Long-Term Effect of Combination of Creatine Monohydrate Plus β-Hydroxy β-Methylbutyrate (HMB) on Exercise-Induced Muscle Damage and Anabolic/Catabolic Hormones in Elite Male Endurance Athletes
Abstract
:1. Introduction
2. Material and Methods
2.1. Participants
2.2. Experimental Protocol and Evaluation Plan
2.3. Blood Collection
2.4. Anthropometry and Body Composition
2.5. Dietary Assessment
2.6. Statistical Analysis
3. Results
4. Discussion
4.1. Limitations, Strengths, and Future Research
4.2. Practical Application
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Meeusen, R.; Duclos, M.; Foster, C.; Fry, A.; Gleeson, M.; Nieman, D.; Raglin, J.; Rietjens, G.; Steinacker, J.; Urhausen, A.; et al. Prevention, diagnosis, and treatment of the overtraining syndrome: Joint consensus statement of the european college of sport science and the American College of Sports Medicine. Med. Sci. Sports Exerc. 2013, 45, 186–205. [Google Scholar] [CrossRef] [Green Version]
- Mielgo-Ayuso, J.; Calleja-González, J.; Urdampilleta, A.; León-Guereño, P.; Córdova, A.; Caballero-García, A.; Fernandez-Lázaro, D. Effects of vitamin D supplementation on haematological values and muscle recovery in elite male traditional rowers. Nutrients 2018, 10, 1968. [Google Scholar] [CrossRef] [Green Version]
- Córdova, A.; Mielgo-Ayuso, J.; Fernandez-Lazaro, C.I.; Caballero-García, A.; Roche, E.; Fernández-Lázaro, D. Effect of iron supplementation on the modulation of iron metabolism, muscle damage biomarkers and cortisol in professional cyclists. Nutrients 2019, 11, 500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terrados, N.; Mielgo-Ayuso, J.; Delextrat, A.; Ostojic, S.M.; Calleja-González, J. Dietetic-nutritional, physical and physiological recovery methods post-competition in team sports. J. Sports Med. Phys. Fitness 2019, 59, 415–428. [Google Scholar] [CrossRef]
- Djaoui, L.; Haddad, M.; Chamari, K.; Dellal, A. Monitoring training load and fatigue in soccer players with physiological markers. Physiol. Behav. 2017, 181, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Brancaccio, P.; Lippi, G.; Maffulli, N. Biochemical markers of muscular damage. Clin. Chem. Lab. Med. 2010, 48, 757–767. [Google Scholar] [CrossRef] [PubMed]
- Orfanos, Z.; Gödderz, M.P.O.; Soroka, E.; Gödderz, T.; Rumyantseva, A.; van der Ven, P.F.M.; Hawke, T.J.; Fürst, D.O. Breaking sarcomeres by in vitro exercise. Sci. Rep. 2016, 6, 19614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urhausen, A.; Gabriel, H.; Kindermann, W. Impaired pituitary hormonal response to exhaustive exercise in overtrained endurance athletes. Med. Sci. Sports Exerc. 1998, 30, 407–414. [Google Scholar] [CrossRef]
- Brancaccio, P.; Maffulli, N.; Limongelli, F.M. Creatine kinase monitoring in sport medicine. Br. Med. Bull. 2007, 81–82, 209–230. [Google Scholar] [CrossRef]
- Martínez, A.; Seco Calvo, J.; Tur Marí, J.; Abecia Inchaurregui, L.; Orella, E.; Biescas, A. Testosterone and cortisol changes in professional basketball players through a season competition. J. Strength Cond. Res. 2010, 24, 1102–1108. [Google Scholar] [CrossRef]
- Häkkinen, K.; Pakarinen, A.; Alén, M.; Komi, P.V. Serum hormones during prolonged training of neuromuscular performance. Eur. J. Appl. Physiol. Occup. Physiol. 1985, 53, 287–293. [Google Scholar] [CrossRef]
- Mielgo-Ayuso, J.; Zourdos, M.; Urdampilleta, A.; Calleja-González, J.; Seco, J.; Córdova, A. Relationship of long-term macronutrients intake on anabolic-catabolic hormones in female elite volleyball players. Nutr. Hosp. 2017, 34, 1155–1162. [Google Scholar] [CrossRef] [Green Version]
- Slimani, M.; Cheoura, F.; Moalla, W.; Baker, J.S. Hormonal responses to a rugby match: A brief review. J. Sports Med. Phys. Fitness 2018, 58, 707–713. [Google Scholar]
- Mastorakos, G.; Pavlatou, M.; Diamanti-Kandarakis, E.; Chrousos, G.P. Exercise and the stress system. Hormones (Athens) 2005, 4, 73–89. [Google Scholar]
- Greenham, G.; Buckley, J.D.; Garrett, J.; Eston, R.; Norton, K. Biomarkers of physiological responses to periods of intensified, non-resistance-based exercise training in well-trained male athletes: A systematic review and meta-analysis. Sport. Med. 2018, 48, 2517–2548. [Google Scholar] [CrossRef]
- Hayes, L.D.; Grace, F.M.; Baker, J.S.; Sculthorpe, N. Exercise-induced responses in salivary testosterone, cortisol, and their ratios in men: A meta-analysis. Sport. Med. 2015, 45, 713–726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vervoorn, C.; Quist, A.; Vermulst, L.; Erich, W.; de Vries, W.; Thijssen, J. The behaviour of the plasma free testosterone/cortisol ratio during a season of elite rowing training. Int. J. Sports Med. 1991, 12, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Urhausen, A.; Gabriel, H.; Kindermann, W. Blood hormones as markers of training stress and overtraining. Sport. Med. 1995, 20, 251–276. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.T.; Erdman, K.A.; Burke, L.M. American College of Sports Medicine Joint Position Statement. Nutrition and Athletic Performance. Med. Sci. Sports Exerc. 2016, 48, 543–568. [Google Scholar]
- Calleja-González, J.; Mielgo-Ayuso, J.; Sampaio, J.; Delextrat, A.; Ostojic, S.M.; Marques-Jiménez, D.; Arratibel, I.; Sánchez-Ureña, B.; Dupont, G.; Schelling, X.; et al. Brief ideas about evidence-based recovery in team sports. J. Exerc. Rehabil. 2018, 14, 545–550. [Google Scholar] [CrossRef]
- Cooke, M.B.; Rybalka, E.; Williams, A.D.; Cribb, P.J.; Hayes, A. Creatine supplementation enhances muscle force recovery after eccentrically-induced muscle damage in healthy individuals. J. Int. Soc. Sports Nutr. 2009, 6, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veggi, K.F.T.; Machado, M.; Koch, A.J.; Santana, S.C.; Oliveira, S.S.; Stec, M.J. Oral creatine supplementation augments the repeated bout effect. Int. J. Sport Nutr. Exerc. Metab. 2013, 23, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Bassit, R.; Pinheiro, C.; Vitzel, K.; Sproesser, A.; Silveira, L.; Curi, R. Effect of short-term creatine supplementation on markers of skeletal muscle damage after strenuous contractile activity. Eur. J. Appl. Physiol. 2010, 108, 945–955. [Google Scholar] [CrossRef] [PubMed]
- Arazi, H.; Rahmaninia, F.; Hosseini, K.; Asadi, A. Effects of short term creatine supplementation and resistance exercises on resting hormonal and cardiovascular responses. Sci. Sport. 2015, 30, 105–109. [Google Scholar] [CrossRef]
- Vatani, D.S.; Faraji, H.; Soori, R.; Mogharnasi, M. The effects of creatine supplementation on performance and hormonal response in amateur swimmers. Sci. Sport. 2011, 26, 272–277. [Google Scholar] [CrossRef]
- Kreider, R.B.; Kalman, D.S.; Antonio, J.; Ziegenfuss, T.N.; Wildman, R.; Collins, R.; Candow, D.G.; Kleiner, S.M.; Almada, A.L.; Lopez, H.L. International Society of Sports Nutrition position stand: Safety and efficacy of creatine supplementation in exercise, sport, and medicine. J. Int. Soc. Sports Nutr. 2017, 14, 18. [Google Scholar] [CrossRef]
- Roberts, P.A.; Fox, J.; Peirce, N.; Jones, S.W.; Casey, A.; Greenhaff, P.L. Creatine ingestion augments dietary carbohydrate mediated muscle glycogen supercompensation during the initial 24 h of recovery following prolonged exhaustive exercise in humans. Amino Acids 2016, 48, 1831–1842. [Google Scholar] [CrossRef]
- van Loon, L.J.; Murphy, R.; Oosterlaar, A.M.; Cameron-Smith, D.; Hargreaves, M.; Wagenmakers, A.J.M.; Snow, R. Creatine supplementation increases glycogen storage but not GLUT-4 expression in human skeletal muscle. Clin. Sci. 2004, 106, 99–106. [Google Scholar] [CrossRef] [Green Version]
- Wilson, J.M.; Lowery, R.P.; Joy, J.M.; Walters, J.A.; Baier, S.M.; Fuller, J.C.; Stout, J.R.; Norton, L.E.; Sikorski, E.M.; Wilson, S.M.C.; et al. β-Hydroxy-β-methylbutyrate free acid reduces markers of exercise-induced muscle damage and improves recovery in resistance-trained men. Br. J. Nutr. 2013, 110, 538–544. [Google Scholar] [CrossRef] [Green Version]
- Panton, L.B.; Rathmacher, J.A.; Baier, S.; Nissen, S. Nutritional supplementation of the leucine metabolite beta-hydroxy-beta-methylbutyrate (hmb) during resistance training. Nutrition 2000, 16, 734–739. [Google Scholar] [CrossRef]
- Knitter, A.E.; Panton, L.; Rathmacher, J.A.; Petersen, A.; Sharp, R. Effects of β-hydroxy-β-methylbutyrate on muscle damage after a prolonged run. J. Appl. Physiol. 2000, 89, 1340–1344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, J.M.; Lowery, R.P.; Joy, J.M.; Andersen, J.C.; Wilson, S.M.C.; Stout, J.R.; Duncan, N.; Fuller, J.C.; Baier, S.M.; Naimo, M.A.; et al. The effects of 12 weeks of beta-hydroxy-beta-methylbutyrate free acid supplementation on muscle mass, strength, and power in resistance-trained individuals: A randomized, double-blind, placebo-controlled study. Eur. J. Appl. Physiol. 2014, 114, 1217–1227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asadi, A.; Arazi, H.; Suzuki, K. Effects of β-hydroxy-β-methylbutyrate-free acid supplementation on strength, power and hormonal adaptations following resistance training. Nutrients 2017, 9, 1316. [Google Scholar] [CrossRef] [Green Version]
- Tinsley, G.M.; Givan, A.H.; Graybeal, A.J.; Villarreal, M.I.; Cross, A.G. β-Hydroxy β-methylbutyrate free acid alters cortisol responses, but not myofibrillar proteolysis, during a 24-h fast. Br. J. Nutr. 2018, 119, 517–526. [Google Scholar] [CrossRef] [Green Version]
- Durkalec-Michalski, K.; Jeszka, J. The effect of β-hydroxy-β-methylbutyrate on aerobic capacity and body composition in trained athletes. J. Strength Cond. Res. 2016, 30, 2617–2626. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, C.; Gerlinger-Romero, F.; Guimarães-Ferreira, L.; de Souza, A.J.; Vitzel, K.; Nachbar, R.; Nunes, M.; Curi, R. Metabolic and functional effects of beta-hydroxy-beta-methylbutyrate (HMB) supplementation in skeletal muscle. Eur. J. Appl. Physiol. 2012, 112, 2531–2537. [Google Scholar] [CrossRef]
- Cross, D.A.E.; Alessi, D.R.; Vandenheede, J.R.; McDowell, H.E.; Hundal, H.S.; Cohen, P. The inhibition of glycogen synthase kinase-3 by insulin or insulin-like growth factor 1 in the rat skeletal muscle cell line L6 is blocked by wortmannin, but not by rapamycin: Evidence that wortmannin blocks activation of the mitogen-activated protein kin. Biochem. J. 1994, 303, 21–26. [Google Scholar] [CrossRef]
- Gerlinger-Romero, F.; Guimarães-Ferreira, L.; Giannocco, G.; Nunes, M.T. Chronic supplementation of beta-hydroxy-beta methylbutyrate (HMβ) increases the activity of the GH/IGF-I axis and induces hyperinsulinemia in rats. Growth Horm. IGF Res. 2011, 21, 57–62. [Google Scholar] [CrossRef]
- Kresta, J.Y.; Oliver, J.M.; Jagim, A.R.; Fluckey, J.; Riechman, S.; Kelly, K.; Meininger, C.; Mertens-Talcott, S.U.; Rasmussen, C.; Kreider, R.B. Effects of 28 days of beta-alanine and creatine supplementation on muscle carnosine, body composition and exercise performance in recreationally active females. J. Int. Soc. Sports Nutr. 2014, 11, 55. [Google Scholar] [CrossRef] [Green Version]
- Stout, J.; Cramer, J.; Mielke, M.; O’Kroy, J.; Torok, D.; Zoeller, F. Effects of twenty-eight days of beta-alanine and creatine monohydrate supplementation on the physical working capacity at neuromuscular fatigue threshold. J. Strength Cond. Res. 2006, 20, 928–931. [Google Scholar]
- Boegman, S.; Dziedzic, C. Nutrition and supplements for elite open-weight rowing. Curr. Sports Med. Rep. 2016, 15, 252–261. [Google Scholar] [PubMed]
- Wilson, J.M.; Fitschen, P.J.; Campbell, B.; Wilson, G.J.; Zanchi, N.; Taylor, L.; Wilborn, C.; Kalman, D.S.; Stout, J.R.; Hoffman, J.R.; et al. International Society of Sports Nutrition Position Stand: Beta-hydroxy-beta-methylbutyrate (HMB). J. Int. Soc. Sports Nutr. 2013, 10, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lloyd, A.; Hodder, S.; Havenith, G. The interactive effect of cooling and hypoxia on forearm fatigue development. Eur. J. Appl. Physiol. 2015, 115, 2007–2018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faramarzi, M.; Nuri, R.; Banitalebi, E.; Sciences, S. The effect of short–term combination of HMB (beta-hydroxy-beta-methylbutyrate) and creatine supplementation on anaerobic performance and muscle injury markers in soccer players. Braz. J. Biomotricity 2009, 3, 366–375. [Google Scholar]
- Crowe, M.J.; O’Connor, D.M.; Lukins, J.E. The effects of β-hydroxy-β-methylbutyrate (HMB) and HMB/creatine supplementation on indices of health in highly trained athletes. Int. J. Sport Nutr. Exerc. Metab. 2003, 13, 184–197. [Google Scholar] [CrossRef]
- Jówko, E.; Ostaszewski, P.; Jank, M.; Sacharuk, J.; Zieniewicz, A.; Wilczak, J.; Nissen, S. Creatine and β-hydroxy-β-methylbutyrate (HMB) additively increase lean body mass and muscle strength during a weight-training program. Nutrition 2001, 17, 558–566. [Google Scholar] [CrossRef]
- Zajac, A.; Waskiewicz, Z.; Poprzecki, S.; Cholewa, J. Effects of creatine and HMß supplementation on anaerobic power and body composition in basketball players. J. Hum. Kinet. 2003, 10, 95–108. [Google Scholar]
- Thomas, D.T.; Erdman, K.A.; Burke, L.M. Position of the Academy of Nutrition and Dietetics, Dietitians of Canada, and the American College of Sports Medicine: Nutrition and Athletic Performance. J. Acad. Nutr. Diet. 2016, 116, 501–528. [Google Scholar] [CrossRef]
- Beelen, M.; Burke, L.M.; Gibala, M.J.; van Loon L, J.C. Nutritional strategies to promote postexercise recovery. Int. J. Sport Nutr. Exerc. Metab. 2010, 20, 515–532. [Google Scholar] [CrossRef] [Green Version]
- Bosquet, L.; Montpetit, J.; Arvisais, D.; Mujika, I. Effects of tapering on performance: A meta-analysis. Med. Sci. Sports Exerc. 2007, 39, 1358–1365. [Google Scholar] [CrossRef] [Green Version]
- Stewart, A.; Marfell-Jones, M.; Olds, T.; de Ridder, H. International Standards for Anthropometric Assessment, 3rd ed.; ISAK: Lower Hutt, New Zealand, 2011. [Google Scholar]
- Carter, J.E.L. Body composition of Montreal Olympic athletes. In Physical Structure of Olympic Athletes. Part I the Montreal Olympic Games Anthropological Project; Karger: Basel, Switzerland, 1982; pp. 107–116. [Google Scholar]
- Lee, R.; Wang, Z.; Heo, M.; Ross, R.; Janssen, I.; Heymsfield, S. Total-body skeletal muscle mass: Development and cross-validation of anthropometric prediction models. Am. J. Clin. Nutr. 2000, 72, 796–803. [Google Scholar] [CrossRef] [PubMed]
- Mielgo-Ayuso, J.; Zourdos, M.C.; Calleja-González, J.; Urdampilleta, A.; Ostojic, S.M. Dietary intake habits and controlled training on body composition and strength in elite female volleyball players during the season. Appl. Physiol. Nutr. Metab. 2015, 40, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Mielgo-Ayuso, J.; Collado, P.S.; Urdampilleta, A.; Martínez-Sanz, J.M.; Seco, J. Changes induced by diet and nutritional intake in the lipid profile of female professional volleyball players after 11 weeks of training. J. Int. Soc. Sports Nutr. 2013, 10, 55. [Google Scholar] [CrossRef] [Green Version]
- Farrán, A.; Zamora, R.; Cervera, P. Tablas de Composición de Alimentos del Centre D’Ensenyament Superior de Nutrició i Dietètica (CESNID), 2nd ed.; McGraw-Hill Interamericana: Barcelona, Spain, 2004. [Google Scholar]
- Ferguson, C.J. An Effect Size Primer: A Guide for Clinicians and Researchers. Prof. Psychol. Res. Pract. 2009, 40, 532–538. [Google Scholar] [CrossRef] [Green Version]
- Kellmann, M.; Bertollo, M.; Bosquet, L.; Brink, M.; Coutts, A.J.; Duf, R.; Erlacher, D.; Halson, S.L.; Hecksteden, A.; Heidari, J.; et al. Recovery and performance in sport : Consensus statement. Int. J. Sports Physiol. Perform. 2018, 13, 240–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banfi, G.; Dolci, A. Free testosterone/cortisol ratio in soccer: Usefulness of a categorization of values. J. Sports Med. Phys. Fitness 2006, 46, 611–616. [Google Scholar] [PubMed]
- Saks, V.A.; Kongas, O.; Vendelin, M.; Kay, L. Role of the creatine/phosphocreatine system in the regulation of mitochondrial respiration. Acta. Physiol. Scand. 2000, 168, 635–641. [Google Scholar] [CrossRef]
- He, X.; Duan, Y.; Yao, K.; Li, F.; Hou, Y.; Wu, G.; Yin, Y. β-Hydroxy-β-methylbutyrate, mitochondrial biogenesis, and skeletal muscle health. Amino Acids 2016, 48, 653–664. [Google Scholar] [CrossRef]
- Baar, K. Nutrition and the adaptation to endurance training. Sport. Med. 2014, 44, 5–12. [Google Scholar] [CrossRef] [Green Version]
- Stallknecht, B.; Vissing, J.; Galbo, H. Lactate production and clearance in exercise. Effects of training. A mini-review. Scand. J. Med. Sci. Sports 1998, 8, 127–131. [Google Scholar] [CrossRef]
- Fernández-Landa, J.; Calleja-González, J.; León-Guereño, P.; Caballero-García, A.; Córdova, A.; Mielgo-Ayuso, J. Effect of the combination of creatine monohydrate plus HMB supplementation on sports performance, body composition, markers of muscle damage and hormone status: A systematic review. Nutrients 2019, 11, 2528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Connor, D.M.; Crowe, M.J. Effects of beta-hydroxy-beta-methylbutyrate and creatine monohydrate supplementation on the aerobic and anaerobic capacity of highly trained athletes. J. Sports Med. Phys. Fitness 2003, 43, 64–68. [Google Scholar] [PubMed]
- Mcneill, C.; Beaven, C.M.; McMaster, D.T.; Gill, N. Eccentric training interventions and team sport athletes. J. Funct. Morphol. Kinesiol. 2019, 4, 67. [Google Scholar] [CrossRef] [Green Version]
- Brownlee, K.K.; Moore, A.W.; Hackney, A.C. Relationship between circulating cortisol and testosterone: Influence of physical exercise. J. Sport. Sci. Med. 2005, 4, 76–83. [Google Scholar]
- Fernández-Landa, J.; Fernández-Lázaro, D.; Calleja-González, J.; Caballero-García, A.; Córdova, A.; León-Guereño, P.; Mielgo-Ayuso, J. Effect of ten weeks of creatine monohydrate plus HMB supplementation on athletic performance tests in elite male endurance athletes. Nutrients 2020, 12, 193. [Google Scholar] [CrossRef] [Green Version]

| PLG | CrMG | HMBG | CrM-HMBG | |
|---|---|---|---|---|
| Energy (kcal/kg) | 44.8 ± 6.2 | 45.0 ± 6.6 | 44.7 ± 6.3 | 45.1 ± 7.0 |
| Protein (g/kg) | 1.9 ± 0.4 | 2.0 ± 0.6 | 1.9 ± 0.7 | 1.9 ± 0.4 |
| Fat (g/kg) | 1.5 ± 0.4 | 1.6 ± 0.5 | 1.5 ± 0.6 | 1.6 ± 0.6 |
| Carbohydrates (g/kg) | 6.0 ± 0.9 | 6.1 ± 1.1 | 6.1 ± 1.3 | 6.0 ± 1.2 |
| Group | T1 | T2 | P (T × G) | η2p |
|---|---|---|---|---|
| Body mass (kg) | ||||
| PLG | 81.9 ± 6.3 | 80.0 ± 5.3 * | 0.883 | 0.028 |
| CrMG | 81.2 ± 5.0 | 78.6 ± 5.4 * | ||
| HMBG | 79.9 ± 12.2 | 77.6 ± 11.1 * | ||
| CrM-HMBG | 78.0 ± 4.7 | 75.5 ± 4.5 * | ||
| BMI (kg/m2) | ||||
| PLG | 24.1 ± 2.4 | 23.6 ± 2.0 | 0.951 | 0.016 |
| CrMG | 24.1 ± 1.5 | 23.4 ± 1.6 * | ||
| HMBG | 24.1 ± 1.8 | 23.3 ± 1.8 * | ||
| CrM-HMBG | 23.7 ± 1.6 | 22.9 ± 1.4 * | ||
| Muscle mass (%) | ||||
| PLG | 40.3 ± 2.5 | 41.0 ± 2.3 | 0.789 | 0.104 |
| CrMG | 40.6 ± 2.4 | 41.7 ± 2.7 | ||
| HMBG | 41.1 ± 2.0 | 41.7 ± 2.2 | ||
| CrM-HMBG | 41.2 ± 2.4 | 42.1 ± 2.3 | ||
| Fat mass (%) | ||||
| PLG | 8.9 ± 1.5 | 8.7 ± 1.4 | 0.884 | 0.030 |
| CrMG | 8.6 ± 1.7 | 8.4 ± 1.8 | ||
| HMBG | 8.7 ± 1.5 | 8.4 ± 1.1 | ||
| CrM-HMBG | 8.5 ± 1.6 | 8.2 ± 1.4 | ||
| Group | T1 | T2 | P (T × G) | η2p |
|---|---|---|---|---|
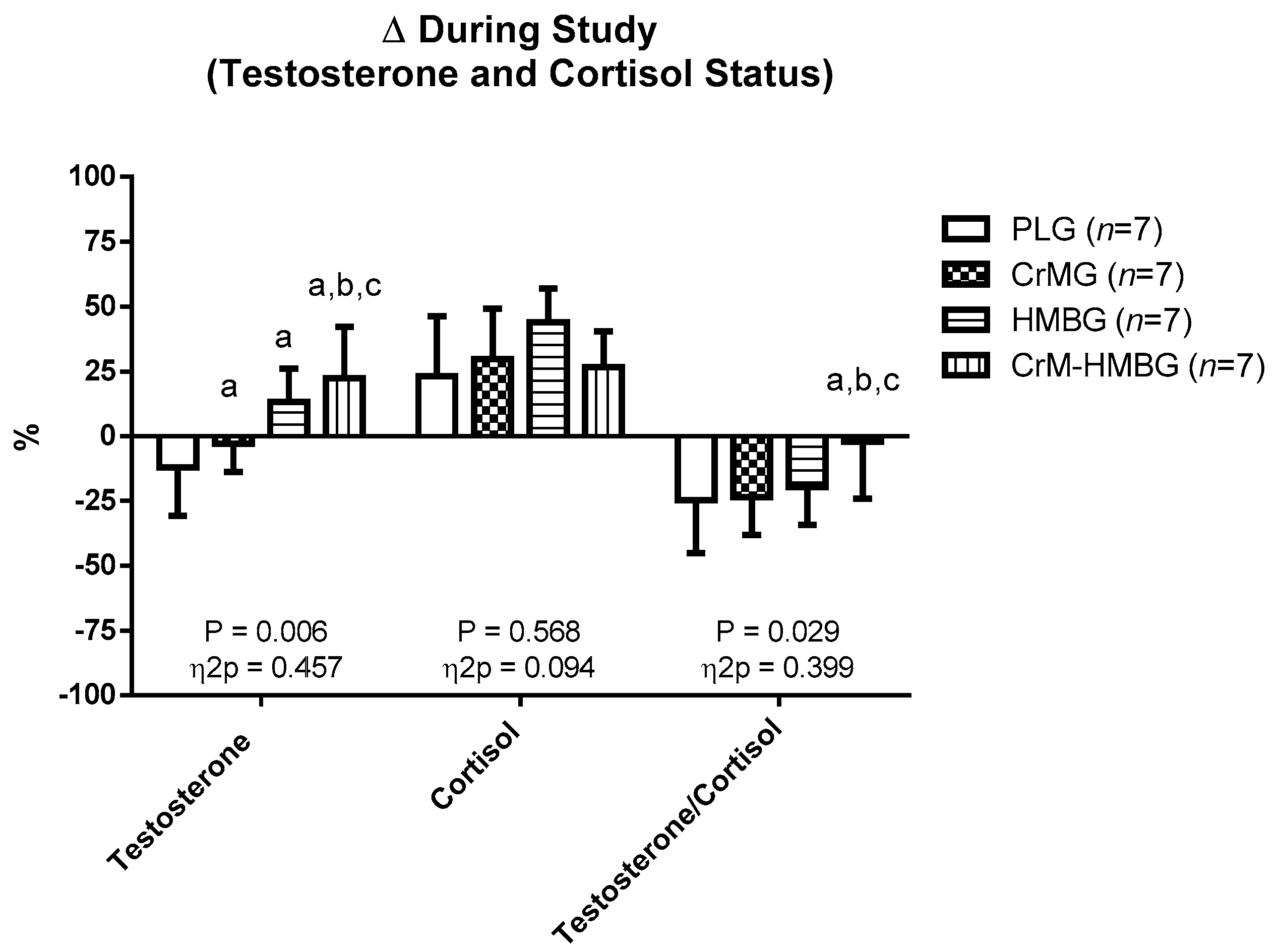
| Testosterone (ng/dL) | ||||
| PLG | 5.22 ± 0.56 | 4.56 ± 0.84 | 0.006 | 0.454 |
| CrMG | 4.27 ± 0.73 | 4.20 ± 1.12 | ||
| HMBG | 4.90 ± 0.95 | 5.60 ± 1.56 | ||
| CrM-HMBG | 4.91 ± 0.87 | 5.97 ± 1.23 * | ||
| Cortisol (μg/dL) | ||||
| PLG | 15.87 ± 2.99 | 17.93 ± 2.08 * | 0.451 | 0.121 |
| CrMG | 15.75 ± 2.78 | 20.80 ± 6.13 * | ||
| HMBG | 16.32 ± 1.29 | 23.30 ± 2.97 * | ||
| CrM-HMBG | 18.18 ± 1.13 | 22.95 ± 1.89 * | ||
| Testosterone/cortisol ratio | ||||
| PLG | 33.77 ± 12.03 | 25.97 ± 6.58 * | 0.032 | 0.349 |
| CrMG | 28.04 ± 7.49 | 22.17 ± 9.90 * | ||
| HMBG | 30.19 ± 6.33 | 23.94 ± 4.98 * | ||
| CrM-HMBG | 27.07 ± 5.12 | 26.07 ± 5.29 | ||
| Group | T1 | T2 | P (T × G) | η2p |
|---|---|---|---|---|
| AST (UI/L) | ||||
| PLG | 17.83 ± 2.79 | 22.00 ± 1.55* | 0.648 | 0.077 |
| CrMG | 22.33 ± 9.65 | 23.50 ± 8.89 | ||
| HMBG | 18.00 ± 3.29 | 19.00 ± 6.96 | ||
| CrM-HMBG | 21.33 ± 4.68 | 24.67 ± 7.30 | ||
| CK (UI/L) | ||||
| PLG | 190.50 ± 94.79 | 216.83 ± 97.35 | 0.641 | 0.079 |
| CrMG | 277.00 ± 171.40 | 256.33 ± 130.38 | ||
| HMBG | 147.50 ± 63.91 | 243.33 ± 286.42 | ||
| CrM-HMBG | 201.67 ± 105.64 | 260.50 ± 159.28 | ||
| LDH (UI/L) | ||||
| PLG | 293.17 ± 36.23 | 310.17 ± 22.98 | 0.792 | 0.049 |
| CrMG | 337.00 ± 47.69 | 337.33 ± 39.20 | ||
| HMBG | 340.17 ± 27.64 | 342.17 ± 45.35 | ||
| CrM-HMBG | 339.17 ± 24.77 | 337.83 ± 60.72 | ||
| Group | % Change to Placebo | Effect |
|---|---|---|
| Cortisol | ||
| CrMG | 145.15% | Antagonistic 131.55 < 389.99 |
| HMBG | 238.84% | |
| CrMG + HMBG | 389.99% | |
| CrM-HMBG | 131.55% | |
| Testosterone | ||
| CrMG | 4.21% | Synergistic −63.85 > −37.85 |
| HMBG | −42.1% | |
| CrMG + HMBG | −37.89% | |
| CrM-HMBG | −63.85% | |
| Testosterone/cortisol ratio | ||
| CrMG | 32.88% | Synergistic 680 > 57.68 |
| HMBG | 24.80% | |
| CrMG + HMBG | 57.68% | |
| CrM-HMBG | 680% | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Landa, J.; Fernández-Lázaro, D.; Calleja-González, J.; Caballero-García, A.; Córdova, A.; León-Guereño, P.; Mielgo-Ayuso, J. Long-Term Effect of Combination of Creatine Monohydrate Plus β-Hydroxy β-Methylbutyrate (HMB) on Exercise-Induced Muscle Damage and Anabolic/Catabolic Hormones in Elite Male Endurance Athletes. Biomolecules 2020, 10, 140. https://doi.org/10.3390/biom10010140
Fernández-Landa J, Fernández-Lázaro D, Calleja-González J, Caballero-García A, Córdova A, León-Guereño P, Mielgo-Ayuso J. Long-Term Effect of Combination of Creatine Monohydrate Plus β-Hydroxy β-Methylbutyrate (HMB) on Exercise-Induced Muscle Damage and Anabolic/Catabolic Hormones in Elite Male Endurance Athletes. Biomolecules. 2020; 10(1):140. https://doi.org/10.3390/biom10010140
Chicago/Turabian StyleFernández-Landa, Julen, Diego Fernández-Lázaro, Julio Calleja-González, Alberto Caballero-García, Alfredo Córdova, Patxi León-Guereño, and Juan Mielgo-Ayuso. 2020. "Long-Term Effect of Combination of Creatine Monohydrate Plus β-Hydroxy β-Methylbutyrate (HMB) on Exercise-Induced Muscle Damage and Anabolic/Catabolic Hormones in Elite Male Endurance Athletes" Biomolecules 10, no. 1: 140. https://doi.org/10.3390/biom10010140
APA StyleFernández-Landa, J., Fernández-Lázaro, D., Calleja-González, J., Caballero-García, A., Córdova, A., León-Guereño, P., & Mielgo-Ayuso, J. (2020). Long-Term Effect of Combination of Creatine Monohydrate Plus β-Hydroxy β-Methylbutyrate (HMB) on Exercise-Induced Muscle Damage and Anabolic/Catabolic Hormones in Elite Male Endurance Athletes. Biomolecules, 10(1), 140. https://doi.org/10.3390/biom10010140











