities and prospects for drug discovery. Curr. Sci. 2010, 99, 922–932. [Google Scholar]
- Arambewela, L.S.R.; Arawwawala, L.D.A.M.; Ratnasooriya, W.D. Antidiabetic activities of aqueous and ethanolic extracts of Piper betle leaves in rats. J. Ethnopharmacol. 2005, 102, 239–245. [Google Scholar] [CrossRef]
- Srividya, S.; Roshana Devi, V.; Subramanian, S. Hypoglycemic and hypolipidemic properties of hydroxychavicol, a major phenolic compound from the leaves of Piper betlelinn. Studied in high fat diet fed- low dose stz induced experimental type 2 diabetes in rats. Der Pharm. Lett. 2015, 7, 130–140. [Google Scholar]
- Safithri, M.E.G.A.; Fahma, F. Potency of Piper crocatum decoction as an antihiperglycemia in rat strain sprague dawley. Hayati J. Biosci. 2008, 15, 45–48. [Google Scholar] [CrossRef]
- Sh Ahmed, A.; Ahmed, Q.U.; Saxena, A.K.; Jamal, P. Evaluation of in vitro antidiabetic and antioxidant characterizations of Elettaria cardamomum (L.) maton (zingiberaceae), Piper cubeba L. F. (piperaceae), and Plumeria rubra L. (apocynaceae). Pak. J. Pharma. Sci. 2017, 30, 113–126. [Google Scholar]
- Srivastava, A.; Karthick, T.; Joshi, B.D.; Mishra, R.; Tandon, P.; Ayala, A.P.; Ellena, J. Spectroscopic (far or terahertz, mid-infrared and Raman) investigation, thermal analysis and biological activity of piplartine. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 184, 368–381. [Google Scholar] [CrossRef] [PubMed]
- Manoharan, S.; Silvan, S.; Vasudevan, K.; Balakrishnan, S. Antihyperglycemic and antilipidperoxidative effects of Piper longum (linn.) dried fruits in alloxan induced diabetic rat. J. Biol. Sci. 2007, 7, 161–168. [Google Scholar]
- Ashish, B.; Swapnil, G. Hypoglycemic effect of polyherbal formulation in alloxan induced diabetic rats. Pharmacologyonline 2011, 3, 764–773. [Google Scholar]
- Zar, C.T.; Teoh, S.L.; Das, S.; Zaiton, Z.; Farihah, H.S. Use Piper sarmentosum as an effective antidiabetic supplement in South East Asia: A review. Clin. Ter. 2012, 163, 505–510. [Google Scholar]
- Fairus, A.; Ima Nirwana, S.; Elvy Suhana, M.R.; Tan, M.H.; Santhana, R.; Farihah, H.S. Piper sarmentosum is comparable to glycyrrhizic acid in reducing visceral fat deposition in adrenalectomised rats given dexamethasone. Clin. Ter. 2013, 164, 5–10. [Google Scholar]
- Uddin, G.; Rauf, A.; Al-Othman, A.M.; Collina, S.; Arfan, M.; Ali, G.; Khan, I. Pistagremic acid, a glucosidase inhibitor from Pistacia integerrima. Fitoterapia 2012, 83, 1648–1652. [Google Scholar] [CrossRef]
- Wang, D.; Qi, M.; Yang, Q.; Tong, R.; Wang, R.; Bligh, S.W.A.; Yang, L.; Wang, Z. Comprehensive metabolite profiling of plantaginis semen using ultra high performance liquid chromatography with electrospray ionization quadrupole time-of-flight tandem mass spectrometry coupled with elevated energy technique. J. Sep. Sci. 2016, 39, 1842–1852. [Google Scholar] [CrossRef]
- Dalar, A.; Konczak, I. Phenolic contents, antioxidant capacities and inhibitory activities against key metabolic syndrome relevant enzymes of herbal teas from Eastern Anatolia. Ind. Crop. Prod. 2013, 44, 383–390. [Google Scholar] [CrossRef]
- Zoua, K.; Batomayena, B.; Kossi, M.; Lawson-Evi, P.; Kwashie, E.G.; Kodjo, A.; Messanvi, G. Effects of Plumeria alba roots hydro alcoholic extract on some parameters of type 2 diabetes. Res. J. Med. Plant 2014, 8, 140–148. [Google Scholar]
- Muruganantham, N.; Solomon, S.; Senthamilselvi, M.M. Anti-oxidant and anti-inflammatory activity of Plumeria rubra (flowers). Int. J. Pharm. Sci. Rev. Res. 2015, 30, 132–135. [Google Scholar]
- Narasimhulu, G.; Reddy, K.K.; Mohamed, J. The genus polygonum (polygonaceae): An ethnopharmacological and phytochemical perspectives—Review. Int. J. Pharmcy Pharm. Sci. 2014, 6, 21–45. [Google Scholar]
- Zhao, Y.; Chen, M.X.; Kongstad, K.T.; Jäger, A.K.; Staerk, D. Potential of Polygonum cuspidatum root as an antidiabetic food: Dual high-resolution α-glucosidase and ptp1b inhibition profiling combined with HPLC-HRMS and NMR for identification of antidiabetic constituents. J. Agric. Food Chem. 2017, 65, 4421–4427. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Wu, C.Q.; Jiang, T.; Wang, Q.J. Progress in microbiome and its application to pharmacological and toxicological research of traditional chinese materia medica. Chin. J. Pharmacol. Toxicol. 2016, 30, 975–982. [Google Scholar]
- Rodrigues, M.J.; Custódio, L.; Lopes, A.; Oliveira, M.; Neng, N.R.; Nogueira, J.M.F.; Martins, A.; Rauter, A.P.; Varela, J.; Barreira, L. Unlocking the in vitro anti-inflammatory and antidiabetic potential of Polygonum maritimum. Pharm. Biol. 2017, 55, 1348–1357. [Google Scholar] [CrossRef]
- Tang, W.; Li, S.; Liu, Y.; Huang, M.T.; Ho, C.T. Anti-inflammatory effects of trans-2,3,5,4′-tetrahydroxystilbene 2-O-β-glucopyranoside (THSG) from Polygonum multiflorum (PM) and hypoglycemic effect of cis-THSG enriched pm extract. J. Funct. Foods 2017, 34, 1–6. [Google Scholar] [CrossRef]
- Bothon, F.T.D.; Debiton, E.; Avlessi, F.; Forestier, C.; Teulade, J.C.; Sohounhloue, D.K.C. In vitro biological effects of two anti-diabetic medicinal plants used in Benin as folk medicine. BMC Complement. Altern. Med. 2013, 13, 51. [Google Scholar] [CrossRef]
- Im, I.; Park, K.R.; Kim, S.M.; Kim, C.; Park, J.H.; Nam, D.; Jang, H.J.; Shim, B.S.; Ahn, K.S.; Mosaddik, A.; et al. The butanol fraction of guava (Psidium cattleianum sabine) leaf extract suppresses MMP-2 and MMP-9 expression and activity through the suppression of the ERK1/2 mapk signaling pathway. Nutr. Cancer 2012, 64, 255–266. [Google Scholar] [CrossRef]
- Deguchi, Y.; Miyazaki, K. Anti-hyperglycemic and anti-hyperlipidemic effects of guava leaf extract. Nutr. Metab. 2010, 7, 9. [Google Scholar] [CrossRef]
- Cheng, J.T.; Yang, R.S. Hypoglycemic effect of guava juice in mice and human subjects. Am. J. Chin. Med. 1983, 11, 74–76. [Google Scholar] [CrossRef]
- Owen, P.L.; Martineau, L.C.; Caves, D.; Haddad, P.S.; Matainaho, T.; Johns, T. Consumption of guava (Psidium guajava L) and noni (Morinda citrifolia L) may protect betel quid-chewing papua new guineans against diabetes. Asia Pac. J. Clin. Nutr. 2008, 17, 635–643. [Google Scholar] [PubMed]
- Bulle, S.; Reddyvari, H.; Nallanchakravarthula, V.; Vaddi, D.R. Therapeutic potential of Pterocarpus santalinus L.: An update. Pharmacogn. Rev. 2016, 10, 43–49. [Google Scholar] [PubMed]
- Modak, M.; Dixit, P.; Londhe, J.; Ghaskadbi, S.; Devasagayam, T.P.A. Indian herbs and herbal drugs used for the treatment of diabetes. J. Clin. Biochem. Nutr. 2007, 40, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Tchamadeu, M.C.; Dzeufiet, P.D.D.; Nana, P.; Kouambou Nouga, C.C.; Ngueguim Tsofack, F.; Allard, J.; Blaes, N.; Siagat, R.; Zapfack, L.; Girolami, J.P.; et al. Acute and sub-chronic oral toxicity studies of an aqueous stem bark extract of Pterocarpus soyauxii taub (papilionaceae) in rodents. J. Ethnopharmacol. 2011, 133, 329–335. [Google Scholar] [CrossRef]
- Hephzibah Christabel, P.; Gopalakrishnan, V.K. Enzyme inhibitors from Prunus persica (L.) batsch: An alternate approach to treat diabetes. Intl. J. Pharma Bio Sci. 2013, 4, B1021–B1029. [Google Scholar]
- Pinto, M.D.S.; Ranilla, L.G.; Apostolidis, E.; Lajolo, F.M.; Genovese, M.I.; Shetty, K. Evaluation of antihyperglycemia and antihypertension potential of native peruvian fruits using in vitro models. J. Med. Food 2009, 12, 278–291. [Google Scholar] [CrossRef]
- Tu, X.; Xie, C.; Wang, F.; Chen, Q.; Zuo, Z.; Zhang, Q.; Wang, X.; Zhong, S.; Jordan, J.B. Fructus mume formula in the treatment of type 2 diabetes mellitus: A randomized controlled pilot trial. Evid.-Based Complement. Altern. Med. 2013, 2013, 787459. [Google Scholar] [CrossRef][Green Version]
- Li, H.; Zhao, L.; Zhang, B.; Jiang, Y.; Wang, X.; Guo, Y.; Liu, H.; Li, S.; Tong, X. A network pharmacology approach to determine active compounds and action mechanisms of ge-gen-qin-lian decoction for treatment of type 2 diabetes. Evid.-Based Complement. Altern. Med. 2014, 2014, 495840. [Google Scholar] [CrossRef]
- Seong, S.H.; Roy, A.; Jung, H.A.; Jung, H.J.; Choi, J.S. Protein tyrosine phosphatase 1b and α-glucosidase inhibitory activities of Pueraria lobata root and its constituents. J. Ethnopharmacol. 2016, 194, 706–716. [Google Scholar] [CrossRef]
- Sook Kim, Y.; Soo Lee, I.; Sook Kim, J. Protective effects of Puerariae radix extract and its single compounds on methylglyoxal-induced apoptosis in human retinal pigment epithelial cells. J. Ethnopharmacol. 2014, 152, 594–598. [Google Scholar] [CrossRef]
- Song, W.; Li, Y.; Qiao, X.; Qian, Y.; Ye, M. Chemistry of the chinese herbal medicine Puerariae radix (ge-gen): A review. J. Chin. Pharm. Sci. 2014, 23, 347–360. [Google Scholar] [CrossRef]
- Wong, K.H.; Razmovski-Naumovski, V.; Li, K.M.; Li, G.Q.; Chan, K. Differentiating puerariae lobatae radix and Puerariae thomsonii radix using HPTLC coupled with multivariate classification analyses. J. Pharm. Biomed. Anal. 2014, 95, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Shin, M.H.; Park, K.Y.; Lee, K.T.; Jung, H.J.; Lee, M.S.; Park, H.J. Effect of kaikasaponin III obtained from Pueraria thunbergiana flowers on serum and hepatic lipid peroxides and tissue factor activity in the streptozotocin-induced diabetic rat. J. Med. Food 2004, 7, 31–37. [Google Scholar] [CrossRef]
- Arvindekar, A.; More, T.; Payghan, P.V.; Laddha, K.; Ghoshal, N.; Arvindekar, A. Evaluation of anti-diabetic and alpha glucosidase inhibitory action of anthraquinones from Rheum emodi. Food. Funct. 2015, 6, 2693–2700. [Google Scholar] [CrossRef]
- Ban, E.; Park, M.; Jeong, S.; Kwon, T.; Kim, E.H.; Jung, K.; Kim, A. Poloxamer-based thermoreversible gel for topical delivery of emodin: Influence of P407 and P188 on solubility of emodin and its application in cellular activity screening. Molecules 2017, 22, 246. [Google Scholar] [CrossRef]
- Gao, J.; Shi, Z.; Zhu, S.; Li, G.Q.; Yan, R.; Yao, M. Influences of processed rhubarbs on the activities of four CYP isozymes and the metabolism of saxagliptin in rats based on probe cocktail and pharmacokinetics approaches. J. Ethnopharmacol. 2013, 145, 566–572. [Google Scholar] [CrossRef]
- Kasabri, V.; Abu-Dahab, R.; Afifi, F.U.; Naffa, R.; Majdalawi, L.; Shawash, H. In vitro modulation of pancreatic MIN6 insulin secretion and proliferation and extrapancreatic glucose absorption by Paronychia argentea, Rheum ribes and Teucrium polium extracts. Jordan J. Pharm. 2012, 5, 203–219. [Google Scholar]
- Naqishbandi, A.M.; Josefsen, K.; Pedersen, M.E.; Jger, A.K. Hypoglycemic activity of iraqi Rheum ribes root extract. Pharm. Biol. 2009, 47, 380–383. [Google Scholar] [CrossRef]
- Shiezadeh, F.; Mousavi, S.H.; Sadegh Amiri, M.; Iranshahi, M.; Tayarani-Najaran, Z.; Karimi, G. Cytotoxic and apoptotic potential of Rheum turkestanicum janisch root extract on human cancer and normal cells. Iran. J. Pharm. Res. 2013, 12, 811–819. [Google Scholar]
- Yoon, S.H.; Hong, M.S.; Chung, J.H.; Chung, S.H. Anti-apoptotic effect of Rheum undulatum water extract in pancreatic β-cell line, HIT-T15. Korean J. Physiol. Pharmacol. 2004, 8, 51–55. [Google Scholar]
- Popescu, R.; Kopp, B. The genus Rhododendron: An ethnopharmacological and toxicological review. J. Ethnopharmacol. 2013, 147, 42–62. [Google Scholar] [CrossRef] [PubMed]
- Tuan, N.Q.; Oh, J.; Park, H.B.; Ferreira, D.; Choe, S.; Lee, J.; Na, M. A grayanotox-9(11)-ene derivative from Rhododendron brachycarpum and its structural assignment via a protocol combining nmr and DP4 plus application. Phytochemistry 2017, 133, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Tam, T.W.; Liu, R.; Arnason, J.T.; Krantis, A.; Staines, W.A.; Haddad, P.S.; Foster, B.C. Actions of ethnobotanically selected cree anti-diabetic plants on human cytochrome P450 isoforms and flavin-containing monooxygenase 3. J. Ethnopharmacol. 2009, 126, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Tam, T.W.; Liu, R.; Arnason, J.T.; Krantis, A.; Staines, W.A.; Haddad, P.S.; Foster, B.C. Cree antidiabetic plant extracts display mechanism-based inactivation of CYP3A4. Can. J. Physiol. Pharmacol. 2011, 89, 13–23. [Google Scholar] [CrossRef]
- Mohammadi, S.; Kouhsari Montasser, S.; Feshani Monavar, A. Antidiabetic properties of the ethanolic extract of Rhus coriaria fruits in rats. DARU J. Pharm. Sci. 2010, 18, 270–275. [Google Scholar]
- Djakpo, O.; Yao, W. Rhus chinensis and galla chinensis—Folklore to modern evidence: Review. Phytother. Res. 2010, 24, 1739–1747. [Google Scholar] [CrossRef]
- Gade, D.R.; Sree Kumar Reddy, G.; Akki, S.N.R.; Vamsi Rajasekhar Reddy, P. Hepatoprotective activity of Rhus mysorensis against carbon tetrachloride induced hepatotoxicity in albino rats. Int. J. Pharm. Sci. Rev. Res. 2010, 4, 46–48. [Google Scholar]
- Kim, M.Y.; Chung, L.M.; Choi, D.C.; Park, H.J. Quantitative analysis of fustin and sulfuretin in the inner and outer heartwoods and stem bark of rhus verniciflua. Nat. Prod. Sci. 2009, 15, 208–212. [Google Scholar]
- Hashem Dabaghian, F.; Abdollahifard, M.; Khalighi Sigarudi, F.; Taghavi Shirazi, M.; Shojaee, A.; Sabet, Z.; Fallah Huseini, H. Effects of Rosa canina L. Fruit on glycemia and lipid profile in type 2 diabetic patients: A randomized, double-blind, placebo-controlled clinical trial. J. Med. Plants 2015, 14, 95–104. [Google Scholar]
- Orhan, N.; Aslan, M.; Hoşbaş, S.; Deliorman Orhan, D. Antidiabetic effect and antioxidant potential of Rosa canina fruits. Pharmacogn. Mag. 2009, 5, 309–315. [Google Scholar] [CrossRef]
- Nam, M.H.; Lee, H.S.; Hong, C.O.; Koo, Y.C.; Seomun, Y.; Lee, K.W. Preventive effects of Rosa rugosa root extract on advanced glycation end product-induced endothelial dysfunction. Korean J. Food Sci. Technol. 2010, 42, 210–216. [Google Scholar]
- Liu, L.; Tang, D.; Zhao, H.; Xin, X.; Aisa, H.A. Hypoglycemic effect of the polyphenols rich extract from Rose rugosa thunb on high fat diet and STZ induced diabetic rats. J. Ethnopharmacol. 2017, 200, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Oda, Y.; Ueda, F.; Kamei, A.; Kakinuma, C.; Abe, K. Biochemical investigation and gene expression analysis of the immunostimulatory functions of an edible salacia extract in rat small intestine. BioFactors 2011, 37, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Duggal, S. Salacia spp: Hypoglycemic principles and possible role in diabetes management. Integr. Med. 2010, 9, 40–43. [Google Scholar]
- Anitha, S.; Martha Leema Rose, A. Comparative evaluation of antihyperglycaemic effect of various parts of Salacia chinensis L. J. Med. Sci. 2013, 13, 493–496. [Google Scholar]
- Nakamura, S.; Matsuda, H.; Yoshikawa, M. Search for antidiabetic constituents of medicinal food. Yakugaku Zasshi 2011, 131, 909–915. [Google Scholar] [CrossRef]
- Tanabe, G.; Sakano, M.; Minematsu, T.; Matusda, H.; Yoshikawa, M.; Muraoka, O. Synthesis and elucidation of absolute stereochemistry of salaprinol, another thiosugar sulfonium sulfate from the ayurvedic traditional medicine Salacia prinoides. Tetrahedron 2008, 64, 10080–10086. [Google Scholar] [CrossRef]
- Im, R.; Mano, H.; Matsuura, T.; Nakatani, S.; Shimizu, J.; Wada, M. Mechanisms of blood glucose-lowering effect of aqueous extract from stems of kothala himbutu (Salacia reticulata) in the mouse. J. Ethnopharmacol. 2009, 121, 234–240. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Murakami, T.; Yashiro, K.; Matsuda, H. Kotalanol, a potent α-glucosidase inhibitor with thiosugar sulfonium sulfate structure, from antidiabetic ayurvedic medicine Salacia reticulata. Chem. Pharm. Bull. 1998, 46, 1339–1340. [Google Scholar] [CrossRef]
- Mohd Ali, N.; Yeap, S.K.; Ho, W.Y.; Beh, B.K.; Tan, S.W.; Tan, S.G. The promising future of chia, Salvia hispanica L. J. Biomed. Biotechnol. 2012, 2012, 171956. [Google Scholar] [CrossRef]
- Javdan, N.; Estakhr, J. Evaluation of the effects of Salvia hypoleuca on the expression of cytokines: IL-6, IL-10 and TNF-α in high fat diet-fed mice towards a cure for diabetes mellitus. Pharmacologyonline 2011, 2, 842–852. [Google Scholar]
- Bassil, M.; Daher, C.F.; Mroueh, M.; Zeeni, N. Salvia libanotica improves glycemia and serum lipid profile in rats fed a high fat diet. BMC Complement. Altern. Med. 2015, 15, 384. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ma, R.; Liu, C.; Liu, H.; Zhu, R.; Guo, S.; Tang, M.; Li, Y.; Niu, J.; Fu, M.; et al. Salvia miltiorrhiza: A potential red light to the development of cardiovascular diseases. Curr. Pharm. Des. 2017, 23, 1077–1097. [Google Scholar] [CrossRef] [PubMed]
- Arya, A.; Abdullah, M.A.; Haerian, B.S.; Mohd, M.A. Screening for hypoglycemic activity on the leaf extracts of nine medicinal plants: In-vivo evaluation. E-J. Chem. 2012, 9, 1196–1205. [Google Scholar] [CrossRef]
- Pawar, R.S.; Kumar, S.; Toppo, F.A.; Pk, L.; Suryavanshi, P. Sida cordifolia linn. Accelerates wound healing process in type 2 diabetic rats. J. Acute Med. 2016, 6, 82–89. [Google Scholar] [CrossRef]
- Narendhirakannan, R.T.; Limmy, T.P. Anti-inflammatory and anti-oxidant properties of Sida rhombifolia stems and roots in adjuvant induced arthritic rats. Immunopharmacol. Immunotoxicol. 2012, 34, 326–336. [Google Scholar] [CrossRef]
- Kang, Y.H.; Lee, Y.S.; Kim, K.K.; Kim, D.J.; Kim, T.W.; Choe, M. Study on antioxidative, antidiabetic and antiobesity activity of solvent fractions of Smilax china L. Leaf extract. J. Nutr. Health 2013, 46, 401–409. [Google Scholar] [CrossRef]
- Sang, H.Q.; Gu, J.F.; Yuan, J.R.; Zhang, M.H.; Jia, X.B.; Feng, L. The protective effect of Smilax glabra extract on advanced glycation end products-induced endothelial dysfunction in HUVECs via RAGE-ERK1/2-NF-κB pathway. J. Ethnopharmacol. 2014, 155, 785–795. [Google Scholar] [CrossRef]
- Aftab, T.B.; Bengir Al, L.; Akter, M.; Kalpana, M.A.; Anwarul Bashar, A.B.M.; Rahmatullah, M. Evaluation of antihyperglycemic activity of Smilax perfoliata lour. (smilacaceae) leaves in swiss albino mice. Adv. Nat. Appl. Sci. 2012, 6, 711–714. [Google Scholar]
- Tavares, D.C.; Munari, C.C.; De Freitas Araújo, M.G.; Beltrame, M.C.; Furtado, M.A.; Gonçalves, C.C.; Jorge Tiossi, R.F.; Bastos, J.K.; Cunha, W.R.; Sola Veneziani, R.C. Antimutagenic potential of Solanum lycocarpum against induction of chromosomal aberrations in V79 cells and micronuclei in mice by doxorubicin. Planta Med. 2011, 77, 1489–1494. [Google Scholar] [CrossRef]
- Ahmad, A.R.; Sakinah, W.; Asrifa, W.O. Study of antioxidant activity and determination of phenol and flavonoid content of pepino’s leaf extract (Solanum muricatum aiton). Int. J. Pharm. Res. 2014, 6, 600–606. [Google Scholar]
- Sohrabipour, S.; Kharazmi, F.; Soltani, N.; Kamalinejad, M. Biphasic effect of Solanum nigrum fruit aqueous extract on vascular mesenteric beds in non-diabetic and streptozotocin-induced diabetic rats. Pharmacogn. Res. 2014, 6, 148–152. [Google Scholar]
- Sathya Meonah, S.T.; Palaniswamy, M.; Immanuel Moses Keerthy, S.T.; Pradeep Rajkumar, L.A.; Usha Nandhini, R. Pharmacognostical and hypoglycemic activity of different parts of Solanum nigrum linn plant. Int. J. Pharmcy Pharm. Sci. 2012, 4, 221–224. [Google Scholar]
- Gandhi, G.R.; Ignacimuthu, S.; Paulraj, M.G.; Sasikumar, P. Antihyperglycemic activity and antidiabetic effect of methyl caffeate isolated from Solanum torvum swartz. Fruit in streptozotocin induced diabetic rats. Eur. J. Pharmacol. 2011, 670, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Perla, V.; Jayanty, S.S. Biguanide related compounds in traditional antidiabetic functional foods. Food Chem. 2013, 138, 1574–1580. [Google Scholar] [CrossRef]
- Aziz, M.A.; Khan, A.H.; Adnan, M.; Izatullah, I. Traditional uses of medicinal plants reported by the indigenous communities and local herbal practitioners of bajaur Agency, Federally Administrated Tribal Areas, Pakistan. J. Ethnopharmacol. 2017, 198, 268–281. [Google Scholar] [CrossRef]
- Kar, D.M.; Maharana, L.; Pattnaik, S.; Dash, G.K. Studies on hypoglycaemic activity of Solanum xanthocarpum schrad. & wendl. Fruit extract in rats. J. Ethnopharmacol. 2006, 108, 251–256. [Google Scholar]
- Fred-Jaiyesimi, A.; Kio, A.; Richard, W. A-amylase inhibitory effect of 3β-olean-12-en-3-yl (9z)-hexadec-9-enoate isolated from Spondias mombin leaf. Food Chem. 2009, 116, 285–288. [Google Scholar] [CrossRef]
- Sujarwo, W.; Saraswaty, V.; Keim, A.P.; Caneva, G.; Tofani, D. Ethnobotanical uses of ‘cemcem’ (Spondias pinnata (L. F.) kurz; anacardiaceae) leaves in bali (Indonesia) and its antioxidant activity. Pharmacologyonline 2017, 1, 113–123. [Google Scholar]
- Attanayake, A.P.; Jayatilaka, K.A.P.W.; Pathirana, C.; Mudduwa, L.K.B. Antihyperglycaemic, antihyperlipidaemic and β cell regenerative effects of Spondias pinnata (linn. F.) kurz. Bark extract on streptozotocin induced diabetic rats. Eur. J. Integr. Med. 2014, 6, 588–596. [Google Scholar] [CrossRef]
- Rani, M.P.; Raghu, K.G.; Nair, M.S.; Padmakumari, K.P. Isolation and identification of α-glucosidase and protein glycation inhibitors from Stereospermum colais. Appl. Biochem. Biotechnol. 2014, 173, 946–956. [Google Scholar] [CrossRef] [PubMed]
- Nag, M.; Mukherjee, P.K.; Chanda, J.; Biswas, R.; Harwansh, R.K.; Al-Dhabi, N.A.; Duraipandiyan, V. Plant developed analytical profile of Stereospermum suaveolens in Indian traditional knowledge. Indian J. Trad. Knowl. 2015, 14, 590–594. [Google Scholar]
- Kumar, V.; Van Staden, J. A review of Swertia chirayita (Gentianaceae) as a traditional medicinal plant. Front. Pharmacol. 2016, 6, 308. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.; Abdulsalam, F.I.; Pandey, D.K.; Bhattacharjee, A.; Eruvaram, N.R.; Malik, T. Evaluation of antioxidant, antibacterial, and antidiabetic potential of two traditional medicinal plants of India: Swertia cordata and Swertia chirayita. Pharmacogn. Res. 2015, 7, S57–S62. [Google Scholar]
- Saeidnia, S.; Ara, L.; Hajimehdipoor, H.; Read, R.W.; Arshadi, S.; Nikan, M. Chemical constituents of Swertia longifolia boiss. With α-amylase inhibitory activity. Res. Pharm. Sci. 2016, 11, 23–32. [Google Scholar]
- Wang, Y.L.; Xiao, Z.Q.; Liu, S.; Wan, L.S.; Yue, Y.D.; Zhang, Y.T.; Liu, Z.X.; Chen, J.C. Antidiabetic effects of Swertia macrosperma extracts in diabetic rats. J. Ethnopharmacol. 2013, 150, 536–544. [Google Scholar] [CrossRef]
- Luo, C.T.; Zheng, H.H.; Mao, S.S.; Yang, M.X.; Luo, C.; Chen, H. Xanthones from Swertia mussotii and their α-glycosidase inhibitory activities. Planta Med. 2014, 80, 201–208. [Google Scholar] [CrossRef]
- Kasetti, R.B.; Nabi, S.A.; Swapna, S.; Apparao, C. Cinnamic acid as one of the antidiabetic active principle(s) from the seeds of Syzygium alternifolium. Food Chem. Toxicol. 2012, 50, 1425–1431. [Google Scholar] [CrossRef]
- Adefegha, S.A.; Oboh, G.; Adefegha, O.M.; Boligon, A.A.; Athayde, M.L. Antihyperglycemic, hypolipidemic, hepatoprotective and antioxidative effects of dietary clove (Szyzgium aromaticum) bud powder in a high-fat diet/streptozotocin-induced diabetes rat model. J. Sci. Food Agric. 2014, 94, 2726–2737. [Google Scholar] [CrossRef]
- Bansode, T.S.; Salalkar, B.K. Phytochemical analysis of some selected indian medicinal plants. Intl. J. Pharma Bio Sci. 2015, 6, P550–P556. [Google Scholar]
- Teixeira, C.C.; Pinto, L.P.; Kessler, F.H.P.; Knijnik, L.; Pinto, C.P.; Gastaldo, G.J.; Fuchs, F.D. The effect of Syzygium cumini (L.) skeels on post-prandial blood glucose levels in non-diabetic rats and rats with streptozotocin-induced diabetes mellitus. J. Ethnopharmacol. 1997, 56, 209–213. [Google Scholar] [CrossRef]
- Sharma, S.; Pathak, S.; Gupta, G.; Sharma, S.K.; Singh, L.; Sharma, R.K.; Mishra, A.; Dua, K. Pharmacological evaluation of aqueous extract of Syzigium cumini for its antihyperglycemic and antidyslipidemic properties in diabetic rats fed a high cholesterol diet—Role of pparγ and pparα. Biomed. Pharmacother. 2017, 89, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Muthusamy, K.; Krishnasamy, G. A computational study on role of 6-(hydroxymethyl)-3-[3,4,5-trihydroxy-6-[(3,4,5-trihydroxyoxan-2-yl)oxymethyl]oxan-2-yl]oxyoxane-2,4,5-triol in the regulation of blood glucose level. J. Biomol. Struct. Dyn. 2016, 34, 2599–2618. [Google Scholar] [CrossRef] [PubMed]
- Baliga, M.S.; Fernandes, S.; Thilakchand, K.R.; D’Souza, P.; Rao, S. Scientific validation of the antidiabetic effects of Syzygium jambolanum DC (black plum), a traditional medicinal plant of India. J. Altern. Complement. Med. 2013, 19, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Zucchi, O.L.A.D.; Moreira, S.; De Jesus, E.F.O.; Salvio Neto, H.; Salvador, M.J. Characterization of hypoglycemiant plants by total reflection X-ray fluorescence spectrometry. Biol. Trace Elem. Res. 2005, 103, 277–290. [Google Scholar] [CrossRef]
- Gavillán-Suárez, J.; Aguilar-Perez, A.; Rivera-Ortiz, N.; Rodríguez-Tirado, K.; Figueroa-Cuilan, W.; Morales-Santiago, L.; Maldonado-Martínez, G.; Cubano, L.A.; Martínez-Montemayor, M.M. Chemical profile and in vivo hypoglycemic effects of Syzygium jambos, Costus speciosus and Tapeinochilos ananassae plant extracts used as diabetes adjuvants in puerto rico. BMC Complement. Altern. Med. 2015, 15, 244. [Google Scholar] [CrossRef]
- Zulkefli, H.N.; Mohamad, J.; Abidin, N.Z. Antioxidant activity of methanol extract of Tinospora crispa and Tabernaemontana corymbosa. Sains Malays. 2013, 42, 697–706. [Google Scholar]
- Sathishkumar, T.; Baskar, R. Renoprotective effect of Tabernaemontana heyneana Wall. Leaves against paracetamol-induced renotoxicity in rats and detection of polyphenols by high-performance liquid chromatography-diode array detector-mass spectrometry analysis. J. Acute Med. 2014, 4, 57–67. [Google Scholar] [CrossRef][Green Version]
- Jin, J.; Cai, D.; Bi, H.; Zhong, G.; Zeng, H.; Gu, L.; Huang, Z.; Huang, M. Comparative pharmacokinetics of paclitaxel after oral administration of Taxus yunnanensis extract and pure paclitaxel to rats. Fitoterapia 2013, 90, 1–9. [Google Scholar] [CrossRef]
- Nguyen, Q.V.; Nguyen, V.B.; Eun, J.B.; Wang, S.L.; Nguyen, D.H.; Tran, T.N.; Nguyen, A.D. Anti-oxidant and antidiabetic effect of some medicinal plants belong to Terminalia species collected in Dak Lak Province, Vietnam. Res Chem Intermed 2016, 42, 5859–5871. [Google Scholar] [CrossRef]
- Raghavan, B.; Kumari, S.K. Effect of Terminalia arjuna stem bark on antioxidant status in liver and kidney of alloxan diabetic rats. Indian J. Physiol. Pharmacol. 2006, 50, 133–142. [Google Scholar] [PubMed]
- Biswas, M.; Kar, B.; Bhattacharya, S.; Kumar, R.B.S.; Ghosh, A.K.; Haldar, P.K. Antihyperglycemic activity and antioxidant role of Terminalia arjuna leaf in streptozotocin-induced diabetic rats. Pharm. Biol. 2011, 49, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Kishimoto, Y.; Saita, E.; Suzuki-Sugihara, N.; Kamiya, T.; Taguchi, C.; Iida, K.; Kondo, K. Terminalia bellirica extract inhibits low-density lipoprotein oxidation and macrophage inflammatory response in vitro. Antioxidants 2016, 5, 20. [Google Scholar] [CrossRef] [PubMed]
- Biswajit, D.; Suvakanta, D.; Chandra, C.R. Pharmaceutical properties of terminalia bellerica (bahera)—An overview. Res. J. Pharm. Technol. 2014, 7, 592–597. [Google Scholar]
- Venkatalakshmi, P.; Brindha, P.; Saralla, R.P. Analytical and chemical standardisation studies on Terminalia catappa bark. Int. J. Pharmcy Pharm. Sci. 2014, 6, 4–8. [Google Scholar]
- Rao, N.K.; Nammi, S. Antidiabetic and renoprotective effects of the chloroform extract of Terminalia chebula Retz. Seeds in streptozotocin-induced diabetic rats. BMC Complement. Altern. Med. 2006, 6, 17. [Google Scholar] [CrossRef]
- Kadir, M.F.; Bin Sayeed, M.S.; Mia, M.M.K. Ethnopharmacological survey of medicinal plants used by indigenous and tribal people in Rangamati, Bangladesh. J. Ethnopharmacol. 2012, 144, 627–637. [Google Scholar] [CrossRef]
- Njomen, G.B.S.N.; Kamgang, R.; Soua, P.R.N.; Oyono, J.L.E.; Njikam, N. Protective effect of methanol-methylene chloride extract of Terminalia glaucescens leaves on streptozotocin-induced diabetes in mice. Trop. J. Pharm. Res. 2009, 8, 19–26. [Google Scholar] [CrossRef][Green Version]
- Pham, A.T.; Malterud, K.E.; Paulsen, B.S.; Diallo, D.; Wangensteen, H. A-glucosidase inhibition, 15-lipoxygenase inhibition, and brine shrimp toxicity of extracts and isolated compounds from Terminalia macroptera leaves. Pharm. Biol. 2014, 52, 1166–1169. [Google Scholar] [CrossRef]
- Nkobole, N.; Houghton, P.J.; Hussein, A.; Lall, N. Antidiabetic activity of Terminalia sericea constituents. Nat. Prod. Comm. 2011, 6, 1585–1588. [Google Scholar] [CrossRef]
- Padmashree; Prabhu, P.P.; Pandey, S. Anti diabetic activity of methanol/methylene chloride extract of Terminalia superba leaves on streptozotocin induced diabetes in rats. Int. J. Pharm. Res. 2010, 2, 2415–2419. [Google Scholar]
- Shahat, A.A.; Alsaid, M.S.; Kotob, S.E.; Husseiny, H.A.; Al-Ghamdi, A.A.M.; Ahmed, H.H. Biochemical and histological evidences for the antitumor potential of Teucrium oliverianum and Rhazya stricta in chemically-induced hepatocellular carcinoma. Afr. J. Trad. Complement. Altern. Med. 2016, 13, 62–70. [Google Scholar] [CrossRef]
- Bahramikia, S.; Yazdanparast, R. Phytochemistry and medicinal properties of Teucrium polium L. (lamiaceae). Phytother. Res. 2012, 26, 1581–1593. [Google Scholar] [CrossRef] [PubMed]
- Afifi, F.U.; Al-Khalidi, B.; Khalil, E. Studies on the in vivo hypoglycemic activities of two medicinal plants used in the treatment of diabetes in Jordanian traditional medicine following intranasal administration. J. Ethnopharmacol. 2005, 100, 314–318. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili-Mahani, S.; Falahi, F.; Yaghoobi, M.M. Proapoptotic and antiproliferative effects of Thymus caramanicus on human breast cancer cell line (MCF-7) and its interaction with anticancer drug vincristine. Evid.-Based Complement. Altern. Med. 2014, 2014, 893247. [Google Scholar] [CrossRef]
- El Kabbaoui, M.; Chda, A.; Mejrhit, N.; Azdad, O.; Farah, A.; Aarab, L.; Bencheikh, R.; Tazi, A. Antidiabetic effect of Thymus satureioides aqueous extract in streptozotocin-induced diabetic rats. Int. J. Pharmcy Pharm. Sci. 2016, 8, 140–145. [Google Scholar] [CrossRef][Green Version]
- Sharma, R.; Amin, H.; Galib; Prajapati, P.K. Antidiabetic claims of Tinospora cordifolia (Willd.) miers: Critical appraisal and role in therapy. Asian Pac. J. Trop. Biomed. 2015, 5, 68–78. [Google Scholar] [CrossRef]
- Patel, M.B.; Mishra, S. Hypoglycemic activity of alkaloidal fraction of Tinospora cordifolia. Phytomedicine 2011, 18, 1045–1052. [Google Scholar] [CrossRef]
- Thomas, A.; Rajesh, E.K.; Kumar, D.S. The significance of tinospora crispa in treatment of diabetes mellitus. Phytother. Res. 2016, 30, 357–366. [Google Scholar] [CrossRef]
- Ahmad, W.; Jantan, I.; Bukhari, S.N.A. Tinospora crispa (L.) Hook. F. & Thomson: A review of its ethnobotanical, phytochemical, and pharmacological aspects. Front. Pharmacol. 2016, 7, 59. [Google Scholar]
- Adnan, A.Z.; Taher, M.; Afriani, T.; Roesma, D.I.; Putra, A.E. Cytotoxic activity assay of tinocrisposide from Tinospora crispa on human cancer cells. Der Pharm. Lett. 2016, 8, 102–106. [Google Scholar]
- Noor, H.; Ashcroft, S.J.H. Antidiabetic effects of Tinospora crispa in rats. J. Ethnopharmacol. 1989, 27, 149–161. [Google Scholar] [CrossRef]
- Xu, Y.; Niu, Y.; Gao, Y.; Wang, F.; Qin, W.; Lu, Y.; Hu, J.; Peng, L.; Liu, J.; Xiong, W. Borapetoside E, a clerodane diterpenoid extracted from Tinospora crispa, improves hyperglycemia and hyperlipidemia in high-fat-diet-induced type 2 diabetes mice. J. Nat. Prod. 2017, 80, 2319–2327. [Google Scholar] [CrossRef] [PubMed]
- Klangjareonchai, T.; Putadechakum, S.; Roongpisuthipong, C. Review of anti-hyperglycemic effect of Tinospora crispa. Walailak J. Sci. Technol. 2015, 12, 403–406. [Google Scholar]
- Hedge, S.; Jayaraj, M.; Bhandarkar, A.V. Pharmacognostic and preliminary phytochemical studies of cold and hot extracts of stem of Tinospora malabarica Miers.—An important medicinal plant. Intl. J. Pharma Bio Sci. 2015, 6, P47–P54. [Google Scholar]
- Sidhu, M.C.; Thaku, S. Documentation of antidiabetic medicinal plants in district mandi of Himachal Pradesh (India). Int. J. Pharm. Res. 2015, 8, 164–169. [Google Scholar]
- Alamin, M.A.; Yagi, A.I.; Yagi, S.M. Evaluation of antidiabetic activity of plants used in Western Sudan. Asian Pac. J. Trop. Biomed. 2015, 5, 395–402. [Google Scholar] [CrossRef]
- Kumar, N.; Singh, S.; Manvi; Gupta, R. Trichosanthes dioica roxb.: An overview. Pharmacogn. Rev. 2012, 6, 61–67. [Google Scholar]
- Lo, H.Y.; Li, T.C.; Yang, T.Y.; Li, C.C.; Chiang, J.H.; Hsiang, C.Y.; Ho, T.Y. Hypoglycemic effects of Trichosanthes kirilowii and its protein constituent in diabetic mice: The involvement of insulin receptor pathway. BMC Complement. Altern. Med. 2017, 17, 53. [Google Scholar] [CrossRef]
- Uchholz, T.B.; Chen, C.; Zhang, X.Y.; Melzig, M.F. Pancreatic lipase and α-amylase inhibitory activities of plants used in Traditional Chinese Medicine (TCM). Pharmazie 2016, 71, 420–424. [Google Scholar]
- Kulandaivel, S.; Bajpai, P.; Sivakumar, T. Anti-hyperglycemic activity of Trichosanthes tricuspidata root extract. Banladesh J. Pharm. 2013, 8, 305–310. [Google Scholar] [CrossRef]
- Zhang, H.; Yan, X.; Jiang, Y.; Han, Y.; Zhou, Y. The extraction, identification and quantification of hypoglycemic active ingredients from Stinging nettle (Urtica angustifolia). Afr. J. Biotechnol. 2011, 10, 9428–9437. [Google Scholar]
- Rezaei Aref, T.; Minaii Zangii, B.; Latifpour, M. Protective effects of urtica dioica extract on the damage of rat small Intestinal mucosa caused by diabetes. J. Babol Univ. Med. Sci. 2012, 14, 31–37. [Google Scholar]
- Hoşbaş, S.; Aslan, M.; Sezik, E. Quality assesment of Urtica dioica L. Samples collected from different locations of Turkey. Turk. J. Pharm. Sci. 2014, 11, 223–230. [Google Scholar]
- Nickavar, B.; Yousefian, N. Evaluation of α-amylase inhibitory activities of selected antidiabetic medicinal plants. J. Verbrauch. Lebensmittelsicherh. 2011, 6, 191–195. [Google Scholar] [CrossRef]
- Nencu, I.; Vlase, L.; Istudor, V.; Mircea, T. Preliminary research regarding Urtica urens L. and Urtica dioica L. Farmacia 2015, 63, 710–715. [Google Scholar]
- Sánchez-Villavicencio, M.L.; Vinqvist-Tymchuk, M.; Kalt, W.; Matar, C.; Alarcón Aguilar, F.J.; Escobar Villanueva, M.C.; Haddad, P.S. Fermented blueberry juice extract and its specific fractions have an anti-adipogenic effect in 3 T3-L1 cells. BMC Complement. Altern. Med. 2017, 17, 24. [Google Scholar] [CrossRef]
- Nickavar, B.; Amin, G. Bioassay-guided separation of an α-amylase inhibitor anthocyanin from Vaccinium arctostaphylos berries. Z. Naturforsch. Sect. C J. Biosci. 2010, 65, 567–570. [Google Scholar] [CrossRef]
- Qian, H.F.; Li, Y.; Wang, L. Vaccinium bracteatum thunb. Leaves’ polysaccharide alleviates hepatic gluconeogenesis via the downregulation of miR-137. Biomed. Pharmacother. 2017, 95, 1397–1403. [Google Scholar] [CrossRef]
- Granfeldt, Y.E.; Björck, I.M.E. A bilberry drink with fermented oatmeal decreases postprandial insulin demand in young healthy adults. Nutr. J. 2011, 10, 57. [Google Scholar] [CrossRef]
- Kellogg, J.; Wang, J.; Flint, C.; Ribnicky, D.; Kuhn, P.; De Mejia, E.G.; Raskin, I.; Lila, M.A. Alaskan wild berry resources and human health under the cloud of climate change. J. Agric. Food Chem. 2010, 58, 3884–3900. [Google Scholar] [CrossRef]
- Beaulieu, L.P.; Harris, C.S.; Saleem, A.; Cuerrier, A.; Haddad, P.S.; Martineau, L.C.; Bennett, S.A.L.; Arnason, J.T. Inhibitory effect of the cree traditional medicine wiishichimanaanh (Vaccinium vitis-idaea) on advanced glycation endproduct formation: Identification of active principles. Phytother. Res. 2010, 24, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Mishra, J.; Dash, A.K.; Mishra, S.N.; Gupta, A.K. Withania coagulans in treatmen of diabetics and some other diseases: A review. Res. J. Pharm., Biol. Chem. Sci. 2013, 4, 1251–1258. [Google Scholar]
- Rehman, K.; Mashwani, Z.U.R.; Khan, M.A.; Ullah, Z.; Chaudhary, H.J. An ethno botanical perspective of traditional medicinal plants from the khattak tribe of Chonthra Karak, Pakistan. J. Ethnopharmacol. 2015, 165, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Maurya, R.; Akanksha; Jayendra; Singh, A.B.; Srivastava, A.K. Coagulanolide, a withanolide from Withania coagulans fruits and antihyperglycemic activity. Bioorg. Med. Chem. Lett. 2008, 18, 6534–6537. [Google Scholar] [CrossRef]
- Jonathan, G.; Rivka, R.; Avinoam, S.; Lumír, H.; Nirit, B. Hypoglycemic activity of withanolides and elicitated Withania somnifera. Phytochemistry 2015, 116, 283–289. [Google Scholar]
- Mukhija, M.; Lal Dhar, K.; Nath Kalia, A. Bioactive lignans from Zanthoxylum alatum Roxb. Stem bark with cytotoxic potential. J. Ethnopharmacol. 2014, 152, 106–112. [Google Scholar] [CrossRef]
- Adebayo, S.A.; Dzoyem, J.P.; Shai, L.J.; Eloff, J.N. The anti-inflammatory and antioxidant activity of 25 plant species used traditionally to treat pain in Southern African. BMC Complement. Altern. Med. 2015, 15, 159. [Google Scholar] [CrossRef]
- Pamhidzai, D.; Isaac, G. Tlc separation, antibacterial and anti-inflammatory activity of extracts derived from Zanthoxylum humile roots. Intern. J. Res. Ayurveda Pharm. 2013, 4, 482–486. [Google Scholar]
- Morakinyo, A.O.; Akindele, A.J.; Ahmed, Z. Modulation of antioxidant enzymes and inflammatory cytokines: Possible mechanism of anti-diabetic effect of ginger extracts. Afr. J. Biomed. Res. 2011, 14, 195–202. [Google Scholar]
- Chen, T.; Cai, J.; Ni, J.; Yang, F. An UPLC-MS/MS application to investigate chemical compositions in the ethanol extract with hypoglycemic activity from Zingiber striolatum diels. J. Chin. Pharm. Sci. 2016, 25, 116–121. [Google Scholar]
- Romero-Castillo, P.A.; Pérez Amador Barron, M.C.; Guevara Fefer, P.; Muñoz Ocotero, V.; Reyes Dorantes, A.; Aguirre Garcia, F.; Amaya Chavez, A. Anti-infammatory activity of Ziziphus amole. Phyton 2013, 82, 75–80. [Google Scholar]
- Sadegh-Nejadi, S.; Aberomand, M.; Ghaffari, M.A.; Mohammadzadeh, G.; Siahpoosh, A.; Afrisham, R. Inhibitory effect of Ziziphus jujuba and Heracleum persicum on the activity of partial purified rat intestinal alpha glucosidase enzyme. J. Maz. Univ. Med. Sci. 2016, 25, 135–146. [Google Scholar]
- Benammar, C.; Hichami, A.; Yessoufou, A.; Simonin, A.; Belarbi, M.; Allali, H.; Khan, N.A. Zizyphus lotus L. (Desf.) modulates antioxidant activity and human T-cell proliferation. BMC Complement. Altern. Med. 2010, 10, 54. [Google Scholar] [CrossRef] [PubMed]
- Diallo, D.; Sanogo, R.; Yasambou, H.; Traoré, A.; Coulibaly, K.; Maïga, A. Study of the chemical compounds of Ziziphus mauritiana Lam. (rhamnaceace) leaves, used traditionally in the treatment of diabetes in mali. C. R. Chim. 2004, 7, 1073–1080. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; Islam, M.S. Effects of butanol fraction of Ziziphus mucronata root ethanol extract on glucose homeostasis, serum insulin and other diabetes-related parameters in a murine model for type 2 diabetes. Pharm. Biol. 2017, 55, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Ahmad, N.; Naqvi, A.A. “Ziziphus oxyphylla”: Ethnobotanical, ethnopharmacological and phytochemical review. Biomed. Pharmacother. 2017, 91, 970–998. [Google Scholar] [CrossRef]
- Glombitza, K.W.; Mahran, G.H.; Mirhom, Y.W.; Michel, K.G.; Motawi, T.K. Hypoglycemic and antihyperglycemic effects of Zizyphus spina-christi in rats. Planta Med. 1994, 60, 244–247. [Google Scholar] [CrossRef]
- Modi, A.; Jain, S.; Kumar, V. Zizyphus xylopyrus (Retz.) willd: A review of its folkloric, phytochemical and pharmacological perspectives. Asian Pac. J. Trop. Dis. 2014, 4, S1–S6. [Google Scholar] [CrossRef]
- Solanki, A.; Zaveri, M. Pharmacognosy, phytochemistry and pharmacology of Abrus precatorius leaf: A review. Int. J. Pharm. Sci. Rev. Res. 2012, 13, 71–76. [Google Scholar]
- Liu, Y.X.; Si, M.M.; Lu, W.; Zhang, L.X.; Zhou, C.X.; Deng, S.L.; Wu, H.S. Effects and molecular mechanisms of the antidiabetic fraction of Acorus calamus L. On GLP-1 expression and secretion in vivo and in vitro. J. Ethnopharmacol. 2015, 166, 168–175. [Google Scholar] [CrossRef]
- Si, M.M.; Lou, J.S.; Zhou, C.X.; Shen, J.N.; Wu, H.H.; Yang, B.; He, Q.J.; Wu, H.S. Insulin releasing and alpha-glucosidase inhibitory activity of ethyl acetate fraction of Acorus calamus in vitro and in vivo. J. Ethnopharmacol. 2010, 128, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Park, H.M.; Son, M.W.; Kim, D.; Kim, S.H.; Kim, S.H.; Kwon, H.C.; Kim, S.Y. Fatty acid components of hardy kiwifruit (Actinidia arguta) as IL-4 production inhibitor. Biomol. Ther. 2011, 19, 126–133. [Google Scholar] [CrossRef]
- Liu, X.; Zhu, L.; Tan, J.; Zhou, X.; Xiao, L.; Yang, X.; Wang, B. Glucosidase inhibitory activity and antioxidant activity of flavonoid compound and triterpenoid compound from Agrimonia pilosa ledeb. BMC Complement. Altern. Med. 2014, 14, 12. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Bhat, Z.A.; Singh, P.; Khatanglakar, V.; Bhujbal, S.S. Antiasthmatic and antiallergic potential of methanolic extract of leaves of Ailanthus excelsa. Braz. J. Pharamacogn. 2011, 21, 139–145. [Google Scholar] [CrossRef]
- Hepcy Kalarani, D.; Dinakar, A.; Senthilkumar, N. Antidiabetic, analgesic and anti-inflammatory activity of aqueous extracts of stem and leaves of Alangium salvifolium and Pavonia zeylanica. Int. J. Drug. Dev. Res. 2012, 4, 298–306. [Google Scholar]
- Hepcy Kalarani, D.; Dinakar, A.; Senthilkumar, N. Hypoglycemic and antidiabetic activity of Alangium salvifolium wang in alloxan induced diabetic rats. Asian J. Pharm. Clin. Res. 2011, 4, 131–133. [Google Scholar]
- Jong-Anurakkun, N.; Bhandari, M.R.; Kawabata, J. A-glucosidase inhibitors from devil tree (Alstonia scholaris). Food Chem. 2007, 103, 1319–1323. [Google Scholar] [CrossRef]
- Babaei, H.; Sadeghpour, O.; Nahar, L.; Delazar, A.; Nazemiyeh, H.; Mansouri, M.R.; Poursaeid, N.; Asnaashari, S.; Moghadam, S.B.; Sarker, S.D. Antioxidant and vasorelaxant activities of flavonoids from Amygdalus lycioides var. Horrida. Turk. J. Biol. 2008, 32, 203–208. [Google Scholar]
- Rao, N.K. Anti-hyperglycemic and renal protective activities of Andrographis paniculata roots chloroform extract. Iran. J. Pharmacol. Ther. 2006, 5, 47–50. [Google Scholar]
- Sani, Y.N.; Haque, M.; Suryati, K.; Mohd, K.W.; Khan, A. Isolation and characterisation of andrographolide from Andrographis paniculata (Burm. F) wall. Ex nees and its total flavonoid effects from Kemaman, Malaysia. Int. J. Pharm. Qual. Assur. 2017, 8, 119–124. [Google Scholar]
- Kim, J.Y.; Shin, J.S.; Ryu, J.H.; Kim, S.Y.; Cho, Y.W.; Choi, J.H.; Lee, K.T. Anti-inflammatory effect of anemarsaponin B isolated from the rhizomes of Anemarrhena asphodeloides in LPS-induced raw 264.7 macrophages is mediated by negative regulation of the nuclear factor-κB and P38 pathways. Food Chem. Toxicol. 2009, 47, 1610–1617. [Google Scholar] [CrossRef] [PubMed]
- Nian, S.H.; Li, H.J.; Liu, E.H.; Li, P. Comparison of α-glucosidase inhibitory effect and bioactive constituents of Anemarrhenae rhizoma and fibrous roots. J. Pharm. Biomed. Anal. 2017, 145, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Golmohammadi, R.; Sabaghzadeh, F.; Mojadadi, M.S. Effect of hydroalcoholic extract of Anethum graveolens leaves on the dentate gyrus of the hippocampus in the epileptic mice: A histopathological and immunohistochemical study. Res. Pharm. Sci. 2016, 11, 259–264. [Google Scholar]
- Goodarzi, M.T.; Khodadadi, I.; Tavilani, H.; Abbasi Oshaghi, E. The role of Anethum graveolens L. (Dill) in the management of diabetes. J. Trop. Med. 2016, 2016, 1098916. [Google Scholar] [CrossRef]
- Devgan, M.; Bhatia, L.; Kumar, H. Anthocephalus cadamba: A comprehensive review. Res. J. Pharm. Technol. 2012, 5, 1478–1483. [Google Scholar]
- Shaikh, S.; Dubey, R.; Dhande, S.; Joshi, Y.M.; Kadam, V.J. Phytochemical and pharmacological profile of Aphanamixis polystachya: An overview. Res. J. Pharm. Technol. 2012, 5, 1260–1263. [Google Scholar]
- Xu, Z.; Ju, J.; Wang, K.; Gu, C.; Feng, Y. Evaluation of hypoglycemic activity of total lignans from Fructus arctii in the spontaneously diabetic goto-kakizaki rats. J. Ethnopharmacol. 2014, 151, 548–555. [Google Scholar] [CrossRef]
- Paulke, A.; Kremer, C.; Wunder, C.; Achenbach, J.; Djahanschiri, B.; Elias, A.; Stefan Schwed, J.; Hübner, H.; Gmeiner, P.; Proschak, E.; et al. Argyreia nervosa (Burm. F.): Receptor profiling of lysergic acid amide and other potential psychedelic LSD-like compounds by computational and binding assay approaches. J. Ethnopharmacol. 2013, 148, 492–497. [Google Scholar] [CrossRef]
- Gupta, V.; Keshari, B.B.; Tiwari, S.K.; Narasimha Murthy, K.H.H.V.S.S. A review on antidiabetic action of Asanadi gana. Intern. J. Res. Ayurveda Pharm. 2013, 4, 638–646. [Google Scholar] [CrossRef]
- Perez-Gutierrez, R.M.; Damian-Guzman, M. Meliacinolin: A potent α-glucosidase and α-amylase inhibitor isolated from Azadirachta indica leaves and in vivo antidiabetic property in streptozotocin-nicotinamide-induced type 2 diabetes in mice. Biol. Pharm. Bull. 2012, 35, 1516–1524. [Google Scholar] [CrossRef]
- Sujarwo, W.; Keim, A.P.; Caneva, G.; Toniolo, C.; Nicoletti, M. Ethnobotanical uses of neem (Azadirachta indica A.Juss.; meliaceae) leaves in bali (Indonesia) and the indian subcontinent in relation with historical background and phytochemical properties. J. Ethnopharmacol. 2016, 189, 186–193. [Google Scholar] [CrossRef]
- Shafie, N.I.; Samsulrizal, N.; Sopian, N.A.; Rajion, M.A.; Meng, G.Y.; Ajat, M.M.M.; Ahmad, H. Qualitative phytochemical screening and GC-MS profiling of Azadirachta excelsa leaf extract. Malays. Appl. Biol. 2015, 44, 87–92. [Google Scholar]
- Kaur, M.; Singh, G.; Mohan, C. Barringtonia acutangula: A traditional medicinal plant. Int. J. Pharm. Sci. Rev. Res. 2013, 23, 168–171. [Google Scholar]
- Jyothi, K.S.N.; Hemalatha, P.; Challa, S. Evaluation of α-amylase inhibitory potential of three medicinally important traditional wild food plants of India. Int. J. Green Pharm. 2011, 5, 95–99. [Google Scholar]
- Bhandari, M.R.; Jong-Anurakkun, N.; Hong, G.; Kawabata, J. A-glucosidase and α-amylase inhibitory activities of nepalese medicinal herb pakhanbhed (Bergenia ciliata, Haw.). Food Chem. 2008, 106, 247–252. [Google Scholar] [CrossRef]
- Puri, D. The insulinotropic activity of a nepalese medicinal plant Biophytum sensitivum: Preliminary experimental study. J. Ethnopharmacol. 2001, 78, 89–93. [Google Scholar] [CrossRef]
- Deepika, S.; Rajagopal, S.V. Evaluation of phytochemical and bioactive screening of Blepharis molluginifolia flower extracts. Intl. J. Pharma Bio Sci. 2014, 5, P204–P211. [Google Scholar]
- Savithramma, N.; Linga Rao, M.; Venkateswarlu, P. Histochemical studies of Boswellia ovalifoliolata Bal. & Henry—An endemic, endangered and threatened medicinal plant of Seshachalam Hill range of Eastern Ghats of India. Int. J. Pharmacogn. Phytochem. Res. 2014, 6, 1–6. [Google Scholar]
- Bljajić, K.; Petlevski, R.; Vujić, L.; Čačić, A.; Šoštarić, N.; Jablan, J.; De Carvalho, I.S.; Končić, M.Z. Chemical composition, antioxidant and α-glucosidase-inhibiting activities of the aqueous and hydroethanolic extracts of Vaccinium myrtillus leaves. Molecules 2017, 22, 703. [Google Scholar] [CrossRef]
- Darsini, I.P.; Shamshad, S.; John Paul, M. Canna indica (L.): A plant with potential healing powers: A review. Intl. J. Pharma Bio Sci. 2015, 6, B1–B8. [Google Scholar]
- Khan, H.U.; Khan, R.A.; Ahmed, M. Cytotoxic, antioxidant, antimicrobial activities of methonol crude extracts of Cardia obaliqua (Linn.). J. Anim. Plant Sci. 2017, 27, 1723–1726. [Google Scholar]
- Sabet, F.; Asgary, S.; Rahimi, P.; Mahzouni, P.; Madani, H. Antidiabetic effect of hydroalcoholic extract of Carthamus tinctorius L. In alloxan-induced diabetic rats. J. Res. Med. Sci. 2012, 17, 386–392. [Google Scholar]
- Takahashi, T.; Miyazawa, M. Potent α-glucosidase inhibitors from safflower (Carthamus tinctorius L.) seed. Phytother. Res. 2012, 26, 722–726. [Google Scholar] [CrossRef] [PubMed]
- Al-Shaqha, W.M.; Khan, M.; Salam, N.; Azzi, A.; Chaudhary, A.A. Anti-diabetic potential of Catharanthus roseus Linn. And its effect on the glucose transport gene (GLUT-2 and GLUT-4) in streptozotocin induced diabetic wistar rats. BMC Complement. Altern. Med. 2015, 15, 379. [Google Scholar] [CrossRef]
- Semenya, S.; Potgieter, M.; Tshisikhawe, M.; Shava, S.; Maroyi, A. Medicinal utilization of exotic plants by Bapedi traditional healers to treat human ailments in Limpopo province, South Africa. J. Ethnopharmacol. 2012, 144, 646–655. [Google Scholar] [CrossRef]
- Nammi, S.; Boini, K.M.; Lodagala, S.D.; Behara, R.B.S. The juice of fresh leaves of Catharanthus roseus Linn. Reduces blood glucose in normal and alloxan diabetic rabbits. BMC Complement. Altern. Med. 2003, 3, 4. [Google Scholar] [CrossRef]
- Rasineni, K.; Bellamkonda, R.; Singareddy, S.R.; Desireddy, S. Antihyperglycemic activity of Catharanthus roseus leaf powder in streptozotocin-induced diabetic rats. Pharmacogn. Res. 2010, 2, 195–201. [Google Scholar]
- Ojewole, J.A.O.; Adewunmi, C.O. Hypoglycaemic effects of methanolic leaf extract of Catharanthus roseus (Linn.) G. Don (Apocynaceae) in normal and diabetic mice. Acta Med. Biol. 2000, 48, 55–58. [Google Scholar]
- Kumar, D.; Kumar, S.; Gupta, J.; Arya, R.; Gupta, A. A review on chemical and biological properties of Cayratia trifolia Linn. (Vitaceae). Pharmacogn. Rev. 2011, 5, 184–188. [Google Scholar] [CrossRef]
- Alagawadi Kallangouda, R.; Shah Amol, S. Analgesic and antipyretic effects of Ceiba pentandra L. Seed extracts. Intl. J. Pharm. Res. 2012, 4, 46–49. [Google Scholar]
- Oyedemi, S.O.; Oyedemi, B.O.; Ijeh, I.I.; Ohanyerem, P.E.; Coopoosamy, R.M.; Aiyegoro, O.A. Alpha-amylase inhibition and antioxidative capacity of some antidiabetic plants used by the traditional healers in Southeastern Nigeria. Sci. World J. 2017, 2017, 3592491. [Google Scholar] [CrossRef] [PubMed]
- Satyaprakash, R.J.; Rajesh, M.S.; Bhanumathy, M.; Harish, M.S.; Shivananda, T.N.; Shivaprasad, H.N.; Sushma, G. Hypoglycemic and antihyperglycemic effect of Ceiba pentandra L. Gaertn in normal and streptozotocin-induced diabetic rats. Ghana Med J 2013, 47, 121–127. [Google Scholar] [PubMed]
- Tang, Y.; Xin, H.L.; Guo, M.L. Review on research of the phytochemistry and pharmacological activities of Celosia argentea. Braz. J. Pharamacogn. 2016, 26, 787–796. [Google Scholar] [CrossRef]
- Fitrianda, E.; Sukandar, E.Y.; Elfahmi; Adnyana, I.K. Antidiabetic activity of extract, fractions, and asiaticoside compound isolated from Centella asiatica Linn. Leaves in alloxan-induced diabetic mice. Asian J. Pharm. Clin. Res. 2017, 10, 268–272. [Google Scholar] [CrossRef]
- Maulidiani; Abas, F.; Khatib, A.; Perumal, V.; Suppaiah, V.; Ismail, A.; Hamid, M.; Shaari, K.; Lajis, N.H. Metabolic alteration in obese diabetes rats upon treatment with Centella asiatica extract. J. Ethnopharmacol. 2016, 180, 60–69. [Google Scholar]
- Zengin, G.; Nithiyanantham, S.; Locatelli, M.; Ceylan, R.; Uysal, S.; Aktumsek, A.; Selvi, P.K.; Maskovic, P. Screening of in vitro antioxidant and enzyme inhibitory activities of different extracts from two uninvestigated wild plants: Centranthus longiflorus subsp. longiflorus and Cerinthe minor subsp. auriculata. Eur. J. Integr. Med. 2016, 8, 286–292. [Google Scholar] [CrossRef]
- Paydar, M.; Moharam, B.A.; Wong, Y.L.; Looi, C.Y.; Wong, W.F.; Nyamathulla, S.; Pandy, V.; Kamalidehghan, B.; Arya, A. Centratherum anthelminticum (L.) kuntze a potential medicinal plant with pleiotropic pharmacological and biological activities. Int. J. Pharmacol. 2013, 9, 211–226. [Google Scholar]
- Thakur, G.S.; Bag, M.; Sanodiya, B.S.; Debnath, M.; Zacharia, A.; Bhadauriya, P.; Prasad, G.B.K.S.; Bisen, P.S. Chlorophytum borivilianum: A white gold for biopharmaceuticals and neutraceuticals. Curr. Pharm. Biotechnol. 2009, 10, 650–666. [Google Scholar] [CrossRef]
- Lai, W.C.; Wu, Y.C.; Dankó, B.; Cheng, Y.B.; Hsieh, T.J.; Hsieh, C.T.; Tsai, Y.C.; El-Shazly, M.; Martins, A.; Hohmann, J.; et al. Bioactive constituents of Cirsium japonicum var. Australe. J. Nat. Prod. 2014, 77, 1624–1631. [Google Scholar] [CrossRef]
- Xiong, W.T.; Gu, L.; Wang, C.; Sun, H.X.; Liu, X. Anti-hyperglycemic and hypolipidemic effects of Cistanche tubulosa in type 2 diabetic db/db mice. J. Ethnopharmacol. 2013, 150, 935–945. [Google Scholar] [CrossRef]
- Barghamdi, B.; Ghorat, F.; Asadollahi, K.; Sayehmiri, K.; Peyghambari, R.; Abangah, G. Therapeutic effects of Citrullus colocynthis fruit in patients with type II diabetes: A clinical trial study. J. Pharm. Bioallied Sci. 2016, 8, 130–134. [Google Scholar]
- Lahfa, F.B.; Azzi, R.; Mezouar, D.; Djaziri, R. Hypoglycemic effect of Citrullus colocynthis extracts. Phytotherapie 2017, 15, 50–56. [Google Scholar] [CrossRef]
- Alam, A.; Ferdosh, S.; Ghafoor, K.; Hakim, A.; Juraimi, A.S.; Khatib, A.; Sarker, Z.I. Clinacanthus nutans: A review of the medicinal uses, pharmacology and phytochemistry. Asian Pac. J. Trop. Med. 2016, 9, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.S.; Ahmad, M.S.; Mamat, A.S. A review on phytochemical constituents and pharmacological activities of Clinacanthus nutans. Int. J. Pharmcy Pharm. Sci. 2015, 7, 30–33. [Google Scholar]
- Kosai, P.; Sirisidthi, K.; Jiraungkoorskul, K.; Jiraungkoorskul, W. Review on ethnomedicinal uses of memory Boosting Herb, Butterfly Pea, Clitoria ternatea. J. Nat. Rem. 2015, 15, 71–76. [Google Scholar] [CrossRef]
- Kavitha, R. Evaluation of hypoglycemic effect of ethanolic extracts of leaf and fruit of T. dioica and leaf of C. ternatea in streptozotocin induced diabetic rats. Intl. J. Pharma Bio Sci. 2014, 5, B1061–B1068. [Google Scholar]
- Ramakrishnan, G.; Kothai, R.; Jaykar, B.; Venkata Rathnakumar, T. In vitro antibacterial activity of different extracts of leaves of Coldenia procumbens. Int. J. Pharm. Res. 2011, 3, 1000–1004. [Google Scholar]
- Shirwaikar, A.; Rajendran, K.; Punitha, I.S.R. Antidiabetic activity of alcoholic stem extract of Coscinium fenestratum in streptozotocin-nicotinamide induced type 2 diabetic rats. J. Ethnopharmacol. 2005, 97, 369–374. [Google Scholar] [CrossRef]
- Rai, R.V.; Rajesh, P.S.; Kim, H.M. Medicinal use of Coscinium fenestratum (Gaertn.) colebr.: An short review. Orient. Pharm. Exp. Med. 2013, 13, 1–9. [Google Scholar] [CrossRef]
- Priyashree, S.; Jha, S.; Pattanayak, S. A review on Cressa cretica linn.: A halophytic plant. Pharmacogn. Rev. 2010, 4, 161–166. [Google Scholar] [CrossRef]
- Mnif, S.; Aifa, S. Cumin (Cuminum cyminum L.) from traditional uses to potential biomedical applications. Chem. Biodivers. 2015, 12, 733–742. [Google Scholar] [CrossRef]
- Selim, S.A.; Adam, M.E.; Hassan, S.M.; Albalawi, A.R. Chemical composition, antimicrobial and antibiofilm activity of the essential oil and methanol extract of the mediterranean cypress (Cupressus sempervirens L.). BMC Complement. Altern. Med. 2014, 14, 179. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Dubey, G.; Kaushik, S. Chemical and medico-biological profile of Cyamopsis tetragonoloba (L) taub: An overview. J. Appl. Pharm. Sci. 2011, 1, 32–37. [Google Scholar]
- Li, Q.; Hu, J.; Xie, J.; Nie, S.; Xie, M.Y. Isolation, structure, and bioactivities of polysaccharides from Cyclocarya paliurus (Batal.) iljinskaja. In Annals of the New York Academy of Science; Blackwell Publishing Inc.: Hoboken, NJ, USA, 2017; Volume 1398, pp. 20–29. [Google Scholar]
- Mustarichie, R.; Warya, S.; Saptarini, N.M.; Musfiroh, I. Acute and subchronic toxicities of indonesian mistletoes Dendrophthoe pentandra L. (miq.) ethanol extract. J. Appl. Pharm. Sci. 2016, 6, 109–114. [Google Scholar] [CrossRef][Green Version]
- Golla, U.; Gajam, P.K.; Solomon Sunder Raj, B. The effect of Desmostachya bipinnata (Linn.) extract on physiologically altered glycemic status in non-diabetic rats. J. Med. Sci. 2013, 13, 221–225. [Google Scholar] [CrossRef]
- Tavana, A.; Pourrajab, F.; Hekmatimoghaddam, S.H.; Khalilzadeh, S.H.; Lotfi, M.H. The hypoglycemic effect of Dorema aucheri (bilhar) extract in diabetic type 2 patients: A first clinical trial. Intl. J. Pharm. Clin. Res. 2015, 7, 343–347. [Google Scholar]
- Geethika, B.; Gayathri, R.; Vishnu Priya, V. Comparative in-vivo free radical scavenging activity of Pineapple and Eclipta alba extracts by no assay. Int. J. Pharm. Sci. Rev. Res. 2016, 39, 69–72. [Google Scholar]
- Kumar, D.; Gaonkar, R.H.; Ghosh, R.; Pal, B.C. Bio-assay guided isolation of α-glucosidase inhibitory constituents from Eclipta alba. Nat. Pro. Comm. 2012, 7, 989–990. [Google Scholar] [CrossRef]
- Hardainiyan, S.; Nandy, B.C.; Kumar, K. Elaeocarpus ganitrus (Rudraksha): A reservoir plant with their pharmacological effects. Int. J. Pharm. Sci. Rev. Res. 2015, 34, 55–64. [Google Scholar]
- Febrinda, A.E.; Yuliana, N.D.; Ridwan, E.; Wresdiyati, T.; Astawan, M. Hyperglycemic control and diabetes complication preventive activities of bawang dayak (Eleutherine palmifolia L. Merr.) bulbs extracts in alloxan-diabetic rats. Int. Food Res. J. 2014, 21, 1405–1411. [Google Scholar]
- Nain, P.; Saini, V.; Sharma, S.; Nain, J. Antidiabetic and antioxidant potential of Emblica officinalis Gaertn. Leaves extract in streptozotocin-induced type-2 diabetes mellitus (T2DM) rats. J. Ethnopharmacol. 2012, 142, 65–71. [Google Scholar] [CrossRef]
- Sarma, U.; Borah, V.V.; Saikia, K.K.R.; Hazarika, N.K. Enhydra fluctuans: A review on its pharmacological importance as a medicinal plant and prevalence and use in North-East India. Int. J. Pharmcy Pharm. Sci. 2014, 6, 48–50. [Google Scholar]
- Asgarpanah, J.; Amin, G.; Parviz, M. In vitro antiglycation activity of Eremurus persicus (Jaub. Et sp.) boiss. Afr. J. Biotechnol. 2011, 10, 11287–11289. [Google Scholar]
- Tian, X.; Chang, L.; Ma, G.; Wang, T.; Lv, M.; Wang, Z.; Chen, L.; Wang, Y.; Gao, X.; Zhu, Y. Delineation of platelet activation pathway of scutellarein revealed its intracellular target as protein kinase C. Biol. Pharm. Bull. 2016, 39, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Kasabri, V.; Afifi, F.U.; Hamdan, I. Evaluation of the acute antihyperglycemic effects of four selected indigenous plants from Jordan used in traditional medicine. Pharm. Biol. 2011, 49, 687–695. [Google Scholar] [CrossRef]
- He, X.; Wang, J.; Li, M.; Hao, D.; Yang, Y.; Zhang, C.; He, R.; Tao, R. Eucommia ulmoides oliv.: Ethnopharmacology, phytochemistry and pharmacology of an important Traditional Chinese Medicine. J. Ethnopharmacol. 2014, 151, 78–92. [Google Scholar] [CrossRef]
- Tatiya, A.U.; Puranik, P.M.; Surana, S.J.; Patil, Y.S.; Mutha, R.E. Evaluation of hypolipidemic, antidiabetic and antioxidant activity of Eulophia herbacea tubers. Banladesh J. Pharm. 2013, 8, 269–275. [Google Scholar] [CrossRef]
- Saleem, S.; Jafri, L.; Haq, I.U.; Chang, L.C.; Calderwood, D.; Green, B.D.; Mirza, B. Plants Fagonia cretica L. and Hedera nepalensis K. Koch contain natural compounds with potent dipeptidyl peptidase-4 (DPP-4) inhibitory activity. J. Ethnopharmacol. 2014, 156, 26–32. [Google Scholar] [CrossRef]
- Samal, P.K.; Dangi, J.S.; Meena, K.P.; Beck, N.R.; Patel, A.; Maheshwari, G. Evaluation of analgesic activity of leaves extracts of Feronia limonia in experimental animal models. Res. J. Pharm. Technol. 2011, 4, 710–714. [Google Scholar]
- Rahimi, R.; Ardekani, M.R.S. Medicinal properties of Foeniculum vulgare Mill. in traditional iranian medicine and modern phytotherapy. Chin. J. Integr. Med. 2013, 19, 73–79. [Google Scholar] [CrossRef]
- Barros, L.; Heleno, S.A.; Carvalho, A.M.; Ferreira, I.C.F.R. Systematic evaluation of the antioxidant potential of different parts of Foeniculum vulgare Mill. from portugal. Food Chem. Toxicol. 2009, 47, 2458–2464. [Google Scholar] [CrossRef]
- Veeraiah, S.; Jaganmohan Reddy, K. Current strategic approaches in ethnomedicinal plants of Tinospora cordifolia and Gloriosa superba—A review. Intl. J. Pharma Bio Sci. 2012, 3, 320–326. [Google Scholar]
- Ramesh Petchi, R.; Vijaya, C. Anti-diabetic and anti-arthritic potential of glycosmis pentaphylla stem bark in FCA induced arthritis and streptozotocin induced diabetic rats. Intl. J. Pharma Bio Sci. 2012, 3, P328–P336. [Google Scholar]
- Kulkarni, Y.; Veeranjaneyulu, A. Toxicological studies on aqueous extract of Gmelina arborea in rodents. Pharm. Biol. 2010, 48, 1413–1420. [Google Scholar] [CrossRef] [PubMed]
- Attanayake, A.P.; Jayatilaka, K.A.P.W.; Pathirana, C.; Mudduwa, L.K.B. Gmelina arborea roxb. (family: Verbenaceae) extract upregulates the β-cell regeneration in stz induced diabetic rats. J. Dia. Res 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Devi, K.; Jain, N. Clinical evaluation of the anti-sweet effects of Gymnema sylvestre extract developed into a dispersable oral tablet. J. Herb. Med. 2015, 5, 184–189. [Google Scholar] [CrossRef]
- Singh, V.K.; Umar, S.; Ansari, S.A.; Iqbal, M. Gymnema sylvestre for diabetics. J. Herbs Spices Med. Plants 2008, 14, 88–106. [Google Scholar] [CrossRef]
- Malik, A.; Mehmood, M.H.; Akhtar, M.S.; Haider, G.; Gilani, A.H. Studies on antihyperlipidemic and endothelium modulatory activities of polyherbal formulation (POL4) and its ingredients in high fat diet-fed rats. Pak. J. Pharma. Sci. 2017, 30, 295–301. [Google Scholar]
- Yadav, M.; Lavania, A.; Tomar, R.; Prasad, G.B.K.S.; Jain, S.; Yadav, H. Complementary and comparative study on hypoglycemic and antihyperglycemic activity of various extracts of Eugenia jambolana seed, Momordica charantia fruits, Gymnema sylvestre, and Trigonella foenum graecum seeds in rats. Appl. Biochem. Biotechnol. 2010, 160, 2388–2400. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Su, G.Y.; Xia, X.Y.; Zhao, Y.Q. Research progress in hypoglycemic effect of natural dammarane saponins. Chin. Trad. Herb. Drugs 2016, 47, 2758–2763. [Google Scholar]
- Huyen, V.T.T.; Phan, D.V.; Thang, P.; Hoa, N.K.; Östenson, C.G. Gynostemma pentaphyllum tea improves insulin sensitivity in type 2 diabetic patients. J. Nutr. Metab. 2013, 2013, 765383. [Google Scholar] [CrossRef]
- Megalli, S.; Davies, N.M.; Roufogalis, B.D. Anti-hyperlipidemic and hypoglycemic effects of Gynostemma pentaphyllum in the zucker fatty rat. J. Pharm. Pharm. Sci. 2006, 9, 281–291. [Google Scholar] [PubMed]
- Mahalingam, G.; Kannabiran, K. Hemidesmus indicus root extract ameliorates diabetes-mediated metabolic changes in rats. Int. J. Green Pharm. 2009, 3, 314–318. [Google Scholar]
- Patra, J.K.; Thatoi, H. Anticancer activity and chromatography characterization of methanol extract of Heritiera fomes buch. Ham., a mangrove plant from Bhitarkanika, India. Orient. Pharm. Exp. Med. 2013, 13, 133–142. [Google Scholar] [CrossRef]
- Wang, B.; Lin, L.; Ni, Q.; Su, C.L. Hippophae rhamnoides Linn. for treatment of diabetes mellitus: A review. J. Med. Plant Res. 2011, 5, 2599–2607. [Google Scholar]
- Naseri, M.; Khalaj Sereshki, Z.; Ghavami, B.; Kamali Nejad, M.; Naderi, G.A.; Faghihzadeh, S. Effect of barley (Hordeum vulgare L.) seed extract on fasting serum glucose level in streptozotocin induced diabetic rats. J. Med. Plants 2010, 9, 57–66. [Google Scholar]
- Doi, K.; Mitoma, C.; Nakahara, T.; Uchi, H.; Hashimoto-Hachiya, A.; Takahara, M.; Tsuji, G.; Nakahara, M.; Furue, M. Antioxidant houttuynia cordata extract upregulates filaggrin expression in an aryl hydrocarbon-dependent manner. Fukuoka Igaku Zasshi 2014, 105, 205–213. [Google Scholar]
- Kumarappan, C.; Mandal, S.C. Antidiabetic effect of polyphenol enriched extract of Ichnocarpus frutescens on key carbohydrate metabolic enzymes. Int. J. Diabetes Dev. Ctries. 2015, 35, 425–431. [Google Scholar] [CrossRef]
- Vijay Simha, G.; Kumar, M.A.; Rajesh, S.; Panda, P.; Rao, M.M. Evaluation of physicochemical parameters of Imperata cylindrica (Linn) beauv root used in ayurvedic formulations. Res. J. Pharm. Technol. 2012, 5, 1352–1355. [Google Scholar]
- Lee, M.R.; Lee, H.Y.; Lee, G.H.; Kim, H.K.; Kim, N.Y.; Kim, S.H.; Kim, H.R.; Chae, H.J. Ixeris dentata decreases ER stress and hepatic lipid accumulation through regulation of ApoB secretion. Am. J. Chin. Med. 2014, 42, 639–649. [Google Scholar] [CrossRef]
- Ravanbakhsh, A.; Mahdavi, M.; Jalilzade-Amin, G.; Javadi, S.; Maham, M.; Mohammadnejad, D.; Rashidi, M.R. Acute and subchronic toxicity study of the median septum of Juglans regia in wistar rats. Adv. Pharm. Bull. 2016, 6, 541–549. [Google Scholar] [CrossRef]
- Boukhari, F.; Tigrine-Kordjani, N.; Youcef Meklati, B. Phytochemical investigation by microwave-assisted extraction of essential oil of the leaves of walnut cultivated in Algeria. Helv. Chim. Acta 2013, 96, 1168–1175. [Google Scholar] [CrossRef]
- Kavalali, G.; Tuncel, H.; Göksel, S.; Hatemi, H.H. Hypoglycemic activity of fruits of Juglans regia L. on streptozotocin diabetic rats. Acta Pharm. Turc. 2002, 44, 243–248. [Google Scholar]
- Pitschmann, A.; Zehl, M.; Atanasov, A.G.; Dirsch, V.M.; Heiss, E.; Glasl, S. Walnut leaf extract inhibits PTP1B and enhances glucose-uptake in vitro. J. Ethnopharmacol. 2014, 152, 599–602. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.; Huseini, H.F.; Larijani, B.; Mohammad, K.; Najmizadeh, A.; Nourijelyani, K.; Jamshidi, L. The hypoglycemic effect of Juglans regia leaves aqueous extract in diabetic patients: A first human trial. DARU J. Pharm. Sci. 2014, 22, 19. [Google Scholar] [CrossRef]
- Tewtrakul, S.; Subhadhirasakul, S.; Kummee, S. Anti-allergic activity of compounds from kaempferia parviflora. J. Ethnopharmacol. 2008, 116, 191–193. [Google Scholar] [CrossRef]
- Park, H.J.; Nam, J.H.; Jung, H.J.; Kim, W.B.; Park, K.K.; Chung, W.Y.; Choi, J. In vivo antinociceptive antiinflamatory and antioxidative effects of the leaf and stem bark of Kalopanax pictus in rats. Korean J. Pharmacogn. 2005, 36, 318–323. [Google Scholar]
- Amin, A.; Tuenter, E.; Foubert, K.; Iqbal, J.; Cos, P.; Maes, L.; Exarchou, V.; Apers, S.; Pieters, L. In vitro and in silico antidiabetic and antimicrobial evaluation of constituents from Kickxia ramosissima (Nanorrhinum ramosissimum). Front. Pharmacol. 2017, 8, 232. [Google Scholar] [CrossRef]
- Kang, D.H.; Kim, M.Y. Antimicrobial activity of Korean camellia mistletoe (Korthalsella japonica (Thunb.) engl.) extracts. J. Appl. Pharm. Sci. 2016, 6, 226–230. [Google Scholar] [CrossRef]
- Prajapati, R.; Kalariya, M.; Parmar, S.; Sheth, N. Phytochemical and pharmacological review of Lagenaria sicereria. J. Ayurveda Integr. Med. 2010, 1, 266–272. [Google Scholar] [CrossRef]
- Teugwa, C.M.; Boudjeko, T.; Tchinda, B.T.; Mejiato, P.C.; Zofou, D. Anti-hyperglycaemic globulins from selected Cucurbitaceae seeds used as antidiabetic medicinal plants in africa. BMC Complement. Altern. Med. 2013, 13, 63. [Google Scholar] [CrossRef]
- Ichikawa, H.; Yagi, H.; Tanaka, T.; Cyong, J.C.; Masaki, T. Lagerstroemia speciosa extract inhibit TNF-induced activation of nuclear factor-κB in rat cardiomyocyte H9c2 cells. J. Ethnopharmacol. 2010, 128, 254–256. [Google Scholar] [CrossRef] [PubMed]
- Tanquilut, N.C.; Tanquilut, M.R.C.; Estacio, M.A.C.; Torres, E.B.; Rosario, J.C.; Reyes, B.A.S. Hypoglycemic effect of Lagerstroemia speciosa (L.) pers. On alloxan-induced diabetic mice. J. Med. Plant Res. 2009, 3, 1066–1071. [Google Scholar]
- Hou, W.; Li, Y.; Zhang, Q.; Wei, X.; Peng, A.; Chen, L.; Wei, Y. Triterpene acids isolated from Lagerstroemia speciosa leaves as α-glucosidase inhibitors. Phytother. Res. 2009, 23, 614–618. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.B.; Kwon, K.R.; Lee, S.H.; Lee, S.H. Lannea coromandelica (Houtt.) merr. Induces heme oxygenase 1 (HO-1) expression and reduces oxidative stress via the p38/c-jun N-terminal kinase-nuclear factor erythroid 2-related factor 2 (p38/JNK-NRF2)-mediated antioxidant pathway. Int. J. Mol. Sci. 2017, 18, 266. [Google Scholar] [CrossRef]
- Pitschmann, A.; Zehl, M.; Heiss, E.; Purevsuren, S.; Urban, E.; Dirsch, V.M.; Glasl, S. Quantitation of phenylpropanoids and iridoids in insulin-sensitising extracts of Leonurus sibiricus L. (Lamiaceae). Phytochem. Anal. 2016, 27, 23–31. [Google Scholar] [CrossRef]
- Rahim, A.A.; Mohamad, J.; Alias, Z. Antidiabetic activity of aqueous extract of leptospermum flavescens in alloxan induced diabetic rats. Sains Malays. 2014, 43, 1295–1304. [Google Scholar]
- Ibrahim, S.R.M.; Mohamed, G.A. Litchi chinensis: Medicinal uses, phytochemistry, and pharmacology. J. Ethnopharmacol. 2015, 174, 492–513. [Google Scholar] [CrossRef]
- Lee, Y.J.; Kang, D.G.; Kim, J.S.; Lee, H.S. Lycopus lucidus inhibits high glucose-induced vascular inflammation in human umbilical vein endothelial cells. Vasc. Pharmacol. 2008, 48, 38–46. [Google Scholar] [CrossRef]
- Bhartiya, A.; Aditya, J.P.; Kant, L. Nutritional and remedial potential of an underutilized food legume horsegram (Macrotyloma uniflorum): A review. J. Anim. Plant Sci. 2015, 25, 908–920. [Google Scholar]
- Poivre, M.; Duez, P. Biological activity and toxicity of the chinese herb Magnolia officinalis Rehder & E. Wilson (Houpo) and its constituents. J. Zhejiang Uni. Sci. B 2017, 18, 194–214. [Google Scholar]
- Ma, J.; Yang, B.; Zhu, W.; Sun, L.; Tian, J.; Wang, X. The complete chloroplast genome sequence of Mahonia bealei (berberidaceae) reveals a significant expansion of the inverted repeat and phylogenetic relationship with other angiosperms. Gene 2013, 528, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Bora, K.S.; Sharma, A. Phytochemical and pharmacological potential of Medicago sativa: A review. Pharm. Biol. 2011, 49, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Qazi Majaz, A.; Molvi Khurshid, I. A comprehensive review on meyna Laxiflora robyns (rubiaceae). Int. J. Pharm. Sci. Rev. Res. 2015, 35, 22–25. [Google Scholar]
- Murdifin, M.; Wahyudin, E.; Lawrence, G.S.; Subehan; Manggau, M.A.; Alam, G. Phytochemical analysis and antioxidant activity of Mezzetia parviflora Becc. Woodbark extract. Pharm. J. 2012, 4, 18–21. [Google Scholar]
- Farah Idayu, N.; Taufik Hidayat, M.; Moklas, M.A.M.; Sharida, F.; Nurul Raudzah, A.R.; Shamima, A.R.; Apryani, E. Antidepressant-like effect of mitragynine isolated from Mitragyna speciosa korth in mice model of depression. Phytomedicine 2011, 18, 402–407. [Google Scholar] [CrossRef]
- Petrus, A.J.A. Mukia maderaspatana (Linn.) M. Roemer: A potentially antidiabetic and vasoprotective functional leafy-vegetable. Pharm. J. 2012, 4, 1–12. [Google Scholar] [CrossRef]
- Kunnaja, P.; Wongpalee, S.P.; Panthong, A. Evaluation of anti-inflammatory, analgesic, and antipyretic activities of the ethanol extract from Murdannia loriformis (Hassk.) Rolla Rao et kammathy. BioImpacts 2014, 4, 183–189. [Google Scholar] [CrossRef]
- Sun, C.; Huang, H.; Xu, C.; Li, X.; Chen, K. Biological activities of extracts from Chinese Bayberry (Myrica rubra Sieb. et Zucc.): A review. Plant Foods Hum. Nutr. 2013, 68, 97–106. [Google Scholar] [CrossRef]
- Sharma, B.R.; Gautam, L.N.S.; Adhikari, D.; Karki, R. A comprehensive review on chemical profiling of Nelumbo nucifera: Potential for drug development. Phytother. Res. 2017, 31, 3–26. [Google Scholar] [CrossRef]
- Mani, S.S.; Subramanian, I.P.; Pillai, S.S.; Muthusamy, K. Evaluation of hypoglycemic activity of inorganic constituents in Nelumbo nucifera seeds on streptozotocin-induced diabetes in rats. Biol. Trace Elem. Res. 2010, 138, 226–237. [Google Scholar] [CrossRef]
- Ahmed, F.; Rahman, S.; Ahmed, N.; Hossain, M.; Biswas, A.; Sarkar, S.; Banna, H.; Khatun, M.A.; Chowdhury, M.H.; Rahmatullah, M. Evaluation of Neolamarckia cadamba (Roxb.) bosser leaf extract on glucose tolerance in glucose-induced hyperglycemic mice. Afr. J. Trad. Complement. Altern. Med. 2011, 8, 79–81. [Google Scholar] [CrossRef] [PubMed]
- Desai, S.D.; Saheb, S.H.; Das, K.K.; Haseena, S. Phytochemical analysis of Nigella sativa and it’s antidiabetic effect. J. Pharm. Sci. Res. 2015, 7, 527–532. [Google Scholar]
- Ahmad, A.; Husain, A.; Mujeeb, M.; Khan, S.A.; Najmi, A.K.; Siddique, N.A.; Damanhouri, Z.A.; Anwar, F. A review on therapeutic potential of Nigella sativa: A miracle herb. Asian Pac. J. Trop. Biomed. 2013, 3, 337–352. [Google Scholar] [CrossRef]
- Jamila, F.; Mostafa, E. Ethnobotanical survey of medicinal plants used by people in Oriental Morocco to manage various ailments. J. Ethnopharmacol. 2014, 154, 76–87. [Google Scholar] [CrossRef]
- Amin, B.; Hosseinzadeh, H. Black cumin (Nigella sativa) and its active constituent, thymoquinone: An overview on the analgesic and anti-inflammatory effects. Planta Med. 2016, 82, 8–16. [Google Scholar] [CrossRef]
- Benhaddou-Andaloussi, A.; Martineau, L.C.; Vallerand, D.; Haddad, Y.; Afshar, A.; Settaf, A.; Haddad, P.S. Multiple molecular targets underlie the antidiabetic effect of Nigella sativa seed extract in skeletal muscle, adipocyte and liver cells. Diabetes Obes. Metab. 2010, 12, 148–157. [Google Scholar] [CrossRef]
- Meddah, B.; Ducroc, R.; El Abbes Faouzi, M.; Eto, B.; Mahraoui, L.; Benhaddou-Andaloussi, A.; Martineau, L.C.; Cherrah, Y.; Haddad, P.S. Nigella sativa inhibits intestinal glucose absorption and improves glucose tolerance in rats. J. Ethnopharmacol. 2009, 121, 419–424. [Google Scholar] [CrossRef]
- Yusoff, N.A.; Yam, M.F.; Beh, H.K.; Abdul Razak, K.N.; Widyawati, T.; Mahmud, R.; Ahmad, M.; Asmawi, M.Z. Antidiabetic and antioxidant activities of Nypa fruticans Wurmb. Vinegar sample from Malaysia. Asian Pac. J. Trop. Med. 2015, 8, 595–605. [Google Scholar] [CrossRef]
- Ojha, D.; Mukherjee, H.; Mondal, S.; Jena, A.; Dwivedi, V.P.; Mondal, K.C.; Malhotra, B.; Samanta, A.; Chattopadhyay, D. Anti-inflammatory activity of Odina wodier Roxb, an indian folk remedy, through inhibition of toll-like receptor 4 signaling pathway. PLoS ONE 2014, 9, e104939. [Google Scholar] [CrossRef]
- Chen, M.H.; Chen, X.J.; Wang, M.; Lin, L.G.; Wang, Y.T. Ophiopogon japonicas—A phytochemical, ethnomedicinal and pharmacological review. J. Ethnopharmacol. 2016, 181, 193–213. [Google Scholar] [CrossRef]
- Ansarullah; Bharucha, B.; Patel, V.; Ramachandran, A.V. Oreocnide integrifolia (Gaud.) miq leaf water extract improves metabolic alterations in high fructose fed insulin resistant and hypertensive rats. Eur. J. Integr. Med. 2010, 2, 79–87. [Google Scholar]
- Dinda, B.; Silsarma, I.; Dinda, M.; Rudrapaul, P. Oroxylum indicum (L.) kurz, an important asian traditional medicine: From traditional uses to scientific data for its commercial exploitation. J. Ethnopharmacol. 2015, 161, 255–278. [Google Scholar] [CrossRef] [PubMed]
- Karthishwaran, K.; Mirunalini, S. Therapeutic potential of Pergularia daemia (Forsk.): The ayurvedic wonder. Int. J. Pharmacol. 2010, 6, 836–843. [Google Scholar]
- Yasir, M.; Das, S.; Kharya, M. The phytochemical and pharmacological profile of Persea americana Mill. Pharmacogn. Rev. 2010, 4, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Odo Christian, E.; Nwodo Okwesili, F.; Joshua Parker, E.; Ugwu Okechukwu, P. Acute toxicity investigation and anti-diarrhoeal effect of the chloroform-methanol extract of the leaves of persea Americana. Iran. J. Pharm. Res. 2014, 13, 651–658. [Google Scholar]
- Xu, Q.; Hu, Y.F.; Wang, D.L.; Xu, G.B.; Wang, N. Analysis on peucedani radix coumarin by UPLC/Q-TOF MS and study on its preliminary pharmacodynamics. Chin. Trad. Herb. Drugs 2015, 46, 3637–3642. [Google Scholar]
- Ateeq, A.; Sunil, S.D.; Varun, S.K.; Santosh, M.K. Phoenix dactylifera linn.(pind kharjura): A review. Intern. J. Res. Ayurveda Pharm. 2013, 4, 447–451. [Google Scholar]
- Batool, A.; Shah, A.; Bahadur, A. Ethnopharmacological relevance of traditional medicinal flora from semi-tribal areas in Khyber Pakhtunkhwa, Punjab, Pakistan. Pak. J. Bot. 2017, 49, 691–705. [Google Scholar]
- Abdelaziz, D.H.A.; Ali, S.A. The protective effect of Phoenix dactylifera L. Seeds against CCL4-induced hepatotoxicity in rats. J. Ethnopharmacol. 2014, 155, 736–743. [Google Scholar] [CrossRef]
- Badr, J.M. Chemical constituents of Phragmanthera austroarabica A. G. Mill and J. A. Nyberg with potent antioxidant activity. Pharmacogn. Res. 2015, 7, 335–340. [Google Scholar] [CrossRef]
- Higa, J.K.; Liang, Z.; Williams, P.G.; Panee, J. Phyllostachys edulis compounds inhibit palmitic acid-induced monocyte chemoattractant protein 1 (MCP-1) production. PLoS ONE 2012, 7, e45082. [Google Scholar] [CrossRef]
- Bansal, P.; Paul, P.; Shankar, G.; Munjal, D.; Nayak, P.G.; Priyadarsini, K.I.; Unnikrishnan, M.K. Flavonoid rich fraction of Pilea microphylla (L.) attenuates metabolic abnormalities and improves pancreatic function in C57BL/KSJ-DB/DB mice. Biomed. Prev. Nutr. 2011, 1, 268–272. [Google Scholar] [CrossRef]
- Sihoglu Tepe, A.; Tepe, B. Traditional use, biological activity potential and toxicity of Pimpinella species. Ind. Crop. Prod. 2015, 69, 153–166. [Google Scholar] [CrossRef]
- Bala Sirisha, K.; Sujathamma, P. Phytochemical and pharmacological properties of Pimpinella tirupatiensis Bal. & Subr.: An endemic important medicinal plant to tirumala hills of Eastern Ghats, India. Med. Plants 2017, 9, 83–87. [Google Scholar]
- Raju, R.; Nambi, S.K.; Gurusamy, M. In vitro propagation of Pisonia grandis R. Br.: An indigenous vegetable and promising medicinal plant. Phytomorphology 2015, 65, 133–138. [Google Scholar]
- Park, N.I.; Tuan, P.A.; Li, X.; Kim, Y.K.; Yang, T.J.; Park, S.U. An efficient protocol for genetic transformation of platycodon grandiflorum with agrobacterium rhizogenes. Mol. Biol. Rep. 2011, 38, 2307–2313. [Google Scholar] [CrossRef]
- Arsiningtyas, I.S.; Gunawan-Puteri, M.D.P.T.; Kato, E.; Kawabata, J. Identification of α-glucosidase inhibitors from the leaves of Pluchea indica (L.) less., a traditional indonesian herb: Promotion of natural product use. Nat. Prod. Res. 2014, 28, 1350–1353. [Google Scholar] [CrossRef]
- Jothy, S.L.; Choong, Y.S.; Saravanan, D.; Deivanai, S.; Latha, L.Y.; Vijayarathna, S.; Sasidharan, S. Polyalthia longifolia sonn: An ancient remedy to explore for novel therapeutic agents. Res. J. Pharm. Biol. Chem. Sci. 2013, 4, 714–730. [Google Scholar]
- Satish Kumar, B.N. Phytochemistry and pharmacological studies of Pongamia pinnata (Linn.) pierre. Int. J. Pharm. Sci. Rev. Res. 2011, 9, 12–19. [Google Scholar]
- Badole, S.L.; Bodhankar, S.L. Antihyperglycaemic activity of cycloart-23-ene-3β, 25-diol isolated from stem bark of Pongamia pinnata in alloxan induced diabetic mice. Res. J. Phytochem. 2009, 3, 18–24. [Google Scholar]
- Chang, C.L.T.; Li, T.H.; Hou, C.C.; Yang, W.C. Anti-hyperglycemic properties of crude extract and triterpenes from Poria cocos. Evid.-Based Complement. Altern. Med. 2011, 2011, 128402. [Google Scholar]
- Chowdhary, C.V.; Meruva, A.; Naresh, K.; Elumalai, R.K.A. A review on phytochemical and pharmacological profile of Portulaca oleracea Linn. (purslane). Intern. J. Res. Ayurveda Pharm. 2013, 4, 34–37. [Google Scholar] [CrossRef]
- Guenzet, A.; Krouf, D.; Berzou, S. Portulaca oleracea extract increases lecithin:Cholesterol acyltransferase and paraoxonase 1 activities and enhances reverse cholesterol transport in streptozotocin-induced diabetic rat. Pharm. J. 2014, 6, 1–9. [Google Scholar]
- Hashem Dabaghian, F.; Kamalinejad, M.; Shojaii, A.; Abdollahi Fard, M.; Ghushegir, S.A. Review of antidiabetic plants in Iranian traditional medicine and their efficacy. J. Med. Plants 2012, 11, 1–11. [Google Scholar]
- Bai, Y.; Zang, X.; Ma, J.; Xu, G. Anti-diabetic effect of Portulaca oleracea L. Polysaccharideandits mechanism in diabetic rats. Int. J. Mol. Sci. 2016, 17, 1201. [Google Scholar] [CrossRef] [PubMed]
- Bautista-Cruz, A.; Arnaud-Viñas, M.R.; Martínez-Gutiérrez, G.A.; Sánchez-Medina, P.S.; Pacheco, R.P. The traditional medicinal and food uses of four plants in oaxaca, mexico. J. Med. Plant Res. 2011, 5, 3404–3411. [Google Scholar]
- Ramadan, B.K.; Schaalan, M.F.; Tolba, A.M. Hypoglycemic and pancreatic protective effects of Portulaca oleracea extract in alloxan induced diabetic rats. BMC Complement. Altern. Med. 2017, 17, 37. [Google Scholar] [CrossRef]
- Padee, P.; Nualkaew, S.; Talubmook, C.; Sakuljaitrong, S. Hypoglycemic effect of a leaf extract of Pseuderanthemum palatiferum (Nees) radlk. In normal and streptozotocin-induced diabetic rats. J. Ethnopharmacol. 2010, 132, 491–496. [Google Scholar] [CrossRef]
- Tayade, P.M.; Chandrasekar, M.J.N.; Borde, S.N.; Joshi, A.S.; Angadi, S.S.; Devdhe, S.J. Effect of Psoralea corylifolia Linn in sexual erectile dysfunction in diabetic rats. Orient. Pharm. Exp. Med. 2013, 13, 35–40. [Google Scholar] [CrossRef]
- Mestry, S.N.; Juvekar, A.R. Aldose reductase inhibitory potential and anti-cataract activity of Punica granatum Linn. Leaves against glucose-induced cataractogenesis in goat eye lens. Orient. Pharm. Exp. Med. 2017, 17, 277–284. [Google Scholar] [CrossRef]
- Pirbalouti, A.G.; Shahrzad, A.; Abed, K.; Hamedi, B. Wound healing activity of Malva sylvestris and Punica granatum in alloxan-induced diabetic rats. Acta Pol. Pharm. Drug Res. 2010, 67, 511–516. [Google Scholar]
- Patel, A.N.; Bandawane, D.D.; Mhetre, N.K. Pomegranate (Punica granatum Linn.) leaves attenuate disturbed glucose homeostasis and hyperglycemia mediated hyperlipidemia and oxidative stress in streptozotocin induced diabetic rats. Eur. J. Integr. Med. 2014, 6, 307–321. [Google Scholar] [CrossRef]
- Salwe, K.J.; Sachdev, D.O.; Bahurupi, Y.; Kumarappan, M. Evaluation of antidiabetic, hypolipedimic and antioxidant activity of hydroalcoholic extract of leaves and fruit peel of Punica granatum in male wistar albino rats. J. Nat. Sci. Biol. Med. 2015, 6, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, N.; Mahernia, S.; Amanlou, M. Comparison of different methods in quercetin extraction from leaves of Raphanus sativus L. Pharm. Sci. 2017, 23, 59–65. [Google Scholar] [CrossRef]
- Sham, T.T.; Yuen, A.C.Y.; Ng, Y.F.; Chan, C.O.; Mok, D.K.W.; Chan, S.W. A review of the phytochemistry and pharmacological activities of raphani semen. Evid.-Based Complement. Altern. Med. 2013, 2013, 636194. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Gao, J.; Yuan, Y.; Zhu, S.; Yao, M. Effect of raw radix rehmanniae on the pharmacokinetics of pioglitazone in rats. Pak. J. Pharma. Sci. 2014, 27, 537–539. [Google Scholar]
- Choi, H.J.; Jang, H.J.; Chung, T.W.; Jeong, S.I.; Cha, J.; Choi, J.Y.; Han, C.W.; Jang, Y.S.; Joo, M.; Jeong, H.S.; et al. Catalpol suppresses advanced glycation end-products-induced inflammatory responses through inhibition of reactive oxygen species in human monocytic THP-1 cells. Fitoterapia 2013, 86, 19–28. [Google Scholar] [CrossRef]
- Algandaby, M.M.; Alghamdi, H.A.; Ashour, O.M.; Abdel-Naim, A.B.; Ghareib, S.A.; Abdel-Sattar, E.A.; Hajar, A.S. Mechanisms of the antihyperglycemic activity of Retama raetam in streptozotocin-induced diabetic rats. Food Chem. Toxicol. 2010, 48, 2448–2453. [Google Scholar] [CrossRef]
- Imam, M.U.; Ismail, M.; Chinnappan, S.M. Effects of the aqueous extracts of Rhodamnia cinerea on metabolic indices and sorbitol-related complications in type 2 diabetic rats. Sains Malays. 2017, 46, 589–595. [Google Scholar] [CrossRef]
- Misra, A.; Srivastava, S.; Verma, S.; Rawat, A.K.S. Nutritional evaluation, antioxidant studies and quantification of poly phenolics, in Roscoea purpurea tubers. BMC Res. Notes 2015, 8, 324. [Google Scholar] [CrossRef]
- Abu-Al-Basal, M.A. Healing potential of Rosmarinus officinalis L. On full-thickness excision cutaneous wounds in alloxan-induced-diabetic BALB/C mice. J. Ethnopharmacol. 2010, 131, 443–450. [Google Scholar] [CrossRef]
- Bakirel, T.; Bakirel, U.; Keleş, O.U.; Ülgen, S.G.; Yardibi, H. In vivo assessment of antidiabetic and antioxidant activities of rosemary (Rosmarinus officinalis) in alloxan-diabetic rabbits. J. Ethnopharmacol. 2008, 116, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Yadav, D.; Asif, M.; Jayasri, M.A.; Agnihotri, V.K.; Ravikumar, P.C. Antidiabetic and antioxidant activities of Roylea cinerea extracts: A comparative study. Indian J. Exp. Biol. 2017, 55, 611–621. [Google Scholar]
- Devi Priya, M.; Siril, E.A. Traditional and modern use of indian madder (Rubia cordifolia L.): An overview. Int. J. Pharm. Sci. Rev. Res. 2014, 25, 154–164. [Google Scholar]
- Rhee, M.H.; Park, H.J.; Cho, J.Y. Salicornia herbacea: Botanical, chemical and pharmacological review of halophyte marsh plant. J. Med. Plant Res. 2009, 3, 548–555. [Google Scholar]
- Hou, Z.; Zhang, Z.; Wu, H. Effect of Sanguis draxonis (a Chinese traditional herb) on the formation of insulin resistance in rats. Diabetes Res. Clin. Pract. 2005, 68, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Park, H.S.; Kim, G.H. Inhibitory effects of Sasa borealis on mechanisms of adipogenesis. J. Korean Soc. Food Sci. Nutr. 2013, 42, 837–843. [Google Scholar] [CrossRef][Green Version]
- Kim, C.J.; Lim, J.S.; Cho, S.K. Anti-diabetic agents from medicinal plants inhibitory activity of Schizonepeta tenuifolia spikes on the diabetogenesis by streptozotocin in mice. Arch. Pharmacal Res. 1996, 19, 441–446. [Google Scholar] [CrossRef]
- Fallah Huseini, H.; Hooseini, P.; Heshmat, R.; Yazdani, D.; Hemati Moqadam, H.R.; Rahmani, M.; Larijani, B.; Alavi, S.H.R. The clinical investigation of Securigera securidaca (L.) (degen & doerfler) seeds in type II diabetic patients; a randomized, double-blind, placebo-controlled study. J. Med. Plants 2006, 5, 75–79. [Google Scholar]
- Suzuki, Y.A.; Tomoda, M.; Muratal, Y.; Inui, H.; Sugiura, M.; Nakano, Y. Antidiabetic effect of long-term supplementation with Siraitia grosvenori on the spontaneously diabetic goto-kakizaki rat. Br. J. Nutr. 2007, 97, 770–775. [Google Scholar] [CrossRef]
- Makhija, I.K.; Richard, L.; Kirti, S.P.; Saleemullah, K.; Jessy, M.; Annie, S. Sphaeranthus indicus: A review of its chemical, pharmacological and ethnomedicinal properties. Int. J. Pharmacol. 2011, 7, 171–179. [Google Scholar] [CrossRef]
- Salunkhe, V.R.; Bhise, S.B. Stevia rebaudiana: An alternative to synthetic sweeteners. Indian Drugs 2010, 47, 5–13. [Google Scholar]
- Jeppesen, P.B.; Gregersen, S.; Alstrup, K.K.; Hermansen, K. Stevioside induces antihyperglycaemic, insulinotropic and glucagonostatic effects in vivo: Studies in the diabetic goto-kakizaki (GK) rats. Phytomedicine 2002, 9, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Abudula, R.; Jeppesen, P.B.; Rolfsen, S.E.D.; Xiao, J.; Hermansen, K. Rebaudioside a potently stimulates insulin secretion from isolated mouse islets: Studies on the dose-, glucose-, and calcium-dependency. Metab. Clin. Exp. 2004, 53, 1378–1381. [Google Scholar] [CrossRef] [PubMed]
- Balijepalli, M.K.; Suppaiah, V.; Chin, A.M.; Buru, A.S.; Sagineedu, S.R.; Pichika, M.R. Acute oral toxicity studies of Swietenia macrophylla seeds in sprague dawley rats. Pharmacogn. Res. 2015, 7, 38–44. [Google Scholar]
- Havinga, R.M.; Hartl, A.; Putscher, J.; Prehsler, S.; Buchmann, C.; Vogl, C.R. Tamarindus indica L. (Fabaceae): Patterns of use in traditional african medicine. J. Ethnopharmacol. 2010, 127, 573–588. [Google Scholar] [CrossRef]
- Costantino, L.; Raimondi, L.; Pirisino, R.; Brunetti, T.; Pessotto, P.; Giannessi, F.; Lins, A.P.; Barlocco, D.; Antolini, L.; El-Abady, S.A. Isolation and pharmacological activities of the Tecoma stans alkaloids. Farmaco 2003, 58, 781–785. [Google Scholar] [CrossRef]
- Palbag, S.; Dey, B.K.; Singh, N.K. Ethnopharmacology, phytochemistry and pharmacology of Tephrosia purpurea. Chin. J. Nat. Med. 2014, 12, 1–7. [Google Scholar] [CrossRef]
- Pavana, P.; Manoharan, S.; Renju, G.L.; Sethupathy, S. Antihyperglycemic and antihyperlipidemic effects of Tephrosia purpurea leaf extract in streptozotocin induced diabetic rats. J. Environ. Biol. 2007, 28, 833–837. [Google Scholar]
- Satyanarayana, T.; Sarita, T.; Balaji, M.; Ramesh, A.; Boini, M.K. Antihyperglycemic and hypoglycemic effect of Thespesia populnea fruit in normal and alloxan-induced diabetes in rabbits. Saudi Pharm. J. 2004, 12, 107–111. [Google Scholar]
- Ajao, A.A.; Moteetee, A.N. Tithonia diversifolia (Hemsl) A. Gray. (asteraceae: Heliantheae), an invasive plant of significant ethnopharmacological importance: A review. S. Afr. J. Bot. 2017, 113, 396–403. [Google Scholar] [CrossRef]
- Wang, X.; Li, W.Z.; Kong, D.; Duan, Y. Ethanol extracts from Toona sinensis seeds alleviate diabetic peripheral neuropathy through inhibiting oxidative stress and regulating growth factor. Indian J. Pharm. Sci. 2016, 78, 307–312. [Google Scholar] [CrossRef]
- Mohamed Farook, S.; Clement Atlee, W. Antidiabetic and hypolipidemic potential of Tragia involucrata Linn. in streptozotocin-nicotinamide induced type II diabetic rats. Int. J. Pharmcy Pharm. Sci. 2011, 3, 103–109. [Google Scholar]
- Nagulapalli Venkata, K.C.; Swaroop, A.; Bagchi, D.; Bishayee, A. A small plant with big benefits: Fenugreek (Trigonella foenum-graecum Linn.) for disease prevention and health promotion. Mol. Nutr. Food Res. 2017, 61, 1600950. [Google Scholar] [CrossRef]
- Hamza, N.; Berke, B.; Cheze, C.; Marais, S.; Lorrain, S.; Abdouelfath, A.; Lassalle, R.; Carles, D.; Gin, H.; Moore, N. Effect of Centaurium erythraea Rafn, Artemisia herba-alba asso and Trigonella foenum-graecum L. On liver fat accumulation in C57BL/6J mice with high-fat diet-induced type 2 diabetes. J. Ethnopharmacol. 2015, 171, 4–11. [Google Scholar] [CrossRef]
- Hasanzadeh, E.; Rezazadeh, S.H.; Shamsa, S.F.; Dolatabadi, R.; Zarringhalam, J. Review on phytochemistry and therapeutic properties of fenugreek (Trigonella foenum-graceum). J. Med. Plants 2010, 9, 1–13. [Google Scholar]
- Zhou, J.; Chan, L.; Zhou, S. Trigonelline: A plant alkaloid with therapeutic potential for diabetes and central nervous system disease. Curr. Med. Chem. 2012, 19, 3523–3531. [Google Scholar] [CrossRef]
- Vats, V.; Yadav, S.P.; Grover, J.K. Effect of T. foenumgraecum on glycogen content of tissues and the key enzymes of carbohydrate metabolism. J. Ethnopharmacol. 2003, 85, 237–242. [Google Scholar] [CrossRef]
- Khlifi, S.; Jemaa, H.B.; Hmad, H.B.; Abaza, H.; Karmous, I.; Abid, A.; Benzarti, A.; Elati, J.; Aouidet, A. Antioxidant, antidiabetic and antihyperlipidemic effects of Trigonella foenum-graecum seeds. Int. J. Pharmacol. 2016, 12, 394–400. [Google Scholar] [CrossRef]
- Hannan, J.M.A.; Ali, L.; Rokeya, B.; Khaleque, J.; Akhter, M.; Flatt, P.R.; Abdel-Wahab, Y.H.A. Soluble dietary fibre fraction of Trigonella foenum-graecum (fenugreek) seed improves glucose homeostasis in animal models of type 1 and type 2 diabetes by delaying carbohydrate digestion and absorption, and enhancing insulin action. Br. J. Nutr. 2007, 97, 514–521. [Google Scholar] [CrossRef]
- Balekar, N.; Nakpheng, T.; Srichana, T. Wedelia trilobata L.: A phytochemical and pharmacological review. Chiang Mai J. Sci. 2014, 41, 590–605. [Google Scholar]
- Patil, R.N.; Patil, R.Y.; Ahirwar, B.; Ahirwar, D. Evaluation of antidiabetic and related actions of some Indian medicinal plants in diabetic rats. Asian Pac. J. Trop. Med. 2011, 4, 20–23. [Google Scholar] [CrossRef]
- Hegazy, G.A.; Alnoury, A.M.; Gad, H.G. The role of Acacia arabica extract as an antidiabetic, antihyperlipidemic, and antioxidant in streptozotocin-induced diabetic rats. Saudi Med J 2013, 34, 727–733. [Google Scholar] [PubMed]
- Geetha, G.; Gopinathapillai, P.K.; Sankar, V. Anti diabetic effect of Achyranthes rubrofusca leaf extracts on alloxan induced diabetic rats. Pak. J. Pharma. Sci. 2011, 24, 193–199. [Google Scholar]
- Ahmed, D.; Kumar, V.; Verma, A.; Gupta, P.S.; Kumar, H.; Dhingra, V.; Mishra, V.; Sharma, M. Antidiabetic, renal/hepatic/pancreas/cardiac protective and antioxidant potential of methanol/dichloromethane extract of Albizzia Lebbeck Benth. Stem bark (ALEx) on streptozotocin induced diabetic rats. BMC Complement Altern Med 2014, 14, 243. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.A.; Parikh, M.P.; Johari, S.; Gandhi, T.R. Antihyperglycemic activity of Albizzia lebbeck bark extract in streptozotocin-nicotinamide induced type II diabetes mellitus rats. Ayu 2015, 36, 335–340. [Google Scholar]
- Kumar, R.; Sharma, B.; Tomar, N.R.; Roy, P.; Gupta, A.K.; Kumar, A. In vivo evaluation of hypoglycemic activity of Aloe spp. And identification of its mode of action on GLUT-4 gene expression in vitro. Appl Biochem Biotechnol 2011, 164, 1246–1256. [Google Scholar] [CrossRef]
- Noor, A.; Gunasekaran, S.; Vijayalakshmi, M.A. Improvement of insulin secretion and pancreatic β-cell function in streptozotocin-induced diabetic rats treated with. Pharmacogn. Res 2017, 9, S99–S104. [Google Scholar] [CrossRef]
- Rahmatullah, M.; Hosain, M.; Rahman, S.; Akter, M.; Rahman, F.; Rehana, F.; Munmun, M.; Kalpana, M.A. Antihyperglycaemic and antinociceptive activity evaluation of methanolic extract of whole plant of Amaranthus tricolour L. (Amaranthaceae). Afr J Tradit Complement Altern Med 2013, 10, 408–411. [Google Scholar]
- Kamtchouing, P.; Sokeng, S.D.; Moundipa, P.F.; Watcho, P.; Jatsa, H.B.; Lontsi, D. Protective role of anacardium occidentale extract against streptozotocin-induced diabetes in rats. J Ethnopharmacol 1998, 62, 95–99. [Google Scholar] [CrossRef]
- Jaiswal, Y.S.; Tatke, P.A.; Gabhe, S.Y.; Vaidya, A.B. Antidiabetic activity of extracts of Anacardium occidentale Linn. leaves on n-streptozotocin diabetic rats. J Tradit Complement Med 2017, 7, 421–427. [Google Scholar] [CrossRef]
- Akhtar, N.; Khan, B.A.; Majid, A.; Khan, H.M.; Mahmood, T.; Gulfishan, S.T. Pharmaceutical and biopharmaceutical evaluation of extracts from different plant parts of indigenous origin for their hypoglycemic responses in rabbits. Acta Pol. Pharm. 2011, 68, 919–925. [Google Scholar] [PubMed]
- Dheer, R.; Bhatnagar, P. A study of the antidiabetic activity of Barleria prionitis Linn. Indian J. Pharmacol. 2010, 42, 70–73. [Google Scholar] [CrossRef] [PubMed]
- Ojezele, M.O.; Abatan, O.M. Hypoglycaemic and coronary risk index lowering effects of Bauhinia thoningii in alloxan induced diabetic rats. Afr. Health Sci. 2011, 11, 85–89. [Google Scholar] [PubMed]
- Vasconcelos, C.F.; Maranhão, H.M.; Batista, T.M.; Carneiro, E.M.; Ferreira, F.; Costa, J.; Soares, L.A.; Sá, M.D.; Souza, T.P.; Wanderley, A.G. Hypoglycaemic activity and molecular mechanisms of Caesalpinia ferrea martius bark extract on streptozotocin-induced diabetes in wistar rats. J. Ethnopharmacol. 2011, 137, 1533–1541. [Google Scholar] [CrossRef] [PubMed]
- Al-Attar, A.M.; Zari, T.A. Influences of crude extract of tea leaves, Camellia sinensis, on streptozotocin diabetic male albino mice. Saudi J. Biol. Sci. 2010, 17, 295–301. [Google Scholar] [CrossRef]
- Prakasam, A.; Sethupathy, S.; Pugalendi, K.V. Influence of Casearia esculenta root extract on protein metabolism and marker enzymes in streptozotocin-induced diabetic rats. Pol. J. Pharm. 2004, 56, 587–593. [Google Scholar]
- Agnihotri, A.; Singh, V. Effect of Tamarindus indica Linn. and Cassia fistula Linn. Stem bark extracts on oxidative stress and diabetic conditions. Acta Pol. Pharm. 2013, 70, 1011–1019. [Google Scholar]
- Lodha, S.R.; Joshi, S.V.; Vyas, B.A.; Upadhye, M.C.; Kirve, M.S.; Salunke, S.S.; Kadu, S.K.; Rogye, M.V. Assessment of the antidiabetic potential of Cassia grandis using an in vivo model. J. Adv. Pharm. Technol. Res. 2010, 1, 330–333. [Google Scholar] [CrossRef]
- Singh, S.N.; Vats, P.; Suri, S.; Shyam, R.; Kumria, M.M.L.; Ranganathan, S.; Sridharan, K. Effect of an antidiabetic extract of catharanthus roseus on enzymic activities in streptozotocin induced diabetic rats. J. Ethnopharmacol. 2001, 76, 269–277. [Google Scholar] [CrossRef]
- Aragão, D.M.; Guarize, L.; Lanini, J.; da Costa, J.C.; Garcia, R.M.; Scio, E. Hypoglycemic effects of Cecropia pachystachya in normal and alloxan-induced diabetic rats. J. Ethnopharmacol. 2010, 128, 629–633. [Google Scholar] [CrossRef]
- Nabeel, M.A.; Kathiresan, K.; Manivannan, S. Antidiabetic activity of the mangrove species Ceriops decandra in alloxan-induced diabetic rats. J. Diabetes 2010, 2, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Gorelick, J.; Kitron, A.; Pen, S.; Rosenzweig, T.; Madar, Z. Anti-diabetic activity of Chiliadenus iphionoides. J. Ethnopharmacol. 2011, 137, 1245–1249. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Jia, Q.; Wang, R.; Wu, X.; Wu, Y.; Huang, C.; Li, Y. Hypoglycemic activities of A- and B-type procyanidin oligomer-rich extracts from different cinnamon barks. Phytomedicine 2011, 18, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, V.; Sharma, A.K.; Upadhyay, A.; Singh, G.; Gupta, R. Hypoglycemic effects of Citrullus colocynthis roots. Acta Pol. Pharm. 2012, 69, 75–79. [Google Scholar] [PubMed]
- Amin, A.; Tahir, M.; Lone, K.P. Effect of Citrullus colocynthis aqueous seed extract on beta cell regeneration and intra-islet vasculature in alloxan induced diabetic male albino rats. J. Pak. Med. Assoc. 2017, 67, 715–721. [Google Scholar]
- Punitha, I.S.R.; Rajendran, K.; Shirwaikar, A. Alcoholic stem extract of Coscinium fenestratum regulates carbohydrate metabolism and improves antioxidant status in streptozotocin-nicotinamide induced diabetic rats. Evid.-Based Complement. Altern. Med. 2005, 2, 375–381. [Google Scholar] [CrossRef]
- Arjun, P.; Shivesh, J.; Alakh, N.S. Antidiabetic activity of aqueous extract of Eucalyptus citriodorahook. in alloxan induced diabetic rats. Pharmacogn. Mag. 2009, 5, 51–54. [Google Scholar]
- Kang, M.H.; Lee, M.S.; Choi, M.K.; Min, K.S.; Shibamoto, T. Hypoglycemic activity of Gymnema sylvestre extracts on oxidative stress and antioxidant status in diabetic rats. J. Agric. Food Chem. 2012, 60, 2517–2524. [Google Scholar] [CrossRef]
- Okokon, J.E.; Umoh, E.E.; Etim, E.I.; Jackson, C.L. Antiplasmodial and antidiabetic activities of ethanolic leaf extract of Heinsia crinata. J. Med. Food 2009, 12, 131–136. [Google Scholar] [CrossRef]
- Venkatesh, S.; Madhava Reddy, B.; Dayanand Reddy, G.; Mullangi, R.; Lakshman, M. Antihyperglycemic and hypolipidemic effects of Helicteres isora roots in alloxan-induced diabetic rats: A possible mechanism of action. J. Nat. Med. 2010, 64, 295–304. [Google Scholar] [CrossRef]
- Tripathi, U.N.; Chandra, D. Anti-hyperglycemic and anti-oxidative effect of aqueous extract of Momordica charantia pulp and trigonella foenum graecum seed in alloxan-induced diabetic rats. Indian J. Biochem. Biophys. 2010, 47, 227–233. [Google Scholar] [PubMed]
- Ma, C.; Yu, H.; Xiao, Y.; Wang, H. Momordica charantia extracts ameliorate insulin resistance by regulating the expression of SOCS-3 and jnk in type 2 diabetes mellitus rats. Pharm. Biol. 2017, 55, 2170–2177. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Mathur, M.; Bajaj, V.K.; Katariya, P.; Yadav, S.; Kamal, R.; Gupta, R.S. Evaluation of antidiabetic and antioxidant activity of Moringa oleifera in experimental diabetes. J. Diabetes 2012, 4, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Villarruel-López, A.; López-de la Mora, D.A.; Vázquez-Paulino, O.D.; Puebla-Mora, A.G.; Torres-Vitela, M.R.; Guerrero-Quiroz, L.A.; Nuño, K. Effect of Moringa oleifera consumption on diabetic rats. BMC Complement. Altern. Med. 2018, 18, 127. [Google Scholar] [CrossRef] [PubMed]
- Pandey, J.; Maurya, R.; Raykhera, R.; Srivastava, M.N.; Yadav, P.P.; Tamrakar, A.K. Murraya koenigii (L.) spreng. ameliorates insulin resistance in dexamethasone-treated mice by enhancing peripheral insulin sensitivity. J. Sci. Food Agric. 2014, 94, 2282–2288. [Google Scholar] [CrossRef]
- Luo, C.; Zhang, W.; Sheng, C.; Zheng, C.; Yao, J.; Miao, Z. Chemical composition and antidiabetic activity of Opuntia milpa alta extracts. Chem. Biodivers. 2010, 7, 2869–2879. [Google Scholar] [CrossRef]
- Vujicic, M.; Nikolic, I.; Kontogianni, V.G.; Saksida, T.; Charisiadis, P.; Orescanin-Dusic, Z.; Blagojevic, D.; Stosic-Grujicic, S.; Tzakos, A.G.; Stojanovic, I. Methanolic extract of Origanum vulgare ameliorates type 1 diabetes through antioxidant, anti-inflammatory and anti-apoptotic activity. Br. J. Nutr. 2015, 113, 770–782. [Google Scholar] [CrossRef]
- Montefusco-Pereira, C.V.; de Carvalho, M.J.; de Araújo Boleti, A.P.; Teixeira, L.S.; Matos, H.R.; Lima, E.S. Antioxidant, anti-inflammatory, and hypoglycemic effects of the leaf extract from Passiflora nitida kunth. Appl. Biochem. Biotechnol. 2013, 170, 1367–1378. [Google Scholar] [CrossRef]
- Jain, S.; Bhatia, G.; Barik, R.; Kumar, P.; Jain, A.; Dixit, V.K. Antidiabetic activity of Paspalum scrobiculatum Linn. in alloxan induced diabetic rats. J. Ethnopharmacol. 2010, 127, 325–328. [Google Scholar] [CrossRef]
- Lima, C.R.; Vasconcelos, C.F.; Costa-Silva, J.H.; Maranhão, C.A.; Costa, J.; Batista, T.M.; Carneiro, E.M.; Soares, L.A.; Ferreira, F.; Wanderley, A.G. Anti-diabetic activity of extract from Persea americana Mill. Leaf via the activation of protein kinase B (PKB/AKT) in streptozotocin-induced diabetic rats. J. Ethnopharmacol. 2012, 141, 517–525. [Google Scholar] [CrossRef]
- Ezejiofor, A.N.; Okorie, A.; Orisakwe, O.E. Hypoglycaemic and tissue-protective effects of the aqueous extract of Persea americana seeds on alloxan-induced albino rats. Malays. J. Med. Sci. 2013, 20, 31–39. [Google Scholar] [PubMed]
- Mard, S.A.; Jalalvand, K.; Jafarinejad, M.; Balochi, H.; Naseri, M.K. Evaluation of the antidiabetic and antilipaemic activities of the hydroalcoholic extract of Phoenix dactylifera palm leaves and its fractions in alloxan-induced diabetic rats. Malays. J. Med. Sci. 2010, 17, 4–13. [Google Scholar] [PubMed]
- Okoli, C.O.; Ibiam, A.F.; Ezike, A.C.; Akah, P.A.; Okoye, T.C. Evaluation of antidiabetic potentials of Phyllanthus niruri in alloxan diabetic rats. Afr. J. Biotechnol. 2010, 9, 248–259. [Google Scholar]
- Giribabu, N.; Karim, K.; Kilari, E.K.; Salleh, N. Phyllanthus niruri leaves aqueous extract improves kidney functions, ameliorates kidney oxidative stress, inflammation, fibrosis and apoptosis and enhances kidney cell proliferation in adult male rats with diabetes mellitus. J. Ethnopharmacol. 2017, 205, 123–137. [Google Scholar] [CrossRef]
- Shabeer, J.; Srivastava, R.S.; Singh, S.K. Antidiabetic and antioxidant effect of various fractions of Phyllanthus simplex in alloxan diabetic rats. J. Ethnopharmacol. 2009, 124, 34–38. [Google Scholar] [CrossRef]
- Teugwa, C.M.; Mejiato, P.C.; Zofou, D.; Tchinda, B.T.; Boyom, F.F. Antioxidant and antidiabetic profiles of two African medicinal plants: Picralima nitida (apocynaceae) and Sonchus oleraceus (asteraceae). BMC Complement. Altern. Med. 2013, 13, 175. [Google Scholar] [CrossRef]
- Nabi, S.A.; Kasetti, R.B.; Sirasanagandla, S.; Tilak, T.K.; Kumar, M.V.; Rao, C.A. Antidiabetic and antihyperlipidemic activity of Piper longum root aqueous extract in stz induced diabetic rats. BMC Complement. Altern. Med. 2013, 13, 37. [Google Scholar] [CrossRef]
- Bhadoriya, S.S.; Ganeshpurkar, A.; Bhadoriya, R.P.S.; Sahu, S.K.; Patel, J.R. Antidiabetic potential of polyphenolic-rich fraction of Tamarindus indica seed coat in alloxan-induced diabetic rats. J. Basic Clin. Physiol. Pharm. 2018, 29, 37–45. [Google Scholar] [CrossRef]
- Nalamolu, K.R.; Nammi, S. Antidiabetic and renoprotective effects of the chloroform extract of Terminalia chebula Retz. Seeds in streptozotocin-induced diabetic rats. BMC Complement. Altern. Med. 2006, 6, 17. [Google Scholar]
- Nagappa, A.N.; Thakurdesai, P.A.; Rao, N.V.; Singh, J. Antidiabetic activity of Terminalia catappa Linn fruits. J. Ethnopharmacol. 2003, 88, 45–50. [Google Scholar] [CrossRef]
- Mowla, A.; Alauddin, M.; Rahman, M.A.; Ahmed, K. Antihyperglycemic effect of Trigonella foenum-graecum (fenugreek) seed extract in alloxan-induced diabetic rats and its use in diabetes mellitus: A brief qualitative phytochemical and acute toxicity test on the extract. Afr. J. Tradit. Complement. Altern. Med. 2009, 6, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Joshi, D.V.; Patil, R.R.; Naik, S.R. Hydroalcohol extract of Trigonella foenum-graecum seed attenuates markers of inflammation and oxidative stress while improving exocrine function in diabetic rats. Pharm. Biol. 2015, 53, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Feshani, A.M.; Kouhsari, S.M.; Mohammadi, S. Vaccinium arctostaphylos, a common herbal medicine in iran: Molecular and biochemical study of its antidiabetic effects on alloxan-diabetic wistar rats. J. Ethnopharmacol. 2011, 133, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Michael, U.A.; David, B.U.; Theophine, C.O.; Philip, F.U.; Ogochukwu, A.M.; Benson, V.A. Antidiabetic effect of combined aqueous leaf extract of Vernonia amygdalina and metformin in rats. J. Basic Clin. Pharm. 2010, 1, 197–202. [Google Scholar] [PubMed]
- Herrera, C.; García-Barrantes, P.M.; Binns, F.; Vargas, M.; Poveda, L.; Badilla, S. Hypoglycemic and antihyperglycemic effect of Witheringia solanacea in normal and alloxan-induced hyperglycemic rats. J. Ethnopharmacol. 2011, 133, 907–910. [Google Scholar] [CrossRef] [PubMed]
- Meenakshi, P.; Bhuvaneshwari, R.; Rathi, M.A.; Thirumoorthi, L.; Guravaiah, D.C.; Jiji, M.J.; Gopalakrishnan, V.K. Antidiabetic activity of ethanolic extract of Zaleya decandra in alloxan-induced diabetic rats. Appl. Biochem. Biotechnol. 2010, 162, 1153–1159. [Google Scholar] [CrossRef]
- Jarald, E.E.; Joshi, S.B.; Jain, D.C. Antidiabetic activity of extracts and fraction of Zizyphus mauritiana. Pharm. Biol. 2009, 47, 328–334. [Google Scholar] [CrossRef]
- Mancha-Ramirez, A.M.; Slaga, T.J. Ursolic acid and chronic disease: An overview of ua’s effects on prevention and treatment of obesity and cancer. In Advances in Experimental Medicine and Biology. Anti-Inflammatory Nutraceuticals and Chronic Diseases; Gupta, S.C., Prasad, S., Aggarwal, B.B., Eds.; Springer International Publishing: Cham, Switzerland, 2016; Volume 928. [Google Scholar]
- Christodoulou, M.; Tchoumtchoua, J.; Skaltsounis, A.; Scorilas, A.; Halabalaki, M. Natural alkaloids intervening the insulin pathway: New hopes for anti-diabetic agents? Curr. Med. Chem. 2019. [Google Scholar] [CrossRef]
- Pan, G.Y.; Huang, Z.J.; Wang, G.J.; Fawcett, J.P.; Liu, X.D.; Zhao, X.C.; Sun, J.G.; Xie, Y.Y. The antihyperglycaemic activity of berberine arises from a decrease of glucose absorption. Planta Med. 2003, 69, 632–636. [Google Scholar]
- Gaikwad, S.B.; Mohan, G.K.; Rani, M.S. Phytochemicals for diabetes management. Pharm. Crop. 2014, 5, 11–28. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Baggioni, A. Berberine and Its Role in Chronic Disease; Springer International Publishing: Cham, Switzerland, 2016; Volume 928. [Google Scholar]
- Oza, M.J.; Kulkarni, Y.A. Phytochemical and complication in type 2 Diabetes—An update. Int. J. Pharm. Sci. Res. 2016, 7, 14–24. [Google Scholar]
- Lau, Y.S.; Tian, X.Y.; Mustafa, M.R.; Murugan, D. Boldine improves endothelial function in diabetic db/db mice through inhibition of angiotensin II-mediated BMP4oxidative stress cascade. Br. J. Pharmacol. 2013, 170, 1190–1198. [Google Scholar] [CrossRef] [PubMed]
- Lopez, P.M.; Mora, P.G.; Wysocka, W.; Maiztegui, B.; Alzugaray, M.E.; Zoto, H.D.; Borelli, M.I. Quinolizidine alkaloids isolated from Lupinus species enhance insulin secretion. Eur. J. Pharmacol. 2004, 504, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Wiedemann, M.; Gurrola-Díaz, C.; Vargas-Guerrero, B.; Wink, M.; García-López, P.; Düfer, M. Lupanine improves glucose homeostasis by influencing KATP channels and insulin gene expression. Molecules 2015, 20, 19085–19100. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Xu, H.; Zhu, S.; Xu, W.; Qin, S.; Liu, S.; Tu, G.; Peng, H.; Qiu, S.; Yu, S.; et al. Effects of neferine on CCL5 and CCR5 expression in SCG of type 2 diabetic rats. Brain Res. Bull. 2013, 90, 79–87. [Google Scholar] [CrossRef]
- Guo, C.; Han, F.; Zhang, C.; Xiao, W.; Yang, Z. Protective effects of oxymatrine on experimental diabetic nephropathy. Planta Med. 2014, 80, 269–276. [Google Scholar] [CrossRef]
- Atal, S.; Atal, S.; Vyas, S.; Phadnis, P. Bio-enhancing effect of piperine with metformin on lowering blood glucose level in alloxan induced diabetic mice. Pharmacogn. Res. 2016, 8, 56–60. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, Z.; Shang, J.; Xia, W. Targets and candidate agents for type 2 diabetes treatment with computational bioinformatics approach. J. Diabetes Res. 2014, 2014, 763936. [Google Scholar] [CrossRef]
- Den Hartogh, D.J.; Tsiani, E. Antidiabetic properties of naringenin: A citrus fruit polyphenol. Biomolecules 2019, 9, 99. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, T.; Leary, L.; Brooks, W.B. The effect of an extract of green and black tea on glucose control in adults with type 2 diabetes mellitus: Double-blind randomized study. Metabolism 2007, 56, 1340–1344. [Google Scholar] [CrossRef]
- Prasath, G.S.; Pillai, S.I.; Subramanian, S.P. Fisetin improves glucose homeostasis through the inhibition of gluconeogenic enzymes in hepatic tissues of streptozotocin induced diabetic rats. Eur. J. Pharmacol. 2014, 740, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Maher, P.; Dargusch, R.; Ehren, J.L.; Okada, S.; Sharma, K.; Schubert, D. Fisetin lowers methylglyoxal dependent protein glycation and limits the complications of diabetes. PLoS ONE 2011, 6, e21226. [Google Scholar] [CrossRef] [PubMed]
- Prasath, G.S.; Subramanian, S.P. Antihyperlipidemic effect of fisetin, a bioflavonoid of strawberries, studied in streptozotocin-induced diabetic rats. J. Biochem. Mol. Toxicol. 2014, 28. [Google Scholar] [CrossRef] [PubMed]
- Alkhalidy, H.; Moore, W.; Zhang, Y.; McMillan, R.; Wang, A.; Ali, M.; Suh, K.-S.; Zhen, W.; Cheng, Z.; Jia, Z. Small molecule kaempferol promotes insulin sensitivity and preserved pancreatic b-cell mass in middle-aged obese diabetic mice. J. Diabetes Res. 2015, 2015, 532984. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.G.; Lu, X.H.; Li, W.; Zhao, X.; Zhang, C. Protective effects of luteolin on diabetic nephropathy in stz-induced diabetic rats. Evid.-Based Complement. Altern. Med. 2011, 2011, 323171. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Li, W.; Lu, X.; Bao, P.; Zhao, X. Luteolin ameliorates cardiac failure in type I diabetic cardiomyopathy. J. Diabetes Its Complicat. 2012, 26, 259–265. [Google Scholar] [CrossRef]
- Tsai, S.J.; Huang, C.S.; Mong, M.C.; Kam, W.Y.; Huang, H.Y.; Yin, M.C. Anti-inflammatory and antifibrotic effects of naringenin in diabetic mice. J. Agric. Food Chem. 2012, 60, 514–521. [Google Scholar] [CrossRef]
- Tang, D.Q.; Wei, Y.Q.; Yin, X.X.; Lu, Q.; Hao, H.H.; Zhai, Y.P.; Wang, J.Y.; Ren, J. In vitro suppression of quercetin on hypertrophy and extracellular matrix accumulation in rat glomerular mesangial cells cultured by high glucose. Fitoterapia 2011, 82, 920–926. [Google Scholar] [CrossRef]
- Li, X.H.; Xin, X.; Wang, Y.; Wu, J.Z.; Jin, Z.D.; Ma, L.N.; Nie, C.J.; Xiao, X.; Hu, Y.; Jin, M.W. Pentamethylquercetin protects against diabetes-related cognitive deficits in diabetic goto-kakizaki rats. J. Alzheimers Dis. 2013, 34, 755–767. [Google Scholar] [CrossRef]
- Prince, P.; Kamalakkannan, N. Rutin improves glucose homeostasis in streptozotocin diabetic tissues by altering glycolytic and gluconeogenic enzymes. J. Biochem. Mol. Toxicol. 2006, 20, 96–102. [Google Scholar] [CrossRef]
- Kappel, V.D.; Cazarolli, L.H.; Pereira, D.F.; Postal, B.G.; Zamoner, A.; Reginatto, F.H.; Silva, F.R.M.B. Involvement of GLUT-4 in the stimulatory effect of rutin on glucose uptake in rat soleus muscle. J. Pharm. Pharmacol. 2013, 65, 1179–1186. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.Y.; Shih, H.Y.; Chia, Y.C.; Lee, C.H.; Ashida, H.; Lai, Y.K.; Weng, C.F. Rutin potentiates insulin receptor kinase to enhance insulin-dependent glucose transporter 4 translocation. Mol. Nutr. Food Res. 2014, 58, 1168–1176. [Google Scholar] [CrossRef] [PubMed]
- Paoli, P.; Cirri, P.; Caselli, A.; Ranaldi, F.; Bruschi, G.; Santi, A.; Camici, G. The insulin-mimetic effect of morin: A promising molecule in diabetes treatment. Biochim. Biophys. Acta 2013, 1830, 3102–3111. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, K.; Hida, M.; Hasegawa, M.; Matsumoto, T.; Kobayashi, T. Dietary polyphenol morin rescues endothelial dysfunction in a diabetic mouse model by activating the AKT/ENOS pathway. Mol. Nutr. Food Res. 2016, 60, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Razavi, T.; Kouhsari, S.M.; Abnous, K. Morin exerts anti-diabetic effects in human HEPG2 cells via down-regulation of miR-29a. Exp. Clin. Endocrinol. Diabetes 2018. [Google Scholar] [CrossRef]
- Pandey, V.K.; Mathur, A.; Khan, M.F.; Kakkar, P. Activation of PERK-eiF2α-ATF4 pathway contributes to diabetic hepatotoxicity: Attenuation of er stress by morin. Cell. Signal. 2019, 59, 41–52. [Google Scholar] [CrossRef]
- Bijak, M. Silybin, a major bioactive component of milk thistle (Silybum marianum L. Gaernt.)—Chemistry, bioavailability, and metabolism. Molecules 2017, 22, 1942. [Google Scholar] [CrossRef]
- Abenavoli, L.; Izzo, A.A.; Milić, N.; Cicala, C.; Santini, A.; Capasso, R. Milk thistle (Silybum marianum): A concise overview on its chemistry, pharmacological, and nutraceutical uses in liver diseases. Phytother. Res. 2018, 32, 2202–2213. [Google Scholar] [CrossRef]
- Jaggi, A.S.; Singh, N. Silymarin and its role in chronic diseases. In Drug Discovery from Mother Nature; Springer: Berlin, Germany, 2016; pp. 25–44. [Google Scholar]
- Sheela, N.; Jose, M.A.; Sathyamurthy, D.; Kumar, B.N. Effect of silymarin on streptozotocin-nicotinamide-induced type 2 diabetic nephropathy in rats. Iran. J. Kidney Dis. 2013, 7, 117–123. [Google Scholar]
- Meng, S.; Yang, F.; Wang, Y.; Qin, Y.; Xian, H.; Che, H.; Wang, L. Silymarin ameliorates diabetic cardiomyopathy via inhibiting TGF-β1/smad signaling. Cell Biol. Int. 2019, 43, 65–72. [Google Scholar] [CrossRef]
- Mani, R.; Natesan, V. Chrysin: Sources, beneficial pharmacological activities, and molecular mechanism of action. Phytochemistry 2018, 145, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Amjid, A.; Ajaz, A.G.; Mohd, M.; Siddiqui, W.A. Chrysin, an antiinflammatory molecule, abrogates renal dysfunction in type 2 diabetic rats. Toxicol. Appl. Pharm. 2014, 279, 1–7. [Google Scholar]
- Taslimi, P.; Kandemir, F.M.; Demir, Y.; İleritürk, M.; Temel, Y.; Caglayan, C.; Gulçin, İ. The antidiabetic and anticholinergic effects of chrysin on cyclophosphamide-induced multiple organ toxicity in rats: Pharmacological evaluation of some metabolic enzyme activities. J. Biochem. Mol. Toxicol. 2019, 33, e22313. [Google Scholar] [CrossRef] [PubMed]
- Ahad, A.; Mujeeb, M.; Ahsan, H.; Siddiqui, W.A. Prophylactic effect of baicalein against renal dysfunction in type 2 diabetic rats. Biochimie 2014, 106, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Hamid, K.; Alqahtani, A.; Kim, M.-S.; Cho, J.-L.; Cui, P.H.; Li, C.G.; Groundwater, P.W.; Li, G.Q. Tetracyclic triterpenoids in herbal medicines and their activities in diabetes and its complications. Curr. Top. Med. Chem. 2015, 15, 2406–2430. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, A.; Hamid, K.; Kam, A.; Wong, K.; Abdelhak, Z.; Razmovski-Naumovski, V.; Chan, K.; Li, K.M.; Groundwater, P.W.; Li, G.Q. The pentacyclic triterpenoids in herbal medicines and their pharmacological activities in diabetes and diabetic complications. Curr. Med. Chem. 2013, 20, 908–931. [Google Scholar] [PubMed]
- Ammon, H.P.T. Use of Boswellic Acids for the Prophylaxis and/or Treatment of Damage to and/or Inflammation of the Islets of Langerhans. U.S. Patent 8975228B2, 10 March 2015. [Google Scholar]
- Jadhav, R.; Puchchakala, G. Hypoglycemic and antidiabetic activity of flavonoids: Boswellic acid, ellagic acid, quercetin, rutin on streptozotocin-nicotamide induced type 2 diabetic rats. Int. J. Pharmcy Pharm. Sci. 2011, 4, 251–256. [Google Scholar]
- Han, L.; Li, C.; Sun, B.; Xie, Y.; Guan, Y.; Ma, Z.; Chen, L. Protective effects of celastrol on diabetic liver injury via TLR4/myd88/NF-κB signaling pathway in type 2 diabetic rats. J. Diabetes Res. 2016, 2016. [Google Scholar] [CrossRef]
- Wang, C.; Shi, C.; Yang, X.; Yang, M.; Sun, H.; Wang, C. Celastrol suppresses obesity process via increasing antioxidant capacity and improving lipid metabolism. Eur. J. Pharmacol. 2015, 744, 52–58. [Google Scholar] [CrossRef]
- Kim, J.E.; Lee, M.H.; Nam, D.H. Celastrol, an nf-κB inhibitor, improves insulin resistance and attenuates renal injury in db/db mice. PLoS ONE 2013, 8, e62068. [Google Scholar] [CrossRef]
- Camer, D.; Yu, Y.; Szabo, A.; Huang, X. The molecular mechanisms underpinning the therapeutic properties of oleanolic acid, its isomer and derivatives for type 2 diabetes and associated complications. Mol. Nutr. Food Res. 2014, 58, 1750–1759. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.Y.; Wang, Y.P.; Cantley, J.; Iseli, T.J.; Molero, J.C.; Hegarty, B.D.; Kraegen, E.W.; Ye, Y.; Ye, J.M. Oleanolic acid reduces hyperglycemia beyond treatment period with Akt/FoxO1-induced suppression of hepatic gluconeogenesis in type-2 diabetic mice. PLoS ONE 2012, 7, e42115. [Google Scholar] [CrossRef] [PubMed]
- Na, M.; Oh, W.K.; Kim, Y.H.; Cai, X.F. Inhibition of protein tyrosine phosphatase 1b by diterpenoids isolated from Acanthopanax koreanum. Bioorg. Med. Chem. Lett. 2006, 16, 3061–3064. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Espinosa, J.J.; Rios, M.Y.; Lopez-Martinez, S.; Lopez-Vallejo, F. Antidiabetic activity of some pentacyclic acid triterpenoids, role of PTP-1B: In vitro, in silico, and in vivo approaches. Eur. J. Med. Chem. 2011, 46, 2243–2251. [Google Scholar] [CrossRef] [PubMed]
- De Melo, C.L.; Queiroz, M.G.; Fonseca, S.G.; Bizerra, A.M. Oleanolic acid, a natural triterpenoid improves blood glucose tolerance in normal mice and ameliorates visceral obesity in mice fed a high-fat diet. Chem. Biol. Interact. 2010, 185, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Ling, C.; Jinping, L.; Xia, L.; Renyong, Y. Ursolic acid provides kidney protection in diabetic rats. Curr. Res. 2013, 75, 59–63. [Google Scholar] [CrossRef]
- Huang, S.H.; Lin, G.J.; Chu, C.H.; Yu, J.C.; Chen, T.W.; Chen, Y.W.; Chien, M.W.; Chu, C.C.; Sytwu, H.K. Triptolide ameliorates autoimmune diabetes and prolongs islet graft survival in nonobese diabetic mice. Pancreas 2013, 42, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Shen, W.; Qin, W.; Zheng, C.; Zhang, M.; Zeng, C.; Wang, S.; Wang, J.; Zhu, X.; Liu, Z. Treatment of db/db diabetic mice with triptolide: A novel therapy for diabetic nephropathy. Nephrol. Dial Transplant. 2010, 25, 3539–3547. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.J.; Goff, D.V.; Leeds, A.R.; Alberti, K.G.; Wolever, T.M.; Gassull, M.A.; Hockaday, T.D. Unabsorbable carbohydrates and diabetes: Decreased postprandial hyperglycaemia. Lancet 1976, 2, 172–177. [Google Scholar] [CrossRef]
- Doi, K.; Matsuura, M.; Kawara, A.; Baba, S. Treatment of diabetes with glucomannan (konjac mannan). Lancet 1979, 1, 987–988. [Google Scholar] [CrossRef]
- Kays, S.J.; Nottingham, S.F. Biology and Chemistry of Jerusalem Artichoke: Helianthus tuberosus L.; CRC Press: Boca Raton, FL, USA, 2007; p. 496. [Google Scholar]
- Ma, X.Y.; Zhang, L.H.; Shao, H.B.; Xu, G.; Zhang, F.; Ni, F.T.; Brestic, M. Jerusalem artichoke (Helianthus tuberosus), a medicinal salt-resistant plant has high adaptability and multiple-use values. J. Med. Plant Res. 2011, 5, 1272–1279. [Google Scholar]
- Baur, J.A.; Pearson, K.J.; Price, N.L.; Jamieson, H.A.; Lerin, C.; Kalra, A.; Prabhu, V.V.; Allard, J.S.; Lopez-Lluch, G.; Lewis, K.; et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 2006, 444, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Minakawa, M.; Miura, Y.; Yagasaki, K. Piceatannol, a resveratrol derivative, promotes glucose uptake through glucose transporter 4 translocation to plasma membrane in l6 myocytes and suppresses blood glucose levels in type 2 diabetic model db/db mice. Biochem. Biophys. Res. Commun. 2012, 422, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Uchida-Maruki, H.; Inagaki, H.; Ito, R.; Kurita, I.; Sai, M.; Ito, T. Piceatannol lowers the blood glucose level in diabetic mice. Biol. Pharm. Bull. 2015, 38, 629–633. [Google Scholar] [CrossRef]
- Oritani, Y.; Okitsu, T.; Nishimura, E.; Sai, M.; Ito, T.; Takeuchi, S. Enhanced glucose tolerance by intravascularly administered piceatannol in freely moving healthy rats. Biochem. Biophys. Res. Commun. 2016, 470, 753–758. [Google Scholar] [CrossRef]
- Jeong, S.O.; Son, Y.; Lee, J.H.; Cheong, Y.K.; Park, S.H.; Chung, H.T.; Pae, H.O. Resveratrol analog piceatannol restores the palmitic acid-induced impairment of insulin signaling and production of endothelial nitric oxide via activation of anti-inflammatory and antioxidative heme oxygenase-1 in human endothelial cells. Mol. Med. Rep. 2015, 12, 937–944. [Google Scholar] [CrossRef]
- Vallianou, N.G.; Evangelopoulos, A.; Kazazis, C. Resveratrol and diabetes. Rev. Diabet. Stud. RDS 2013, 10, 236. [Google Scholar] [CrossRef]
- Szkudelski, T.; Szkudelska, K. Resveratrol and diabetes: From animal to human studies. Biochim. Et Biophys. Acta (BBA)-Mol. Basis Dis. 2015, 1852, 1145–1154. [Google Scholar] [CrossRef]
- Bagul, P.; Banerjee, S. Application of resveratrol in diabetes: Rationale, strategies and challenges. Curr. Mol. Med. 2015, 15, 312. [Google Scholar] [CrossRef]
- Öztürk, E.; Arslan, A.K.K.; Yerer, M.B.; Bishayee, A. Resveratrol and diabetes: A critical review of clinical studies. Biomed. Pharmacother. 2017, 95, 230–234. [Google Scholar] [CrossRef]
- Benzler, J.; Ganjam, G.K.; Pretz, D.; Oelkrug, R.; Koch, C.E.; Legler, K.; Stöhr, S.; Culmsee, C.; Williams, L.M.; Tups, A. Central inhibition of ikkβ/nf-κb signaling attenuates high-fat diet–induced obesity and glucose intolerance. Diabetes 2015, 64, 2015–2027. [Google Scholar] [CrossRef] [PubMed]
- Kunwar, A.; Priyadarsini, K.I. Curcumin and its role in chronic diseases. In Advances in Experimental Medicine and Biology. Anti-Inflammatory Nutraceuticals and Chronic Diseases; Gupta, S.C., Prasad, S., Aggarwal, B.B., Eds.; Springer International Publishing: Cham, Switzerland, 2016; Volume 928, pp. 1–26. [Google Scholar]
- Zhang, D.W.; Fu, M.; Gao, S.H.; Liu, J.L. Curcumin and diabetes: A systematic review. Evid. Complement. Altern. Med. 2013, 16, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Meng, B.I.; Li, J.; Cao, H. Antioixidant and anti-inflammatory activities of curcumin on diabetes mellitus and its complications. Curr. Pharm. Des. 2013, 19, 2101–2103. [Google Scholar] [PubMed]
- Chin, K.Y.; Pang, K.L.; Soelaiman, I.N. Tocotrienol and its role in chronic diseases. In Anti-Inflammatory Nutraceuticals and Chronic Diseases; Gupta, S.C., Prasad, S., Aggarwal, B.B., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 97–130. [Google Scholar]
- Haghighat, N.; Vafa, M.; Eghtesadi, S.; Heidari, I.; Hosseini, A.; Rostami, A. The effects of tocotrienols added to canola oil on microalbuminuria, inflammation, and nitrosative stress in patients with type 2 diabetes: A randomized, double-blind, placebo-controlled trial. Int. J. Prev. Med. 2014, 5, 617–623. [Google Scholar]
- Kuhad, A.; Chopra, K. Tocotrienol attenuates oxidative-nitrosative stress and inflammatory cascade in experimental model of diabetic neuropathy. Neuropharmacology 2009, 57, 456–462. [Google Scholar] [CrossRef]
- Licznerska, B.; Baer-Dubowska, W. Indole-3-carbinol and its role in chronic diseases. In Advances in Experimental Medicine and Biology. Anti-Inflammatory Nutraceuticals and Chronic Diseases; Gupta, S.C., Prasad, S., Aggarwa, B.B., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 131–154. [Google Scholar]
- Jayakumar, P.; Pugalendi, K.V.; Sankaran, M. Attenuation of hyperglycemia-mediated oxidative stress by indole-3-carbinol and its metabolite 3, 3′-diindolylmethane in c57bl/6j mice. J. Physiol. Biochem. 2014, 70, 525–534. [Google Scholar] [CrossRef]
- Ong, K.W.; Hsu, A.; Tan, B.K.H. Chlorogenic acid stimulates glucose transport in skeletal muscle via ampk activation: A contributor to the beneficial effects of coffee on diabetes. PLoS ONE 2012, 7, e32718. [Google Scholar] [CrossRef]
- Ong, K.W.; Hsu, A.; Tan, B.K.H. Anti-diabetic and anti-lipidemic effects of chlorogenic acid are mediated by ampk activation. Biochem. Pharmacol. 2013, 85, 1341–1351. [Google Scholar] [CrossRef]
- Bassoli, B.K.; Cassolla, P.; Borba-Murad, G.R.; Constantin, J.; Salgueiro-Pagadigorria, C.L.; Bazotte, R.B.; da Silva, R.S.; de Souza, H.M. Chlorogenic acid reduces the plasma glucose peak in the oral glucose tolerance test: Effects on hepatic glucose release and glycaemia. Cell Biochem. Funct. 2008, 26, 320–328. [Google Scholar] [CrossRef]
- Fatima, N.; Hafizur, R.M.; Hameed, A.; Ahmed, S.; Nisar, M.; Kabir, N. Ellagic acid in Emblica officinalis exerts anti-diabetic activity through the action on β-cells of pancreas. Eur. J. Nutr. 2017, 56, 591–601. [Google Scholar] [CrossRef]
- Mehta, V.; Verma, P.; Sharma, N.; Sharma, A.; Thakur, A.; Malairaman, U. Quercetin, ascorbic acid, caffeine and ellagic acid are more efficient than rosiglitazone, metformin and glimepiride in interfering with pathways leading to the development of neurological complications associated with diabetes: A comparative in-vitro study. Bull. Fac. Pharm. Cairo Univ. 2017, 55, 115–121. [Google Scholar]
- Ahad, A.; Ganai, A.A.; Mujeeb, M.; Siddiqui, W.A. Ellagic acid, an nf-κb inhibitor, ameliorates renal function in experimental diabetic nephropathy. Chem. Biol. Interact. 2014, 219, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Naik, S.R.; Niture, N.T.; Ansari, A.A.; Shah, P.D. Anti-diabetic activity of embelin: Involvement of cellular inflammatory mediators, oxidative stress and other biomarkers. Phytomedicine 2013, 20, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Durg, S.; Veerapur, V.P.; Neelima, S.; Dhadde, S.B. Antidiabetic activity of Embelia ribes, embelin and its derivatives: A systematic review and meta-analysis. Biomed. Pharmacother. 2017, 86, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Zhang, T.; Gong, C.; Sheng, Y.; Lu, B.; Zhou, L.; Ji, L.; Wang, Z. Erianin inhibits high glucose-induced retinal angiogenesis via blocking erk1/2-regulated hif-1α-vegf/vegfr2 signaling pathway. Sci. Rep. 2016, 6, 34306. [Google Scholar] [CrossRef]
- Cui, J.; Gong, R.; Hu, S.; Cai, L.; Chen, L. Gambogic acid ameliorates diabetes-induced proliferative retinopathy through inhibition of the hif-1α/vegf expression via targeting pi3k/akt pathway. Life Sci. 2018, 192, 293–303. [Google Scholar] [CrossRef]
- Madhuri, K.; Naik, P.R. Modulatory effect of garcinol in streptozotocin-induced diabetic wistar rats. Arch. Physiol. Biochem. 2017, 123, 322–329. [Google Scholar] [CrossRef]
- Mali, K.K.; Dias, R.J.; Havaldar, V.D.; Yadav, S.J. Antidiabetic effect of garcinol on streptozotocin-induced diabetic rats. Indian J. Pharm. Sci. 2017, 79, 463–468. [Google Scholar] [CrossRef]
- Sun, J.; Fu, X.; Liu, Y.; Wang, Y.; Huo, B.; Guo, Y.; Gao, X.; Li, W.; Hu, X. Hypoglycemic effect and mechanism of honokiol on type 2 diabetic mice. Drug Des. Devel. Ther. 2015, 9, 6327–6342. [Google Scholar]
- Wang, J.; Zhao, R.; Liang, J.; Yong, C. Antidiabetic and anti-oxidative effects of honokiol on diabetic rats induced by high-fat diet and streptozotocin. Chin. Herb. Med. 2014, 6, 42–46. [Google Scholar]
- Li, C.-G.; Ni, C.-L.; Yang, M.; Tang, Y.-Z.; Li, Z.; Zhu, Y.-J.; Jiang, Z.-H.; Sun, B.; Li, C.-J. Honokiol protects pancreatic β cell against high glucose and intermittent hypoxia-induced injury by activating nrf2/are pathway in vitro and in vivo. Biomed. Pharmacother. 2018, 97, 1229–1237. [Google Scholar] [CrossRef] [PubMed]
- Udayakumar, R.; Kasthurirengan, S.; Mariashibu, T.S.; Rajesh, M.; Anbazhagan, V.R.; Kim, S.C.; Ganapathi, A.; Choi, C.W. Hypoglycaemic and hypolipidaemic effects of Withania somnifera root and leaf extracts on alloxan-induced diabetic rats. Int. J. Mol. Sci. 2009, 10, 2367–2382. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Xing, H.; Ye, J. Efficacy of berberine in patients with type 2 diabetes. Metabolism 2008, 57, 712–717. [Google Scholar] [CrossRef]
- Yin, J.; Ye, J.; Ji, W. Effects and mechanisms of berberine in diabetes treatment. Acta Pharm. Sin. B 2012, 2, 327–334. [Google Scholar] [CrossRef]
- Samarghandian, S.; Azimi-Nezhad, M.; Farkhondeh, T. Catechin treatment ameliorates diabetes and its complications in streptozotocin-induced diabetic rats. Dose-Response 2017. [Google Scholar] [CrossRef]
- Nagao, T.; Meguro, S.; Hase, T.; Otsuka, K.; Komikado, M.; Tokimitsu, I.; Yamamoto, T.; Yamamoto, K. A catechin-rich beverage improves obesity and blood glucose control in patients with type 2 diabetes. Obesity 2009, 17, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Atal, S.; Agrawal, R.P.; Vyas, S.; Phadnis, P.; Rai, N. Evaluation of the effect of piperine per se on blood glucose level in alloxan-induced diabetic mice. Acta Pol. Pharm. 2012, 69, 965–969. [Google Scholar] [PubMed]
- Szkudelski, T.; Szkudelska, K. Anti-diabetic effects of resveratrol. Ann. N. Y. Acad. Sci. 2011, 1215, 34–39. [Google Scholar] [CrossRef]
- Szkudelska, K.; Szkudelski, T. Resveratrol, obesity and diabetes. Eur. J. Pharmacol. 2010, 635, 1–8. [Google Scholar] [CrossRef]
- Szkudelski, T. The insulin-suppressive effect of resveratrol—An in vitro and in vivo phenomenon. Life Sci. 2008, 82, 430–435. [Google Scholar] [CrossRef]
- Do, G.M.; Jung, U.J.; Park, H.J.; Kwon, E.Y.; Jeon, S.M.; McGregor, R.A.; Choi, M.S. Resveratrol ameliorates diabetes-related metabolic changes via activation of amp-activated protein kinase and its downstream targets in db/db mice. Mol. Nutr. Food Res. 2012, 56, 1282–1291. [Google Scholar] [CrossRef]
- Lee, J.; Lee, H.I.; Seo, K.I.; Cho, H.W.; Kim, M.J.; Park, E.M.; Lee, M.K. Effects of ursolic acid on glucose metabolism, the polyol pathway and dyslipidemia in non-obese type 2 diabetic mice. Indian J. Exp. Biol. 2014, 52, 683–691. [Google Scholar] [PubMed]
- Kazmi, I.; Rahman, M.; Afzal, M.; Gupta, G.; Saleem, S.; Afzal, O.; Shaharyar, M.A.; Nautiyal, U.; Ahmed, S.; Anwar, F. Anti-diabetic potential of ursolic acid stearoyl glucoside: A new triterpenic gycosidic ester from Lantana camara. Fitoterapia 2012, 83, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Castro, A.J.; Frederico, M.J.; Cazarolli, L.H.; Mendes, C.P.; Bretanha, L.C.; Schmidt, E.C.; Bouzon, Z.L.; de Medeiros Pinto, V.A.; da Fonte Ramos, C.; Pizzolatti, M.G.; et al. The mechanism of action of ursolic acid as insulin secretagogue and insulinomimetic is mediated by cross-talk between calcium and kinases to regulate glucose balance. Biochim. Biophys. Acta 2015, 1850, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Sai, K.S.; Nagarajan, S. Blood glucose lowering effect of the leaves of Tinospora cordifolia and Sauropus androgynus in diabetic subjects. J. Nat. Remedies 2002, 2, 28–32. [Google Scholar]
- Singh, S.; Gupta, S.K.; Sabir, G.; Gupta, M.K.; Seth, P.K. A database for anti-diabetic plants with clinical/experimental trials. Bioinformation 2009, 4, 263–268. [Google Scholar] [CrossRef][Green Version]
- Bunyapraphatsara, N.; Yongchaiyudha, S.; Rungpitarangsi, V.; Chokechaijaroenporn, O. Antidiabetic activity of Aloe vera L. Juice ii. Clinical trial in diabetes mellitus patients in combination with glibenclamide. Phytomedicine 1996, 3, 245–248. [Google Scholar] [CrossRef]
- Yagi, A.; Hegazy, S.; Kabbash, A.; Wahab, E.A.-E. Possible hypoglycemic effect of Aloe vera L. High molecular weight fractions on type 2 diabetic patients. Saudi Pharm. J. 2009, 17, 209–215. [Google Scholar] [CrossRef]
- Choi, H.-C.; Kim, S.-J.; Son, K.-Y.; Oh, B.-J.; Cho, B.-L. Metabolic effects of aloe vera gel complex in obese prediabetes and early non-treated diabetic patients: Randomized controlled trial. Nutrition 2013, 29, 1110–1114. [Google Scholar] [CrossRef]
- Cárdenas-Ibarra, L.; Villarreal-Pérez, J.Z.; Lira-Castillo, J.C.; Nava-Alemán, A. Randomized double blind crossover trial of aloe vera, cnidoscolus chayamansa and placebo for reducing hyperglycemia in women with early metabolic syndrome. Clin. Nutr. Exp. 2017, 14, 1–12. [Google Scholar] [CrossRef]
- Kirkham, S.; Akilen, R.; Sharma, S.; Tsiami, A. The potential of cinnamon to reduce blood glucose levels in patients with type 2 diabetes and insulin resistance. DiabetesObes. Metab. 2009, 11, 1100–1113. [Google Scholar] [CrossRef]
- Hasanzade, F.; Toliat, M.; Emami, S.A.; Emamimoghaadam, Z. The effect of cinnamon on glucose of type ii diabetes patients. J. Tradit. Complementary Med. 2013, 3, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Mang, B.; Wolters, M.; Schmitt, B.; Kelb, K.; Lichtinghagen, R.; Stichtenoth, D.O.; Hahn, A. Effects of a cinnamon extract on plasma glucose, hba, and serum lipids in diabetes mellitus type 2. Eur. J. Clin. Investig. 2006, 36, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Ziegenfuss, T.N.; Hofheins, J.E.; Mendel, R.W.; Landis, J.; Anderson, R.A. Effects of a water-soluble cinnamon extract on body composition and features of the metabolic syndrome in pre-diabetic men and women. J. Int. Soc. Sports Nutr. 2006, 3, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, J.L.; Bowden, R.G.; Willoughby, D.S. Cassia cinnamon supplementation reduces peak blood glucose responses but does not improve insulin resistance and sensitivity in young, sedentary, obese women. J. Diet. Suppl. 2016, 13, 461–471. [Google Scholar] [CrossRef]
- Vanschoonbeek, K.; Thomassen, B.J.; Senden, J.M.; Wodzig, W.K.; van Loon, L.J. Cinnamon supplementation does not improve glycemic control in postmenopausal type 2 diabetes patients. J. Nutr. 2006, 136, 977–980. [Google Scholar] [CrossRef]
- Altschuler, J.A.; Casella, S.J.; MacKenzie, T.A.; Curtis, K.M. The effect of cinnamon on a1c among adolescents with type 1 diabetes. Diabetes Care 2007, 30, 813–816. [Google Scholar] [CrossRef]
- Kudolo, G.B. The effect of 3-month ingestion of ginkgo biloba extract on pancreatic beta-cell function in response to glucose loading in normal glucose tolerant individuals. J. Clin. Pharmacol. 2000, 40, 647–654. [Google Scholar] [CrossRef]
- Kudolo, G.B. Effect of ginkgo biloba extract ingestion on plasma total cortisol levels during an oral glucose tolerance test in normal glucose tolerant individuals. Food Nutr. Sci. 2014, 5, 1561–1567. [Google Scholar] [CrossRef]
- Kudolo, G.B. The effect of 3-month ingestion of ginkgo biloba extract (egb 761) on pancreatic beta-cell function in response to glucose loading in individuals with non-insulin-dependent diabetes mellitus. J. Clin. Pharmacol. 2001, 41, 600–611. [Google Scholar] [CrossRef]
- Hosseini, S.; Jamshidi, L.; Mehrzadi, S.; Mohammad, K.; Najmizadeh, A.R.; Alimoradi, H.; Huseini, H.F. Effects of juglans regia l. Leaf extract on hyperglycemia and lipid profiles in type two diabetic patients: A randomized double-blind, placebo-controlled clinical trial. J. Ethnopharmacol. 2014, 152, 451–456. [Google Scholar] [CrossRef]
- Tharavanij, T.; Pawa, K.K.; Maungboon, P.; Panpitpat, P.; Porntisan, S.; Thangcharoende, W.; Tasanarong, A.; Ritthidej, G.C.; Jesadanont, S. Glucose-lowering efficacy of water extract of malvastrum coromandelianum in type 2 diabetes subjects: A double blind, randomized controlled trial. J. Med. Assoc. Thail. Chotmaihet Thangphaet 2015, 98 (Suppl. 3), S75–S80. [Google Scholar]
- Suparmi, S.; Fasitasari, M.; Martosupono, M.; Mangimbulude, J.C. Comparisons of curative effects of chlorophyll from Sauropus androgynus (L.) merr leaf extract and cu-chlorophyllin on sodium nitrate-induced oxidative stress in rats. J. Toxicol. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Bunawan, H.; Bunawan, S.N.; Baharum, S.N.; Noor, N.M. Sauropus androgynus (L.) merr. Induced bronchiolitis obliterans: From botanical studies to toxicology. Evid.-Based. Complement. Altern. Med. 2015, 2015, 7. [Google Scholar] [CrossRef]
- Mishra, S.; Verma, N.; Bhattacharya, S.; Usman, K.; Reddy, H.; Verma, N.; Anjum, B.; Singh, P.; Bharadwaj, S.; Bharadwaj, K. Efficacy and safety of tinospora cordifolia (tc) as an add-on therapy in patients with type-2 diabetes. IJMRS 2017, 3, 5. [Google Scholar] [CrossRef]
- Chakraborty, S.K.; Barman, N.N. Clinical Evaluation of Tinospora Cordifolia (Wild) Miers (Guduci) in the Management of Diabetic Foot Ulcer; University of Gauhati, Government Ayurvedic College: Gauhati, India, 2012. [Google Scholar]
- Karkal, Y.R.; Bairy, L.K. Safety of Aqueous Extract of Tinospora cordifolia (Tc) in Healthy Volunteers: A Double Blind Randomised Placebo Controlled Study. Iran. J. Pharmacol. Ther. 2007, 6, 59–61. [Google Scholar]
- Baquer, N.Z.; Kumar, P.; Taha, A.; Kale, R.K.; Cowsik, S.M.; McLean, P. Metabolic and molecular action of trigonella foenum-graecum (fenugreek) and trace metals in experimental diabetic tissues. J. Biosci. 2011, 36, 383–396. [Google Scholar] [CrossRef]
- Neelakantan, N.; Narayanan, M.; de Souza, R.J.; van Dam, R.M. Effect of fenugreek (Trigonella foenum-graecum L.) intake on glycemia: A meta-analysis of clinical trials. Nutr. J. 2014, 13, 7. [Google Scholar] [CrossRef] [PubMed]
- Neeraja, A.; Rajyalakshmi, P. Hypoglycemic effect of processed fenugreek seeds in humans. J. Food Sci. Technol. 1996, 33, 427–430. [Google Scholar]
- Madar, Z.; Abel, R.; Samish, S.; Arad, J. Glucose-lowering effect of fenugreek in non-insulin dependent diabetics. Eur. J. Clin. Nutr. 1988, 42, 51–54. [Google Scholar]
- Sharma, R.D.; Raghuram, T.C. Hypoglycemic effect of fenugreek seeds in non-insulin-dependent diabetic subjects. Nutr. Res. 1988, 10, 731–739. [Google Scholar]
- Sharma, R.D.; Sarkar, A.; Hazra, D.K.; Mishra, B.; Singh, J.B.; Sharma, S.K.; Maheshwari, B.B.; Maheshwari, P.K. Use of fenugreek seed powder in the management of non-insulin dependent diabetes mellitus. Nutr. Res. 1996, 16, 1331–1339. [Google Scholar] [CrossRef]
- Zargar, A.H.; Anjli Nehr, B.A.; Laway, F.A.D. Effect of consumption of powdered fenugreek seeds on blood sugar and hbaic levels in patients with type ii diabetes mellitus. Int. J. Diabetes Dev. Ctries. 1992, 12, 49–55. [Google Scholar]
- Gupta, A.; Gupta, R.; Lal, B. Effect of trigonella foenum-graecum (fenugreek) seeds on glycaemic control and insulin resistance in type 2 diabetes mellitus: A double blind placebo controlled study. J. Assoc. Physicians India 2001, 49, 1057–1061. [Google Scholar] [PubMed]
- Hokayem, M.; Blond, E.; Vidal, H.; Lambert, K.; Meugnier, E.; Feillet-Coudray, C.; Coudray, C.; Pesenti, S.; Luyton, C.; Lambert-Porcheron, S.; et al. Grape polyphenols prevent fructose-induced oxidative stress and insulin resistance in first-degree relatives of type 2 diabetic patients. Diabetes Care 2013, 36, 1454–1461. [Google Scholar] [CrossRef] [PubMed]
- Arablou, T.; Aryaeian, N.; Valizadeh, M.; Sharifi, F.; Hosseini, A.; Djalali, M. The effect of ginger consumption on glycemic status, lipid profile and some inflammatory markers in patients with type 2 diabetes mellitus. Int. J. Food Sci. Nutr. 2014, 65, 515–520. [Google Scholar] [CrossRef]
- Mahluji, S.; Attari, V.E.; Mobasseri, M.; Payahoo, L.; Ostadrahimi, A.; Golzari, S.E.J. Effects of ginger (Zingiber officinale) on plasma glucose level, hba1c and insulin sensitivity in type 2 diabetic patients. Int. J. Food Sci. Nutr. 2013, 64, 682–686. [Google Scholar] [CrossRef]
- Rotman-Pikielny, P.; Ness-Abramof, R.; Charach, G.; Roitman, A.; Zissin, R.; Levy, Y. Efficacy and safety of the dietary supplement dbcare(r) in patients with type 2 diabetes mellitus and inadequate glycemic control. J. Am. Coll. Nutr. 2014, 33, 55–62. [Google Scholar] [CrossRef]
| Genus | Species | Geographic Zone | Activity | Reference |
|---|---|---|---|---|
| Acacia | Acacia nilotica | antidiabetic | [148] | |
| Acacia catechu | Nepal, India | antihyperglycemic | [149,150,151] | |
| Acacia farnesiana | Bangladesh | antidiabetic | [133,152] | |
| Acacia tortilis | antidiabetic | [153] | ||
| Acacia senegal | Sudan | antidiabetic | [154] | |
| Acacia ferruginea | antidiabetic | [155] | ||
| Acacia nilotica | antidiabetic | [156] | ||
| Acacia modesta | India and Pakistan | antihyperglycemic | [157] | |
| Acacia arabica | India | hypoglycemic and antihyperglycemic | [158] | |
| Acalypha | Acalypha indica | India | antidiabetic | [135,159] |
| Acalypha langiana | antidiabetic | [160] | ||
| Acalypha wilkesiana | Nigeria | antidiabetic | [161] | |
| Acanthopanax | Acanthopanax gracilistylus | Korea | antidiabetic | [162] |
| Acanthopanax koreanum | Korea | antidiabetic | [163] | |
| Acanthopanax senticosus | China (TCM) | antidiabetic | [164] | |
| Acanthopanax sessiliflorus | Southeast Asia | antidiabetic | [165] | |
| Achillea | Achillea millefolium | India | antidiabetic | [151,166] |
| Achillea santolina | Iraq and Jordan | antidiabetic | [167,168] | |
| Alisma | Alisma orientale | China | antidiabetic | [169] |
| Alisma orientale | China | hypoglycemic | [170] | |
| Allium | Allium ampeloprasum | Iran | antidiabetic | [171] |
| Allium cepa | Mauritius, Algeria | antihyperglycemic | [172,173,174,175] | |
| Allium porrum | Turkey | hypoglycemic | [176] | |
| Allium sativum | India (Ayurveda), Indonesia, Iran, Cuba, Mauritius, Togo, China (TCM) | α-amylase inhibitor, hypoglycemic, α-glucosidase inhibitor, antihyperglycemic | [128,173,175,177,178,179,180,181] | |
| Allium stipitatum | Iran | hypoglycemic, α-glucosidase inhibitor | [178] | |
| Aloe | Aloe ferox | India (Ayurveda) | antidiabetic | [182] |
| Aloe marlothii | South Africa | antidiabetic | [183] | |
| Aloe vera | India (Ayurveda), Ghana, Mauritius, Uganda, Tanzania, Traditional Chinese medicines, Trinidad and Tobago, Iran, Pakistan, Philippines, Saudi Arabia | α-amylase inhibitor, hypoglycemic | [52,61,63,128,138,181,184,185,186,187,188,189,190] | |
| Alpinia | Alpinia calcarata | India, Sri Lanka | antidiabetic | [191,192] |
| Alpinia galanga | India | antidiabetic | [193] | |
| Alpinia officinarum | China | hypoglycemic | [109] | |
| Amaranthus | Amaranthus cruentus | Kenya | antidiabetic | [194] |
| Amaranthus hybridus | Mauritius | antidiabetic | [186] | |
| Amaranthus spinosus | Taiwan | α-glucosidase inhibitor | [195,196] | |
| Angelica | Angelica hirsutiflora | Taiwan | antidiabetic | [197] |
| Angelica keiskei | Japan | antidiabetic | [198] | |
| Angelica sinensis | China (TCM) | antidiabetic | [199] | |
| Aralia | Aralia cachemirica | antidiabetic | [200] | |
| Aralia cortex | antidiabetic | [201] | ||
| Aralia elata | China, Korea, Japan | α-glucosidase inhibitor | [146,202] | |
| Aralia taibaiensis | China | α-glucosidase and α-amylase inhibitor | [203,204] | |
| Artemisia | Artemisia absinthium | antidiabetic | [120,205] | |
| Artemisia afra | Africa | antidiabetic | [121] | |
| Artemisia campestris | Morocco | antidiabetic | [206] | |
| Artemisia capillaris | antidiabetic | [207] | ||
| Artemisia dracunculus | antidiabetic | [208] | ||
| Artemisia judaica | Jordan | antidiabetic | [209] | |
| Artemisia herba-alba | Iraq, Algeria, Jordan | hypoglycemic | [122,123,210] | |
| Artemisia ludoviciana | Mexico | hypoglycemic | [211] | |
| Artemisia pallens | antidiabetic | [212] | ||
| Artemisia parviflora | India | antidiabetic | [213] | |
| Artemisia princeps | Asia | antidiabetic | [214] | |
| Artemisia roxburghiana | antidiabetic | [215] | ||
| Artemisia sacrorum | China | antidiabetic | [216] | |
| Artocarpus | Artocarpus altilis | Indonesia, Trinidad and Tobago, Mauritius | antidiabetic | [186,189,217] |
| Artocarpus communis | Nigeria | antidiabetic | [218] | |
| Artocarpus heterophyllus | India (Ayurveda), Mauritius | hypoglycemic, α-amylase inhibitor | [186,219,220] | |
| Artocarpus mariannensis | Marshall Islands | antidiabetic | [221] | |
| Astragalus | Astragalus complanatus | China | antidiabetic | [221] |
| Astragalus membranaceus | China | antidiabetic | [222] | |
| Astragalus propinquus | China | α-glucosidase inhibitor | [223] | |
| Averrhoa | Averrhoa bilimbi | antidiabetic | [224] | |
| Averrhoa carambola | Bangladesh | antihyperglycemic | [116] | |
| Berberis | Berberis aristata | India (Ayurveda) | antidiabetic | [225,226] |
| Berberis asiatica | India | antidiabetic | [227] | |
| Berberis vulgaris | Iran, China | antidiabetic | [228,229] | |
| Brassica | Brassica juncea | India (Ayurveda) | antidiabetic | [172] |
| Brassica oleracea | antihyperglycemic | [175] | ||
| Brassica rapa | India | antidiabetic | [229] | |
| Buddleja | Buddleja asiatica | India | antidiabetic | [230] |
| Buddleja cordata | Mexico | antidiabetic | [231] | |
| Buddleja officinalis | Korea | antidiabetic | [232] | |
| Butea | Butea monosperma | India | antidiabetic | [151] |
| Butea frondosa | India | antidiabetic | [233] | |
| Caesalpinia | Caesalpinia bonducella | India | α-amylase inhibitor | [234] |
| Caesalpinia ferrea | Brazil | antidiabetic | [235] | |
| Calamus | Calamus tenuis | India | antidiabetic | [125] |
| Calamus erectus | India | antidiabetic | [236] | |
| Calotropis | Calotropis gigantea | Bangladesh | antihyperglycemic | [237] |
| Calotropis procera | antidiabetic | [238] | ||
| Capparis | Capparis aphylla | antihyperglycemic | [239] | |
| Capparis decidua | India, Pakistan | antidiabetic | [240,241] | |
| Capparis sepiaria | India | antidiabetic | [242] | |
| Capparis spinosa | India (Ayurveda and Unani) | antidiabetic | [243] | |
| Caralluma | Caralluma adscendens | India | antidiabetic | [244,245] |
| Caralluma umbellata | India | antihyperglycemic | [246] | |
| Carissa | Carissa carandas | India (Ayurveda, Unani, and Homoeopathy) | antidiabetic | [247] |
| Carissa spinarum | Kenya | antidiabetic | [248] | |
| Cassia | Cassia auriculata | India, Tanzania | antidiabetic | [249,250] |
| Cassia fistula | India | antidiabetic | [251] | |
| Cassia obtusifolia | China | antidiabetic | [252] | |
| Cassia sieberiana | Nigeria | antidiabetic | [253] | |
| Cassia spectabilis | Diabetes | antidiabetic | [254] | |
| Centaurea | Centaurea karduchorum | Turkey | antidiabetic | [255] |
| Centaurea repens | Persia | antidiabetic | [256] | |
| Centaurea virgata | Turkey | antidiabetic | [257] | |
| Cichorium | Cichorium pumilum | Jordan | antidiabetic | [258] |
| Cichorium intybus | Turkey | antidiabetic | [259] | |
| Cinnamomum | Cinnamomum burmannii | antidiabetic | [260] | |
| Cinnamomum cassia | India (Unani, Ayurveda) Japan, China, South Africa | antidiabetic | [261,262] | |
| Cinnamomum impressinervium | India | antidiabetic | [104] | |
| Cinnamomum iners | Malaysia | antidiabetic | [263] | |
| Cinnamomum japonicum | Korea | antidiabetic | [264] | |
| Cinnamomum obtusifolium | Bangladesh | antidiabetic | [133] | |
| Cinnamomum tamala | India (Ayurveda) | hypoglycemic | [113] | |
| Cinnamomum verum | India (Ayurveda) | α-amylase inhibitor | [128] | |
| Cinnamomum zeylanicum | α-glucosidase | [147,265] | ||
| Cistus | Cistus laurifolius | Turkey | antidiabetic | [266] |
| Cistus ladaniferus | Morocco | antidiabetic | [267] | |
| Cistus monspeliensis | Morocco | antidiabetic | [268] | |
| Cistus salviifolius | Morocco | antidiabetic | [268] | |
| Citrus | Citrus aurantium | antidiabetic | [269] | |
| Citrus grandis | China | antidiabetic | [270] | |
| Citrus paradisi | Nigeria, Cuba, Trinidad and Tobago | antidiabetic | [179,189,271] | |
| Citrus reticulata | China | antidiabetic | [199] | |
| Citrus sinensis | India | antidiabetic | [272] | |
| Clerodendrum | Clerodendrum glandulosum | India | antidiabetic | [273] |
| Clerodendrum colebrookianum | India | antidiabetic | [230] | |
| Clerodendrum capitatum | Africa | antidiabetic | [274] | |
| Clerodendrum inerme | antidiabetic | [275] | ||
| Clerodendrum infortunatum | India | antidiabetic | [276] | |
| Clerodendrum phlomidis | India (Ayurveda) | antidiabetic | [277] | |
| Coccinia | Coccinia cordifolia | India | antidiabetic | [278] |
| Coccinia grandis | India (Ayurveda), Sri Lanka | antihyperglycemic, α-glucosidase inhibitor, α-amylase inhibitor | [128,279,280,281] | |
| Coccinia indica | India (Ayurveda) | antidiabetic | [113,172] | |
| Coptis | Coptis chinensis | China | antidiabetic | [282] |
| Coptis deltoidea | China | antidiabetic | [282] | |
| Coptis japonica | China | antidiabetic | [282] | |
| Cordyceps | Cordyceps sinensis | China | antidiabetic | [283] |
| Cordyceps militaris | antidiabetic | [284] | ||
| Cornus | Cornus officinalis | China | antidiabetic, α-glucosidase inhibitor | [285,286] |
| Cornus kousa | China | antidiabetic | [287] | |
| Cornus mas | China | antidiabetic | [288] | |
| Cornus nuttallii | Canada | antidiabetic | [289] | |
| Cornus stolonifera | Canada | antidiabetic | [290] | |
| Costus | Costus igneus | India | antidiabetic | [291] |
| Costus pictus | India | antidiabetic | [141] | |
| Costus speciosus | Sri Lanka | antidiabetic | [279] | |
| Croton | Croton cajucara | antidiabetic | [292] | |
| Croton celtidifolius | Brazil | antidiabetic | [293] | |
| Croton guatemalensis | Guatemala | antidiabetic | [124] | |
| Croton klozchianus | India (Ayurveda) | antidiabetic | [294] | |
| Croton zambesicus | antidiabetic | [295] | ||
| Cucumis | Cucumis callosus | India | antidiabetic | [296] |
| Cucumis sativus | Malaysia | antidiabetic | [297] | |
| Cucurbita | Cucurbita ficifolia | Iran, Mexico | hypoglycemic | [175,298,299,300] |
| Cucurbita pepo | South Africa | antidiabetic | [262] | |
| Curculigo | Curculigo latifolia | antidiabetic | [301] | |
| Curculigo orchioides | India (Ayurveda) | antidiabetic | [302] | |
| Curculigo recurvata | Bangladesh | antidiabetic | [133] | |
| Curcuma | Curcuma angustifolia | India | antidiabetic | [303] |
| Curcuma domestica | India | antidiabetic | [151] | |
| Curcuma longa | China, Bangladesh, India (Ayurveda), Indonesia, Laos | antidiabetic | [177,181,226,304,305,306] | |
| Curcuma xanthorrhiza | Bangladesh, Indonesia, Laos | antidiabetic | [306,307,308] | |
| Cuscuta | Cuscuta reflexa | India, Bangladesh | antidiabetic | [125,126] |
| Cuscuta chinensis | China | antidiabetic | [309] | |
| Cuscuta americana | Trinidad and Tobago | antidiabetic | [189] | |
| Cynomorium | Cynomorium coccineum | Saudi Arabia, China, Afghanistan, Mongolia, Iran | antidiabetic | [310] |
| Cynomorium songaricum | Saudi Arabia, China, Afghanistan, Mongolia, Iran | antidiabetic | [310] | |
| Cyperus | Cyperus kyllinga | India (Ayurveda) | antidiabetic | [311] |
| Cyperus laevigatus | India (Ayurveda) | antidiabetic | [312] | |
| Cyperus rotundus | India (Ayurveda) | antidiabetic | [313] | |
| Delonix | Delonix regia | Bangladesh | antidiabetic | [314] |
| Delonix elata | antidiabetic | [315] | ||
| Dendrobium | Dendrobium nobile | Korea | antidiabetic | [316] |
| Dendrobium loddigesii | China | α-glucosidase inhibitor | [317] | |
| Desmodium | Desmodium gangeticum | India (Ayurveda), Sri Lanka | antidiabetic | [279,318] |
| Desmodium gyrans | China (TCM) | antidiabetic | [319] | |
| Desmodium styracifolium | China (TCM) | antidiabetic | [319] | |
| Dioscorea | Dioscorea alata | antidiabetic | [320] | |
| Dioscorea bulbifera | α-amylase, α-glucosidase inhibitor | [321] | ||
| Dioscorea japonica | Korea | antidiabetic | [322] | |
| Dioscorea nipponica | Korea | antidiabetic | [323] | |
| Dioscorea opposita | China, India (Ayurveda), China (TCM) | antidiabetic | [181,226,324] | |
| Diospyros | Diospyros canaliculata | Cameroon | antidiabetic | [325] |
| Diospyros crassiflora | Cameroon | antidiabetic | [325] | |
| Diospyros lotus | antidiabetic | [326] | ||
| Diospyros melanoxylon | India, Sri Lanka | antidiabetic | [327] | |
| Diospyros peregrina | India | antidiabetic | [328] | |
| Elephantopus | Elephantopus scaber | India | antidiabetic | [329] |
| Elephantopus mollis | antidiabetic | [330] | ||
| Embelia | Embelia madagascariensis | hypoglycemic | [331] | |
| Embelia ribes | India (Ayurveda) | antidiabetic | [332] | |
| Enicostema | Enicostema axillare | India (Ayurveda) | antidiabetic | [333] |
| Enicostema littorae | antidiabetic | [334] | ||
| Erica | Erica arborea | Turkey | antidiabetic | [335] |
| Erica bocquetii | Turkey | antidiabetic | [335] | |
| Erica sicula | Turkey | antidiabetic | [335] | |
| Erythrina | Erythrina indica | India | antidiabetic | [336] |
| Erythrina variegeta | India | antidiabetic | [315] | |
| Eucalyptus | Eucalyptus globulus | Iran | antihyperglycemic | [337,338] |
| Eucalyptus torreliana | Nigeria | antihyperglycemic | [339,340] | |
| Eugenia | Eugenia cumini | α-amylase inhibitor | [127] | |
| Eugenia jambolana | India (Ayurveda) | α-amylase inhibitor | [172,341] | |
| Eugenia polyantha | India, Indonesia | antidiabetic | [96,144] | |
| Eugenia uniflora | Paraguay | α-glucosidase inhibitor | [342] | |
| Euonymus | Euonymus laxiflorus | Vietnam | antidiabetic | [343] |
| Euonymus alatus | China (TCM) | antidiabetic | [344] | |
| Euphorbia | Euphorbia caducifolia | India | antidiabetic | [132] |
| Euphorbia dioeca | α-glucosidase inhibitor | [345] | ||
| Euphorbia drumondii | India (Ayurveda) | hyperglycemic | [136,346] | |
| Euphorbia hirta | India, Bangladesh, Nepal | α-glucosidase | [93,133,150,347] | |
| Euphorbia humifusa | Mongolia | antidiabetic | [60] | |
| Euphorbia kansui | antidiabetic | [134] | ||
| Euphorbia ligularia | India | antidiabetic | [104] | |
| Euphorbia neriifolia | India (Ayurveda) | antidiabetic | [131] | |
| Euphorbia prostrata | antihyperglycemic | [348] | ||
| Euphorbia thymifolia | Bangladesh | antihyperglycemic | [116] | |
| Ferula | Ferula assa-foetida | India (Ayurveda), Iran, Afghanistan | antidiabetic | [349,350] |
| Ferula feruloides | Mongolia | antidiabetic | [60] | |
| Ferula hermonis | Lebanon, Syria | antidiabetic | [351] | |
| Ferula persica | Jordan | hypoglycemic | [352] | |
| Ficus | Ficus amplissima | India (Ayurveda, Siddha, Unani) | antidiabetic | [353] |
| Ficus benghalensis | India (Ayurveda, Siddha, Unani, homoeopathy), Southeast Asia | antidiabetic | [114,354,355,356] | |
| Ficus carica | India (Ayurveda, Siddha, Unani, homoeopathy) | antidiabetic | [357,358] | |
| Ficus cunia | India | α-glucosidase inhibitor | [359] | |
| Ficus deltoidea | Malaysia, Southeast Asia | α-glucosidase inhibitor | [360,361,362] | |
| Ficus elastica | Philippines | antidiabetic | [62] | |
| Ficus exasperata | Nigeria, Cameroon, Ivory Coast, Sierra Leone | antidiabetic | [253,363] | |
| Ficus glomerata | India (Ayurveda, Siddha, Unani, homoeopathy) | antidiabetic | [113,364] | |
| Ficus glumosa | Nigeria, Cameroon | hypoglycemic | [365,366,367] | |
| Ficus hispida | Bangladesh | antihyperglycemic | [116,368] | |
| Ficus lutea | Africa | antidiabetic | [119] | |
| Ficus microcarpa | in south Asia | antidiabetic | [369,370] | |
| Ficus palmata | antidiabetic | [371] | ||
| Ficus racemosa | India (Ayurveda, Siddha, Unani, homoeopathy), Bangladesh, Southeast Asia | antihyperglycemic, hypoglycemic, α-glucosidase and α-amylase inhibitor | [83,356,372,373,374,375,376] | |
| Ficus religiosa | India (Ayurveda) | antidiabetic | [354,377] | |
| Ficus sansibarica | Africa | antidiabetic | [378] | |
| Ficus thonningii | Africa | antidiabetic | [363] | |
| Ficus virens | India (Ayurveda) | antidiabetic | [379] | |
| Gardenia | Gardenia gasminoides | China | antidiabetic | [380] |
| Gardenia ternifolia | Togo | antidiabetic | [180] | |
| Gentiana | Gentiana crassicaulis | antidiabetic | [366] | |
| Gentiana scabra | Korea | antidiabetic | [381] | |
| Geranium | Geranium dielsianum | antidiabetic | [382] | |
| Geranium graveolens | Jordan | antidiabetic | [383] | |
| Glycyrrhiza | Glycyrrhiza glabra | China, India | antidiabetic | [181,384] |
| Glycyrrhiza uralensis | India | antidiabetic | [385] | |
| Grewia | Grewia asiatica | India (Ayurveda) | antidiabetic | [386] |
| Grewia hirsuta | India | antidiabetic | [387] | |
| Grewia nervosa | antidiabetic | [388] | ||
| Gynura | Gynura divaricata | China | antidiabetic | [389] |
| Gynura formosana | China | antidiabetic | [390] | |
| Gynura procumbens | Indonesia, Malaysia, Thailand, Southeast Asia, Korea | antidiabetic | [391,392,393,394] | |
| Gynura segetum | antidiabetic | [395] | ||
| Hedysarum | Hedysarum limprichtii | China | antidiabetic | [396] |
| Hedysarum polybotrys | China | antidiabetic | [396] | |
| Hedysarum smithianum | China | antidiabetic | [396] | |
| Hedysarum vicioider | China | antidiabetic | [396] | |
| Helichrysum | Helichrysum caespititium | South Africa | antidiabetic | [183] |
| Helichrysum graveolens | Turkey | α-amylase inhibitor | [142] | |
| Helichrysum italicum | Europe | antidiabetic | [397] | |
| Helicteres | Helicteres hirsuta | Southeast Asia | antidiabetic | [398] |
| Helicteres isora | India (Ayurveda) | antidiabetic | [399] | |
| Holarrhena | Holarrhena antidysenterica | India (Ayurveda) | antidiabetic | [400] |
| Holarrhena floribunda | Nigeria | α-amylase inhibitor | [401] | |
| Hydnocarpus | Hydnocarpus alpina | hypoglycemic | [402] | |
| Hydnocarpus wightiana | India (Ayurveda) | antidiabetic | [403] | |
| Juniperus | Juniperus oxycedrus | Turkey | α-amylase inhibitor, hypoglycemic activity | [142,404] |
| Juniperus communis | Turkey | α-glucosidase inhibitor | [142] | |
| Justicia | Justicia adhatoda | Pakistan | antidiabetic | [405] |
| Justicia gendarussa | antidiabetic | [406] | ||
| Justicia secunda | antidiabetic | [407] | ||
| Justicia spicigera | antidiabetic | [408] | ||
| Leucas | Leucas aspera | India, Bangladesh | antidiabetic | [193,409] |
| Leucas cephalotes | India (Ayurveda), Nepal, Pakistan | antidiabetic | [410] | |
| Liriope | Liriope platyphylla | China | antidiabetic | [411] |
| Liriope spicata | China | antidiabetic | [412] | |
| Lonicera | Lonicera caerulea | northern Russia, China, Japan | antidiabetic | [413] |
| Lonicera japonica | China | antidiabetic | [414] | |
| Luffa | Luffa acutangula | antidiabetic | [415] | |
| Luffa cylindrica | antidiabetic | [416] | ||
| Luffa echinata | India | antidiabetic | [417] | |
| Lycium | Lycium barbarum | China | antidiabetic | [181,418] |
| Lycium chinense | China | antidiabetic, antihyperglycemic | [418,419,420] | |
| Lycium ruthenicum | China | antidiabetic | [421] | |
| Mangifera | Mangifera indica | India (Ayurveda), Nigeria | α-amylase inhibitor, antihyperglycemic | [128,422] |
| Mangifera mekongensis | Vietnam | α-glucosidase inhibitor | [423] | |
| Marrubium | Marrubium alysson | α-glucosidase inhibitor | [424] | |
| Marrubium deserti | Tunisia | antidiabetic | [425] | |
| Marrubium radiatum | Lebanon | α-amylase inhibitor | [137] | |
| Marrubium vulgare | Mexico, Jordan, Algeria | antidiabetic | [231,426,427] | |
| Melia | Melia azadirachta | Mexico | antidiabetic | [231] |
| Melia dubia | India | antidiabetic | [428] | |
| Melia orientalis | India (Ayurveda) | antidiabetic | [429] | |
| Mentha | Mentha arvensis | India | antidiabetic | [151] |
| Mentha longifolia | India | antidiabetic | [151] | |
| Mentha piperita | antidiabetic | [430] | ||
| Mimosa | Mimosa invisa | Nigeria | hypoglycemic | [431] |
| Mimosa pigra | Bangladesh | antihyperglycemic | [432] | |
| Mimosa pudica | Sri Lanka, Thailand | hypoglycemic | [279,433] | |
| Mimusops | Mimusops elengi | India (Ayurveda) | antidiabetic | [434] |
| Mimusops zeyheri | South Africa | antidiabetic | [183] | |
| Momordica | Momordica balsamina | South Africa | antidiabetic | [183] |
| Momordica charantia | Philippines, Vietnam, Mauritius, Trinidad and Tobago, India (Ayurveda), Nigeria, Bangladesh, Taiwan, central America | α-amylase inhibitor, hypoglycemic, antihyperglycemic | [61,85,113,129,186,189,435,436,437,438,439] | |
| Momordica cymbalaria | antidiabetic | [440] | ||
| Momordica foetida | South Africa | antidiabetic | [441] | |
| Momordica grosvenori | China (TCM) | antidiabetic | [442] | |
| Moringa | Moringa oleifera | South Africa, Kenya, Mexico, India (Ayurveda), Nigeria, Mauritius, Senegal | hypoglycemic | [113,183,194,231,443,444,445] |
| Moringa peregrina | antidiabetic | [446] | ||
| Moringa stenopetala | Ethiopia | α-glucosidase inhibitor | [139,444] | |
| Morus | Morus alba | Iran, Philippines, Trinidad and Tobago, India (Ayurveda), China (TCM), Pakistan, Korea, Chile | antidiabetic, hypoglycemic, α-glucosidase and α-amylase inhibition | [53,62,189,447,448,449,450,451,452,453] |
| Morus nigra | Iran, Jordon | antidiabetic | [53,57] | |
| Mucuna | Mucuna gigantea | India | antidiabetic | [454] |
| Mucuna pruriens | India (Ayurveda) | antidiabetic | [172] | |
| Murraya | Murraya koenigii | India (Ayurveda) | α amylase inhibitor, hypoglycemic effects, antihyperglycemic | [455,456,457,458,459] |
| Murraya panicutata | Nigeria | α-glucosidase inhibitor | [339] | |
| Musa | Musa acuminata | antidiabetic | [460] | |
| Musa paradisiaca | antidiabetic | [460] | ||
| Musa Sapientum | India | antihyperglycemic | [348,461] | |
| Nymphaea | Nymphaea nouchali | Bangladesh, India (Ayurveda) | antidiabetic | [133,462] |
| Nymphaea stellata | India (Ayurveda) | α-glucosidase inhibitor, hypoglycemic, antihyperglycemic | [463,464,465] | |
| Ocimum | Ocimum campechianum | Trinidad and Tobago | antidiabetic | [189] |
| Ocimum canum | Ghana | lowers blood glucose | [466,467] | |
| Ocimum gratissimum | Bangladesh, Nigeria | hypoglycemic | [133,436,468] | |
| Ocimum sanctum | India (Ayurveda), China, Bangladesh | hypoglycemic | [469,470,471,472] | |
| Ocimum tenuiflorum | India (Ayurveda) | α-amylase inhibitor, hypoglycemic, antihyperglycemic | [128,473] | |
| Oplopanax | Oplopanax elatus | China, Russia, and Korea | antidiabetic | [474] |
| Oplopanax horridus | antidiabetic | [475] | ||
| Origanum | Origanum onites | Turkey | antidiabetic | [476] |
| Origanum vulgare | antidiabetic | [477] | ||
| Orthosiphon | Orthosiphon aristatus | antidiabetic | [478,479] | |
| Orthosiphon stamineus | Indonesia and Malaysia | antidiabetic | [480] | |
| Otostegia | Otostegia persica | Iran | antidiabetic | [481] |
| Otostegia integrifolia | antidiabetic | [482] | ||
| Oxalis | Oxalis corniculata | India | antidiabetic | [151] |
| Oxalis griffithii | India | antidiabetic | [125] | |
| Paederia | Paederia foetida | China, Vietnam, India Japan | antidiabetic | [483] |
| Paederia scandens | China, Vietnam, India, Japan | antidiabetic | [483] | |
| Paeonia | Paeonia lactiflora | Korea, China, Japan | hypoglycemic | [484] |
| Paeonia suffruticosa | China, Korea, Japan | antidiabetic | [471,485] | |
| Pandanus | Pandanus amaryllifolius | antihyperglycemic | [486] | |
| Pandanus fascicularis | India (Ayurveda) | antihyperglycemic | [487] | |
| Pandanus tectorius | antidiabetic | [488] | ||
| Panax | Panax ginseng | Korea | antidiabetic | [489] |
| Panax notoginseng | China | antihyperglycemic | [490,491] | |
| Panax quinquefolius | antidiabetic | [492] | ||
| Phaleria | Phaleria cumingii | antidiabetic | [493] | |
| Phaleria macrocarpa | Indonesia, Malaysia, Papua | α-glucosidase inhibitor | [494,495,496,497] | |
| Phaleria nishidae | antidiabetic | [498] | ||
| Phyllanthus | Phyllanthus amarus | Vietnam, India (Ayurveda, Siddha, Unani and homeopathy), Nigeria, Malaysia | α-glucosidase inhibitor, hypoglycemic, α-amylase inhibitor | [83,499,500,501,502] |
| Phyllanthus emblica | Thailand, Southeast Asia, India (Ayurveda) | antidiabetic | [75,356,503] | |
| Phyllanthus engleri | Tanzania | antidiabetic | [504] | |
| Phyllanthus fraternus | antidiabetic | [505] | ||
| Phyllanthus gardnerianus | India | antidiabetic | [506] | |
| Phyllanthus niruri | hypoglycemic | [507,508] | ||
| Phyllanthus urinaria | Vietnam | α-glucosidase and α-amylase inhibitor | [83] | |
| phyllanthus virgatus | α-amylase inhibitor | [509] | ||
| Phyllanthus watsonii | antidiabetic | [510] | ||
| Physalis | Physalis angulata | India | antidiabetic | [511] |
| Physalis minima | India | antidiabetic | [193] | |
| Physalis peruviana | India | antidiabetic | [248] | |
| Piper | Piper angustifolium | Latin America | antidiabetic | [512] |
| Piper betle | Asia | hypoglycemic | [513,514,515] | |
| Piper crocatum | antihyperglycemic | [516] | ||
| Piper cubeba | α-amylase and α-glucosidase | [517] | ||
| Piper guineense | Nigeria | α-amylase inhibitor | [401] | |
| Piper longum | Bangladesh, India (Ayurveda) | antihyperglycemic | [305,518,519] | |
| Piper nigrum | α-amylase inhibitor, hypoglycemic | [128,226,520] | ||
| Piper sarmentosum | South East Asia | antidiabetic | [521,522] | |
| Pistacia | Pistacia atlantica | Jordan | hypoglycemic | [168,352] |
| Pistacia integerrima | antidiabetic | [523] | ||
| Plantago | Plantago asiatica | antidiabetic | [524] | |
| Plantago lanceolata | Turkey | α-amylase and α-glucosidase inhibitor | [525] | |
| Plantago ovata | India | antidiabetic | [341] | |
| Plumeria | Plumeria alba | Togo | antidiabetic | [526] |
| Plumeria obtusa | South Africa | antidiabetic | [183] | |
| Plumeria rubra | India | α-amylase and α-glucosidase inhibitor | [517,527] | |
| Polygonum | Polygonum cuspidatum | Japan, Korea, China | α-glucosidase inhibitor | [528,529] |
| Polygonum hydropiper | India | antidiabetic | [230] | |
| Polygonum multiflorum | China, Asia, Europe, Africa | hypoglycemic | [530,531,532] | |
| Polygonum senegalensis | antidiabetic | [533] | ||
| Psidium | Psidium cattleianum | east Asia | antidiabetic | [534] |
| Psidium guajava | Mauritius, Togo, Sri Lanka, central America, Japan, China (TCM), Papua New Guinea | antihyperglycemic, hypoglycemic | [173,180,279,438,535,536,537] | |
| Pterocarpus | Pterocarpus santalinus | India (Ayurveda) | antidiabetic | [538] |
| Pterocarpus marsupium | India | antidiabetic | [539] | |
| Pterocarpus soyauxii | antidiabetic | [540] | ||
| Prunus | Prunus persica | India | antidiabetic | [541] |
| Prunus capuli | Peru | antidiabetic | [542] | |
| Prunus emarginata | Canada | antidiabetic | [289] | |
| Prunus mume | China | antidiabetic | [543] | |
| Pueraria | Pueraria lobata | Korea, China (TCM) | antidiabetic, α-glucosidase inhibitor | [544,545,546,547] |
| Pueraria thomsonii | antidiabetic | [548] | ||
| Pueraria thunbergiana | Korea | antidiabetic | [549] | |
| Rheum | Rheum emodi | India (Ayurveda), China | antidiabetic | [550] |
| Rheum officinale | China | antidiabetic | [551] | |
| Rheum palmatum | China | antidiabetic | [552] | |
| Rheum ribes | Iran, Jordon | hypoglycemic | [52,553,554] | |
| Rheum tanguticum | China | antidiabetic | [552] | |
| Rheum turkestanicum | Iran | antidiabetic | [555] | |
| Rheum undulatum | Korea | antidiabetic | [556] | |
| Rhododendron | Rhododendron brachycarpum | Korea | antidiabetic | [557,558] |
| Rhododendron groenlandicum | antidiabetic | [559] | ||
| Rhododendron tomentosum | Canada | antidiabetic | [560] | |
| Rhus | Rhus coriaria | Iran | antidiabetic | [561] |
| Rhus chinensis | antidiabetic | [562] | ||
| Rhus hirta | antidiabetic | [290] | ||
| Rhus mysorensis | antidiabetic | [563] | ||
| Rhus verniciflua | Korea | antidiabetic | [564] | |
| Rhus virens | Mexico | antidiabetic | [231] | |
| Rosa | Rosa canina | Iran, Turkey | antidiabetic | [565,566] |
| Rosa rugosa | Korea, China | hypoglycemic | [109,567,568] | |
| Salacia | Salacia chinensis | India (Ayurveda, Unani), Japan, Korea | hypoglycemic, antihyperglycaemic | [569,570,571] |
| Salacia oblonga | India (Ayurveda, Unani), Japan, Korea | hypoglycemic | [569,570,572] | |
| Salacia prinoides | India (Ayurveda), Sri Lanka, Southeast Asia | antidiabetic | [573] | |
| Salacia reticulata | India (Ayurveda, Unani), Japan, Korea, Sri Lanka | hypoglycemic, α-glucosidase inhibitor | [569,570,574,575] | |
| Salvia | Salvia acetabulosa | Lebanon | α-amylase inhibitor | [137] |
| Salvia hispanica | Central and South America | antidiabetic | [576] | |
| Salvia hypoleuca | Iran | antidiabetic | [577] | |
| Salvia officinalis | Iran | hypoglycemic, α-glucosidase inhibitor | [178] | |
| Salvia libanotica | antidiabetic | [578] | ||
| Salvia limbata | Turkey | α-amylase and α-glucosidase inhibitor | [525] | |
| Salvia miltiorrhiza | China | antidiabetic | [181,579] | |
| Sida | Sida acuta | India | antidiabetic | [580] |
| Sida cordifolia | Bangladesh, India (Ayurveda) | antidiabetic | [471,581] | |
| Sida rhombifolia | antidiabetic | [582] | ||
| Smilax | Smilax china | Korea | antidiabetic | [583] |
| Smilax glabra | China | antidiabetic | [584] | |
| Smilax officinalis | Latin America | antidiabetic | [512] | |
| Smilax perfoliata | Bangladesh | antihyperglycemic | [585] | |
| Solanum | Solanum americanum | Guatemala | antidiabetic | [124] |
| Solanum indicum | Uganda, India | antidiabetic | [104,187] | |
| Solanum lycocarpum | Brazil | antidiabetic | [586] | |
| Solanum muricatum | antidiabetic | [587] | ||
| Solanum nigrum | Asia | hypoglycemic | [588,589] | |
| Solanum torvum | antihyperglycemic | [590] | ||
| Solanum trilobatum | India (Ayurveda, Siddha) | antidiabetic | [118] | |
| Solanum tuberosum | antidiabetic | [591] | ||
| Solanum viarum | India | antidiabetic | [125] | |
| Solanum virginianum | Pakistan | antidiabetic | [592] | |
| Solanum xanthocarpum | hypoglycemic | [593] | ||
| Spondias | Spondias mombin | Nigeria | α-amylase inhibition, hypoglycemic | [594] |
| Spondias pinnata | Indonesia, Sri Lanka | antihyperglycemic | [595,596] | |
| Stereospermum | Stereospermum colais | α-glucosidase inhibitor | [597] | |
| Stereospermum suaveolens | India | antidiabetic | [598] | |
| Swertia | Swertia chirata | Bangladesh | antidiabetic | [126] |
| Swertia chirayita | India (Ayurveda) | hypoglycemic | [113,599] | |
| Swertia cordata | antidiabetic | [600] | ||
| Swertia longifolia | α-amylase inhibitor | [601] | ||
| Swertia macrosperma | Tibet, China | antidiabetic | [602] | |
| Swertia mussotii | China | α-glycosidase inhibitor | [603] | |
| Syzygium | Syzygium alternifolium | antidiabetic | [604] | |
| Syzygium aromaticum | antihyperglycemic, hypoglycemic | [605] | ||
| Syzygium cumini | Bangladesh, India (Ayurveda), Brazil | α-glucosidase and α-amylase inhibitor, antihyperglycemic | [83,172,220,376,606,607,608] | |
| Syzygium densiflorum | India | antidiabetic | [609] | |
| Syzygium jambolanum | India (Ayurveda) | hypoglycemic | [610,611] | |
| Syzygium jambosa | Puerto Rico | hypoglycemic | [612] | |
| Syzygium samarangense | Bangladesh | antihyperglycemic | [116] | |
| Tabernaemontana | Tabernaemontana corymbosa | Malaysia | antidiabetic | [613] |
| Tabernaemontana divaricata | India | antidiabetic | [104] | |
| Tabernaemontana heyneana | antidiabetic | [614] | ||
| Taxus | Taxus baccata | India | antidiabetic | [151] |
| Taxus yunnanensis | China | antidiabetic | [615] | |
| Terminalia | Terminalia alata | Vietnam | antidiabetic | [616] |
| Terminalia arjuna | Bangladesh, India (Ayurveda) | α-amylase inhibitor, antihyperglycemic | [126,127,617,618] | |
| Terminalia bellirica | Bangladesh, Vietnam, India (Ayurveda, Siddha, Unani), Sri Lanka, Southeast Asia | antidiabetic | [133,616,619,620] | |
| Terminalia catappa | antidiabetic | [621] | ||
| Terminalia chebula | Thailand, India (Ayurveda), Bangladesh, Iran | α-amylase inhibitor | [75,128,130,622,623] | |
| Terminalia citrina | Bangladesh | antidiabetic | [133] | |
| Terminalia corticosa | Vietnam | antidiabetic | [616] | |
| Terminalia glaucescens | Cameroon | antidiabetic | [624] | |
| Terminalia macroptera | Africa | α-glucosidase inhibitor | [625] | |
| Terminalia sericea | antidiabetic | [626] | ||
| Terminalia superba | antidiabetic | [627] | ||
| Teucrium | Teucrium oliverianum | antidiabetic | [628] | |
| Teucrium polium | Jordan, Iran | hypoglycemic | [553,629,630] | |
| Thymus | Thymus caramanicus | Iran | antidiabetic | [631] |
| Thymus satureioides | Morocco | antidiabetic | [632] | |
| Tinospora | Tinospora cordifolia | Southeast Asia, India (Ayurveda), Thailand, Malaysia, Guyana, Bangladesh | α-amylase inhibitors, hypoglycemic, antihyperglycemic | [113,128,135,356,619,633,634,635] |
| Tinospora crispa | Malaysia, Thailand, Malaysia, Guyana, Bangladesh, Indonesia, Malaysia | hypoglycemic, antihyperglycemic | [613,635,636,637,638,639,640] | |
| Tinospora malabarica | antidiabetic | [641] | ||
| Tinospora sinensis | Nepal, India | antidiabetic | [150,642] | |
| Tinospora bakis | Sudan | antidiabetic | [643] | |
| Trichosanthes | Trichosanthes cucumerina | India (Ayurveda) | hypoglycemic | [113] |
| Trichosanthes dioica | India (Ayurveda) | antidiabetic | [644] | |
| Trichosanthes kirilowii | China (TCM) | hypoglycemic, α-amylase inhibitor | [645,646] | |
| Trichosanthes tricuspidata | hyperglycemic | [647] | ||
| Urtica | Urtica angustifolia | hypoglycemic | [648] | |
| Urtica dioica | Kenya, Iran, Turkey | α-amylase inhibitor | [248,649,650,651] | |
| Urtica urens | antidiabetic | [652] | ||
| Vaccinium | Vaccinium angustifolium | antidiabetic | [653] | |
| Vaccinium arctostaphylos | Iran | α-amylase inhibitor | [654] | |
| Vaccinium bracteatum | China | antidiabetic | [655] | |
| Vaccinium myrtillus | antidiabetic | [656] | ||
| Vaccinium ovalifolium | antidiabetic | [657] | ||
| Vaccinium uliginosum | antidiabetic | [657] | ||
| Vaccinium vitis | antidiabetic | [658] | ||
| Withania | Withania coagulans | India (Ayurveda), Pakistan | antihyperglycemic | [659,660,661] |
| Withania somnifera | India (Ayurveda) | hypoglycemic | [96,662] | |
| Zanthoxylum | Zanthoxylum alatum | antidiabetic | [663] | |
| Zanthoxylum armatum | India (Ayurveda) | antidiabetic | [251] | |
| Zanthoxylum capense | South African | antidiabetic | [664] | |
| Zanthoxylum chalybeum | Tanzania | antidiabetic | [188] | |
| Zanthoxylum humile | India (Ayurveda) | antidiabetic | [665] | |
| Zingiber | Zingiber officinale | India (Ayurveda), Latin America Africa | α-amylase inhibitor, hypoglycemic | [113,128,512,666] |
| Zingiber striolatum | China (TCM) | hypoglycemic | [667] | |
| Ziziphus | Ziziphus amole | antidiabetic | [668] | |
| Ziziphus jujuba | Turkey | α glucosidase inhibitor | [76,669] | |
| Ziziphus lotus | Algeria | antidiabetic | [670] | |
| Ziziphus mauritiana | Southeast Asia, Mali | antidiabetic | [356,671] | |
| Ziziphus mucronata | Nigeria | antidiabetic | [672] | |
| Ziziphus nummularia | India | antidiabetic | [132] | |
| Ziziphus oxyphylla | Pakistan | antidiabetic | [673] | |
| Ziziphus spina-christi | Egypt | hypoglycemic and anti-hyperglycemic | [674] | |
| Ziziphus xylopyrus | India (Ayurveda), Pakistan, China | antidiabetic | [675] |
| Plant Name | Country/Region | Activity | Reference |
|---|---|---|---|
| Abrus precatorius | India (Ayurveda, Unani, Siddha) | antidiabetic | [676] |
| Acorus calamus | India, Indonesia, America | α-glucosidase inhibitor | [93,677,678] |
| Actinidia arguta | Korea | antidiabetic | [679] |
| Adansonia digitata | India (Ayurveda) | α-amylase inhibitor | [128] |
| Adiantum capillus-veneris | India | antidiabetic | [151] |
| Ageratum conyzoides | Bangladesh | antidiabetic | [126] |
| Agrimonia pilosa | China | α-glucosidase inhibitor | [680] |
| Ailanthus excelsa | India | antidiabetic | [681] |
| Alangium salvifolium | India (Ayurveda) | hypoglycemic | [682,683] |
| Alstonia scholaris | India, Thailand | α-glucosidase inhibitor | [87,684] |
| Amomum villosum | China | antidiabetic | [109] |
| Amygdalus lycioides | Iran | antidiabetic | [685] |
| Andrographis paniculata | India (Ayurveda), Bangladesh, Nepal, Malaysia, Southeast Asia | antihyperglycemic | [126,150,356,686,687] |
| Anemarrhena asphodeloides | China | antidiabetic, α-glucosidase inhibitor | [181,688,689] |
| Anethum graveolens | Iran, Asia | antidiabetic | [690,691] |
| Anogeissus acuminate | Thailand | hypoglycemic | [433] |
| Anthocephalus cadamba | India (Ayurveda), Australia, China, Indonesia, Malaysia, Papua New Guinea, Philippines, Singapore, Vietnam | antidiabetic | [692] |
| Aphanamixis polystachya | India (Ayurveda) | antidiabetic | [693] |
| Arctium lappa | China | hypoglycemic | [694] |
| Argyreia nervosa | India (Ayurveda) | antidiabetic | [695] |
| Asanadi gana | India (Ayurveda) | antidiabetic | [696] |
| Azadirachta indica | India (Ayurveda), Nigeria, Pakistan, Mexico, Bangladesh, Nepal, Saudi Arabia, South East Asia, Mauritius, Malaysia, Indonesia | α-glucosidase and α-amylase inhibitor, hypoglycemic | [65,113,126,135,150,190,220,231,253,356,697,698,699] |
| Barringtonia acutangula | India (Ayurveda) | antidiabetic | [700] |
| Basella rubra | India | α-amylase inhibitor | [701] |
| Begonia roxburghii | India | antidiabetic | [125] |
| Bergenia ciliata | Nepal | α-glucosidase, α-amylase inhibitor | [702] |
| Biophytum sensitivum | Nepal | antidiabetic | [703] |
| Blepharis molluginifolia | India | antidiabetic | [704] |
| Boerhavia diffusa | India (Ayurveda) | antidiabetic | [226] |
| Boswellia ovalifoliolata | India | antidiabetic | [705] |
| Caccinium myrtillus | Europe | α-glucosidase inhibitor | [706] |
| Cajanus cajan | India (Ayurveda) | antidiabetic | [172] |
| Callicarpa arborea | India | antidiabetic | [125] |
| Camellia sinensis | Iran | α-amylase inhibitor | [651] |
| Canna indica | antidiabetic | [707] | |
| Cardia obaliqua | Pakistan | antidiabetic | [708] |
| Carthamus tinctorius | Iran | α-glucosidase inhibitor | [709,710] |
| Casia fistula | India (Ayurveda) | α-amylase inhibitor | [128] |
| Catharanthus roseus | India (Ayurveda), South Africa, China, Malaysia, South East Asian Countries, South Africa, Trinidad, Tobago | α amylase inhibitor, antihyperglycemic, hypoglycemic | [113,189,234,356,711,712,713,714,715] |
| Catunaregam tormentosa | Thailand | hypoglycemic | [433] |
| Cayratia trifolia | India | antidiabetic | [716] |
| Ceiba pentandra | India, Nigeria | α-amylase inhibition, hypoglycemic, antihyperglycemic | [717,718,719] |
| Celosia argentea | China | antidiabetic | [720] |
| Centella asiatica | India (Ayurveda), Bangladesh, Malaysia, Laos, Southeast Asia | antidiabetic | [133,306,356,721,722] |
| Centranthus longiflorus | Turkey | antidiabetic | [723] |
| Centratherum anthelminticum | India (Ayurveda) | hypoglycemic | [580,724] |
| Cerinthe minor | Turkey | antidiabetic | [723] |
| Chlorophytum borivilianum | India (Ayurveda) | antidiabetic | [725] |
| Cirsium japonicum | Taiwan | antidiabetic | [726] |
| Cistanche tubulosa | China | antihyperglycemic | [727] |
| Citrullus colocynthis | Iran, Algeria, Southeast Asia | hypoglycemic | [356,728,729] |
| Clinacanthus nutans | Indonesia, Malaysia, Thailand | antidiabetic | [730,731] |
| Clitoria ternatea | India (Ayurveda) | α-glucosidase, α-amylase inhibitor hypoglycemic | [452,732,733] |
| Cocculus hirsutus | India | α-amylase inhibitor | [701] |
| Coldenia procumbens | India | antidiabetic | [734] |
| Commiphora wightii | India (Ayurveda) | antidiabetic | [226] |
| Coscinium fenestratum | India, Sri Lanka | antidiabetic | [735,736] |
| Cressa cretica | Bahrain | antidiabetic | [737] |
| Crossostephium chinense | China | antidiabetic | [289] |
| Cuminum cyminum | India | antidiabetic | [738] |
| Cupressus sempervirens | Cyprus | antidiabetic | [739] |
| Cyamopsis tetragonoloba | India (Ayurveda) | antidiabetic | [740] |
| Cyclocarya paliurus | China | antidiabetic | [741] |
| Cydonia oblonga | Turkey | hypoglycemic | [176] |
| Dendrocalamus hamiltonii | India (Ayurveda) | hypoglycemic | [113] |
| Dendrophthoe pentandra | Indonesia | antidiabetic | [742] |
| Desmostachya bipinnata | India (Ayurveda) | antidiabetic | [743] |
| Dillenia indica | India | antidiabetic | [125] |
| Dioecrescis erythroclada | Thailand | hypoglycemic | [433] |
| Diplazium esculentum | India | antidiabetic | [125] |
| Dorema aucheri | Iran | hypoglycemic | [744] |
| Eclipta alba | Bangladesh, India (Ayurveda) | α-glucosidase inhibitor | [409,745,746] |
| Elaeocarpus ganitrus | India (Ayurveda), Nepal | antidiabetic | [747] |
| Eleutherine palmifolia | Indonesia | hyperglycemic | [748] |
| Emblica officinalis | India (Ayurveda), Bangladesh | antidiabetic | [89,409,749] |
| Enhydra fluctuans | India | antidiabetic | [750] |
| Eremurus persicus | Iran | antidiabetic | [751] |
| Erigeron breviscapus | China | antidiabetic | [752] |
| Eryngium creticum | Jordan | antidiabetic | [753] |
| Eucommia ulmoides | China, Japan, Korea | antidiabetic | [754] |
| Eulophia herbacea | Bangladesh | antidiabetic | [755] |
| Fagonia cretica | Pakistan | antidiabetic | [143,756] |
| Fagopyrum cymosum | China | hypoglycemic | [109] |
| Feronia limonia | India | antidiabetic | [757] |
| Foeniculum vulgare | Sudan, Iran, Portugal | antidiabetic | [154,758,759] |
| Gloriosa superba | India (Ayurveda) | antidiabetic | [760] |
| Glycosmis pentaphylla | Siddha, India (Ayurveda) | antidiabetic | [761] |
| Gmelina arborea | India, Sri Lanka | antidiabetic | [762,763] |
| Gymnema sylvestre | Ayurveda, Pakistan, Southeast Asia | hypoglycemic and antihyperglycemic | [356,764,765,766,767] |
| Gynostemma pentaphyllum | China, Vietnam | hypoglycemic | [768,769,770] |
| Helianthus tuberosus | Turkey | hypoglycemic | [176] |
| Hemidesmus indicus | India (Ayurveda) | antidiabetic | [771] |
| Heritiera fomes | India | antidiabetic | [772] |
| Hippophae rhamnoides | China | antidiabetic | [773] |
| Hordeum vulgare | Iran | antidiabetic | [774] |
| Houttuynia cordata | Japan | antidiabetic | [775] |
| Ichnocarpus frutescens | India (Ayurveda) | antidiabetic | [776] |
| Imperata cylindrica | India (Ayurveda) | antidiabetic | [777] |
| Ixeris dentata | Korea, Japan, and China | antidiabetic | [778] |
| Juglans regia | Iran, Algeria, Turkey, Austria | hypoglycemic | [779,780,781,782,783] |
| Kaempferia parviflora | Thailand | antidiabetic | [784] |
| Kalopanax pictus | Korea | antidiabetic | [785] |
| Kickxia ramosissima | Pakistan | antidiabetic | [786] |
| Korthalsella japonica | Korea | antidiabetic | [787] |
| Lagenaria sicereria | Mauritius, India (Ayurveda) | antihyperglycemic | [186,788,789] |
| Lagerstroemia speciosa | Philippines | hypoglycemic, α-glucosidase inhibitor | [790,791,792] |
| Lannea coromandelica | Bangladesh | antidiabetic | [793] |
| Lactuca gracilis | India | antidiabetic | [125] |
| Leonurus sibiricus | Mongolia | antidiabetic | [794] |
| Leptospermum flavescens | Malaysia | antidiabetic | [795] |
| Linum usitatisumum | India (Ayurveda) | α-amylase inhibitor | [128] |
| Litchi chinensis | Indonesia | antidiabetic | [796] |
| Lycopus lucidus | China (TCM), Korea | α-amylase inhibitor | [646,797] |
| Macrotyloma uniflorum | Asia, Africa | antidiabetic | [798] |
| Magnolia officinalis | China, Japan | antidiabetic | [799] |
| Mahonia bealei | China | antidiabetic | [800] |
| Medicago sativa | China | antidiabetic | [801] |
| Meyna laxiflora | India | antidiabetic | [802] |
| Mezzetia parviflora | Indonesia | antidiabetic | [803] |
| Millingtonia hortensis | India | antidiabetic | [125] |
| Mitragyna speciosa | Malaysia, Thailand, Southeast Asia | antidiabetic | [804] |
| Mukia maderaspatana | India (Ayurveda, Siddha) | antidiabetic | [805] |
| Murdannia loriformis | China | antidiabetic | [806] |
| Myrica rubra | China | antidiabetic | [807] |
| Nelumbo nucifera | India (Ayurveda), China (TCM), Southeast Asia | α-glucosidase, α-amylase inhibitor, hypoglycemic | [140,356,808,809] |
| Neolamarckia cadamba | Bangladesh | antidiabetic | [810] |
| Nicotiana plumbaginifolia | India | antidiabetic | [151] |
| Nigella sativa | Algeria, India (Ayurveda, Siddha, Unani), Pakistan, Morocco, Middle East, Mediterranean, North Africa | antidiabetic | [174,766,811,812,813,814,815,816] |
| Nycantus arbor-tristis | India (Ayurveda), Sri Lanka | hypoglycemic | [117] |
| Nypa fruticans | Malaysia | antidiabetic | [817] |
| Odina wodier | India | antidiabetic | [818] |
| Ophiopogon japonicus | China, Japan, Southeast Asia | antidiabetic | [181,819] |
| Oreocnide integrifolia | India | antidiabetic | [820] |
| Oroxylum indicum | Bangladesh, India (Ayurveda) | antidiabetic | [133,821] |
| Paronychia argentea | Jordan | hypoglycemic | [352,553] |
| Pavonia zeylanica | India (Ayurveda) | antidiabetic | [682] |
| Pergularia daemia | India (Ayurveda) | antidiabetic | [822] |
| Persea americana | Togo, Tanzania, Trinidad and Tobago, Central America, India (Ayurveda), Nigeria | antidiabetic | [180,188,189,438,823,824] |
| Peucedanum praeruptorum | India (Ayurveda), China | antidiabetic | [825] |
| Phaseolus vulgaris | Jordan | antihyperglycemic | [175,258] |
| Phlomis armeniaca | Turkey | α-amylase and an α-glucosidase inhibitor | [525] |
| Phoenix dactylifera | Jordan, India (Ayurveda), Pakistan, Egypt | antidiabetic | [258,826,827,828] |
| Phragmanthera austroarabica | Saudi Arabia | antidiabetic | [829] |
| Phyllostachys edulis | China | antidiabetic | [830] |
| Pilea microphylla | China | antidiabetic | [831] |
| Pimpinella tirupatiensis | Turkey, China, Korea, Iran, Egypt, Palestine, Lebanon, Europe | antidiabetic | [832,833] |
| Pisonia grandis | India | antidiabetic | [834] |
| Platycodon grandiflorum | Korea | antidiabetic | [835] |
| Pluchea indica | Indonesia | α-glucosidase inhibitor | [836] |
| Plumbago zeylanica | India | antidiabetic | [151] |
| Polyalthia longifolia | India | antidiabetic | [837] |
| Polygonatum sibiricum | China | antidiabetic | [181] |
| Pongamia pinnata | India (Ayurveda) | antihyperglycemic | [838,839] |
| Poria cocos | China | antidiabetic | [840] |
| Portulaca oleracea | Trinidad and Tobago, India (Ayurveda), Algeria, Iran, China (TCM), Mexico | hypoglycemic | [189,841,842,843,844,845,846] |
| Premna integrifolia | India (Ayurveda) | hypoglycemic | [113] |
| Pseuderanthemum palatiferum | Vietnam, Thailand | hypoglycemic | [847] |
| Psoralea corylifolia | India (Ayurveda) | antidiabetic | [848] |
| Punica granatum | India (Ayurveda, unani) | antidiabetic | [849,850,851,852] |
| Raphanus sativus | Iran, China | antidiabetic | [853,854] |
| Rauwolfia serpentina | Thailand | hypoglycemic | [433] |
| Rehmannia glutinosa | China, Korea | antidiabetic | [855,856] |
| Retama raetam | Saudi Arabia | antihyperglycemic | [857] |
| Rhodamnia cinerea | Malaysia | antidiabetic | [858] |
| Roscoea purpurea | Nepal | antidiabetic | [859] |
| Rosmarinus officinalis | Algeria, Jordan, Turkey | antidiabetic | [174,860,861] |
| Roylea cinerea | India | antidiabetic | [862] |
| Rubia cordifolia | India | antidiabetic | [863] |
| Saccharum spontaneum | India | antidiabetic | [125] |
| Salicornia herbacea | Korea | antidiabetic | [864] |
| Sanguis draxonis | China | antidiabetic | [865] |
| Sasa borealis | Korea | antidiabetic | [866] |
| Schisandra chinensis | China | antidiabetic | [181] |
| Schizonepeta tenuifolia | Korea | antidiabetic | [867] |
| Securigera securidaca | Iran | antidiabetic | [868] |
| Sesbenia aegyptiaca | India (Ayurveda) | hypoglycemic | [113] |
| Siraitia grosvenori | China | antidiabetic | [869] |
| Sphaeranthus indicus | India | antidiabetic | [870] |
| Stevia rebaudiana | India, Paraguay, Brazil, south America | antidiabetic | [871,872,873] |
| Swietenia macrophylla | Malaysia | antidiabetic | [874] |
| Tamarindus indica | India (Ayurveda), Trinidad and Tobago, Africa | α amylase inhibitor | [189,234,875] |
| Tecoma stans | Jordan, Central America, Egypt, Mexico | α-glucosidase inhibitor | [145,258,438,876] |
| Tephrosia purpurea | India (Ayurveda) | antihyperglycemic | [877,878] |
| Thespesia populnea | India (Ayurveda) | antihyperglycemic and hypoglycemic | [879] |
| Tithonia diversifolia | Costa Rica, Democratic Republic of Congo, Kenya, Nigeria, Mexico, the Philippines, São Tomé and Príncipe, Taiwan, Uganda, Venezuela | antidiabetic | [880] |
| Toona sinensis | China | antidiabetic | [881] |
| Tragia involucrata | India (Ayurveda) | antidiabetic | [882] |
| Trichosanthis kirilowii | China | antidiabetic | [181] |
| Trigonella foenum-graecum | Iran, Turkey, Algeria, Bangladesh, Pakistan, Morocco, Algeria, Mediterranean, China, India (Ayurveda) | antidiabetic, α-amylase inhibitor, antihyperlipidemic effect, hypoglycemic | [50,76,128,129,174,181,651,766,767,813,883,884,885,886,887,888,889] |
| Varthemia iphionoides | Jordan | antidiabetic | [753] |
| Vinca major | South Africa | antidiabetic | [441] |
| Viola odorata | India | antidiabetic | [151] |
| Wedelia trilobata | South America, China, Japan, India | antidiabetic | [890] |
| Species | Extract | Part of the Plant | Dosage (mg/kg) | Experimental Model | Induction of Diabetes | Reference |
|---|---|---|---|---|---|---|
| Acacia arabica | chloroform | bark | 250, 500 | male Wistar rats and albino mice | alloxan | [891] |
| chloroform | bark | 100, 200 | female albino rats | streptozotocin | [892] | |
| Achyranthes rubrofusca | aqueous and ethanolic | leaves | 200 | rats | alloxan | [893] |
| Albizzia lebbeck | methanol/dichloro-methane | stem bark | 100, 200, 300, 400 | male albino Wistar rats | streptozotocin | [894] |
| methanolic | bark | 200, 350, 620 | female Sprague–Dawley rats | streptozotocin-nicotinamide | [895] | |
| Aloe vera | aqueous | leaves | 130 | swiss albino mice | streptozotocin | [896] |
| ethanolic | leaves | 300 | male albino Wistar rats | streptozotocin | [897] | |
| Amaranthus tricolor | methanolic | whole plant | 50, 100, 200, 400 | male swiss albino mice | glucose-induced hyperglycemia | [898] |
| Anacardium occidentale | aqueous | leaves | 175 | male albino Wistar rats | streptozotocin | [899] |
| methanolic | leaves | 100 | female albino mice | streptozotocin | [900] | |
| Azadirachta indica | ethanolic | leaves | 200 | adult rabbits | alloxan | [901] |
| Barleria prionitis | ethanolic | leaves and root | 200 | adult albino rats | alloxan | [902] |
| Bauhinia thoningii | aqueous | leaves | 500 | Wistar albino rats | alloxan | [903] |
| Caesalpinia ferrea | aqueous | stem bark | 300, 450 | male Wistar rats | streptozotocin | [904] |
| Camellia sinensis | crude tea | leaves | 0.5 mL/day | male albino mice | streptozotocin | [905] |
| Casearia esculenta Roxb | aqueous | root | 200, 300 | male albino Wistar rats | streptozotocin | [906] |
| Cassia fistula | ethanolic | stem bark | 250, 500 | Wistar rats | alloxan | [907] |
| Cassia grandis | aqueous and ethanolic | stem | 150 | male albino Wistar rats | alloxan | [908] |
| Catharanthus roseus | dichloromethane-methanol | leaves and twigs | 500 | male Sprague–Dawley rats | streptozotocin | [909] |
| ethanolic | leaves | 100, 200 | male Wistar rats | streptozotocin | [711] | |
| Cecropia pachystachya | methanolic | leaves | 80 | male Wistar rats | alloxan | [910] |
| Ceriops decandra | ethanolic | leaves | 30, 60, 120 | male albino Wistar rats | alloxan | [911] |
| Chiliadenus iphionoides | ethanolic | aerial parts | 1000 | male and female diabetes-prone Psammomys obesus | - | [912] |
| Cinnamomum cassia | ethanolic | bark | 200, 300 | male Kunming mice | streptozotocin | [913] |
| Cinnamomum japonica | ethanolic | bark | 200, 300 | male Kunming mice | streptozotocin | [913] |
| Citrullus colocynthis | aqueous | root | 2000 | male and female Wistar rats and Swiss albino mice | alloxan | [914] |
| aqueous | seed | 1, 2 mL/kg | male Wistar albino rats | alloxan | [915] | |
| Coscinium fenestratum | ethanolic | stem | 250 | male albino Wistar rats | streptozotocin-nicotinamide | [916] |
| Eucalyptus citriodora | aqueous | leaves | 250, 500 | albino rats | alloxan | [917] |
| Gymnema sylvestre | ethanolic | leaves | 100 | male Sprague–Dawley rats | streptozotocin | [918] |
| Heinsia crinata | ethanolic | leaves | 450–1350 | rats | alloxan | [919] |
| Helicteres isora | butanol and aqueous ethanol | roots | 250 | male Wistar rats | alloxan | [920] |
| Momordica charantia | aqueous | pulp | 13.33 g pulp/kg | male albino Wistar rats | alloxan | [921] |
| ethanolic | fruit | 200 | adult rabbits | alloxan | [901] | |
| ethanolic | fruit | 400 | male Sprague–Dawley rats | streptozotocin | [922] | |
| Moringa oleifera | methanolic | pod | 150, 300 | Wistar albino rats | streptozotocin | [923] |
| - | leaves | 50 | male Sprague–Dawley rats | alloxan | [924] | |
| Murraya koenigii | aqueous | leaves | 200, 300, 400 | male albino rabbits | alloxan | [458] |
| ethanolic | leaves | 100, 250 | male albino Swiss mice | dexamethasone | [925] | |
| Opuntia ficus-indica | petroleum ether | stems | 200 | male ICR mice | streptozotocin | [926] |
| Origanum vulgare | methanolic | leaves | 5 | male C57BL/6 mice | streptozotocin | [927] |
| Passiflora nitida | hydro-ethanolic | leaves | 50 | female Wistar rats | streptozotocin | [928] |
| Paspalum scrobiculatum | aqueous and ethanolic | grains | 250, 500 | male Wistar albino rats | alloxan | [929] |
| Persea americana | hydro-alcoholic | leaves | 150, 300 | male Wistar rats | streptozotocin | [930] |
| aqueous | seed | 20, 30, 40 g/L | male Wistar albino rats | alloxan | [931] | |
| Phoenix dactylifera | ethanolic | leaves | 50-400 | male Wistar rats | alloxan | [932] |
| Phyllanthus niruri | aqueous | leaves | 200, 400 | male Wistar rats | streptozotocin-nicotinamide | [934] |
| Phyllanthus simplex | petroleum ether, ethyl acetate, methanol and water fraction | 100–400 | rats | alloxan | [935] | |
| Picralima nitida | methanolic | steam bark and leaves | 75, 150, 300 | Wistar rats | streptozotocin | [936] |
| Piper longum | aqueous | root | 200, 300, 400 | male Wistar albino rats | streptozotocin | [937] |
| Sonchus oleraceus | hydro-alcoholic | whole plant | 75, 150, 300 | Wistar rats | streptozotocin | [936] |
| Syzygium jambolana | ethanolic | seed | 200 | adult rabbits | alloxan | [901] |
| Tamarindus indica | ethanolic | stem bark | 250, 500 | Wistar rats | alloxan | [907] |
| ethanolic | seed coat | 500 | Wistar albino rats | alloxan | [938] | |
| Terminalia chebula | chloroform | seed | 100, 200, 300 | male Sprague–Dawley rats | streptozotin | [939] |
| Terminalia catappa | petroleum ether, methanol and aqueous | fruit | 68, 40, 42 | Wistar albino rats and mice | alloxan | [940] |
| Trigonella foenum-graecum | ethanolic | seed | 100, 500, 1000, 2000 | male Wistar albino rats | alloxan | [941] |
| hydro-alcoholic | seed | 500, 1000, 2000 | Sprague–Dawley rats | alloxan | [942] | |
| Vaccinium arctostaphylos | ethanolic | fruit | 200, 400 | male Wistar rats | alloxan | [943] |
| Vernonia amygdalina | aqueous | leaves | 100 | Wistar albino rats | alloxan | [944] |
| Witheringia solanacea | aqueous | leaves | 500, 1000 | male Sprague–Dawley rats | GTT | [945] |
| Zaleya decandra | ethanolic | roots | 200 | Wistar albino rats | alloxan | [946] |
| Zizyphus mauritiana | petroleum ether, chloroform, acetone, ethanol and aqueous | fruit | 200, 400 | female Wistar rats | alloxan | [947] |
| Compound | Sources | Structure | Target | Reference |
|---|---|---|---|---|
| Baicalein | Oroxylum indicum, Scutellaria baicalensis |  | mitigates renal oxidative stress, suppresses activation of NF-κB, decreases expression of iNOS and TGF-β1, ameliorates structural changes in renal tissues, and normalizes the levels of serum proinflammatory cytokines and liver function enzymes | [953,987] |
| Berberine | Argemone mexicana, Berberis aquifolium, Berberis aristata, Berberis vulgaris, Coptis chinensis, Eschscholzia californica, Hydrastis canadensis, Tinospora cordifolia, Xanthorhiza simplicissima, Phellodendron amurense |  | regulates glucose and lipid metabolism | [1041,1042] |
| Boldine | Peumus boldus |  | reduces overproduction of reactive oxygen species by inhibiting Ang II-stimulated BMP4 expression | [953,954] |
| Boswellic acids | the oleo gum resin from the trees of different Boswellia species (Boswellia serrata, Boswellia carteri) |  | for the prophylaxis and/or treatment of damage to and/or inflammation of the islets of langerhans; stimulates β cells to release more insulin | [990,991] |
| Butein | Toxicodendron vernicifluum, Dalbergia odorifera, Cyclopia subternata, Semecarpus anacardium, Creopsis tungtoria |  | inhibits central NF-κB signaling and improves glucose homeostasis | [1016] |
| Catechins (catechin, epicatechin and epigallocatechin gallate (EGCG)) | tea and cocoa, Camellia sinensis, Theobroma cacao |  | antioxidative; by protective effects against oxidative damage; by modification of oxidative stress; reduces lipid peroxidation by enhancing the SOD, GST, and CAT activities | [1043,1044] |
| Celastrol | Tripterygium wilfordii, Celastrus orbiculatus, Celastrus aculeatus, Celastrus reglii, Celastrus scandens | 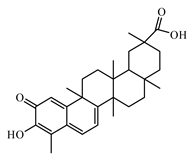 | protective effects on diabetic liver injury via TLR4/MyD88/NF-kB signaling pathway in T2DM; suppresses obesity process via increase in antioxidant capacity and improves lipid metabolism; an NF-κB inhibitor; improves insulin resistance and attenuates renal injury | [992,993,994] |
| Chlorogenic acid | in many varieties of plant species |  | stimulates glucose transport in skeletal muscle via AMPK activation; effects on hepatic glucose release and glycemia | [1025,1026,1027] |
| Chrysin | Passiflora caerulea, Passiflora incarnata, Oroxylum indicum |  | suppresses transforming growth factor-beta (TGF-β), fibronectin, and collagen-IV protein expressions in renal tissues; reduces the serum levels of pro-inflammatory cytokines, interleukin-1beta (IL-1β), and IL-6 | [953,985] |
| Curcumin | Zingiberaceae plants, Curcuma longa |  | blood glucose-lowering effect; lowers glycosylated hemoglobin levels | [1017,1018,1019] |
| Ellagic acid | in fruits (pomegranates, persimmon, raspberries, black raspberries, strawberries, peach, plums), nuts (walnuts, almonds), vegetables, wine |  | by the action on β cells of the pancreas that stimulates insulin secretion and decreases glucose intolerance; possesses superior antioxidant properties and genotoxicitypreventive; inhibits a-amylase activity; reduces hyperglycemia and insulin resistance in T2DM | [1028,1029,1030] |
| Embelin | Embelia ribes, Lysimachia punctata, Lysimachia erythrorhiza |  | reduces the elevated plasma glucose, glycosylated hemoglobin, and pro-inflammatory mediators | [1031,1032] |
| Erianin | Dendrobium chrysotoxum |  | inhibits high glucose-induced retinal angiogenesis via blocking ERK1/2-regulated HIF-1α-VEGF/VEGFR2 signaling pathway | [1033] |
| Fisetin | Acacia greggii, Acacia berlandieri, Gleditschia triacanthow, Butea fronds, Gleditsia triacanthos, Quebracho colorado, Rhus cotinus, Rhus vemiciflua Cotinus coggygria, Callitropsis Nootkatensis |  | improves glucose homeostasis through the inhibition of gluconeogenic enzymes; increases the level and activity of glyoxalase 1; significantly reduces blood glucose | [963,964,965] |
| Galactomannan gum | Cyamopsis tetragonolobus Amorphophallus konjac |  | delays the rate of glucose absorption and thereby helps to reduce postprandial hyperglycemia | [1003,1004] |
| Gambogic acid | Garcinia hanburyi. Garcinia indica, Garcinia cambogia |  | ameliorates diabetes-induced proliferative retinopathy through inhibition of the HIF-1α/VEGF expression via targeting the PI3K/AKT pathway | [1034] |
| Garcinol | Garcinia spp. plants (Garcinia indica) |  | decreases plasma insulin, HOMA-β-cell functioning index, glycogen, high-density lipoprotein cholesterol, body weight, and antioxidant enzyme activities, viz. SOD, CAT, and glutathione; causes a significant reduction in elevated levels of blood glucose, glycosylated hemoglobin, and lipids | [1035,1036] |
| Honokiol | Magnolia plant spp. (Magnolia officinalis) |  | significantly increases phosphorylations of the IRβ and the downstream insulin signaling factors including AKT and ERK1/2; potential binding mode of honokiol to PTP1B; protects pancreatic β cells against high glucose and intermittent hypoxia-induced injury by activating the Nrf2/ARE pathway | [1037,1038] |
| Kaempferol | in a variety of plants and plant-derived foods |  | promotes insulin sensitivity and preserves pancreatic β-cell mass | [966] |
| Lupanine | Lupinus species (Lupinus perennis) |  | enhances insulin secretion; improves glucose homeostasis by influencing KATP channels and insulin gene | [955] |
| Luteolin | Lamiaceae plant family |  | diabetic nephropathy; ameliorates cardiac failure in T1DM cardiomyopathy | [967,968] |
| Indole-3-Carbinol | in cruciferous vegetables |  | increases the antioxidant-scavenging action by increasing levels of SOD, CAT, GPx, vitamin C, vitamin E, and glutathione | [1023,1024] |
| Inulin | the Helianthus tuberosus tubers contain 75 to 80% of carbohydrates in the form of inulin |  | acts as a biogenetic factor for the development of natural intestinal microflora after dysbacteriosis; in the modulation of blood metabolites and liver enzymes | [1005,1006] |
| Morin | Morus alba, Maclura pomifera, Psidium guajava, Chlorophora tinctoria, Prunus dulcis, Maclura tinctoria, Castanea sativa |  | as an activator and sensitizer of the insulin receptor stimulating the metabolic pathways; rescues endothelial dysfunction in a diabetic mouse model by activating the Akt/eNOS pathway; downregulation of the miR-29a level; attenuates ER stress throughout the downregulation of the PERK-eIF2α-ATF4 pathway by interacting with the PERK protein | [975,976] |
| Naringenin | Grapefruit (Citrus × paradisi) |  | attenuates diabetic nephropathy via its anti-inflammatory and anti-fibrotic activities | [953,969] |
| Neferine | Nelumbo nucifera | 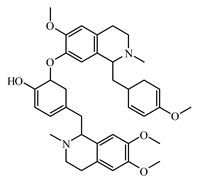 | reduces expression of CCL5 and CCR5 mRNA in the superior cervical ganglion of T2D; prevents hyperglycemia-induced endothelial cell apoptosis through suppressing the OS/Akt/NF-κB signal | [953,957] |
| Oxymatrine | Sophora flavescens |  | prevents oxidative stress and reduces the contents of renal advanced glycation end products, transforming growth factor-β1, connective tissue growth factor, and inflammatory cytokines in diabetic rats | [953,958] |
| Piceatannol | in a variety of plant sources (grapes, rhubarb, peanuts, sugarcane, white tea) and in the seeds of Passiflora edulis |  | lowers the blood glucose level; promotes glucose uptake through glucose transporter 4 translocation to the plasma membrane in L6 myocytes; and suppresses blood glucose levels in T2DM | [1008,1009] |
| Piperine | Piper species (Piper nigrum, Piper longum) |  | bio-enhancing effect of piperine with metformin in lowering blood glucose levels; blood glucose-lowering effect | [959,1045] |
| Quercetin | in many fruits, vegetables, leaves, grains |  | decreases the cell percentages of G(0)/G(1) phase, Smad 2/3 expression, laminin and type IV collagen, and TGF-β(1) mRNA level; activates the Akt/cAMP response element-binding protein pathway | [970,971] |
| Resveratrol | wine and grape (Vitis vinifera) juice, peanuts (Arachis hypogaea), pistachios (Pistacia vera), blueberries (Vaccinium corymbosum) |  | decreases blood insulin levels; reduces adiposity, changes in gene expression, and changes in the activities of some enzymes; enhances GLUT-4 translocation; activates SIRT1 and AMPK; affects insulin secretion and blood insulin concentration; reduces blood insulin; diabetes-related metabolic changes via activation of AMP-activated protein kinase | [1046,1047,1048,1049] |
| Rutin | present in certain fruits and vegetables |  | improves glucose homeostasis by altering glycolytic and gluconeogenic enzymes; involvement of GLUT-4 in the stimulatory effect on glucose uptake; potentiates insulin receptor kinase to enhance insulin-dependent glucose transporter 4 translocation | [972,973,974] |
| Sanguinarine | Sanguinaria canadensis |  | was targets and candidate agent for T2DM treatment with a computational bioinformatics approach | [960] |
| Silymarin | the milk thistle plant (Silybum marianum) |  | reduction in levels of blood glucose, glycosylated hemoglobin, urine volume, serum creatinine, serum uric acid, and urine albumin; nephroprotective effects in T2DM; ameliorates diabetic cardiomyopathy through the inhibition of TGF-β1/Smad signaling | [953,982] |
| Tocotrienol | in a wide variety of plants; Bixa orellana, Zea mays, Garcinia mangostana, Elaeis guineensis, Hevea brasiliensis |  | reduced the high-sensitivity C-reactive protein in a group of patients with T2DM; involved in the NF-κB signaling pathway, oxidative-nitrosative stress, and inflammatory cascade in an experimental model | [1021,1022] |
| Triptolide | Tripterygium wilfordii |  | levels of phosphorylated protein kinase B and phosphorylated inhibitor of kappa B in splenocytes were reduced, and caspases 3, 8, and 9 were increased; diabetic nephropathy; triptolide treatment, accompanied with alleviated glomerular hypertrophy and podocyte injury | [1001,1002] |
| Ursolic acid, ursolic acid stearoyl glucoside | Calluna vulgaris, Crataegus laevigata, Eriobotrya japonica, Eugenia jambolana, Melissa officinalis, Mentha piperita, Ocimum sanctum, Rosmarinus officinalis, Thymus vulgaris Dracocephalum heterrophyllum, Hyssopus seravshanicus |  | decreased hepatic glucose-6-phosphatase activity and increased glucokinase activity; reduced blood glucose levels; insulin secretagogue and insulinomimetic is mediated by cross-talk between calcium and kinases to regulate glucose balance | [1050,1051,1052] |
| Withanolides | Withania somnifera in plant sources from the Dioscoreaceae, Fabaceae, Lamiaceae, Myrtaceae, Taccaceae families |  | hypoglycaemic and hypolipidaemic activities | [1040] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salehi, B.; Ata, A.; V. Anil Kumar, N.; Sharopov, F.; Ramírez-Alarcón, K.; Ruiz-Ortega, A.; Abdulmajid Ayatollahi, S.; Valere Tsouh Fokou, P.; Kobarfard, F.; Amiruddin Zakaria, Z.; et al. Antidiabetic Potential of Medicinal Plants and Their Active Components. Biomolecules 2019, 9, 551. https://doi.org/10.3390/biom9100551
Salehi B, Ata A, V. Anil Kumar N, Sharopov F, Ramírez-Alarcón K, Ruiz-Ortega A, Abdulmajid Ayatollahi S, Valere Tsouh Fokou P, Kobarfard F, Amiruddin Zakaria Z, et al. Antidiabetic Potential of Medicinal Plants and Their Active Components. Biomolecules. 2019; 9(10):551. https://doi.org/10.3390/biom9100551
Chicago/Turabian StyleSalehi, Bahare, Athar Ata, Nanjangud V. Anil Kumar, Farukh Sharopov, Karina Ramírez-Alarcón, Ana Ruiz-Ortega, Seyed Abdulmajid Ayatollahi, Patrick Valere Tsouh Fokou, Farzad Kobarfard, Zainul Amiruddin Zakaria, and et al. 2019. "Antidiabetic Potential of Medicinal Plants and Their Active Components" Biomolecules 9, no. 10: 551. https://doi.org/10.3390/biom9100551
APA StyleSalehi, B., Ata, A., V. Anil Kumar, N., Sharopov, F., Ramírez-Alarcón, K., Ruiz-Ortega, A., Abdulmajid Ayatollahi, S., Valere Tsouh Fokou, P., Kobarfard, F., Amiruddin Zakaria, Z., Iriti, M., Taheri, Y., Martorell, M., Sureda, A., N. Setzer, W., Durazzo, A., Lucarini, M., Santini, A., Capasso, R., ... Sharifi-Rad, J. (2019). Antidiabetic Potential of Medicinal Plants and Their Active Components. Biomolecules, 9(10), 551. https://doi.org/10.3390/biom9100551