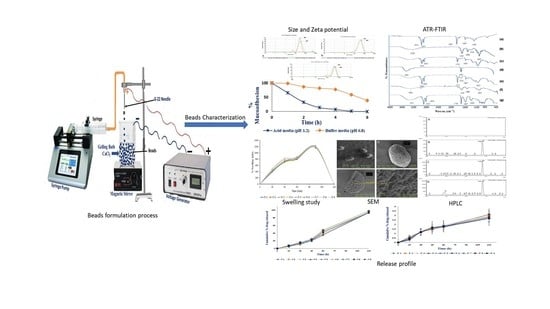
Encapsulation of Black Seed Oil in Alginate Beads as a pH-Sensitive Carrier for Intestine-Targeted Drug Delivery: In Vitro, In Vivo and Ex Vivo Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Alginate-BSO Emulsion
2.3. Characterisation of Alginate-BSO Emulsion
2.4. Bead Preparation
2.5. Bead Characterization
2.5.1. Size and Shape
2.5.2. Encapsulation Efficiency (EE%)
2.5.3. Analysis of Bead Weight Uniformity
2.5.4. Scanning Electron Microscopy (SEM)
2.5.5. Differential Scanning Calorimetry (DSC)
2.5.6. ATR-FTIR
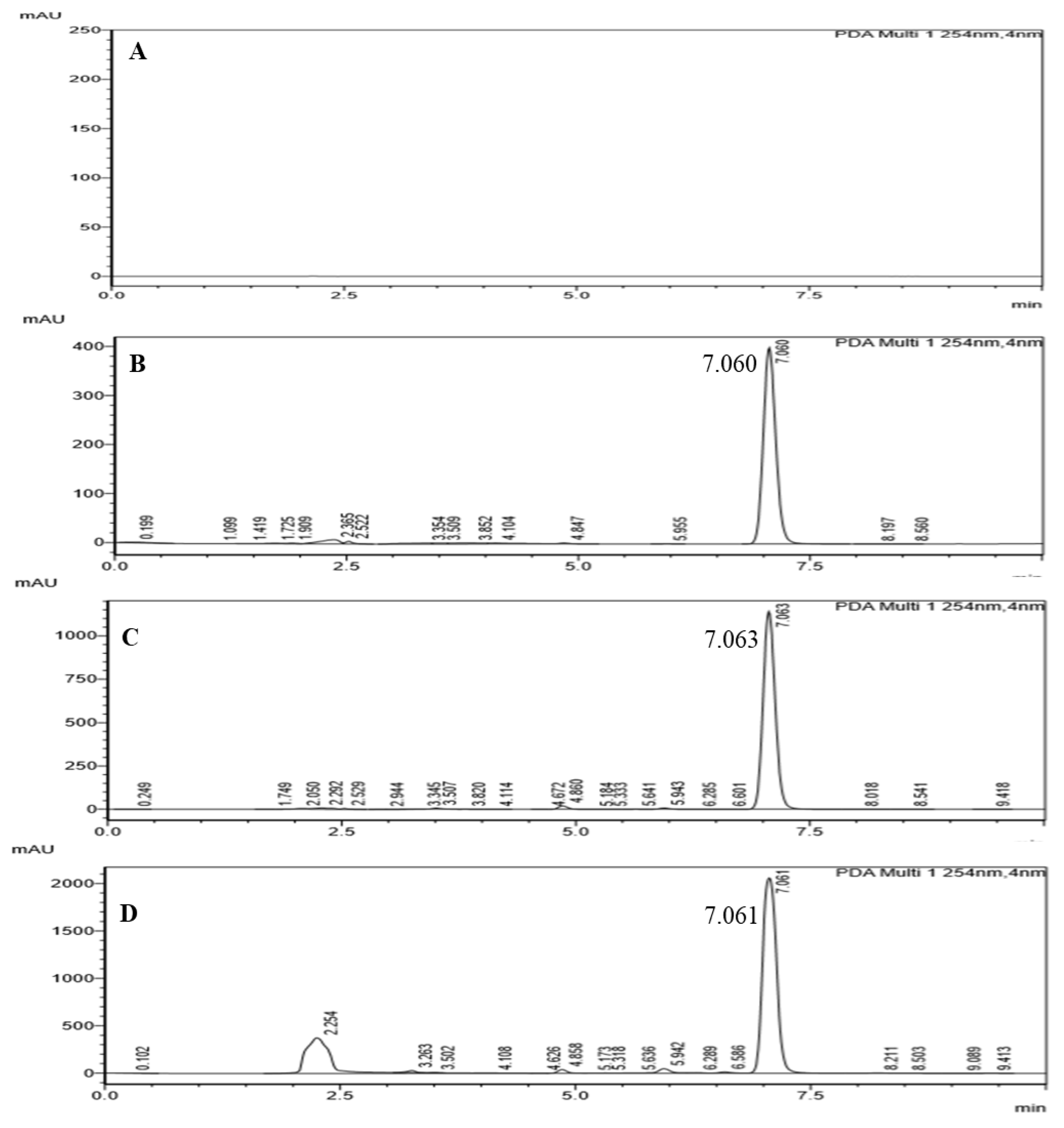
2.5.7. Determination of Thymoquinone
2.5.8. Swelling Characteristics of Beads
2.5.9. In Vitro Drug Release
2.5.10. Ex Vivo Mucoadhesion Test
2.5.11. Gastrointestinal Tract (GIT) Distribution
2.6. Statistical Analysis
3. Results and Discussion
3.1. Alginate-BSO Emulsion
3.2. Percentage of Yield (Y%)
3.3. Characterization of Beads
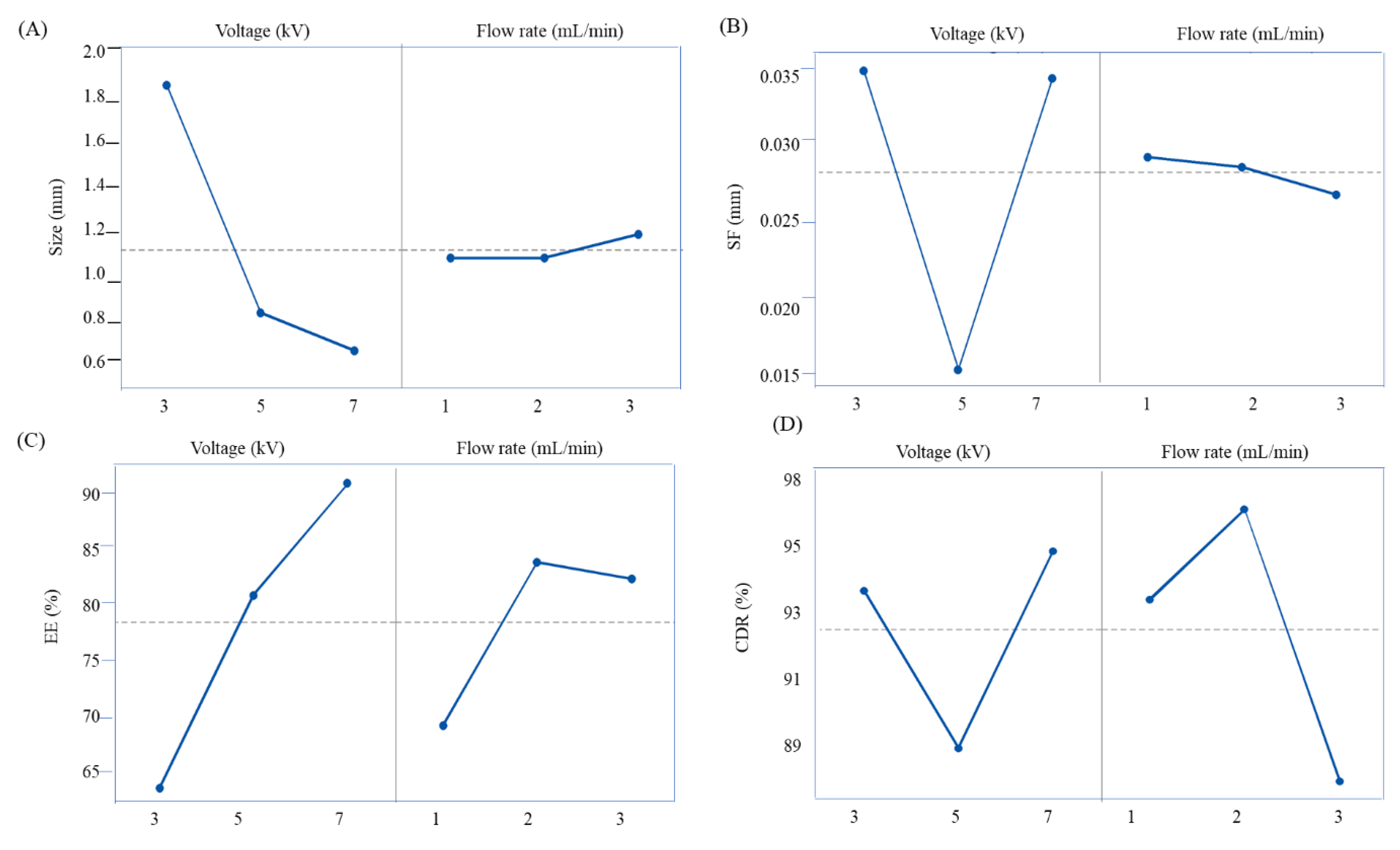
3.3.1. Size and Shape
3.3.2. Encapsulation Efficiency
3.3.3. Analysis of Beads Weight Uniformity
3.3.4. ATR-FTIR
3.3.5. DSC
3.3.6. SEM
3.3.7. Quantification of TQ
3.3.8. Bead Swelling Behaviour
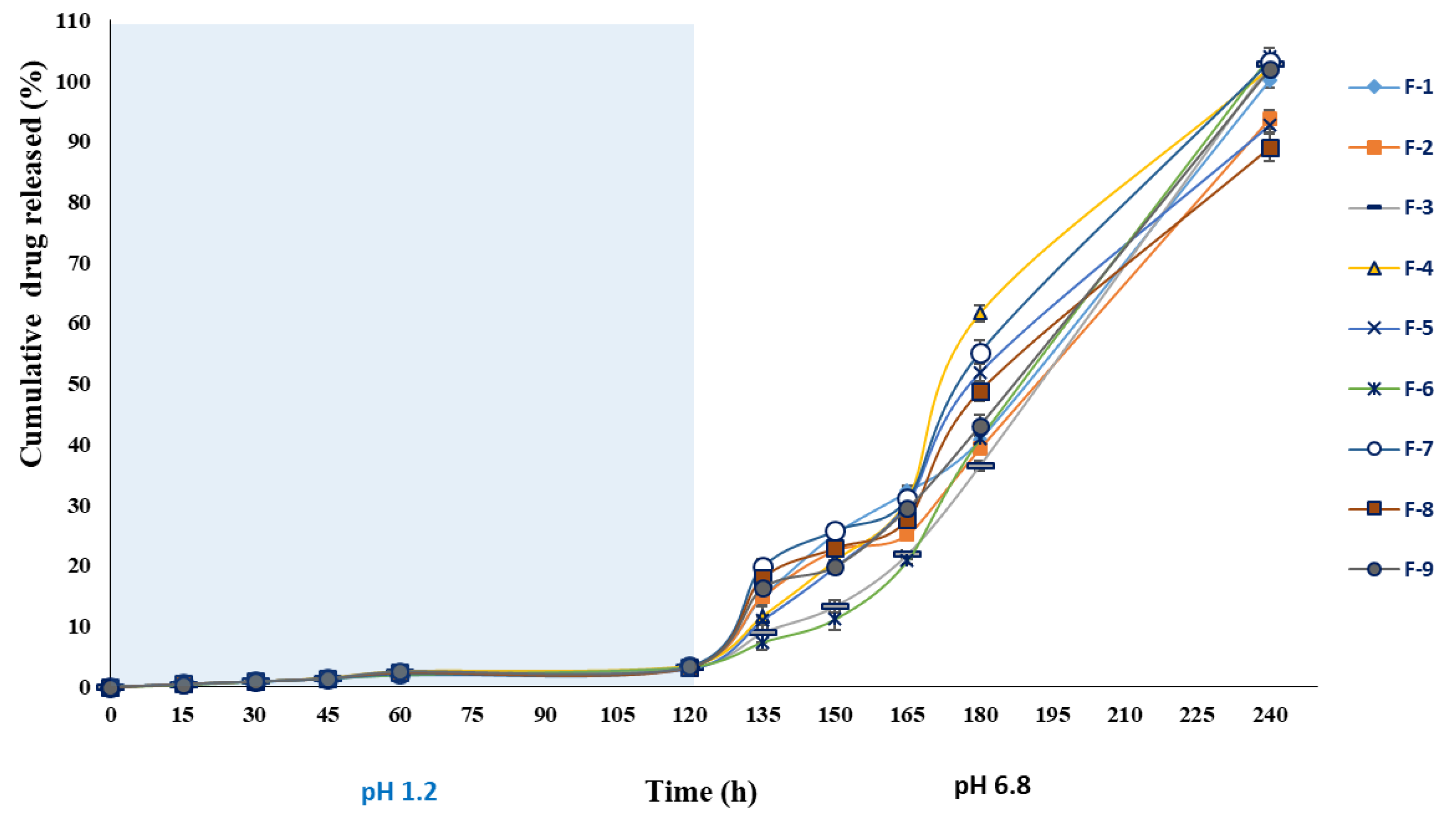
3.3.9. In Vitro Drug Release
3.3.10. Kinetics Modelling and Mechanism of Drug Release
3.3.11. Ex Vivo Mucoadhesion Test of Beads
3.3.12. GIT Distribution Pattern of BSO-Loaded Alginate Beads
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Benavides, S.; Cortés, P.; Parada, J.; Franco, W. Development of alginate microspheres containing thyme essential oil using ionic gelation. Food Chem. 2016, 204, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Agbaria, R.; Gabarin, A.; Dahan, A.; Ben-Shabat, S. Anticancer activity of Nigella sativa (black seed) and its relationship with the thermal processing and quinone composition of the seed. Drug Des. Dev. Ther. 2015, 9, 3119. [Google Scholar]
- Wang, D.; Qiao, J.; Zhao, X.; Chen, T.; Guan, D. Thymoquinone inhibits IL-1β-induced inflammation in human osteoarthritis chondrocytes by suppressing NF-κB and MAPKs signaling pathway. Inflammation 2015, 38, 2235–2241. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.M.; Hosseini, H.; Mohammadifar, M.A.; Mortazavian, A.M.; Mohammadi, A.; Khosravi-Darani, K.; Khaksar, R. Incorporation of essential oil in alginate microparticles by multiple emulsion/ionic gelation process. Int. J. Biol. Macromol. 2013, 62, 582–588. [Google Scholar] [CrossRef]
- Lim, L.Y.; Wan, L.S. Propranolol hydrochloride binding in calcium alginate beads. Drug Dev. Ind. Pharm. 1997, 23, 973–980. [Google Scholar] [CrossRef]
- Dima, C.; Cotârlet, M.; Alexe, P.; Dima, S. Microencapsulation of essential oil of pimento [Pimenta dioica (L) Merr.] by chitosan/k-carrageenan complex coacervation method. Innov. Food Sci. Emerg. 2014, 22, 203–211. [Google Scholar] [CrossRef]
- Chan, E.S. Preparation of Ca-alginate beads containing high oil content: Influence of process variables on encapsulation efficiency and bead properties. Carbohydr. Polym. 2011, 84, 1267–1275. [Google Scholar] [CrossRef]
- Singh, M.N.; Hemant, K.S.Y.; Ram, M.; Shivakumar, H.G. Microencapsulation: A promising technique for controlled drug delivery. Res. Pharm. Sci. 2010, 2, 65. [Google Scholar]
- Zamani, M.; Prabhakaran, M.P.; Ramakrishna, S. Advances in drug delivery via electrospun and electrosprayed nanomaterials. Int. J. Nanomed. 2013, 8, 2997. [Google Scholar]
- Wan, L.Q.; Jiang, J.; Arnold, D.E.; Guo, X.E.; Lu, H.H.; Mow, V.C. Calcium concentration effects on the mechanical and biochemical properties of chondrocyte-alginate constructs. Cell. Mol. Bioeng. 2008, 1, 93–102. [Google Scholar] [CrossRef] [Green Version]
- Husain, O.; Lau, W.; Edirisinghe, M.; Parhizkar, M. Investigating the particle to fibre transition threshold during electrohydrodynamic atomization of a polymer solution. Mater. Sci. Eng. C 2016, 65, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Jiang, J.; Davoodi, P.; Srinivasan, M.P.; Wang, C.H. Electrohydrodynamic atomization: A two-decade effort to produce and process micro-/nanoparticulate materials. Chem. Eng. Sci. 2015, 125, 32–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baimark, Y.; Srisuwan, Y. Preparation of alginate microspheres by waterin-oil emulsion method for drug delivery: Effect of Ca2+ post-cross-linking. Adv. Powder Technol. 2014, 25, 1541–1546. [Google Scholar] [CrossRef]
- Paques, J.P.; Sagis, L.M.C.; van Rijn, C.J.M.; van der Linden, E. Nanospheres of alginate prepared through w/o emulsification and internal gelation with nanoparticles of CaCO3. Food Hydrocoll. 2014, 40, 182–188. [Google Scholar] [CrossRef]
- Suksamran, T.; Opanasopit, P.; Rojanarata, T.; Ngawhirunpat, T.; Ruktanonchai, U.; Supaphol, P. Biodegradable alginate microparticles developed by electrohydrodynamic spraying techniques for oral delivery of protein. J. Microencapsul. 2009, 26, 563–570. [Google Scholar] [CrossRef]
- Szekalska, M.; Puciłowska, A.; Szymańska, E.; Ciosek, P.; Winnicka, K. Alginate: Current use and future perspectives in pharmaceutical and biomedical applications. Int. J. Polym. Sci. 2016. [Google Scholar] [CrossRef] [Green Version]
- Esteban, P.P.; Toby, A.; Jenkins, A.; Arnot, T.C. Elucidation of the mechanisms of action of Bacteriophage K/nano-emulsion formulations against S. aureus via measurement of particle size and zeta potential. Colloids Surf. B 2016, 139, 87–94. [Google Scholar] [CrossRef] [Green Version]
- Doolaanea, A.A.; Mansor, N.I.; Mohd Nor, N.H.; Mohamed, F. Co-encapsulation of Nigella sativa oil and plasmid DNA for enhanced gene therapy of Alzheimer’s disease. J. Microencapsul. 2016, 33, 114–126. [Google Scholar] [CrossRef]
- Osman, M.T.; Taha, B.I.; Al-Duboni, G.; Muhamed, L.A. Immunomodulatory effect of Nigella sativa oil treatment in iron deficiency anemia caused by refractory coeliac disease. Res. J. Pharm. Biol. Chem. Sci. 2012, 3, 887–895. [Google Scholar]
- Işık, H.; Çevikbaş, A.; Gürer, Ü.S.; Kıran, B.; Üresin, Y.; Rayaman, P.; Rayaman, E.; Gürbüz, B.; Büyüköztürk, S. Potential adjuvant effects of Nigella sativa seeds to improve specific immunotherapy in allergic rhinitis patients. Med. Princ. Pract. 2010, 19, 206–211. [Google Scholar] [CrossRef]
- Salem, A.M.; Bamosa, A.O.; Qutub, H.O.; Gupta, R.K.; Badar, A.; Elnour, A.; Afzal, M.N. Effect of Nigella sativa supplementation on lung function and inflammatory mediators in partly controlled asthma: A randomized controlled trial. Ann. Saudi. Med. 2017, 37, 64–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohtashami, R.; Huseini, H.F.; Heydari, M.; Amini, M.; Sadeqhi, Z.; Ghaznavi, H.; Mehrzadi, S. Efficacy and safety of honey based formulation of Nigella sativa seed oil in functional dyspepsia: A double blind randomized controlled clinical trial. J. Ethnopharmacol. 2015, 175, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Gheita, T.A.; Kenawy, S.A. Effectiveness of Nigella sativa oil in the management of rheumatoid arthritis patients: A placebo controlled study. Phytother. Res. 2012, 26, 1246–1248. [Google Scholar] [CrossRef] [PubMed]
- Nikakhlagh, S.; Rahim, F.; Aryani, F.H.N.; Syahpoush, A.; Brougerdnya, M.G.; Saki, N. Herbal treatment of allergic rhinitis: The use of Nigella sativa. Am. J. Otolaryng. 2011, 32, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Kalus, U.; Pruss, A.; Bystron, J.; Jurecka, M.; Smekalova, A.; Lichius, J.J.; Kiesewetter, H. Effect of Nigella sativa (black seed) on subjective feeling in patients with allergic diseases. Phytother. Res. 2003, 17, 1209–1214. [Google Scholar] [CrossRef]
- Malakar, J.; Datta, P.K.; Purakayastha, S.D.; Dey, S.; Nayak, A.K. Floating capsules containing alginate-based beads of salbutamol sulfate: In vitro–In vivo evaluations. Int. J. Biol. Macromol. 2014, 64, 181–189. [Google Scholar] [CrossRef]
- Yilmaztekin, M.; Lević, S.; Kalušević, A.; Cam, M.; Bugarski, B.; Rakić, V.; Nedović, V. Characterization of peppermint (Mentha piperita L.) essential oil encapsulates. J. Microencapsul. 2019, 1–30. [Google Scholar] [CrossRef]
- Nikoo, A.M.; Kadkhodaee, R.; Ghorani, B.; Razzaq, H.; Tucker, N. Electrospray-assisted encapsulation of caffeine in alginate microhydrogels. Int. J. Biol. Macromol. 2018, 116, 208–216. [Google Scholar] [CrossRef]
- Alkhatib, H.; Mohamed, F.; Doolaanea, A.A. ATR-FTIR and spectroscopic methods for analysis of black seed oil from alginate beads. Int. J. Appl. Pharm. 2018, 10, 147–152. [Google Scholar] [CrossRef] [Green Version]
- Zeeb, B.; Saberi, A.H.; Weiss, J.; McClements, D.J. Retention and release of oil-in-water emulsions from filled hydrogel beads composed of calcium alginate: Impact of emulsifier type and pH. Soft Matter. 2015, 11, 2228–2236. [Google Scholar] [CrossRef]
- Nayak, A.K.; Das, B.; Maji, R. Calcium alginate/gum Arabic beads containing glibenclamide: Development and in vitro characterization. Int. J. Biol. Macromol. 2012, 51, 1070–1078. [Google Scholar] [CrossRef] [PubMed]
- Nayak, A.K.; Pal, D.; Santra, K. Development of calcium pectinate-tamarind seed polysaccharide mucoadhesive beads containing metformin HCl. Carbohydr. Polym. 2014, 101, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Aboul-Enein, H.Y.; Abou-Basha, L.I. Simple HPLC method for the determination of thymoquinone in black seed oil (Nigella sativa Linn). J. Liquid Chromatogr. Relat. Technol. 1995, 18, 895–902. [Google Scholar] [CrossRef]
- Nayak, A.K.; Pal, D.; Das, S. Calcium pectinate-fenugreek seed mucilage mucoadhesive beads for controlled delivery of metformin HCl. Carbohydr. Polym. 2013, 96, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Bera, H.; Boddupalli, S.; Nayak, A.K. Mucoadhesive-floating zinc-pectinate–sterculia gum interpenetrating polymer network beads encapsulating ziprasidone HCl. Carbohydr. Polym. 2015, 131, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Bera, H.; Abbasi, Y.F.; Yoke, F.F.; Seng, P.M.; Kakoti, B.B.; Ahmmed, S.M.; Bhatnagar, P. Ziprasidone-loaded arabic gum modified montmorillonite-tailor-made pectin based gastroretentive composites. Int. J. Biol. Macromol. 2019, 129, 552–563. [Google Scholar] [CrossRef] [PubMed]
- Dhaliwal, S.; Jain, S.; Singh, H.P.; Tiwary, A.K. Mucoadhesive microspheres for gastroretentive delivery of acyclovir: In vitro and in vivo evaluation. AAPS J. 2008, 10, 322. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Gong, X.; Guo, X.; Liu, C.; Fan, Y.Y.; Zhang, J.; Li, W. Characterization, release, and antioxidant activity of curcumin-loaded sodium alginate/ZnO hydrogel beads. Int. J. Biol. Macromol. 2019, 121, 1118–1125. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Davarani, H.F.; Javanmard, R.; Dokhani, A.; Mozafari, M.R. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [Green Version]
- Schulz, A.; Katsen-Globa, A.; Huber, E.J.; Mueller, S.C.; Kreiner, A.; Pütz, N.; Gepp, M.M.; Fischer, B.; Stracke, F.; von Briesen, H.; et al. Poly (amidoamine)-alginate hydrogels: Directing the behavior of mesenchymal stem cells with charged hydrogel surfaces. J. Mater. Sci. Mater. Med. 2018, 29, 105. [Google Scholar] [CrossRef] [Green Version]
- Piornos, J.A.; Burgos-Díaz, C.; Morales, E.; Rubilar, M.; Acevedo, F. Highly efficient encapsulation of linseed oil into alginate/lupin protein beads: Optimization of the emulsion formulation. Food Hydrocoll. 2017, 63, 139–148. [Google Scholar] [CrossRef]
- Morales, E.; Rubilar, M.; Burgos-Díaz, C.; Acevedo, F.; Penning, M.; Shene, C. Alginate/Shellac beads developed by external gelation as a highly efficient model system for oil encapsulation with intestinal delivery. Food Hydrocoll. 2017, 70, 321–328. [Google Scholar] [CrossRef]
- US Pharmacopoeia National Formulary, USP 23/NF 18; United States Pharmaccopoeia Convention Inc.: Rockville, MD, USA, 2000.
- Qiusheng, Z.; Xiaoyan, L.; Jin, Q.; Jing, W.; Xuegang, L. Porous zirconium alginate beads adsorbent for fluoride adsorption from aqueous solutions. RSC Adv. 2015, 5, 2100–2112. [Google Scholar] [CrossRef]
- Guru, P.R.; Nayak, A.K.; Sahu, R.K. Oil-entrapped sterculia gum–alginate buoyant systems of aceclofenac: Development and in vitro evaluation. Colloids Surf. B 2013, 104, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Treesinchai, S.; Puttipipatkhachorn, S.; Pitaksuteepong, T.; Sungthongjeen, S. Development of curcumin floating beads with low density materials and solubilizers. J. Drug Deliv. Sci. Technol. 2019, 51, 542–551. [Google Scholar] [CrossRef]
- Mallappa, M.K.; Kesarla, R.; Banakar. S. Calcium alginate-neusilin US2 nanocomposite microbeads for oral sustained drug delivery of poor water-soluble drug aceclofenac sodium. J. Drug Deliv. 2015, 2015. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.C.; Ho, H.O.; Liu, D.Z.; Siow, W.S.; Sheu, M.T. Swelling/floating capability and drug release characterizations of gastroretentive drug delivery system based on a combination of hydroxyethyl cellulose and sodium carboxymethyl cellulose. PLoS ONE 2015, 10, e0116914. [Google Scholar] [CrossRef]
- Badri, W.; El Asbahani, A.; Miladi, K.; Baraket, A.; Agusti, G.; Nazari, Q.A.; Elaissari, A. Poly (ε-caprolactone) nanoparticles loaded with indomethacin and Nigella sativa L. essential oil for the topical treatment of inflammation. J. Drug Deliv. Sci. Technol. 2018, 46, 234–242. [Google Scholar] [CrossRef]
- Sriamornsak, P.; Thirawong, N.; Korkerd, K. Swelling, erosion and release behaviour of alginate-based matrix tablets. Eur. J. Pharm. Biopharm. 2007, 66, 435–450. [Google Scholar] [CrossRef]
- Seeli, D.S.; Dhivya, S.; Selvamurugan, N.; Prabaharan, M. Guar gum succinate-sodium alginate beads as a pH-sensitive carrier for colon-specific drug delivery. Int. J. Biol. Macromol. 2016, 91, 45–50. [Google Scholar] [CrossRef]
- Pasparakis, G.; Bouropoulos, N. Swelling studies and in vitro release of verapamil from calcium alginate and calcium alginate–chitosan beads. Int. J. Pharm. 2006, 323, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, S.K.; Sharma, S. Investigation of swelling/degradation behaviour of alginate beads crosslinked with Ca2+ and Ba2+ ions. React. Funct. Polym. 2004, 59, 129–140. [Google Scholar] [CrossRef]
- Bae, S.B.; Nam, H.C.; Park, W.H. Electrospraying of environmentally sustainable alginate microbeads for cosmetic additives. Int. J. Biol. Macromol. 2019, 133, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Schuh, R.S.; Bruxel, F.; Teixeira, H.F. Physicochemical properties of lecithin-based nanoemulsions obtained by spontaneous emulsification or high-pressure homogenization. Química Nova 2014, 7, 1193–1198. [Google Scholar]
- Sarmento, B.; Ferreira, D.; Veiga, F.; Ribeiro, A. Characterization of insulin-loaded alginate nanoparticles produced by ionotropic pre-gelation through DSC and FTIR studies. Carbohydr. Polym. 2006, 66, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Bhopatkar, D.; Anal, A.K.; Stevens, W.F. Ionotropic alginate beads for controlled intestinal protein delivery: Effect of chitosan and barium counter-ions on entrapment and release. J. Microencapsul. 2005, 22, 91–100. [Google Scholar] [CrossRef]
- Corstens, M.N.; Berton-Carabin, C.C.; Elichiry-Ortiz, P.T.; Hol, K.; Troost, F.J.; Masclee, A.A.; Schroën, K. Emulsion-alginate beads designed to control in vitro intestinal lipolysis: Towards appetite control. J. Funct. Foods 2017, 34, 319–328. [Google Scholar] [CrossRef]
- Li, Y.; Hu, M.; Du, Y.; Xiao, H.; McClements, D.J. Control of lipase digestibility of emulsified lipids by encapsulation within calcium alginate beads. Food Hydrocoll. 2011, 25, 122–130. [Google Scholar] [CrossRef]
- Zeeb, B.; Saberi, A.H.; Weiss, J.; McClements, D.J. Formation and characterization of filled hydrogel beads based on calcium alginate: Factors influencing nanoemulsion retention and release. Food Hydrocoll. 2015, 50, 27–36. [Google Scholar] [CrossRef]
- Wright, P.J.; Ciampi, E.; Hoad, C.L.; Weaver, A.C.; van Ginkel, M.; Marciani, L.; Rayment, P. Investigation of alginate gel inhomogeneity in simulated gastro-intestinal conditions using magnetic resonance imaging and transmission electron microscopy. Carbohydr. Polym. 2009, 77, 306–315. [Google Scholar] [CrossRef]
- Apninder, K.; Amarjeet, K.; Vishav, P.K.; Manpreet, K.; Murthy, R.S.R. Polymeric drug delivery approaches for colon targeting: A review. Drug Deliv. Lett. 2014, 4, 38–48. [Google Scholar]
- Wu, Y.; Zhang, W.; Huang, J.; Luo, Z.; Li, J.; Wang, L.; Di, L. Mucoadhesive improvement of alginate microspheres as potential gastroretentive delivery carrier by blending with Bletilla striata polysaccharide. Int. J. Biol. Macromol. 2019, in press. [Google Scholar] [CrossRef] [PubMed]











| Lecithin Concentration (% w/v) | Particle Size (nm) | PDI | Zeta Potential (mV) | ES (%) | |||
|---|---|---|---|---|---|---|---|
| Before Centrifugation | After Centrifugation | Before Centrifugation | After Centrifugation | Before Centrifugation | After Centrifugation | ||
| 1 | 253.32 ± 4.6 | 282.93 ± 14.1 * | 0.392 ± 0.0 | 0.333 ± 0.1 | −43.1 ± 0.3 | −37.6 ± 2.2 * | 92.50 ± 0.2 |
| 3 | 334.51 ± 12.8 | 347.44 ± 15.1 | 0.315 ± 0.1 | 0.358 ± 0.1 * | −46.9 ± 2.2 | −43.5 ± 1.3 * | 94.83 ± 0.4 |
| 5 | 430.13 ± 14.2 | 463.23 ± 8.9 * | 0.422 ± 0.0 | 0.548 ± 0.1 * | −51.4 ± 2.7 | −45 ± 1.0 * | 96.50 ± 0.4 |
| Code | Voltage (kV) | Flow Rate (mL/min) | Yield (%) | Bead Size (mm) | Sphericity Factor (mm) | Encapsulation Efficiency (%) | Coefficient of Weight Variation (%) = (SD/mean) ×100 | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Wet | Dry | Wet | Dry | Wet | Dry | |||||
| F1 | 3 | 1 | 90.23 ± 4.19 | 4.47 ± 0.01 | 1.82 ± 0.01 | 0.02 ± 0.00 | 0.04 ± 0.00 | 67.20 ± 1.46 | 59.92 ± 0.90 | 2.97 |
| F2 | 3 | 2 | 92.11 ± 2.41 | 4.52 ± 0.03 | 1.86 ± 0.02 | 0.01 ± 0.00 | 0.03 ± 0.00 | 68.22 ± 3.09 | 65.89 ± 1.57 | 2.61 |
| F3 | 3 | 3 | 93.33 ± 3.22 | 4.76 ± 0.01 | 1.86 ± 0.01 | 0.02 ± 0.01 | 0.04 ± 0.00 | 70.76 ± 0.86 | 66.34 ± 1.89 | 2.43 |
| F4 | 5 | 1 | 91.78 ± 2.65 | 3.12 ± 0.01 | 0.99 ± 0.04 | 0.02 ± 0.01 | 0.01 ± 0.01 | 70.11 ± 0.51 | 70.11 ± 0.51 | 2.88 |
| F5 | 5 | 2 | 93.33 ± 2.08 | 2.83 ± 0.05 | 0.80 ± 0.02 | 0.02 ± 0.01 | 0.02 ± 0.02 | 86.89 ± 1.67 | 86.89 ± 1.67 | 3.93 |
| F6 | 5 | 3 | 92.98 ± 3.11 | 3.30 ± 0.01 | 0.90 ± 0.02 | 0.03 ± 0.01 | 0.01 ± 0.01 | 86.63 ± 0.83 | 86.12 ± 0.15 | 2.97 |
| F7 | 7 | 1 | 93.98 ± 2.84 | 1.86 ± 0.02 | 0.72 ± 0.01 | 0.01 ± 0.00 | 0.04 ± 0.00 | 82.16 ± 2.50 | 78.67 ± 0.90 | 2.90 |
| F8 | 7 | 2 | 94.87 ± 2.11 | 1.50 ± 0.02 | 0.58 ± 0.01 | 0.02 ± 0.00 | 0.03 ± 0.00 | 104.50 ± 4.04 | 90.13 ± 0.93 | 1.37 |
| F9 | 7 | 3 | 93.88 ± 4.32 | 2.58 ± 0.05 | 0.93 ± 0.01 | 0.02 ± 0.00 | 0.03 ± 0.01 | 96.04 ± 3.61 | 89.82 ± 154 | 2.99 |
| Code | Swelling Exponent (ns) | Swelling kinetic Constant (ks) (h−1) | Correlation Coefficient (r2) | Swelling Rate (%/h) | Correlation Coefficient (r2) | Water Penetration Velocity (V) (cm/s) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pH 1.2 | pH 6.8 | pH 1.2 | pH 6.8 | pH 1.2 | pH 6.8 | pH 1.2 | pH 6.8 | pH 1.2 | pH 6.8 | pH 1.2 | pH 6.8 | |
| F-1 | 0.74 | 0.64 | 12.173 | 820.55 | 0.941 | 0.946 | 12.15 | 799.50 | 0.944 | 0.981 | 0.024 | 1.540 |
| F-2 | 1.23 | 0.67 | 44.47 | 899.84 | 0.932 | 0.976 | 39.98 | 876.20 | 0.887 | 0.986 | 0.074 | 1.533 |
| F-3 | 1.32 | 0.72 | 45.15 | 827.47 | 0.928 | 0.946 | 40.02 | 798.54 | 0.871 | 0.973 | 0.027 | 0.507 |
| F-4 | 1.49 | 0.82 | 53.52 | 848.16 | 0.957 | 0.949 | 46.76 | 807.35 | 0.854 | 0.959 | 0.182 | 2.942 |
| F-5 | 1.15 | 0.81 | 48.54 | 818.20 | 0.823 | 0.964 | 43.70 | 781.67 | 0.835 | 0.965 | 0.219 | 3.897 |
| F-6 | 1.35 | 0.58 | 58.78 | 814.15 | 0.917 | 0.965 | 51.80 | 800.48 | 0.863 | 0.990 | 0.082 | 1.178 |
| F-7 | 1.38 | 0.59 | 59.90 | 850.94 | 0.972 | 0.916 | 53.39 | 832.97 | 0.869 | 0.977 | 0.306 | 4.553 |
| F-8 | 1.04 | 0.58 | 48.05 | 994.99 | 0.892 | 0.988 | 44.15 | 982.23 | 0.900 | 0.996 | 0.357 | 7.620 |
| F-9 | 1.19 | 0.66 | 58.00 | 930.56 | 0.982 | 0.964 | 53.15 | 905.95 | 0.900 | 0.984 | 0.163 | 2.595 |
| Code | DE (%) | MDT (min) | Correlation Coefficient (r2) | Release Exponent (n) | Gel Characteristic Constant (kKP) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pH 1.2 | pH 6.8 | pH 1.2 | pH 6.8 | Zero order | First order | Higuchi | Hixson crowell | Korsemeyer-Peppas | pH 1.2 | pH 6.8 | pH 1.2 | pH 6.8 | ||||||
| pH 1.2 | pH 6.8 | pH 1.2 | pH 6.8 | pH 1.2 | pH 6.8 | pH 1.2 | pH 6.8 | pH 1.2 | pH 6.8 | |||||||||
| F-1 | 1.10 | 38.75 | 54.71 | 70.29 | 0.953 | 0.968 | 0.954 | 0.856 | 0.879 | 0.757 | 0.648 | 0.876 | 0.763 | 0.997 | 0.66 | 1.47 | 0.01 | 0.34 |
| F-2 | 1.48 | 38.59 | 34.88 | 71.51 | 0.815 | 0.958 | 0.818 | 0.838 | 0.964 | 0.738 | 0.506 | 0.884 | 0.912 | 0.995 | 0.49 | 1.44 | 0.02 | 0.33 |
| F-3 | 1.03 | 41.19 | 54.75 | 67.76 | 0.937 | 0.926 | 0.938 | 0.867 | 0.870 | 0.713 | 0.711 | 0.891 | 0.846 | 0.909 | 1.03 | 1.66 | 0.01 | 0.29 |
| F-4 | 1.82 | 38.92 | 38.13 | 71.29 | 0.862 | 0.956 | 0.864 | 0.835 | 0.971 | 0.738 | 0.547 | 0.871 | 0.958 | 0.979 | 0.61 | 1.29 | 0.02 | 0.34 |
| F-5 | 1.73 | 47.76 | 43.06 | 61.16 | 0.893 | 0.928 | 0.895 | 0.885 | 0.947 | 0.763 | 0.618 | 0.838 | 0.924 | 0.917 | 0.88 | 1.46 | 0.02 | 0.38 |
| F-6 | 1.28 | 42.13 | 66.28 | 68.33 | 0.969 | 0.939 | 0.968 | 0.840 | 0.817 | 0.726 | 0.759 | 0.872 | 0.956 | 0.900 | 1.00 | 1.39 | 0.01 | 0.33 |
| F-7 | 2.22 | 44.86 | 43.06 | 63.31 | 0.858 | 0.989 | 0.861 | 0.879 | 0.913 | 0.833 | 0.633 | 0.779 | 0.851 | 0.972 | 1.11 | 1.02 | 0.02 | 0.43 |
| F-8 | 2.07 | 43.36 | 55.06 | 53.72 | 0.980 | 0.951 | 0.981 | 0.955 | 0.896 | 0.868 | 0.707 | 0.701 | 0.946 | 0.901 | 0.88 | 0.86 | 0.02 | 0.41 |
| F-9 | 1.67 | 39.97 | 45.23 | 69.63 | 0.860 | 0.961 | 0.862 | 0.840 | 0.881 | 0.762 | 0.652 | 0.825 | 0.884 | 0.962 | 0.99 | 1.10 | 0.02 | 0.37 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azad, A.K.; Al-Mahmood, S.M.A.; Chatterjee, B.; Wan Sulaiman, W.M.A.; Elsayed, T.M.; Doolaanea, A.A. Encapsulation of Black Seed Oil in Alginate Beads as a pH-Sensitive Carrier for Intestine-Targeted Drug Delivery: In Vitro, In Vivo and Ex Vivo Study. Pharmaceutics 2020, 12, 219. https://doi.org/10.3390/pharmaceutics12030219
Azad AK, Al-Mahmood SMA, Chatterjee B, Wan Sulaiman WMA, Elsayed TM, Doolaanea AA. Encapsulation of Black Seed Oil in Alginate Beads as a pH-Sensitive Carrier for Intestine-Targeted Drug Delivery: In Vitro, In Vivo and Ex Vivo Study. Pharmaceutics. 2020; 12(3):219. https://doi.org/10.3390/pharmaceutics12030219
Chicago/Turabian StyleAzad, Abul Kalam, Sinan Mohammed Abdullah Al-Mahmood, Bappaditya Chatterjee, Wan Mohd Azizi Wan Sulaiman, Tarek Mohamed Elsayed, and Abd Almonem Doolaanea. 2020. "Encapsulation of Black Seed Oil in Alginate Beads as a pH-Sensitive Carrier for Intestine-Targeted Drug Delivery: In Vitro, In Vivo and Ex Vivo Study" Pharmaceutics 12, no. 3: 219. https://doi.org/10.3390/pharmaceutics12030219
APA StyleAzad, A. K., Al-Mahmood, S. M. A., Chatterjee, B., Wan Sulaiman, W. M. A., Elsayed, T. M., & Doolaanea, A. A. (2020). Encapsulation of Black Seed Oil in Alginate Beads as a pH-Sensitive Carrier for Intestine-Targeted Drug Delivery: In Vitro, In Vivo and Ex Vivo Study. Pharmaceutics, 12(3), 219. https://doi.org/10.3390/pharmaceutics12030219