Natural Compounds with Potential to Modulate Cancer Therapies and Self-Reactive Immune Cells
Abstract
:1. Introduction
The Link between Immunotherapies, Adverse Events and Self-Reactive T Cells
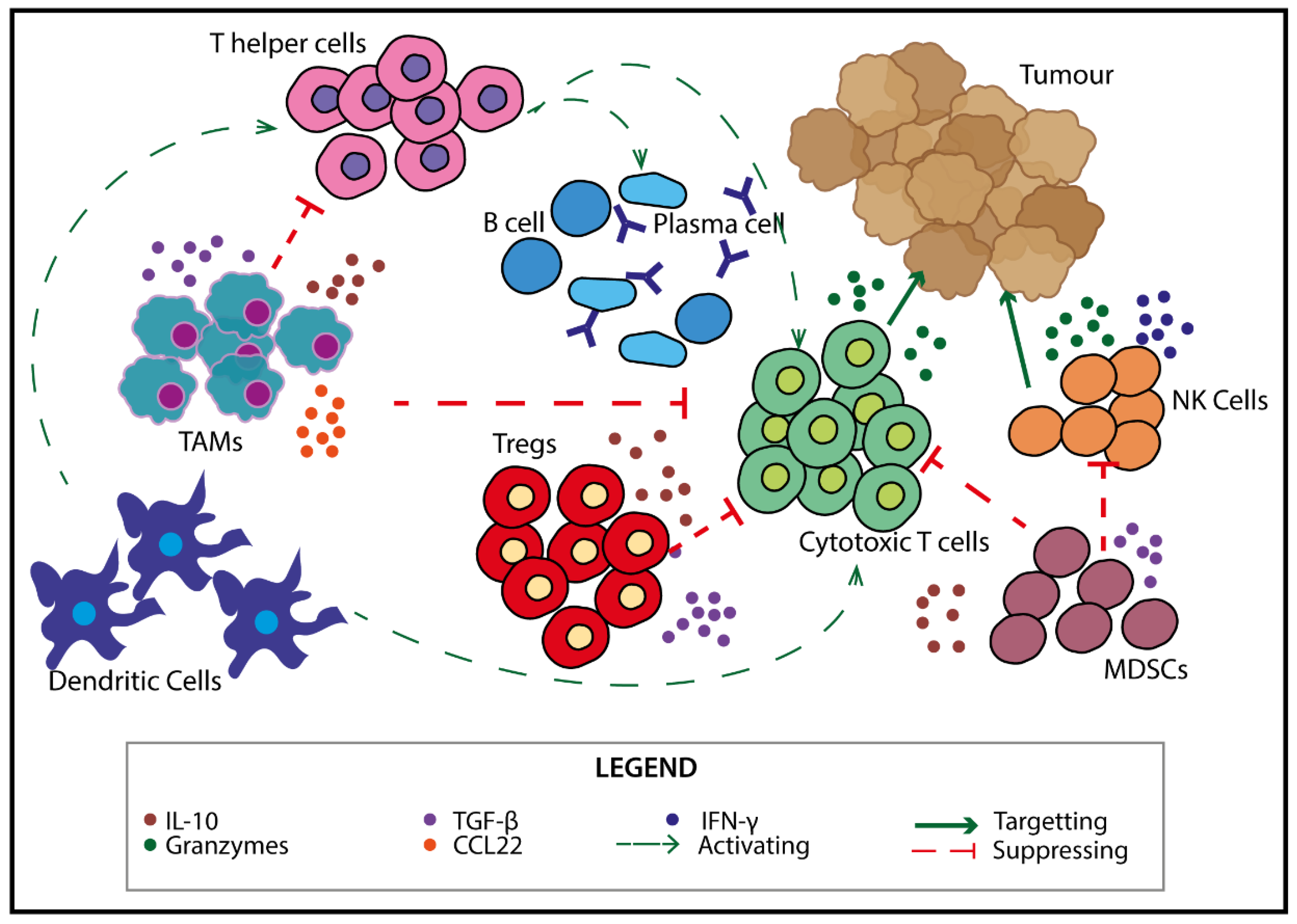
2. Immune Cells in the TME
3. Self-Reactive Cells—Targets for Novel Therapeutics?
Utilising Self-Reactive T Cells to Modulate the Anti-Tumour Response
4. Treating Cancer and the High Risk of Recurrence
Emerging Combination Therapies for Cancer
5. Natural Compounds as Anti-Cancer and Autoimmune Mediators
5.1. Immune Cell Modulation by Natural Compounds
5.1.1. Resveratrol
5.1.2. Curcumin
5.1.3. EGCG
5.2. Bioavailability of Natural Compounds
6. Natural Compounds from Native Australian Plants
6.1. Phenolic Content of Native Australian Plants
6.2. Therapeutic Activity of Whole Extracts from Native Australian Plants
7. Therapeutic Benefits of Isolated Phenolic Compounds
7.1. Hesperetin
7.2. Myricetin
7.3. Quercetin
7.4. Cyanidin-3-glucoside
8. Future of Natural Compounds as Potential Anti-Cancer Therapeutics
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, N.; Baby, D.; Rajguru, J.P.; Patil, P.B.; Thakkannavar, S.S.; Pujari, V.B. Inflammation and cancer. Ann. Afr. Med. 2019, 18, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Greten, F.R.; Grivennikov, S.I. Inflammation and cancer: Triggers, mechanisms, and consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Kazandjian, D.; Khozin, S.; Blumenthal, G.; Zhang, L.; Tang, S.; Libeg, M.; Kluetz, P.; Sridhara, R.; Keegan, P.; Pazdur, R. Benefit-risk summary of nivolumab for patients with metastatic squamous cell lung cancer after platinum-based chemotherapy: A report from the us food and drug administration. JAMA Oncol. 2016, 2, 118–122. [Google Scholar] [CrossRef] [Green Version]
- Necchi, A.; Joseph, R.W.; Loriot, Y.; Hoffman-Censits, J.; Perez-Gracia, J.L.; Petrylak, D.P.; Derleth, C.L.; Tayama, D.; Zhu, Q.; Ding, B.; et al. Atezolizumab in platinum-treated locally advanced or metastatic urothelial carcinoma: Post-progression outcomes from the phase ii imvigor210 study. Ann. Oncol. 2017, 28, 3044–3050. [Google Scholar] [CrossRef]
- Fallarino, F.; Fields, P.E.; Gajewski, T.F. B7-1 engagement of cytotoxic t lymphocyte antigen 4 inhibits t cell activation in the absence of cd28. J. Exp. Med. 1998, 188, 205–210. [Google Scholar] [CrossRef]
- Parry, R.V.; Chemnitz, J.M.; Frauwirth, K.A.; Lanfranco, A.R.; Braunstein, I.; Kobayashi, S.V.; Linsley, P.S.; Thompson, C.B.; Riley, J.L. Ctla-4 and pd-1 receptors inhibit t-cell activation by distinct mechanisms. Mol. Cell. Biol. 2005, 25, 9543–9553. [Google Scholar] [CrossRef] [Green Version]
- Trinh, S.; Le, A.; Gowani, S.; La-Beck, N.M. Management of immune-related adverse events associated with immune checkpoint inhibitor therapy: A minireview of current clinical guidelines. Asia Pac. J. Oncol. Nurs. 2019, 6, 154–160. [Google Scholar]
- Yoest, J.M. Clinical features, predictive correlates, and pathophysiology of immune-related adverse events in immune checkpoint inhibitor treatments in cancer: A short review. Immunotargets 2017, 6, 73–82. [Google Scholar] [CrossRef] [Green Version]
- Baxi, S.; Yang, A.; Gennarelli, R.L.; Khan, N.; Wang, Z.; Boyce, L.; Korenstein, D. Immune-related adverse events for anti-pd-1 and anti-pd-l1 drugs: Systematic review and meta-analysis. BMJ 2018, 360, k793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michot, J.M.; Bigenwald, C.; Champiat, S.; Collins, M.; Carbonnel, F.; Postel-Vinay, S.; Berdelou, A.; Varga, A.; Bahleda, R.; Hollebecque, A.; et al. Immune-related adverse events with immune checkpoint blockade: A comprehensive review. Eur. J. Cancer 2016, 54, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Gettinger, S.N.; Horn, L.; Gandhi, L.; Spigel, D.R.; Antonia, S.J.; Rizvi, N.A.; Powderly, J.D.; Heist, R.S.; Carvajal, R.D.; Jackman, D.M.; et al. Overall survival and long-term safety of nivolumab (anti-programmed death 1 antibody, bms-936558, ono-4538) in patients with previously treated advanced non-small-cell lung cancer. J. Clin. Oncol. 2015, 33, 2004–2012. [Google Scholar] [CrossRef]
- Pillai, R.N.; Behera, M.; Owonikoko, T.K.; Kamphorst, A.O.; Pakkala, S.; Belani, C.P.; Khuri, F.R.; Ahmed, R.; Ramalingam, S.S. Comparison of the toxicity profile of pd-1 versus pd-l1 inhibitors in non-small cell lung cancer: A systematic analysis of the literature. Cancer 2018, 124, 271–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weber, J.S.; D’Angelo, S.P.; Minor, D.; Hodi, F.S.; Gutzmer, R.; Neyns, B.; Hoeller, C.; Khushalani, N.I.; Miller, W.H., Jr.; Lao, C.D.; et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-ctla-4 treatment (checkmate 037): A randomised, controlled, open-label, phase 3 trial. Lancet. Oncol. 2015, 16, 375–384. [Google Scholar]
- Ribas, A.; Puzanov, I.; Dummer, R.; Schadendorf, D.; Hamid, O.; Robert, C.; Hodi, F.S.; Schachter, J.; Pavlick, A.C.; Lewis, K.D.; et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (keynote-002): A randomised, controlled, phase 2 trial. Lancet Oncol. 2015, 16, 908–918. [Google Scholar] [CrossRef]
- Hinchcliff, E.M.; Hong, D.; Le, H.; Chisholm, G.; Iyer, R.; Naing, A.; Jazaeri, A.A. Adverse events and responses in patients with recurrent ovarian cancer undergoing early-phase immune checkpoint inhibitor clinical trials. Gynecol. Oncol. 2018, 149, 8. [Google Scholar] [CrossRef]
- Johnson, C.; Jazaeri, A.A. Diagnosis and management of immune checkpoint inhibitor-related toxicities in ovarian cancer: A series of case vignettes. Clin. Ther. 2018, 40, 389–394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Postow, M.A.; Sidlow, R.; Hellmann, M.D. Immune-related adverse events associated with immune checkpoint blockade. N. Engl. J. Med. 2018, 378, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Cruz, C.R.Y.; Bollard, C.M. Chapter 16—T-cell immunotherapy for cancer. In Novel Approaches and Strategies for Biologics, Vaccines and Cancer Therapies; Singh, M., Salnikova, M., Eds.; Academic Press: Cambridge, MA, USA, 2015; pp. 389–410. [Google Scholar]
- Fratta, E.; Coral, S.; Covre, A.; Parisi, G.; Colizzi, F.; Danielli, R.; Nicolay, H.J.M.; Sigalotti, L.; Maio, M. The biology of cancer testis antigens: Putative function, regulation and therapeutic potential. Mol. Oncol. 2011, 5, 164–182. [Google Scholar] [CrossRef] [Green Version]
- Hollingsworth, R.E.; Jansen, K. Turning the corner on therapeutic cancer vaccines. NPJ Vaccines 2019, 4, 7. [Google Scholar] [CrossRef]
- Maeda, Y.; Nishikawa, H.; Sugiyama, D.; Ha, D.; Hamaguchi, M.; Saito, T.; Nishioka, M.; Wing, J.B.; Adeegbe, D.; Katayama, I.; et al. Detection of self-reactive cd8(+) t cells with an anergic phenotype in healthy individuals. Science 2014, 346, 1536–1540. [Google Scholar] [CrossRef] [PubMed]
- Makkouk, A.; Weiner, G.J. Cancer immunotherapy and breaking immune tolerance: New approaches to an old challenge. Cancer Res. 2015, 75, 5–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richards, D.M.; Kyewski, B.; Feuerer, M. Re-examining the nature and function of self-reactive t cells. Trends Immunol. 2016, 37, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.L.; Moffitt, L.R.; Duffield, N.; Rainczuk, A.; Jobling, T.W.; Plebanski, M.; Stephens, A.N. Autoantibodies against hsf1 and ccdc155 as biomarkers of early-stage, high-grade serous ovarian cancer. Cancer Epidemiol. Biomark. Prev. 2018, 27, 183–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hardwick, N.R.; Frankel, P.; Ruel, C.; Kilpatrick, J.; Tsai, W.; Kos, F.; Kaltcheva, T.; Leong, L.; Morgan, R.; Chung, V.; et al. P53-reactive t cells are associated with clinical benefit in patients with platinum-resistant epithelial ovarian cancer after treatment with a p53 vaccine and gemcitabine chemotherapy. Clin. Cancer Res. 2018, 24, 1315–1325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersen, M.H. Novel understanding of self-reactive t cells. Oncoimmunology 2015, 5, e1083672. [Google Scholar] [CrossRef] [Green Version]
- Ohashi, P.S.; DeFranco, A.L. Making and breaking tolerance. Curr. Opin. Immunol. 2002, 14, 744–759. [Google Scholar] [CrossRef]
- Jackson, S.R.; Yuan, J.; Berrien-Elliott, M.M.; Chen, C.L.; Meyer, J.M.; Donlin, M.J.; Teague, R.M. Inflammation programs self-reactive cd8+ t cells to acquire t-box-mediated effector function but does not prevent deletional tolerance. J. Leukoc. Biol. 2014, 96, 397–410. [Google Scholar] [CrossRef] [Green Version]
- Zaenker, P.; Lo, J.; Pearce, R.; Cantwell, P.; Cowell, L.; Lee, M.; Quirk, C.; Law, H.; Gray, E.; Ziman, M. A diagnostic autoantibody signature for primary cutaneous melanoma. Oncotarget 2018, 9, 30539–30551. [Google Scholar] [CrossRef] [Green Version]
- Chapman, C.J.; Thorpe, A.J.; Murray, A.; Parsy-Kowalska, C.B.; Allen, J.; Stafford, K.M.; Chauhan, A.S.; Kite, T.A.; Maddison, P.; Robertson, J.F. Immunobiomarkers in small cell lung cancer: Potential early cancer signals. Clin. Cancer Res. 2011, 17, 1474–1480. [Google Scholar] [CrossRef] [Green Version]
- Anderson, K.S.; Sibani, S.; Wallstrom, G.; Qiu, J.; Mendoza, E.A.; Raphael, J.; Hainsworth, E.; Montor, W.R.; Wong, J.; Park, J.G.; et al. Protein microarray signature of autoantibody biomarkers for the early detection of breast cancer. J. Proteome Res. 2011, 10, 85–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zayakin, P.; Ancans, G.; Silina, K.; Meistere, I.; Kalnina, Z.; Andrejeva, D.; Endzelins, E.; Ivanova, L.; Pismennaja, A.; Ruskule, A.; et al. Tumor-associated autoantibody signature for the early detection of gastric cancer. Int. J. Cancer 2013, 132, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.S.; Cramer, D.W.; Sibani, S.; Wallstrom, G.; Wong, J.; Park, J.; Qiu, J.; Vitonis, A.; LaBaer, J. Autoantibody signature for the serologic detection of ovarian cancer. J. Proteome Res. 2015, 14, 578–586. [Google Scholar] [CrossRef] [Green Version]
- Lambeck, A.; Leffers, N.; Hoogeboom, B.N.; Sluiter, W.; Hamming, I.; Klip, H.; ten Hoor, K.; Esajas, M.; van Oven, M.; Drijfhout, J.W.; et al. P53-specific t cell responses in patients with malignant and benign ovarian tumors: Implications for p53 based immunotherapy. Int. J. Cancer 2007, 121, 606–614. [Google Scholar] [CrossRef] [PubMed]
- AIHW. Cancer in Australia 2019; Australian Institute of Health and Welfare: Canberra, Australia, 2019.
- Masuda, K.; Shoji, H.; Nagashima, K.; Yamamoto, S.; Ishikawa, M.; Imazeki, H.; Aoki, M.; Miyamoto, T.; Hirano, H.; Honma, Y.; et al. Correlation between immune-related adverse events and prognosis in patients with gastric cancer treated with nivolumab. BMC Cancer 2019, 19, 974. [Google Scholar] [CrossRef] [PubMed]
- Xing, P.; Zhang, F.; Wang, G.; Xu, Y.; Li, C.; Wang, S.; Guo, Y.; Cai, S.; Wang, Y.; Li, J. Incidence rates of immune-related adverse events and their correlation with response in advanced solid tumours treated with nivo or nivo+ipi: A systematic review and meta-analysis. J. Immunother. Cancer 2019, 7, 341. [Google Scholar] [CrossRef]
- Sato, K.; Akamatsu, H.; Murakami, E.; Sasaki, S.; Kanai, K.; Hayata, A.; Tokudome, N.; Akamatsu, K.; Koh, Y.; Ueda, H.; et al. Correlation between immune-related adverse events and efficacy in non-small cell lung cancer treated with nivolumab. Lung Cancer 2018, 115, 71–74. [Google Scholar] [CrossRef] [Green Version]
- Horvat, T.Z.; Adel, N.G.; Dang, T.-O.; Momtaz, P.; Postow, M.A.; Callahan, M.K.; Carvajal, R.D.; Dickson, M.A.; D’Angelo, S.P.; Woo, K.M.; et al. Immune-related adverse events, need for systemic immunosuppression, and effects on survival and time to treatment failure in patients with melanoma treated with ipilimumab at memorial sloan kettering cancer center. J. Clin. Oncol. 2015, 33, 3193–3198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.T.; Jeong, H.; Woo, O.H.; Seo, J.H.; Kim, A.; Lee, E.S.; Shin, S.W.; Kim, Y.H.; Kim, J.S.; Park, K.H. Tumor-infiltrating lymphocytes, tumor characteristics, and recurrence in patients with early breast cancer. Am. J. Clin. Oncol. 2013, 36, 224–231. [Google Scholar] [CrossRef]
- Mony, J.T.; Schuchert, M.J. Prognostic implications of heterogeneity in intra-tumoral immune composition for recurrence in early stage lung cancer. Front. Immunol. 2018, 9, 2298. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Conejo-Garcia, J.R.; Katsaros, D.; Gimotty, P.A.; Massobrio, M.; Regnani, G.; Makrigiannakis, A.; Gray, H.; Schlienger, K.; Liebman, M.N.; et al. Intratumoral t cells, recurrence, and survival in epithelial ovarian cancer. N. Engl. J. Med. 2003, 348, 203–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, W.-T.; Adams, S.F.; Tahirovic, E.; Hagemann, I.S.; Coukos, G. Prognostic significance of tumor-infiltrating t cells in ovarian cancer: A meta-analysis. Gynecol. Oncol. 2012, 124, 192–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clarke, B.; Tinker, A.V.; Lee, C.H.; Subramanian, S.; van de Rijn, M.; Turbin, D.; Kalloger, S.; Han, G.; Ceballos, K.; Cadungog, M.G.; et al. Intraepithelial t cells and prognosis in ovarian carcinoma: Novel associations with stage, tumor type, and brca1 loss. Mod. Pathol. 2009, 22, 393–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voskoboinik, I.; Smyth, M.J.; Trapani, J.A. Perforin-mediated target-cell death and immune homeostasis. Nat. Rev. Immunol. 2006, 6, 940–952. [Google Scholar] [CrossRef] [PubMed]
- Lopez, J.A.; Susanto, O.; Jenkins, M.R.; Lukoyanova, N.; Sutton, V.R.; Law, R.H.; Johnston, A.; Bird, C.H.; Bird, P.I.; Whisstock, J.C.; et al. Perforin forms transient pores on the target cell plasma membrane to facilitate rapid access of granzymes during killer cell attack. Blood 2013, 121, 2659–2668. [Google Scholar] [CrossRef] [Green Version]
- Walankiewicz, M.; Grywalska, E.; Polak, G.; Kotarski, J.; Siwicka-Gieroba, D.J.; Roliński, J. Myeloid-derived suppressor cells in ovarian cancer: Friend or foe? Cent. -Eur. J. Immunol. 2017, 42, 383–389. [Google Scholar] [CrossRef] [Green Version]
- Curiel, T.J.; Coukos, G.; Zou, L.; Alvarez, X.; Cheng, P.; Mottram, P.; Evdemon-Hogan, M.; Conejo-Garcia, J.R.; Zhang, L.; Burow, M.; et al. Specific recruitment of regulatory t cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat. Med. 2004, 10, 942–949. [Google Scholar] [CrossRef]
- Chen, X.; Subleski, J.J.; Hamano, R.; Howard, O.M.; Wiltrout, R.H.; Oppenheim, J.J. Co-expression of tnfr2 and cd25 identifies more of the functional cd4+foxp3+ regulatory t cells in human peripheral blood. Eur. J. Immunol. 2010, 40, 1099–1106. [Google Scholar] [CrossRef] [Green Version]
- Taylor, A.; Verhagen, J.; Blaser, K.; Akdis, M.; Akdis, C.A. Mechanisms of immune suppression by interleukin-10 and transforming growth factor-beta: The role of t regulatory cells. Immunology 2006, 117, 433–442. [Google Scholar] [CrossRef]
- Viel, S.; Marcais, A.; Guimaraes, F.S.; Loftus, R.; Rabilloud, J.; Grau, M.; Degouve, S.; Djebali, S.; Sanlaville, A.; Charrier, E.; et al. Tgf-beta inhibits the activation and functions of nk cells by repressing the mtor pathway. Sci. Signal. 2016, 9, ra19. [Google Scholar] [CrossRef]
- Truxova, I.; Kasikova, L.; Hensler, M.; Skapa, P.; Laco, J.; Pecen, L.; Belicova, L.; Praznovec, I.; Halaska, M.J.; Brtnicky, T.; et al. Mature dendritic cells correlate with favorable immune infiltrate and improved prognosis in ovarian carcinoma patients. J. Immunother. Cancer 2018, 6, 139. [Google Scholar] [CrossRef] [PubMed]
- Hoogstad-van Evert, J.S.; Maas, R.J.; van der Meer, J.; Cany, J.; van der Steen, S.; Jansen, J.H.; Miller, J.S.; Bekkers, R.; Hobo, W.; Massuger, L.; et al. Peritoneal nk cells are responsive to il-15 and percentages are correlated with outcome in advanced ovarian cancer patients. Oncotarget 2018, 9, 34810–34820. [Google Scholar] [PubMed] [Green Version]
- Kroeger, D.R.; Milne, K.; Nelson, B.H. Tumor-infiltrating plasma cells are associated with tertiary lymphoid structures, cytolytic t-cell responses, and superior prognosis in ovarian cancer. Clin. Cancer Res. 2016, 22, 3005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milne, K.; Kobel, M.; Kalloger, S.E.; Barnes, R.O.; Gao, D.; Gilks, C.B.; Watson, P.H.; Nelson, B.H. Systematic analysis of immune infiltrates in high-grade serous ovarian cancer reveals cd20, foxp3 and tia-1 as positive prognostic factors. PLoS ONE 2009, 4, e6412. [Google Scholar] [CrossRef] [Green Version]
- Bevan, M.J. Helping the cd8(+) t-cell response. Nat. Rev. Immunol. 2004, 4, 595–602. [Google Scholar] [CrossRef]
- Zhu, J.; Paul, W.E. Cd4 t cells: Fates, functions, and faults. Blood 2008, 112, 1557–1569. [Google Scholar] [CrossRef] [Green Version]
- Sato, E.; Olson, S.H.; Ahn, J.; Bundy, B.; Nishikawa, H.; Qian, F.; Jungbluth, A.A.; Frosina, D.; Gnjatic, S.; Ambrosone, C.; et al. Intraepithelial cd8+ tumor-infiltrating lymphocytes and a high cd8+/regulatory t cell ratio are associated with favorable prognosis in ovarian cancer. Proc. Natl. Acad. Sci. USA 2005, 102, 18538–18543. [Google Scholar] [CrossRef] [Green Version]
- Lan, C.; Huang, X.; Lin, S.; Huang, H.; Cai, Q.; Wan, T.; Lu, J.; Liu, J. Expression of m2-polarized macrophages is associated with poor prognosis for advanced epithelial ovarian cancer. Technol. Cancer Res. Treat. 2013, 12, 259–267. [Google Scholar] [CrossRef] [Green Version]
- Horikawa, N.; Abiko, K.; Matsumura, N.; Hamanishi, J.; Baba, T.; Yamaguchi, K.; Yoshioka, Y.; Koshiyama, M.; Konishi, I. Expression of vascular endothelial growth factor in ovarian cancer inhibits tumor immunity through the accumulation of myeloid-derived suppressor cells. Clin. Cancer Res. 2017, 23, 587–599. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Deavers, M.; Patenia, R.; Bassett, R.L., Jr.; Mueller, P.; Ma, Q.; Wang, E.; Freedman, R.S. Monocyte/macrophage and t-cell infiltrates in peritoneum of patients with ovarian cancer or benign pelvic disease. J. Transl. Med. 2006, 4, 30. [Google Scholar] [CrossRef] [Green Version]
- Läubli, H.; Koelzer, V.H.; Matter, M.S.; Herzig, P.; Dolder Schlienger, B.; Wiese, M.N.; Lardinois, D.; Mertz, K.D.; Zippelius, A. The t cell repertoire in tumors overlaps with pulmonary inflammatory lesions in patients treated with checkpoint inhibitors. Oncoimmunology 2017, 7, e1386362. [Google Scholar] [CrossRef]
- Johnson, D.B.; Balko, J.M.; Compton, M.L.; Chalkias, S.; Gorham, J.; Xu, Y.; Hicks, M.; Puzanov, I.; Alexander, M.R.; Bloomer, T.L.; et al. Fulminant myocarditis with combination immune checkpoint blockade. N. Engl. J. Med. 2016, 375, 1749–1755. [Google Scholar] [CrossRef] [PubMed]
- Disis, M.L.; Wallace, D.R.; Gooley, T.A.; Dang, Y.; Slota, M.; Lu, H.; Coveler, A.L.; Childs, J.S.; Higgins, D.M.; Fintak, P.A.; et al. Concurrent trastuzumab and her2/neu-specific vaccination in patients with metastatic breast cancer. J. Clin. Oncol. 2009, 27, 4685–4692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antonia, S.J.; Mirza, N.; Fricke, I.; Chiappori, A.; Thompson, P.; Williams, N.; Bepler, G.; Simon, G.; Janssen, W.; Lee, J.H.; et al. Combination of p53 cancer vaccine with chemotherapy in patients with extensive stage small cell lung cancer. Clin. Cancer Res. 2006, 12, 878–887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, R.; Zaman, S.; Sharpe, S.; Helenowski, I.; Shaw, C.; Han, H.; Soliman, H.; Czerniecki, B. A brief report of toxicity end points of her2 vaccines for the treatment of patients with her2(+) breast cancer. Drug Des. Dev. Ther. 2019, 13, 309–316. [Google Scholar] [CrossRef] [Green Version]
- Gadducci, A.; Ferdeghini, M.; Buttitta, F.; Cosio, S.; Fanucchi, A.; Annicchiarico, C.; Gagetti, O.; Bevilacqua, G.; Genazzani, A.R. Assessment of the prognostic relevance of serum anti-p53 antibodies in epithelial ovarian cancer. Gynecol. Oncol. 1999, 72, 76–81. [Google Scholar] [CrossRef]
- Peoples, G.E.; Goedegebuure, P.S.; Smith, R.; Linehan, D.C.; Yoshino, I.; Eberlein, T.J. Breast and ovarian cancer-specific cytotoxic t lymphocytes recognize the same her2/neu-derived peptide. Proc. Natl. Acad. Sci. USA 1995, 92, 432. [Google Scholar] [CrossRef] [Green Version]
- Linehan, D.C.; Peoples, G.E.; Hess, D.T.; Summerhayes, I.C.; Parikh, A.S.; Goedegebuure, P.S.; Eberlein, T.J. In vitro stimulation of ovarian tumour-associated lymphocytes with a peptide derived from her2/neu induces cytotoxicity against autologous tumour. Surg. Oncol. 1995, 4, 41–49. [Google Scholar] [CrossRef]
- Chu, C.S.; Boyer, J.; Schullery, D.S.; Gimotty, P.A.; Gamerman, V.; Bender, J.; Levine, B.L.; Coukos, G.; Rubin, S.C.; Morgan, M.A.; et al. Phase i/ii randomized trial of dendritic cell vaccination with or without cyclophosphamide for consolidation therapy of advanced ovarian cancer in first or second remission. Cancer Immunol. Immunother. 2012, 61, 629–641. [Google Scholar] [CrossRef]
- Bowen, W.S.; Svrivastava, A.K.; Batra, L.; Barsoumian, H.; Shirwan, H. Current challenges for cancer vaccine adjuvant development. Expert Rev. Vaccines 2018, 17, 207–215. [Google Scholar] [CrossRef]
- Bookman, M.A.; Brady, M.F.; McGuire, W.P.; Harper, P.G.; Alberts, D.S.; Friedlander, M.; Colombo, N.; Fowler, J.M.; Argenta, P.A.; De Geest, K.; et al. Evaluation of new platinum-based treatment regimens in advanced-stage ovarian cancer: A phase iii trial of the gynecologic cancer intergroup. J. Clin. Oncol. 2009, 27, 1419–1425. [Google Scholar] [CrossRef] [PubMed]
- Pomel, C.; Jeyarajah, A.; Oram, D.; Shepherd, J.; Milliken, D.; Dauplat, J.; Reynolds, K. Cytoreductive surgery in ovarian cancer. Cancer Imaging 2007, 7, 210–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trimbos, J.B. Surgical treatment of early-stage ovarian cancer. Best Pract. Res. Clin. Obstet. Gynaecol. 2017, 41, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Stenvers, K.L. Getting to know ovarian cancer ascites: Opportunities for targeted therapy-based translational research. Front. Oncol. 2013, 3, 256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooke, S.L.; Brenton, J.D. Evolution of platinum resistance in high-grade serous ovarian cancer. Lancet Oncol. 2011, 12, 1169–1174. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, S.; Yang, Y.; Liu, T. Resveratrol induces immunogenic cell death of human and murine ovarian carcinoma cells. Infect. Agent Cancer 2019, 14, 27. [Google Scholar] [CrossRef] [Green Version]
- Zong, X.; Nephew, K.P. Ovarian cancer stem cells: Role in metastasis and opportunity for therapeutic targeting. Cancers 2019, 11, 934. [Google Scholar] [CrossRef] [Green Version]
- Pieterse, Z.; Amaya-Padilla, M.A.; Singomat, T.; Binju, M.; Madjid, B.D.; Yu, Y.; Kaur, P. Ovarian cancer stem cells and their role in drug resistance. Int. J. Biochem. Cell Biol. 2019, 106, 117–126. [Google Scholar] [CrossRef]
- Chihara, D.; Fanale, M.A.; Miranda, R.N.; Noorani, M.; Westin, J.R.; Nastoupil, L.J.; Hagemeister, F.B.; Fayad, L.E.; Romaguera, J.E.; Samaniego, F.; et al. The survival outcome of patients with relapsed/refractory peripheral t-cell lymphoma-not otherwise specified and angioimmunoblastic t-cell lymphoma. Br. J. Haematol. 2017, 176, 750–758. [Google Scholar] [CrossRef] [Green Version]
- Rockberg, J.; Amelio, J.M.; Taylor, A.; Jorgensen, L.; Ragnhammar, P.; Hansson, J. Epidemiology of cutaneous melanoma in sweden-stage-specific survival and rate of recurrence. Int. J. Cancer 2016, 139, 2722–2729. [Google Scholar] [CrossRef]
- Prat, J.; Oncology, F.C.o.G. Figo’s staging classification for cancer of the ovary, fallopian tube, and peritoneum: Abridged republication. J. Gynecol. Oncol. 2015, 26, 87–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez, G.M.; Galpin, K.J.C.; McCloskey, C.W.; Vanderhyden, B.C. The tumor microenvironment of epithelial ovarian cancer and its influence on response to immunotherapy. Cancers 2018, 10, 242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González-Martín, A.; Sánchez-Lorenzo, L. Immunotherapy with checkpoint inhibitors in patients with ovarian cancer: Still promising? Cancer 2019, 125, 4616–4622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hellmann, M.D.; Callahan, M.K.; Awad, M.M.; Calvo, E.; Ascierto, P.A.; Atmaca, A.; Rizvi, N.A.; Hirsch, F.R.; Selvaggi, G.; Szustakowski, J.D.; et al. Tumor mutational burden and efficacy of nivolumab monotherapy and in combination with ipilimumab in small-cell lung cancer. Cancer Cell 2018, 33, 853–861.e854. [Google Scholar] [CrossRef]
- Rizvi, N.A.; Hellmann, M.D.; Snyder, A.; Kvistborg, P.; Makarov, V.; Havel, J.J.; Lee, W.; Yuan, J.; Wong, P.; Ho, T.S.; et al. Cancer immunology. Mutational landscape determines sensitivity to pd-1 blockade in non-small cell lung cancer. Science 2015, 348, 124–128. [Google Scholar] [CrossRef] [Green Version]
- Goodman, A.M.; Kato, S.; Bazhenova, L.; Patel, S.P.; Frampton, G.M.; Miller, V.; Stephens, P.J.; Daniels, G.A.; Kurzrock, R. Tumor mutational burden as an independent predictor of response to immunotherapy in diverse cancers. Mol. Cancer Ther. 2017, 16, 2598–2608. [Google Scholar] [CrossRef] [Green Version]
- Alexandrov, L.B.; Nik-Zainal, S.; Wedge, D.C.; Aparicio, S.A.; Behjati, S.; Biankin, A.V.; Bignell, G.R.; Bolli, N.; Borg, A.; Borresen-Dale, A.L.; et al. Signatures of mutational processes in human cancer. Nature 2013, 500, 415–421. [Google Scholar] [CrossRef] [Green Version]
- Rouleau, M.; Patel, A.; Hendzel, M.J.; Kaufmann, S.H.; Poirier, G.G. Parp inhibition: Parp1 and beyond. Nat. Rev. Cancer 2010, 10, 293–301. [Google Scholar] [CrossRef] [Green Version]
- Sen, T.; Rodriguez, B.L.; Chen, L.; Corte, C.M.D.; Morikawa, N.; Fujimoto, J.; Cristea, S.; Nguyen, T.; Diao, L.; Li, L.; et al. Targeting DNA damage response promotes antitumor immunity through sting-mediated t-cell activation in small cell lung cancer. Cancer Discov. 2019, 9, 646–661. [Google Scholar] [CrossRef] [Green Version]
- Ding, L.; Kim, H.J.; Wang, Q.; Kearns, M.; Jiang, T.; Ohlson, C.E.; Li, B.B.; Xie, S.; Liu, J.F.; Stover, E.H.; et al. Parp inhibition elicits sting-dependent antitumor immunity in brca1-deficient ovarian cancer. Cell Rep. 2018, 25, 2972–2980.e2975. [Google Scholar] [CrossRef] [Green Version]
- Shen, J.; Zhao, W.; Ju, Z.; Wang, L.; Peng, Y.; Labrie, M.; Yap, T.A.; Mills, G.B.; Peng, G. Parpi triggers the sting-dependent immune response and enhances the therapeutic efficacy of immune checkpoint blockade independent of brcaness. Cancer Res. 2019, 79, 311–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higuchi, T.; Flies, D.B.; Marjon, N.A.; Mantia-Smaldone, G.; Ronner, L.; Gimotty, P.A.; Adams, S.F. Ctla-4 blockade synergizes therapeutically with parp inhibition in brca1-deficient ovarian cancer. Cancer Immunol. Res. 2015, 3, 1257–1268. [Google Scholar] [CrossRef] [Green Version]
- McCann, K.E.; Hurvitz, S.A. Advances in the use of parp inhibitor therapy for breast cancer. Drugs Context 2018, 7, 212540. [Google Scholar] [CrossRef]
- Eckschlager, T.; Plch, J.; Stiborova, M.; Hrabeta, J. Histone deacetylase inhibitors as anticancer drugs. Int. J. Mol. Sci. 2017, 18, 1414. [Google Scholar] [CrossRef]
- Mishra, N.; Reilly, C.M.; Brown, D.R.; Ruiz, P.; Gilkeson, G.S. Histone deacetylase inhibitors modulate renal disease in the mrl-lpr/lpr mouse. J. Clin. Investig. 2003, 111, 539–552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magner, W.J.; Kazim, A.L.; Stewart, C.; Romano, M.A.; Catalano, G.; Grande, C.; Keiser, N.; Santaniello, F.; Tomasi, T.B. Activation of mhc class i, ii, and cd40 gene expression by histone deacetylase inhibitors. J. Immunol. 2000, 165, 7017. [Google Scholar] [CrossRef] [PubMed]
- Skov, S.; Pedersen, M.T.; Andresen, L.; Thor Straten, P.; Woetmann, A.; Ødum, N. Cancer cells become susceptible to natural killer cell killing after exposure to histone deacetylase inhibitors due to glycogen synthase kinase-3–dependent expression of mhc class i–related chain a and b. Cancer Res. 2005, 65, 11136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bandolik, J.J.; Hamacher, A.; Schrenk, C.; Weishaupt, R.; Kassack, M.U. Class i-histone deacetylase (hdac) inhibition is superior to pan-hdac inhibition in modulating cisplatin potency in high grade serous ovarian cancer cell lines. Int. J. Mol. Sci. 2019, 20, 3052. [Google Scholar] [CrossRef] [Green Version]
- Desai, V.; Bhushan, A. Natural bioactive compounds: Alternative approach to the treatment of glioblastoma multiforme. Biomed. Res. Int. 2017, 2017, 9363040. [Google Scholar] [CrossRef] [Green Version]
- Vuong, Q.V.; Hirun, S.; Phillips, P.A.; Chuen, T.L.; Bowyer, M.C.; Goldsmith, C.D.; Scarlett, C.J. Fruit-derived phenolic compounds and pancreatic cancer: Perspectives from australian native fruits. J. Ethnopharmacol. 2014, 152, 227–242. [Google Scholar] [CrossRef]
- Sudhakaran, M.; Sardesai, S.; Doseff, A.I. Flavonoids: New frontier for immuno-regulation and breast cancer control. Antioxidants 2019, 8, 103. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.G.; Kim, H.; Liu, C.; Yu, S.; Wang, J.; Grizzle, W.E.; Kimberly, R.P.; Barnes, S. Curcumin reverses breast tumor exosomes mediated immune suppression of nk cell tumor cytotoxicity. Biochim. Biophys. Acta 2007, 1773, 1116–1123. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Sun, P.; Wang, Q.; Zhang, P.; Wang, Y.; Zi, C.; Wang, X.; Sheng, J. (-)-epigallocatechin-3-gallate derivatives combined with cisplatin exhibit synergistic inhibitory effects on non-small-cell lung cancer cells. Cancer Cell Int. 2019, 19, 266. [Google Scholar] [CrossRef]
- Mokbel, K.; Wazir, U.; Mokbel, K. Chemoprevention of prostate cancer by natural agents: Evidence from molecular and epidemiological studies. Anticancer. Res. 2019, 39, 5231–5259. [Google Scholar] [CrossRef] [Green Version]
- Mileo, A.M.; Nistico, P.; Miccadei, S. Polyphenols: Immunomodulatory and therapeutic implication in colorectal cancer. Front. Immunol. 2019, 10, 729. [Google Scholar] [CrossRef]
- Rejhova, A.; Opattova, A.; Cumova, A.; Sliva, D.; Vodicka, P. Natural compounds and combination therapy in colorectal cancer treatment. Eur. J. Med. Chem. 2018, 144, 582–594. [Google Scholar] [CrossRef]
- Chinembiri, T.N.; du Plessis, L.H.; Gerber, M.; Hamman, J.H.; du Plessis, J. Review of natural compounds for potential skin cancer treatment. Molecules 2014, 19, 11679–11721. [Google Scholar] [CrossRef] [Green Version]
- Ijaz, S.; Akhtar, N.; Khan, M.S.; Hameed, A.; Irfan, M.; Arshad, M.A.; Ali, S.; Asrar, M. Plant derived anticancer agents: A green approach towards skin cancers. Biomed. Pharm. 2018, 103, 1643–1651. [Google Scholar] [CrossRef] [PubMed]
- Bill, M.A.; Bakan, C.; Benson, D.M., Jr.; Fuchs, J.; Young, G.; Lesinski, G.B. Curcumin induces proapoptotic effects against human melanoma cells and modulates the cellular response to immunotherapeutic cytokines. Mol. Cancer Ther. 2009, 8, 2726–2735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The immunomodulatory and anti-inflammatory role of polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giada, M. Food Phenolic Compounds: Main Classes, Sources and Their Antioxidant Power, Oxidative Stress and Chronic Degenerative Diseases – A Role for Antioxidants. IntechOpen 2013, 87–112. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.J.; Deng, G.F.; Xu, X.R.; Wu, S.; Li, S.; Xia, E.Q.; Li, F.; Chen, F.; Ling, W.H.; Li, H.B. Antioxidant capacities, phenolic compounds and polysaccharide contents of 49 edible macro-fungi. Food Funct. 2012, 3, 1195–1205. [Google Scholar] [CrossRef]
- Ahn, W.S.; Kim, D.J.; Chae, G.T.; Lee, J.M.; Bae, S.M.; Sin, J.I.; Kim, Y.W.; Namkoong, S.E.; Lee, I.P. Natural killer cell activity and quality of life were improved by consumption of a mushroom extract, agaricus blazei murill kyowa, in gynecological cancer patients undergoing chemotherapy. Int. J. Gynecol. Cancer 2004, 14, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Wang, J.; Pae, M.; Meydani, S.N. Green tea egcg, t cells, and t cell-mediated autoimmune diseases. Mol. Asp. Med. 2012, 33, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Jung, Y.O.; Ryu, J.G.; Oh, H.J.; Son, H.J.; Lee, S.H.; Kwon, J.E.; Kim, E.K.; Park, M.K.; Park, S.H.; et al. Epigallocatechin-3-gallate ameliorates autoimmune arthritis by reciprocal regulation of t helper-17 regulatory t cells and inhibition of osteoclastogenesis by inhibiting stat3 signaling. J. Leukoc. Biol. 2016, 100, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Negri, A.; Naponelli, V.; Rizzi, F.; Bettuzzi, S. Molecular targets of epigallocatechin-gallate (egcg): A special focus on signal transduction and cancer. Nutrients 2018, 10, 1936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malaguarnera, L. Influence of resveratrol on the immune response. Nutrients 2019, 11, 946. [Google Scholar] [CrossRef] [Green Version]
- Czop, M.; Bogucka-Kocka, A.; Kubrak, T.; Knap-Czop, K.; Makuch-Kocka, A.; Galkowski, D.; Wawer, J.; Kocki, T.; Kocki, J. Imaging flow cytometric analysis of stilbene-dependent apoptosis in drug resistant human leukemic cell lines. Molecules 2019, 24, 1896. [Google Scholar] [CrossRef] [Green Version]
- Pan, J.; Shen, J.; Si, W.; Du, C.; Chen, D.; Xu, L.; Yao, M.; Fu, P.; Fan, W. Resveratrol promotes mica/b expression and natural killer cell lysis of breast cancer cells by suppressing c-myc/mir-17 pathway. Oncotarget 2017, 8, 65743–65758. [Google Scholar] [CrossRef] [Green Version]
- Noh, K.T.; Chae, S.H.; Chun, S.H.; Jung, I.D.; Kang, H.K.; Park, Y.M. Resveratrol suppresses tumor progression via the regulation of indoleamine 2,3-dioxygenase. Biochem. Biophys. Res. Commun. 2013, 431, 348–353. [Google Scholar] [CrossRef]
- Jeong, S.K.; Yang, K.; Park, Y.S.; Choi, Y.J.; Oh, S.J.; Lee, C.W.; Lee, K.Y.; Jeong, M.H.; Jo, W.S. Interferon gamma induced by resveratrol analog, hs-1793, reverses the properties of tumor associated macrophages. Int. Immunopharmacol. 2014, 22, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Lee-Chang, C.; Bodogai, M.; Martin-Montalvo, A.; Wejksza, K.; Sanghvi, M.; Moaddel, R.; de Cabo, R.; Biragyn, A. Inhibition of breast cancer metastasis by resveratrol-mediated inactivation of tumor-evoked regulatory b cells. J. Immunol. 2013, 191, 4141–4151. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Paik, J.H.; Cho, D.; Cho, J.A.; Kim, C.W. Resveratrol induces the suppression of tumor-derived cd4+cd25+ regulatory t cells. Int. Immunopharmacol. 2008, 8, 542–547. [Google Scholar] [CrossRef] [PubMed]
- Pan, P.; Huang, Y.W.; Oshima, K.; Yearsley, M.; Zhang, J.; Arnold, M.; Yu, J.; Wang, L.S. The immunomodulatory potential of natural compounds in tumor-bearing mice and humans. Crit. Rev. Food Sci. Nutr. 2019, 59, 992–1007. [Google Scholar] [CrossRef] [PubMed]
- Jagetia, G.C.; Aggarwal, B.B. "Spicing up" of the immune system by curcumin. J. Clin. Immunol. 2007, 27, 19–35. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Lee, H.G.; Choi, J.M. Curcumin elevates tfh cells and germinal center b cell response for antibody production in mice. Immune Netw. 2019, 19, e35. [Google Scholar] [CrossRef]
- Focaccetti, C.; Izzi, V.; Benvenuto, M.; Fazi, S.; Ciuffa, S.; Giganti, M.G.; Potenza, V.; Manzari, V.; Modesti, A.; Bei, R. Polyphenols as immunomodulatory compounds in the tumor microenvironment: Friends or foes? Int. J. Mol. Sci. 2019, 20, 1714. [Google Scholar] [CrossRef] [Green Version]
- Bahrami, A.; Fereidouni, M.; Pirro, M.; Bianconi, V.; Sahebkar, A. Modulation of regulatory t cells by natural products in cancer. Cancer Lett. 2019, 459, 72–85. [Google Scholar] [CrossRef]
- Ou, Y.; Cannon, M.J.; Nakagawa, M. Regulatory t cells in gynecologic cancer. MOJ Immunol. 2018, 6, 34–42. [Google Scholar] [CrossRef]
- Kampan, N.C.; Madondo, M.T.; McNally, O.M.; Stephens, A.N.; Quinn, M.A.; Plebanski, M. Interleukin 6 present in inflammatory ascites from advanced epithelial ovarian cancer patients promotes tumor necrosis factor receptor 2-expressing regulatory t cells. Front. Immunol. 2017, 8, 1482. [Google Scholar] [CrossRef]
- Govindaraj, C.; Scalzo-Inguanti, K.; Madondo, M.; Hallo, J.; Flanagan, K.; Quinn, M.; Plebanski, M. Impaired th1 immunity in ovarian cancer patients is mediated by tnfr2+tregs within the tumor microenvironment. Clin. Immunol. 2013, 149, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Shafabakhsh, R.; Pourhanifeh, M.H.; Mirzaei, H.R.; Sahebkar, A.; Asemi, Z.; Mirzaei, H. Targeting regulatory t cells by curcumin: A potential for cancer immunotherapy. Pharm. Res. 2019, 147, 104353. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.Y.; Su, C.H.; Luo, H.H.; Lei, Y.Y.; Zeng, B.; Zhu, H.S.; Chen, Z.G. Curcumin converts foxp3+ regulatory t cells to t helper 1 cells in patients with lung cancer. J. Cell Biochem. 2018, 119, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Yu, L.; Zhao, L.Z. Curcumin up regulates t helper 1 cells in patients with colon cancer. Am. J. Transl. Res. 2017, 9, 1866–1875. [Google Scholar] [PubMed]
- Liao, F.; Liu, L.; Luo, E.; Hu, J. Curcumin enhances anti-tumor immune response in tongue squamous cell carcinoma. Arch. Oral Biol. 2018, 92, 32–37. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Md Sakib Hossain, D.; Mohanty, S.; Sankar Sen, G.; Chattopadhyay, S.; Banerjee, S.; Chakraborty, J.; Das, K.; Sarkar, D.; Das, T.; et al. Curcumin reverses t cell-mediated adaptive immune dysfunctions in tumor-bearing hosts. Cell. Mol. Immunol. 2010, 7, 306–315. [Google Scholar] [CrossRef]
- D’Arena, G.; Simeon, V.; De Martino, L.; Statuto, T.; D’Auria, F.; Volpe, S.; Deaglio, S.; Maidecchi, A.; Mattoli, L.; Mercati, V.; et al. Regulatory t-cell modulation by green tea in chronic lymphocytic leukemia. Int. J. Immunopathol. Pharm. 2013, 26, 117–125. [Google Scholar] [CrossRef]
- Liu, L.; Ju, Y.; Wang, J.; Zhou, R. Epigallocatechin-3-gallate promotes apoptosis and reversal of multidrug resistance in esophageal cancer cells. Pathol. Res. Pract. 2017, 213, 1242–1250. [Google Scholar] [CrossRef]
- Wubetu, G.Y.; Shimada, M.; Morine, Y.; Ikemoto, T.; Ishikawa, D.; Iwahashi, S.; Yamada, S.; Saito, Y.; Arakawa, Y.; Imura, S. Epigallocatechin gallate hinders human hepatoma and colon cancer sphere formation. J. Gastroenterol. Hepatol. 2016, 31, 256–264. [Google Scholar] [CrossRef]
- Kim, Y.W.; Bae, S.M.; Lee, J.M.; Namkoong, S.E.; Han, S.J.; Lee, B.R.; Lee, I.P.; Kim, S.H.; Lee, Y.J.; Kim, C.K.; et al. Activity of green tea polyphenol epigallocatechin-3-gallate against ovarian carcinoma cell lines. Cancer Res. Treat. 2004, 36, 315–323. [Google Scholar] [CrossRef]
- Huh, S.W.; Bae, S.M.; Kim, Y.W.; Lee, J.M.; Namkoong, S.E.; Lee, I.P.; Kim, S.H.; Kim, C.K.; Ahn, W.S. Anticancer effects of (-)-epigallocatechin-3-gallate on ovarian carcinoma cell lines. Gynecol. Oncol. 2004, 94, 760–768. [Google Scholar] [CrossRef] [PubMed]
- Suganuma, M.; Saha, A.; Fujiki, H. New cancer treatment strategy using combination of green tea catechins and anticancer drugs. Cancer Sci. 2011, 102, 317–323. [Google Scholar] [CrossRef]
- Rawangkan, A.; Wongsirisin, P.; Namiki, K.; Iida, K.; Kobayashi, Y.; Shimizu, Y.; Fujiki, H.; Suganuma, M. Green tea catechin is an alternative immune checkpoint inhibitor that inhibits pd-l1 expression and lung tumor growth. Molecules 2018, 23, 2071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, B.N.; Shankar, S.; Srivastava, R.K. Green tea catechin, epigallocatechin-3-gallate (egcg): Mechanisms, perspectives and clinical applications. Biochem. Pharm. 2011, 82, 1807–1821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henning, S.M.; Wang, P.; Carpenter, C.L.; Heber, D. Epigenetic effects of green tea polyphenols in cancer. Epigenomics 2013, 5, 729–741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, J.Y.; Lee, J.K.; Jeon, Y.K.; Kim, C.W. Exosome derived from epigallocatechin gallate treated breast cancer cells suppresses tumor growth by inhibiting tumor-associated macrophage infiltration and m2 polarization. BMC Cancer 2013, 13, 421. [Google Scholar] [CrossRef] [Green Version]
- Pae, M.; Wu, D. Immunomodulating effects of epigallocatechin-3-gallate from green tea: Mechanisms and applications. Food Funct. 2013, 4, 1287–1303. [Google Scholar] [CrossRef]
- Mukherjee, S.; Baidoo, J.N.E.; Sampat, S.; Mancuso, A.; David, L.; Cohen, L.S.; Zhou, S.; Banerjee, P. Liposomal tricurin, a synergistic combination of curcumin, epicatechin gallate and resveratrol, repolarizes tumor-associated microglia/macrophages, and eliminates glioblastoma (gbm) and gbm stem cells. Molecules 2018, 23, 201. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Miao, L.; Wang, Y.; Xu, Z.; Zhao, Y.; Shen, Y.; Xiang, G.; Huang, L. Curcumin micelles remodel tumor microenvironment and enhance vaccine activity in an advanced melanoma model. Mol. Ther. 2016, 24, 364–374. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Tian, W.; Cai, X.; Wang, X.; Dang, W.; Tang, H.; Cao, H.; Wang, L.; Chen, T. Hydrazinocurcumin encapsuled nanoparticles "re-educate" tumor-associated macrophages and exhibit anti-tumor effects on breast cancer following stat3 suppression. PLoS ONE 2013, 8, e65896. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Zhang, X.; Tian, W.; Feng, W.; Chen, T. The curcumin analogue hydrazinocurcumin exhibits potent suppressive activity on carcinogenicity of breast cancer cells via stat3 inhibition. Int. J. Oncol. 2012, 40, 1189–1195. [Google Scholar] [CrossRef] [PubMed]
- Refat, M.S. Synthesis and characterization of ligational behavior of curcumin drug towards some transition metal ions: Chelation effect on their thermal stability and biological activity. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 105, 326–337. [Google Scholar] [CrossRef] [PubMed]
- Davatgaran-Taghipour, Y.; Masoomzadeh, S.; Farzaei, M.H.; Bahramsoltani, R.; Karimi-Soureh, Z.; Rahimi, R.; Abdollahi, M. Polyphenol nanoformulations for cancer therapy: Experimental evidence and clinical perspective. Int. J. Nanomed. 2017, 12, 2689–2702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tyagi, N.; De, R.; Begun, J.; Popat, A. Cancer therapeutics with epigallocatechin-3-gallate encapsulated in biopolymeric nanoparticles. Int. J. Pharm 2017, 518, 220–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Granja, A.; Pinheiro, M.; Reis, S. Epigallocatechin gallate nanodelivery systems for cancer therapy. Nutrients 2016, 8, 307. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, I.A.; Bharali, D.J.; Nihal, M.; Adhami, V.M.; Khan, N.; Chamcheu, J.C.; Khan, M.I.; Shabana, S.; Mousa, S.A.; Mukhtar, H. Excellent anti-proliferative and pro-apoptotic effects of (-)-epigallocatechin-3-gallate encapsulated in chitosan nanoparticles on human melanoma cell growth both in vitro and in vivo. Nanomedicine 2014, 10, 1619–1626. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Peng, Y.; Li, Y.; Yao, J.; Zhang, G.; Chen, J.; Wang, J.; Sui, L. Cell death pathway induced by resveratrol-bovine serum albumin nanoparticles in a human ovarian cell line. Oncol. Lett. 2015, 9, 1359–1363. [Google Scholar] [CrossRef] [Green Version]
- Cock, I.E.; Kukkonen, L. An examination of the medicinal potential of scaevola spinescens: Toxicity, antibacterial, and antiviral activities. Pharmacogn. Res. 2011, 3, 85–94. [Google Scholar] [CrossRef] [Green Version]
- Tang, K.S.; Konczak, I.; Zhao, J. Identification and quantification of phenolics in australian native mint (mentha australis r. Br.). Food Chem. 2016, 192, 698–705. [Google Scholar] [CrossRef]
- Konczak, I.; Roulle, P. Nutritional properties of commercially grown native australian fruits: Lipophilic antioxidants and minerals. Food Res. Int. 2011, 44, 2339–2344. [Google Scholar] [CrossRef]
- Sommano, S.; Caffin, N.; Kerven, G. Screening for antioxidant activity, phenolic content, and flavonoids from australian native food plants. Int. J. Food Prop. 2013, 16, 1394–1406. [Google Scholar] [CrossRef] [Green Version]
- Tan, A.C.; Konczak, I.; Ramzan, I.; Sze, D.M. Native australian fruit polyphenols inhibit cell viability and induce apoptosis in human cancer cell lines. Nutr. Cancer 2011, 63, 444–455. [Google Scholar] [CrossRef] [PubMed]
- Netzel, M.; Netzel, G.; Tian, Q.; Schwartz, S.; Konczak, I. Sources of antioxidant activity in australian native fruits. Identification and quantification of anthocyanins. J. Agric. Food Chem. 2006, 54, 9820–9826. [Google Scholar] [CrossRef]
- Konczak, I.; Zabaras, D.; Dunstan, M.; Aguas, P. Antioxidant capacity and phenolic compounds in commercially grown native australian herbs and spices. Food Chem. 2010, 122, 260–266. [Google Scholar] [CrossRef]
- Guo, Y.; Sakulnarmrat, K.; Konczak, I. Anti-inflammatory potential of native australian herbs polyphenols. Toxicol. Rep. 2014, 1, 385–390. [Google Scholar] [CrossRef] [Green Version]
- Cock, I.E.; Winnett, V.; Sirdaarta, J.; Matthews, B. The potential of selected australian medicinal plants with anti-proteus activity for the treatment and prevention of rheumatoid arthritis. Pharm. Mag. 2015, 11, S190–S208. [Google Scholar] [CrossRef] [Green Version]
- Sakulnarmrat, K.; Fenech, M.; Thomas, P.; Konczak, I. Cytoprotective and pro-apoptotic activities of native australian herbs polyphenolic-rich extracts. Food Chem. 2013, 136, 9–17. [Google Scholar] [CrossRef]
- Tan, A.C.; Konczak, I.; Ramzan, I.; Zabaras, D.; Sze, D.M. Potential antioxidant, antiinflammatory, and proapoptotic anticancer activities of kakadu plum and illawarra plum polyphenolic fractions. Nutr. Cancer 2011, 63, 1074–1084. [Google Scholar] [CrossRef]
- Symonds, E.L.; Konczak, I.; Fenech, M. The australian fruit illawarra plum (podocarpus elatus endl., podocarpaceae) inhibits telomerase, increases histone deacetylase activity and decreases proliferation of colon cancer cells. Br. J. Nutr. 2013, 109, 2117–2125. [Google Scholar] [CrossRef] [Green Version]
- Tan, A.C.; Hou, D.-X.; Konczak, I.; Tanigawa, S.; Ramzan, I.; Sze, D.M.Y. Native australian fruit polyphenols inhibit cox-2 and inos expression in lps-activated murine macrophages. Food Res. Int. 2011, 44, 2362–2367. [Google Scholar] [CrossRef]
- Tan, A.C.; Konczak, I.; Sze, D.M.Y.; Ramzan, I. Molecular pathways for cancer chemoprevention by dietary phytochemicals. Nutr. Cancer 2011, 63, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Parhiz, H.; Roohbakhsh, A.; Soltani, F.; Rezaee, R.; Iranshahi, M. Antioxidant and anti-inflammatory properties of the citrus flavonoids hesperidin and hesperetin: An updated review of their molecular mechanisms and experimental models. Phytother. Res. 2015, 29, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.-Y.; Park, J.; Han, Y.-S.; Lee, Y.H.; Shin, S.Y.; Lim, Y. Synthesis and biological evaluation of hesperetin derivatives as agents inducing apoptosis. Bioorganic Med. Chem. 2017, 25, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Palit, S.; Kar, S.; Sharma, G.; Das, P.K. Hesperetin induces apoptosis in breast carcinoma by triggering accumulation of ros and activation of ask1/jnk pathway. J. Cell. Physiol. 2015, 230, 1729–1739. [Google Scholar] [CrossRef]
- Wu, D.; Zhang, J.; Wang, J.; Li, J.; Liao, F.; Dong, W. Hesperetin induces apoptosis of esophageal cancer cells via mitochondrial pathway mediated by the increased intracellular reactive oxygen species. Tumour Biol. 2016, 37, 3451–3459. [Google Scholar] [CrossRef]
- Wolfram, J.; Scott, B.; Boom, K.; Shen, J.; Borsoi, C.; Suri, K.; Grande, R.; Fresta, M.; Celia, C.; Zhao, Y.; et al. Hesperetin liposomes for cancer therapy. Curr. Drug Deliv. 2016, 13, 711–719. [Google Scholar] [CrossRef]
- Semwal, D.K.; Semwal, R.B.; Combrinck, S.; Viljoen, A. Myricetin: A dietary molecule with diverse biological activities. Nutrients 2016, 8, 90. [Google Scholar] [CrossRef] [Green Version]
- Shiomi, K.; Kuriyama, I.; Yoshida, H.; Mizushina, Y. Inhibitory effects of myricetin on mammalian DNA polymerase, topoisomerase and human cancer cell proliferation. Food Chem. 2013, 139, 910–918. [Google Scholar] [CrossRef]
- Ko, C.H.; Shen, S.C.; Lee, T.J.; Chen, Y.C. Myricetin inhibits matrix metalloproteinase 2 protein expression and enzyme activity in colorectal carcinoma cells. Mol. Cancer Ther. 2005, 4, 281–290. [Google Scholar]
- Knickle, A.; Fernando, W.; Greenshields, A.L.; Rupasinghe, H.P.V.; Hoskin, D.W. Myricetin-induced apoptosis of triple-negative breast cancer cells is mediated by the iron-dependent generation of reactive oxygen species from hydrogen peroxide. Food Chem. Toxicol. 2018, 118, 154–167. [Google Scholar] [CrossRef]
- Ghassemi-Rad, J.; Maleki, M.; Knickle, A.F.; Hoskin, D.W. Myricetin-induced oxidative stress suppresses murine t lymphocyte activation. Cell Biol. Int. 2018, 42, 1069–1075. [Google Scholar] [CrossRef]
- Cho, Y.C.; Yoon, G.; Lee, K.Y.; Choi, H.J.; Kang, B.Y. Inhibition of interleukin-2 production by myricetin in mouse el-4 t cells. Arch. Pharm. Res. 2007, 30, 1075–1079. [Google Scholar] [CrossRef]
- Kang, B.Y.; Kim, S.H.; Cho, D.; Kim, T.S. Inhibition of interleukin-12 production in mouse macrophages via decreased nuclear factor-kappab DNA binding activity by myricetin, a naturally occurring flavonoid. Arch. Pharm. Res. 2005, 28, 274–279. [Google Scholar] [CrossRef]
- Fu, R.-H.; Liu, S.-P.; Chu, C.-L.; Lin, Y.-H.; Ho, Y.-C.; Chiu, S.-C.; Lin, W.-Y.; Shyu, W.-C.; Lin, S.-Z. Myricetin attenuates lipopolysaccharide-stimulated activation of mouse bone marrow-derived dendritic cells through suppression of ikk/nf-κb and mapk signalling pathways. J. Sci. Food Agric. 2013, 93, 76–84. [Google Scholar] [CrossRef]
- Khan, F.; Niaz, K.; Maqbool, F.; Ismail Hassan, F.; Abdollahi, M.; Nagulapalli Venkata, K.C.; Nabavi, S.M.; Bishayee, A. Molecular targets underlying the anticancer effects of quercetin: An update. Nutrients 2016, 8, 529. [Google Scholar] [CrossRef]
- Dihal, A.A.; de Boer, V.C.J.; van der Woude, H.; Tilburgs, C.; Bruijntjes, J.P.; Alink, G.M.; Rietjens, I.M.C.M.; Woutersen, R.A.; Stierum, R.H. Quercetin, but not its glycosidated conjugate rutin, inhibits azoxymethane-induced colorectal carcinogenesis in f344 rats. J. Nutr. 2006, 136, 2862–2867. [Google Scholar] [CrossRef]
- Khanduja, K.L.; Gandhi, R.K.; Pathania, V.; Syal, N. Prevention of n-nitrosodiethylamine-induced lung tumorigenesis by ellagic acid and quercetin in mice. Food Chem. Toxicol. 1999, 37, 313–318. [Google Scholar] [CrossRef]
- Kim, H.; Moon, J.Y.; Ahn, K.S.; Cho, S.K. Quercetin induces mitochondrial mediated apoptosis and protective autophagy in human glioblastoma u373mg cells. Oxid. Med. Cell. Longev. 2013, 2013, 596496. [Google Scholar] [CrossRef]
- Maurya, A.K.; Vinayak, M. Anticarcinogenic action of quercetin by downregulation of phosphatidylinositol 3-kinase (pi3k) and protein kinase c (pkc) via induction of p53 in hepatocellular carcinoma (hepg2) cell line. Mol. Biol. Rep. 2015, 42, 1419–1429. [Google Scholar] [CrossRef]
- Duo, J.; Ying, G.G.; Wang, G.W.; Zhang, L. Quercetin inhibits human breast cancer cell proliferation and induces apoptosis via bcl-2 and bax regulation. Mol. Med. Rep. 2012, 5, 1453–1456. [Google Scholar]
- Nair, M.P.; Kandaswami, C.; Mahajan, S.; Chadha, K.C.; Chawda, R.; Nair, H.; Kumar, N.; Nair, R.E.; Schwartz, S.A. The flavonoid, quercetin, differentially regulates th-1 (ifngamma) and th-2 (il4) cytokine gene expression by normal peripheral blood mononuclear cells. Biochim. Biophys. Acta 2002, 1593, 29–36. [Google Scholar] [CrossRef] [Green Version]
- Kandere-Grzybowska, K.; Kempuraj, D.; Cao, J.; Cetrulo, C.L.; Theoharides, T.C. Regulation of il-1-induced selective il-6 release from human mast cells and inhibition by quercetin. Br. J. Pharm. 2006, 148, 208–215. [Google Scholar] [CrossRef] [Green Version]
- Huang, R.Y.; Yu, Y.L.; Cheng, W.C.; OuYang, C.N.; Fu, E.; Chu, C.L. Immunosuppressive effect of quercetin on dendritic cell activation and function. J. Immunol. 2010, 184, 6815–6821. [Google Scholar] [CrossRef] [Green Version]
- Lu, X. Impact of il-12 in cancer. Curr. Cancer Drug Targets 2017, 17, 682–697. [Google Scholar] [CrossRef]
- Waters, J.P.; Pober, J.S.; Bradley, J.R. Tumour necrosis factor and cancer. J. Pathol. 2013, 230, 241–248. [Google Scholar] [CrossRef]
- Gerspach, J.; Pfizenmaier, K.; Wajant, H. Improving tnf as a cancer therapeutic: Tailor-made tnf fusion proteins with conserved antitumor activity and reduced systemic side effects. Biofactors 2009, 35, 364–372. [Google Scholar] [CrossRef]
- Lasek, W.; Zagozdzon, R.; Jakobisiak, M. Interleukin 12: Still a promising candidate for tumor immunotherapy? Cancer Immunol. Immunother. 2014, 63, 419–435. [Google Scholar] [CrossRef] [Green Version]
- Ding, M.; Feng, R.; Wang, S.Y.; Bowman, L.; Lu, Y.; Qian, Y.; Castranova, V.; Jiang, B.H.; Shi, X. Cyanidin-3-glucoside, a natural product derived from blackberry, exhibits chemopreventive and chemotherapeutic activity. J. Biol. Chem. 2006, 281, 17359–17368. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Li, S.; Zhang, G.; Wu, H.; Chang, X. Potential therapeutic effects of cyanidin-3-o-glucoside on rheumatoid arthritis by relieving inhibition of cd38+ nk cells on treg cell differentiation. Arthritis Res. Ther. 2019, 21, 220. [Google Scholar] [CrossRef] [Green Version]
- Kotecha, R.; Takami, A.; Espinoza, J.L. Dietary phytochemicals and cancer chemoprevention: A review of the clinical evidence. Oncotarget 2016, 7, 52517–52529. [Google Scholar] [CrossRef] [Green Version]
- Rahman, I.; Chung, S. Dietary polyphenols, deacetylases and chromatin remodeling in inflammation. World Rev. Nutr. Diet. 2010, 101, 84–94. [Google Scholar] [PubMed]
- Panda, A.K.; Chakraborty, D.; Sarkar, I.; Khan, T.; Sa, G. New insights into therapeutic activity and anticancer properties of curcumin. J. Exp. Pharm. 2017, 9, 31–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ko, J.H.; Sethi, G.; Um, J.Y.; Shanmugam, M.K.; Arfuso, F.; Kumar, A.P.; Bishayee, A.; Ahn, K.S. The role of resveratrol in cancer therapy. Int. J. Mol. Sci. 2017, 18, 2589. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, S.; Hussaini, R.; White, R.; Atwi, D.; Fried, A.; Sampat, S.; Piao, L.; Pan, Q.; Banerjee, P. Tricurin, a synergistic formulation of curcumin, resveratrol, and epicatechin gallate, repolarizes tumor-associated macrophages and triggers an immune response to cause suppression of hpv+ tumors. Cancer Immunol. Immunother. 2018, 67, 761–774. [Google Scholar] [CrossRef]
- Guldiken, B.; Ozkan, G.; Catalkaya, G.; Ceylan, F.D.; Ekin Yalcinkaya, I.; Capanoglu, E. Phytochemicals of herbs and spices: Health versus toxicological effects. Food Chem. Toxicol. 2018, 119, 37–49. [Google Scholar] [CrossRef]
- Williams, D.J.; Edwards, D.; Pun, S.; Chaliha, M.; Burren, B.; Tinggi, U.; Sultanbawa, Y. Organic acids in kakadu plum (terminalia ferdinandiana): The good (ellagic), the bad (oxalic) and the uncertain (ascorbic). Food Res. Int. 2016, 89, 237–244. [Google Scholar] [CrossRef] [Green Version]
| Common Name | Botanical Name | Known Therapeutic Potential | Phenolic Compounds Identified | Refs |
|---|---|---|---|---|
| Kakadu plum | Terminalia ferdinandiana | Antioxidant and induces apoptosis and inhibits proliferation in cancer cell lines | Catechin Naringenin Quercetin/hesperitin glucosides Kaempferol/luteolin glycosides | [164,165] |
| Illawara plum | Podocarpus elatus | Antioxidant and induces apoptosis and inhibits proliferation in cancer cell lines | Cyanidin 3-glucoside pelargonidin 3-glucoside | [165,166] |
| Davidson’s plum | Davidsonia pruriens | Antioxidant | Naringenin Hesperetin delphinidin 3-sambubioside cyanidin 3-sambubioside peonidin 3-sambubioside petunidin 3-sambubioside | [164,166] |
| Native river mint | Mentha australis | Antioxidant | Neoponcirin Rosmarinic acid Narirutin Chlorogenic acid Biochanin A | [162] |
| Muntries | Kunzea pomifera | Antioxidant and induces apoptosis and inhibits proliferation in cancer cell lines | Delphinidin 3-glucoside cyanidin 3-glucoside | [165,166] |
| Tasmanian pepper berry | Tasmannia lanceolata | Antioxidant | Cyanidin 3-rutinoside Cyanidin 3-glucoside Rutin Chlorogenic acid Caffeic acid Quercetin | [166,167] |
| Tasmanian pepper leaf | Tasmannia lanceolata | Antioxidant | Chlorogenic acid Quercetin p-Coumaric acid Cyanidin 3-glucoside | [167] |
| Anise myrtle | Syzygium anisatum | Antioxidant and anti-inflammatory | Chlorogenic acid Myricetin Quercetin Quercetin pentoside Ellagic acid Ellagic acid derivatives Catechin Hesperetin | [167,168] |
| Lemon myrtle | Backhousia citriodora | Antioxidant and anti-inflammatory | Catechin Epicatechin Vanilic acid Myricetin Hesperetin rhamnoside Hesperetin hexoside Quercetin Ellagic acid Ellagic acid derivatives | [164,167,168] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moody, R.; Wilson, K.; Jaworowski, A.; Plebanski, M. Natural Compounds with Potential to Modulate Cancer Therapies and Self-Reactive Immune Cells. Cancers 2020, 12, 673. https://doi.org/10.3390/cancers12030673
Moody R, Wilson K, Jaworowski A, Plebanski M. Natural Compounds with Potential to Modulate Cancer Therapies and Self-Reactive Immune Cells. Cancers. 2020; 12(3):673. https://doi.org/10.3390/cancers12030673
Chicago/Turabian StyleMoody, Rhiane, Kirsty Wilson, Anthony Jaworowski, and Magdalena Plebanski. 2020. "Natural Compounds with Potential to Modulate Cancer Therapies and Self-Reactive Immune Cells" Cancers 12, no. 3: 673. https://doi.org/10.3390/cancers12030673
APA StyleMoody, R., Wilson, K., Jaworowski, A., & Plebanski, M. (2020). Natural Compounds with Potential to Modulate Cancer Therapies and Self-Reactive Immune Cells. Cancers, 12(3), 673. https://doi.org/10.3390/cancers12030673






