Effects of Phytochemicals on Blood Pressure and Neuroprotection Mediated Via Brain Renin-Angiotensin System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Experimental Design and Treatment
2.3. Weight Gain, Feed Intake, and Assay Sampling Procedure
2.4. Blood Pressure Measurement
2.5. Renin Angiotensin System (RAS) Measurement
2.5.1 Angiotensin converting enzyme (ACE), angiotensin II, and aldosterone
2.5.2 Transcription of renin and ACE
2.6. Cholinergic System Measurement
2.7. Statistical Analysis
3. Results
3.1. Weight Change, Food Intake, and Food Efficiency Ratio (FER)
3.2. Phytochemical Treatments and Changes in Blood Pressure
3.3. Effect on the Renin Angiotensin System (RAS)
3.3.1. Angiotensin Converting Enzyme (ACE) Concentration and ACE Activity in Brain
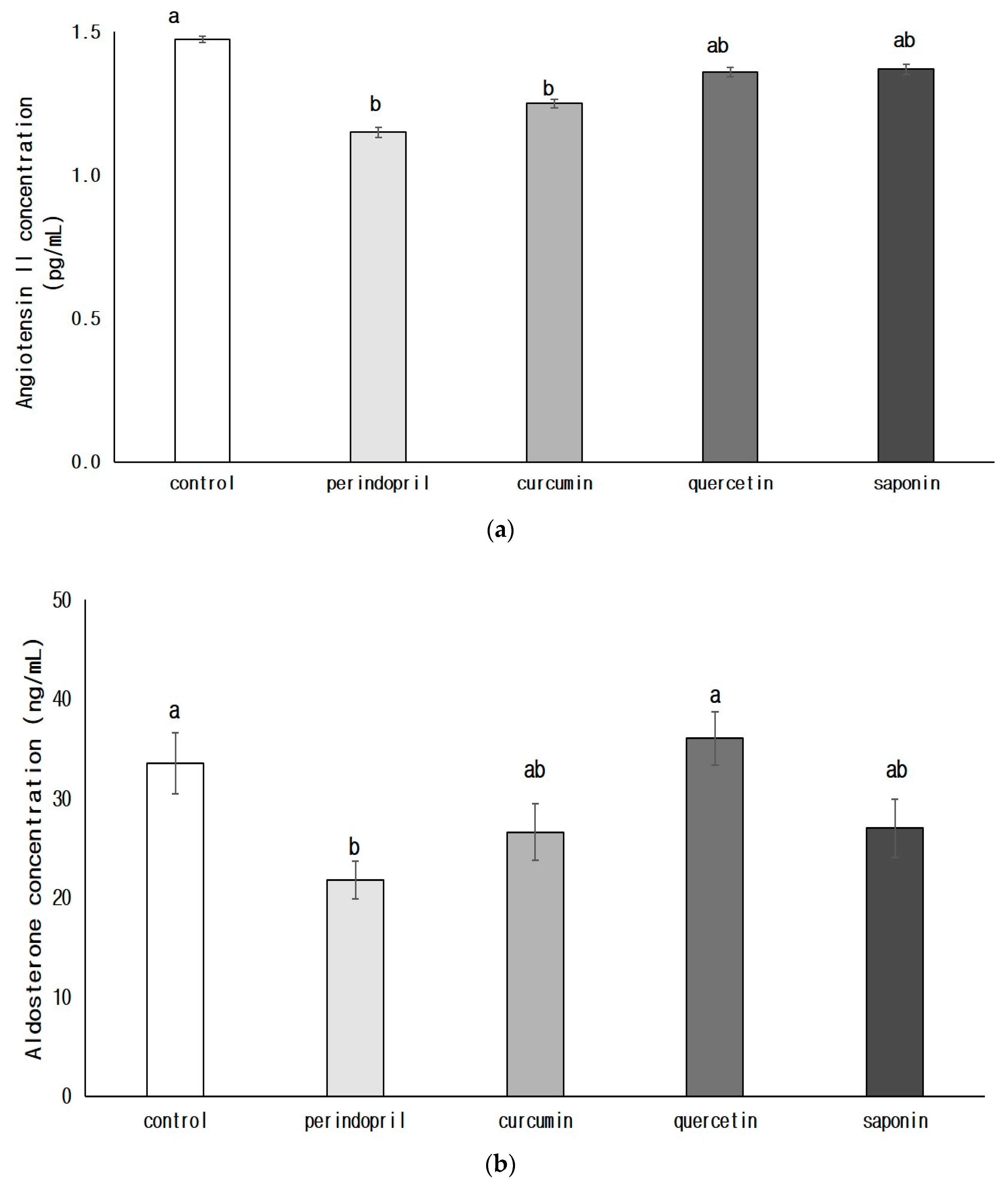
3.3.2. Concentrations of Angiotensin II (AngII, a) and Aldosterone (b) in Brain
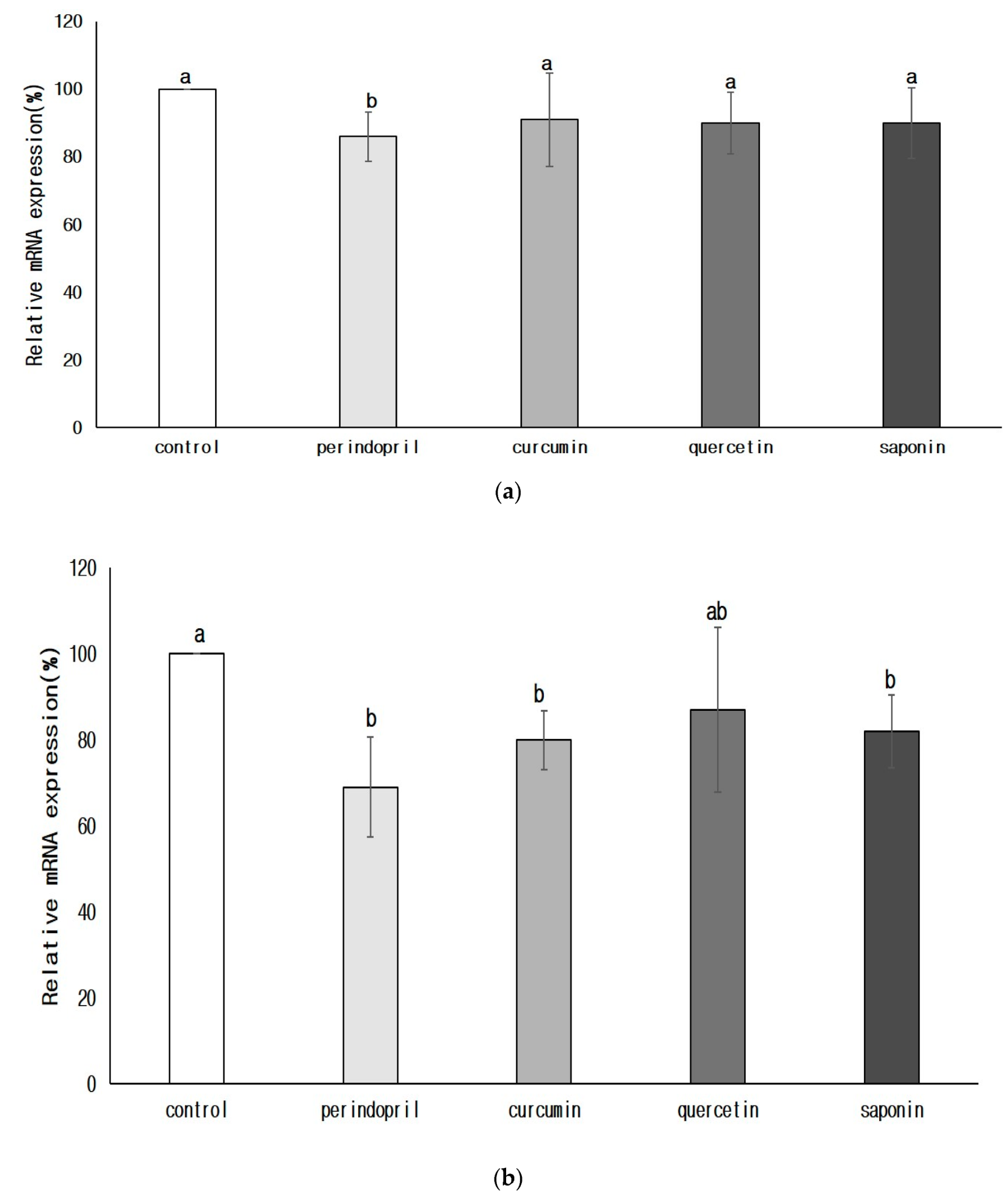
3.3.3. Transcription of Renin and ACE in the Brain
3.4. Cholinergic Expression in the Nervous System
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ferrario, C.M. Importance of the renin-angiotensin-aldosterone system (RAS) in the physiology and pathology of hypertension. An overview. Drugs 1990, 39, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Farag, E.; Sessler, D.I.; Ebrahim, Z.; Kurz, A.; Morgan, J.; Ahuja, S.; Maheshwari, K.; John Doyle, D. The renin angiotensin system and the brain: New developments. J. Clin. Neurosci. 2017, 46, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, S.; Sigmund, C.D. Angiotensin mutant mice: A focus on the brain renin-angiotensin system. Neuropeptides 2002, 36, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Jackson, L.; Eldahshan, W.; Fagan, S.C.; Ergul, A. Within the Brain: The Renin Angiotensin System. Int. J. Mol. Sci. 2018, 19, 876. [Google Scholar] [CrossRef] [PubMed]
- Tzourio, C.; Anderson, C.; Chapman, N.; Woodward, M.; Neal, B.; MacMahon, S.; Chalmers, J. Progress Collaborative Group. Effects of blood pressure lowering with perindopril and indapamide therapy on dementia and cognitive decline in patients with cerebrovascular disease. Arch. Intern. Med. 2003, 163, 1069–1075. [Google Scholar] [PubMed]
- Skoog, I.; Lithell, H.; Hansson, L.; Elmfeldt, D.; Hofman, A.; Olofsson, B.; Trenkwalder, P.; Zanchetti, A. Scope Study Group. Effect of baseline cognitive function and antihypertensive treatment on cognitive and cardiovascular outcomes: Study on Cognition and Prognosis in the Elderly (Scope). Am. J. Hypertens. 2005, 18, 1052–1059. [Google Scholar] [CrossRef]
- Poon, I.O. Effects of antihypertensive drug treatment on the risk of dementia and cognitive impairment. Pharmacotherapy 2008, 28, 366–375. [Google Scholar] [CrossRef]
- Picciotto, M.R.; Higley, M.J.; Mineur, Y.S. Acetylcholine as a neuromodulator: Cholinergic signaling shapes nervous system function and behavior. Neuron 2012, 76, 116–129. [Google Scholar] [CrossRef]
- Lazarevic-Pasti, T.; Leskovac, A.; Momic, T.; Petrovic, S.; Vasic, V. Modulators of acetylcholinesterase activity: From Alzheimer’s disease to anti-cancer drugs. Curr. Med. Chem. 2017, 24, 3283–3309. [Google Scholar] [CrossRef]
- Kumaran, D.; Udayabanu, M.; Kumar, M.; Aneja, R.; Katyal, A. Involvement of angiotensin converting enzyme in cerebral hypoperfusion induced anterograde memory impairment and cholinergic dysfunction in rats. Neuroscience 2008, 155, 626–639. [Google Scholar] [CrossRef]
- Dong, Y.F.; Kataoka, K.; Tokutomi, Y.; Nako, H.; Nakamura, T.; Toyama, K.; Sueta, D.; Koibuchi, N.; Yamamoto, E.; Ogawa, H.; et al. Perindopril, a centrally active angiotensin-converting enzyme inhibitor, prevents cognitive impairment in mouse models of Alzheimer’s disease. FASEB J. 2011, 25, 2911–2920. [Google Scholar] [CrossRef] [PubMed]
- Manschot, S.M.; Biessels, G.J.; Cameron, N.E.; Cotter, M.A.; Kamal, A.; Kappelle, L.J.; Gispen, W.H. Angiotensin converting enzyme inhibition partially prevents deficits in water maze performance, hippocampal synaptic plasticity and cerebral blood flow in streptozotocin-diabetic rats. Brain Res. 2003, 966, 274–282. [Google Scholar] [CrossRef]
- Yamada, K.; Horita, T.; Takayama, M.; Takahashi, S.; Takaba, K.; Nagata, Y.; Suzuki, N.; Kanda, T. Effect of a centrally active angiotensin converting enzyme inhibitor, perindopril, on cognitive performance in chronic cerebral hypo-perfusion rats. Brain Res. 2011, 3, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Tedla, Y.G.; Bautista, L.E. Drug Side Effect Symptoms and Adherence to Antihypertensive Medication. Am. J. Hypertens. 2016, 29, 772–779. [Google Scholar] [CrossRef]
- Bakoyiannis, I.; Daskalopoulou, A.; Pergialiotis, V.; Perrea, D. Phytochemicals and cognitive health: Are lavonoids doing the trick? Biomed. Pharmacother. 2019, 109, 1488–1497. [Google Scholar] [CrossRef]
- Wang, J.; Song, Y.; Gao, M.; Bai, X.; Chen, Z. Neuroprotective effect of several phytochemicals and its potential application in the prevention of neurodegenerative diseases. Geriatrics 2016, 1, 29. [Google Scholar] [CrossRef]
- Kim, M.H.; Kim, S.H.; Yang, W.M. Mechanisms of action of phytochemicals from medicinal herbs in the treatment of Alzheimer’s disease. Planta Med. 2014, 80, 1249–1258. [Google Scholar]
- Wang, L.; Zhao, H.; Zhai, Z.Z.; Qu, L.X. Protective effect and mechanism of ginsenoside Rg1 in cerebral ischaemia-reperfusion injury in mice. Biomed. Pharmacother. 2018, 99, 876–882. [Google Scholar] [CrossRef]
- Huber, G.; Schuster, F.; Raasch, W. Brain renin-angiotensin system in the pathophysiology of cardiovascular diseases. Pharmacol. Res. 2017, 125, 72–90. [Google Scholar] [CrossRef]
- Li, C.; Miao, X.; Li, F.; Adhikari, B.K.; Liu, Y.; Sun, J.; Zhang, R.; Cai, L.; Liu, Q.; Wang, Y. Curcuminoids: Implication for inflammation and oxidative stress in cardiovascular diseases. Phytother. Res. 2019, 33, 1302–1317. [Google Scholar] [CrossRef]
- Karimian, M.S.; Pirro, M.; Johnston, T.P.; Majeed, M.; Sahebkar, A. Curcumin and endothelial function: Evidence and mechanisms of protective effects. Curr. Pharm. Des. 2017, 23, 2462–2473. [Google Scholar] [CrossRef] [PubMed]
- Sompamit, K.; Kukongviriyapan, U.; Nakmareong, S.; Pannangpetch, P.; Kukongviriyapan, V. Curcumin improves vascular function and alleviates oxidative stress in non-lethal lipopolysaccharide-induced endotoxaemia in mice. Eur. J. Pharmacol. 2009, 616, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ye, Q.; Tu, J.; Zhang, M.; Ji, B. Curcumin protects against hypertension aggravated retinal ischemia/reperfusion in a rat stroke model. Clin. Exp. Hypertens. 2017, 39, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Lan, C.; Chen, X.; Zhang, Y.; Wang, W.; Wang, W.E.; Liu, Y.; Cai, Y.; Ren, H.; Zheng, S.; Zhou, L.; et al. Curcumin prevents strokes in stroke-prone spontaneously hypertensive rats by improving vascular endothelial function. BMC Cardiovasc. Disord. 2018, 18, 43. [Google Scholar] [CrossRef]
- Zhuang, X.D.; Liao, L.Z.; Dong, X.B.; Hu, X.; Guo, Y.; Du, Z.M.; Liao, X.X.; Wang, L.C. Design, synthesis, and antihypertensive activity of curcumin-inspired compounds via ACE inhibition and vasodilation, along with a bioavailability study for possible benefit in cardiovascular diseases. Drug Des. Dev. Ther. 2016, 5, 129–139. [Google Scholar]
- Fazal, Y.; Fatima, S.N.; Shahid, S.M.; Mahboob, T. Effects of curcumin on angiotensin-converting enzyme gene expression, oxidative stress and antioxidant status in thioacetamide-induced hepatotoxicity. J. Renin Angiotensin Aldosterone Syst. 2015, 16, 1046–1051. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, W.; Li, M.; Ren, H.; Chen, C.; Wang, J.; Wang, W.E.; Zeng, C. Curcumin exerts its anti-hypertensive effect by down-regulating the AT1 receptor in vascular smooth muscle Cells. Sci. Rep. 2016, 5, 25579. [Google Scholar] [CrossRef]
- Nurullahoglu, O.Z.; Atalik, K.E.; Yerlikaya, F.H.; Demir, E.A. Curcumin alleviates cisplatin-induced learning and memory impairments. Neurobiol. Learn. Mem. 2015, 123, 43–49. [Google Scholar]
- Bhutani, M.K.; Bishnoi, M.; Kulkarni, S.K. Anti-depressant like effect of curcumin and its combination with piperine in unpredictable chronic stress-induced behavioral, biochemical and neurochemical changes. Pharmacol. Biochem. Behav. 2009, 92, 39–43. [Google Scholar] [CrossRef]
- Abu-Taweel, G.M. Neurobehavioral protective properties of curcumin against the mercury chloride treated mice offspring. Saudi. J. Biol. Sci. 2019, 26, 736–743. [Google Scholar]
- Chen, M.; Long, Z.; Wang, Y.; Liu, J.; Pian, H.; Wang, L.; Chen, Z. Protective effects of saponin on a hypertension target organ in spontaneously hypertensive rats. Exp. Ther. Med. 2013, 5, 429–432. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, C.H.; Wang, B.W.; Lee, T.F. Effects of ginseng saponins on ß-amyloid-induced amnesia in rats. J. Ethnopharmacol. 2006, 103, 103–108. [Google Scholar]
- Yuan, Q.L.; Yang, C.X.; Xu, P.; Gao, X.O.; Deng, L.; Chen, P.; Sun, Z.L.; Chen, Q.Y. Neuroprotective effects of ginsenoside Rb1 on transient cerebral ischemia in rats. Brain Res. 2007, 1167, 1–12. [Google Scholar] [PubMed]



| Group 1) | Initial Weight (g) | Final Weight (g) | Weight Gain (g/4 Weeks) | Food Intake (g/4 Weeks) | FER 2) |
|---|---|---|---|---|---|
| Control | 26.4 ± 0.9 3)NS4) | 34.6 ± 1.2 a5) | 8.2 ± 1.9 a | 105.8 ± 4.4 a | 0.08 ± 0.02 a |
| Perindopril | 26.8 ± 1.1 | 33.8 ± 2.1 a | 7.1 ± 2.1 b | 100.8 ± 8.6 a | 0.06 ± 0.02 ab |
| Curcumin | 26.7 ± 1.1 | 32.5 ± 1.9 ab | 6.0 ± 1.6 bc | 97.7 ± 7.4 ab | 0.06 ± 0.02 ab |
| Quercetin | 26.9 ± 0.9 | 34.2 ± 2.1 a | 7.3 ± 1.8 ab | 101.2 ± 4.8 a | 0.06 ± 0.02 ab |
| Saponin | 26.7 ± 1.0 | 31.5 ± 2.4 b | 5.6 ± 2.0 c | 96.0 ± 8.4 b | 0.05 ± 0.02 b |
| Group 1) | Systolic Blood Pressure (mmHg) | Diastolic Blood Pressure (mmHg) | ||||
|---|---|---|---|---|---|---|
| 0 Weeks | 2 Weeks | 4 Weeks | 0 Weeks | 2 Weeks | 4 Weeks | |
| Control | 98.8 ± 19.3 2)NS3) | 125.0 ± 9.1 a4) | 113.3 ± 10.4 a | 53.2 ± 11.4 NS | 63.6 ± 26.8 NS | 57.7 ± 13.7 NS |
| Perindopril | 111.1 ± 20.4 | 104.2 ± 5.4 b | 78.1 ± 17.9 b | 65.4 ± 22.8 | 46.1 ± 13.0 | 61.1 ± 19.9 |
| Curcumin | 103.3 ± 11.6 | 108.8 ± 15.2 b | 95.4 ± 21.3 c | 44.5 ± 16.9 | 54.4 ± 16.9 | 48.3 ± 25.8 |
| Quercetin | 106.6 ± 9.0 | 118.5 ± 14.5 b | 102.6 ± 1.3 ac | 50.0 ± 22.6 | 57.6 ± 22.0 | 58.9 ± 24.7 |
| Saponin | 99.1 ± 14.5 | 109.6 ± 10.4 b | 97.6 ± 7.3 c | 62.9 ± 20.0 | 58.3 ± 21.3 | 48.3 ± 12.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.L.; Kim, W.K.; Ha, A.W. Effects of Phytochemicals on Blood Pressure and Neuroprotection Mediated Via Brain Renin-Angiotensin System. Nutrients 2019, 11, 2761. https://doi.org/10.3390/nu11112761
Kim HL, Kim WK, Ha AW. Effects of Phytochemicals on Blood Pressure and Neuroprotection Mediated Via Brain Renin-Angiotensin System. Nutrients. 2019; 11(11):2761. https://doi.org/10.3390/nu11112761
Chicago/Turabian StyleKim, Hye Lin, Woo Kyoung Kim, and Ae Wha Ha. 2019. "Effects of Phytochemicals on Blood Pressure and Neuroprotection Mediated Via Brain Renin-Angiotensin System" Nutrients 11, no. 11: 2761. https://doi.org/10.3390/nu11112761




