Abstract
Pyrrolidino[3,4-c]pyrazoline and pyrazole derivatives were synthesized via reactions of a substituted hydrazonoyl bromide with N-arylmaleimides and active methylene reagents, respectively. Synthesized pyrazoles were reacted with hydrazine hydrate to give the corresponding pyrazolo[3,4-d]pyridazines.
Introduction
Hydrazonoyl halides have been widely employed in the synthesis of heterocyclic derivatives [2,3,4,5]. In continuation of our interest in the synthesis of heterocyclic systems containing a pyrazole moiety [6,7,8,9,10], we report herein a facile synthesis of pyrrolidino[3,4-c]pyrazoline, pyrazole, and pyrazolo[3,4-d]pyridazine derivatives.
Results and Discussion
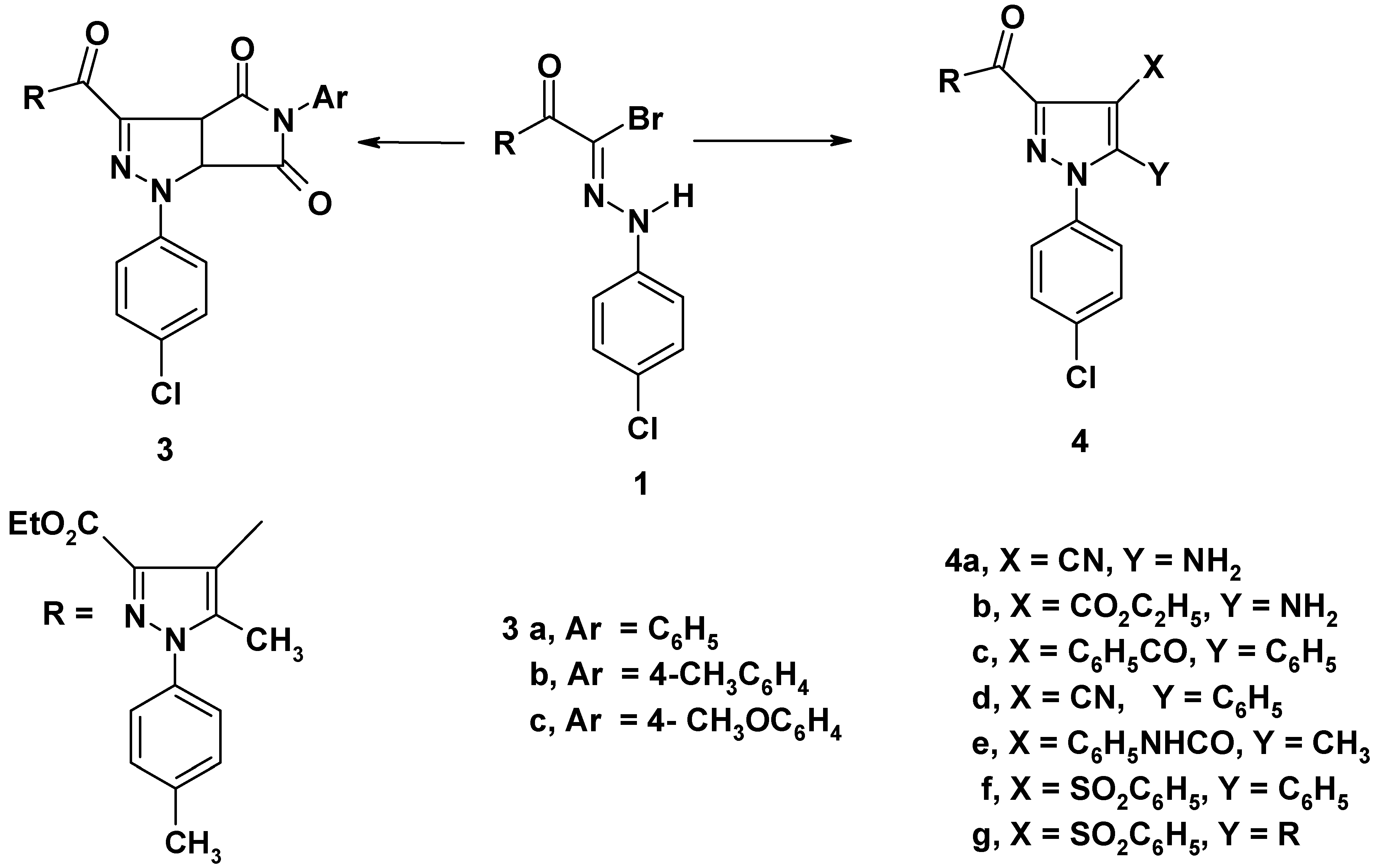
Treatment of the hydrazonoyl bromide 1 [10] with the appropriate N-arylmaleimides 2a-c in benzene containing triethylamine afforded pyrrolidino[3,4-d]pyrazolines 3a-c (cf. Scheme 1). The structures of compounds 3a-c were confirmed by their spectroscopic data. For example, the 1H NMR spectrum of 3a showed signals at δ = 1.11 (t, 3H, CH3CH2); 2.33 (s, 3H, CH3); 2.41 (s, 3H, CH3); 4.12 (q, 2H, CH2CH3); 5.20 (d, J=7Hz, 1H, pyrazoline H-4); 5.49 (d, J=7Hz, 1H, pyrazoline H-5) and 7.16-7.46 (m, 13H, ArH). The IR spectra of 3a-c were characterized by two widely separated bands in the region 1790-1690 cm-1 assigned the CO-NAr-CO grouping [11].
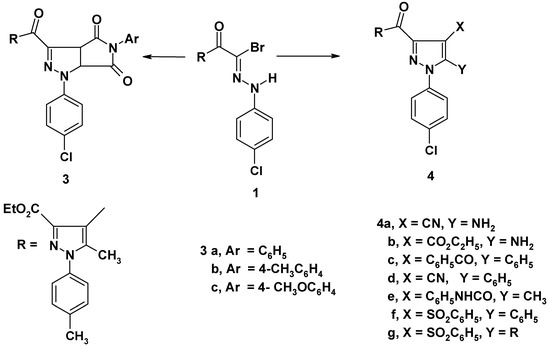
Scheme 1.
Also, the hydrazonoyl bromide 1 was reacted with each of malononitrile, ethyl cyanoacetate, dibenzoylmethane, benzoylacetonitrile, acetoacetanilide, and ω-benzenesulfonylacetophenone in ethanolic sodium ethoxide to give substituted pyrazoles 4a-g (Scheme 1). Structures 4a-g were confirmed on the basis of spectral data. For example, the IR spectrum of compound 4c revealed bands at 1720, 1685, 1660 cm-1 (CO groups) and its 1H NMR spectrum showed signals at δ = 1.23 (t, 3H, CH3CH2); 2.32 (s, 3H, CH3); 2.39 (s, 3H, CH3); 4.24 (q, 2H, CH2CH3); 7.19-8.09 (m, 18H, ArH).
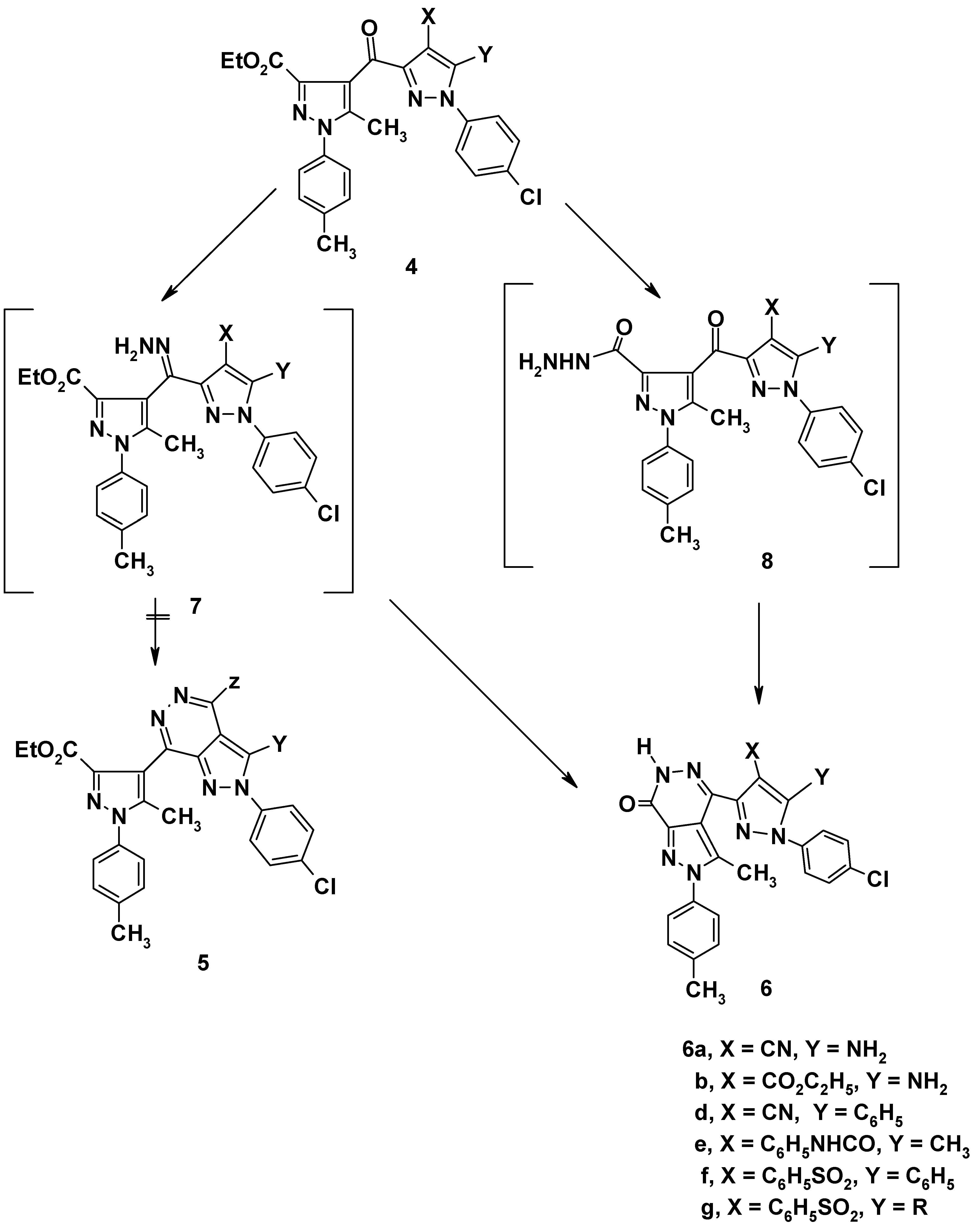
Pyrazoles 4a,b and 4d-g were converted to the corresponding pyrazolo[3,4-d]pyridazines 6a,b and 6d-g, respectively, on boiling under reflux with hydrazine hydrate in ethanol. The structure of the prod- ucts could conceivably be formulated as either 5 or 6. Based on analytical analyses and spectral data, structures 5 were ruled out. The formation of the products of type 6 can be explained via elimination of water, to give the hydrazone intermediate 7, which then readily cyclized to product 6 (cf. Scheme 2). Alternatively, the formation of the product can be explained by formation of the hydrazide 8, followed by elimination of water to give pyrazolo[3,4-d]pyridazines 6. All attempts to isolate intermediates 7 and 8 were unsuccessful.
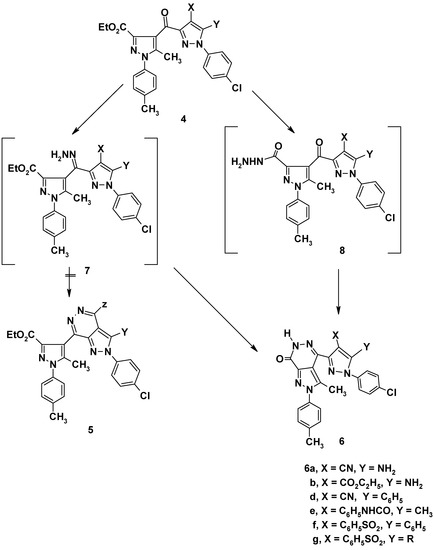
Scheme 2.
Experimental
General
All melting points were determined on an Electrothermal apparatus and are uncorrected. IR spectra were recorded (KBr discs) on a Shimadzu FT-IR 8201 PC spectrophotometer. 1H NMR spectra were recorded in CDCl3 on a Varian Gemini 200 MHz spectrometer and chemical shifts are expressed in δ units using TMS as an internal reference. Elemental analyses were carried out at the Microanalytical Center of the University of Cairo, Giza, Egypt. Hydrazonoyl bromides 1 [10] and N-arylmaleimides 2a-c [12] were prepared as previously reported.
General procedure for the synthesis of 5-aryl-1-(4-chlorophenyl)-3-[3'-ethoxycarbonyl-5'-methyl-1'- (4-tolyl)-4'-pyrazoloyl]pyrrolidino[3,4-c]pyrazoline-4,6-diones (3a-c)
A solution of the substituted hydrazonoyl bromide 1 (2.5 g, 0.005 mmol), the appropriate N-arylmaleimides 2a-c (0.005 mol) and triethylamine (0.7 mL, 0.005 mol) in dry benzene (20 mL) was refluxed for 3 h. The solvent was removed under vacuum and the residue triturated with petroleum ether (b.p. 40/60°C, 10 mL). The resulting solid was collected, washed and crystallized from acetic acid or ethanol to give 3a-c (cf. Table 1).

Table 1.
Analytical data of the newly synthesized compounds.
General procedure for the synthesis of 4,5-disubstituted 1-(4-chlorophenyl)- 3-[3'-ethoxy-carbonyl-5'- methyl-1'-(4-tolyl)-4'-pyrazoloyl]pyrazoles (4a-g)
The appropriate active methylene compound (malononitrile, ethyl cyanoacetate dibenzoylmethane, benzoylacetonitrile, acetoacetanilide, ω-benzenesulfonylacetophenone, or ketosulfone. (0.005 mol) was added to an ethanolic sodium ethoxide solution [prepared from sodium metal (0.11 g-atom) in absolute ethanol (20 mL)]. After stirring for 10 minutes, the hydrazonoyl bromide 1 (2.5 g, 0.005 mol) was added and stirring was continued for an additional 30 minutes. The reaction mixture was left overnight at room temperature and the precipitated product was collected by filtration. The solid was washed with water and recrystallized from ethanol to give the corresponding pyrazoles 4a-g, respectively (cf. Table 1).
General procedure for the synthesis of 4,5-disubstituted 4-[1-(4-chlorophenyl)-3-pyrazolyl]-2,6- dihydro-3-methyl-2-(4-tolyl)-pyrazolo[3,4-d]pyridazin-7-ones (6a,b,d-g)
The appropriate pyrazoles (4a,b and 4d-g) (0.005 mol) in a mixture of ethanol (20 mL) and hydrazine hydrate (0.75 mL, 0.015 mol) were refluxed for 4h, during which time the pyrazole dissolved and the corresponding pyrazolo[3,4-d]pyridazine precipitated. The latter was collected, washed with water and recrystallized from ethanol or dimethylformamide to give 6a,b,d-g (cf. Table 1).
References and Notes
- Abdelhamid, A.O.; Rateb, N.M.; Dawood, K.M. Phosph., Sulfur, Silicon, and Related Elements 2000, in press.
- Ulrich, H. The Chemistry of Imidoyl Halides; Plenum Press: New York, 1968; p. 173. [Google Scholar]
- Huisgen, R.; Garashey, R.; Sauer, J. The Chemistry of Alkenes; Patai, S., Ed.; Wiley-Interscience: New York, N.Y, 1964; Vol. 1, p. 739. [Google Scholar]
- Abdelhamid, A.O. J. Chem. Res. 1993, (S) 208, (M) 1239.
- Butler, R.N.; Scott, F.L. Chem. Ind. (London) 1970, 1216.
- Hassan, N.M.; Abdelhamid, A.O. J. Chem. Res. 1997, (S) 350, (M) 2254 (1997).
- Hassan, N.M.; Fahmi, A.A.; Abd-El-mageid, F.F.; Abdelhamid, A.O. J. Chin. Chem. Soc. 1996, 43, 493.
- Abdelhamid, A.O.; Abd-El-mageid, F.F.; Hassan, N.M.; Zohdi, H.F. J. Chem. Res. 1995 (S), 492, (M) 3036.
- Abdelhamid, A.O.; Attaby, F.A.; Khalifa, F.A.; Ghabrial, S. S. Arch. Pharm. Res. 1992, 15, 14.
- Abdelhamid, A.O.; Zohdi, H.F.; Sallam, M.M.M.; Ahmed, N.A. Phosph., Sulfur, Silicon, and Related Elements 2000, in press.
- Abdelhamid, A.O.; Ghabrial, S.S. Sulfur Letters 1987, 7, 19.
- Searle, N.F.E. U.S. U.S. Pat, 2,444,536 (to E.I. Dupont De Nemours and Co. Inc.). 1948. [Chem. Abstr. 1949, 42, 7340c].
- Sample Availability: Available from MDPI.
© 2000 by MDPI (http://www.mdpi.org).